Abstract
Endocannabinoid sex differences are present in the rat hippocampus. Specifically, at perisomatic GABAergic synapses, tonic anandamide (AEA) and estrogenic-AEA signaling are active in females but not males. Furthermore, in males, hippocampal eCB function varies along the CA1 pyramidal somatodendritic axis. Constitutive CB1 and tonic 2-AG activity are present at perisomatic GABAergic synapses and lacking at dendritic GABAergic synapses. It is unknown if these eCB somatodendritic differences occur at female GABAergic synapses. Moreover, it is unclear whether eCB sex differences occur at hippocampal glutamatergic synapses. In vitro, field potential (fEPSP) recordings were performed to assess eCB sex differences at rat CA3-CA1 dendritic synapses. At female GABAergic synapses, we observed: 1) constitutive CB1 function, 2) tonic AEA, 3) tonic 2-AG and 3) estrogen (ERα)-driven 2-AG activity. In contrast, only constitutive CB1 and tonic 2-AG activity was observed in males. Sex differences in eCB/CB1 signaling at dendritic synapses appear to shift the basal excitatory/inhibitory balance towards excitation in females and towards inhibition in males. Chronic Mild Stress (CMS) exposure (21 days) in female rats reverses CB1constitutive function and impairs both tonic and ERα-driven eCB signaling. Endocannabinoid sex differences under both normal and stress conditions may contribute to sexual disparities in stress-related neurobehavioral disorders.
Keywords: CB1 receptor/female//hippocampus/estrogen
1. Introduction
The endocannabinoids (eCBs), N-arachidonylethanolamine (anandamide, AEA) and 2-Arachidonylglycerol (2-AG), modulate a variety of behavioral phenomena including learning, memory, cognition, mood, stress and anxiety (Lee et al., 2015; McEwen et al., 2015; Moreira and Wotjak, 2010). These lipid messengers are particularly involved in the processing of context-dependent emotional valence (Lee et al., 2015; McEwen et al., 2015; Moreira and Wotjak, 2010). eCBs exert much of their action through the cannabinoid receptor (CB1), the most abundant G-protein-coupled receptor in the central nervous system. It is densely located in many brain areas associated with the limbic system, such as the hippocampus, cortex, amygdala, basal ganglia, nucleus accumbens, the hypothalamus and throughout the Hypothalamic-Pituitary-Adrenal axis (HPA). This widespread influence in both the limbic system and HPA axis implicates eCB signaling in the pathophysiology of stress-related mental disorders such as Major Depressive Disorder and Post-Traumatic Stress Disorder (Lee et al., 2015; McEwen et al., 2015; Moreira and Wotjak, 2010). Prevalence rates for these disorders are twice as high for women than men (Kessler et al, 2010, 2012) and eCB sex differences are associated with this disparity (Reich et al., 2009; Rubino and Parlolaro, 2011). Thus, understanding the neurobiology of eCB sex differences may provide insight into the etiology of sexual disparities in stress-related mental disorders.
Studies in both humans and animals report a general trend whereby males possess greater CB1 densities than females in most cortical areas (Gorzalka and Dang, 2012; Rubino and Parlolaro, 2011). Female CB1 receptors may, however, function more efficiently as evidenced by increased G-protein activation compared to male CB1 (Craft et al., 2013; Gorzalka and Dang, 2012; Rubino and Parlolaro, 2011). Interestingly, the difference in the hippocampus is reversed following exposure to chronic stress with male CB1 levels decreasing and female levels increasing (Reich et al., 2009); suggesting that the eCB system is organized in male and female animals to respond differentially to chronic stress. Furthermore, sex-specific effects of exogenous cannabinoid exposure during adolescence appear to produce longer lasting effects on emotional behavior in adult females compared to male rodents with overlapping data in humans (Craft et al., 2013; Rubino and Parolaro, 2011, 2015).
Despite a growing literature supporting sex differences in the eCB system, few studies have addressed how this difference translates neurophysiologically. The most notable discovery is an estrogen-AEA pathway that suppresses GABA release from perisomatic CB1 containing interneurons in the female rat hippocampus (Huang and Woolley, 2012; Tabatadze et al., 2015). Estrogenic-AEA production is mediated via a sex-specific ERα-mGLuR1 pathway (Tabatadze et al., 2015). Furthermore, females show both tonic AEA and 2-AG (Huang and Woolley, 2012; Tabatadze et al., 2015) activity at CA1 perisomatic synapses whereas males show tonic 2-AG signaling only (Huang and Woolley, 2012; Lee et al., 2010, 2015; Tabatadze et al., 2015). Additionally, CB1 is persistently active at perisomatic CCK-GABAergic-CA1 pyramidal cell synapses in male rats and mice and in female rats (Huang and Woolley, 2012; Lee et al., 2010, 2015; Tabatadze et al., 2015).
In male rats and mice, CA1 eCB function varies along the somatodendritic axis. At perisomatic GABAergic synapses, CB1 is constitutively active and tonic 2-AG activity is present; whereas neither of these occur at dendritic GABAergic synapses (Lee et al., 2010, 2015). Phasic 2-AG signaling also is more prominent at perisomatic synapses than those on the dendrite, although tonic AEA activity does not occur at either synapse in males (Lee et al., 2010, 2015). Moreover, CB1 is present on both GABAergic and glutamatergic terminals in CA1 Stratum Radiatum (SR). In contrast, perisomatic synapses are almost exclusively GABAergic (Kano, 2014; Younts and Castillo, 2014). Given that male CA1 eCB signaling is distinct between dendritic and perisomatic compartments, we hypothesize that 1) eCB signaling varies along the somatodendritic axis in females and 2) that eCB signaling sex differences are present at dendritic synapses as well as at perisomatic synapses.
Exposure to a 21 day CMS protocol in male rats alters CB1-mediated excitatory/inhibitory balance by increasing CB1 function on GABAergic terminals, while sparing glutamatergic CB1 function at dendritic synapses (Reich et al., 2013b). The observations that CMS also downregulates CB1 (Reich et al., 2009), suggests that CB1 function is inversely proportional to receptor density. Because CMS exposure upregulates CB1 in female rats (Reich et al., 2009), we also hypothesize that CMS may lead to the reversal of normal CB1/eCB function at female dendritic synapses.
2. Materials and methods
2.1. Subjects
Female (67) and male (15) Sprague-Dawley rats (Charles River, Boston, MA) were group-caged (3 per cage) and allowed to acclimate and handled for a minimum of 7 days prior to experimental testing. All animals were 40–65 days old at the beginning of neurophysiological investigations and maintained on a 12-h/12-h light–dark cycle with lights on at 8:00 am Food and water were available ad libitum in the home cages, unless otherwise noted. All experimental procedures were carried out in accordance with protocols established by the Institutional Animal Care and Use Committee of the Ramapo College of New Jersey.
2.2. Electrophysiology
Animals were deeply anesthetized with halothane and decapitated from approximately 9:00 a.m.-11:00 a.m. Stress animals were sacrificed between 14 and 42 h following the last stressor. The brain was rapidly removed and hippocampi dissected. Transverse hippocampal slices, 400 μM thick, were cut on a Vibrotome (Leica-Microsystems). Slices were kept in a holding chamber at room temperature at the interface of artificial cerebrospinal fluid (ACSF) and a humidified 95/5% O2/CO2 atmosphere for >1 h. The slices were then transferred to a submerged recording chamber and perfused with warm (30 °C) ACSF: (mM), NaCl, 120; KCl, 3; MgSO4, 2; NaH2PO4, 1; NaHCO3, 25; CaCl2, 2.5; and glucose, 10 and saturated with 95% O2–5% CO2 (pH 7.4). Field potentials (fEPSPs) were recorded from Stratum Radiatum (SR) of CA1 with glass microelectrodes (tip diameter ~4–8 μM) filled with extracellular saline. Stimuli were delivered via a bipolar stimulating electrode located in SR, between CA3 and CA1. Signals were recorded with an amplifier (Model 773, WPI Inc. or Model IE-251A, Warner Instruments Inc.), digitized at 10 kHz with an AD interface (Digidata 1440A, Axon Instruments or PCI-6259, National Instruments) and analyzed with either pClamp 10.0 (Axon Instruments) or WinLTP software. Only fEPSPs that could achieve or exceed initial amplitude of 1.0 mV were recorded. Stable baseline responses (10 min) were recorded prior to experimental manipulation. During pharmacological experiments, fEPSPs were adjusted to ~0.8 mV prior to beginning a baseline. Following the baseline period, drugs were bath-applied for 20–45 min or delivered continuously throughout the entire experiment.
2.3. Data analysis
The initial slope of the fEPSP (mV/ms) was measured within the first 2 ms of the response immediately after the negative peak of the fiber volley. To standardize responses across experimental conditions, all slope measurements within a given experiment were divided by the average baseline response (10 min). Then, individual slice experiments within each experiment were averaged across each 0.33 s time point (i.e. data were averaged into 0.33 s bins). The listed sample sizes (n) represent the number of slices per experiment. No more than 3 slices per animal were used for a given experiment, however many rats serviced two or more types of experiments. For statistical analyses, the mean baseline value (last 5 min; fifteen consecutive responses) and the mean experimental value (last 5 min; fifteen consecutive responses) were computed from the averaged time-binned group data. All data are reported as mean ± SEM (fifteen consecutive responses) (Reich et al., 2013b). Statistical comparisons were performed using one-way, two-way mixed repeated measures ANOVAs and student t-tests, p = 0.05 (SPSS).
2.4. Pharmacological treatment
R-(+)-(2,3-dihydro-5- methyl-3-[(4-morpholinyl)methyl]pyrol [1,2,3-de]-1,4-benzoxazin-6-yl)[1-napht- halenyl] (WIN55,212-2), N-(Piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl- 1H-pyrazole-3-carboxamide (AM251), Cyclohexyl Carbamic acid 3'-(Aminocarbonyl) - [1,1' -biphenyl]-3-yl ester (URB597), 4-[Bis(1,3-benzodioxol-5-yl) hydroxymethyl]-1- piperidinecarboxylic acid 4-nitrophenyl ester (JZL184), 4,4′,4''-(4-Propyl- [1H]-pyrazole -1,3,5-triyl) trisphenol (PPT), (17β)-Estra-1,3,5(10)-triene-3,17-diol (17-β Estradiol, E2), Picrotoxin (PTX), (E)-N-[(4-Hydroxy-3-methoxyphenyl)methyl] -8- methyl-6-nonenamide (Capsaicin), 8-chloro-1- (2,4-dichlorophenyl)-N-piperidin-1-yl-5, 6-dihydro-4H-benzo2,3]cyclohepta[2,4-b] pyrazole-3-carboxamine (NESS0327) and 2-Benzyl-4-{[(2-methyl-2-propanyl)oxy]carbonyl}piperazinyl){4-[(4-trifluoromethoxy)phenyl]-1H-1,2,3-triazol-1-yl}methanone,3-(Phenylmethyl)-4-[[4-[4(trifluoromethoxy)phenyl]-1H-1,2,3-triazol-1-yl]carbonyl]-1-piperazinecarboxylic acid, 1,1-dimethylethyl ester (DO34) were dissolved in dimethyl sulfoxide (DMSO) and prepared from stock solutions prior to being diluted in ACSF (1:1000) to achieve final concentrations. Final DMSO concentrations in extracellular saline did not exceed 0.1%. All drugs except NESS0327 and THL were purchased from Tocris Cookson Inc. (St. Louis, Mo.). NESS0327 was purchased from Cayman Chemicals (Ann Arbor, MI) and DO34 was purchased from AOBIOUS (Gloucester, MA).
2.5. Chronic mild stress protocol (CMS)
A subgroup of female rats was subjected to either the CMS protocol or the non-stress protocol (handled daily). Rats began CMS between 33 and 40 days old. Each day, 1–3 stressors were administered according to a set schedule. The complete regimen lasted 7 days/wk for 3 wks. This protocol is modeled after Willner (2005) in that no individual stressor is considered severe but that the unpredictability of the protocol constitutes much of the stress. The stressors were: 1) 5 min swim in 20 °C water, 2) cage rotation (social stress), 3) 18-hr social isolation with damp bedding, 4) 14-hr food deprivation, 14-hr water deprivation or 14-hr food and water deprivation, 5) 30 min physical restraint, 6) 30 min strobe light exposure and 7) 3-hr cage tilt. In previous studies, we observed that this CMS protocol resulted in decreased body weight gain in both male and female animals and reduced sucrose preference in male animals (Reich et al., 2009, 2013a, 2013b). These effects are in accordance with the published behavioral effects of CMS protocols (Willner, 2005).
3. Results
3.1. CB1 receptor activation increases excitatory neurotransmission in adolescent female rats
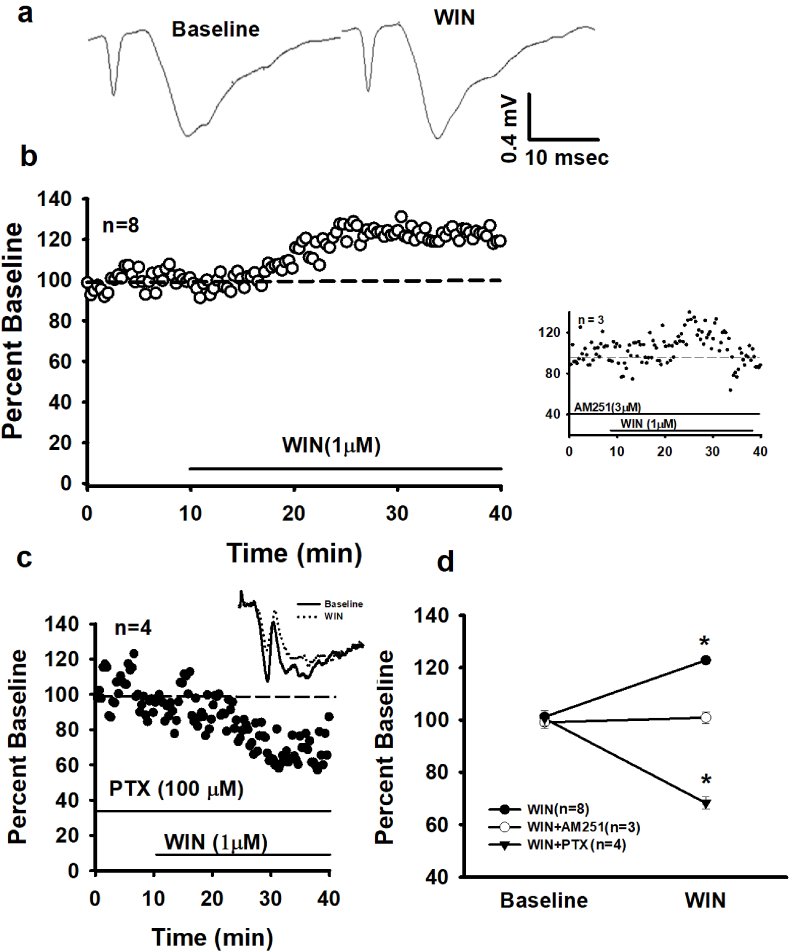
A 30 min WIN55, 212-2 (WIN, 1 μM) bath application significantly increases female CA1 fEPSPs compared to baseline activity (122.81 ± 0.88% vs. 101.18 ± 0.93%, respectively; F (1, 14) = 350.87, p < 0.00; Fig. 1a, b, d). This WIN-induced increase in female fEPSPs starkly contrasts to the consistently observed WIN decrease (~35%) in male CA1 fEPSPs (Hoffman et al., 2010; Reich et al., 2013b; Takahashi and Castillo, 2006). The WIN-induced increase was blocked by continuous application of the CB1 antagonist/inverse agonist, AM251 (3 μM); baseline: 99.06 ± 2.29% vs WIN + AM251: 100.86 ± 2.20%, F (1, 28) = 70.39, p < 0.00. See Fig. 1b, d. To isolate glutamatergic and GABAergic CB1 function, the WIN experiments are repeated in the continuous presence of the GABA (A) antagonist, Picrotoxin (PTX, 100 μM). A one-way rmANOVA reveals a significant fEPSP decrease (~30%) in the presence of WIN + PTX compared to the PTX baseline condition (F (1, 14) = 181.78, p < 0.00, Fig. 1c and d). Thus, CB1 activation on CA1 glutamatergic terminals is classically suppressing glutamate neurotransmission in females. These findings suggest that the WIN-induced fEPSP increase in females involves CB1-mediated suppression of GABAergic neurotransmission; thereby shifting CA1 dendritic excitatory/inhibitory balance in favor of excitation.
Fig. 1.
CB1 activation at female CA1 dendritic synapses enhances fEPSPs. a) Raw traces from a single experiment and b) time course of averaged experiments show WIN (1 μM) significantly increasing female fEPSPs. Inset: AM251 blocks the WIN-induced increase. c) WIN in the presence of picrotoxin (PTX 100 μM) decreases female fEPSPs, revealing CB1 function on glutamatergic terminals. d) Summary of the WIN, WIN + AM251 and WIN + PTX experiments comparing mean ± SEM baseline responses (last 5 min of the baseline period) and mean ± SEM post-WIN responses (last 5 min of the experiment). Each n = number of slices per experiment. Each * indicates significant differences from baseline responses (p ≤ 0.05), one-way rmANOVAs.
3.2. Sex differences in dendritic constitutive CB1 activity
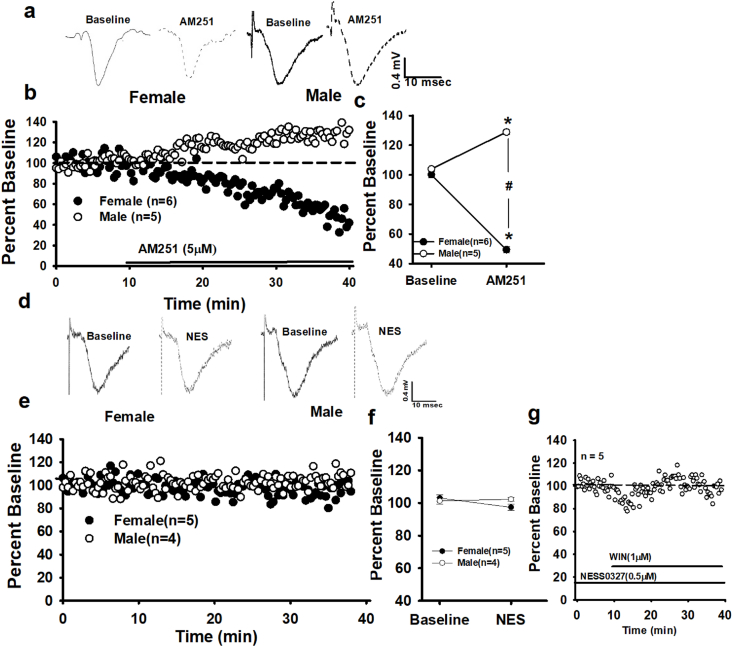
A 30 min bath application of the CB1 inverse agonist, AM251 (5 μM), decreased fEPSPs (49.42 ± 2.24% vs 100.07 ± 2.20%, baseline) in female slices while increasing male fEPSPs (128.87 ± 1.63% vs 103.98 ± 1.00%, baseline). See Fig. 2 a-c. A mixed, two-way rmANOVA (sex x time) shows a main effect of time, in that AM251 significantly changed fEPSP magnitudes compared to baseline responses (F (1, 28) = 30.53, p < 0.00). Importantly, there is an interaction effect between females and males (F (1, 28) = 456.54, p < 0.00; see Fig. 2c). Thus, persistent CB1 activity is present in both female and male rat CA1 dendritic synapses. This activity may result from the receptor's own constitutive conformations, tonic ligand binding or a combination of both (Lee et al., 2015). To differentiate AM251's inverse agonistic and antagonistic actions, the neutral CB1 antagonist, NESS0327 (NES, 0.5 μM) (Lee et al., 2015), was bath-applied (30 min) to both male and female slices. As observed by Lee et al., NES did not significantly affect fEPSPs in males (baseline: 101.26 ± 2.14% vs. NES: 102.22 ± 1.28% (n = 3); p = 0.10, paired t-test; Fig. 2 d-f). Nor did NES significantly affect female fEPSPs (baseline: 102.98 ± 1.98% vs. NES: 97.30 ± 1.91% (n = 5); p = 0.09, paired t-test; Fig. 2 d-f). Moreover, female slices preincubated and continuously perfused with NES blocked the effect of WIN (NES_baseline: 99.24 ± 1.31% vs. NES + WIN: 93.07 ± 1.45% (n = 5); p = 0.17, paired t-test; Fig. 2 g); demonstrating NES as an effective CB1 antagonist. AM251's sexually divergent effects on fEPSPs are inversely proportional to the WIN-based sex differences on fEPSP magnitudes, suggesting distinct CB1 physiologies between males and females. Specifically, reversing CB1 activity at GABAergic synapses in females presumably increases GABA neurotransmission leading to a decreased fEPSP. However, in males, the increase in fEPSP magnitude suggests that constitutive CB1 activity at dendritic glutamatergic synapses is more salient than CB1 function at GABAergic synapses.
Fig. 2.
Sex differences in CB1 constitutive activity at CA1 dendritic synapses. a) Raw traces from single experiments and b) time course of averaged experiments show that the CB1 inverse agonist/antagonist AM251 (1 μM) decreases female fEPSPs while increasing male fEPSPs. c) Summary of the AM251 experiments comparing mean ± SEM baseline responses (last 5 min of the baseline period) and mean ± SEM post-AM251 responses (last 5 min of the experiment). Each * indicates significant differences from baseline responses; #s indicate significant differences between females and males (p ≤ 0.05), mixed, two-way rmANOVA). d) Raw traces from single experiments and e) time course of averaged experiments show the CB1 silent antagonist NESS0327 (0.5 μM) does not affect females or males. f) Summary of the NESS0327 experiments. g) NESS0327 blocks the WIN effect in female slices. Each n = number of slices per experiment.
3.3. Sex differences in dendritic tonic AEA activity
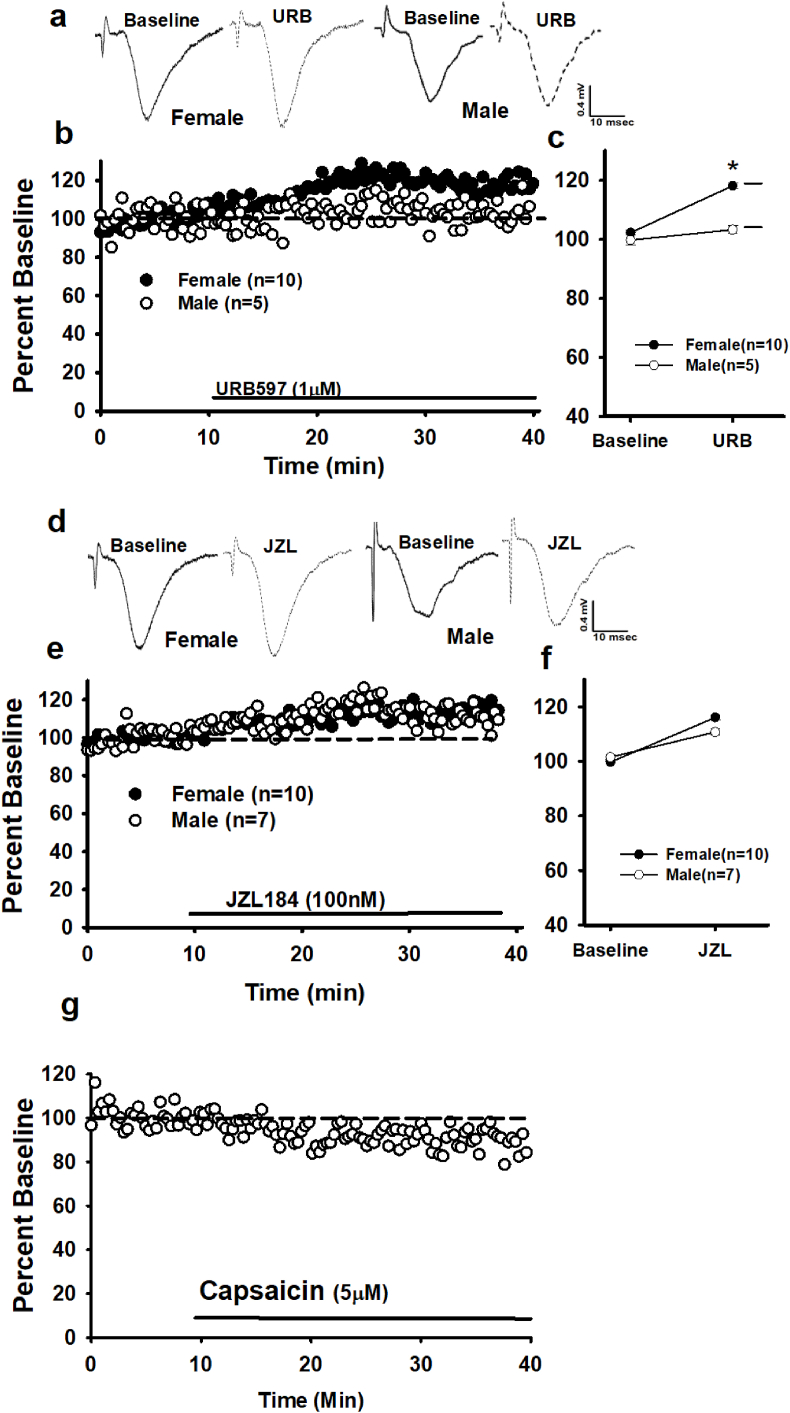
Despite the evidence of constitutive CB1 activity, tonic ligand signaling also may contribute to persistent CB1 function. To test for presence of a basal AEA tone at female and male dendritic synapses, AEA degradation was inhibited. A two-way rmANOVA (sex x time) shows that a 30 min application of the fatty-acid-amide-hydrolase (FAAH) inhibitor, URB597 (URB, 1 μM), significantly increases female fEPSPs (118.14 ± 0.95% vs. 102.22 ± 0.80%, baseline), but not in males (103.37.14 ± 1.27% vs. 99.71 ± 1.61%, baseline), F (1, 28) = 20.63, p < 0.00, Fig. 3a–c. The increased fEPSP magnitude in females suggests constitutive AEA activation of CB1 contributes to a tonic suppression of neurotransmission from SCA (CCK)-GABAergic interneurons. However, AEA is a full agonist at the transient-receptor-potential, TRPV1 (Kano, 2014; Lee et al., 2015); thus tonic AEA signaling may act via TRPV1 rather than CB1. A 30 min application of the TRPV1 agonist, capsaicin (1 μM), significantly decreased female fEPSPs (89.64 ± 1.36% vs 100.03 ± 1.03% -baseline, n = 5; t (14) = 7.20, p < 0.00, Student's paired t-test, Fig. 3g). This observation indicates that in females direct TRPV1 activation decreases dendritic glutamatergic neurotransmission, further suggesting that tonic AEA acts via CB1.
Fig. 3.
Sex differences in tonic eCB activity at CA1 dendritic synapses. a) Raw traces from single experiments and b) time course of averaged experiments demonstrate that FAAH inhibition (URB597, 1 μM) increases female but not male fEPSP, thus revealing tonic AEA activity at female synapses. c) Summary of the URB experiments comparing mean ± SEM baseline responses (last 5 min of the baseline period) and mean ± SEM post-URB responses (last 5 min of the experiment). *s indicate significant differences from baseline responses; #s indicate significant differences between females and males (p ≤ 0.05), mixed, two-way rmANOVA). d) Raw traces from single experiments and e) time course of averaged experiments showing increases in both female and male fEPSPs following MAGL inhibition (JZL184, 100 nM). f) Summary of the JZL experiments. g) Activation of TRPV1 with Capsaicin (5 μM) decreases female fEPSPs. n = number of slices per experiment. Each * indicates significant differences from baseline responses; #s indicate significant differences between females and males (p ≤ 0.05), mixed, two-way rmANOVA.
3.4. Dendritic tonic 2-AG activity is present in both males and females
The monoacylglycerol lipase (MAGL) inhibitor, JZL184 (JZL, 100 nm) was applied to female and male slices to assess the presences of tonic 2-AG activity. JZL significantly increases fEPSPs in both females (116.08 ± 0.93% vs. 99.72 ± 0.53%, baseline) and males (110.76 ± 1.15% vs. 101.54 ± 0.84%, baseline); F (1, 28) = 291.05, p = 0.000. A significant interaction effect (F (1, 28) = 22.78, p = 0.000) suggests that JZL had a stronger effect on fEPSPs in females compared to males. See Fig. 3d–f. Our observations of tonic 2-AG signaling in the female dendritic layer is consistent with both AEA and 2-AG tonic activity at female perisomatic synapses (Huang and Woolley, 2012; Tabatadze et al., 2015), although tonic 2-AG in males contradicts previous research (Lee et al., 2010, 2015). Potential reasons for this discrepancy are addressed in the Discussion.
3.5. Dendritic estrogen receptor alpha (ERα) drives 2-AG signaling in females
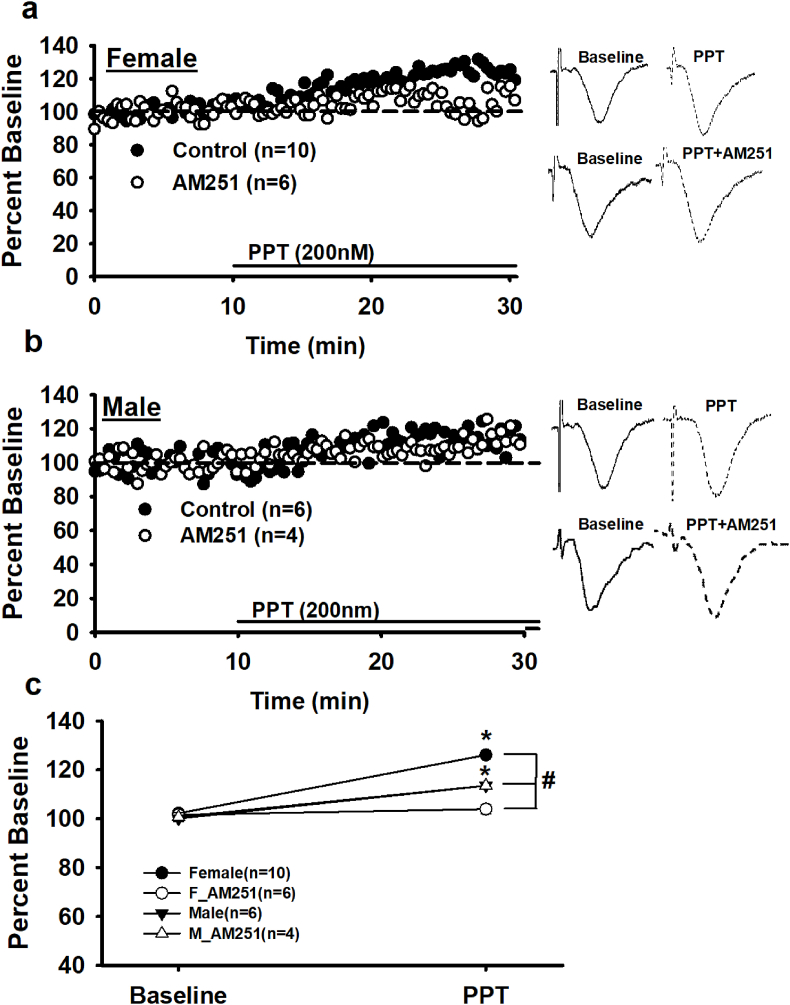
At female GABAergic-CA1 perisomatic synapses, AEA signaling is driven via stimulation of an ERα-mGluR1 complex (Tabatadze et al., 2015). Bath application of the ERα agonist, PPT (200 nm, 20 min) was used to test if ERα-AEA signaling exists at dendritic synapses. PPT application produces increases in fEPSP magnitude in females (126.08 ± 0.81% vs.101.87 ± 0.70%, baseline; Fig. 4a) and in males (113.54 ± 1.41% vs. 100.10 ± 1.30%, baseline; Fig. 4b). A two-way rmANOVA indicates both a significant main effect of drug, (F (1, 28) = 634.17, p < 0.00, and interaction effect (sex x drug), F (1, 28) = 52.45, p < 0.00 (Fig. 4c). The ERα-driven increase in fEPSPs is consistent with estrogenic modulation of hippocampal excitatory and inhibitory neurotransmission in both sexes (Oberlander and Woolley, 2016). Indeed, a 20 min exposure to E2 (100 nm) also significantly increases female fEPSPs (121.86 ± 1.05% vs 103.50 ± 1.16%; baseline; t (14) = −15.44, p < 0.00, n = 5, data not shown).
Fig. 4.
Dendritic ERαdrives 2-AG signaling in females only. a) Time course of averaged experiments (left) and single experiment raw traces (right) demonstrate that activation of ERα (PPT, 200 nM) increases female fEPSPs. The PPT effect is blocked by AM251, indicating that ERα stimulation drives eCB signaling at female synapses. b) Time course of averaged experiments (left) and single experiment raw traces (right) demonstrate that activation of ERα (PPT, 200 nM) also increases male fEPSPs. However, the PPT effect is not blocked by AM251, indicating that ERα stimulation does not drive eCB signaling at male synapses. c) Summary of the PPT and PPT + AM251 experiments comparing mean ± SEM baseline responses (last 5 min of the baseline period) and mean ± SEM post-PPT responses (last 5 min of the experiment). n = number of slices per experiment. Each * indicates significant differences from baseline responses; #s indicate significant differences between females and males (p ≤ 0.05), mixed, two-way rmANOVA.
Prior incubation (>1 h) and continuous perfusion of AM251 (5 μM) impairs the PPT-induced increase in female fEPSPs (Fig. 4a, c). An interaction effect (two-way rmANOVA) reveals a significant difference between PPT (126.08 ± 0.81%) and PPT + AM251 (103.91 ± 1.91%); thus indicating that ERα activation drives eCB signaling at female dendritic synapses. In contrast to females, CB1 antagonism does not prevent the PPT-induced increase in males (PPT: 113.54 ± 1.41% vs. PPT + AM25:115.30 ± 1.45%, F (1, 28) = 0.436, p > 0.5; Fig. 4b and c). The ERα-eCB effect on female fEPSPs is likely the result of CB1-mediated suppression of GABAergic neurotransmission. An alternative mechanism is that ERα-eCB production activates astrocytic CB1 that facilitates glutamatergic neurotransmission (Navarrete et al., 2014). Isolation of glutamatergic neurotransmission through continuous application of the GABA(A) antagonist, PTX (100 μM), impairs the ERα-mediated fEPSP increase in females (baseline (PTX): 100.30 ± 1.28% vs. 110.92 ± 1.25%: PPT + PTX, t(14) = -5.79, p < 0.00, data not shown). The ~10% increase following PPT (with PTX) in females suggests either that ERα-eCB also acts via astrocytic CB1 to increase excitatory neurotransmission or ERα can affect fEPSPs through an eCB-independent mechanism.
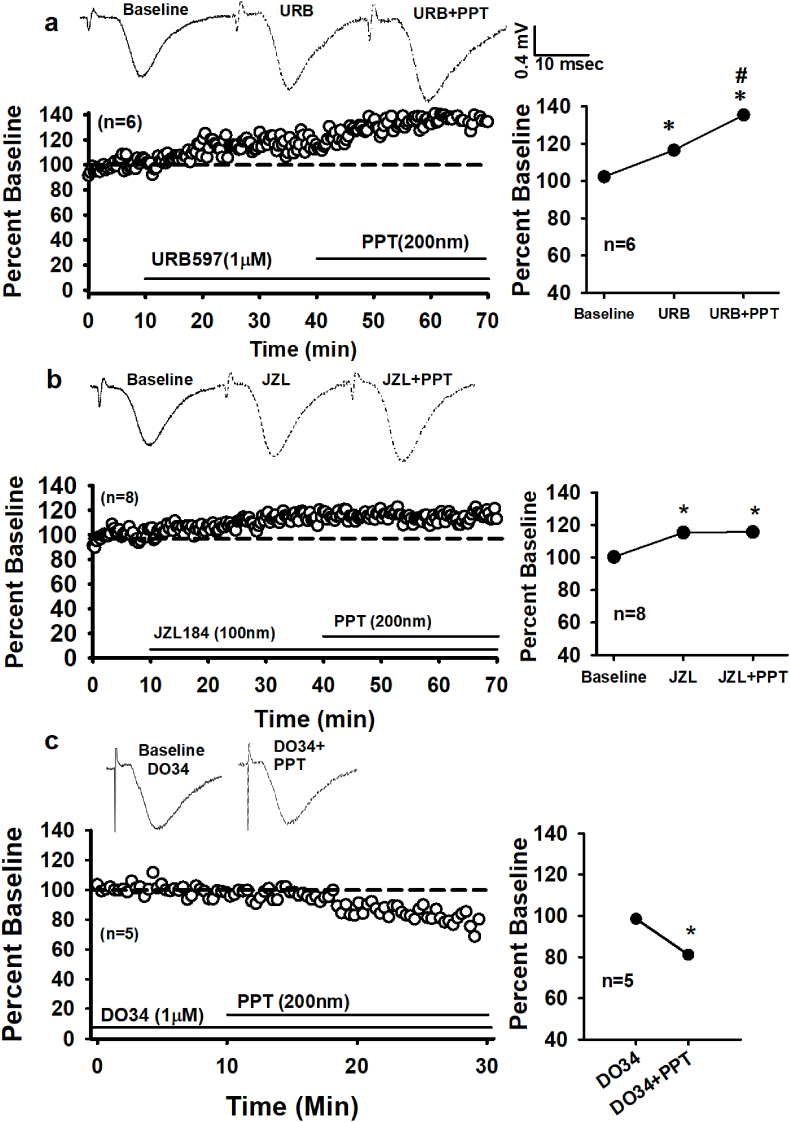
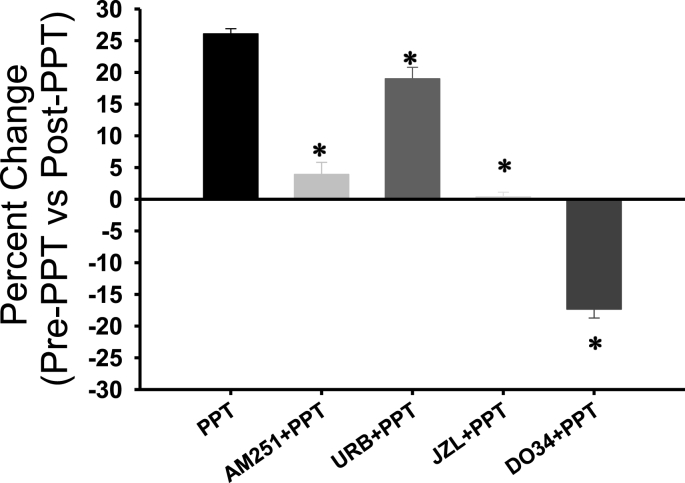
Our dendritic observations are consistent with sex differences with ERα-AEA-mediated suppression of perisomatic inhibitory neurotransmission (Huang and Woolley, 2012; Tabatadze et al., 2015). However, occlusion experiments, in which the FAAH inhibitor URB (1 μM) is applied (30 min) followed by PPT (30 min), fail to show occlusion in female slices. URB increases fEPSPs compared to baseline (116.53 ± 1.38% vs. 101.93 ± 1.12%) and PPT further increases fEPSPs (137.28 ± 1.21%), F (2, 28) = 191.63, p < 0.00 (one-way rmANOVA). See Fig. 5a. These findings suggest in females either 1) dendritic ERα- AEA production is robust enough to act synergistically with tonic AEA or 2) ERα stimulation recruits 2-AG production rather than AEA. Repeating the occlusion experiments with the MAGL inhibitor, JZL (100 nM), demonstrates that enhancing tonic 2-AG blocks the PPT-induced fEPSP increase in females. A one-way rmANOVA reveals that JZL significantly increases fEPSPs compared to baseline responses (115.42 ± 0.95% vs 100.40 ± 1.14%; F (2, 28) = 81.44, p < 0.00; Fig. 5b), however the addition of PPT does not augment the JZL effect (116.92 ± 0.84%), F (1, 14) = 2.72, p > 0.12 (planned pair-wise contrast, Fig. 5b). Furthermore, blocking the synthesis of 2-AG with the diacylglycerol lipase (DAGL) inhibitor, DO34 (1 μM), not only blocked but reversed the direction of the PPT effect (Fig. 5c). Female slices were pre-incubated (>1hr) and continuously perfused with DO34 throughout the experiment. Following the baseline period, a 20 min bath application of PPT (200 nM) decreased fEPSPs (DO34_baseline: 99.54% ± 0.75% vs 81.10 ± 1.38%; t (14) = 10.76. p = 0.000), paired-samples t-test). Collectively, 1) the failed occlusion of tonic AEA and ERα stimulation, 2) the occlusion between tonic 2-AG and ERα activation and 3) the blocked PPT-increase during inhibition of DAGL, all indicate that dendritic ERα activation drives 2-AG production in females. Fig. 6 provides a summary comparison of the female PPT experiments, represented as the difference between fEPSPs following PPT application (last 5 min) and responses prior to PPT application (5 min prior). A one-way ANOVA revealed significant differences among all the groups, F(4,74) = 318.05, p = 0.05.
Fig. 5.
Dendritic ERαdrives 2-AG signaling in females. a) Raw traces from single experiments (top) and time course of averaged experiments (bottom left) show that increasing tonic AEA via inhibition of FAAH (URB, 1 μM) does not occlude eCB signaling via ERα (PPT, 200 nM). A summary of the URB_PPT occlusion experiments is shown bottom, right. These suggest that ERα stimulation does not drive AEA production. Each * indicates significant differences from baseline responses; #s indicate significant differences between URB (last 5 min and URB + PPT (last 5 min) (p ≤ 0.05), one-way rmANOVAs. b) Raw traces from single experiments (top) and time course of averaged experiments (bottom left) show that increasing tonic 2-AG via inhibition of MAGL (JZL, 100 nM) does occlude eCB signaling via ERα (PPT, 200 nM). A summary of the JZL_PPT occlusion experiments is shown right; indicating that ERα stimulation does drive 2-AG production. Each * indicates significant differences from baseline responses (p ≤ 0.05), one-way rmANOVAs. c) Raw traces from single experiments (top) and time course of averaged experiments (bottom left) show that decreasing tonic 2-AG via inhibition of DAGL (DO34, 1 μM) not only blocks eCB signaling via ERα (PPT, 200 nM), but leads to a decrease in female fEPSPs. A summary of the DO34_PPT occlusion experiments is shown right. n = number of slices per experiment. Each * indicates significant differences from baseline responses; #s indicate significant differences between DO34 (last 5 min and DO34 (last 5 min) (p ≤ 0.05), one-way rmANOVAs.
Fig. 6.
Summary of female ERα(PPT) experiments. The percent change in fEPSP magnitudes following PPT application (last 5 min of experiment) from either the last 5 min of baseline activity or a previous drug application is shown (AM251,URB, JZL or DO34). A one-way ANOVA revealed significant differences among PPT and the other drugs applied in combination with PPT. Differences indicated by *.
3.6. Chronic mild stress (CMS) inverts female eCB signaling at dendritic synapses
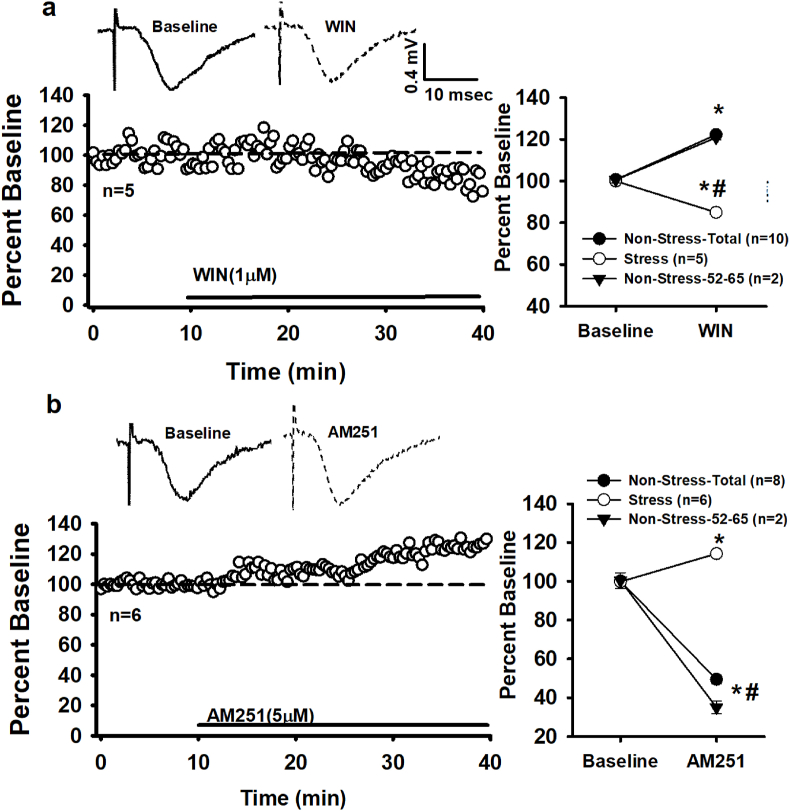
In males, exposure to a 21 day CMS protocol produces a WIN-induced increase in fEPSPs compared to the normal observed decrease (Reich et al., 2013b). Based on these male data, we hypothesized that CMS exposure in females may lead to the reversal of CB1/eCB function at female dendritic synapses. Indeed, we now observe that CMS results in a WIN-induced fEPSP reduction and reverses CB1 constitutive activity. Specifically, a 30 min WIN (1 μM) application results in a ~15% decrease in fEPSPs from female stressed slices (84.94 ± 1.62% vs. 100.44 ± 1.83%, baseline; t (14) = 6.44, p = 0.000). See Fig. 7a. A one-way ANOVA confirms that the ~22% WIN-induced increase in normal (non-stress) females is significantly different from the ~15% decrease in stress females (F (1, 28) = 450.71, p = 0.000, Fig. 5a). For the stress experiments, data was collected from a small cohort of age-matched non-stress cohorts (52–65 days old; 2–3 rats per experiment with an average of 1–3 slices per animal). Recordings from these small samples exhibited the same trends as slices from females in the earlier experiments with ages ranging from 40 to 55 days (mean = 48 days old). See Fig. 7, Fig. 8. Therefore, the following analyses representing non-stress females are pooled across all ages.
Fig. 7.
Chronic Mild Stress reverses female CB1 function. a) Raw traces from a single experiment (top) and time course of averaged experiments show that WIN activation of CB1 in stress females decreases fEPSPs. Right: Summary comparison of stress and non-stress females. b) Raw traces from a single experiment (top) and time course of averaged experiments reveal that in stress females inverse agonism (AM251) of CB1 increases fEPSPs. Right: comparison of stress and non-stress females. n = number of slices per experiment. Note data for non-stress animals shown comparing the total number of slices across all age ranges (40–65 days old) and representative data from slices aged 52–65 days old(each slice is from different rat). Each * indicates significant differences from baseline responses; #s indicate significant differences between stress and non-stress slices (p ≤ 0.05), mixed, two-way rmANOVA.
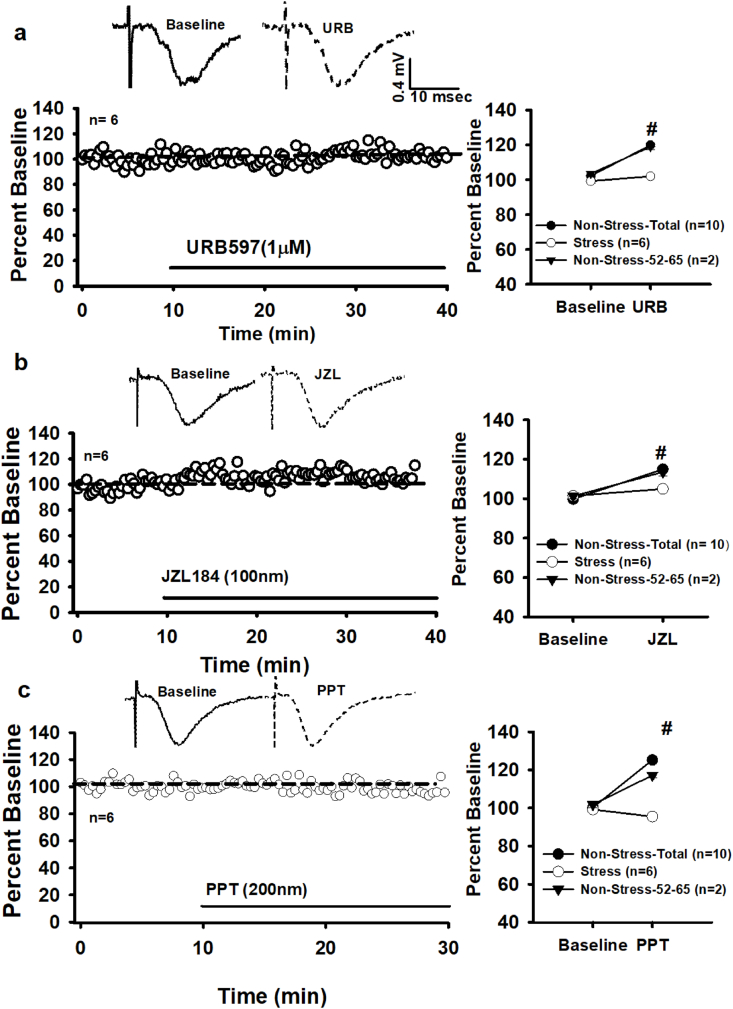
Fig. 8.
Chronic Mild Stress impairs female tonic and ERα-driven eCB signaling. a) Raw traces from a single experiment (top) and time course of averaged experiments demonstrate that FAAH inhibition (URB597) stress females has no effect on fEPSPs. Right: comparison of stress and non-stress females. b) Raw traces from a single experiment (top) and time course of averaged experiments show that in stress females MAGL inhibition (JZL184) marginally increases fEPSPs. Right: comparison of stress and non-stress females. c) Raw traces from a single experiment (top) and time course of averaged experiments show that in stress females ERα-driven eCB signaling is absent. Right: comparison of stress and non-stress females. n = number of slices per experiment. Note data for non-stress animals shown comparing the total number of slices across all age ranges (40–65 days old) and representative data from slices aged 52–65 days old (each slice is from different rat). Each * indicates significant differences from baseline responses; #s indicate significant differences between stress and non-stress slices (p ≤ 0.05), mixed, two-way rmANOVA.
CMS exposure also results in a significant increase in female fEPSPs upon application of AM251 (5 μM) compared to baseline responses (125 ± 0.69% vs. 99.71 ± 0.49%; t (14) = 23.81, p = 0.00, Fig. 7b). This AM251-driven increase is significant from the decrease observed (~50%) in non-stress females (F (1, 28) = 1040.83, p = 0.000, Fig. 7b) and reflects a reversal of CB1 constitutive activity.
CMS modulation of CB1 activity is reflected in alterations in female tonic eCB signaling. A 30 min application of the FAAH inhibitor, URB (1 μM), demonstrates that CMS abolishes tonic AEA activity (102.00 ± 0.79% vs. 99.28 ± 1.31%, baseline; t (14) = 1.59, p > 0.05). See Fig. 8a. The failure of URB to alter fEPSPs in stressed slices is markedly different to the ~16% increase in response magnitudes of non-stressed slices (F (1, 28) = 170.08, p = 0.000, Fig, 8a). In regards to tonic 2-AG signaling, a 30 min application of JZL (100 nM) produced a small, but significant increase in stressed fEPSPs (104.74 ± 0.96% vs. 101.44 ± 0.96%, baseline; t (14) = 2.50, p = 0.03, Fig. 8b). However, the ~4% fEPSP increase in stress females is a significant reduction from the ~16% JZL-induced increase in non-stress females (F (1, 28) = 69.08, p = 0.00, Fig. 8b). Thus, CMS robustly attenuates tonic eCB signaling in the female CA1 dendritic layer. Furthermore, CMS impairs ERα-eCB signaling. In stressed slices, a 20 min application of PPT (200 nM) failed to increase fEPSPs (98 ± 1.13% vs. 99.23 ± 1.12%, baseline; t (14) = 0.650, p > 0.05, Fig. 8c) and is significantly different from the ~25% PPT-driven increase in non-stress fEPSPs (F (1, 28) = 531.58, p = 0.000, Fig. 8c).
4. Discussion
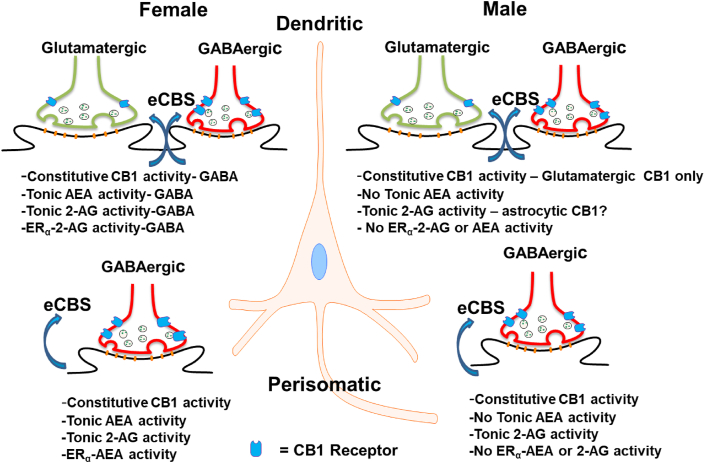
Sexually distinct eCB signaling occurs at rodent CA1 GABAergic perisomatic synapses. Specifically, tonic AEA and ERα-mGluR1-driven AEA are present at female synapses, but not in males (Huang and Woolley, 2012; Lee et al., 2010, 2015; Tabatadze et al., 2015). However, tonic 2-AG and constitutive CB1 activity occur in both sexes at perisomatic synapses (Lee et al., 2010, 2015). We presently report that sex-specific eCB signaling also occurs at dendritic GABAergic synapses. Our observations demonstrate sex differences 1) in both ligand-driven and constitutive CB1 receptor function, 2) in tonic AEA and 2-AG signaling and 3) ERα-driven eCB signaling. See Fig. 9 for a summary of eCB sex differences across the somatodendritic axis. Additionally, we report that exposure to chronic mild stress in females reverses the direction of CB1 and eCB signaling.
Fig. 9.
Summary of eCB Sex Differences at CA3-CA1 Synapses. This model represents the current data on sex differences across the somatodendritic axis of CA1 pyramidal cells.
4.1. Sex differences in dendritic CB1 receptor function
In male CA1-SR, exogenous CB1 activation classically decreases fEPSPs through activation of CB1 on glutamatergic terminals (Hoffman et al., 2010; Reich et al., 2013b; Takahashi and Castillo, 2006). We initially reasoned that this effect would be attenuated in females due to lower hippocampal CB1 densities (Reich et al., 2009). Surprisingly, WIN application caused a significant increase in female fEPSPs. After verifying that WIN was acting via CB1, we isolated glutamatergic neurotransmission and revealed that WIN did attenuate fEPSPs in females similar to males. Thus, CB1-modulation of glutamatergic neurotransmission is present in females, although CB1-mediated suppression of GABAergic neurotransmission appears to be more robust. In males, the opposite occurs whereby CB1 regulation of dendritic glutamatergic neurotransmission outperforms regulation of GABAergic transmission. Indeed, eCB/CB1-modulation at CA1 dendritic GABAergic synapses is relatively weak compared to that of perisomatic synapses (Lee et al., 2010, 2015). The functional implication is that sex differences in CA1 dendritic excitatory/inhibitory balance favor excitation in females and inhibition in males.
Sex-specific CB1 function could be attributed to differences in ligand-receptor efficacy or constitutive receptor activity. We first assessed constitutive activity and observed that the CB1 inverse agonist/antagonist produced sexually divergent effects on fEPSP magnitudes. In females, AM251 attenuated the response; demonstrating constitutive CB1 activity at GABAergic synapses and unmasking a tonic inhibitory tone on SCA-CCK GABA release. The AM251-driven increase observed in males also shows constitutive CB1 activity, which suggests that male CB1 is preferentially active at glutamatergic synapses. This interpretation is buttressed by a lack of constitutive CB1 activity at male dendritic GABAergic synapses (Lee et al., 2010, 2015). These findings complement the WIN data and clearly demonstrate sex differences in CB1 function at the dendritic layer of hippocampal CA1.
4.2. Sex differences in dendritic tonic AEA signaling
Tonic AEA signaling occurs at female CA1 GABAergic-pyramidal perisomatic synapses (Huang and Woolley, 2012; Tabatadze et al., 2015), however is not observed in males at either GABAergic dendritic or perisomatic synapses (Lee et al., 2015). Our current findings that tonic AEA activity exists at female, but not at male, GABAergic dendritic synapses supplements the established sex-specific pattern of tonic AEA signaling in CA1. Furthermore, our observations that activation of TRPV1 results in a decrease of female fEPSPs argues that tonic AEA is acting primarily as an endocannabinoid and not an endovanilloid. Combined with constitutive CB1 activity, female tonic AEA signaling presents a basal eCB/CB1 inhibition of GABA release in CA1 SR. The presumed targets of this inhibition are the CB1-containing SCA-CCK-interneurons (Kano, 2014; Lee et al., 2010; S.-H. Lee et al., 2015; Younts and Castillo, 2014).
4.3. Sex differences in dendritic tonic 2-AG signaling
As with AEA, tonic 2-AG signaling is present at both male and female CA1 perisomatic GABAergic synapses (Huang and Woolley, 2012; Lee et al., 2010, 2015; Tabatadze et al., 2015), but not at male dendritic SCA-CCK-GABAergic synapses (Lee et al., 2010, 2015). We currently observe tonic 2-AG activity in both females and males. The female data complements perisomatic tonic 2-AG signaling and furthers evidence of a basal inhibition of CCK-GABA activity in female CA1. Conversely, the male findings contradict previous research that report no dendritic tonic 2-AG activity at GABAergic synapses. Differences in methodology may account for this discrepancy. Lee et al. (2010, 2015) interrogated CA1 perisomatic and dendritic targeting CCK interneuron-pyramidal cell synapses using a paired whole-cell patch clamp technique. While paired-recordings is an elegant technique allowing for fine-grained dissection and analysis of neural circuitry, it may not be sensitive to population synaptic events that require a much greater number of participating synapses to detect. Thus, a weak 2-AG tone at GABAergic synapses may be better resolved through the indirect but larger population-based field potential. A more plausible explanation for the discrepancy in male dendritic 2-AG tone is that 2-AG exerts a direct excitatory effect on glutamate release rather than reducing GABAergic inhibition. We posit that male dendritic tonic 2-AG activates astrocytic CB1, thereby increasing extracellular glutamate levels and augmenting fEPSPs (Navarrete et al., 2014). This scenario is consistent with both the reported lack of tonic 2-AG signaling at male GABAergic synapses and that key precursor enzymes necessary for 2-AG synthesis are present in CA1 pyramidal cell dendrites (Kano, 2014; Lee et al., 2010, 2015).
4.4. Dendritic ERα drives 2-AG signaling in females
Activation of a postsynaptic ERα- mGLuR1 pathway induces AEA production and release at perisomatic GABAergic synapses in females (Tabatadze et al., 2015). In agreement with an estrogenic-eCB pathway, we observed that ERα activation caused a CB1-dependent increase in female fEPSP magnitude. In males, ERα activation increased fEPSPs, however the effect was non-CB1 dependent. Surprisingly, tonic AEA- and ERα-mediated increases in female fEPSPs did not occlude, although tonic 2-AG and ERα signaling did occlude each other. Furthermore, inhibiting DAGL blocked the ERα effect on fEPSPs. These findings indicate that at CA1 dendritic synapses, ERα drives a form of 2-AG signaling. Thus, estrogenic-eCB signaling is present along the CA1 somatodendritic axis in a compartment-specific fashion. The functional implications of this signaling dichotomy are unclear. AEA is considered to provide more tonic signaling and 2-AG is the predominant phasic eCB (Kano, 2014; Lee et al., 2015; Younts and Castillo, 2014). Perhaps dendritic estrogenic-eCB signaling acts in a rapid, activity-driven fashion whereas in the perisomatic region, it behaves in a slower, tonic manner; providing the female eCB system a dynamic operating range to gate compartment-specific synaptic plasticity. ERα-driven and tonic 2-AG signaling may also activate astrocytic CB1, subsequently resulting in higher synaptic glutamate levels, which drive the observed fEPSP increase. Blocking GABA (A) neurotransmission significantly reduced the ERα effect, demonstrating that ERα-2-AG signaling is mainly targeting GABAergic CB1. However, ERα stimulation in the presence of picrotoxin still produces ~10% increase in fEPSPs; suggesting that ERα -2-AG signaling either includes astrocytic CB1 or non-eCB ERα signaling pathways. The observations that ERα activation still increases fEPSPs in the presence of AM251 and that in males, ERα-driven fEPSP facilitation is similar in magnitude to that in females with AM251 supports the latter. Moreover, observing that PPT application with the DAGL inhibitor DO34 decreased female fEPSPs suggests that in the absence of DAGL, ERα may recruit multiple signaling pathways depending on biochemical availability within the cell.
Our present findings and those from the Woolley lab (Huang and Woolley, 2012; Tabatadze et al., 2015) provide reinforcing evidence for an estrogenic-eCB signaling pathway in the female hippocampus. The estrogen source driving these pathways remains unclear. Huang et al. (2012) argue that ovarian E2 levels (100 pM) in the hippocampus are not sufficiently high enough to drive ERα-AEA production, although local de novo E2 synthesis (5–10 nM) would be sufficient. They performed their experiments in ovariectomized rats and the current study used intact, natural cycling rats. Despite this difference in hormone status, our results are in agreement regarding an ERα-eCB pathway; suggesting that ovarian estrogen is not primarily driving ERα. However, CB1 receptor levels and binding affinities fluctuate across the estrous cycle, implicating ovarian hormonal modulation on CB1 function (Craft et al., 2013; Gorzalka and Dang, 2012; Rubino and Parlolaro, 2011). Furthermore, ovarian E2 replacement reverses ovariectomy-induced changes in CB1 levels in several brain regions such as the limbic forebrain, amygdala and hippocampus (Gorzalka and Dang, 2012). It is plausible that the ERα-eCB pathways may be ovarian-independent while CB1 function itself may be regulated by ovarian estrogen. The effects of ovarian estrogen could be subtle and easily masked in studies using either ovariectomized or intact (without estrous cycle tracking) animals. Given that we did not track our data across the estrous cycle, the contribution of fluctuating ovarian hormones was not assessed. This will be an important consideration in future studies.
4.5. CMS adversely affects female eCB signaling
At the receptor level, exogenous activation (WIN) resulted in a modest fEPSP decrease in stressed slices. Furthermore, inverse agonism of CB1 produced a significant fEPSP increase. These observations are the inverse of the data from normal (non-stress) slices. This represents a stress-induced shift in the excitatory/inhibitory influence of tonic eCB signaling in the female hippocampus wherein less eCB regulation of GABA neurotransmission allows more inhibition of CA1 dendrites. Interestingly, stressed female dendritic eCB signaling appears to resemble the non-stressed male system in that eCB-glutamatergic regulation is dominant over eCB-GABAergic control (Lee et al., 2010, 2015; Reich et al., 2013b). Moreover, the reverse occurs in both non-stress females and stress males (Reich et al., 2013b). These current findings parallel observations that CMS reverses hippocampal CB1 densities compared to control rats. Notably, in non-stress rats, males possess higher CB1 levels than females and CMS reverses this relationship (Reich et al., 2009).
CMS also abolishes tonic AEA and ERα-driven 2-AG activity while significantly attenuating tonic 2-AG signaling at female dendritic synapses. The reduction of AEA is consistent with the well documented chronic stress-induced AEA decrease in hippocampus, amygdala, hypothalamus and medial prefrontal cortex (mPFC) in male rodents (McEwen et al., 2015; Morena et al., 2016). An important caveat is that stress-regulation of AEA is sensitive to a given stress protocol. For example, in homotypic restraint stress, where animals are subjected to the same stressor (restraint) for 10–21 days, AEA reduction is quite reliable (Hill et al., 2010; Morena et al., 2016). However, the use of heterotypic chronic stress protocols (using multiple types of stressors), like in the current study, are less reliable in producing alterations in eCB/CB1 signaling (Hill et al., 2010; Morena et al., 2016). In our hands, we have observed consistently reliable effects of the CMS protocol on the hippocampal eCB system (Reich et al., 2009, 2013a, 2013b) including this study. Regardless of stress protocol, upregulation of FAAH via corticosterone and corticotropin-releasing hormone (CRH) signaling during stress are the putative mechanisms underlying the stress-induced AEA reduction (McEwen et al., 2015; Morena et al., 2016). The reduction in 2-AG in females again aligns with heterotypic stress associated with 2-AG decreases in the male hippocampus; although increases in 2-AG occur following homotypic stress exposure in male amygdala, hippocampus and hypothalamus (Hill et al., 2010; Morena et al., 2016). Homotypic stress-induced 2-AG increases are linked to reductions in MAGL, while no changes in MAGL are reported or measured in studies using heterotypic stressors (Hill et al., 2010; Morena et al., 2016). Thus, increases in MAGL activity could account for both our observed reductions in tonic and ERα-driven 2-AG. Future studies will need to assess sex differences in FAAH and MAGL activity under basal and stress conditions.
These current data demonstrate that a 21-day CMS exposure has a profound effect on female CA1 tonic eCB/CB1 signaling. These findings provide further evidence for our earlier hypothesis that the hippocampal eCB system is organized in male and female animals to respond differentially to chronic stress (Reich et al., 2009). Both our current and previous work (Reich et al., 2013b) exposed adolescent rats to chronic stress. Adolescence is a lengthy period of neuronal maturation and pruning that is vulnerable to toxic insults such a stress (Lee and Gorzalka, 2012; Spear, 2000). Our combined work indicates a clear sexual dimorphism in adolescent basal hippocampal eCB signaling that is modulated by chronic stress. Given the known roles of the hippocampus and eCBs in the emotional modulation of learning and memory, it is plausible that sexually-divergent hippocampal eCB signaling in both non-stress and stress conditions significantly contributes to sex-specific emotional processing and behaviors during adolescence. Perturbations of the neurobiology underlying adolescent emotional processing may contribute to development of adult stress-related mental disorders.
CRediT authorship contribution statement
Angelica Ferraro: Investigation, Formal analysis, Writing - original draft. Philip Wig: Investigation. Joseph Boscarino: Investigation. Christian G. Reich: Conceptualization, Methodology, Investigation, Formal analysis, Writing - original draft.
Declaration of competing interest
None
Acknowledgements
Research supported by NIH R15-MH085280-01, Ramapo College of New Jersey Foundation Grants and Ramapo College of New Jersey Faculty Development Fund to CGR. We thank Alana Acciardi, Shane O'Sullivan, Zachary Mall, Alexandra Kemp, Brian Haner and Cecilia Xu for their excellent technical assistance. In addition, we thank Michele Reich for proofreading and editing.
References
- Craft R.M., Marusich J.A., Wiley J.L. Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life Sci. 2013;92:476–481. doi: 10.1016/j.lfs.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzalka B.B., Dang S.S. Minireview: endocannabinoids and gonadal hormones: bidirectional interactions in physiology and behavior. Endocrinology. 2012;153:1016–1024. doi: 10.1210/en.2011-1643. [DOI] [PubMed] [Google Scholar]
- Hill M.N., McLaughlin R.J., Bingham B., Shrestha L., Lee T.T.Y., Gray J.M., Hillard C.J., Gorzalka B.B., Viau V. Endogenous cannabinoid signaling is essential for stress adaptation. Proc. Natl. Acad. Sci. U.S.A. 2010;107:9406–9411. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman A.F., Laaris N., Kawamura M., Masino S. a, Lupica C.R. Control of cannabinoid CB1 receptor function on glutamate axon terminals by endogenous adenosine acting at A1 receptors. J. Neurosci. 2010;30:545–555. doi: 10.1523/JNEUROSCI.4920-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G.Z., Woolley C.S. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74:801–808. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M. Control of synaptic function by endocannabinoid-mediated retrograde signaling. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2014;90:235–250. doi: 10.2183/pjab.90.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Birnbaum H.G., Shahly V., Bromet E., Hwang I., McLaughlin K.A., Sampson N., Andrade L.H., de Girolamo G., Demyttenaere K., Haro J.M., Karam A.N., Kostyuchenko S., Kovess V., Lara C., Levinson D., Matschinger H., Nakane Y., Browne M.O., Ormel J., Posada-Villa J., Sagar R., Stein D.J. Age differences in the prevalence and co-morbidity of DSM-IV major depressive episodes: results from the WHO World Mental Health Survey Initiative. Depress. Anxiety. 2010;27:351–364. doi: 10.1002/da.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Petukhova M., Sampson N.A., Zaslavsky A.M., Wittchen H.-U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int. J. Methods Psychiatr. Res. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-H., Földy C., Soltesz I. Distinct endocannabinoid control of GABA release at perisomatic and dendritic synapses in the hippocampus. J. Neurosci. 2010;30:7993–8000. doi: 10.1523/JNEUROSCI.6238-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-H., Ledri M., Tóth B., Marchionni I., Henstridge C.M., Dudok B., Kenesei K., Barna L., Szabó S.I., Renkecz T., Oberoi M., Watanabe M., Limoli C.L., Horvai G., Soltesz I., Katona I. Multiple forms of endocannabinoid and endovanilloid signaling regulate the tonic control of GABA release. J. Neurosci. 2015;35:10039–10057. doi: 10.1523/JNEUROSCI.4112-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.T.-Y., Gorzalka B.B. Timing is everything: evidence for a role of corticolimbic endocannabinoids in modulating hypothalamic-pituitary-adrenal axis activity across developmental periods. Neuroscience. 2012;204:17–30. doi: 10.1016/j.neuroscience.2011.10.006. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Bowles N.P., Gray J.D., Hill M.N., Hunter R.G., Karatsoreos I.N., Nasca C. Mechanisms of stress in the brain. Nat. Neurosci. 2015;18:1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira F.A., Wotjak C.T. Cannabinoids and anxiety. Curr. Top. Behav. Neurosci. 2010;2:429–450. doi: 10.1007/7854_2009_16. [DOI] [PubMed] [Google Scholar]
- Morena M., Patel S., Bains J.S., Hill M.N. Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology. 2016;41:80–102. doi: 10.1038/npp.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M., Díez A., Araque A. Astrocytes in endocannabinoid signalling. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:20130599. doi: 10.1098/rstb.2013.0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander J.G., Woolley C.S. 17 -Estradiol acutely potentiates glutamatergic synaptic transmission in the Hippocampus through distinct mechanisms in males and females. J. Neurosci. 2016 doi: 10.1523/JNEUROSCI.4437-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich C.G., Iskander A.N., Weiss M.S. Cannabinoid modulation of chronic mild stress-induced selective enhancement of trace fear conditioning in adolescent rats. J. Psychopharmacol. 2013;27 doi: 10.1177/0269881113499207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich C.G., Mihalik G.R., Iskander A.N., Seckler J.C., Weiss M.S. Adolescent chronic mild stress alters hippocampal CB1 receptor-mediated excitatory neurotransmission and plasticity. Neuroscience. 2013;253 doi: 10.1016/j.neuroscience.2013.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich C.G., Taylor M.E., McCarthy M.M. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav. Brain Res. 2009;203 doi: 10.1016/j.bbr.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Parlolaro D. Sexually dimorphic effects of cannabinoid compounds on emotion and cognition. Front. Behav. Neurosci. 2011;5(64) doi: 10.3389/fnbeh.2011.00064. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T., Parolaro D. Sex-dependent vulnerability to cannabis abuse in adolescence. Front. psychiatry. 2015;6:56. doi: 10.3389/fpsyt.2015.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Tabatadze N., Huang G., May R.M., Jain A., Woolley C.S. Sex differences in molecular signaling at inhibitory synapses in the Hippocampus. J. Neurosci. 2015;35:11252–11265. doi: 10.1523/JNEUROSCI.1067-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K. a, Castillo P.E. The CB1 cannabinoid receptor mediates glutamatergic synaptic suppression in the hippocampus. Neuroscience. 2006;139:795–802. doi: 10.1016/j.neuroscience.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Younts T.J., Castillo P.E. Endogenous cannabinoid signaling at inhibitory interneurons. Curr. Opin. Neurobiol. 2014;26:42–50. doi: 10.1016/j.conb.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]