Abstract
Background
Maternal depression and anxiety during pregnancy may enhance fetal exposure to glucocorticoids (GCs) and harm neurodevelopment. We tested whether a novel cross-tissue polyepigenetic biomarker indicative of in utero exposure to GC is associated with mental and behavioral disorders and their severity in children, possibly mediating the associations between maternal prenatal depressive and anxiety symptoms and these child outcomes.
Methods
Children (n = 814) from the Prediction and Prevention of Preeclampsia and Intrauterine Growth Restriction (PREDO) study were followed-up from birth to age 7.1–10.7 years. A weighted polyepigenetic GC exposure score was calculated based on the methylation profile of 24 CpGs from umbilical cord blood. Child diagnosis of mental and behavioral disorder (n = 99) and its severity, defined as the number of days the child had received treatment (all 99 had received outpatient treatment and 8 had been additionally in inpatient treatment) for mental or behavioral disorder as the primary diagnosis, came from the Care Register for Health Care. Mothers (n = 408) reported on child total behavior problems at child's age of 2.3–5.8 years and their own depressive and anxiety symptoms during pregnancy (n = 583).
Results
The fetal polyepigenetic GC exposure score at birth was not associated with child hazard of mental and behavioral disorder (HR = 0.82, 95% CI 0.54; 1.24, p = 0.35) or total behavior problems (unstandardized beta = −0.10, 95% CI -0.31; 0.10, p = 0.33). However, for one standard deviation decrease in the polyepigenetic score, the child had spent 2.94 (95%CI 1.59; 5.45, p < 0.001) more days in inpatient or outpatient treatment with any mental and behavioral disorder as the primary diagnosis. Criteria for mediation tests were not met.
Conclusions
These findings suggest that fetal polyepigenetic GC exposure score at birth was not associated with any mental or behavioral disorder diagnosis or mother-rated total behavior problems, but it may contribute to identifying children at birth who are at risk for more severe mental or behavioral disorders.
Keywords: Cord blood methylation, Glucocorticoids, Polyepigenetic biomarker, Childhood mental health, Prenatal psychopathology, Prospective study
Abbreviations: 11β-HSD2, 11-beta-hydroxysteroid-dehydrogenase type 2; ADHD, Attention deficit/hyperactivity disorder; BMI, Body-mass index; CES‐D, Center for epidemiologic studies depression scale; DNAm, DNA methylation; GC, Glucocorticoid; GR, Glucocorticoid receptor; GRE, Glucocorticoid response element; HILMO, Care register for health care; HPA-axis, Hypothalamic-pituitary-adrenal axis; PREDO, Prediction and prevention of preeclampsia and intrauterine growth restriction; STAI, Spielberger state anxiety inventory; ZINB, Zero-inflated negative binomial regression
1. Introduction
Many women experience clinically significant symptoms of depression and anxiety during pregnancy, with an estimated prevalence of 7%–20% (Bennett et al., 2004; Marcus et al., 2003). These maternal mental health problems not only complicate the mother's wellbeing and health during pregnancy, but are also associated with increased risk of preterm birth, lower birth weight and neurodevelopmental and mental health adversities of the offspring later in life (Grote et al., 2010; Madigan et al., 2018; Robinson et al., 2019; Van den Bergh et al., 2017). The offspring neurodevelopmental and mental health adversities include developmental delay (Graignic-Philippe et al., 2014; Tuovinen et al., 2018), poorer cognitive functioning (Stein et al., 2014), internalizing and externalizing (Kingston et al., 2018; Lahti et al., 2017), attention deficit/hyperactivity (ADHD) (Van Batenburg-Eddes et al., 2013; Wolford et al., 2017) and sleep problems (Toffol et al., 2019). In addition, neuroimaging studies have shown that maternal depressive and anxiety symptoms during pregnancy are associated with alterations in offspring structural and functional brain connectivity across various brain regions and networks (Adamson et al., 2018; Van den Bergh et al., 2017).
Mechanisms that could explain these associations remain uncertain. It has been postulated that enhanced fetal exposure to glucocorticoids (GCs) may be one of the underpinning mechanisms (Reynolds, 2013; Wolford et al., 2019; Räikkönen et al., 2020). A number of studies report that maternal depression and anxiety during pregnancy are associated with higher circulating levels of GC hormone cortisol (Evans et al., 2008; O'Connor et al., 2014). This is suggestive of an increased fetal exposure to maternal cortisol as the maternal and fetal levels are highly correlated (Gitau et al., 1998). However, studies testing associations between maternal cortisol levels during pregnancy and offspring neurodevelopmental and mental health outcomes still remain limited (O'Donnell and Meaney, 2017). Yet, there is evidence on the effects of maternal antenatal administration of exogenous synthetic GCs which are transferred through the placenta or increased transfer of cortisol through inhibition of the placental GC barrier enzyme 11-beta-hydroxysteroid-dehydrogenase type 2 (11β-HSD2) by, for example, licorice or its derivatives (Reynolds, 2013). These exposures are associated with poorer learning and memory, anxiety- and depression-like behaviors and altered hypothalamic-pituitary-adrenal (HPA)-axis functioning in the animal offspring, and poorer cognition, mental health problems and altered HPA-axis functioning in the human offspring (Painter et al., 2012; Räikkönen et al, 2009, 2017, 2020).
At the molecular level, enhanced fetal glucocorticoid exposure may become embedded in fetal epigenetic alterations, such as alterations in DNA methylation (DNAm) (Kundakovic and Jaric, 2017; Van Den Bergh, 2011). In fact, a number of studies have shown that GC exposure can lead to changes in DNA methylation, both dynamic and lasting, in part mediated by local de-methylation at glucocorticoid receptor (GR) binding sites, so called GC response elements (GREs) (Bartlett et al., 2019; Kress et al., 2006). Studies in animals as well as humans suggest that these effects may have cross-tissue impact, with at least some overlap of methylation sites responsive to GC in peripheral blood and brain tissue (Ewald et al., 2014; Klengel et al., 2013; Wiechmann et al., 2019). We have recently shown that exposure to GC can lead to lasting changes in DNA methylation at CpG sites in a human hippocampal progenitor cell line (Provençal et al., 2020), especially when exposure happens early in neurodevelopment. These lasting changes in DNA methylation are associated with an enhanced transcriptional responsiveness of the target genes to a second GC exposure while not changing baseline gene expression. This suggests that GC exposure early during brain development could leave an epigenetic mark that is associated with an altered set point for molecular and cellular responses to future stress exposure and thus possibly the individual's risk or resilience to psychiatric disorders (Clayton et al., 2020). We also tested whether a biomarker derived from these GC responsive methylation sites showing a lasting change in the neuronal cell culture model, would be indicative of increased prenatal GC exposure in the newborn (Provençal et al., 2020). For this, we first identified the overlap of these CpGs with CpG sites that showed changes in DNA methylation in peripheral blood cells with exposure to the synthetic GC dexamethasone and identified 496 common CpGs. These cross-tissue GC responsive CpGs were significantly enriched among CpGs measured in cord blood that were associated with prenatal exposure to synthetic glucocorticoids as well as prenatal depression and anxiety. As described above, these latter states have been associated with increased maternal cortisol levels.
Finally, a cross-tissue polyepigenetic score was computed using these overlapping 496 CpGs and elastic-net regression, which selected 24 CpGs within 24 different loci and weighted by GC-induced methylation changes in peripheral blood. Measured in fetal cord blood, this score correlated inversely with maternal depressive and anxiety symptoms during pregnancy. The direction of the association suggested that lower scores reflecting more de-methylation after GC, associated with exposure to higher prenatal depression or anxiety symptoms. However, it remains unknown if this polyepigenetic GC exposure score at birth might allow to identify offspring at risk for neurodevelopmental and mental health adversities later in life (Provençal et al., 2020).
We examined associations between this novel polyepigenetic fetal GC exposure score at birth and any mental or behavioral disorder diagnosis of the child in a follow-up of from birth to the child's age of 7.1–10.7 years. We also examined if the GC exposure score was associated with the severity of any mental or behavioral disorder diagnosis, measured as the number of days the child had spent in inpatient or outpatient treatment in public specialized medical care with any mental disorder as the primary diagnosis (Wolff et al., 2015), and if the score was associated with the mother-rated total behavior problems of the child. We further tested if the GC exposure score mediated the associations between maternal depressive and anxiety symptoms during pregnancy and child outcomes.
2. Methods and materials
2.1. Participants
The PREDO study comprises 1079 pregnant women who gave birth to a singleton live child in Finland between 2006 and 2010 (Girchenko et al., 2017a). Recruitment took place in consecutive order when women attended their first ultrasound screening between 12 + 0 and 13 + 6 gestational weeks + days at one of the ten study hospitals in Southern and Eastern Finland. Of the recruited women, 969 had one or more clinical risk factors for preeclampsia and/or intrauterine growth restriction (IUGR), and 110 had no known risk factors, including maternal pre-pregnancy body mass index (BMI)≥30 kg/m2), preeclampsia or gestational diabetes in previous pregnancy, type 1 diabetes, systemic lupus erythematosus, Sjögren's syndrome, age below 20 or above 40 years, intrauterine growth restriction or demise of the fetus in previous pregnancy (Girchenko et al., 2017a).
In total, 817 fetal umbilical cord blood samples with full information on genome-wide DNAm and genotype were available after quality control (see below). 807 umbilical cord blood samples came from the 1079 pregnancies and 10 from an additional 88 pregnancies from whom we sampled placental biopsies (Girchenko et al., 2017a; Lahti-Pulkkinen et al., 2018).
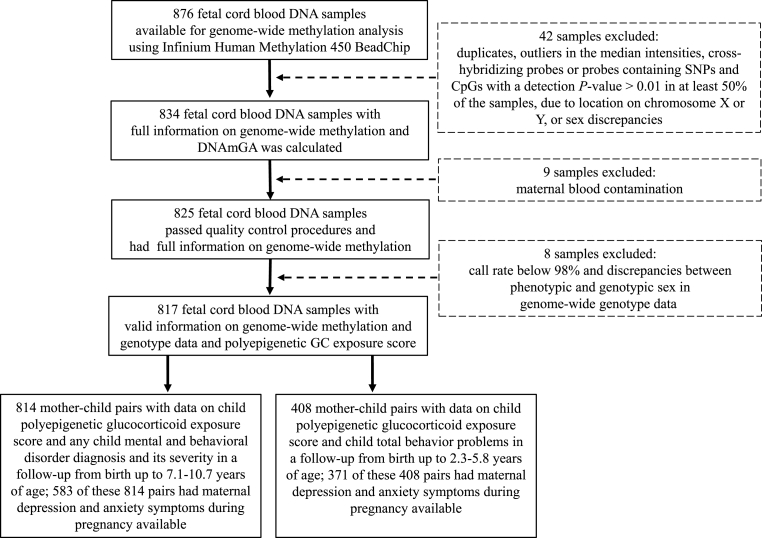
Data on child mental and behavioral disorder diagnoses from birth to December 31, 2016 were available for 814 children (99.6%). At the end of the follow-up, the children were 7.1–10.7 years of age [Median = 8.4 (Interquartile range 7.9–9.0)]. Of the 814 children with DNAm, genotype and mental and behavioral disorders data, 408 had information on mother-rated total behavior problems at the child's age of 2.3–5.8 years [Median = 3.5 (Interquartile range 3.1–4.2)] and 583 had information on maternal depressive and anxiety symptoms during pregnancy. See Fig. 1 for sample and attrition.
Fig. 1.
Flow chart of the PREDO study participants and sample attrition.
In comparison to children who were not included in the analytic sample (n = 262), those who were included were born at a later gestational age (Mean Difference (MD) = 0.66 weeks, 95% Confidence Interval (95% CI) 0.41; 0.91, p < 0.001), were younger at the end of the follow-up (MD = −0.16 years, 95% CI -0.29; −0.03, p = 0.005), and their mothers had type 1 diabetes less frequently (1.4% vs 4.0%, χ2(1) = 7.29, p = 0.007). The groups did not differ on any other characteristics (p > 0.16).
The Ethics Committees of the Helsinki and Uusimaa Hospital District and the participating hospitals approved the study protocol. All participating women signed written informed consents.
2.2. Child DNA methylation and polyepigenetic biomarker of fetal GC exposure at birth
Fetal cord blood samples were collected according to standard procedures. DNA was extracted at the National Institute for Health and Welfare, Helsinki, Finland, and the Institute for Molecular Medicine Finland, University of Helsinki, Finland. Methylation analyses were performed at the Max Planck Institute of Psychiatry (MPIP) in Munich, Germany. DNA was bisulfite converted using the EZ-96 DNA Methylation kit (Zymo Research, Irvine, CA). Genome-wide methylation status of over 485,000 CpG sites was measured using the Infinium Human Methylation 450 BeadChip (Illumina Inc., San Diego, CA) according to the manufacturer's protocol. The arrays were scanned using the iScan System (Illumina Inc., San Diego, CA). The quality control pipeline was set up using the R-package minfi (Aryee et al., 2014). Samples with maternal blood contamination were excluded (n = 9) (Morin et al., 2017). The final dataset contained 428,619 CpG sites. Methylation β values were normalized using the funnorm function in the R-package minfi (Aryee et al., 2014).
Weighted polyepigenetic GC exposure score was calculated from the selected 24 CpG sites as described previously (Provençal et al., 2020). The methylation level of each site was multiplied by the weight and summed to get the score for each sample. The weights represent the coefficients from the elastic-net regression using dexamethasone associated changes in DNA methylation of the CpG sites in peripheral blood in the MPIP cohort (Provençal et al., 2020). Appendix A, Figure A1 shows the distribution of the weighted polyepigenetic GC exposure score in the sample (Fig. A1).
2.3. Child cord blood cell counts at birth
Cord blood cell composition was estimated for seven cell types (nucleated red blood cells, granulocytes, monocytes, natural killer cells, B cells, CD4(+)T cells, and CD8(+)T cells) following the Bakulski et al. method (Bakulski et al., 2016) using the R-package minfi (Aryee et al., 2014).
2.4. Child genotyping and multidimensional scaling analysis
Genotyping was performed on Illumina Human Omni Express Exome Arrays (Illumina Inc., San Diego, CA). Only markers with a call rate over 98%, minor allele frequency of 1% and a p-value for deviation from Hardy–Weinberg equilibrium > × 10–06 were kept in the analysis. We performed multidimensional scaling (MDS) analysis on the identity by state matrix of quality-controlled genotypes and identified two MDS components depicting the population structure.
2.5. Child mental and behavioral disorders and total behavior problems
We identified any mental and behavioral disorder diagnosis in children from the Care Register for Health Care (HILMO) between the child's birth in 07.11.2006–24.07.2010 and December 31, 2016. The HILMO includes primary and subsidiary diagnoses of all inpatient treatments and of outpatient treatments in public specialized medical care settings in Finland. We included diagnoses coded F00–F99 according to International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) in the mental and behavioral disorder category. Validation studies have indicated that HILMO has high validity for psychiatric diagnoses (Sund, 2012). To study severity of any mental and behavioral disorder diagnosis, we summed up the number of days the child had been receiving inpatient or outpatient treatment for mental or behavioral disorder as the primary diagnosis.
Total behavior problems were mother-rated using the Child Behavior Checklist for ages 1½-5 (Achenbach and Rescorla, 2000). The score captures problems in behaviors related to aggression, anxiety, depression, attention, rule breaking, somatic complains, social problems, thought problems and withdrawn behavior.
2.6. Maternal depressive and anxiety symptoms during pregnancy
The women completed the questionnaires on depressive and anxiety symptoms biweekly up to 14 times throughout pregnancy between 12-13 and 38–39 gestational weeks/delivery. Depressive symptoms during the past week were measured using the Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff, 1977). The 20 CES-D items were rated from none (0) to all of the time (3), and a sum score was calculated ranging from 0 to 60.
Anxiety symptoms, reflecting the present state, were measured using the Spielberger State Anxiety Inventory (STAI) (Spielberg et al., 1970). The 20 STAI items were rated from not at all (1) to very much (4), and a sumscore was calculated ranging from 20 to 80.
We used the 14 time-points raw data to calculate the mean of the CES-D and STAI scores to represent the overall depressive and anxiety symptoms across pregnancy.
2.7. Covariates
All analyses were adjusted for cord blood cell type composition, the first two genetic MDS components, child's sex, child's birth year, gestational age at birth, maternal age at delivery (years), maternal smoking during pregnancy (yes/no), maternal education (primary or secondary/tertiary), having any of the cardiometabolic conditions during pregnancy (hypertensive disorders, gestational diabetes mellitus, type 1 diabetes, or pre-pregnancy BMI≥25 kg/m2]), and maternal lifetime diagnosis of any mental disorder. Maternal lifetime diagnosis of any mental disorder were derived from HILMO in- and outpatient visits (any/no; ICD-9: 290–319, ICD-10: F00–F99 diagnosis codes with inpatient data available between 1987 and 2016 and outpatient data available between 1998 and 2016).
In the analysis of the severity of the mental and behavioral disorders and total behavior problems, we made additional adjustments for follow-up time.
2.8. Statistical analyses
With Cox Proportional Hazards models, we estimated the associations between the GC exposure score and any mental and behavioral disorder in children. Before applying the Cox regression analyses, we tested if the hazard ratios (HR) changed across time. The time-dependent Cox regression analysis detected no time-dependent effects (p = 0.87). The proportional hazards assumptions were met, there were no outliers, and there was no non-linearity.
We studied the association between the GC exposure score and severity of any mental and behavioral disorder in children using Zero-Inflated Negative Binomial (ZINB) regression analysis (Zaninotto and Falaschetti, 2011) to account for the excessive number of zeros in the outcome count variable. Multiple linear regression tested associations between the GC exposure score and total behavior problems in children.
We pursued to test whether polyepigenetic GC exposure score mediated the association between maternal depressive and anxiety symptoms during pregnancy and any mental and behavioral disorder, its severity and total behavior problems in children using Sobel test. For the Sobel test, we derived effect size estimates from Cox Proportional Hazards Model (any mental and behavioral disorder as the outcome), ZINB (severity of mental and behavioral disorders as the outcome) and linear regression (total behavior problems and polyepigenetic GC exposure score as the outcome). Mediation tests were conducted pending that the criteria for mediation were met, i.e., that the predictor, mediator and the outcome variables were significantly interrelated.
As effect sizes from Cox models, we present HRs and from linear regression unstandardized regression coefficients. From the ZINB regression, we report the hurdle negative binomial count model estimates, which reflect the exponential value per unit increase of the predictor variable. We further present contrast estimates that reflect the inverse number of the exponential values and represent the number of days in inpatient or outpatient hospital treatment in our analyses. We also present the 95% CIs for all estimates; two-sided p-values < 0.05 were regarded as significant.
In Cox models, we stratified the analysis by child's sex and then added all the other covariates into the models. In the ZINB, logit and linear regression analysis, we made adjustments for all the covariates. In addition, the ZINB model was adjusted for the length of follow-up to the child's last hospital discharge, the logit model was adjusted for child's birth year, and linear model predicting child outcome for child's age at testing.
Statistical analyses were performed with SAS 9.4 and IBM SPSS Statistics 25.0.
3. Results
Characteristics of the sample are presented in Table 1. There were 99 (12.2%) children diagnosed with any mental or behavioral disorder during the follow-up. Of them, all 99 children received outpatient treatment in public specialized medical care settings and 8 were additionally hospitalized to inpatient treatment (Table 1). Of the mental and behavioral disorder diagnoses, psychological development disorders (n = 64, 64.6%) and emotional and behavioral disorders (n = 49, 49.5%) were the most common (Table A1). Of the 24 comorbid mental and behavioral disorder diagnoses (Table A1), 22 (91.7%) were comorbid psychological development and emotional and behavioral disorders. Compared with children with no mental disorder, those with any mental disorder diagnosis were more often boys and had higher total behavior problems scores, their mothers had lower education, higher early pregnancy BMI, higher anxiety symptoms during pregnancy, and more often a lifetime diagnosis of any mental disorder (p-values<0.05). There were no significant differences in the other characteristics (Table 1).
Table 1.
Characteristics of the sample.
| Total Sample (N = 814) |
Сhild Has a Diagnosis of Any Mental or Behavioral Disorder (N = 99) |
Сhild Has No Diagnosis of Mental or Behavioral Disorder (N = 715) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean/N | SD/% | Range | Mean/N | SD/% | Range | Mean/N | SD/% | Range | P | |
| Child characteristics | ||||||||||
| Sex (Boys) | 432 | 53.1 | 72 | 72.7 | 360 | 50.3 | <0.001 | |||
| Age at Follow-Up (until December 31, 2016) (Years) | 8.46 | 0.77 | 7.06–10.66 | 8.43 | 0.77 | 7.09–10.53 | 8.46 | 0.77 | 7.06–10.66 | 0.73 |
| Polyepigenetic GC Exposure Score | −1.13 | 0.21 | −1.78–−0.49 | −1.14 | 0.19 | −1.72–−0.75 | −1.13 | 0.21 | −1.78–−0.49 | 0.59 |
| Gestational Age (weeks) | 39.77 | 1.60 | 31.00–42.71 | 39.80 | 1.66 | 31.57–42.14 | 39.76 | 1.59 | 31.00–42.71 | 0.84 |
| Inpatient Treatment for Any Mental or Behavioral Disorder (yes) | 8 | 1.0 | 8 | 8.1 | NA | NA | NA | NA | ||
| Outpatient Treatment for Any Mental or Behavioral Disorder (yes) | 99 | 100 | 99 | 100 | NA | NA | NA | NA | ||
| Number of Days the Child Has Been in In- or Outpatient Treatment for Any Mental or Behavioral Disorder as the Primary Diagnosis (days) | 1.77 | 8.89 | 0.00–108.00 | 14.57 | 21.63 | 1.00–108.00 | NA | NA | NA | NA |
| Inpatient | 0.07 | 0.96 | 0.00–18.00 | 0.60 | 2.69 | 0.00–18.00 | NA | NA | NA | NA |
| Outpatient | 1.70 | 8.76 | 0.00–108.00 | 13.97 | 21.52 | 1.00–108.00 | NA | NA | NA | NA |
| Total Behavior Problems | 24.93 | 15.67 | 0.00–91.00 | 33.44 | 20.16 | 4.00–91.00 | 23.87 | 14.72 | 0.00–88.00 | p < 0.001 |
| Maternal Characteristics | ||||||||||
| Age at Delivery (years) | 33.29 | 5.78 | 17.17–47.39 | 32.90 | 5.83 | 19.47–44.56 | 33.34 | 5.78 | 17.17–47.39 | 0.48 |
| Education | 0.002 | |||||||||
| Primary/Secondary | 358 | 45.3 | 59 | 60.2 | 299 | 43.2 | ||||
| Tertiary | 432 | 54.7 | 39 | 39.8 | 393 | 56.8 | ||||
| Smoking during Pregnancy (yes) | 34 | 4.2 | 5 | 5.1 | 29 | 4.0 | 0.64 | |||
| Hypertension Disorder During Pregnancy (yes) | 276 | 33.9 | 41 | 41.4 | 235 | 32.8 | 0.092 | |||
| Gestational Diabetes Mellitus (yes) | 182 | 22.4 | 27 | 27.6 | 155 | 21.7 | 0.20 | |||
| Type 1 Diabetes Mellitus (yes) | 11 | 1.4 | 3 | 3.1 | 8 | 1.1 | 0.12 | |||
| Pre-Pregnancy BMI (kg/m2) | 27.27 | 6.78 | 17.30–55.00 | 28.63 | 6.46 | 17.51–49.82 | 27.24 | 6.40 | 17.30–55.00 | 0.044 |
| Lifetime Diagnosis of Any Mental Disorder (yes) | 130 | 16.0 | 27 | 27.3 | 103 | 14.4 | 0.001 | |||
| Antenatal Mean Score of Depressive Symptoms (CES-D) | 11.32 | 6.47 | 0.50–44.69 | 12.70 | 7.62 | 0.57–44.69 | 11.13 | 6.28 | 0.50–38.64 | 0.055 |
| Antenatal Mean Score of State-Trait Anxiety (STAI) | 33.91 | 7.90 | 20.36–67.76 | 35.97 | 8.70 | 20.43–58.07 | 33.62 | 7.75 | 20.36–67.76 | 0.019 |
P refers to p-values derived from the t-test and Chi-square test when comparing the children with any mental or behavioral disorder diagnosis (N = 99) with the children with no diagnosis for mental or behavioral disorder (N = 715).
For total behavior problems the sample size is n = 408: children with diagnosis, n = 45, children without diagnosis n = 363.
Abbreviations: GC, glucocorticoid; BMI, body mass index; CES-D, Center for epidemiologic studies depression scale; STAI, Spielberger state anxiety inventory.
Among the 99 children with a diagnosis of any mental and behavioral disorder, the median number of days spent in inpatient or outpatient treatment for any mental and behavioral disorder as the primary diagnosis was 7 (Interquartile Range = 2.00–18.00) days. Table A1 demonstrates that children who had spent 7 days or more in inpatient or outpatient treatment had more often a psychological development or emotional and behavioral disorder diagnosis and less often a diagnosis of behavioral syndromes associated with physiological disturbances and physical factors than children who had spent less than 7 days in treatment (p-values <0.035). They also more often had comorbid diagnosis in more than one broad category (p < 0.001) (Table A1).
Of the covariates, child's sex, gestational age at birth, birth year, maternal age at delivery, education, smoking during pregnancy, having any cardiometabolic condition and maternal lifetime diagnosis of any mental disorder were not significantly associated with the child's polyepigenetic GC exposure score (p > 0.09) (Table A2).
3.1. Polyepigenetic GC exposure score and child mental and behavioral disorders
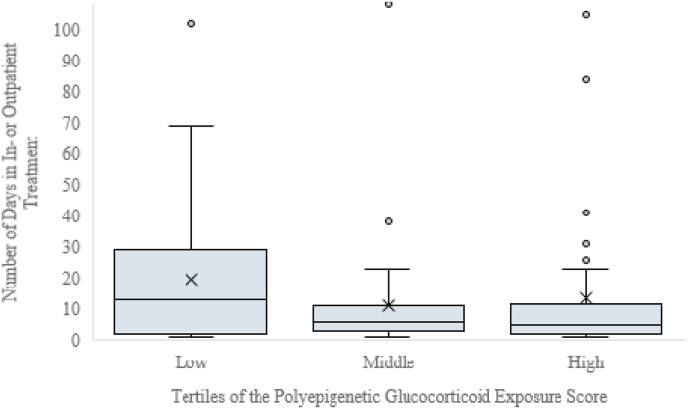
Polyepigenetic GC exposure score was not significantly associated with the hazard of being diagnosed with any mental and behavioral disorder in children (HR = 0.82, 95% CI 0.54; 1.24, p = 0.35). It was not significantly associated with the mother-rated total behavioral problems either (unstandardized beta = −0.10, 95% CI -0.31; 0.10, p = 0.33). However, lower polyepigenetic score was significantly associated with more days spent in inpatient or outpatient treatment for any mental or behavioral disorder as the primary diagnosis (hurdle model estimate = -1.08 natural logarithm units per each standard deviation (SD) unit increase in polyepigenetic score; 95% CI -1.70; −0.46, p = 0.001). This translated into 2.94 (95% CI 1.59; 5.45, p = 0.001) more days spent in in- or outpatient treatment per each SD unit decrease in the polyepigenetic score according to contrast estimate results (Fig. 2). A scatterplot with a smooth fitted line (loess fit) indicated that the association between the GC exposure score and the number of days the child had spent in inpatient or outpatient treatment was not non-linear and that there was no indication of a clear threshold effect (Figure A2).
Fig. 2.
The number of days the child has spent in any inpatient treatment or in outpatient treatment in public specialized medical care with any mental or behavioral disorder as the primary diagnosis (N = 99) according to the polyepigenetic glucocorticoid exposure score at birth categorized into tertiles. Horizontal lines refer to the medians and inter-quartile ranges and cross marks to the mean values.
3.2. Polyepigenetic GC exposure score as a mediator
Before proceeding to mediation analyses, we tested if the criteria for mediation were met. We have previously shown in a subsample of the current sample, namely in women who reported depressive and anxiety symptoms during pregnancy that higher maternal anxiety and depressive symptoms were associated with a lower polyepigenetic score in the offspring (Provençal et al., 2020), and the above analyses showed that the score was also associated with a higher number of days spent in inpatient or outpatient treatment. However, in the subsample of the 583 women who reported depressive and anxiety symptoms during pregnancy, these symptoms were not significantly associated with the number of days the child had spent in the inpatient or outpatient treatment (depressive symtpoms: hurdle model estimate = 0.003; 95% CI -0.06; 0.07, p = 0.94; anxiety symptoms: hurdle model estimate = 0.02; 95% CI -0.04; 0.07, p = 0.58). Hence, we did not pursue mediation.
However, in post hoc analyses in the entire PREDO cohort with data available on maternal depressive (n = 3404) and anxiety symptoms (n = 3405) during pregnancy, higher maternal depressive and anxiety symptoms were associated with 1.31 (95% CI 1.07; 1.61; (hurdle model estimate = 0.27, 95% CI 0.06; 0.48, p = 0.011) and 1.32 (95% CI 1.08; 1.62; hurdle model estimate = 0.28, 95% CI 0.08; 0.48, p = 0.007) more days spent in inpatient or outpatient treatment, respectively, per each SD unit increase in the symptomatology. This is suggestive of a possible mediation effect in a cohort with greater statistical power.
4. Discussion
We showed here that a novel polyepigenetic biomarker based on 24 CpG sites reflecting fetal GC exposure at birth was not significantly associated with higher hazard for any childhood mental or behavioral disorder or with mother-reported total behavior problems. However, it was significantly associated with the severity of any mental or behavioral disorder diagnosis, such that lower polyepigenetic GC exposure score at birth was associated with more days spent in inpatient or outpatient treatment for any mental and behavioral disorder as the primary diagnosis. For each SD unit decrease in this score, the child had spent almost three more days in inpatient or outpatient treatment. These findings, thus, suggest that this novel polyepigenetic biomarker may contribute to identification of children at birth who are at risk for more severe mental and behavioral disorders, enabling timely targeted preventive interventions, before any manifest symptoms or disorders occur.
In our study sample, we have previously shown that the fetal polyepigenetic GC exposure score correlated with maternal depressive and anxiety symptoms during pregnancy (Provençal et al., 2020). However, in the subsample studied here, maternal depressive and anxiety symptoms were not associated with the severity of child mental and behavioral disorders. Hence, our study did not allow us to test for mediation. However, in a larger sample of the PREDO study, for whom we missed cord blood samples, maternal higher depressive and anxiety symptoms were significantly associated with 1.3 more days spent in in- or outpatient treatment, suggesting insufficient power in the current subset to detect this association.
The fact that a lower polyepigenetic GC exposure score was associated with higher severity of child mental and behavioral disorders is in line with an increased prenatal GC exposure in the severely affected children. We have previously shown that a lower polyepigenetic GC exposure score is reflective of DNA de-methylation with GR activation (Provençal et al., 2020). In fact, exposure to GC has been associated with DNA de-methylation specifically at glucocorticoid-responsive elements (Wiechmann et al., 2019). These findings would suggest that children with more severe symptoms have been exposed to more GC prenatally or are more sensitive, which is reflected in their epigenetic profiles. This may be indicative of an increased priming of target genes to subsequent stress exposure, as suggested by our findings in hippocampal progenitor cells (Provençal et al., 2020). Furthermore, combining GC exposure of human cerebral organoids as a model of early brain development and single cell sequencing, we showed that GC target genes in the developing brain are enriched for genes that have been associated with neurodevelopmental disorders and psychiatric disease, including major depression, schizophrenia as well as cross disorders risk in large genome-wide association studies (Cruceanu et al., 2020). The strongest disease enrichments were found for transcripts regulated in late neuronal progenitors and neurons. This would suggest that prenatal GC exposure may contribute to the increased risk for more severe mental and behavioral disorders observed in the offspring via lasting epigenetic changes in relevant neuronal target genes and that this risk maybe exacerbated in with additional genetic risk (O'Donnell and Meaney, 2017).
Our study has several strengths. First, our study design was prospective, and our mother–child cohort was well-characterized. This allowed us to account for several important covariates in our analyses that have been shown to alter cord blood methylation levels, including cell-type composition, genetic background, child's sex, gestational age at birth and age at follow-up, maternal age at delivery, smoking during pregnancy, level of education, cardiometabolic conditions during pregnancy, and maternal lifetime diagnosis of any mental disorder (Alfano et al., 2019; Bakulski et al., 2016; Barfield et al., 2014; Girchenko et al., 2017b; Rauschert et al., 2019).
Second, our study utilized data from a validated nationwide healthcare registry on child mental and behavioral disorder diagnoses as well as on the number of days the child had been in in- or outpatient treatment for these disorders as the primary diagnosis (Sund, 2012). This is likely to decrease the common-method bias present in studies where both the predictor and the outcome are reported by the same person (Podsakoff et al., 2003). In addition, since the registry data has almost 100% coverage, registry data attrition in our study was nearly zero (0.4%), which increases generalizability from our findings. However, data attrition was almost 50% for mother-reported total behavior problems in children, which limits generalizability and statistical power.
Furthermore, the polyepigenetic score was derived from cross-tissue overlap of GC responsive CpG sites of neuronal cell lines and peripheral blood. In fact, DNA methylation patterns are largely tissue specific, so a cross-tissue score is relevant as a biomarker for deciphering the potential long-term impact of stress. In addition, the use of a pharmacological stimulus allows better interpretation of the directionality of the observed association in a biological context – lower scores are reflective of more de-methylation with GC and thus increased prenatal GC exposure or increased epigenetic sensitivity to their effects.
The main limitation of our study relates to the comparatively low number of children with mental and behavioral disorders leading to decreased statistical power to study any mental and behavioral disorder in children. In proportion (12.1%) this number is, however, slightly higher in magnitude than that found in the general population of Finnish children (Räikkönen et al., 2020). Moreover, our study was limited to disorders carrying higher prevalence in childhood, namely psychological development and behavioral and emotional disorders. These disorders were also more prevalent in the children who had spent 7 or more days, the median number of, days in in- or outpatient treatment for mental and behavioral disorder as the primary diagnosis. As many psychiatric disorders manifest in adolescence and later in life (Paus et al., 2008) further follow-up of the PREDO cohort as the children age is warranted.
Another limitation is the epidemiological design, restricting causal inferences. Furthermore, since the vast majority of our sample came from the high-risk sample for preeclampsia and IUGR, population-wide studies are needed to confirm if our findings may extend to all pregnant women and their children. Furthermore, our sample was ethnically homogeneous with the majority of Caucasians, therefore, generalizability is limited.
Finally, even though we accounted for a number of important covariates, we cannot rule out that some other unmeasured factor might explain our findings, and hence cannot rule out residual confounding. Other factors operating in the postnatal environment may be driving the discovered associations. Such postnatal factors may include diet, neurotoxic exposures, use of corticosteroids, and stress, which may influence child DNAm, and thereby increase the child's risk for mental and behavioral disorders (Barker et al., 2018). It is yet unclear whether the initial prenatal insult may induce immediate pathophysiological outcomes and/or whether the initial exposure may lead to cellular reprogramming via DNAm and other biological mechanisms that prime differential responses to the same environmental conditions later on that then lead to pathology (McGowan and Matthews, 2018). Therefore, more longitudinal studies with multiple DNAm measurements and evaluation of polyepigenetic GC exposure scores, which account for both pre- and postnatal adversities in relation to child neurodevelopment, are needed.
5. Conclusions
Our findings showed that fetal polyepigenetic GC exposure score at birth was not significantly associated with a higher hazard for any mental or behavioral disorder or with mother-reported total behavior problems, but a lower fetal polyepigenetic GC exposure score at birth was associated with more severe mental or behavioral disorder as indicated by more days spent in inpatient or outpatient treatment with any mental or behavioral disorder as the primary diagnosis. The polyepigenetic biomarker of fetal GC exposure holds potential for early identification of children at risk for mental and behavioral disorders for timely targeted interventions and may also provide opportunities for developing therapeutic targets.
CRediT authorship contribution statement
Anna Suarez: Conceptualization, Formal analysis, Writing - original draft, Visualization. Jari Lahti: Supervision, Writing - review & editing, Funding acquisition. Marius Lahti-Pulkkinen: Formal analysis, Investigation, Data curation, Writing - review & editing. Polina Girchenko: Formal analysis. Darina Czamara: Methodology, Writing - review & editing. Janine Arloth: Methodology, Software. Anni LK. Malmberg: Formal analysis. Esa Hämäläinen: Conceptualization, Investigation, Writing - review & editing. Eero Kajantie: Conceptualization, Investigation, Writing - review & editing. Hannele Laivuori: Conceptualization, Investigation, Writing - review & editing. Pia M. Villa: Conceptualization, Investigation. Rebecca M. Reynolds: Conceptualization, Investigation, Writing - review & editing. Nadine Provençal: Methodology, Software, Writing - review & editing. Elisabeth B. Binder: Conceptualization, Methodology, Writing - review & editing. Katri Räikkönen: Supervision, Conceptualization, Project administration, Funding acquisition, Writing - review & editing.
Declaration of competing interest
None.
Acknowledgements
This work was supported by the Academy of Finland, European Union's Horizon 2020 Award (grant number SC1-2016-RTD-733280) for RECAP, European Commission Dynamics of Inequality Across the Life-course: structures and processes (DIAL) (grant number 724363) for PremLife, EVO (special state subsidy for research), Signe and Ane Gyllenberg Foundation, Orion Research Foundation, Emil Aaltonen Foundation, Finnish Medical Foundation, Jane and Aatos Erkko Foundation, Novo Nordisk Foundation, Päivikki and Sakari Sohlberg Foundation, Sigrid Juselius Foundation, Sir Jules Thorn Charitable Trust, Doctoral School of Psychology, Learning and Education, and University of Helsinki Research Funds.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2020.100275.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Achenbach T.M., Rescorla L.A. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2000. Manual for the ASEBA Preschool Forms & Profiles. [Google Scholar]
- Adamson B., Letourneau N., Lebel C. Prenatal maternal anxiety and children's brain structure and function: a systematic review of neuroimaging studies. J. Affect. Disord. 2018;241:117–126. doi: 10.1016/j.jad.2018.08.029. [DOI] [PubMed] [Google Scholar]
- Alfano R., Guida F., Galobardes B., Chadeau-Hyam M., Delpierre C., Ghantous A., Henderson J., Herceg Z., Jain P., Nawrot T.S., Relton C., Vineis P., Castagne R., Plusquin M. Socioeconomic position during pregnancy and DNA methylation signatures at three stages across early life: epigenome-wide association studies in the ALSPAC birth cohort. Int. J. Epidemiol. 2019;48:30–44. doi: 10.1093/ije/dyy259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryee M.J., Jaffe A.E., Corrada-Bravo H., Ladd-Acosta C., Feinberg A.P., Hansen K.D., Irizarry R.A. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics. 2014;30:1363–1369. doi: 10.1093/bioinformatics/btu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakulski K.M., Feinberg J.I., Andrews S.V., Yang J., Brown S., McKenney L., Witter S.F., Walston J., Feinberg A.P., Fallin M.D. DNA methylation of cord blood cell types: applications for mixed cell birth studies. Epigenetics. 2016;11:354–362. doi: 10.1080/15592294.2016.1161875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barfield R.T., Almli L.M., Kilaru V., Smith A.K., Mercer K.B., Duncan R., Klengel T., Mehta D., Binder E.B., Epstein M.P., Ressler K.J., Conneely K.N. Accounting for population stratification in DNA methylation studies. Genet. Epidemiol. 2014;38:231–241. doi: 10.1002/gepi.21789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker E.D., Walton E., Cecil C.A.M. Annual Research Review: DNA methylation as a mediator in the association between risk exposure and child and adolescent psychopathology. J. Child Psychol. Psychiatry Allied Discip. 2018;59:303–322. doi: 10.1111/jcpp.12782. [DOI] [PubMed] [Google Scholar]
- Bartlett A.A., Lapp H.E., Hunter R.G. Epigenetic mechanisms of the glucocorticoid receptor. Trends Endocrinol. Metab. 2019;30:807–818. doi: 10.1016/j.tem.2019.07.003. [DOI] [PubMed] [Google Scholar]
- Bennett H.A., Einarson A., Taddio A., Koren G., Einarson T.R. Prevalence of depression during pregnancy: systematic review. Obstet. Gynecol. 2004;103:698–709. doi: 10.1097/01.AOG.0000116689.75396.5f. [DOI] [PubMed] [Google Scholar]
- Clayton D.F., Anreiter I., Aristizabal M., Frankland P.W., Binder E.B., Citri A. The role of the genome in experience-dependent plasticity: extending the analogy of the genomic action potential. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117:23252–23260. doi: 10.1073/pnas.1820837116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruceanu A.C., Dony L., Krontira A.C., Fischer D.S., Roeh S., Koedel M., Sauer S., Rex-haffner M., Cappello S., Theis F.J., Elisabeth B. bioRxiv; 2020. Cell-type Specific Impact of Glucocorticoid Receptor Activation on the Developing Brain. [DOI] [PubMed] [Google Scholar]
- Evans L.M., Myers M.M., Monk C. Pregnant women's cortisol is elevated with anxiety and depression - but only when comorbid. Arch. Womens. Ment. Health. 2008;11:239–248. doi: 10.1007/s00737-008-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald E.R., Wand G.S., Seifuddin F., Yang X., Tamashiro K.L., Potash J.B., Zandi P., Lee R.S. Alterations in DNA methylation of Fkbp5 as a determinant of blood-brain correlation of glucocorticoid exposure. Psychoneuroendocrinology. 2014;44:112–122. doi: 10.1016/j.psyneuen.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girchenko P., Hämäläinen E., Kajantie E., Pesonen A.-K., Villa P., Laivuori H., Räikkönen K. Prediction and prevention of preeclampsia and intrauterine growth restriction (PREDO) study. Int. J. Epidemiol. 2017;46:1380–1381. doi: 10.1093/ije/dyw154. [DOI] [PubMed] [Google Scholar]
- Girchenko P., Lahti J., Czamara D., Knight A.K., Jones M.J., Suarez A., Hämäläinen E., Kajantie E., Laivuori H., Villa P.M., Reynolds R.M., Kobor M.S., Smith A.K., Binder E.B., Räikkönen K. Associations between maternal risk factors of adverse pregnancy and birth outcomes and the offspring epigenetic clock of gestational age at birth. Clin. Epigenet. 2017;9:49. doi: 10.1186/s13148-017-0349-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitau R., Cameron A., Fisk N.M., Glover V. Fetal exposure to maternal cortisol. Lancet. 1998;352:707–708. doi: 10.1016/S0140-6736(05)60824-0. [DOI] [PubMed] [Google Scholar]
- Graignic-Philippe R., Dayan J., Chokron S., Jacquet A.Y., Tordjman S. Effects of prenatal stress on fetal and child development: a critical literature review. Neurosci. Biobehav. Rev. 2014;43:137–162. doi: 10.1016/j.neubiorev.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Grote N.K., Bridge J.A., Gavin A.R., Melville J.L., Iyengar S., Katon W.J. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch. Gen. Psychiatr. 2010;67:1012–1024. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston D., Kehler H., Austin M.P., Mughal M.K., Wajid A., Vermeyden L., Benzies K., Brown S., Stuart S., Giallo R. Trajectories of maternal depressive symptoms during pregnancy and the first 12 months postpartum and child externalizing and internalizing behavior at three years. PloS One. 2018;13 doi: 10.1371/journal.pone.0195365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T., Mehta D., Anacker C., Rex-haffner M., Jens C., Pariante C.M., Pace T.W.W., Mercer K.B., Helen S., Ressler K.J., Rein T., Binder E.B. Allele-specific FKBP5 DNA demethylation mediates gene–childhood trauma interactions. Nat. Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress C., Thomassin H., Grange T. Active cytosine demethylation triggered by a nuclear receptor involves DNA strand breaks. Proc. Natl. Acad. Sci. U.S.A. 2006;103:11112–11117. doi: 10.1073/pnas.0601793103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundakovic M., Jaric I. The epigenetic link between prenatal adverse environments and neurodevelopmental disorders. Genes. 2017;8:104. doi: 10.3390/genes8030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti-Pulkkinen M., Cudmore M.J., Haeussner E., Schmitz C., Pesonen A.K., Hämäläinen E., Villa P.M., Mehtälä S., Kajantie E., Laivuori H., Reynolds R.M., Frank H.G., Räikkönen K. Placental morphology is associated with maternal depressive symptoms during pregnancy and toddler psychiatric problems. Sci. Rep. 2018;8:1–12. doi: 10.1038/s41598-017-19133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti M., Savolainen K., Tuovinen Soile, Pesonen A.-K., Lahti J., Heinonen K., Hämäläinen E., Laivuori H., Villa P.M., Reynolds R.M., Kajantie E., Räikkönen K. Maternal depressive symptoms during and after pregnancy and psychiatric problems in children. J. Am. Acad. Child Adolesc. Psychiatry. 2017;56:698–709. doi: 10.1016/j.jaac.2016.10.007. [DOI] [PubMed] [Google Scholar]
- Madigan S., Oatley H., Racine N., Fearon R.M.P., Schumacher L., Akbari E., Cooke J.E., Tarabulsy G.M. A meta-analysis of maternal prenatal depression and anxiety on child socioemotional development. J. Am. Acad. Child Adolesc. Psychiatry. 2018;57:645–657. doi: 10.1016/j.jaac.2018.06.012. e8. [DOI] [PubMed] [Google Scholar]
- Marcus S.M., Flynn H. a, Blow F.C., Barry K.L. Depressive symptoms among pregnant women screened in obstetrics settings. J. Womens. Health (Larchmt). 2003;12:373–380. doi: 10.1089/154099904322836528. [DOI] [PubMed] [Google Scholar]
- McGowan P.O., Matthews S.G. Prenatal stress, glucocorticoids, and developmental programming of the stress response. Endocrinology. 2018;159:69–82. doi: 10.1210/en.2017-00896. [DOI] [PubMed] [Google Scholar]
- Morin A.M., Gatev E., McEwen L.M., MacIsaac J.L., Lin D.T.S., Koen N., Czamara D., Räikkönen K., Zar H.J., Koenen K., Stein D.J., Kobor M.S., Jones M.J. Maternal blood contamination of collected cord blood can be identified using DNA methylation at three CpGs. Clin. Epigenet. 2017;9:75. doi: 10.1186/s13148-017-0370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor T.G., Tang W., Gilchrist M.A., Moynihan J.A., Pressman E.K., Blackmore E.R. Diurnal cortisol patterns and psychiatric symptoms in pregnancy: short-term longitudinal study. Biol. Psychol. 2014;96:35–41. doi: 10.1016/j.biopsycho.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell K.J., Meaney M.J. Fetal origins of mental health: the developmental origins of health and disease hypothesis. Am. J. Psychiatr. 2017;174:319–328. doi: 10.1176/appi.ajp.2016.16020138. [DOI] [PubMed] [Google Scholar]
- Painter R.C., Roseboom T.J., de Rooij S.R. Long-term effects of prenatal stress and glucocorticoid exposure. Birth Defects Res. Part C Embryo Today - Rev. 2012;96:315–324. doi: 10.1002/bdrc.21021. [DOI] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsakoff P.M., MacKenzie S.B., Lee J.Y., Podsakoff N.P. Common method biases in behavioral research: a critical review of the literature and recommended remedies. J. Appl. Psychol. 2003;88:879–903. doi: 10.1037/0021-9010.88.5.879. [DOI] [PubMed] [Google Scholar]
- Provençal N., Arloth J., Cattaneo A., Anacker C., Cattane N., Wiechmann T., Röh S., Ködel M., Klengel T., Czamara D., Müller N.S., Lahti J., Räikkönen K., Pariante C.M., Binder E.B. Glucocorticoid exposure during hippocampal neurogenesis primes future stress response by inducing changes in DNA methylation. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117:23280–23285. doi: 10.1073/pnas.1820842116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L.S. The CES-D scale: a self report depression scale for research in the general. Appl. Psychol. Meas. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Räikkönen K., Gissler M., Kajantie E. Associations between maternal antenatal corticosteroid treatment and mental and behavioral disorders in children supplemental content. J. Am. Med. Assoc. 2020;323:1924–1933. doi: 10.1001/jama.2020.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räikkönen K., Martikainen S., Pesonen A.K., Lahti J., Heinonen K., Pyhälä R., Lahti M., Tuovinen S., Wehkalampi K., Sammallahti S., Kuula L., Andersson S., Eriksson J.G., Ortega-Alonso A., Reynolds R.M., Strandberg T.E., Seckl J.R., Kajantie E. Maternal licorice consumption during pregnancy and pubertal, cognitive, and psychiatric outcomes in children. Am. J. Epidemiol. 2017;185:317–328. doi: 10.1093/aje/kww172. [DOI] [PubMed] [Google Scholar]
- Räikkönen K., Pesonen A.K., Heinonen K., Lahti J., Komsi N., Eriksson J.G., Seckl J.R., Järvenpää A.L., Strandberg T.E. Maternal licorice consumption and detrimental cognitive and psychiatric outcomes in children. Am. J. Epidemiol. 2009;170:1137–1146. doi: 10.1093/aje/kwp272. [DOI] [PubMed] [Google Scholar]
- Rauschert S., Melton P.E., Burdge G., Craig J., Godfrey K.M., Holbrook J.D., Lillycrop K., Mori T.A., Beilin L.J., Oddy W.H., Pennell C., Huang R.C. Maternal smoking during pregnancy induces persistent epigenetic changes into adolescence, independent of postnatal smoke exposure and is associated with cardiometabolic risk. Front. Genet. 2019;10:770. doi: 10.3389/fgene.2019.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R.M. Glucocorticoid excess and the developmental origins of disease: two decades of testing the hypothesis - 2012 Curt Richter Award Winner. Psychoneuroendocrinology. 2013;38:1–11. doi: 10.1016/j.psyneuen.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Robinson R., Lahti-Pulkkinen M., Heinonen K., Reynolds R.M., Räikkönen K. Fetal programming of neuropsychiatric disorders by maternal pregnancy depression: a systematic mini review. Pediatr. Res. 2019;85:134–145. doi: 10.1038/s41390-018-0173-y. [DOI] [PubMed] [Google Scholar]
- Spielberg C.D., Gorsuch R.L., Lushene R.E. Consulting Psychologists Press; Palo Alto, California: 1970. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- Stein A., Pearson R.M., Goodman S.H., Rapa E., Rahman A., McCallum M., Howard L.M., Pariante C.M. Effects of perinatal mental disorders on the fetus and child. Lancet. 2014;384:1800–1819. doi: 10.1016/S0140-6736(14)61277-0. [DOI] [PubMed] [Google Scholar]
- Sund R. Quality of the Finnish hospital discharge register: a systematic review. Scand. J. Publ. Health. 2012;40:505–515. doi: 10.1177/1403494812456637. [DOI] [PubMed] [Google Scholar]
- Toffol E., Lahti-Pulkkinen M., Lahti J., Lipsanen J., Heinonen K., Pesonen A.K., Hämäläinen E., Kajantie E., Laivuori H., Villa P.M., Räikkönen K. Maternal depressive symptoms during and after pregnancy are associated with poorer sleep quantity and quality and sleep disorders in 3.5-year-old offspring. Sleep Med. 2019;56:201–210. doi: 10.1016/j.sleep.2018.10.042. [DOI] [PubMed] [Google Scholar]
- Tuovinen S., Lahti-Pulkkinen M., Girchenko P., Lipsanen J., Lahti J., Heinonen K., Reynolds R.M., Hämäläinen E., Kajantie E., Laivuori H., Pesonen A.K., Villa P.M., Räikkönen K. Maternal depressive symptoms during and after pregnancy and child developmental milestones. Depress. Anxiety. 2018;35:732–741. doi: 10.1002/da.22756. [DOI] [PubMed] [Google Scholar]
- Van Batenburg-Eddes T., Brion M.J., Henrichs J., Jaddoe V.W.V., Hofman A., Verhulst F.C., Lawlor D.A., Davey Smith G., Tiemeier H. Parental depressive and anxiety symptoms during pregnancy and attention problems in children: a cross-cohort consistency study. J. Child Psychol. Psychiatry Allied Discip. 2013;54:591–600. doi: 10.1111/jcpp.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Bergh B.R.H. Developmental programming of early brain and behaviour development and mental health: a conceptual framework. Dev. Med. Child Neurol. 2011;53:19–23. doi: 10.1111/j.1469-8749.2011.04057.x. [DOI] [PubMed] [Google Scholar]
- Van den Bergh B.R.H., van den Heuvel M.I., Lahti M., Braeken M., de Rooij S.R., Entringer S., Hoyer D., Roseboom T., Räikkönen K., King S., Schwab M. Prenatal developmental origins of behavior and mental health: the influence of maternal stress in pregnancy. Neurosci. Biobehav. Rev. 2017;S0149–7634(16):30734–30735. doi: 10.1016/j.neubiorev.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Wiechmann T., Röh S., Sauer S., Czamara D., Arloth J., Ködel M., Beintner M., Knop L., Menke A., Binder E.B., Provençal N. Identification of dynamic glucocorticoid-induced methylation changes at the FKBP5 locus. Clin. Epigenet. 2019;11:83. doi: 10.1186/s13148-019-0682-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J., McCrone P., Patel A., Kaier K., Normann C. Predictors of length of stay in psychiatry: analyses of electronic medical records. BMC Psychiatr. 2015;15:238. doi: 10.1186/s12888-015-0623-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolford E., Lahti-Pulkkinen M., Girchenko P., Lipsanen J., Tuovinen S., Lahti J., Heinonen K., Hämäläinen E., Kajantie E., Pesonen A.K., Villa P.M., Laivuori H., Reynolds R.M., Räikkönen K. Associations of antenatal glucocorticoid exposure with mental health in children. Psychol. Med. 2019;50:247–257. doi: 10.1017/S0033291718004129. [DOI] [PubMed] [Google Scholar]
- Wolford E., Lahti M., Tuovinen S., Lahti J., Lipsanen J., Savolainen K., Heinonen K., Hämäläinen E., Kajantie E., Pesonen A.K., Villa P.M., Laivuori H., Reynolds R.M., Räikkönen K. Maternal depressive symptoms during and after pregnancy are associated with attention-deficit/hyperactivity disorder symptoms in their 3- to 6-year-old children. PloS One. 2017;12:1–13. doi: 10.1371/journal.pone.0190248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaninotto P., Falaschetti E. Comparison of methods for modelling a count outcome with excess zeros: application to Activities of Daily Living (ADL-s) J. Epidemiol. Community Health. 2011;65:205–210. doi: 10.1136/jech.2008.079640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.