Abstract
Objective
Exacerbated autonomic responses to acute stress are prevalent in posttraumatic stress disorder (PTSD). The purpose of this study was to assess the effects of transcutaneous cervical VNS (tcVNS) on autonomic responses to acute stress in patients with PTSD. The authors hypothesized tcVNS would reduce the sympathetic response to stress compared to a sham device.
Methods
Using a randomized double-blind approach, we studied the effects of tcVNS on physiological responses to stress in patients with PTSD (n = 25) using noninvasive sensing modalities. Participants received either sham (n = 12) or active tcVNS (n = 13) after exposure to acute personalized traumatic script stress and mental stress (public speech, mental arithmetic) over a three-day protocol. Physiological parameters related to sympathetic responses to stress were investigated.
Results
Relative to sham, tcVNS paired to traumatic script stress decreased sympathetic function as measured by: decreased heart rate (adjusted β = −5.7%; 95% CI: ±3.6%, effect size d = 0.43, p < 0.01), increased photoplethysmogram amplitude (peripheral vasodilation) (30.8%; ±28%, 0.29, p < 0.05), and increased pulse arrival time (vascular function) (6.3%; ±1.9%, 0.57, p < 0.0001). Similar (p < 0.05) autonomic, cardiovascular, and vascular effects were observed when tcVNS was applied after mental stress or without acute stress.
Conclusion
tcVNS attenuates sympathetic arousal associated with stress related to traumatic memories as well as mental stress in patients with PTSD, with effects persisting throughout multiple traumatic stress and stimulation testing days. These findings show that tcVNS has beneficial effects on the underlying neurophysiology of PTSD. Such autonomic metrics may also be evaluated in daily life settings in tandem with tcVNS therapy to provide closed-loop delivery and measure efficacy.
ClinicalTrials.gov Registration # NCT02992899.
Keywords: Posttraumatic stress disorder, Wearable bioelectronic medicine, Vagus nerve stimulation, Transcutaneous cervical stimulation, Stress, Electroceuticals
Highlights
-
•
We studied the effects of tcVNS on physiological responses to stress in patients with posttraumatic stress disorder (PTSD).
-
•
TcVNS modulates physiologic reactivity to acute traumatic and mental stress.
-
•
Peripheral autonomic measures could serve as real-time measures to evaluate the therapy response in longitudinal settings.
Introduction
Posttraumatic stress disorder (PTSD, a list of abbreviations was provided in Table 1) has a lifetime prevalence of 8% and is associated with considerable morbidity, loss of productivity, and treatment costs (Kilpatrick et al., 2013). Less than half of patients with PTSD seek or receive treatment, with existing treatment options exhibiting high (24%) dropout rates due to insufficient time with the mental health professional, treatment ineffectiveness, work interference, personal problems, or discomfort with how the medical professional interacted (Hoge et al., 2014). Cognitive behavioral therapy with prolonged exposure is an effective method to improve the PTSD symptoms in some patients, but requires considerable expertise, time, and resources (Bradley et al., 2005; Cukor et al., 2010). In addition, psychiatrists may hesitate to employ exposure therapies due to concerns about decompensation, discomfort in using exposure, and patients’ reluctance regarding re-exposure to traumatic reminders (Becker et al., 2004; Cahill et al., 2006). Pharmacological treatments represent another standard of treatment although questions persist regarding their efficacy (Hoskins et al., 2015; Kelmendi et al., 2016; Krystal et al., 2017). New, evidence-based treatments that align well with the clinical needs of those with PTSD are needed (Medicine Io, 2014; Frayne et al., 2011; Steenkamp et al., 2015).
Table 1.
Table of abbreviations.
| Term | Abbreviation | Term | Abbreviation |
|---|---|---|---|
| AC | Alternating current | PEP | Pre-ejection period |
| BOLD signal | Blood-oxygen-level-dependent signal | PNS | Parasympathetic nervous system |
| BP | Blood pressure | PP | Pulse pressure |
| BRS | Baroreflex sensitivity | PPG | Photoplethysmography |
| CAPS | Clinician-administered PTSD scale | PTSD | Posttraumatic stress disorder |
| CI | Confidence interval | PTT | Pulse transit time |
| CONSORT | Consolidated standards of reporting trials | RP | Respiration prominence |
| DBP | Diastolic blood pressure | RR | Respiratory rate |
| DC | Direct current | RSP | Respiration |
| ECG | Electrocardiography | RW | Respiration width |
| EDA | Electrodermal activity | SBP | Systolic blood pressure |
| fc | Cutoff frequency | SCG | Seismocardiography |
| fNSSCR | frequency of non-specific skin conductance responses | DSM | Diagnostic and statistical manual of mental disorders |
| GAD | Generalized anxiety disorder | SCID | Structured clinical interview for DSM disorders |
| (HF/LF) HRV | (High frequency/low frequency) heart rate variability | SCL | Skin conductance level |
| HR | Heart rate | SCR | Skin conductance response |
| HR-PET | High resolution positron emission tomography | SD1 | Poincare-based short-term HRV |
| (k)Hz | (kilo)Hertz | SD2 | Poincare-based short- and long-term HRV |
| LSCR | Latency of skin conductance response | SD | Standard deviation |
| MAP | Mean arterial pressure | SIAD | Substance induced anxiety disorder |
| MDD | Major depressive disorder | SNS | Sympathetic nervous system |
| OCD | Obsessive compulsive disorder | SPAWAR | Naval Information Warfare Systems Command |
| PAT | Pulse arrival time | (tc/ta) VNS | (Transcutaneous cervical/auricular) vagus nerve stimulation |
In patients with PTSD, exposure to events or stress—particularly those with salient characteristics related to previously experienced trauma—can elicit symptoms such as hyperarousal, intrusive thoughts, avoidance behaviors, and dissociation (Nemeroff et al., 2006). This adverse response can lead to elevated inflammatory marker concentrations, impaired autonomic modulation, memory deficits, changes in brain morphology, and increased neural reactivity in emotion-specific brain areas (Nemeroff et al., 2006; Sherin and Nemeroff, 2011; Yehuda and LeDoux, 2007; Shah et al., 2013; Bremner et al., 1993, 1997, 1999a, 1999b; Kitayama et al., 2005; Gill et al., 2009). Vagus nerve stimulation (VNS) is a potential treatment method for PTSD as it modulates sympathetic tone and related cardiovascular reactivity (Ardell et al., 2015). Moreover, it was shown that it enhances fear extinction in rodents trained with conditional fear paradigm (Peña et al., 2013a; Pena et al., 2014). However, VNS implants are limited by the cost and inconvenience of surgical procedures as revealed by the high rates of non-compliance in multi-year VNS studies due to the biological and psychological effects of the surgical implantation (Bremner and Rapaport, 2017; Aaronson et al., 2017). Recent advances in noninvasive neuromodulation technologies are promising for widespread use of VNS.
Transcutaneous VNS devices noninvasively target vagal projections in the ear (auricular branch of the vagus) and neck (cervical branch in the carotid sheath). Auricular tVNS (taVNS) devices modulate central and peripheral physiology, as observed with the monitoring of peripheral physiological parameters (Badran et al., 2018a; Bretherton et al., 2019; Clancy et al., 2014), inflammatory cytokines (Stavrakis et al., 2015, 2020,bib_Stavrakis_et_al_2015; Yu et al., 2017,bib_Stavrakis_et_al_2020), hormonal indices (Warren et al., 2019), brain imaging (Frangos et al., 2015; Yakunina et al., 2017; Garcia et al., 2017; Badran et al., 2018b), and a case study comparing VNS implant and taVNS for an epilepsy patient (Assenza et al., 2017). taVNS also has been shown to ameliorate tinnitus (Steenerson and Cronin, 2003; Yakunina et al., 2018), atrial fibrillation (Stavrakis et al., 2020), episodic migraine (Garcia et al., 2017), seizure frequency (Hamer and Bauer, 2019), cluster headache (Reuter et al., 2019), and major depression (Fang et al., 2016; Rong et al., 2016). In addition, it was shown to improve vagal tone (Badran et al., 2018a; Bretherton et al., 2019; Clancy et al., 2014), to deactivate limbic (amygdala, hippocampus, parahippocampal gyrus) and temporal (middle and superior temporal gyrus) brain areas as measured by BOLD-signal in functional magnetic resonance imaging (Kraus et al., 2007), and to improve mood (Kraus et al., 2007) in young healthy or old individuals. In a pilot study with patients with PTSD and mild traumatic brain injury (Lamb et al., 2017), studied that autonomic state and acoustic startle reflex were dampened with taVNS. Fewer studies have looked at cervical tVNS (tcVNS), although they have been shown to reliably activate vagal nerve fibers (Frangos and Komisaruk, 2017; Mourdoukoutas et al., 2018; Nonis et al., 2017), produce anti-inflammatory effects (Brock et al., 2017; Lerman et al., 2016; Tarn et al., 2019), reduce neural and physiologic responses to noxious thermal stimuli (Lerman et al., 2019) with possible clinical utility in migraine and trigeminal allodynia (Chen et al., 2016; Oshinsky et al., 2014). We have recently explored pairing of tcVNS with personalized traumatic script stress and non-personalized mental stress (public speaking, mental arithmetic) in healthy individuals with a history of exposure to psychological trauma without the current diagnosis of PTSD, and shown a reduction in cardiovascular reactivity and peripheral sympathetic activity both for personalized traumatic stress and neutral mental stress as well as in the absence of stress exposure (Gurel et al., 2020a). In this healthy sample, we also investigated high resolution positron emission tomography (HR-PET) findings, and our results suggested attenuation in the increased neural activity in the fear memory circuitry with tcVNS (Wittbrodt et al., 2020) in limbic and other brain areas (cortex areas, temporal lobe, parahippocampal gyrus, insula, and left anterior cingulate). Finally, we recently explored computational methods to model and predict the transient stimulation response (Gazi et al., 2020). In the current study, we examined the physiological effects of tcVNS in patients with PTSD to traumatic and mental stress. The physiological measurements included the collection of electrocardiography (ECG), seismocardiography (SCG), photoplethysmography (PPG), respiration (RSP), electrodermal activity (EDA), and blood pressure (BP). We hypothesized that tcVNS (compared to sham) would attenuate the physiological responses to stress in PTSD.
Materials and methods
Participants & assessments
The study was approved by the Institutional Review Boards of Georgia Institute of Technology, Emory University, SPAWAR Systems Center Pacific, and the Department of Navy Human Research Protection Program and was conducted at the Emory University School of Medicine between May 2017 and October 2019. It should be noted that the study registration (ClinicalTrials.gov # NCT02992899) included autonomic peripheral measures examined in this study, however these were not listed as primary or secondary outcomes, rather the outcomes focused on previously examined blood biomarkers by our group in PTSD populations (Lima et al., 2019). The physiological reactivity measures examined in this study are exploratory research questions that align well with the effects of tcVNS. Participants in the current study aged between 18 and 70 years with current PTSD as determined by the Structured Interview for Diagnostic and Statistical Manual of Mental Disorders (SCID) (First et al., 2004) and provided written, informed consent. Exclusion criteria were: pregnancy; meningitis; traumatic brain injury; neurological disorder; organic mental disorder; history of loss of consciousness greater than 1 min; alcohol abuse or substance abuse based on the SCID within the past 12 months; current or lifetime history of schizophrenia, schizoaffective disorder, or bulimia, based on the SCID; a history of serious medical or neurological illness, such as cardiovascular, gastrointestinal, hepatic, renal, neurologic or other systemic illness; evidence of a major medical or neurological illness on physical examination or as a result of laboratory studies; active implantable device (i.e. pacemaker); carotid atherosclerosis; cervical vagotomy. The Clinician Administered PTSD Scale (CAPS) was administered to establish current PTSD diagnosis and quantitate severity of symptoms (Blake et al., 1995). Although the results presented here focused on participants with PTSD, the overall study involved both PTSD and non-PTSD traumatized controls and the recruitments were made regardless of the PTSD status, depending on the availability of the participant pool. The physiological reactivity results regarding the non-PTSD population were published in (Gurel et al., 2020a, 2020b). Within the main (PTSD and non-PTSD) study, among 129 who were screened for eligibility (See Fig. S1 for Consolidated Standards of Reporting Trials (CONSORT) diagram), 69 were excluded due to lack of consent or not meeting the inclusion criteria. The remaining 60 were randomized to active stimulation or sham. Among 60 randomized participants, 9 (15% loss) were excluded due to either technical reasons or participant did not come. Among the remaining 51, 26 participants were traumatized controls without PTSD and have recently been published (Gurel et al., 2020a, 2020b, 2020c; Gazi et al., 2020). 25 participants were patients with PTSD, which are the focus of the current study. Table S1 presents the demographics data, Table S2 presents the comorbid and past diagnoses data. The mean age was 35 (±13 SD) with 19 females. The active group participants (n = 13) had a mean age of 33 (±12 SD) and included 12 females; sham group participants (n = 12) had a mean age of 38 (±13 SD), with seven females. SCID was used to evaluate for possible comorbid psychiatric diagnosis. In this sample, 11 (44%) met criteria for major depressive disorder (MDD, four current, seven past), seven (28%) for generalized anxiety disorder (GAD), four (16%) for panic disorder, two (8%) for social phobia, two (8%) for current obsessive compulsive disorder (OCD), one (4%) for agoraphobia without panic disorder, one (4%) for body dysmorphia, three (12%) for past alcohol abuse or dependence, and one (4%) for a past substance induced anxiety disorder (SIAD).
Study protocol
The three day protocol is summarized in Fig. S2. Participants were instructed to withhold any stimulant (i.e. coffee) throughout the entire protocol. Participants provided their traumatic experiences in written form, later, personalized traumatic stress scripts were prepared for each participant. On day one, participants were prepared with noninvasive sensing modalities (explained in physiological monitoring section), dedicated neuromodulation devices, and a headphone, by the researchers. The first day took place at Emory University Hospital at Wesley Woods (Atlanta, GA), at the same dedicated HR-PET room at ~20 °C temperature, approximately starting at 8:00 A M. The protocol started when they lay down in an HR-PET scanner bed (head inside the scanner) for 14 HR-PET scans, each took approximately 8 min. Before the scans started, baseline physiological and blood data were collected in the same posture. In the first two scans, “neutral” pleasant scenery recordings (1 min each, without stimulation) were delivered audibly. These were presented before traumatic stress scans for calculation of net activations in brain areas, as required by recently published brain imaging investigations (Wittbrodt et al., 2020). In scans three and four, traumatic stress recordings (1 min each) were delivered immediately followed by stimulation (2 min each). In scans five and six, stimulation was applied in the absence any acute stressor (2 min each). From scans seven to ten, two neutral recordings (1 min each, no stimulation) and two traumatic stress recordings (1 min each) followed by stimulation were applied. Then, the participants took a 90-min break. After the break, four more scans were taken that included two neutral recordings (1 min each) were and two traumatic stress recordings (1 min each) were followed by stimulation (2 min each), respectively. In short, the first day included audible delivery of six neutral scripts, six traumatic stress scripts followed by stimulation, and two stimulations without acute stress in 14 HR-PET scans. All neutral/traumatic recordings were approximately 1 min in duration. The second and third days were the same as each other: they did not include brain imaging, and they focused on non-personalized mental stressors. They took place in Emory Brain Health Center (Atlanta, GA) at the same dedicated room (~25 °C) with start time around 8:30 A M. Baseline signals were recorded from the participants, and they underwent a public speech (5 min) and a mental arithmetic task (3 min), both immediately followed by stimulation (2 min each). In the public speech task, the participants were given 2 min preparation time to prepare a defense statement in a scenario they were accused of theft at a shopping mall. Their speech was immediately followed by stimulation. Following 8 min in silence after stimulation stopped, the participants were required to answer a series of arithmetic questions as fast as possible for 3 min. Immediately after the arithmetic task, another stimulation was applied, and the participants waited for eight more minutes in silence (post-stimulation period). For both mental stress tasks, negative feedback was provided for incorrect answers and delayed response times to exaggerate the stress effect. After this mental stress paired with stimulation paradigm, a 90-min break was given. After the break, a third stimulation was applied without any acute stressor.
Transcutaneous cervical vagal nerve stimulation & blinding
Both active tcVNS and sham stimuli were administered using hand-held devices (GammaCore, ElectroCore, Basking Ridge, New Jersey) with identical appearance, placement, and operation. The researcher identified the carotid pulsation on the left neck, and collar electrodes were placed at this location, using conductive electrode gel (Gammacore, ElectroCore, Basking Ridge, New Jersey). Fig. S3b shows tcVNS electrode placement. Active tcVNS produces an AC voltage signal consisting of five 5 kHz sine waves, repeating at a rate of 25 Hz (once every 40 ms). The sham produces a biphasic, stepped voltage signal consisting of 0.2 Hz pulses (once every 5 s). The peak voltage amplitudes for active and sham device are 30 V and 14 V, respectively. Fig. S4 presents the active and sham stimulus waveforms. Throughout the protocol, the researcher gradually increased the stimulation intensity with a roll switch to the maximum the participant can tolerate, without pain. The active group received 20.3 V (±7.5 SD), and sham group received 13.6 V (±1.4 SD) averaged across all uses over three days. No participants reported lack of sensation. An active stimulation amplitude higher than 15 V using the studied device was previously reported to create vagal somatosensory evoked potentials associated with vagal afferent activation reliably, that are also activated with VNS implants (Nonis et al., 2017).
High frequency voltage signals (such as the active stimulus) pass through the skin with minimal power dissipation due to the low skin-electrode impedance at kHz frequencies; in contrast, lower frequency signals (such as the sham stimulus) are mainly attenuated at the skin-electrode interface due to the high impedance (Rosell et al., 1988). Accordingly, the active device operating at higher frequencies can deliver substantial energy to the vagus nerve to facilitate stimulation, while the voltage levels appearing at the vagus would be expected to be orders of magnitude lower for the sham device and thus vagal stimulation is highly unlikely. Nevertheless, since the sham device does deliver relatively high voltage and current levels directly to the skin, it activates skin nociceptors, causing a similar feeling to a pinch. This sensation is necessary for blinding of the participants, and is thought as a critical detail by the authors for the valuation of the potential treatment in psychiatric populations.
The randomization of the active tcVNS or sham stimulus groups were conducted with an online tool using simple randomization with group allocation completed by an individual who was dissociated from enrollment, data collection, or analysis. The devices were pre-numbered by the manufacturer who were not involved in the research design. The participants, clinical staff, and researchers were blinded to the stimulus type. Later, stimulus grouping (active or sham) was un-blinded for the interpretation of statistical analysis, after data processing was completed.
Physiological monitoring
Physiological data were collected by the measurement of ECG, PPG, SCG, EDA, RSP, and BP, Fig. S3a details the electrode placement for each participant. Wireless 3-lead ECG and piezoresistive strap-based RSP were collected through RSPEC-R amplifiers; transmissive, index finger-based PPG and inner palm-based EDA were collected through PPGED-R amplifiers from Biopac Systems (Goleta, CA). A low noise 356A32 accelerometer (PCB Electronics, Depew, NY) was taped with a Kinesio tape on mid-sternum for SCG monitoring, with Z-axis surface touching the sternum, aligning with the dorsoventral movement of the heart. For EDA measurement, an isotonic electrode gel (GEL101) and pre-gelled isotonic electrodes (EL507) were used (Biopac Systems, Goleta, CA). Continuous ECG, PPG, SCG, EDA, and RSP data were simultaneously transmitted to a 16-bit MP150 data acquisition system at 2 kHz sampling rate (Biopac Systems, Goleta, CA). Non-continuous, cuff-based systolic (SBP) and diastolic blood pressure (DBP) values were recorded periodically with an Omron blood pressure cuff for baseline, stress, stimulation, and post-stimulation intervals.
Signal processing & parameter extraction
The physiological signals were processed in MATLAB (R2020a, Mathworks, Natick MA) and the following parameters were extracted: heart rate (HR), pre-ejection period, amplitude of PPG, pulse arrival time (PAT), respiratory rate (RR), width (RW), respiration prominence (RP), low frequency and high frequency heart rate variability (LF HRV, HF HRV), non-linear heart rate variability (SD1, SD2), skin conductance level (SCL), skin conductance response (Bradley et al., 2008), frequency of non-specific skin conductance responses (fNSSCR), and latency of skin conductance response (LSCR). Table 2 presents information on these physiological parameters. Signal processing steps were matched to the previous work investigating non-PTSD population (Gurel et al., 2020a).
Table 2.
Physiological parameter definitions.
| Term | Abbreviation |
|---|---|
| DBP | Cuff-based diastolic blood pressure |
| SBP | Cuff-based systolic blood pressure |
| MAP | Average pressure in patient's arteries during one cardiac cycle ((SBP+2*DBP)/3) |
| PP | Difference between SBP and DBP |
| HR | Instantaneous heart rate calculated by locating the R-peaks of ECG signal |
| HF HRV | The power in the high-frequency range (0.15–0.4 Hz), obtained from the non-constant R-R intervals from ECG R-peaks. |
| LF HRV | The power in the low-frequency range (0.04–0.15 Hz), obtained from the non-constant R-R intervals from ECG R-peaks. |
| LF/HF HRV | The ratio of the two power bands, LF and HF HRV |
| SD1 | Poincare-based short-term HRV: Standard deviation of points perpendicular to the axis of line-of-identity of the scatter plot (R–Rn versus R–Rn+1) |
| SD2 | Poincare-based short- and long-term HRV: Standard deviation of points along the axis of line-of-identity of the scatter plot (R–Rn versus R–Rn+1) |
| SD1/SD2 | The unpredictability of R-R intervals based on Poincaré-based HRV |
| fNSSCR | Frequency of non-specific skin conductance responses |
| SCL | The slow tonic component of EDA signal |
| SCR | The faster phasic component of EDA signal |
| LSCR | Latency from the start of the interval to the first peak appearance in SCR signal |
| PAT | Time delay from the electrical depolarization of the heart to the arrival of the pressure wave |
| PPG Amplitude | Beat-by-beat amplitude of the peripheral blood volume pulse |
| PTT | The time it takes a pulse wave to travel between two arterial sites |
| PEP | Latency between the start of electrical depolarization of the ventricles to the opening of the aortic valve. |
| RR | Instantaneous rate of respiration peak appearance |
| RP | Prominence of each respiration peak. i.e., how much the peak stands out due to its intrinsic height and its location relative to other peaks |
| RW | The width of each respiration cycle |
Pre-processing
ECG, PPG, SCG signals were band-pass filtered with cut-off frequencies (fc) 0.6–40 Hz for ECG, 0.4–8 Hz for PPG, and 0.6–25 Hz for SCG to cancel the noise out of the bandwidth. EDA was high-pass filtered (fc = 0.15 Hz) to obtain the fast varying AC component. Its DC level was calculated from the signal's trend. The R-peaks of ECG were detected using thresholding (i.e. findpeaks MATLAB function), and were used to calculate HR and HRV. PPG and SCG signals were segmented referenced to the R-peaks of ECG signal. SCG and feature points related to continuous blood pressure on PPG are prone to artifacts: SCG captures heart motion, body motion, and vibration due to talking; motion artifacts in PPG might instantaneously push or move back the pulse arrival point on the waveform to calculate PAT. To mitigate these, an additional exponential moving averaging step (Inan et al., 2015) was carried out after segmenting for these signals, as described below. The extracted parameters are summarized as follows:
Heart rate and heart rate variability measure
Frequency-domain analysis and joint time-frequency analysis (Poincaré Method) were used to extract the autonomic measures LF HRV, HF HRV, LF/HF HRV, SD1, SD2, SD1/SD2 values. Based on previous studies, the LF HRV and SD2 are a reflection of baroreflex sensitivity (BRS) (Sleight et al., 1995). Individuals with PTSD are reported to have reduced BRS in previous studies (Shah et al., 2013; Park et al., 2017; Hughes et al., 2007; Ulmer et al., 2009), therefore these biomarkers of BRS have been computed. As low-frequency HRV measures require at least 5 min of continuous ECG signal, the comparisons from the start to the end of that days (during rest periods) were the focus of our analyses using a MATLAB toolbox that has been reliably validated with different datasets before (Vest et al., 2018).
Pre-ejection period
PEP is determined by the ventricular electromechanical delay of isovolumic contraction period. As a cardiac timing interval inversely related to cardiac contractility, decreased PEP reflects increased cardiac β1 receptor stimulation and cardiac sympathetic activity, also termed as “effort” in the literature (Kelsey, 2012; Newlin and Levenson, 1979; Gurel et al., 2019). It can be obtained from simultaneously collected ECG (reference for electrical depolarization of the heart) and SCG (reference for aortic valve opening of the heart) signals by calculating the time delay from ECG R-peaks to the aortic opening point on SCG beats with a three-beat exponential moving averaging paradigm (Inan et al., 2015; Etemadi and Inan, 2018). A decrease in PEP denotes increased cardiac contractility and sympathetic activity.
PPG amplitude and pulse arrival times
Calculated the beat-by-beat amplitude of the peripheral blood volume pulse, photoplethysmogram (PPG) amplitude is a peripheral sympathetic activity index, reflecting the dilation or constriction of peripheral blood vessels. An increase in PPG amplitude suggests vasodilation at the local area where the signal is acquired from (index finger). PAT is the time delay between the electrical stimulation of the heart (obtained from ECG R-peaks) to the “foot” (or trough) of a distal arterial waveform (taken from the index finger). It is the sum of the pulse transit time (PTT), which is inversely related to blood pressure, and the PEP (Mukkamala et al., 2015). PPG amplitude and PAT are vascular-dominated measures among other factors (Allen, 2007; Millasseau et al., 2006). PAT values were calculated with a five-beat exponential moving averaging.
Respiratory measure
Due to the role of the vagus nerve in efferent parasympathetic activity, respiratory measures that take part in the regulation of parasympathetic activity were extracted: respiratory rate (RR), width (RW), prominence (RP). As the RSP signal was taken with a piezoresistive strap, it had varying DC offset due to tightening/loosening of the strap. To detrend the signal, a sixth order polynomial was fit to the signal at each interval of interest, and points representing inhalation and exhalation were located. RR was calculated as the instantaneous rate of peak appearance, RW was calculated as the width of each respiration cycle, and RP was calculated as the prominence of each respiration peak.
Electrodermal activity measures
Skin conductance level (SCL) and its slope, skin conductance response, frequency of non-specific peaks (fNSSCR), and latency of SCR (LSCR) were extracted as sweat gland activity measures (Hernandez et al., 2014; Boucsein et al., 2012).
Blood pressure measure
Periodic values of SBP and DBP were used to find pulse pressure (PP) and mean arterial pressure (MAP) using a clinical grade upper arm BP cuff (Omron Healthcare, Kyoto, Japan).
Statistical analysis
The baseline characteristics between the device groups were compared in Table S3 and first traumatic stress reactivity between the device groups was examined in Table S4. For these comparisons, normality was assessed using Shapiro-Wilk test. Student's t-test, Wilcoxon rank-sum tests, and chi-squared tests were used for normal continuous, non-normal continuous, and categorical variables, respectively. Stimulation-only administrations (n = 2 on day one, n = 1 on days two and three) were expressed as change values (from baseline) for stimulation and post-stimulation intervals and subsequently averaged across days. For stress tasks, data during stress, stimulation, and post-stimulation intervals were extracted. Data were then averaged across stress types (six traumatic, two public speech, two mental arithmetic stressors) and change values (from baseline) of intervals (stress, stimulation, post-stimulation) were computed. For mental tasks that require speaking (public speech, mental arithmetic), stress data were extracted during times without vocalizations (immediately before task) to avoid unwanted signal noise while talking. Longer intervals of ECG (exceeding 3 min), as extracted as baseline, following a break, and end of day were used to assess HRV changes. Four essential comparisons were performed to assess differences between the device groups: stimulation without stress, stimulation following traumatic stress, public speech stress, mental arithmetic stress. Data in bar plots represent raw (unadjusted) mean ± 95% confidence interval (95% CI). Mixed models with repeated measures included random effect for each participant, used an unstructured correlation matrix, and were adjusted for age. The beta coefficients (β) from the mixed models indicate the adjusted average percent or absolute differences in active group compared to sham group. β were reported along with adjusted 95% CI, p-values, and effect sizes (d, based on Cohen's d for independent observations) (Lakens, 2013) in results and figure captions in β (±CI, d, p) format. A two-sided p < 0.05 denoted statistical significance. Lastly, to compare the statistical power for reported parameters in PTSD and previously published non-PTSD groups, effect sizes were calculated based on Cohen's d in Table S5 for stimulation and post-stimulation intervals. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) and MATLAB (R2020a, Natick, MA).
Results
TcVNS consistently decreases SNS in absence of stress over multiple days.
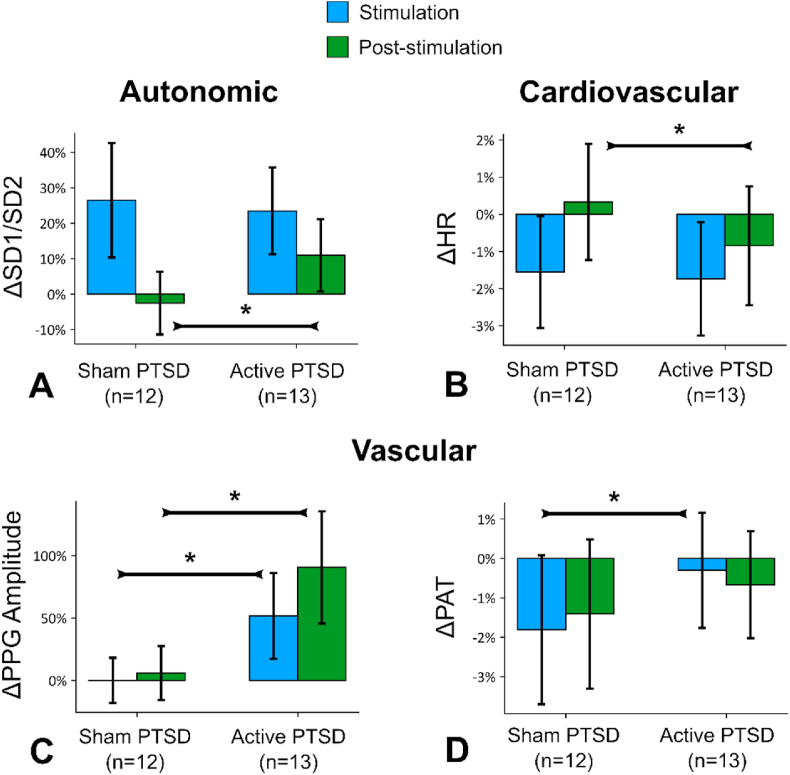
Fig. 1 presents the raw values for autonomic (SD1/SD2), cardiovascular (HR), and vascular (PPG Amplitude and PAT) tone. Compared to sham, active tcVNS increased SD1/SD2 (Fig. 1A, post-stimulation, β (±CI, d, p) 14.1% (±11.6%, d = 0.43, p = 0.019)), decreased HR (Fig. 1B, following stimulation, 2.7% (±2.0%, d = 0.21, p = 0.009)), increased PPG amplitude (inversely associated with peripheral sympathetic activity, Fig. 1C, during stimulation, 43.4% (±43.4%, d = 0.53, p = 0.049); following stimulation 73.1% (±63.2%, d = 0.7, p = 0.025)), and increased PAT (inversely associated with peripheral sympathetic activity, Fig. 1D, during stimulation, 2.5% (±2.2%, d = 0.26, p = 0.026)). The results were similar when the days were evaluated separately (Figs. S5 and S6). In summary, the active group experienced change in autonomic variables towards parasympathetic dominance (increased SD1/SD2, PPG amplitude, PAT, decreased HR), compared to sham, when tcVNS was applied without acute stress. We did not observe any significant changes in other measures analyzed: frequency domain HRV measures (LF, HF, LF/HF), EDA measures (SCLMEAN, SCLSLOPE, LSCR, fNSSCR), RSP measures (RR, RW, RP), SCG measures (Sheps et al., 2002), and BP measures (SBP, DBP, PP) for tcVNS without stress in any of the days or in the longer intervals (>3 min) that belong to the start and end of the day (p > 0.05).
Fig. 1.
tcVNS without acute stress: Outcomes for stimulation without acute stress, merged from all days. Bars represent the unadjusted mean changes from baseline, error bars: 95% confidence interval (CI), values calculated from raw data, * indicates p < 0.05. β coefficients, adjusted CI, effect sizes (d), and p-values were reported in β (±CI, d, p) format. Active tcVNS group experienced the following relative to sham after adjustments: (A) The ratio of short-term variability to long-term variability (SD1/SD2) increased following stimulation by 14.1% (±11.6%, d = 0.43, p = 0.019). (B) Heart rate (HR) decreased following stimulation by 2.7% (±2.0%, d = 0.21, p = 0.009). (C) Photoplethysmogram (PPG) amplitude increased during stimulation by 43.4% (±43.4%, d = 0.53, p = 0.049) and following stimulation by 73.1% (±63.2%, d = 0.67, p = 0.025). (D) Pulse arrival time (PAT) increased during stimulation by 2.5% (±2.2%, d = 0.26, p = 0.026).
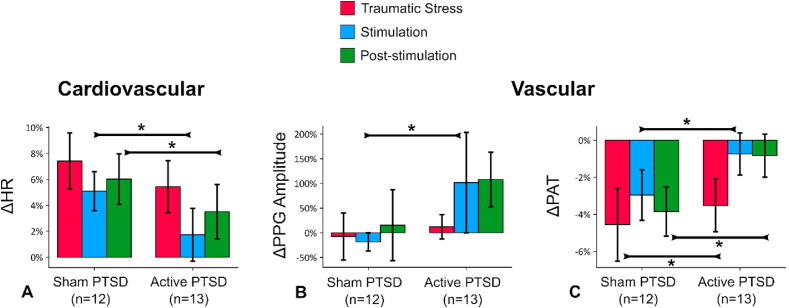
TcVNS reduces sympathetic tone following exposure to personalized traumatic scripts.
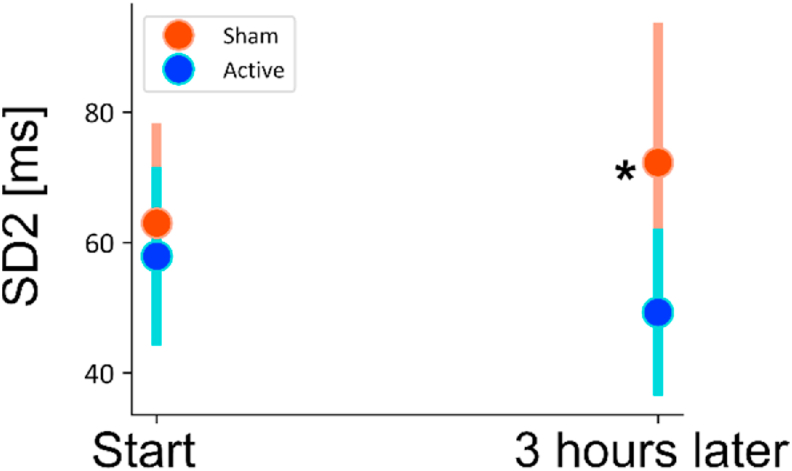
Stimulation following exposure to personalized traumatic scripts resulted in marked changes in autonomic reactivity, similarly to stimulation without stress. Fig. 2A–C illustrate changes in from the baseline state for the three intervals: traumatic stress, stimulation, and post-stimulation, merged from all six traumatic stressors. Relative to sham, active tcVNS decreased HR (Fig. 2A, during stimulation, 5.6% (±3.6%, d = 0.43, p = 0.003); following stimulation, 3.9% (±3.0%, d = 0.29, p = 0.013)), increased PPG amplitude (Fig. 2B, during stimulation, 30.8% (±28.0%, d = 0.41, p = 0.032)), less reduction in PAT (Fig. 2C, during combined traumatic stress, 9.2% (±3.0%, d = 0.15, p < 0.0001); during stimulation, 2.2% (±2.2%, d = 0.42, p = 0.045); following stimulation, 6.2% (±1.9%, d = 0.57, p < 0.0001)), indicating attenuation in the elevated autonomic tone due to stress. These effects were not initially observed, as no differences (p > 0.05) were found between active and sham during the first traumatic script. Figs. S7D–F presents the stress reactivity across each traumatic script for these biomarkers. tcVNS also decreased long-term heart rate variability after multiple traumatic stress and stimulation protocol: Following the repeated traumatic stress protocol, tcVNS decreased SD2 obtained from Poincaré plot (31.7 ms (±15.5 ms, d = 1.02, p = 0.026); Fig. 3). In summary, the active group experienced dampening in autonomic reactivity when stimulation was paired with traumatic recall (increased PPG amplitude, PAT, decreased HR, decreased SD2), compared to sham. We did not observe any significant changes in other parameters (PEP, RR, RW, RP, SCLMEAN, SCLSLOPE, LSCR, fNSSCR, LF, HF, LF/HF, SD1, SD1/SD2, SBP, DBP, PP) for tcVNS paired with traumatic stress (p > 0.05).
Fig. 2.
tcVNS after traumatic stress: Outcomes for stimulation following traumatic stress (all six scripts). Bars represent the unadjusted mean changes from baseline, error bars: 95% confidence interval (CI), values calculated from raw data, * indicates p < 0.05. β coefficients, adjusted CI, effect sizes (d), and p-values were reported in β (±CI, d, p) format. Active tcVNS group experienced the following relative to sham after traumatic stress after adjustments: (A) Heart rate (HR) decreased during stimulation by 5.6% (±3.6%, d = 0.43, p = 0.003), and following stimulation by 3.9% (±3.0%, d = 0.29, p = 0.013). (B) Photoplethysmogram (PPG) amplitude increased during stimulation by 30.8% (±28.0%, d = 0.41, p = 0.032). (C) Pulse arrival time (PAT) decreased less during traumatic stress by 9.2% (±3.0%, d = 0.15, p < 0.0001), stimulation by 2.2% (±2.2%, d = 0.42, p = 0.045), and following stimulation by 6.2% (±1.9%, d = 0.57, p < 0.0001).
Fig. 3.
Change in long-term heart rate variability (SD2) for multiple stimulation protocol following traumatic stress (four traumatic stress and six stimulation administrations on the first day). Bars represent the unadjusted mean changes from baseline, error bars: 95% confidence interval (CI), values calculated from raw data, * indicates p < 0.05. β coefficients, adjusted CI, effect sizes (d), and p-values were reported in β (±CI, d, p) format. Active tcVNS group experienced decrease in SD2 after the multiple stress protocol by 31.7 ms (±15.5 ms, d = 1.02, p = 0.026) after adjustments.
TcVNS affects cardiac contractility and heart rate variability following mental stress.
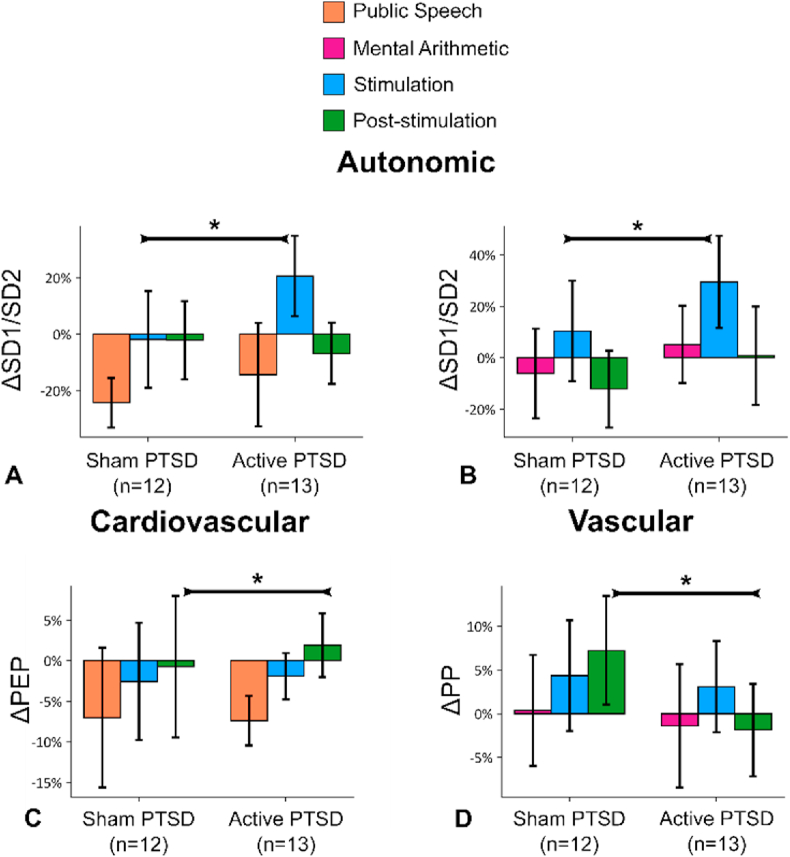
Fig. 4A–D summarize the effects of tcVNS when applied after two different mental stress tasks: public speech and mental arithmetic on the second and third days. SD1/SD2 increased during stimulation right after speech task (Fig. 4A, inversely related to sympathetic activity, during stimulation, 23.1% (±21.1%, d = 0.71, p = 0.033)) and after mental arithmetic test (Fig. 4B, during stimulation, 41.2% (±22.5%, d = 0.44, p = 0.001)).
Fig. 4.
tcVNS after mental stress: Outcomes for stimulation following two types of mental stress, public speech and mental arithmetic. Bars represent the unadjusted mean changes from baseline, error bars: 95% confidence interval (CI), values calculated from raw data, * indicates p < 0.05. β coefficients, adjusted CI, effect sizes (d), and p-values were reported in β (±CI, d, p) format. Active tcVNS group experienced the following relative to sham after adjustments: (A) SD1/SD2 increased during stimulation right after speech task by 23.1% (±21.1%, d = 0.71, p = 0.033). (B) Similar to the speech task, SD1/SD2 increased by 41.2% (±22.5%, d = 0.44, p = 0.001). (C) Pre-ejection period (83) increased following stimulation after speech task by 6.8% (±5%, d = 0.16, p = 0.009). (D) Pulse pressure (PP) decreased following stimulation after mental arithmetic by 9.6% (±9.7%, d = 0.68, p = 0.049).
Active tcVNS increased PEP (Fig. 4C, inversely related to cardiac sympathetic activity) following stimulation after public speech compared to sham (6.8% (±5%, d = 0.16, p = 0.009)), indicating a decrease in cardiac contractility and sympathetic activity. Active tcVNS also decreased PP following stimulation after the mental arithmetic task compared to sham (9.6% (±9.7%, d = 0.68, p = 0.049); Fig. 3D), indicating a decrease in vascular reactivity. In summary, the active group experienced dampening in autonomic reactivity when stimulation was paired with mental stress (increased SD1/SD2, PEP, decreased PP), compared to sham. We did not observe any significant changes in other measures (LF, HF, LF/HF, SD1, SD2, HR, PPG Amplitude, RR, RW, RP, PAT, SBP, DBP, PP) for tcVNS paired with any of the mental stressors (p > 0.05).
Discussion
This study showed that tcVNS modulates autonomic, cardiovascular, and vascular measures in PTSD with or without exposure to traumatic and mental stress. The broad interpretation of the changes due to tcVNS are similar to the study involving non-PTSD controls (Gurel et al., 2020a) (i.e., reduction of sympathetic tone at baseline and blocking sympathetic responses to stress). Additionally, the effects of tcVNS on vascular measures (i.e., PPG amplitude) persist as markers of autonomic changes with tcVNS independent of disease status.
Active tcVNS decreased sympathetic arousal as measured by autonomic, cardiovascular, and vascular measures across multiple days and types of stressors. Results were seen on the first day after multiple exposures to personalized traumatic script stress, and on the afternoons of the second and third days after exposure to mental stress challenges (public speech, mental arithmetic) in the morning (Figs. S5–S6). PPG amplitude was a persistent biomarker of stimulation regardless of the disease status. We found greater SD1/SD2 response to tcVNS than sham, while the other frequency domain metrics were not affected by tcVNS. While the biological significance of this measure/finding is not clear, it is complemented by auricular VNS studies in which frequency-domain HRV improved (Bretherton et al., 2019; Clancy et al., 2014; Brock et al., 2017) (Bretherton et al., 2019; Clancy et al., 2014; Brock et al., 2017). SD1/SD2 is a non-linear Poincaré-based HRV, which is less studied in literature, although another study noted that it is negatively associated with diabetes (Roy and Ghatak, 2013). Hemodynamic measures (BP, HR) have been studied more, and been mixed throughout tcVNS studies: tcVNS decreased HR in humans (Brock et al., 2017) and rats, albeit only momentarily (Chen et al., 2016). Other studies, however, have not observed any autonomic or cardiovascular changes (Oshinsky et al., 2014).
TcVNS improved recovery from traumatic stress (reduced HR, increased PAT) and decreased peripheral sympathetic activity (decreased PPG amplitude). When PTSD and non-PTSD groups are compared based on effect sizes (Table S5), PPG amplitude (except stimulation paired with mental arithmetic) and PAT (except stimulation without stress) effect sizes appear to be higher for PTSD group, however HR, PEP, SCLSLOPE and RR effect sizes are variable depending on the case.
PAT reactivity to traumatic stress was comparable between the groups when data from only the first script was analyzed (Figs. S7A–C). With the merged data (Fig. 2C), sham group experienced more reactivity to stress. Patients with PTSD fail to habituate to repeated exposure of stress (Lissek and van Meurs, 2015; Wessa and Flor, 2007). The therapeutic potential of tcVNS is shown by its effect in decreasing stress reactivity. These results suggest that repeated tcVNS enhances resilience in the face of repeated stress in patients with PTSD.
It is important to point out that stimulation leads to dampening in autonomic activity, regardless of the stress presence. This is of interest in PTSD population: Autonomic activity is known to be impaired in patients with PTSD. Indeed, the impairment in autonomic activity is associated with the severity of PTSD symptoms based on a twin study (Shah et al., 2013). Besides dampening in baseline activity, in the protocol, we simulate the expected use of the therapy with acute traumatic stress applications as the patients would use the therapy upon traumatic flashbacks.
An interesting outcome was the decrease in SD2 in the active tcVNS group with PTSD (Fig. 3), which might indicate increased BRS. Reduced BRS is associated with increased mortality, higher inflammation (Lampert et al., 2008), increased depressive symptoms (Vaccarino et al., 2008), and increased risk of myocardial infarction (Bigger et al., 1992; La Rovere et al., 1998), and lower BRS has been proposed as a risk stratification for multiple cardiac mortality conditions (La Rovere, 2000; La Rovere and Schwartz, 1997). It is also known that patients with PTSD have impaired autonomic modulation and autonomic inflexibility as measured by frequency-domain HRV (Shah et al., 2013). Other studies noted impaired baroreflex sensitivity in women with PTSD compared to women without PTSD (Hughes et al., 2007; Ulmer et al., 2009), and in male veterans with PTSD compared to twins without (Shah et al., 2013). We observed dampened SD2, which may also support this concept regarding BRS as a moderating factor.
Active tcVNS improved cardiac contractility recovery following the speech task, as evidenced by PEP. PEP is an index of effort-related cardiac activity (Kelsey, 2012), with greater PEP indicating decreased effort and cardiac sympathetic activation. PEP is responsive to tasks requiring effortful active coping (Kelsey, 2012), similar to the speech task, potentially indicating tcVNS helps to recover increased cardiac contractility. PEP has been shown previously to respond differently to challenge or threat conditions, specifically resulting in decreases with challenge and minimal changes with threat (Wormwood et al., 2019). In the current study, PEP decreased with the speech task in both groups, and the decrease was mitigated with active tcVNS. The traumatic stress perhaps could be regarded as threat for patients with PTSD. PEP outcomes for speech tasks and (the lack of) PEP outcomes for traumatic stress might be due to the perceptional differences for challenge versus threat (Seery, 2013). That said, whether stimulations from previous day or the acute application right after speech task modified PEP reactivity remains as a question that needs to be answered in neuroscience based study designs in a more controlled fashion.A comparison of previous works in PTSD notes more than double cortisol release with cognitive challenge (Bremner et al., 2003), compared to the cortisol levels with traumatic stress (Elzinga et al., 2003). Although there is no direct statistical comparison between these studies, the magnitude differences in cortisol levels are apparent.
Several aspects of this study might limit the generalization of the results. The active group was female-dominated due to the small sample size. However it is important to note that PTSD is twice more prevalent in females than males (Kessler et al., 1995). In addition, we further evaluated possible confounds in device groups to determine whether active and sham groups differ during baseline and during stress. Table S3 presents physiological parameter information during baseline conditions for both groups. We did not find significant difference between active and sham groups in any of the physiological parameters analyzed. Next, we explored stress reactivity between device groups before the stimulation took place on day one, to understand whether the stress reactivity was comparable between the groups. Table S4 presents the first traumatic stress reactivity. There does not seem to be significant difference between device groups during the first stress reaction.
Our non-significant findings regarding non-acute measures (such as HRV) should be approached with caution as the study is an acute study: in this investigation, stressors and stimulations take short amounts of time (such as 1–2 min). For instance, as frequency-domain HRV measures do not have a continuous nature, LF/HF HRV computation requires at least three to 5 min of clean ECG data (Camm et al., 1996). Furthermore, lower HRV frequencies (very low frequency, VLF; or ultra low frequency, ULF) assessed from short-term recordings such as ours (≤5 min) should be avoided due to the lack of power in short recordings (Camm et al., 1996). In VNS literature, some auricular stimulation studies noted changes in HRV with long-term recordings (Bretherton et al., 2019; Clancy et al., 2014), while others did not (Burger et al., 2016, 2018,bib_Burger_et_al_2016; Verkuil, 2019,bib_Burger_et_al_2018).
Though our results indicate active tcVNS causes physiologic changes in stress reactivity, these results should not necessarily be interpreted as reduction in perceived stress. That said, recent neuroscience studies using implanted VNS in rat models trained for fear conditioning reported enhancement of fear extinction based on freezing time (Peña et al., 2013b, 2014,bib_Peña_et_al_2013b). Along similar lines, fear conditioning studies on human subjects reported acceleration in fear extinction (Burger et al., 2016) and enhanced processing of safety cues (Burger et al., 2018) based on US expectancy ratings.
The stimulation timing employed in this study may not be generalizable to other previously published data. This study applied stimulation immediately following the traumatic script, as this usage reflects potential clinical application where a patient with PTSD applies tcVNS following an intrusive memory. Previous studies in animals have applied stimulation both during — which also may be applicable in humans —and before traumatic stress (Pena et al., 2014; Lamb et al., 2017; Burger et al., 2016, 2017; Engineer et al., 2011) (Pena et al., 2014; Lamb et al., 2017; Burger et al., 2016, 2017; Engineer et al., 2011). No current study has examined the efficacy and/or changes in physiological arousal when stimulation is applied either during or following traumatic stress, and therefore it is unclear how the timing affects the findings in the current study. Given that the effects of traumatic stress, as autonomic parameters, persists beyond the initial presentation of the adverse stimulus, it is possible that stimulation during or after a traumatic script elicits similar changes in arousal. This is consistent with findings across various studies reporting autonomic dampening with implanted of noninvasive VNS, despite variations within stimulation timing and protocols. (Peña et al., 2013a; Pena et al., 2014; Bretherton et al., 2019; Lamb et al., 2017; Noble et al., 2017).
In summary, using a multimodal sensing approach, we found that tcVNS at rest and paired with various stressors modulates the autonomic nervous system and cardiovascular reactivity. We demonstrated feasibility of use of wearable sensing devices for measurement of novel physiological markers in patients with PTSD. These modalities can be used in a home setting to assess target engagement and treatment efficacy for personalized neuromodulation. Future work should focus on methods to evaluate longitudinal outcomes (Gurel et al., 2020c) and parameter determination studies (Badran et al., 2018a) for selective modulation of autonomic tone and to utilize these modalities in assessment of response to neuromodulation treatments in PTSD.
CRediT authorship contribution statement
Nil Z. Gurel: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Visualization, Writing - original draft, Writing - review & editing. Matthew T. Wittbrodt: Validation, Writing - review & editing. Hewon Jung: Investigation, Data curation, Writing - review & editing. Md. Mobashir H. Shandhi: Investigation, Writing - review & editing. Emily G. Driggers: Project administration, Resources, Writing - review & editing. Stacy L. Ladd: Project administration, Resources, Writing - review & editing. Minxuan Huang: Methodology, Formal analysis. Yi-An Ko: Methodology, Writing - review & editing. Lucy Shallenberger: Project administration, Resources. Joy Beckwith: Project administration, Resources. Jonathon A. Nye: Project administration, Resources. Bradley D. Pearce: Funding acquisition, Resources, Writing - review & editing. Viola Vaccarino: Funding acquisition, Resources, Supervision, Writing - review & editing. Amit J. Shah: Funding acquisition, Resources, Supervision, Writing - review & editing. Omer T. Inan: Funding acquisition, Resources, Supervision, Writing - review & editing. J. Douglas Bremner: Funding acquisition, Resources, Supervision, Writing - review & editing.
Acknowledgments
This work was supported by the Defense Advanced Research Projects Agency (DARPA), Arlington, VA, under Cooperative Agreement N66001-16-2-4054. Dr. Shah is sponsored by the National Institutes of Health, Award K23 HL127251. We would like to acknowledge and thank Margie Jones, CNMT, Steven Rhodes, RN from Emory University, and Javier Hernandez, PhD from Microsoft Research for their assistance with clinical research and affective computing discussions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2020.100264.
Disclosures
JDB is currently conducting research on noninvasive vagus nerve stimulation with applications to posttraumatic stress disorder with research support from an investigator-initiated research contract with ElectroCore LLC and a Distinguished Investigator Award from the Brain and Behavior Research Foundation (BBRF)/National Alliance for Research on Schizophrenia and Affective Disorders (NARSAD). There are no other relevant royalty, advisory board, consulting, patents, or stock ownership to disclose.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aaronson S.T., Sears P., Ruvuna F., Bunker M., Conway C.R. A 5-year observational study of patients with treatment-resistant depression treated with vagus nerve stimulation or treatment as usual: comparison of response, remission, and suicidality. Am. J. Psychiatr. 2017;174(7):640–648. doi: 10.1176/appi.ajp.2017.16010034. [DOI] [PubMed] [Google Scholar]
- Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007;28(3):R1–R39. doi: 10.1088/0967-3334/28/3/R01. [DOI] [PubMed] [Google Scholar]
- Ardell J.L., Rajendran P.S., Nier H.A., KenKnight B.H., Armour J.A. Central-peripheral neural network interactions evoked by vagus nerve stimulation: functional consequences on control of cardiac function. Am. J. Physiol. Heart Circ. Physiol. 2015;309(10):H1740–H1752. doi: 10.1152/ajpheart.00557.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assenza G., Campana C., Colicchio G., Tombini M., Assenza F., Di Pino G. Transcutaneous and invasive vagal nerve stimulations engage the same neural pathways: in-vivo human evidence. Brain Stimulation. 2017;10(4):853–854. doi: 10.1016/j.brs.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Badran B.W., Mithoefer O.J., Summer C.E., LaBate N.T., Glusman C.E., Badran A.W. Short trains of transcutaneous auricular vagus nerve stimulation (taVNS) have parameter-specific effects on heart rate. Brain Stimul. 2018;11(4):699–708. doi: 10.1016/j.brs.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badran B.W., Dowdle L.T., Mithoefer O.J., LaBate N.T., Coatsworth J., Brown J.C. Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: a concurrent taVNS/fMRI study and review. Brain Stimul. 2018;11(3):492–500. doi: 10.1016/j.brs.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C.B., Zayfert C., Anderson E. A survey of psychologists' attitudes towards and utilization of exposure therapy for PTSD. Behav. Res. Ther. 2004;42(3):277–292. doi: 10.1016/S0005-7967(03)00138-4. [DOI] [PubMed] [Google Scholar]
- Bigger J.T., Jr., Fleiss J.L., Steinman R.C., Rolnitzky L.M., Kleiger R.E., Rottman J.N. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85(1):164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- Blake D.D., Weathers F.W., Nagy L.M., Kaloupek D.G., Gusman F.D., Charney D.S. The development of a clinician-administered PTSD Scale. J. Trauma Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Boucsein W., Fowles D.C., Grimnes S., Ben-Shakhar G., roth W.T., Dawson M.E. Publication recommendations for electrodermal measurements. Psychophysiology. 2012;49(8):1017–1034. doi: 10.1111/j.1469-8986.2012.01384.x. [DOI] [PubMed] [Google Scholar]
- Bradley R., Greene J., Russ E., Dutra L., Westen D. A multidimensional meta-analysis of psychotherapy for PTSD. Am. J. Psychiatr. 2005;162(2):214–227. doi: 10.1176/appi.ajp.162.2.214. [DOI] [PubMed] [Google Scholar]
- Bradley M.M., Miccoli L., Escrig M.A., Lang P.J. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008;45(4):602–607. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Rapaport M.H. Vagus nerve stimulation: back to the future. Am. J. Psychiatr. 2017;124(7):609–610. doi: 10.1176/appi.ajp.2017.17040422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Scott T.M., Delaney R.C., Southwick S.M., Mason J.W., Johnson D.R. Deficits in short-term memory in posttraumatic stress disorder. Am. J. Psychiatr. 1993;150(7):1015–1019. doi: 10.1176/ajp.150.7.1015. [DOI] [PubMed] [Google Scholar]
- Bremner J.D., Licinio J., Darnell A., Krystal J.H., Owens M.J., Southwick S.M. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am. J. Psychiatr. 1997;154(5):624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Narayan M., Staib L.H., Southwick S.M., McGlashan T., Charney D.S. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am. J. Psychiatr. 1999;156(11):1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Staib L.H., Kaloupek D., Southwick S.M., Soufer R., Charney D.S. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol. Psychiatr. 1999;45(7):806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Vythilingam M., Vermetten E., Adil J., Khan S., Nazeer A. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 2003;28(6):733–750. doi: 10.1016/s0306-4530(02)00067-7. [DOI] [PubMed] [Google Scholar]
- Bretherton B., Atkinson L., Murray A., Clancy J., Deuchars S., Deuchars J. Effects of transcutaneous vagus nerve stimulation in individuals aged 55 years or above: potential benefits of daily stimulation. Aging (Albany NY) 2019;11(14):4836–4857. doi: 10.18632/aging.102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock C., Brock B., Aziz Q., Moller H.J., Pfeiffer Jensen M., Drewes A.M. Transcutaneous cervical vagal nerve stimulation modulates cardiac vagal tone and tumor necrosis factor-alpha. Neuro Gastroenterol. Motil. 2017;29(5) doi: 10.1111/nmo.12999. [DOI] [PubMed] [Google Scholar]
- Burger A.M., Verkuil B., Van Diest I., Van der Does W., Thayer J.F., Brosschot J.F. The effects of transcutaneous vagus nerve stimulation on conditioned fear extinction in humans. Neurobiol. Learn. Mem. 2016;132:49–56. doi: 10.1016/j.nlm.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Burger A.M., Verkuil B., Fenlon H., Thijs L., Cools L., Miller H.C. Mixed evidence for the potential of non-invasive transcutaneous vagal nerve stimulation to improve the extinction and retention of fear. Behav. Res. Ther. 2017;97:64–74. doi: 10.1016/j.brat.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Burger A.M., Van Diest I., van der Does W., Hysaj M., Thayer J.F., Brosschot J.F. Transcutaneous vagus nerve stimulation and extinction of prepared fear: a conceptual non-replication. Sci. Rep. 2018;8(1):11471. doi: 10.1038/s41598-018-29561-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill S.P., Foa E.B., Hembree E.A., Marshall R.D., Nacash N. Dissemination of exposure therapy in the treatment of posttraumatic stress disorder. J. Trauma Stress. 2006;19(5):597–610. doi: 10.1002/jts.20173. [DOI] [PubMed] [Google Scholar]
- Camm A.J., Malik M., Bigger J., Breithardt G., Cerutti S., Cohen R.J. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996;17(3):354–381. [PubMed] [Google Scholar]
- Chen S.P., Ay I., de Morais A.L., Qin T., Zheng Y., Sadeghian H. Vagus nerve stimulation inhibits cortical spreading depression. Pain. 2016;157(4):797–805. doi: 10.1097/j.pain.0000000000000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy J.A., Mary D.A., Witte K.K., Greenwood J.P., Deuchars S.A., Deuchars J. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul. 2014;7(6):871–877. doi: 10.1016/j.brs.2014.07.031. [DOI] [PubMed] [Google Scholar]
- Cukor J., Olden M., Lee F., Difede J. Evidence-based treatments for PTSD, new directions, and special challenges. Ann. N. Y. Acad. Sci. 2010;1208:82–89. doi: 10.1111/j.1749-6632.2010.05793.x. [DOI] [PubMed] [Google Scholar]
- Elzinga B.M., Schmahl C.G., Vermetten E., van Dyck R., Bremner J.D. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology. 2003;28(9):1656–1665. doi: 10.1038/sj.npp.1300226. [DOI] [PubMed] [Google Scholar]
- Engineer N.D., Riley J.R., Seale J.D., Vrana W.A., Shetake J.A. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470(7332):101. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemadi M., Inan O.T. Wearable ballistocardiogram and seismocardiogram systems for health and performance. J. Appl. Physiol. 2018;124(2):452–461. doi: 10.1152/japplphysiol.00298.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J., Rong P., Hong Y., Fan Y., Liu J., Wang H. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol. Psychiatr. 2016;79(4):266–273. doi: 10.1016/j.biopsych.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Gibbon M. The structured clinical Interview for DSM-IV Axis I disorders (SCID-I) and the structured clinical Interview for DSM-IV Axis II disorders (SCID-II) In: Segal M.J.H.D.L., editor. Comprehensive Handbook of Psychological Assessment. 2. John Wiley & Sons Inc.; Hoboken, NJ, US: 2004. pp. 134–143. [Google Scholar]
- Frangos E., Komisaruk B.R. Access to vagal projections via cutaneous electrical stimulation of the neck: fMRI evidence in healthy humans. Brain Stimul. 2017;10(1):19–27. doi: 10.1016/j.brs.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Frangos E., Ellrich J., Komisaruk B.R. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul. 2015;8(3):624–636. doi: 10.1016/j.brs.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayne S.M., Chiu V.Y., Iqbal S., Berg E.A., Laungani K.J., Cronkite R.C. Medical care needs of returning veterans with PTSD: their other burden. J. Gen. Intern. Med. 2011;26(1):33–39. doi: 10.1007/s11606-010-1497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R.G., Lin R.L., Lee J., Kim J., Barbieri R., Sclocco R. Modulation of brainstem activity and connectivity by respiratory-gated auricular vagal afferent nerve stimulation in migraine patients. Pain. 2017;158(8):1461–1472. doi: 10.1097/j.pain.0000000000000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazi A.H., Gurel N.Z., Richardson K.L.S., Wittbrodt M.T., Shah A.J., Vaccarino V. Investigating digital cardiovascular biomarker responses to transcutaneous cervical vagus nerve stimulation: state-space modeling, prediction, and simulation. JMIR mHealth and uHealth. 2020:20488. doi: 10.2196/20488. (forthcoming/(in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J.M., Saligan L., Woods S., Page G. PTSD is associated with an excess of inflammatory immune activities. Psychiatr. Care. 2009;45(4):262–277. doi: 10.1111/j.1744-6163.2009.00229.x. [DOI] [PubMed] [Google Scholar]
- Gurel N.Z., Jung H., Hersek S., Inan O.T. Fusing near-infrared spectroscopy with wearable hemodynamic measurements improves classification of mental stress. IEEE Sensor. J. 2019;19(19):8522–8531. doi: 10.1109/jsen.2018.2872651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel N.Z., Huang M., Wittbrodt M.T., Jung H., Ladd S.L., Shandhi M.M.H. Quantifying acute physiological biomarkers of transcutaneous cervical vagal nerve stimulation in the context of psychological stress. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation. 2020;13(1):47–59. doi: 10.1016/j.brs.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel N.Z., Gazi A.H., Scott K.L., Wittbrodt M.T., Shah A.J., Vaccarino V. Timing considerations for noninvasive vagal nerve stimulation in clinical studies. AMIA Annu Symp Proc. 2020;2019:1061–1070. [PMC free article] [PubMed] [Google Scholar]
- Gurel N.Z., Wittbrodt M.T., Jung H., Ladd S.L., Shah A.J., Vaccarino V. Automatic detection of target engagement in transcutaneous cervical vagal nerve stimulation for traumatic stress triggers. IEEE Journal of Biomedical and Health Informatics. 2020:1. doi: 10.1109/JBHI.2020.2981116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer H.M., Bauer S. Lessons learned from transcutaneous vagus nerve stimulation (tVNS) Epilepsy Res. 2019;153:83–84. doi: 10.1016/j.eplepsyres.2019.02.015. [DOI] [PubMed] [Google Scholar]
- Hernandez J., Riobo I., Rozga A., Abowd G.D., Picard R.W., editors. Using Electrodermal Activity to Recognize Ease of Engagement in Children during Social Interactions. Proceedings of the 2014 ACM International Joint Conference on Pervasive and Ubiquitous Computing. 2014. [Google Scholar]
- Hoge C.W., Grossman S.H., Auchterlonie J.L., Riviere L.A., Milliken C.S., Wilk J.E. PTSD treatment for soldiers after combat deployment: low utilization of mental health care and reasons for dropout. Psychiatr. Serv. 2014;65(8):997–1004. doi: 10.1176/appi.ps.201300307. [DOI] [PubMed] [Google Scholar]
- Hoskins M., Pearce J., Bethell A., Dankova L., Barbui C., Tol W.A. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br. J. Psychiatry. 2015;206(2):93–100. doi: 10.1192/bjp.bp.114.148551. [DOI] [PubMed] [Google Scholar]
- Hughes J.W., Dennis M.F., Beckham J.C. Baroreceptor sensitivity at rest and during stress in women with posttraumatic stress disorder or major depressive disorder. J. Trauma Stress. 2007;20(5):667–676. doi: 10.1002/jts.20285. [DOI] [PubMed] [Google Scholar]
- Inan O.T., Migeotte P.F., Park K.S., Etemadi M., Tavakolian K., Casanella R. Ballistocardiography and seismocardiography: a review of recent advances. IEEE J Biomed Health Inform. 2015;19(4):1414–1427. doi: 10.1109/JBHI.2014.2361732. [DOI] [PubMed] [Google Scholar]
- Kelmendi B., Adams T.G., Yarnell S., Southwick S., Abdallah C.G., Krystal J.H. PTSD: from neurobiology to pharmacological treatments. Eur. J. Psychotraumatol. 2016;7:31858. doi: 10.3402/ejpt.v7.31858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey R.M. American Psychological Association; Washington, DC, US: 2012. Beta-adrenergic Cardiovascular Reactivity and Adaptation to Stress: the Cardiac Pre-ejection Period as an Index of Effort. How Motivation Affects Cardiovascular Response: Mechanisms and Applications; pp. 43–60. [Google Scholar]
- Kessler R.C., Sonnega A., Bromet E., Hughes M., Nelson C.B. Posttraumatic stress disorder in the national comorbidity survey. Arch. Gen. Psychiatr. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D.G., Resnick H.S., Milanak M.E., Miller M.W., Keyes K.M., Friedman M.J. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J. Trauma Stress. 2013;26(5):537–547. doi: 10.1002/jts.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama N., Vaccarino V., Kutner M., Weiss P., Bremner J.D. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J. Affect. Disord. 2005;88(1):79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Kraus T., Hosl K., Kiess O., Schanze A., Kornhuber J., Forster C. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J. Neural. Transm. 2007;114(11):1485–1493. doi: 10.1007/s00702-007-0755-z. [DOI] [PubMed] [Google Scholar]
- Krystal J.H., Davis L.L., Neylan T.C., Raskind M A., Schnurr P.P., Stein M.B. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD psychopharmacology working group. Biol. Psychiatr. 2017;82(7):e51–e59. doi: 10.1016/j.biopsych.2017.03.007. [DOI] [PubMed] [Google Scholar]
- La Rovere M.T. Baroreflex sensitivity as a new marker for risk stratification. Z. Kardiol. 2000;89(Suppl. 3):44–50. doi: 10.1007/s003920070082. [DOI] [PubMed] [Google Scholar]
- La Rovere M.T., Schwartz P.J. Baroreflex sensitivity as a cardiac and arrhythmia mortality risk stratifier. Pacing Clin. Electrophysiol. 1997;20(10 Pt 2):2602–2613. doi: 10.1111/j.1540-8159.1997.tb06110.x. [DOI] [PubMed] [Google Scholar]
- La Rovere M.T., Bigger J.T., Jr., Marcus F.I., Mortara A., Schwartz P.J. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes after Myocardial Infarction) Investigators. Lancet. 1998;351(9101):478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front. Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb D.G., Porges E.C., Lewis G.F., Williamson J.B. Non-invasive vagal nerve stimulation effects on hyperarousal and autonomic state in patients with posttraumatic stress disorder and history of mild traumatic brain injury: preliminary evidence. Front. Med. 2017;4:124. doi: 10.3389/fmed.2017.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert R., Bremner J.D., Su S., Miller A., Lee F., Cheema F. Decreased heart rate variability is associated with higher levels of inflammation in middle-aged men. Am. Heart J. 2008;156(4):759. doi: 10.1016/j.ahj.2008.07.009. e1-.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman I., Hauger R., Sorkin L., Proudfoot J., Davis B., Huang A. Noninvasive transcutaneous vagus nerve stimulation decreases whole blood culture-derived cytokines and chemokines: a randomized, blinded, healthy control pilot trial. Neuromodulation. 2016;19(3):283–290. doi: 10.1111/ner.12398. [DOI] [PubMed] [Google Scholar]
- Lerman I., Davis B., Huang M., Huang C., Sorkin L., Proudfoot J. Noninvasive vagus nerve stimulation alters neural response and physiological autonomic tone to noxious thermal challenge. PloS One. 2019;14(2) doi: 10.1371/journal.pone.0201212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima B.B., Hammadah M., Wilmot K., Pearce B.D., Shah A., Levantsevych O. Posttraumatic stress disorder is associated with enhanced interleukin-6 response to mental stress in subjects with a recent myocardial infarction. Brain Behav. Immun. 2019;75:26–33. doi: 10.1016/j.bbi.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S., van Meurs B. Learning models of PTSD: theoretical accounts and psychobiological evidence. Int. J. Psychophysiol. 2015;98(3 Pt 2):594–605. doi: 10.1016/j.ijpsycho.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicine Io Treatment for posttraumatic stress disorder in military and veteran populations: final assessment. Mil. Med. 2014;179(12):1401–1403. doi: 10.7205/MILMED-D-14-00418. [DOI] [PubMed] [Google Scholar]
- Millasseau S.C., Ritter J.M., Takazawa K., Chowienczyk P.J. Contour analysis of the photoplethysmographic pulse measured at the finger. J. Hypertens. 2006;24(8):1449–1456. doi: 10.1097/01.hjh.0000239277.05068.87. [DOI] [PubMed] [Google Scholar]
- Mourdoukoutas A.P., Truong D.Q., Adair D.K., Simon B.J., Bikson M. High-resolution multi-scale computational model for non-invasive cervical vagus nerve stimulation. Neuromodulation. 2018;21(3):261–268. doi: 10.1111/ner.12706. Epub 2017 Oct 27. PMID: 29076212; PMCID: PMC5895480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukkamala R., Hahn J.O., Inan O.T., Mestha L.K., Kim C.S., Toreyin H. Toward ubiquitous blood pressure monitoring via pulse transit time: theory and practice. IEEE Trans. Biomed. Eng. 2015;62(8):1879–1901. doi: 10.1109/TBME.2015.2441951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff C.B., Bremner J.D., Foa E.B., Mayberg H.S., North C.S., Stein M.B. Posttraumatic stress disorder: a state-of-the-science review. J. Psychiatr. Res. 2006;40(1):1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Newlin D.B., Levenson R.W. Pre-ejection period: measuring beta-adrenergic influences upon the heart. Psychophysiology. 1979;16(6):546–553. doi: 10.1111/j.1469-8986.1979.tb01519.x. [DOI] [PubMed] [Google Scholar]
- Noble I.J., Gonzalez I.J., Meruva V.B., Callahan K.A., Belfort B.D., Ramanathan K.R. Effects of vagus nerve stimulation on extinction of conditioned fear and post-traumatic stress disorder symptoms in rats. Transl. Psychiatry. 2017;7(e1217):1–8. doi: 10.1038/tp.2017.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonis R., D'Ostilio K., Schoenen J., Magis D. Evidence of activation of vagal afferents by non-invasive vagus nerve stimulation: an electrophysiological study in healthy volunteers. Cephalalgia. 2017;37(13):1285–1293. doi: 10.1177/0333102417717470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshinsky M.L., Murphy A.L., Hekierski H., Jr., Cooper M., Simon B.J. Noninvasive vagus nerve stimulation as treatment for trigeminal allodynia. Pain. 2014;155(5):1037–1042. doi: 10.1016/j.pain.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Marvar P.J., Liao P., Kankam M.L., Norrholm S.D., Downey R.M. Baroreflex dysfunction and augmented sympathetic nerve responses during mental stress in veterans with post-traumatic stress disorder. J. Physiol. 2017;595(14):4893–4908. doi: 10.1113/JP274269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña D.F., Engineer N.D., McIntyre C.K. Rapid remission of conditioned fear expression with extinction training paired with vagus nerve stimulation. Biol. Psychiatr. 2013;73(11):1071–1077. doi: 10.1016/j.biopsych.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña D.F., Engineer N.D., McIntyre C.K. Rapid remission of conditioned fear expression with extinction training paired with vagus nerve stimulation. Biol. Psychiatr. 2013;73(11):1071–1077. doi: 10.1016/j.biopsych.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena D.F., Childs J.E., Willett S., Vital A., McIntyre C.K., Kroener S. Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the amygdala. Front. Behav. Neurosci. 2014;8:327. doi: 10.3389/fnbeh.2014.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña D.F., Childs J.E., Willett S., Vital A., McIntyre C.K., Kroener S. Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the amygdala. Front. Behav. Neurosci. 2014;8:327. doi: 10.3389/fnbeh.2014.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter U., McClure C., Liebler E., Pozo-Rosich P. Non-invasive neuromodulation for migraine and cluster headache: a systematic review of clinical trials. J. Neurol. Neurosurg. Psychiatry. 2019;90(7):796–804. doi: 10.1136/jnnp-2018-320113. PMID: 30824632; PMCID: PMC6585264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong P., Liu J., Wang L., Liu R., Fang J., Zhao J. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. J. Affect. Disord. 2016;195:172–179. doi: 10.1016/j.jad.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell J., Colominas J., Riu P., Pallas-Areny R., Webster J.G. Skin impedance from 1 Hz to 1 MHz. IEEE (Inst. Electr. Electron. Eng.) Trans. Biomed. Eng. 1988;35(8):649–651. doi: 10.1109/10.4599. [DOI] [PubMed] [Google Scholar]
- Roy B., Ghatak S. Nonlinear methods to assess changes in heart rate variability in type 2 diabetic patients. Arq. Bras. Cardiol. 2013;101(4):317–327. doi: 10.5935/abc.20130181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seery M.D. The biopsychosocial model of challenge and threat: using the heart to measure the mind. Social and Personality Psychology Compass. 2013;7(9):637–653. [Google Scholar]
- Shah A.J., Lampert R., Goldberg J., Veledar E., Bremner J.D., Vaccarino V. Posttraumatic stress disorder and impaired autonomic modulation in male twins. Biol. Psychiatr. 2013;73(11):1103–1110. doi: 10.1016/j.biopsych.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheps D.S., McMahon R.P., Becker L., Carney R.M., Freedland K.E., Cohen J.D. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation. 2002;105(15):1780–1784. doi: 10.1161/01.cir.0000014491.90666.06. [DOI] [PubMed] [Google Scholar]
- Sherin J.E., Nemeroff C.B. Post-traumatic stress disorder: the neurobiological impact of psychological trauma. Dialogues Clin. Neurosci. 2011;13(3):263–278. doi: 10.31887/DCNS.2011.13.2/jsherin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleight P., La Rovere M.T., Mortara A., Pinna G., Maestri R., Leuzzi S. Physiology and pathophysiology of heart rate and blood pressure variability in humans: is power spectral analysis largely an index of baroreflex gain? Clin. Sci. (Lond.) 1995;88(1):103–109. doi: 10.1042/cs0880103. [DOI] [PubMed] [Google Scholar]
- Stavrakis S., Humphrey M.B., Scherlag B.J., Hu Y., Jackman W.M., Nakagawa H. Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J. Am. Coll. Cardiol. 2015;65(9):867–875. doi: 10.1016/j.jacc.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrakis S., Stoner J.A., Humphrey M.B., Morris L., Filiberti A., Reynolds J.C. TREAT af (transcutaneous electrical vagus nerve stimulation to suppress atrial fibrillation): a randomized clinical trial. JACC (J. Am. Coll. Cardiol.): Clinical Electrophysiology. 2020;6(3):282–291. doi: 10.1016/j.jacep.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenerson R.L., Cronin G.W. Tinnitus reduction using transcutaneous electrical stimulation. Otolaryngol. Clin. 2003;36(2):337–344. doi: 10.1016/s0030-6665(02)00164-0. [DOI] [PubMed] [Google Scholar]
- Steenkamp M.M., Litz B.T., Hoge C.W., Marmar C.R. Psychotherapy for military-related PTSD: a review of randomized clinical trials. J. Am. Med. Assoc. 2015;314(5):489–500. doi: 10.1001/jama.2015.8370. [DOI] [PubMed] [Google Scholar]
- Tarn J., Legg S., Mitchell S., Simon B., Ng W.-F. The effects of noninvasive vagus nerve stimulation on fatigue and immune responses in patients with primary sjögren's syndrome. Neuromodulation: Technology at the Neural Interface. 2019;22(5):580–585. doi: 10.1111/ner.12879. [DOI] [PubMed] [Google Scholar]
- Ulmer C.S., Calhoun P.S., Edinger J.D., Wagner H.R., Beckham J.C. Sleep disturbance and baroreceptor sensitivity in women with posttraumatic stress disorder. J. Trauma Stress. 2009;22(6):643–647. doi: 10.1002/jts.20464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V., Lampert R., Bremner J.D., Lee F., Su S., Maisano C. Depressive symptoms and heart rate variability: evidence for a shared genetic substrate in a study of twins. Psychosom. Med. 2008;70(6):628–636. doi: 10.1097/PSY.0b013e31817bcc9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkuil B. Transcutaneous vagus nerve stimulation does not affect attention to fearful faces in high worriers. Behav. Res. Ther. 2019;1(113):25–31. doi: 10.1016/j.brat.2018.12.009. [DOI] [PubMed] [Google Scholar]
- Vest A.N., Da Poian G., Li Q., Liu C., Nemati S., Shah A. 2018. An Open Source Benchmarked Toolbox for Cardiovascular Waveform and Interval Analysis. Physiological Measurement. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren C.M., Tona K.D., Ouwerkerk L., van Paridon J., Poletiek F., van Steenbergen H. The neuromodulatory and hormonal effects of transcutaneous vagus nerve stimulation as evidenced by salivary alpha amylase, salivary cortisol, pupil diameter, and the P3 event-related potential. Brain Stimul. 2019;12(3):635–642. doi: 10.1016/j.brs.2018.12.224. [DOI] [PubMed] [Google Scholar]
- Wessa M., Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am. J. Psychiatr. 2007;164(11):1684–1692. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- Wittbrodt M.T., Gurel N.Z., Nye J.A., Ladd S., Shandhi M.M.H., Huang M. Non-invasive vagal nerve stimulation decreases brain activity during trauma scripts. Brain Stimulation. 2020;13(5):1333–1348. doi: 10.1016/j.brs.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormwood J.B., Khan Z., Siegel E., Lynn S.K., Dy J., Barrett L.F. Physiological indices of challenge and threat: a data-driven investigation of autonomic nervous system reactivity during an active coping stressor task. Psychophysiology. 2019;56(12) doi: 10.1111/psyp.13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakunina N., Kim S.S., Nam E.C. Optimization of transcutaneous vagus nerve stimulation using functional MRI. Neuromodulation. 2017;20(3):290–300. doi: 10.1111/ner.12541. [DOI] [PubMed] [Google Scholar]
- Yakunina N., Kim S.S., Nam E.C. BOLD fMRI effects of transcutaneous vagus nerve stimulation in patients with chronic tinnitus. PloS One. 2018;13(11) doi: 10.1371/journal.pone.0207281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R., LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56(1):19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Yu L., Huang B., Po S.S., Tan T., Wang M., Zhou L. Low-level tragus stimulation for the treatment of ischemia and reperfusion injury in patients with ST-segment elevation myocardial infarction: a proof-of-concept study. JACC Cardiovasc. Interv. 2017;10(15):1511–1520. doi: 10.1016/j.jcin.2017.04.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.