Abstract
Adverse experiences in early life have a long-term impact on the development of brain, which in turn increases the susceptibility to mental illness during adulthood, especially in female subjects. However, whether and how the visual cortex is affected by these adverse experiences as well as the mechanisms underlying the sex difference are largely unknown. Here, we established a new mouse model of early-life chronic mild stress (ECMS) without anxiety or depression-like behavior in adulthood. ECMS mice showed normal maturation of visual acuity and orientation/direction selectivity, whereas their visual cortical neurons preferred lower spatial frequency (SF) and higher temporal frequency (TF) than control mice. Meanwhile the development of ocular dominance (OD) plasticity was delayed. Specifically, compared with control mice, ECMS mice in the early stage of the critical period (CP) showed a reduction in GABA synthesis enzyme expression as well as lower OD plasticity which could be occluded by diazepam. In contrast, ECMS mice in the late stage of CP showed stronger OD plasticity, accompanied by higher expression of N-methyl-D-aspartate (NMDA) receptor NR2B subunit. Interestingly, only female ECMS mice at adulthood maintained juvenile-like OD plasticity as well as high NR2B expressions. Artificial increase in estradiol level in ECMS males via estradiol supplementary diminished this sex difference. Lastly, OD plasticity was abolished in adult ECMS females either performed with the bilateral ovariectomy in prepuberty, or directly infused with NR2B antagonist Ro 25–6981 into the visual cortex. Overall, our study demonstrates that early adverse experiences have a lasting effect on visual development of mice in a sex-dependent manner, which is mediated by the estradiol-NR2B pathway.
Keywords: Early-life chronic mild stress, Visual cortex, Ocular dominance plasticity, Sex difference, Estradiol-NR2B
1. Introduction
Childhood is an important period for the establishment and maturation of cortical functions, making the central nervous system vulnerable to early stressful experiences. Early adverse experience is an important risk factor for major depression, anxiety disorders, cognitive behavioral disorders, and even suicidal behavior (Cromheeke et al., 2014; Felitti et al., 1998). Decreases in the total brain volume and grey matter abnormalities in hippocampal, prefrontal cortex (PFC) and orbitofrontal cortex are believed to be associated with some of those mood disorders (Frodl et al., 2010; Hanson et al., 2010). The experience of childhood maltreatment not only interferes with development of the cognitive function, but also induces a lower perceived competence (Toth and Cicchetti, 1996). A volume decrease in left visual cortex is observed in patients with reactive attachment disorder, a severe social functioning disorder associated with early childhood maltreatment (Fujisawa et al., 2018). Therefore, development of perceptual cortices may also be under the influence of early-childhood adversity.
It has been speculated that the opening and closure of CP in perceptual cortex may be accelerated by early adversity, based on the hippocampal results (Bath et al., 2016; Callaghan et al., 2013). CP of OD plasticity during the development of primary visual cortex is one of the most popular characterized models to investigate the underlying molecular mechanism. However, the alterations of several signaling proteins (Leon Rodriguez and Duenas, 2013; Lippmann et al., 2007; Roceri et al., 2002), which are crucial to the development of visual cortex, following early adversity seem contrary to this hypothesis. For example, stressful experiences during early life reduce the level of brain-derived neurotrophic factor (BDNF) and GABAergic inhibition in the hippocampus, prefrontal cortex and other brain regions (Leon Rodriguez and Duenas, 2013; Lippmann et al., 2007). Meanwhile, a down-regulation of BDNF expression and a delayed maturation of GABAergic inhibition observed in dark-reared rodents induce a slower visual acuity maturation and a delayed CP of OD plasticity (Gianfranceschi et al., 2003; Iwai et al., 2003). In addition, 24-h maternal separation on postnatal day 9 (P9) triggers a reduced expression of NMDA receptor subunits (NR2A and NR2B) in adult rats (Roceri et al., 2002). Suitable NMDA receptor-mediated excitatory post-synaptic currents are required for the opening of the CP (Roberts and Ramoa, 1999). Thus it is worthy to investigate molecular mechanisms by which early adverse experiences accelerate or delay the maturation of visual system.
Clinically, women are more sensitive to early life stress and twice as likely to develop stress-related disorders (such as major depression MDD) in later life (Koenen and Widom, 2009), which has been proven in animal experiments (Goodwill et al., 2019). The underlying mechanism of females’ susceptibility to early experiences is still elusive. Hippocampal neurogenesis is significantly decreased in female but increased in male after maternal separation, which may explain the vulnerability of females to depression (Oomen et al., 2009). Dendritic arborization and the spine density in medial prefrontal cortex are affected only in females with maternal separation experiences (Farrell et al., 2016). Estradiol is believed to be one of the key factors for the sex-differences in response to early life stress. Early limited nesting stress exerts a more prominent effect on hypothalamic-pituitary-adrenal (HPA) axis in females than in males (Moussaoui et al., 2017), which might be mediated by estradiol for its activation function in the female HPA response to stress (Seale et al., 2005). Higher DNA methylation of estrogen receptor shores is observed in adult females subjected to early life adversity (Fiacco et al., 2019). Early life stress also alters neural plasticity in a sex-dependent manner. Female rats experiencing infant isolation show a significantly longer duration of long-term potentiation in hippocampus than males, in which estradiol is supposed to be involved (Kehoe and Bronzino, 1999). The downstream target of estrogen is largely unknown and requires further investigated.
Maternal separation and limited bedding are popular protocols to establish rodent models of early adverse experience. These model animals show anxiety and/or depression-like behavior in adulthood (Reus et al., 2011; Wang et al., 2012), while anxiety/depression itself affects visual functions like visual attention, visual learning and memory, and visual working memory (Kalogerakou et al., 2015; Qi et al., 2014). To exclude the interference of anxiety/depression, we established a new ECMS model with 1-h random stimulation from P2-8 in mice. The stimuli were selected from mild chronic stressors used in previous studies (Karst and Joels, 2003; Mineur et al., 2006). In our study, adult ECMS mice did not have any anxiety/depression-like syndromes, but showed abnormal development of both visual function and OD plasticity. Moreover, our findings suggest that early adverse experiences have long-lasting impact on NR2B expression which is maintained by estradiol in adult female mice, resulting in sex-dependent delayed closure of CP in visual cortex.
2. Materials and methods
2.1. Animals
Male and female C57BL/6J mice were purchased from Vital River Laboratories (Beijing, China). One male and two females were reared in a standard cage, placed in a 12-h reversed light–dark cycle with food and water ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Science and Technology of China. The mice were divided into four time periods(the early stage of CP, Early-CP, P21/22; the peak stage of CP, Peak-CP, P28/29; the late stage of CP, Late-CP, P38/39; Adult stage, > P60). A total of 599 litters (285 females, 314 males) were used in this study.
2.2. Early chronic mild stress model
Dam and litters as a whole, were subjected to unpredictable chronic mild stress such as cage tilting, keeping lights on for a short period of time during the dark phase, damp sawdust and shaken on a horizontal shaker (40 rpm), for 1 h daily from P2-8 in the morning (Karst and Joels, 2003; Mineur et al., 2006). One stressor was selected randomly per day. After receiving stress, the dam and litters were returned to the original cage. Males and females were fed separately after P21.
2.3. Blood sample and measurement of plasma corticosterone levels
Blood samples were collected from the retro-orbital sinus on P16 and P60-70 under 1–2% isoflurane anesthesia. All samples were collected between 8:00-11:00 to control for circadian variation in hormonal levels. Samples were centrifuged at 4000 rpm for 10 min to extract plasma which was then stored at −80 °C for subsequent analysis with a corticosterone ELISA kit (Enzo life sciences, NY, USA), according to the manufacturer's protocols (Choi et al., 2017).
2.4. Behavioral tests
All behavioral tests were performed on adult mice (>P60) between 13:00 and 17:00 by the same experimenter.
2.4.1. Open-field test (OFT)
The open-field apparatus was applied to analyze spontaneous exploratory activity and curiosity of animals to a novel environment (Cai et al., 2010). The open field was a square Plexiglas box (50 cm × 50 cm × 38 cm) painted with black lines to form 16 equal squares. The four central squares were defined as the center area. The test was performed under bright ambient room light. Each mouse was placed in the center of the open field and left free to explore the unfamiliar arena for 5 min while their behavior was recorded by a video camera.
2.4.2. Elevated plus maze (EPM)
The apparatus elevated 50 cm from the floor consists of two open arms and two closed arms (Hu et al., 2013). Open arms and closed arms are perpendicular to each other. Mice were placed in the center of the maze facing an open arm and allowed to explore for 5 min while their behavior was recorded by a video camera.
2.4.3. Sucrose preference test (SPT)
Sucrose intake is a measure of anhedonia, a core symptom of depression. After a 12-h water deprivation, the mouse was given two bottles, one with 200 ml of 2% sucrose (w/v) and the other with 200 ml of normal water, for 12 h. Then the amount of liquid consumed was measured. The measurement experiment lasted for 4 days. To avoid left/right preference, bottle position was alternated during the testing period. The average percentage of sucrose solution in the total liquid ingested is used as a measure for mouse's sensitivity to reward.
2.5. Intrinsic signal optical imaging
The performance of intrinsic signal optical imaging in the visual cortex of mice is described in a recent literature (Zhang et al., 2015). After anesthetized with a mixture (i.p.) of ketamine (0.1 mg/g) and xylazine (0.01 mg/g), the mouse was fixed on the stereotaxic instrument. Anesthesia was maintained by isoflurane (0.5–1%). The mouse's visual cortex was illuminated with green (550 nm) and red (720 nm) light to obtain a cerebral vascular map and evoked signal, respectively. Images were taken by a Dalsa Pantera 1M60 CCD camera with a resolution of approximately 17 μm/pixel. In order to detect the cut-off SF of the mouse, sinusoidal gratings were displayed in binocular vision (−5 to +15° horizontal by −15 to 45° vertical) at a wide range of SFs (0.01, 0.02, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5 cycle per degree (cpd)), for a total of 32 trials. The cut-off SF of each animal was calculated according to previous work (Zhang et al., 2015).
2.6. Behavioral assessment of visual acuity
Visual recognition tasks were applied to assess the visual acuity of mice (Prusky and Douglas, 2003). The apparatus used in this study is the same with previous study (Wang et al., 2016). Two sessions consisting of 24 trials were executed per day. First, the mice must learn to find the hidden escape platform which was placed on the side of the positive stimulus (vertical sinusoidal grating, 0.12 cpd). Negative stimulus was a horizontal sinusoidal grating of the same SF. Which side the platform was placed on was pseudo-randomly picked, and consecutive selection of one single side was limited to two times. If the mouse swam across the choice line towards the negative direction, the trial was considered incorrect. When correct rate at starting SF reached 100%, the SF of the gratings would be progressively increased. If no less than 6 correct choices in 8 consecutive tests or 4 correct choices in 4 consecutive tests were performed, the SF of the gratings would be increased by 0.03 cpd. If the accuracy decreased below 70% in 10 successive trials, the SF of the grating was decreased. The threshold of SF was repeatedly assessed. Finally, an accuracy-SF curve was fitted and the visual acuity of the mouse was determined as the grating SF corresponding to 70% accuracy.
2.7. Animal surgery and drug administration
2.7.1. Eyelid suture
Monocular deprivation (MD) was performed by suturing the eyelid of mice at different ages (four days before in vivo electrophysiology) under 0.5–3% isoflurane anesthesia. After surgery, we checked the mice and applied erythromycin ophthalmic ointment to the sutured eye daily to prevent eye opacity due to inflammation. For MD animals, eyelids were kept closed for 4 days. The mice with eye's coming open before recording were excluded from subsequent experiments.
2.7.2. Osmotic minipump implantation and delivery of NR2B antagonist
One day before MD, the mice were anesthetized with 1–2% isoflurane and fixed on the stereotaxic instrument. Erythromycin ophthalmic ointment was applied to protect eyes. Body temperature was kept constant at 37 °C. In the monocular area of the primary visual cortex, a 0.5 mm hole was drilled at 2.0 mm lateral to the midline and 1.0 mm anterior to the lambda. Then the infusion cannula was inserted 1 mm below the surface of the skull. We placed the connected minipump (0.5 μl/h, ALZET 1007D) under the skin on the back of the neck. The exposed skull was covered with cyanoacrylate and dental cement. NR2B antagonist Ro 25–6981 (0.88 mM, diluted with PBS, Sigma) or 0.01 M PBS were directly infused into visual cortex through osmotic minipump for 5 days.
2.7.3. Ovariectomy and estradiol supplementation
At P16, ECMS female mice were anesthetized with a mixture (i.p.) of ketamine (0.1 mg/g) and xylazine (0.01 mg/g). Removal of the ovaries was performed bilaterally as previously described (Lu et al., 2018). We also set up a sham operation group that only performed surgery without removing the ovaries. After suturing, the mice were placed on an electric blanket. After awakening, the mice were returned to their mother's cage. In estradiol supplementation experiment in ECMS male mice, estradiol (diluted with sesame oil, 5 μg/day, s.c., MCE) was infused from P39 to adulthood (>P60) once a day in the morning.
2.7.4. Diazepam administration
On P16, mice were anesthetized with xylazine and stereotaxically injected with 1.5 μl of diazepam (DZ, 2 mg/ml in saline) into the lateral ventricles of both hemispheres (coordinates from bregma: anteroposterior, −0.3 mm; mediolateral, 1 mm; dorsoventral, 2–2.5 mm), and each injection was finished within 10 min. After the injection, the scalp was sutured with a disposable suture needle, then the wound was coated with erythromycin ointment to eliminate inflammation. The mice were placed in separate cages, placed on an electric blanket. After fully awakening, they were returned to the cage of the dam. DZ or vehicle (Veh) solution was injected in the morning for 2 consecutively days. In adult mice, DZ (diluted with saline, 5 mg/kg, i.p.) was administrated for 5 days.
2.8. In vivo electrophysiology
Mice were anesthetized, maintained with urethane (2 g/kg, i.p.) and chlorprothixene (5 mg/kg, i.m.) and placed in a stereotaxic frame. Body temperature was continuously monitored and maintained at 37 °C. A craniotomy was performed over primary visual cortex (V1b) for recording. For single-unit recording, a computer-generated moving bar was presented on a monitor that was positioned 23 cm from the mouse's eyes. Cortical responses to each eye were recorded within the entire thickness of primary visual cortex. The mean firing rates of spontaneous and visually evoked activities were computed from peristimulus time histograms. In each mouse, 18–25 cells were recorded in 4–5 vertical penetrations which were evenly spaced (at least a 200 μm interval) across the mediolateral extent of V1b to avoid sampling bias. Only the cells with a receptive field within 20° from the vertical meridian were included in our sample. Cells were assigned to OD categories according to the seven category scheme of Hubel and Wiesel (1970). The CBI value of each mouse was calculated as follows: ( = the total number of cells, = the number of cells corresponding to an OD score of ). Normalized OD scores of single neurons were calculated using the following formula: , where and were the evoked contralateral and ipsilateral responses, respectively.
The electrophysiological properties of the cells in V1b were recorded in the adult mice without MD. Orientation/direction selectivity was measured with sinusoidal drifting gratings presented between 0° and 330° (12 steps at 30° spacing, SF: 0.05 cpd, TF: 2 Hz). Then, the SF and TF tuning curve was obtained with drifting gratings at the cell's optimal direction. All stimuli were presented in a pseudorandom sequence. The tuning curves were fitted with Gaussian distribution in Matlab to get the optimal SF and TF of each cell. The responses to 12 orientations were fitted with a bimodal Gaussian distribution as previous work (Zhao et al., 2013). The Orientation Selection Index (OSI) was calculated as , where was the response at the preferred direction, represented the mean response at the two directions orthogonal to the preferred one; the Direction Selection Index (DSI) was , where was the average response at the opposite direction.
2.9. Western blot analysis
The primary visual cortex of the mouse was quickly dissected under deep anesthesia with diethyl ether and placed in an EP tube containing RIPA lysis buffer (Li et al., 2018). After electrophoresis, the protein was transferred onto polyvinylidene fluoride (PVDF) membrane. After blocking with 5% no-fat milk (wt/vol, in 20 mM TBST) for 1 h at room temperature, the membrane was incubated with rabbit anti-NR2B (1:1,000, Cell Signaling Technology, 4212S), rabbit anti-GAD65 (1:2,000, Proteintech, 20746-1-AP), mouse anti-GAD67 (1:5,000, Millipore, MAB5406), and mouse anti-β-actin (1:15,000, Abcam, ab6276) antibodies at 4 °C overnight. Then, the membrane was incubated with the secondary antibody conjugated with the corresponding HRP (1:2500-1:10,000, Promega) for 1 h at room temperature. Super Signal West Pico Chemiluminescent Substrate (Pierce) was used to develop the membrane. After repeating the procedure above multiple times, the optical density of each band was determined using ImageJ, and normalized according to β-actin.
2.10. Hoechst staining
After anesthetizing with a mixture (i.p.) of ketamine (0.1 mg/g) and xylazine (0.01 mg/g), ECMS and control mice were transcardially perfused with ice-cold PBS, followed by 4% paraformaldehyde in PBS. Brains were dissected and post-fixed in 4% paraformaldehyde overnight at 4 °C, and then cryoprotected with 30% sucrose in 0.1M PBS for 2 days at 4 °C 40 mm thick coronal sections were cut with a cryostat microtome (Leica CM1950). After stained with Hoechst 33342 (2 mg/ml, Sigma, B2261) for 10min, slices were transferred to slides. Images were obtained under constant parameters of the LSM 710 confocal laser scanning microscope (Zeiss).
2.11. Statistical analysis
All data are presented as mean ± SEM. Statistical significance between two groups was analyzed by student t-test or one-way ANOVA with post hoc Turkey. To detrmine differences among multiple groups, two-way ANOVA with post hoc Turkey was performed. Kolmogorov-Smirnov test (K-S test) was used to compare the cumulative distributions of two groups. Differences were considered to be significant with a P value less than 0.05.
3. Results
3.1. ECMS mice do not show anxiety and/or depression-like behavior
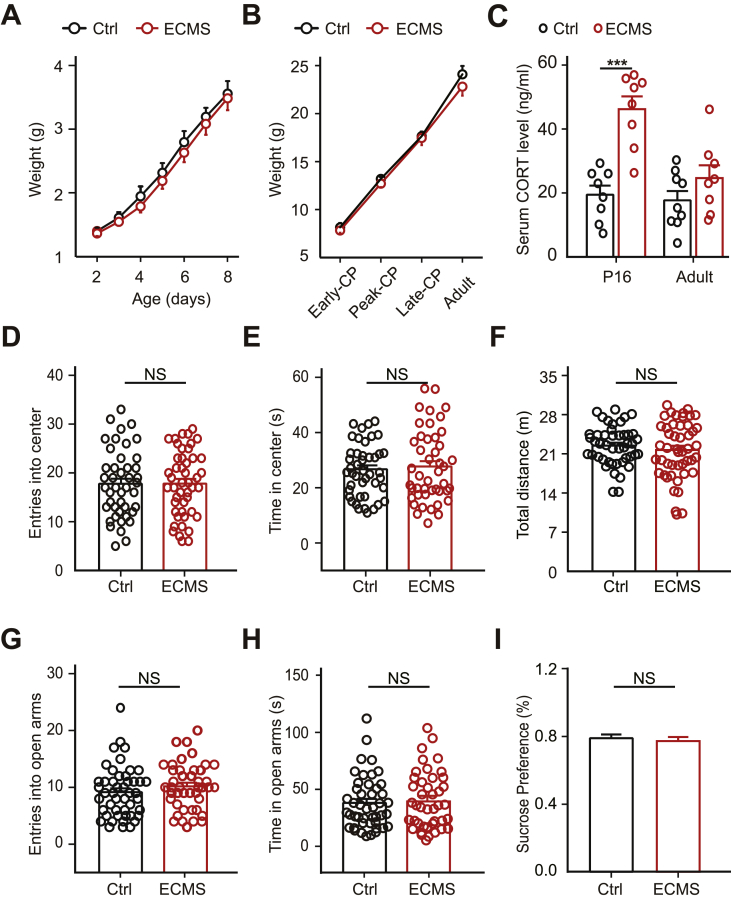
We established early chronic mild stress model as described in methods. The weights of ECMS mice were not significantly different from those of control mice at the same age (Fig. 1A–B, P > 0.05, t-test), excluding malnutrition-induced effects on brain structure and function (Prado and Dewey, 2014). The corticosterone level in the serum of ECMS mice was significantly higher than that in control mice one week after ECMS (P16, t-test, P < 0.001), indicating that these animals had a stressful experience. However, the corticosterone level reduced to a normal level in adulthood (Fig. 1C, P = 0.29, t-test). We then investigated whether ECMS induces emotional abnormalities in adulthood. In open field test, the frequencies of entering the central area (Fig. 1D) and the retention time in the central zone (Fig. 1E) in the ECMS group were similar to those in the control group (P = 0.97 and 0.69 respectively, one-way ANOVA). In the elevated-plus maze test, the entries (Fig. 1G) and the total time (Fig. 1H) in open arms were identical between ECMS and control mice (P = 0.23 and 0.67 respectively, one-way ANOVA). These results indicated that adult ECMS mice did not have anxiety-like behavior. The total distance traveled by ECMS mice in the open field was similar to control (Fig. 1F, P = 0.39, one-way ANOVA), indicating that the early adversity did not affect the ability of mice to move. The proportion of sucrose consumption in the ECMS group was similar to that in the control group in sucrose preference test (Fig. 1I, P = 0.60, t-test), suggesting an absence of depression-like behavior. These findings indicate that early chronic mild stress has a limited effect on the stress hormone, and does not trigger anxiety/depression-like behavior in adulthood.
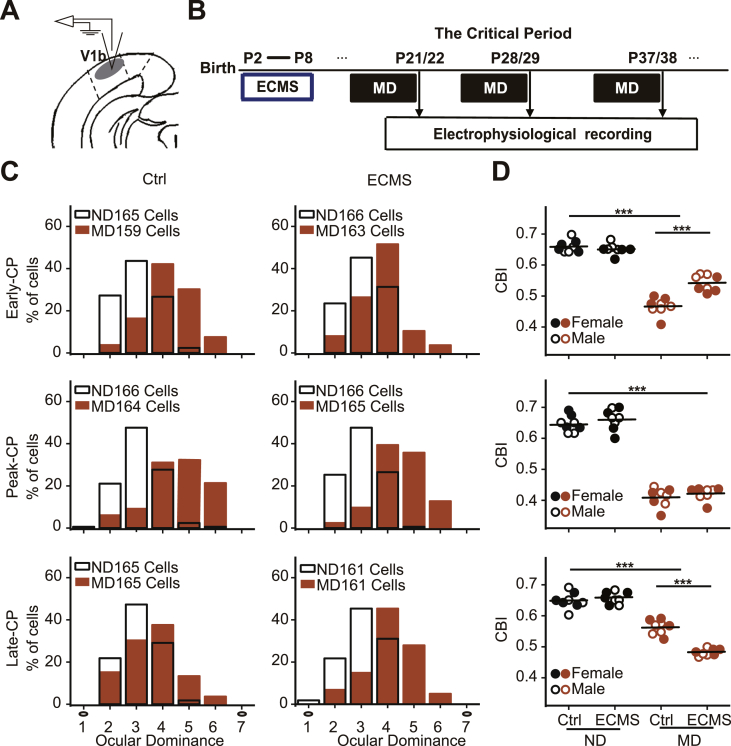
Fig. 3.
ECMS has a long-term impact on the development of OD plasticity among CP.
A. Schematic of in vivo electrophysiological recording. B. Schematic of the experimental procedure. The time displayed above the black arrow is the time point of the electrophysiological recording, and MD was carried out 4 days before the electrophysiological recording. C. OD distributions in control (Ctrl, left column) and ECMS (right column) mice were shown during CP (top: Early-CP; middle: Peak-CP; bottom: Late-CP). Orange column represents mouse with MD, White column represents mouse without MD (ND), n = 4 females and males/group. The number of cells in each group is shown in the figure. Left hollow black circles represent the contralateral eye and right hollow black circles represent the ipsilateral eye. D. Summary of CBI of each group shown in C. The horizontal line represents the average of the data, each symbol represents an animal. The hollow circle symbol represents male mouse, and the solid circle is female mouse. ***P < 0.001, two-way ANOVA with post hoc Tukey's test.
Fig. 1.
Adult ECMS mice have no anxiety and/or depression-like behavior.
A. The weight of the control mice and the ECMS mice during the period of stress (P2-8). B. The weight of mice at four tested developmental stages (Early-CP, Peak-CP, Late-CP, Adult). C. Basal plasma corticosterone (CORT) concentrations in serum samples were measured in the ECMS and control groups at P16 (Ctrl, 19.50 ± 2.83, n = 8 mice; ECMS, 46.23 ± 3.99, n = 8 mice; P < 0.001) and adult age (Ctrl, 17.72 ± 2.83, n = 9 mice; ECMS, 24.68 ± 3.98, n = 8 mice; P = 0.17). D-F. Anxiety-like behavior assessed by open field test for 5 min in adulthood. Ctrl mice (n = 46 mice) and ECMS mice (n = 49 mice) movement related to anxiety was assessed based on the number of entries into the center zone (D), time in center zone (E), and total travel distance (F). G-H. Anxiety-like behavior assessed by elevated-plus maze test for 5 min. Ctrl mice (n = 47 mice) and ECMS mice (n = 42 mice) movement related to anxiety was assessed based on the number of entries into the open arms (G) and time in open arms (H). I. The percentage of sucrose consumption in sucrose preference test in adult ECMS and Ctrl mice (Ctrl, 78.93% ± 2.19%, n = 35 mice; ECMS, 77.28% ± 2.40%, n = 34 mice; P = 0.6, t-test). A-I. Black hollow circle represents Ctrl mouse, red hollow circle represents ECMS mouse. Error bars, data represents mean ± SEM. A-C. ***P < 0.001, Student's t-test. D-I. P > 0.05, one-way ANOVA with post hoc Tukey's test.
3.2. ECMS does not affect the development of visual acuity, but alters the functional properties of visual cortical neurons
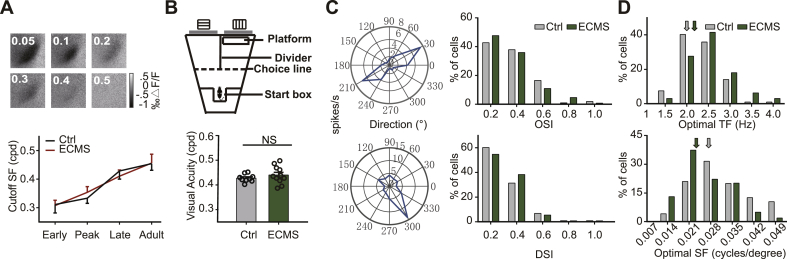
We next investigated whether ECMS affects the development of the functional properties of primary visual cortex. We used transcranial optical imaging of intrinsic signals to repeatedly sample the cutoff SF of ECMS and control mice at four stages: Early-CP (P21-22), Peak-CP (P28-29), Late-CP (P37-38), and Adult stage (>P60). According to others’ and our work (Heimel et al., 2007; Zhang et al., 2015), the cutoff SF gradually increased with the maturation of the visual system and remained comparable in control and ECMS mice (Fig. 2A right). We further measured the visual acuity behaviorally with a two-alternative forced choice visual detection water task in adult mice. The ECMS and control groups showed similar visual acuity thresholds (Fig. 2B right, P = 0.96, t-test), which were identical to those in previous studies (Wang et al., 2016). These findings suggest that ECMS does not affect the development of visual acuity of mice.
Fig. 2.
ECMS has no effect on the maturation of visual acuity and orientation/direction selectivity.
A. The left column shows the response intensity to different SFs in one control mouse at Late-CP. The right column shows the maturation of the cutoff SF of mouse during the development (Early-CP (Early): Ctrl, 0.31 ± 0.03 cpd, n = 8 mice; ECMS, 0.31 ± 0.02 cpd,n = 8 mice; Peak-CP (Peak): Ctrl, 0.33 ± 0.02 cpd, n = 8 mice; ECMS, 0.35 ± 0.02 cpd,n = 9 mice; Late-CP (Late): Ctrl, 0.43 ± 0.03 cpd, n = 10 mice; ECMS, 0.41 ± 0.02 cpd, n = 9 mice; Adult: Ctrl, 0.45 ± 0.02 cpd,n = 12 mice; ECMS,0.46 ± 0.03 cpd,n = 9 mice. P > 0.05, t-test). B. Schematic of the visual water task is shown in the left column, and the visual acuity of each mouse is shown in the right column (Ctrl, n = 9 mice; ECMS, n = 11 mice, P = 0.35). C. The left column illustrates a cell with high orientation-selectivity and a cell with high direction-selectivity. The right column shows the distribution of OSI (top) and DSI (bottom) in all cells of the control (Ctrl, 103 cells, n = 5 mice) and ECMS groups (128 cells, n = 6 mice). D. The distribution of the optimal TF (top) and SF (bottom) for all cells in the control (Ctrl, 95 cells, n = 5 mice) and ECMS mice (100 cells, n = 6 mice). The arrows indicate the median value of each group. B-D. Grey column represents Ctrl mouse, green column represents ECMS mouse. Error bars, data represents mean ± SEM. A-B. P > 0.05, Student's t-test. C-D. Kolmogorov-Smirnov test.
Orientation and direction selectivity emerging around the time of eye opening are important and unique properties of V1 neurons. We performed single-unit recording in binocular visual cortex and calculated the orientation and direction selectivity index (OSI and DSI) of each cell according to previous study (Zhao et al., 2013). Most of cells exhibited strong orientation or directional selectivity to the moving sinusoidal grating (Fig. 2C right). The distributions of both OSI and DSI in the ECMS group were identical to those in the control group (OSI: P = 0.23; DSI: P = 0.69, K-S test). Next, we examined the spatial-temporal tuning property of V1 neurons. Interestingly, the mean value of the optimal SF of neurons in ECMS mice (0.023 ± 0.001 cpd) was significantly lower than that in control mice (0.028 ± 0.001 cpd, P = 0.004, K-S test, Fig. 2D bottom). Meanwhile, V1 neurons in ECMS mice preferred higher TF than those in control mice (Fig. 2D top, P = 0.012, K-S test). These results indicate that early adversity does not alter the development of orientation and direction selectivity, while V1 neurons in adult ECMS mice mainly tuned to low SF and high TF gratings.
3.3. ECMS delays the development of ocular dominance plasticity during CP
Previous studies indicate that adverse experiences in early life have long-term effect on neurogenesis and plasticity in hippocampus (Lucassen et al., 2015). We thus investigated whether OD plasticity would be altered by ECMS during CP (Fig. 3B). The OD histograms in binocular visual cortex of control and ECMS mice were biased in favor of the contralateral eye and similar at all tested ages (the non-deprived animals, blank bars, Fig. 3C), indicating that adverse experiences did not affect the formation of the OD column during development. However, OD plasticity induced by 4 days of MD was altered. At the Early-CP (Fig. 3C and D, top), the OD shift in ECMS mice was less than that in control mice, and the mean CBI value of ECMS mice (0.54 ± 0.01) was significantly higher than that of control mice (0.47 ± 0.01, two-way ANOVA, P < 0.001). At the eak-CP (Fig. 3C and D, middle), the OD distributions in both groups strongly shifted to the non-deprived eye and the mean CBIs were identical (P = 0.37, two-way ANOVA). At the Late-CP, the trend of OD shift was reduced in control mice while ECMS mice still kept a relatively large shift (Fig. 3C and D, bottom). The mean CBI of ECMS mice with MD was significantly lower than that of deprived control mice (P < 0.001, two-way ANOVA). These data strongly suggest that early chronic mild stress delayed the development of OD plasticity.
3.4. Adult ECMS females maintain juvenile-like visual plasticity
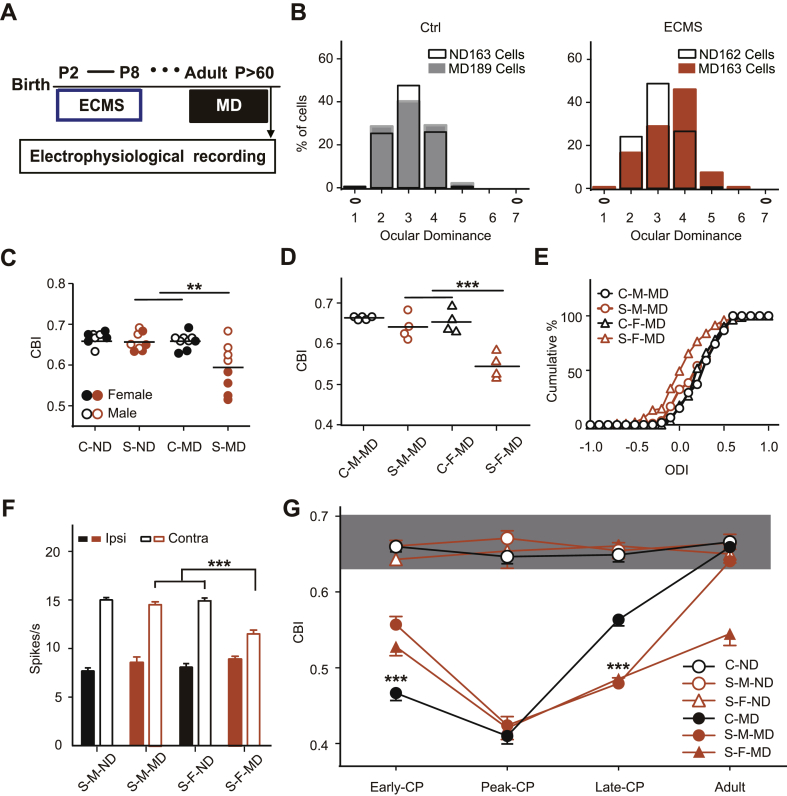
In naive adult mice (>P60), 4 days of MD no longer results in OD shift. Instead, up to 7 days of MD can induce a type of plasticity, which is mediated by the enhancement of the non-deprived eye input (Sato and Stryker, 2008). In the control group, the OD distribution favored the contralateral eye in non-deprived animals and this bias was impervious to 4 days of MD (Fig. 4B left), consistent with previous studies (Morishita et al., 2010). In adult ECMS mice, however, 4 days of MD induced an OD shift (Fig. 4B right). The mean CBI value in ECMS mice with MD was significantly lower than that in deprived control mice (control 0.66 ± 0.01 vs ECMS 0.59 ± 0.02, two-way ANOVA, P = 0.006, Fig. 4C).
Fig. 4.
Adult ECMS females maintain juvenile-like visual plasticity.
A. Schematic of the experimental procedure. B. OD distribution for adult control (left column) and ECMS (right column) mice with MD (Ctrl, n = 5 males and 4 females; ECMS, n = 4 males and 4 females) and ND (Ctrl, n = 4 males and 4 females; ECMS, n = 4 males and 4 females). Left hollow black circles represent the contralateral eye and right hollow black circles represent the ipsilateral eye. C. Summary of CBI of each group shown in (B). Each symbol represents an animal. The hollow circle symbol represents male mouse, and the solid circle is female mouse. D. Summary of CBI of MD mice in Ctrl and ECMS group after separation of males and females in (C). E. Cumulative distribution of ODI of each group in (D). F. Stimulation-evoked firing rates response to the ipsilateral and contralateral eyes of ND and MD in ECMS mice (Contra, contralateral eye; Ipsi, ipsilateral eye). G. The fold line summarizes the mean CBI of control mice and ECMS mice during the development. Male and female mice in the ECMS group are separately counted, while the control group does not. C-G. “C” represents control and “S” means the ECMS. "M" represents male and "F" represents female. Error bars, data represents mean ± SEM. **P < 0.01, ***P < 0.001 two-way ANOVA with post hoc Tukey's test. E. Kolmogorov-Smirnov test.
Gender differences in stress-related disorders and dendritic morphology in adult rodents following early adverse experiences have been widely reported (Farrell et al., 2016; Goodwill et al., 2019). Noticing that the range of CBI values in adult ECMS mice with MD was broader than other groups (Fig. 4c), we re-analyzed the data and divided it into male and female groups (Fig. 4d). Following MD, the OD histograms remained partial to the contralateral eye in male ECMS mice, while shifted significantly to the non-deprived eye in females. The CBI values of the ECMS females with MD were significantly lower than those of other MD groups (Fig. 4D, P < 0.001, two-way ANOVA), which was supported by the cumulative ocular dominance index (ODI) distribution curve (Fig. 4E, P < 0.001, K-S test). Juvenile-like OD plasticity induced by short-term MD during CP is characterized by a decrease in the response to the deprived eye (Sato and Stryker, 2008). As shown in Fig. 4F, the evoked firing rates in response to the deprived eye in the ECMS females reduced significantly following MD (contralateral eye: the non-deprived females 14.9 ± 0.3, the deprived females 11.5 ± 0.4, P < 0.001; ipsilateral eye: the non-deprived females, 8.1 ± 0.4; the deprived females, 8.9 ± 0.3, P > 0.05, two-way ANOVA). All results indicate that female ECMS mice retain juvenile-like plasticity in adulthood.
We summarized all data of OD plasticity during the development by gender (Fig. 4G). Visual plasticity of control mice showed a tendency to rise first and then decline with the gradual maturity of the visual cortex, which was consistent with previous studies (Gordon and Stryker, 1996). ECMS mice showed lower plasticity in Early-CP, but higher plasticity in Late-CP. No significant difference was observed between male and female mice during CP. In adult ECMS mice, however, the females maintained a high level of plasticity, while males did not (Fig. 4G). These data illustrate that ECMS can effectively alter the development of OD plasticity in a sex-dependent manner.
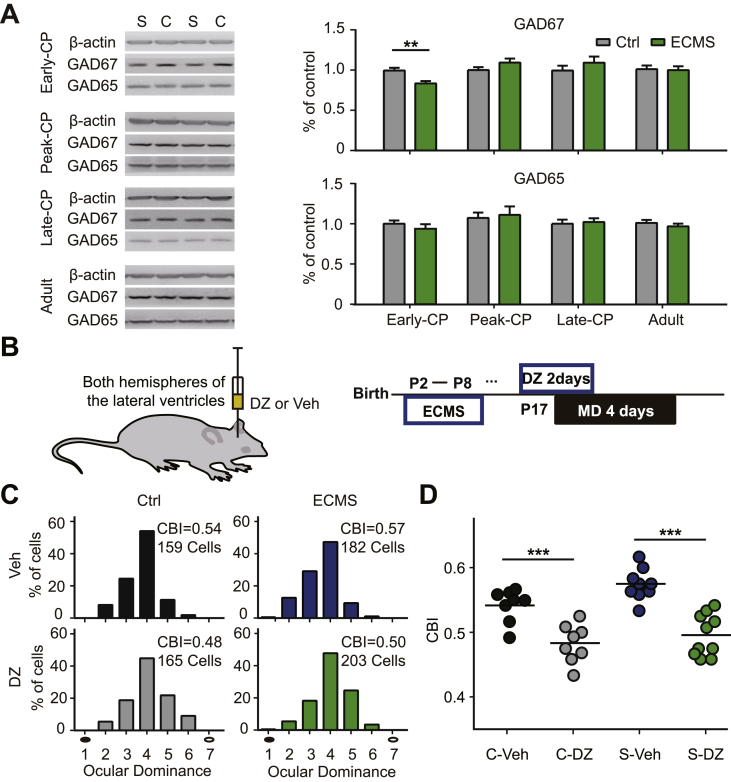
3.5. Diazepam occludes the low plasticity in the Early-CP of ECMS mice
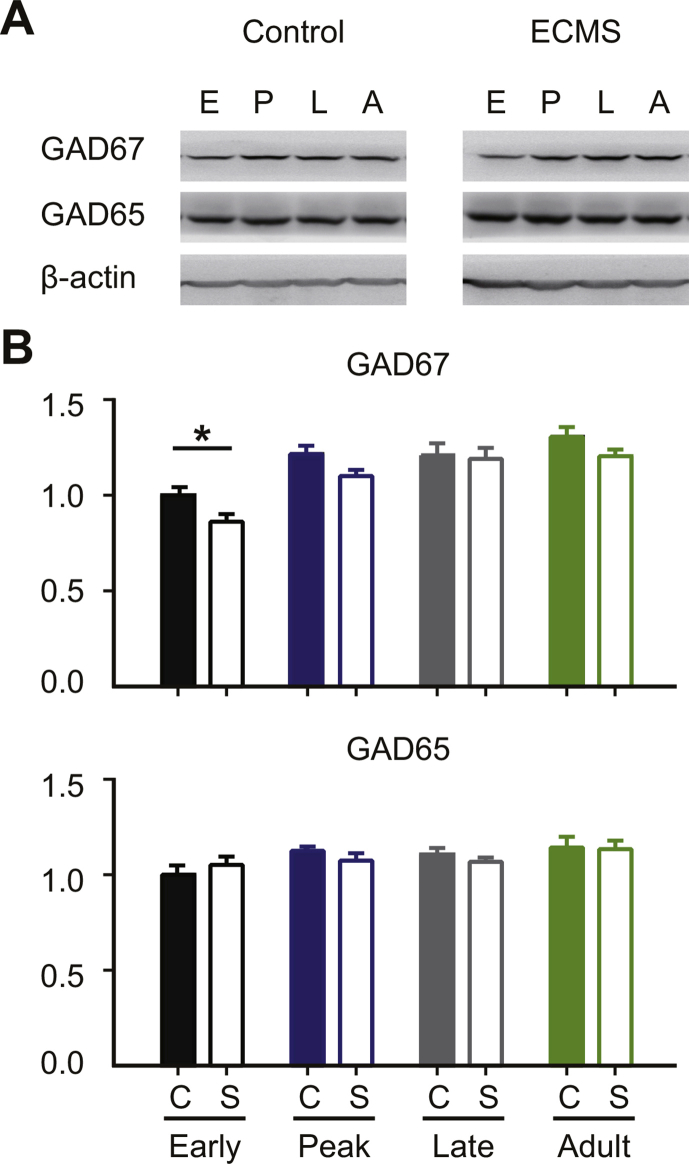
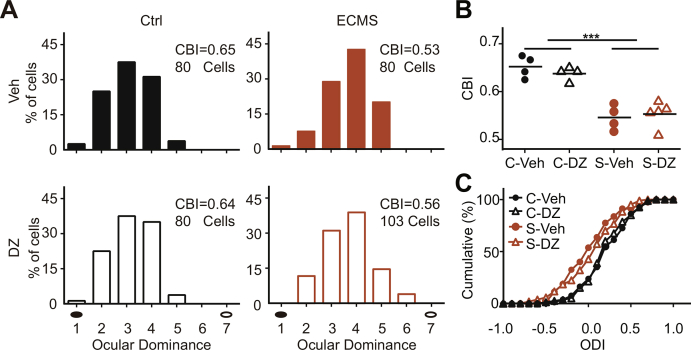
Early adverse experiences induce a decreased level of inhibition in adolescents (Leon Rodriguez and Duenas, 2013). Down-regulated GABAergic inhibition delays the opening of CP (Hensch et al., 1998), and restores the plasticity in adulthood (Harauzov et al., 2010). To reveal the molecular mechanisms underlying the abnormal visual plasticity in ECMS mice, we measured the expression levels of two main GABA synthesizing enzymes (GAD65 and GAD67) during the development. Only in the Early-CP, the expression levels of GAD67 subunits in visual cortex were significantly lower in ECMS mice compared with control mice (Fig. 5A, P < 0.01, t-test). In contrast, the GAD65 expression levels were similar in ECMS and control mice at all stages (Fig. 5A). Thus, the reduced GABAergic inhibition may underlie the reduced OD plasticity in the Early-CP. To validate this hypothesis, we injected diazepam (DZ) to the lateral ventricles of both hemispheres in ECMS and control mice in the Early-CP. DZ administration significantly enhanced the OD plasticity in both ECMS and control groups (Fig. 5C). The mean CBI values of the ECMS and control groups with DZ treatment were similar (Fig. 5D, P = 0.32, two-way ANOVA). These results suggest that reduced GABAergic inhibition may contribute to a lower plasticity in the Early-CP in ECMS mice.
Fig. 5.
Diazepam restores the impaired plasticity induced by ECMS in the Early-CP.
A. The protein expression levels of GAD67 and GAD65 in V1b of control (C, n = 12/group) and the ECMS (S, n = 14/group) mice of four periods (Early-CP, Peak-CP, Late-CP, Adult). Grey column represents the Ctrl mouse, green column represents the ECMS mouse. Error bars, data represents mean ± SEM. **P < 0.01, student t-test. B. Schematic of DZ perfusion (left) and the experimental procedure (right). C. OD distribution of the Ctrl and ECMS mice treated with diazepam (DZ, Ctrl:n = 8 mice; ECMS:n = 9 mice) or vehicle (Veh,Ctrl:n = 8 mice; ECMS:n = 10 mice) with 4 days of MD in Early-CP. Filled black circles represent the deprived eye and open black circles represent the non-deprived eye. D. Summary of the CBI of each group shown in (C). “C” represents control and “S” represents ECMS. The horizontal line represents the average of the data, each symbol represents an animal, ***P < 0.001 two-way ANOVA with post hoc Tukey's test.
3.6. Estradiol and NR2B subunit play an important role in the sex difference in adult ocular dominance plasticity caused by ECMS
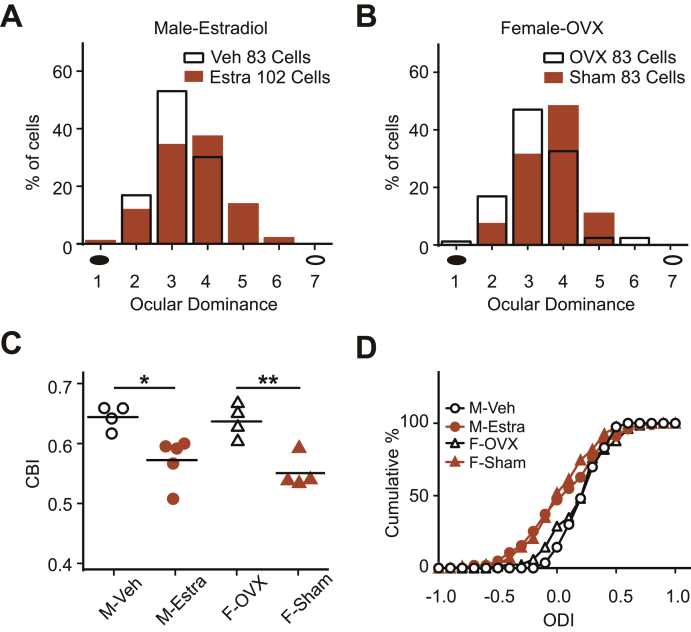
The levels of estradiol in males and females are similar at early age, but divergence occurs after sexual maturation. Estradiol levels are significantly down-regulated in males, but not in females (Overpeck et al., 1978). Thus, high estradiol level in adult female might be the reason for the late-onset gender difference in OD plasticity after ECMS. To test this hypothesis, we continuously supplied ECMS males with estradiol from P39 to adult age. As expected, ECMS males with estradiol supplementary became sensitive to visual experience and showed significant OD shift after 4 days of MD (Fig. 6A), similar to the results in ECMS females. Male ECMS mice with estradiol supplementation showed significantly lower CBI values than those with vehicle (Fig. 6C, P < 0.05, one-way ANOVA). Next, we performed bilateral ovariectomy (OVX) at P16 to reduce the estradiol level in adult female mice. Afterwards, four days of MD no longer induced an OD shift in adult ECMS females with OVX (Fig. 6B). The CBI values of the OVX group were significantly higher than those of the sham group (Fig. 6C, P < 0.01, one-way ANOVA). The cumulative curve of ODI scores in adult ECMS mice following MD (Fig. 6D) further indicated that males treated with estradiol and control females had similar plasticity, so did OVX females and control males. These results indicate that the estradiol level is sufficient to determine gender differences in adult OD plasticity induced by ECMS.
Fig. 6.
Estradiol plays a key role in the gender differences in OD plasticity induced by ECMS.
A. OD distribution for male-Estradiol (orange column, n = 5 mice) and male-vehicle (white column, n = 4 mice) mice with 4 days of MD. B. OD distribution for female-OVX (white column, n = 4 mice) and female-Sham (orange column, n = 4 mice) mice with 4 days of MD. A-B. Filled black circles represent the deprived eye and open black circles represent the non-deprived eye. C. Summary of CBI of each group shown in (A) and (B). The horizontal line represents the average of the data, each symbol represents an animal. In C **P < 0.01, *P < 0.05 two-way ANOVA with post hoc Tukey's test. D. Cumulative distribution of ODI shown in (C). “Estra” represents estradiol. P = 0.018, Kolmogorov-Smirnov test. C-D. “F” means female and “M” means male.
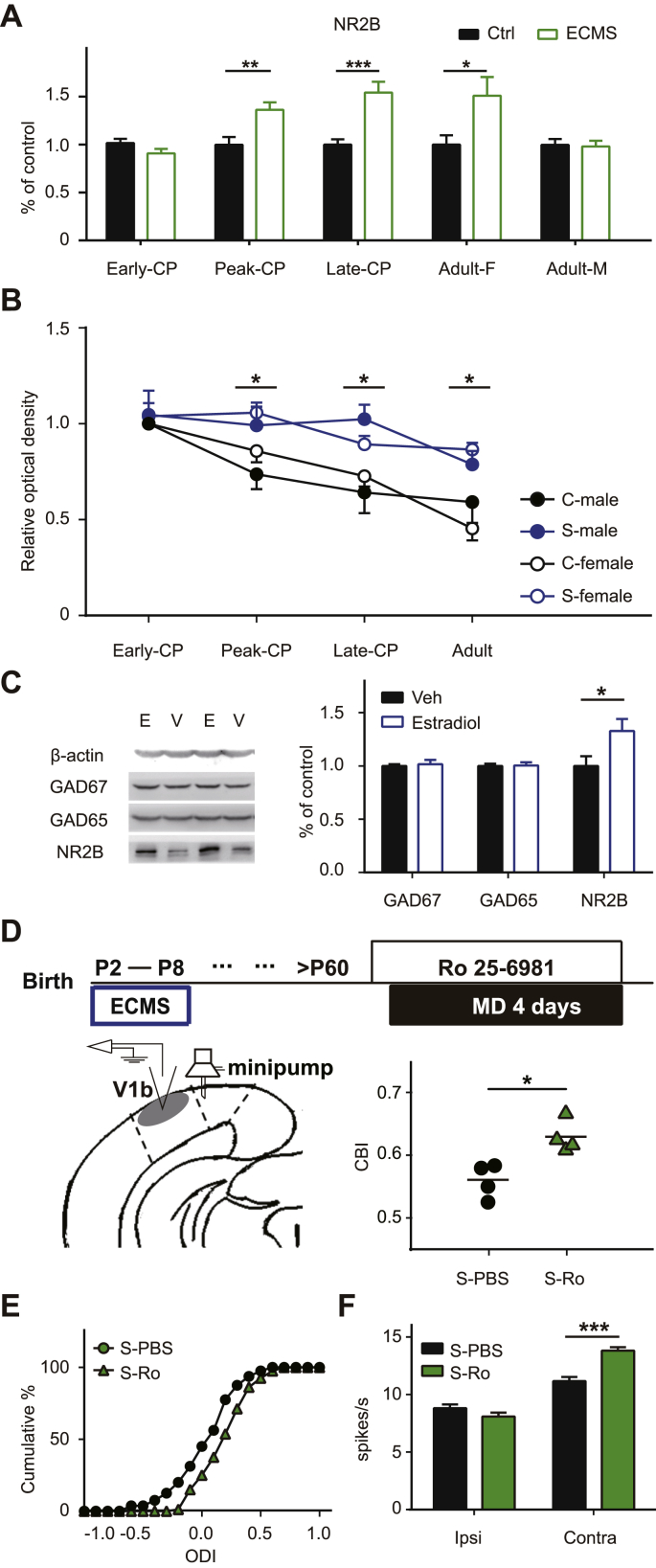
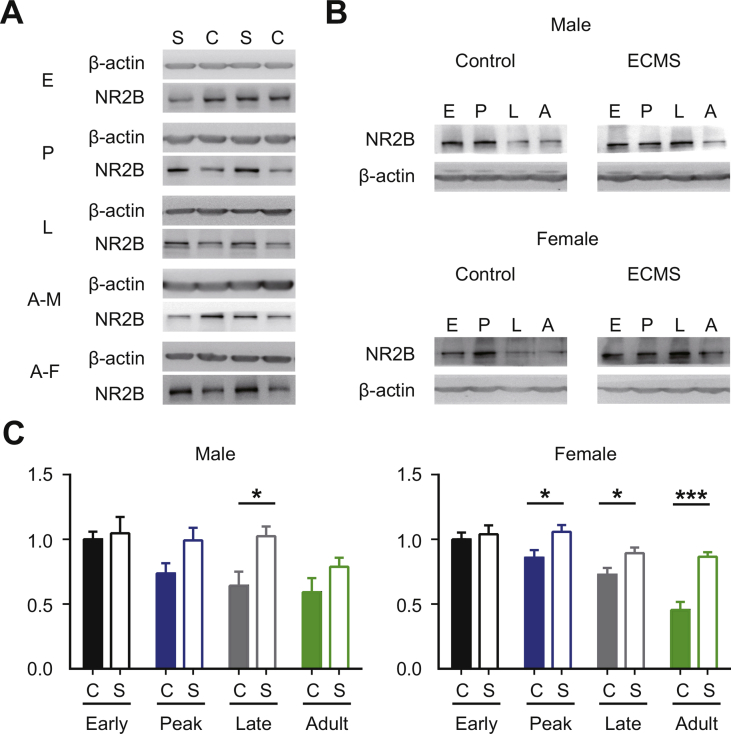
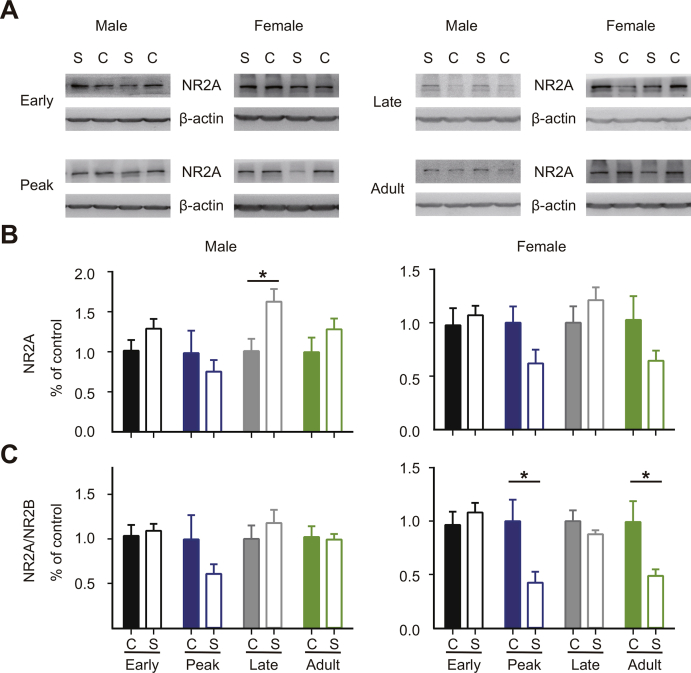
During development, the transition from NR2B to NR2A regulates visual experience-dependent transformation (Quinlan et al., 1999). Consistent with previous studies (Roberts and Ramoa, 1999), the expression of NR2B in the visual cortex of control mice showed a downward trend throughout CP (Fig. S2). ECMS significantly enhanced the expression of NR2B subunit at each time point beyond the Peak-CP (Fig. 7A, P < 0.001, t-test). Interestingly, the elevated NR2B expression also showed sex-dependent manner in adult ECMS mice, in which females had higher NR2B protein levels than males (Fig. 7A, females, 1.54 ± 0.11, P = 0.046; males, 0.98 ± 0.06, P = 0.85, t-test). We also examined the expression levels of GAD65, GAD67, and NR2B in the visual cortex of the adult ECMS males with estradiol supplementation since this treatment was able to restore the plasticity. The expression level of NR2B in the estradiol group was significantly higher than that in the vehicle group, while no significant change was observed in GADs expression (Fig. 7C). We did not observe a significant change in NR2A levels except that ECMS males had a higher NR2A expression at the Late-CP (Fig. S3). To confirm the contribution of NR2B to the observed adult plasticity, we locally infused Ro 25–6981 or vehicle into the visual cortex of adult ECMS female mice with an osmotic mini-pump one day prior to and throughout 4 days of MD (Fig. 7D). The CBI values of deprived female ECMS mice treated with Ro 25–6981 were significantly higher compared to those with vehicle (Fig. 7D, P < 0.05, t-test), indicating that the OD shift was blocked by NR2B antagonist. To rule out the possibility that cortical inhibition is reduced without the alteration of GAD expression and consequently contributes to the observed plasticity in adulthood, we administrated DZ in adult ECMS females. DZ did not block the plasticity in adult ECMS females (Fig. S4). These findings demonstrate that estradiol-NR2B pathway is crucial to the abnormal OD plasticity in adult ECMS females.
Fig. 7.
Activation of NR2B-containing NMDA receptors is tightly correlated with the high plasticity in ECMS mice.
A. The protein expression levels of NR2B in V1b of Ctrl and ECMS mice of four periods (Early-CP, Peak-CP, Late-CP, Adult). Black column represents Ctrl mouse (n = 6 females and males/group), green hollow column represents ECMS mouse (n = 7 females and males/group). B. The expression of NR2B in the visual cortex of male and female mice in the Ctrl and ECMS groups during development. Relative optical densities are the ratios of band densities at each stage divided by the band densities of Ctrl mice at Early-CP of the sample from the same immunoblot. Error bars, Data represent mean ± SEM. *P < 0.05, Student's t-test. C. The protein expression levels of NR2B, GAD67 and GAD65 in V1b of adult ECMS male mice administrated with estradiol (blue hollow column,n = 7 mice) or vehicle (Veh, black column, n = 6 mice). D. NR2B antagonist Ro 25–6981 blocks the plasticity in adult ECMS females (n = 4 mice/group). Schematic of the experimental procedure (top), the position of the implanted minipump and electrode recording site (left), summary of CBI of ECMS females treated with and without Ro25-6981 (right). The horizontal line represents the average of the data; each symbol represents an animal. E. Cumulative distribution of ODI. P = 0.018, Kolmogorov-Smirnov test. F. Stimulation-evoked firing rates driven by the ipsilateral and contralateral eyes of each group. Contra, contralateral eye; Ipsi, ipsilateral eye. In A, B, F, error bars, data represents mean ± SEM. *P < 0.05, ***P < 0.001, student t-test.
4. Discussion
Childhood maltreatment has been shown to cause psychiatric problems among the children. The present study provides the first evidence that even chronic mild stress experiences in early life are sufficient to postpone the development of visual cortex in a sex-dependent manner. This may explain the higher prevalence of vision and hearing problems among international adoptees (Eckerle et al., 2014). We further show that the delayed visual plasticity is accompanied by lower GAD67 level at the Early-CP and higher NR2B level at the Late-CP. Moreover, we identify that both estradiol and NR2B are crucial for the gender-related OD plasticity in adult animals. These findings indicate that early adversities interrupt the maturation of excitation and inhibition balance in visual cortex, and the abnormality of NR2B expression may account for the vulnerability to external stimuli in adult females.
4.1. ECMS modulates the maturation of visual cortical function
A large and growing body of literature indicates that early adverse experiences disturb the development of cognitive and emotional function as well as related brain regions (Fareri and Tottenham, 2016; Hanson et al., 2015), and the abnormality of mental health is directly proportional to the severity of adverse experiences in childhood. However, less attention was paid to the impact of early adversity on the functional maturation of perceptual cortex. One study shows that rats living in impoverished conditions have less body weight and a delayed maturation of visual acuity compared with normal rearing control rats (Narducci et al., 2018), which is supported by a recent study (Demaestri et al., 2020) showing that mice with maternal separation or limited bedding from P4-P11 have lower body weight gain and a delayed eye opening. This seems contrary to our results that ECMS mice have similar developmental trajectories of body weight and visual acuity to the controls. The discrepancy is most likely due to the severity of stress. Animals reared in impoverished rearing environment experience reduced maternal physical activity and diversity of visual stimuli for all day long from birth to adult age, while our mild chronic stressor is only given 1 h daily from P2-8. Consistently, our ECMS mice had no depression or anxiety-like behavior in adulthood, which is distinct from the maternal separation and limited bedding mice (Demaestri et al., 2020). Nevertheless, we do not rule out the possibility of species difference. Meanwhile, mild adversity in early life does modulate some visual properties of neurons in primary visual cortex. V1 neurons of adult ECMS mice preferred lower SF and higher TF than those of control mice. It is well known that multiple cortical areas receive V1 input with distinct visual properties, which reflects the abilities of the visual system to spatial pattern recognition. Anterolateral (AL) neurons prefer relatively low SF and high TF, whereas posteromedial (PM) neurons prefer high SF and low TF (Glickfeld et al., 2013). Taking together, our work suggests that ECMS may make different impacts on the information process from V1 to AL and PM, respectively.
To our knowledge, our data provide the first demonstration that early mild adversity delays the development of OD plasticity. OD plasticity in ECMS mice was lower at the Early-CP but higher at the Late-CP, as compared with that in control mice at the same age. Likewise, long term depression, which can be readily induced in hippocampal slices of juvenile but not adult animals, is observed in adult rat subjected to low maternal care (Bagot et al., 2009). The delayed maturation of visual cortex in our study does not support the hypothesis of accelerated maturation of CP of perceptual cortex with early adversity, which is proposed basing on the hippocampal data (Callaghan et al., 2013). The hippocampal-dependent memory system undergoes a developmental CP similar to sensory systems (Hensch and Bilimoria, 2012). Maternal separation in infant rats accelerates the maturation of hippocampal CP and leads to an early transition into adult-like fear retention, allowing infant memories to have a long-lasting influence (Callaghan and Richardson, 2012). Thus, how early adverse experiences accelerate or delay the development of distinct brain regions and whether they share common mechanisms need to be further explored.
The morphology of visual cortex is known to be affected by early adverse experience, similar to that of hippocampus and PFC. Subjects with child maltreatment, such as sexual abuse or witnessing domestic violence, show the reduction in grey matter volumes and thickness of V1 and visual association cortices (Fujisawa et al., 2018; Tomoda et al., 2009, 2012). We did not observe a significant alteration of cortical thickness in V1 of ECMS mice (Fig.S5), suggesting that the relatively mild adversity in our model does not result in long-term changes in cortical structure. These findings imply that a mild early adverse experience affects the development of cortical function without structural abnormality.
4.2. ECMS affects the maturation of inhibition and excitation in visual cortex at different time window
Excitatory-inhibitory balance plays an important role in the development of primary visual cortex. ECMS down-regulated the expression of GAD67, but not GAD65, in mouse visual cortex at the early stage of development (Fig. 5a). Consistently, rats experienced early impoverished environment demonstrate a reduced GAD67 expression in visual cortex at P18 and P21, but not P28, as compared with controls (Narducci et al., 2018). In agreement with previous studies (Hensch et al., 1998), the reduction of GABAergic inhibition caused low OD plasticity in the Early-CP of ECMS mice, which could be rescued by GABAA receptor agonist DZ. Eye opening, a natural but important event in the maturation of the visual system, increases the inhibitory inputs from fast-spiking interneurons to excitatory neurons (Guan et al., 2017). Thus ECMS may delay the maturation of cortical inhibition through the alteration of eye opening. Together, our findings support the theory that maturation of cortical inhibition is required for the occurrence of OD plasticity. The mechanisms limiting adult cortical plasticity are still unclear. It is believed that high GABAergic inhibitory level in adult cortex is crucial for the closure of CP for OD plasticity. A reduction of GABAergic transmission can restore OD plasticity in adult rats (Harauzov et al., 2010). In this study, we observed a juvenile-like OD plasticity in adult ECMS females. However, the expression of GADs was not significantly altered at adult age, and the plasticity could not be blocked by DZ, indicating that other mechanisms are involved.
Animals undergo a visually dependent shift in the expression of NMDA receptor subunits NR2B and NR2A during development (Quinlan et al., 1999). ECMS significantly delayed the developmental decline of NR2B expression at the late stage of development (Fig.S2-S3). ECMS mice showed higher NR2B expression level and OD plasticity than controls in the Late-CP. The abnormal expression of NR2B and OD plasticity was maintained in adult females, but not males. The plasticity in adult ECMS females was blocked by NR2B antagonist, indicating a tight correlation between NR2B expression and OD plasticity as reported in previous studies. Visual deprivation reactivated juvenile-like plasticity in adult rat visual cortex, which was accompanied by an increase in NR2B (He et al., 2006). Chronic magnesium treatment enhances the expression of NR2B in visual cortex and restores OD plasticity in normal and amblyopic adult mice (Liu et al., 2015). Thus, NR2B-containing NMDA receptor may represent another important factor for gating the termination of CP. Taken together, mild chronic stress experiences in infancy affect the maturation of inhibition and excitation in visual cortex at different time window, while the underlying mechanism remains to be elucidated.
4.3. Estradiol and NR2B pathway may mediate the sex-dependent phenomena after ECMS
Addressing sex-differences caused by early experiences in adulthood has long been considered an attractive challenge. Consistent with previous studies (Goodwill et al., 2019), our study revealed that female mice were more significantly affected by ECMS in adult age. Furthermore, we showed that the sex-dependent effect of ECMS on adult OD plasticity was correlated with the estradiol level. Adult ECMS female mice no longer showed visual plasticity induced by short MD if estradiol level was reduced by ovariectomy, while adult ECMS males with estradiol supplementary became sensitive to MD. Therefore, we proved that estradiol level is an important factor for the sex-dependent difference following early life adverse experiences. Estradiol has various downstream targets. Estradiol administration not only regulates the expression levels of glucocorticoid receptor mRNA and serotonin 1A receptor mRNA in the hippocampus (Wada et al., 2018), but also restores the NR2B in older CA1 to a younger level (Adams et al., 2004). In this study, we further identified that the NR2B level in visual cortex was correlated with estradiol level. Male ECMS mice had lower NR2B levels than females in adulthood, but this was reversed by supplementing estradiol to male mice. This finding was consistent with previous study that administration of estrogen to ovariectomized females increases the expression of NR2B and NR1 in hippocampal CA1 (Cyr et al., 2001). Notably, a previous study suggests that the modulation of estradiol on the NR2B expression depends on age. The expression of NR2B mRNA in hypothalamus is enhanced in female rats receiving estradiol on P30, but not on P15 (Kanamaru et al., 2001). These may explain our late-onset gender-differences of the expression of NR2B and OD plasticity in the visual cortex. Taken together,our findings suggest that high estradiol levels in females after sex maturation maintain abnormally high NR2B expression levels in mice with early adverse experiences. NR2B-containing NMDA receptor is believed to be crucial to the high level of plasticity in hippocampus and amygdala (Duan et al., 2015; Oh-Nishi et al., 2009). The estradiol-NR2B pathway may contribute to the high sensitivity of adult females to the external stimuli not only in visual cortex, but also in other brain regions.
With the new model, we point out that low to mild early adverse experiences which may not induce brain structural changes are sufficient to make impact on brain functional development. The effectiveness of preventive interventions for early adversity-induced mental disorders depends on early diagnosis. Thus these visual functional changes can be used as a potential sensitive indicator for the brain abnormality. The delayed CP of OD plasticity in primary visual cortex after ECMS gives further theoretical support for the long-lasting changes induced by early stress in brain development.
CRediT authorship contribution statement
Yueqin Liu: Designed the experiments, built animal model, in vivo electrophysiology, intrinsic signal optical imaging, T-Water Maze, biochemical experiments, behavior test, Data curation, Writing - original draft. Zhenni Wang: Modified the paper. Xinxin Zhang: Built animal model, behavior test. Sitong Li: Modified the paper. Wei Wu: Monocular deprivation and double-blind experimental design. Xin Li: Designed the experiments, built animal model, in vivo electrophysiology. Yupeng Yang: Designed the experiments, Writing - original draft, modified the paper.
Declaration of competing interest
There is no conflict of interest between the authors.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31871054).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ynstr.2020.100256.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
figs1.
figs2.
figs3.
figs4.
figs5.
References
- Adams M.M., Fink S.E., Janssen W.G., Shah R.A., Morrison J.H. Estrogen modulates synaptic N-methyl-D-aspartate receptor subunit distribution in the aged hippocampus. J. Comp. Neurol. 2004;474:419–426. doi: 10.1002/cne.20148. [DOI] [PubMed] [Google Scholar]
- Bagot R.C., van Hasselt F.N., Champagne D.L., Meaney M.J., Krugers H.J., Joels M. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol. Learn. Mem. 2009;92:292–300. doi: 10.1016/j.nlm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Bath K.G., Manzano-Nieves G., Goodwill H. Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Horm. Behav. 2016;82:64–71. doi: 10.1016/j.yhbeh.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L., Yan X.B., Chen X.N., Meng Q.Y., Zhou J.N. Chronic all-trans retinoic acid administration induced hyperactivity of HPA axis and behavioral changes in young rats. Eur. Neuropsychopharmacol. 2010;20:839–847. doi: 10.1016/j.euroneuro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Callaghan B.L., Graham B.M., Li S., Richardson R. From resilience to vulnerability: mechanistic insights into the effects of stress on transitions in critical period plasticity. Front. Psychiatr. 2013;4:90. doi: 10.3389/fpsyt.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B.L., Richardson R. Early-life stress affects extinction during critical periods of development: an analysis of the effects of maternal separation on extinction in adolescent rats. Stress. 2012;15:671–679. doi: 10.3109/10253890.2012.667463. [DOI] [PubMed] [Google Scholar]
- Choi J.E., Park D.M., Chun E., Choi J.J., Seo J.H., Kim S., Son J., Do M., Kim S.Y., Park Y.C. Control of stress-induced depressive disorders by So-ochim-tang-gamibang, a Korean herbal medicine. J. Ethnopharmacol. 2017;196:141–150. doi: 10.1016/j.jep.2016.12.025. [DOI] [PubMed] [Google Scholar]
- Cromheeke S., Herpoel L.A., Mueller S.C. Childhood abuse is related to working memory impairment for positive emotion in female university students. Child. Maltreat. 2014;19:38–48. doi: 10.1177/1077559513511522. [DOI] [PubMed] [Google Scholar]
- Cyr M., Ghribi O., Thibault C., Morissette M., Landry M., Di Paolo T. Ovarian steroids and selective estrogen receptor modulators activity on rat brain NMDA and AMPA receptors. Brain Res Brain Res Rev. 2001;37:153–161. doi: 10.1016/s0165-0173(01)00115-1. [DOI] [PubMed] [Google Scholar]
- Demaestri C., Pan T., Critz M., Ofray D., Gallo M., Bath K.G. Type of early life adversity confers differential, sex-dependent effects on early maturational milestones in mice. Horm. Behav. 2020;124 doi: 10.1016/j.yhbeh.2020.104763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y., Zhou S., Ma J., Yin P., Cao X. Forebrain NR2B overexpression enhancing fear acquisition and long-term potentiation in the lateral amygdala. Eur. J. Neurosci. 2015;42:2214–2223. doi: 10.1111/ejn.13008. [DOI] [PubMed] [Google Scholar]
- Eckerle J.K., Hill L.K., Iverson S., Hellerstedt W., Gunnar M., Johnson D.E. Vision and hearing deficits and associations with parent-reported behavioral and developmental problems in international adoptees. Matern. Child Health J. 2014;18:575–583. doi: 10.1007/s10995-013-1274-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri D.S., Tottenham N. Effects of early life stress on amygdala and striatal development. Dev Cogn Neurosci. 2016;19:233–247. doi: 10.1016/j.dcn.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M.R., Holland F.H., Shansky R.M., Brenhouse H.C. Sex-specific effects of early life stress on social interaction and prefrontal cortex dendritic morphology in young rats. Behav. Brain Res. 2016;310:119–125. doi: 10.1016/j.bbr.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Felitti V.J., Anda R.F., Nordenberg D., Williamson D.F., Spitz A.M., Edwards V., Koss M.P., Marks J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fiacco S., Gardini E.S., Mernone L., Schick L., Ehlert U. DNA methylation in healthy older adults with a history of childhood adversity-findings from the women 40+ healthy aging study. Front. Psychiatr. 2019;10:777. doi: 10.3389/fpsyt.2019.00777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T., Reinhold E., Koutsouleris N., Reiser M., Meisenzahl E.M. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J. Psychiatr. Res. 2010;44:799–807. doi: 10.1016/j.jpsychires.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Fujisawa T.X., Shimada K., Takiguchi S., Mizushima S., Kosaka H., Teicher M.H., Tomoda A. Type and timing of childhood maltreatment and reduced visual cortex volume in children and adolescents with reactive attachment disorder. Neuroimage Clin. 2018;20:216–221. doi: 10.1016/j.nicl.2018.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfranceschi L., Siciliano R., Walls J., Morales B., Kirkwood A., Huang Z.J., Tonegawa S., Maffei L. Visual cortex is rescued from the effects of dark rearing by overexpression of BDNF. Proc. Natl. Acad. Sci. U. S. A. 2003;100:12486–12491. doi: 10.1073/pnas.1934836100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickfeld L.L., Andermann M.L., Bonin V., Reid R.C. Cortico-cortical projections in mouse visual cortex are functionally target specific. Nat. Neurosci. 2013;16:219–226. doi: 10.1038/nn.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwill H.L., Manzano-Nieves G., Gallo M., Lee H.I., Oyerinde E., Serre T., Bath K.G. Early life stress leads to sex differences in development of depressive-like outcomes in a mouse model. Neuropsychopharmacology. 2019;44:711–720. doi: 10.1038/s41386-018-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J.A., Stryker M.P. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J. Neurosci. 1996;16:3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W., Cao J.W., Liu L.Y., Zhao Z.H., Fu Y., Yu Y.C. Eye opening differentially modulates inhibitory synaptic transmission in the developing visual cortex. Elife. 2017;6 doi: 10.7554/eLife.32337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Chung M.K., Avants B.B., Shirtcliff E.A., Gee J.C., Davidson R.J., Pollak S.D. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J. Neurosci. 2010;30:7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Nacewicz B.M., Sutterer M.J., Cayo A.A., Schaefer S.M., Rudolph K.D., Shirtcliff E.A., Pollak S.D., Davidson R.J. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol. Psychiatr. 2015;77:314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harauzov A., Spolidoro M., DiCristo G., De Pasquale R., Cancedda L., Pizzorusso T., Viegi A., Berardi N., Maffei L. Reducing intracortical inhibition in the adult visual cortex promotes ocular dominance plasticity. J. Neurosci. 2010;30:361–371. doi: 10.1523/JNEUROSCI.2233-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H.Y., Hodos W., Quinlan E.M. Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. J. Neurosci. 2006;26:2951–2955. doi: 10.1523/JNEUROSCI.5554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimel J.A., Hartman R.J., Hermans J.M., Levelt C.N. Screening mouse vision with intrinsic signal optical imaging. Eur. J. Neurosci. 2007;25:795–804. doi: 10.1111/j.1460-9568.2007.05333.x. [DOI] [PubMed] [Google Scholar]
- Hensch T.K., Bilimoria P.M. Re-opening windows: manipulating critical periods for brain development. Cerebrum 2012. 2012:11. [PMC free article] [PubMed] [Google Scholar]
- Hensch T.K., Fagiolini M., Mataga N., Stryker M.P., Baekkeskov S., Kash S.F. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P., Liu J., Zhao J., Qi X.R., Qi C.C., Lucassen P.J., Zhou J.N. All-trans retinoic acid-induced hypothalamus-pituitary-adrenal hyperactivity involves glucocorticoid receptor dysregulation. Transl. Psychiatry. 2013;3:e336. doi: 10.1038/tp.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D.H., Wiesel T.N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J. Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai Y., Fagiolini M., Obata K., Hensch T.K. Rapid critical period induction by tonic inhibition in visual cortex. J. Neurosci. 2003;23:6695–6702. doi: 10.1523/JNEUROSCI.23-17-06695.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalogerakou S., Oulis P., Anyfandi E., Konstantakopoulos G., Papakosta V.M., Kontis D., Theochari E., Angelopoulos E., Zervas I.M., Mellon R.C. Episodic visual learning/memory and attentional flexibility in patients with major depressive disorder after clinically effective electroconvulsive therapy. J. ECT. 2015;31:246–252. doi: 10.1097/YCT.0000000000000238. [DOI] [PubMed] [Google Scholar]
- Kanamaru H., Kakeyama M., Seki T., Arai Y. Estrogen potentiates N-methyl-D-aspartate receptor subunit R2B mRNA expression during the late prepubertal period in female rats. Neurosci. Lett. 2001;300:9–12. doi: 10.1016/s0304-3940(01)01527-0. [DOI] [PubMed] [Google Scholar]
- Karst H., Joels M. Effect of chronic stress on synaptic currents in rat hippocampal dentate gyrus neurons. J. Neurophysiol. 2003;89:625–633. doi: 10.1152/jn.00691.2002. [DOI] [PubMed] [Google Scholar]
- Kehoe P., Bronzino J.D. Neonatal stress alters LTP in freely moving male and female adult rats. Hippocampus. 1999;9:651–658. doi: 10.1002/(SICI)1098-1063(1999)9:6<651::AID-HIPO6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Koenen K.C., Widom C.S. A prospective study of sex differences in the lifetime risk of posttraumatic stress disorder among abused and neglected children grown up. J. Trauma Stress. 2009;22:566–574. doi: 10.1002/jts.20478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon Rodriguez D.A., Duenas Z. Maternal separation during breastfeeding induces gender-dependent changes in anxiety and the GABA-A receptor alpha-subunit in adult wistar rats. PloS One. 2013;8 doi: 10.1371/journal.pone.0068010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wang L., Zhang X., Huang M., Li S., Wang X., Chen L., Jiang B., Yang Y. Inhibition of Cdk5 rejuvenates inhibitory circuits and restores experience-dependent plasticity in adult visual cortex. Neuropharmacology. 2018;128:207–220. doi: 10.1016/j.neuropharm.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Lippmann M., Bress A., Nemeroff C.B., Plotsky P.M., Monteggia L.M. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur. J. Neurosci. 2007;25:3091–3098. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- Liu H., Li Y., Wang Y., Wang X., An X., Wang S., Chen L., Liu G., Yang Y. The distinct role of NR2B subunit in the enhancement of visual plasticity in adulthood. Mol. Brain. 2015;8:49. doi: 10.1186/s13041-015-0141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Ma K., Jin L., Zhu H., Cao R. 17beta-estradiol rescues damages following traumatic brain injury from molecule to behavior in mice. J. Cell. Physiol. 2018;233:1712–1722. doi: 10.1002/jcp.26083. [DOI] [PubMed] [Google Scholar]
- Lucassen P.J., Oomen C.A., Naninck E.F., Fitzsimons C.P., van Dam A.M., Czeh B., Korosi A. Regulation of adult neurogenesis and plasticity by (early) stress, glucocorticoids, and inflammation. Cold Spring Harb Perspect Biol. 2015;7 doi: 10.1101/cshperspect.a021303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur Y.S., Belzung C., Crusio W.E. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav. Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Morishita H., Miwa J.M., Heintz N., Hensch T.K. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science. 2010;330:1238–1240. doi: 10.1126/science.1195320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussaoui N., Jacobs J.P., Larauche M., Biraud M., Million M., Mayer E., Tache Y. Chronic early-life stress in rat pups alters basal corticosterone, intestinal permeability, and fecal microbiota at weaning: influence of sex. J Neurogastroenterol Motil. 2017;23:135–143. doi: 10.5056/jnm16105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narducci R., Baroncelli L., Sansevero G., Begenisic T., Prontera C., Sale A., Cenni M.C., Berardi N., Maffei L. Early impoverished environment delays the maturation of cerebral cortex. Sci. Rep. 2018;8:1187. doi: 10.1038/s41598-018-19459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh-Nishi A., Saji M., Satoh S.Z., Ogata M., Suzuki N. Late phase of long-term potentiation induced by co-application of N-methyl-d-aspartic acid and the antagonist of NR2B-containing N-methyl-d-aspartic acid receptors in rat hippocampus. Neuroscience. 2009;159:127–135. doi: 10.1016/j.neuroscience.2008.10.037. [DOI] [PubMed] [Google Scholar]
- Oomen C.A., Girardi C.E., Cahyadi R., Verbeek E.C., Krugers H., Joels M., Lucassen P.J. Opposite effects of early maternal deprivation on neurogenesis in male versus female rats. PloS One. 2009;4 doi: 10.1371/journal.pone.0003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overpeck J.G., Colson S.H., Hohmann J.R., Applestine M.S., Reilly J.F. Concentrations of circulating steroids in normal prepubertal and adult male and female humans, chimpanzees, rhesus monkeys, rats, mice, and hamsters: a literature survey. J. Toxicol. Environ. Health. 1978;4:785–803. doi: 10.1080/15287397809529700. [DOI] [PubMed] [Google Scholar]
- Prado E.L., Dewey K.G. Nutrition and brain development in early life. Nutr. Rev. 2014;72:267–284. doi: 10.1111/nure.12102. [DOI] [PubMed] [Google Scholar]
- Prusky G.T., Douglas R.M. Developmental plasticity of mouse visual acuity. Eur. J. Neurosci. 2003;17:167–173. doi: 10.1046/j.1460-9568.2003.02420.x. [DOI] [PubMed] [Google Scholar]
- Qi S., Chen J., Hitchman G., Zeng Q., Ding C., Li H., Hu W. Reduced representations capacity in visual working memory in trait anxiety. Biol. Psychol. 2014;103:92–99. doi: 10.1016/j.biopsycho.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Quinlan E.M., Olstein D.H., Bear M.F. Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc. Natl. Acad. Sci. U. S. A. 1999;96:12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reus G.Z., Stringari R.B., Ribeiro K.F., Cipriano A.L., Panizzutti B.S., Stertz L., Lersch C., Kapczinski F., Quevedo J. Maternal deprivation induces depressive-like behaviour and alters neurotrophin levels in the rat brain. Neurochem. Res. 2011;36:460–466. doi: 10.1007/s11064-010-0364-3. [DOI] [PubMed] [Google Scholar]
- Roberts E.B., Ramoa A.S. Enhanced NR2A subunit expression and decreased NMDA receptor decay time at the onset of ocular dominance plasticity in the ferret. J. Neurophysiol. 1999;81:2587–2591. doi: 10.1152/jn.1999.81.5.2587. [DOI] [PubMed] [Google Scholar]
- Roceri M., Hendriks W., Racagni G., Ellenbroek B.A., Riva M.A. Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Mol. Psychiatr. 2002;7:609–616. doi: 10.1038/sj.mp.4001036. [DOI] [PubMed] [Google Scholar]
- Sato M., Stryker M.P. Distinctive features of adult ocular dominance plasticity. J. Neurosci. 2008;28:10278–10286. doi: 10.1523/JNEUROSCI.2451-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale J.V., Wood S.A., Atkinson H.C., Harbuz M.S., Lightman S.L. Postnatal masculinization alters the HPA axis phenotype in the adult female rat. J. Physiol. 2005;563:265–274. doi: 10.1113/jphysiol.2004.078212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda A., Navalta C.P., Polcari A., Sadato N., Teicher M.H. Childhood sexual abuse is associated with reduced gray matter volume in visual cortex of young women. Biol. Psychiatr. 2009;66:642–648. doi: 10.1016/j.biopsych.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda A., Polcari A., Anderson C.M., Teicher M.H. Reduced visual cortex gray matter volume and thickness in young adults who witnessed domestic violence during childhood. PloS One. 2012;7 doi: 10.1371/journal.pone.0052528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth S.L., Cicchetti D. Patterns of relatedness, depressive symptomatology, and perceived competence in maltreated children. J. Consult. Clin. Psychol. 1996;64:32–41. doi: 10.1037//0022-006x.64.1.32. [DOI] [PubMed] [Google Scholar]
- Wada T., Sameshima A., Yonezawa R., Morita M., Sawakawa K., Tsuneki H., Sasaoka T., Saito S. Impact of central and peripheral estrogen treatment on anxiety and depression phenotypes in a mouse model of postmenopausal obesity. PloS One. 2018;13 doi: 10.1371/journal.pone.0209859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.D., Labermaier C., Holsboer F., Wurst W., Deussing J.M., Muller M.B., Schmidt M.V. Early-life stress-induced anxiety-related behavior in adult mice partially requires forebrain corticotropin-releasing hormone receptor 1. Eur. J. Neurosci. 2012;36:2360–2367. doi: 10.1111/j.1460-9568.2012.08148.x. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wu W., Zhang X., Hu X., Li Y., Lou S., Ma X., An X., Liu H., Peng J. A mouse model of visual perceptual learning reveals alterations in neuronal coding and dendritic spine density in the visual cortex. Front. Behav. Neurosci. 2016;10:42. doi: 10.3389/fnbeh.2016.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., An X., Liu H., Peng J., Cai S., Wang W., Lin D.T., Yang Y. The topographical arrangement of cutoff spatial frequencies across lower and upper visual fields in mouse V1. Sci. Rep. 2015;5:7734. doi: 10.1038/srep07734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Chen H., Liu X., Cang J. Orientation-selective responses in the mouse lateral geniculate nucleus. J. Neurosci. 2013;33:12751–12763. doi: 10.1523/JNEUROSCI.0095-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]