Abstract
Background
Epigenetic changes are considered the main mechanisms behind the interplay of environment and genetic susceptibility in major depressive disorder (MDD). However, studies focusing on epigenetic dysregulation of the HPA axis stress response in MDD are lacking. Our objective was to simultaneously asses DNA methylation of the glucocorticoid receptor gene (NR3C1) and serotonin transporter gene (SLC6A4) and HPA axis response to stress in MDD.
Methods
We recruited 80 depressed inpatients and 58 gender and age matched healthy controls. All participants underwent the Trier Social Stress Test (TSST) and salivary cortisol was repeatedly measured to assess HPA axis reactivity. DNA methylation of the NR3C1 (exon 1 F) and SLC6A4 CpG islands was quantified from whole blood DNA. In the MDD group, clinical assessment was repeated at 8-week follow-up to test the predictive potential of DNA methylation for symptom improvement.
Results
Depressed patients had blunted cortisol reactivity to TSST compared to healthy controls (p = 0.01). In addition, they presented with increased average SLC6A4 (p = 0.003) and NR3C1 methylation (p = 0.03), as well as methylation of two individual NR3C1 CpG loci overlapping with the NGFI-A-binding sites (CpG12 and CpG20). Methylation of one of these two loci (CpG20) predicted lower symptom improvement at the follow-up (p = 0.007). Both, average NR3C1 and SLC6A4 methylation were associated with lower cortisol reactivity in the MDD group and explained about 16% of variability in cortisol response to TSST.
Conclusions
We provide evidence of the role of NR3C1 and SLC6A4 DNA methylation in HPA axis dysregulation in MDD, which needs to be further explored.
Keywords: Depression, Epigenetics, Stress reactivity, HPA axis, NR3C1, SLC6A4
1. Introduction
Major depressive disorder (MDD) is a multifactorial disease and it is well established that the interplay between genetic susceptibility and adverse environmental factors occurring across lifespan contributes to its development (Klengel et al., 2014). A recent meta-analysis indicates that about 40% of depression risk can be attributed to genetics, whereas the other 60% are associated with individual environmental factors (Sullivan et al., 2000). Epigenetic mechanisms, particularly DNA methylation, are considered to be the main mechanism by which this gene-environment interplay takes place (Kular and Kular, 2018). Briefly, DNA methylation involves the addition of a methyl group at the 5’ position of cytosines in CpG dinucleotides, which leads to gene silencing, mainly when located in a gene promoter region (Newell-price et al., 2000). In the context of MDD, DNA methylation changes have been investigated with regard to diagnosis, symptom severity as well as predictors of treatment response and remission (Li et al., 2019).
In addition, relevance of DNA methylation in dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis response to stress in MDD has attracted a growing interest.
Among the potential gene-candidates that could contribute to epigenetic dysregulation of the HPA axis and MDD in general, two gained particular interest: glucocorticoid receptor gene (NR3C1) and serotonin transporter gene (SLC6A4) (Bakusic et al., 2017). NR3C1 encodes for the glucocorticoid receptor (GR), responsible for the effects of cortisol on peripheral tissues, but also the self-regulatory negative feedback of the axis (Lee and Sawa, 2014). DNA methylation changes in NR3C1 have been extensively investigated with regard to early adverse environment (Oberlander et al., 2008) (McGowan et al., 2009) (Hompes et al., 2013), suggesting that early life stress induces hypermethylation of the NR3C1 region overlapping with the nerve growth factor-inducible protein A (NGFI-A) binding site (promoter of exon 1 F) (Weaver et al., 2004a). As NGFI-A regulates GR expression, these alterations are further associated with the impaired regulation of the HPA axis (Liu and Nusslock, 2018). Even though there is some evidence that NR3C1 methylation changes linked to early life adversity can persist into adulthood (Liu and Nusslock, 2018), it is not clear yet whether this would correspond to phenotypic modifications. In fact, NR3C1 methylation changes in MDD were only investigated in a few studies (Nantharat et al., 2015) (Na et al., 2014) (Bustamante et al., 2016) (Roy et al., 2017), without any functional measurement of the HPA axis activity. In addition, longitudinal studies exploring the potential role of NR3C1 methylation in predicting symptoms improvement and recovery in MDD are missing.
In contrast to NR3C1, DNA methylation changes of SLC6A4 have been more systematically explored in MDD, however the results were inconsistent (Li et al., 2019). While cross-sectional and case-control studies somewhat consistently reported hypermethylation of this gene in depression, outcomes of longitudinal studies are not so uniform, reporting both increased and decreased SLC6A4 methylation to be associated with treatment response as well as absence of any correlation (Li et al., 2019). SLC6A4 encodes for the serotonin transporter (SERT) involved in the reuptake of serotonin or 5-hydroxytryptamine (5-HT) from the synaptic cleft (Mohammad-Zadeh et al., 2008). Apart from the role of 5-HT in brain development and mood (dys)regulation, the serotonergic system also plays an important role in the control of our stress response system (Booij et al., 2013a). Despite this interplay, little is known about the link between the epigenetic regulation of SLC6A4 and disruption in the HPA axis reactivity. To the best of our knowledge, only a couple of studies investigated neuroendocrine correlates of SLC6A4 methylation. One of them was done on monozygotic twin pairs discordant for bullying victimization, where hypermethylation of SLC6A4 was associated with blunted stress reactivity (Ouellet-Morin et al., 2012). The other two studies were done on healthy adults and similarly reported a link between increased methylation of SLC6A4 and disrupted stress reactivity (Alexander et al., 2014a) Alexander et al., 2019 . However, in the context of MDD, no such research was performed.
The fact that methylation changes of NR3C1 and SLC6A4 in depression are mostly investigated in separate studies and without functional measurements of the HPA axis reactivity is hindering our understanding of their interplay. Therefore, with the present study, we aimed to bridge this gap by assessing DNA methylation of these two genes, together with the HPA axis response to the Trier Social Stress Test (TSST) (Kudielka et al., 2007) in depressed patients and healthy controls. In addition, we used a longitudinal design in order to test whether DNA methylation changes in NR3C1 and SLC6A4 couldpredict symptom improvement in depressed patients at follow-up assessment.
2. Methods and materials
2.1. Study population
Eighty depressed patients and 58 age and gender matched control subjects were included in the study at baseline. All patients were hospitalized at the University Psychiatric Centre of the University of Leuven in Belgium. A detailed recruitment procedure and inclusion/exclusion criteria were described previously (Vrieze et al., 2013) (Vrieze et al., 2014). Briefly, patients with a current MDD diagnosis were included in the study. MDD diagnosis was set by a psychiatrist based on DSM-IV criteria (First et al., 2002). Patients with psychotic comorbidity, substance abuse or unstable medical conditions were excluded. Almost all MDD patients started antidepressant treatment before admission to the hospital. During follow-up, patients were treated with psychotropic medication and/or psychotherapy and were clinically assessed after 8 weeks. Healthy participants did not have any current or past psychiatric disorder or unstable medical condition and were assessed only at the baseline.
The study was approved by the UZ Leuven Medical Ethics Committee and all participants signed the informed consent.
2.2. Design and procedure
All patients were evaluated within the first week of admission to the hospital. All appointments started at the same hour (1:00 p.m.). Upon arrival, patients underwent a psychiatric interview after which they were asked to fill out the questionnaires and blood samples were drawn. At 2:20 p.m., patients rested for 40 min and at 3:00 p.m., they underwent the TSST. TSST and blood collections were performed at the baseline for both groups. For depressed patients, follow-up appointments were made after 8 weeks where collection of clinical data was repeated.
2.3. Clinical assessment
The Structured Clinical Interview for DSM-IV-TR (SCID-I) (First et al., 2002) was used to set the MDD diagnosis, according to DSM-IV criteria. The 17-item Hamilton Rating Scale for Depression (HDRS) (Hamilton, 1960) was used to assess the severity of depression. Early life stress (ELS) was assessed using the Structured Trauma Interview (STI), which is focused on childhood experience with sexual and physical violence (Draijer and Langeland, 1999). Positive and negative affect were measured using the Positive and Negative Affect Scale (PANAS) (Watson et al., 1988) and anhedonia was assessed using the Snaith-Hamilton Pleasure Scale (SHAPS) (Snaith et al., 1995). In addition, the personality trait neuroticism was assessed using the NEO-Five Factor Inventory (NEO-FFI) (Costa and McCrae, 1992). In the depression group, clinical assessment was repeated at the 8-week follow-up. Symptom improvement was calculated as the difference between HDRS score at 8-week follow-up and baseline.
2.4. Stress induction and cortisol measurements
The Trier Social Stress Test (TSST) was used to induce the endocrine response of the HPA axis to stress (Allen et al., 2017) and was performed at the baseline. A detailed protocol of the procedure has been described elsewhere (Kudielka et al., 2007). Briefly, after a 40-min rest and preparation phase, all participants were asked to perform a public speech and arithmetic tasks in front of two experts, who also recorded the procedure with a video camera. Participants were informed that they would be judged on their performance, but they were not aware of the real objective before completing the whole protocol.
Saliva samples were collected before the task (−2 min) and repeatedly during the recovery period after completing the stress task (+15, +25, +35, +45, +60 and + 105 min). Saliva samples were collected using Salivettes (Sarstedt, Numbrecht, Germany). Cortisol concentrations were determined using radio-immunoassay (Sulon et al., 1978) and the analyses were performed in duplicates.
2.5. DNA methylation analysis
DNA methylation analysis was performed at the baseline. DNA was extracted from whole blood and the quantity and purity of DNA were determined using a NanoDrop spectrophotometer. DNA samples were bisulphite-converted using the EZ-96 DNA Methylation-Gold™ Kit (#D5008, Zymo Research) according to the manufacturer's protocol. Next, bisulphite-converted DNA was amplified by PCR and pyrosequencing was performed on the PyroMark Q24 instrument (Qiagen) following the manufacturer's instructions. Pyrosequencing results were analyzed using the PyroMark analysis 2.0.7 software (Qiagen) with standard quality-control settings. All samples were randomized prior to DNA methylation analysis to reduce any potential bias during different steps in the methylation analysis and ensure that each reaction plate includes both patients and controls in random order. The primer sequences and the PCR protocols were based on those previously published by Alexander et al. (2018) and Vangeel et al. (2015) for NR3C1 and Wankerl et al. (2014) for SLC6A4. The primers were designed to target the whole CpG island of NR3C1 1 F region and part of the CpG island overlapping with the SLC6A4 promoter region (Figure A1 and A2). Positive controls (highly methylated) were used in each plate to validate the analysis and test variability between the plates. A detailed protocol with all analyzed amplicons, PCR and sequencing primers is provided in the Appendix.
2.6. Statistical analysis
Socio-demographic and clinical characteristics of the depression and the control group were compared using an independent sample t-test for continuous variables, and Chi-Square test for categorical variables. Because NR3C1 1 F and SLC6A4 methylation data were not normally distributed, a non-parametric Mann-Whitney U test was applied to compare DNA methylation levels between groups.
Since cortisol data were skewed, we performed log-transformation of the data to achieve normal distribution. In order to test the effect of the group and methylation on cortisol stress reactivity over time, we used a general linear model for repeated measurements, using cortisol as the outcome variable and time (−2, 15, 25, 35, 45, 60 and 105 min) as a fixed factor (categorical variable). Group, methylation data and other covariates (such as age, gender, and early life stress) were added to the model as explanatory variables. To test potential moderation effects, interactions between explanatory variables were added (e.g. methylation and early life stress). Since the sphericity assumption was violated, we used the Huynh-Feldt adjustment when interpreting the outcomes.
In addition to the analysis using linear model for repeated measures, we computed the cortisol area under the curve with respect to the ground (AUCg) and the increase (AUCi), using the trapezoid model formula described by Pruessner (Pruessner et al., 2003). These variables were computed for the sake of data visualization and confirmatory analysis.
Spearman correlation was used to test basic associations between continuous variables. The identified associations were further explored in linear regression models, adding covariates and testing potential interactions.
All statistical analyses were performed using the SPSS software package, version 26.0. All tests were two-sided, and the significance level was set at 0.05.
3. Results
3.1. Sample characteristics
At baseline, 80 depressed patients and 58 control subjects were tested. Socio-demographic and clinical characteristics of the participants included at the baseline are presented in Table 1. Participants from the control and the depression group differed in education level, as well as the presence of an early life stress event, which was more common in the depression group. As shown in Table 1, most depressed patients were taking either selective serotonin reuptake inhibitors (SSRIs) or serotonin–norepinephrine reuptake inhibitors (SNRIs) at the moment of inclusion and a smaller number of patients were taking antidepressants other than SSRIs and SNRIs (tricyclic antidepressants (TCA), mirtazapine or bupropion). DNA methylation analyses were done in all participants. Salivary cortisol data were missing for two participants in the MDD group. At 8-week follow-up, fifteen depressed patients dropped out. The MDD subsample that dropped out did not differ from the rest of the MDD group in basic socio-demographic and clinical characteristics (Table A6).
Table 1.
Socio-demographic and clinical characteristics of participants at baseline.
| Control group (N = 58) | MDD group (baseline, N = 80) | Significance | |
|---|---|---|---|
| Age (year, mean ± SD) | 45.5 ± 12.0 | 45.4 ± 12.1 |
t = 0.08 p = 0.93 |
| Gender (female/male) | 31/27 | 47/33 | X2 = 0.38 p = 0.53 |
| Educational level (low/high)a | 13/45 | 48/32 | X2 = 19.3 p < 0.001** |
| Age of onset (year, mean ± SD) | – | 36.1 ± 13.2 | – |
| Number of episodes (%) | |||
| First | – | 33.8 | – |
| Second | 32.4 | ||
| Third or higher | 33.8 | ||
| Early life stress event (% yes)b | 10.3 | 38.0 | X2 = 13.18 p < 0.001** |
| Antidepressant use (%) | |||
| None | 96.6 | 4 | – |
| SSRI | 1.7 | 41 | |
| SNRI | 0 | 34 | |
| Other (TCA, mirtazapine, bupropion) | 1.7 | 21 | |
| Depression severity (HDRS) | 0.6 ± 1.3 | 16.9 ± 5.0 | p < 0.001** |
| Positive affect (PANAS) | 36.3 ± 5.6 | 18.1 ± 5.8 | p < 0.001** |
| Negative affect (PANAS) | 14.7 ± 4.2 | 33.2 ± 8.7 | p < 0.001** |
| Anhedonia (SHAPS) | 19.2 ± 4.1 | 35.7 ± 7.6 | p < 0.001** |
| Neuroticism (NEO-FFI) | 3.4 ± 1.6 | 8.1 ± 1.3 | p < 0.001** |
P-values are derived from statistical analysis using independent sample t-test for continuous variables or Chi-Square test for categorical variables.
HDRS: Hamilton Rating Scale for Depression; PANAS: Positive and Negative Affect Scale; SHAPS: Snaith¬Hamilton Pleasure Scale; NEO-FFI: NEO-Five Factor Inventory Scale.
Low education = finished secondary school or less; High education = any additional education after secondary school.
Early life stress event: assessed by Structured Trauma Interview (STI).
3.2. Cortisol reactivity to stress
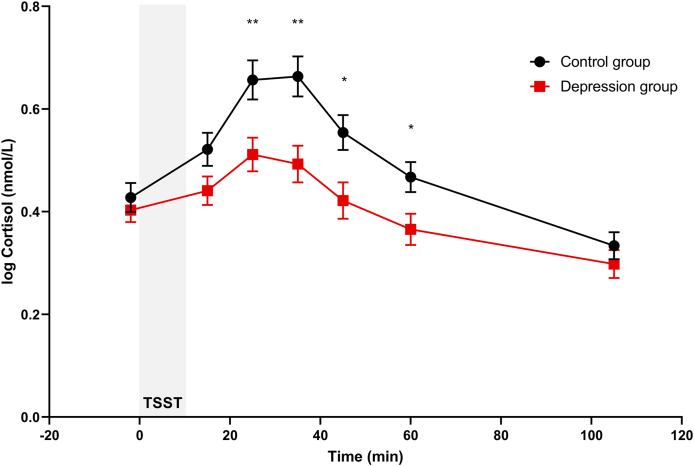
As expected, TSST induced an increase in cortisol in the overall sample over the different time measurements (F (6,750) = 74.50, p < 0.001, ηp2 = 0.37) as well as in each group separately (all ps < 0.05). When comparing the stress response between the groups, we observed a significant Group (F (1,124) = 5.86, p = 0.017, ηp2 = 0.05) and Group*Time effect (F (6,750) = 6.09, p = 0.001, ηp2 = 0.05), indicative of a blunted cortisol reactivity in depressed patients. Bonferroni-adjusted post hoc tests revealed that depressed patients had significantly lower cortisol levels at four out of seven time points (t = 25, 35, 45 and 60 min, all p-values <0.05), as illustrated in Fig. 1. The observed differences in cortisol reactivity were confirmed when comparing AUCg and AUCi. Depressed patients had significantly lower overall cortisol output (AUCg = 42.3 ± 25.9 nmol/L) compared to healthy controls (AUCg = 51.7 ± 22.6 nmol/L; mean difference 9.45, p = 0.029) as well as lower increase in cortisol over time (depressed patients: AUCi = −1.1 ± 18.8 nmol/L, healthy controls: AUCi = 6.0 ± 19.9 nmol/L; mean difference 7.11, p = 0.037). Among depressed patients, type of antidepressant (SSRI, SNRI or other) did not have an impact on cortisol levels (p = 0.224).
Fig. 1.
Cortisol concentrations (mean ± SEM) before and after a social stressor (TSST) in the control group and the depression group. P-values are derived from generalized linear model for repeated measurements, using Bonferroni-adjusted post hoc tests (significance: p < 0.05*, p < 0.01**).
3.3. NR3C1 and SLC6A4 methylation
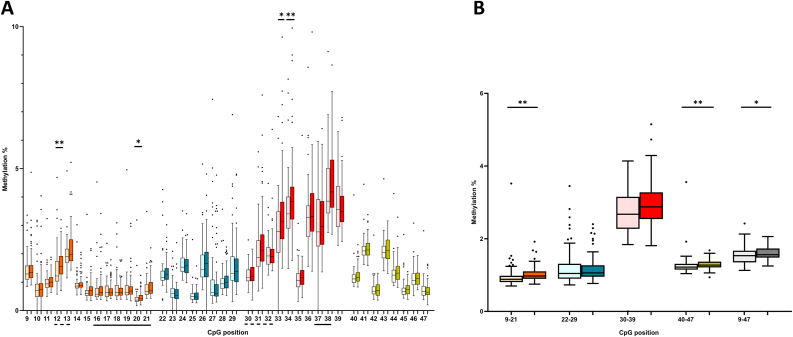
Group comparison between all analyzed regions of both genes is provided in Table A4. Comparing the average NR3C1 methylation of the whole CpG island between the groups (Fig. 2B), we observed significantly higher methylation % in the depression group (p = 0.030). Next, we compared average methylation levels of the four analyzed amplicons, which revealed a significant increase in Amplicon1 (p < 0.001) and Amplicon 4 (p = 0.006). Looking at the individual CpGs within the individual amplicons (Fig. 2A), CpGs in Amplicon 1 overlapping with the NGFI-A binding site (CpG12 and CpG20) had higher methylation levels in the depression group. However, after applying Bonferroni correction, only the difference in CpG 12 remained significant. To reduce multiple testing, we kept the differentially methylated averages as well as the 2 CpGs overlapping with the NGFI-A binding site in the further exploratory analysis.
Fig. 2.
Overview of NR3C1 1 F methylation per individual CpG (A) and as average (B) in the control group (lighter shade) and the depression group (darker shade). Different colors correspond to the different analyzed amplicons and total average of the whole CpG island is presented in grey (B). Data are presented as the median (central line), 25th-75th percentile (box), 2.5th and 97.5th percentile (whiskers) and outliers (individual points). NGFI-A binding sites are underlined (canonical - solid line and noncanonical - broken line). Significance: p < 0.05*, p < 0.01**
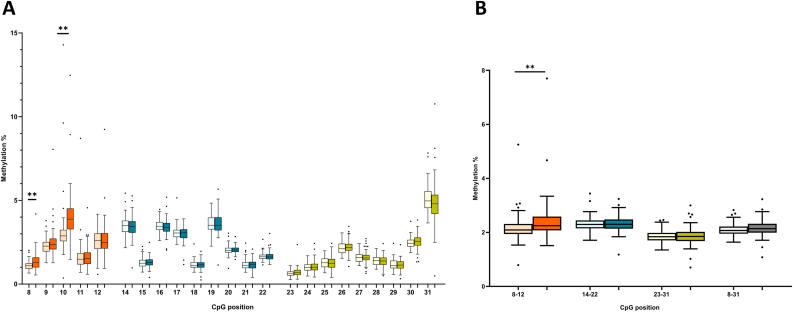
Group comparison of the total methylation level of the SLC6A4 CpG island (Fig. 3B) showed no significant difference (p = 0.161). However, when comparing the averages of the three analyzed amplicons, we observed significantly higher methylation levels in Amplicon 1 (p = 0.003). In the same amplicon, two individual CpGs (CpG8 and CpG10) were hypermethylated in depressed patients, and this remained significant after Bonferroni correction for multiple testing (Fig. 3A). In Amplicon 2 and 3, there were no significant difference in methylation levels between the groups (all ps > 0.05). To avoid the multiple testing issue, we kept CpG8, CpG10 and the average methylation of Amplicon 1 in further exploratory analysis.
Fig. 3.
Overview of SLC6A4 methylation per individual CpG (A) and as average (B) in the control group (lighter shade) and the depression group (darker shade). Different colors correspond to the different analyzed amplicons and total average of the whole CpG island is presented in grey (B). Data are presented as the median (central line), 25th-75th percentile (box), 2.5th and 97.5th percentile (whiskers) and outliers (individual points). Significance: p < 0.01**
As a confirmatory analysis, we applied a dimensional approach to test whether the observed methylation differences were associated with clinical symptoms (presented in Table A5). In the overall sample, we observed significant correlations between most of the differentially methylated regions and the depression measures in the expected direction (higher methylation indicating higher severity). However, within the depression group, no significance was observed (all ps > 0.05), indicating that methylation of these regions is not an indicator of depression severity. Finally, age, gender, early life stress and type of antidepressant (SSRI, SNRI or other) did not have a direct effect on any of the differentially methylated regions (all ps > 0.05).
3.4. Associations between methylation and cortisol reactivity
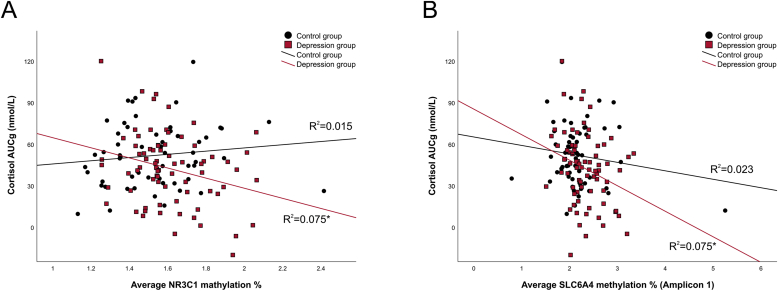
Associations between methylation and cortisol response to TSST are presented in Table 2. Looking at the association between the average NR3C1 methylation and cortisol response to stress, we observed a significant Methylation*Group interaction effect (Methylation*Group: F (1,123) = 5.72, p = 0.018, ηp2 = 0.04, Methylation*Group*Time: F (6,738) = 3.38, p = 0.022, ηp2 = 0.03), indicating that higher NR3C1 methylation was associated with lower cortisol response to stress in depressed patients. Repeating the analysis in the depression group confirmed this association, also when age, gender, early life stress and neuroticism were added to the model as covariates (Methylation: F (1,65) = 5.45, p = 0.023, ηp2 = 0.08, Methylation*Time: F (6,390) = 3.14, p = 0.010). Early life stress did not have a moderation effect on the association between NR3C1 methylation and cortisol (F (1,64) = 0.26, p = 0.613, ηp2 = 0.004). Confirmatory linear regression analyses using cortisol AUC as the dependent variable and NR3C1 methylation as the predictor yielded the same outcome, confirming the negative impact of NR3C1 methylation on both AUCg (B = −37.49, p = 0.017, R2 = 0.076) and AUCi (B = −22.51, p = 0.049, R2 = 0.052) in the depression group (Fig. 4A).
Table 2.
Associations between NR3C1 and SLC6A4 methylation and cortisol response to TSST in both groups and in MDD patients Data are analyzed using linear model for repeated measures with cortisol as the outcome measure and methylation as a predictor. All analyses were corrected for age, gender, early life stress and neuroticism. Significance: p < 0.05*
| Both groups (N = 138) |
MDD group (N = 80) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | p | ηp2 | df | F | p | ηp2 | ||
| NR3C1 | |||||||||
| CpG12 | 1, 116 | 1.033 | 0.312 | 0.009 | 1, 63 | 0.143 | 0.706 | 0.002 | |
| CpG20 | 1, 117 | 0.859 | 0.356 | 0.007 | 1, 63 | 2.294 | 0.135 | 0.035 | |
| Amplicon 1 (CpG9-21) | 1, 117 | 0.649 | 0.422 | 0.006 | 1, 63 | 0.716 | 0.401 | 0.011 | |
| Amplicon 4 (CpG40-47) | 1, 119 | 1.039 | 0.310 | 0.009 | 1, 65 | 0.002 | 0.968 | 0.000 | |
| Total average | 1, 119 | 0.907 | 0.343 | 0.008 | 1, 65 | 5.451 | 0.023* | 0.077 | |
| SLC6A4 | |||||||||
| CpG8 | 1, 118 | 0.004 | 0.952 | 0.000 | 1, 65 | 0.007 | 0.936 | 0.000 | |
| CpG10 | 1, 118 | 4.081 | 0.046* | 0.033 | 1, 65 | 2.873 | 0.095 | 0.042 | |
| Amplicon 1 (CpG8-12) | 1, 118 | 6.963 | 0.010* | 0.056 | 1, 65 | 5.180 | 0.026* | 0.074 | |
Fig. 4.
Association between average NR3C1 methylation (4 A) and SLC6A4 (Amplicon 1) methylation (4 B) with cortisol response to TSST (AUCg) in healthy controls (black) and depressed patients (red). R2 values are derived from linear regression analyses and are presented for both groups separately. Significance: p < 0.05*
Methylation of specific differentially methylated regions (CpG12, CpG20 and averages of Amplicons 1 and 4) did not have a significant effect on cortisol levels (all ps > 0.05, data not shown).
Next, testing the effect of SLC6A4 methylation (Amplicon 1) on cortisol response to stress, we observed a significant negative association in the overall sample (F (1,124) = 6.83, p = 0.01, ηp2 = 0.052) without the Methylation*Group interaction. When we analyzed each group separately, this association was significant in the depression group (F (1,71) = 5.02, p = 0.028, ηp2 = 0.066), but not in the healthy control group (F (1,51) = 1.34, p = 0.253, ηp2 = 0.026). In the depression group, this effect was still significant when covariates (age, gender, early life stress and neuroticism) were added to the model (F (1,65) = 5.18, p = 0.026, ηp2 = 0.07) and early life stress did not moderate this association (F (1,64) = 0.18, p = 0.668, ηp2 = 0.003). Linear regression analysis with AUC as the outcome confirmed a significant negative impact of SLC6A4 methylation (Amplicon 1) on AUCg (B = −36.48, p = 0.017, R2 = 0.075), but not AUCi (B = −20.88, p = 0.063, R2 = 0.047) (Fig. 4B).
In multivariate analysis, methylation of NR3C1 and SLC6A4 (Amplicon 1) could explain 16.4% of variability in cortisol response to stress in depressed patients (R2 = 0.164, p = 0.002).
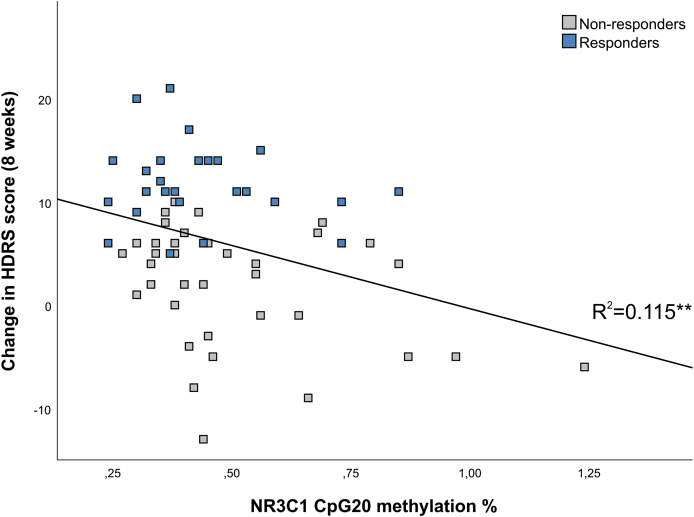
3.5. Association between baseline methylation and symptom improvement
Basic correlation analysis between baseline methylation and symptom improvement revealed a significant negative correlation between CpG20 of NR3C1 and symptom improvement at 8-weeks (Spearman's Rho = −0.275, p = 0.028). As presented in Fig. 5, linear regression analysis confirmed that baseline methylation of NR3C1 CpG20 was a significant predictor of symptom improvement as the outcome variable in univariate model (R2 = 0.115, B = −12.22, p = 0.006) but also in the multivariate model with baseline depression severity, age, gender and early life stress as covariates (B = −11.23, p = 0.008). This association was not significantly different in treatment responders (≥50% improvement in HDRS score at 8 weeks) and non-responders (<50% improvement in HDRS score at 8 weeks), presented by non-significant Methylation*Response interaction (B = −3.47, p = 0.61). The other NR3C1 and SLC6A4 regions were not significantly associated with the symptom improvement (all ps > 0.05, data not presented).
Fig. 5.
Association between NR3C1 methylation (CpG20) at the baseline and change in HDRS score in depressed patients at the 8-week follow-up. Responders to treatment (≥50% improvement in HDRS score) are presented in blue and non-responders (<50% improvement in HDRS score) in grey. R2 value is derived from linear regression analysis and is presented for both groups together since there was no significant group difference.
HDRS: Hamilton Rating Scale for Depression. Significance: p < 0.01**
4. Discussion
In the present study, we aimed to investigate the relevance of DNA methylation changes of NR3C1 and SLC6A4 for HPA axis dysregulation in MDD. In addition, we tested whether differentially methylated patterns at baseline have a predictive value for symptoms improvement in depressed patients at the 8-week follow-up.
First, we identified lower cortisol release after the TSST in patients with MDD compared to the healthy control group, which is in line with the previous literature. Whereas studies investigating diurnal cortisol or HPA axis response to a chemical stressor (such as dexamethasone suppression test) in MDD more commonly reported HPA axis hyperactivity (Pariante and Lightman, 2008) (Stetler and Miller, 2011) (Knorr et al., 2010), literature indicates that depressed patients are more likely presented with blunted cortisol when exposed to a psychosocial stressor (Burke et al., 2005) (Ahrens et al.,) (Booij et al., 2013b). In particular, a meta-analysis showed that depressed patients express blunted cortisol in the recovery phase, which starts approximately 25 min after the stress task (Burke et al., 2005). Our results support this finding, as our post-hoc analysis revealed that depressed patients had lower cortisol levels at four measurement points, all of which fall within the recovery period (25, 35, 45 and 60 min after the TSST). The same meta-analysis showed that chronically depressed patients and those who were hospitalized are more likely to have blunted cortisol reactivity. In our study, all depressed participants were inpatients and therefore potentially more prone to a chronic course of illness. The main postulated mechanism behind the blunted HPA axis reactivity assumes that this bluntness reflects the adaptation of the stress system to a prolonged period of stressful events and elevated cortisol levels and is linked to inefficient coping with stressors (Miller et al., 2013) (Heim et al., 2000).
Further, we observed hypermethylation of NR3C1 and SLC6A4 in depressed patients, which could explain about 16% of the blunted cortisol response to TSST. NR3C1 methylation differences were reflected in the average values of the whole 1 F CpG island, as well as 2 specific CpGs overlapping with NGFI-A binding site. In line with our findings, two authors reported hypermethylation of this gene in depressed patients (Nantharat et al., 2015) (Roy et al., 2017), which was associated with lower NR3C1 expression in one of them (Roy et al., 2017). In contrast, two other studies reported lower NR3C1 methylation in patients with MDD (Bustamante et al., 2016) (Na et al., 2014). However, in these two studies, patients with a lifetime MDD prevalence were included and only one part of the CpG island was assessed, in which we observed no significant differences between the groups. Both hypo- and hypermethylation of NR3C1 are considered maladaptive and different direction of these changes might depend on the illness phase, severity and other clinical features (Palma-gudiel et al., 2015). Interestingly, in our study, hypermethylation of one particular site (CpG12) was the most pronounced, which is the same site previously reported to moderate the effect of childhood trauma on cortisol reactivity to stress (Alexander et al., 2018). In our study, blunted reactivity to stress was associated with the average methylation of the whole island, and not this specific CpG, indicating that this might be a rather cumulative effect. There are no other studies assessing the link between NR3C1 methylation and cortisol reactivity in MDD to support this hypothesis, however NR3C1 hypermethylation was previously associated with blunted cortisol reactivity in healthy adults with a history of childhood maltreatment (Tyrka et al., 2012). Hypermethylation at the NGFI-A binding sites of this region was shown to reduce hippocampal GR expression in rodents (Weaver et al., 2004b) and humans (McGowan et al., 2009). If we assume that blunted HPA axis reactivity is a consequence of a prolonged period of HPA axis hyperactivity, it could be hypothesized that chronically elevated cortisol levels lead to NR3C1 hypermethylation and lower GR expression, promoting glucocorticoid resistance, finally resulting in a failure to adequately respond to novel stressors. In addition, literature indicates the existence of intermediary mechanisms such as neural atrophy in hippocampus (Dranovsky and Hen, 2006) and dendritic remodelling in amygdala (Mitra and Sapolsky, 2008), which make this link more complex and difficult to elucidate.
In addition to NR3C1, higher SLC6A4 methylation was also a significant predictor of blunted HPA axis reactivity in our MDD group. These findings are in line with two other studies that showed the higher SLC6A4 methylation to be a significant predictor of blunted cortisol reactivity to stress in victims of childhood bullying (Ouellet-Morin et al., 2012) as well as low hair cortisol levels in healthy adults (Alexander et al., 2019). In another study, SLC6A4 methylation was investigated as a predictor of cortisol response to TSST in healthy adults, relative to the 5-HTTLPR polymorphism (Alexander et al., 2014b). The main findings indicated a dose-dependent interaction between 5-HTTLPR and methylation in a sense that 5-HTTLPR had an impact on stress reactivity only if SLC6A4 methylation was low, whereas high methylation outweighed the genotype effect. However, in this study, higher methylation of the SERT gene predicted higher cortisol response to stress, which is opposite to what we found. As previously mentioned, these differences could be linked to different type and timing of stressors and phases in the HPA axis disruption, which were previously identified as important contributors to the potentially variable effects of SLC6A4 methylation changes (Palma-Gudiel and Fananas, 2017). In addition, the association of SLC6A4 hypermethylation and blunted stress reactivity seems logical looking at our cross-sectional comparison, where we observed increased SLC6A4 methylation in depressed patients compared to healthy controls, which is also in line with previous studies (Philibert et al., 2008) (Van Der Knaap et al., 2015) (Iga et al., 2016) (Okada et al., 2014) (Zhao et al.,). Even though hypermethylation of SLC6A4 was previously associated with lower expression of SERT (Palma-Gudiel and Fananas, 2017), whether this is the main functional effect in depression is not so evident and still remains to be explored. Since in our study we did not assess mRNA expression of SERT, we cannot make further conclusions on this.
In our study, we tested the predictive potential of NR3C1 and SLC6A4 methylation for treatment response at the 8-week follow-up and found that NR3C1 methylation of a specific CpG site could predict symptom improvement in depressed patients after 8 weeks. To the best of our knowledge, this is the first study to report such findings in patients with MDD. Previously, in a study on combat veterans with PTSD, pre-treatment NR3C1 1 F methylation predicted treatment response to psychotherapy at 12-week follow-up (Yehuda et al., 2013). In the same study, treatment responders had higher post-treatment GR expression, but NR3C1 methylation did not change in response to treatment, indicating potentially enduring nature and stability of NR3C1 epigenetic signature. Unlike some previous studies (Iga et al., 2016) (Domschke et al., 2014), we did not observe any predictive value of baseline SLC6A4 methylation in depressed patients for treatment response at the follow-up. This could be due to fact that our participants took antidepressants prior to inclusion, which might have had an effect on SLC6A4 methylation and its predictive potential for treatment outcome. Anyhow, the predictive potential of NR3C1 and SLC6A4 methylation for treatment response in MDD deserves further exploration.
When interpreting the results of the present study, several limitations need to be taken into account. First, almost all depressed patients were taking antidepressant medication for approximately 2 weeks at the moment of inclusion, which could have an impact on cortisol stress response (Mckay and Zakzanis, 2010) and methylation (Webb et al., 2020) (Booij et al., 2015). However, we tested whether the cortisol response to TSST and the differentially methylated regions were associated with the type of antidepressant and found no significant association. In addition, even if the blunting of the cortisol response to the TSST in depressed patients could be partly due to the use of antidepressants, this would not explain the association with NR3C1 and SLC6A4 methylation levels. Next, even though the observed differences in DNA methylation between depressed patients and healthy controls were statistically significant, they were very small effect sizes, especially in the case of NR3C1 and therefore their biological plausibility can be questioned. However, these subtle differences are consistent with previous findings, especially for NR3C1 methylation and were previously shown to affect mRNA expression or functional HPA axis tests (Oberlander et al., 2008) (Nantharat et al., 2015) (Na et al., 2014) (Bustamante et al., 2016) (Roy et al., 2017) (Yehuda et al., 2015) (Vangeel et al., 2018) (Perroud et al., 2011). The fact that the 1 F region of NR3C1 is uniformly unmethylated (methylation <5%) suggests that small methylation increases in functional CpG sites can have the potential to influence transcription (Palma-gudiel et al., 2015). In our study, we did not collect RNA samples and therefore mRNA expression analysis could not be performed, which is another limitation of the study as these data would help get a clearer picture of the functional effects of the observed DNA methylation changes. However, we found an association between methylation of both NR3C1 and SLC6A4 and cortisol response to TSST, which supports the idea of biological relevance of these subtle methylation changes, at least in patients with MDD. Finally, we used whole blood for DNA methylation analysis and it is possible that DNA methylation profiles of different cell types had a confounding effect for which we did not account. Future studies focusing on epigenetic signatures of specific cell types could provide more insight into potentially more pronounced vulnerability of specific cell types to stress and their role in stress-related disorders such as MDD.
5. Conclusions
To conclude, we found hypermethylation of NR3C1 and SLC6A4 in depressed patients, which was associated with blunted cortisol reactivity to TSST in the same population and was predictive of poorer recovery. These findings, together with the previous literature, prove that the role of methylation of these two genes in HPA axis dysregulation in MDD deserves to be further explored. We recommend future studies to apply an integrative approach including simultaneous assessment of different genes and potentially other epigenetic mechanisms (histone modifications and/or microRNAs) together with mRNA expression and cortisol reactivity in order to learn more about their role in MDD.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Manosij Ghosh is a postdoctoral research fellow (12W7718N) funded by the Research Foundation – Flanders (FWO).
CRediT authorship contribution statement
Jelena Bakusic: Conceptualization, Methodology, Investigation, Formal analysis, Writing - original draft. Elske Vrieze: Conceptualization, Methodology, Investigation, Writing - review & editing. Manosij Ghosh: Formal analysis, Writing - review & editing, Supervision. Bram Bekaert: Resources, Writing - review & editing, Supervision. Stephan Claes: Conceptualization, Writing - review & editing, Supervision. Lode Godderis: Conceptualization, Resources, Writing - review & editing, Supervision.
Declaration of competing interest
None.
Acknowledgements
We would like to acknowledge all people who participated in the study. We would also like to thank to Dr. Annouschka Laenen from Leuven Biostatistics and Statistical Bioinformatics Centre (L-BioStat), who helped with the statistical analyses. Manosij Ghosh is a postdoctoral research fellow (12W7718N) funded by the Research Foundation – Flanders (FWO).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2020.100272.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- Ahrens T, Deuschle M, Krumm B, van der Pompe G, den Boer JA, Lederbogen F (n.d.): Pituitary-adrenal and sympathetic nervous system responses to stress in women remitted from recurrent major depression. Psychosom. Med. 70: 461–467. [DOI] [PubMed]
- Alexander N., Wankerl M., Hennig J., Miller R., Zänkert S., Steudte-Schmiedgen S. DNA methylation profiles within the serotonin transporter gene moderate the association of 5-HTTLPR and cortisol stress reactivity. Transl. Psychiatry. 2014;4:e443. doi: 10.1038/tp.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander N., Wankerl M., Hennig J., Miller R., Zänkert S., Steudte-Schmiedgen S. DNA methylation profiles within the serotonin transporter gene moderate the association of 5-HTTLPR and cortisol stress reactivity. Transl. Psychiatry. 2014;4:e443. doi: 10.1038/tp.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander N., Kirschbaum C., Wankerl M., Stauch B., Stalder T., Steudte-Schmiedgen S. Glucocorticoid receptor gene methylation moderates the association of childhood trauma and cortisol stress reactivity. Psychoneuroendocrinology. 2018;90:68–75. doi: 10.1016/j.psyneuen.2018.01.020. [DOI] [PubMed] [Google Scholar]
- Alexander N., Illius S., Stalder T., Wankerl M., Muehlhan M., Kirschbaum C. Serotonin transporter gene methylation predicts long-term cortisol concentrations in hair. Psychoneuroendocrinology. 2019;106:179–182. doi: 10.1016/j.psyneuen.2019.03.033. [DOI] [PubMed] [Google Scholar]
- Allen A.P., Kennedy P.J., Dockray S., Cryan J.F., Dinan T.G., Clarke G. The trier social stress test: principles and practice. Neurobiol Stress. 2017;6:113–126. doi: 10.1016/j.ynstr.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakusic J., Schaufeli W., Claes S., Godderis L. Stress, burnout and depression: a systematic review on DNA methylation mechanisms. J. Psychosom. Res. 2017;92:34–44. doi: 10.1016/j.jpsychores.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Booij L., Wang D., Lévesque M.L., Tremblay R.E., Szyf M. Looking beyond the DNA sequence: the relevance of DNA methylation processes for the stress-diathesis model of depression. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;368 doi: 10.1098/rstb.2012.0251. 20120251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij S.H., Bouma E.M.C., Jonge P De, Ormel J., Oldehinkel A.J. Chronicity of depressive problems and the cortisol response to psychosocial stress in adolescents: the TRAILS study. Psychoneuroendocrinology. 2013;38:659–666. doi: 10.1016/j.psyneuen.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Booij L., Szyf M., Carballedo A., Frey E.-M., Morris D., Dymov S. DNA methylation of the serotonin transporter gene in peripheral cells and stress-related changes in hippocampal volume: a study in depressed patients and healthy controls. PloS One. 2015;10 doi: 10.1371/journal.pone.0119061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke H.M., Davis M.C., Otte C., Mohr D.C. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Bustamante A.C., Aiello A.E., Galea S., Ratanatharathorn A., Noronha C., Wildman D.E., Uddin M. Glucocorticoid receptor DNA methylation, childhood maltreatment and major depression. J. Affect. Disord. 2016;206:181–188. doi: 10.1016/j.jad.2016.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa P.T., McCrae R.R. Psychological Assessment Resources; Florida, USA: Odessa: 1992. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) Manual. [Google Scholar]
- Domschke K., Tidow N., Schwarte K., Deckert J., Lesch K., Arolt V. Serotonin transporter gene hypomethylation predicts impaired antidepressant treatment response. Int. J. Neuropsychopharmacol. 2014;17:1167–1176. doi: 10.1017/S146114571400039X. [DOI] [PubMed] [Google Scholar]
- Draijer N., Langeland W. Childhood trauma and perceived parental dysfunction in the etiology of dissociative symptoms in psychiatric inpatients. Am. J. Psychiatr. 1999;156:379–385. doi: 10.1176/ajp.156.3.379. [DOI] [PubMed] [Google Scholar]
- Dranovsky A., Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol. Psychiatr. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB et, Spitzer R.L., Gibbon M., Williams J.B.W. Non-patient Edition. For DSMIV; 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C., Ehlert U., Hellhammer D.H. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Hompes T., Izzi B., Gellens E., Morreels M., Fieuws S., Pexsters A. Investigating the influence of maternal cortisol and emotional state during pregnancy on the DNA methylation status of the glucocorticoid receptor gene (NR3C1) promoter region in cord blood. J. Psychiatr. Res. 2013;47:880–891. doi: 10.1016/j.jpsychires.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Iga J., Watanabe S., Numata S., Umehara H., Nishi A., Kinoshita M. Association study of polymorphism in the serotonin transporter gene promoter, methylation profiles, and expression in patients with major depressive disorder. Hum. Psychopharmacol. Clin. Exp. 2016;31:193–199. doi: 10.1002/hup.2527. [DOI] [PubMed] [Google Scholar]
- Klengel T., Pape J., Binder E.B., Mehta D. The role of DNA methylation in stress-related psychiatric disorders. Neuropharmacology. 2014;80:115–132. doi: 10.1016/j.neuropharm.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Knorr U., Vinberg M., Kessing L.V., Wetterslev J. Salivary cortisol in depressed patients versus control persons: a systematic review and meta-analysis. Psychoneuroendocrinology. 2010;35:1275–1286. doi: 10.1016/j.psyneuen.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Kudielka B.M., Hellhammer D., Kirschbaum C. Ten years of research with the trier social stress test — revisited. In: Harmon-Jones E., Winkielman P., editors. Social Neuroscience. The Guilford Press; New York: 2007. pp. 56–83. [Google Scholar]
- Kular L., Kular S. Epigenetics applied to psychiatry: clinical opportunities and future challenges. Psychiatr. Clin. Neurosci. 2018;72:195–211. doi: 10.1111/pcn.12634. [DOI] [PubMed] [Google Scholar]
- Lee R.S., Sawa A. Environmental stressors and epigenetic control of the hypothalamic-pituitary-adrenal axis. Neuroendocrinology. 2014;100:278–287. doi: 10.1159/000369585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., D'Arcy C., Li X., Zhang T., Joober R., Meng X. What do DNA methylation studies tell us about depression? A systematic review. Transl. Psychiatry. 2019;9:68. doi: 10.1038/s41398-019-0412-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.Z., Nusslock R. How stress gets under the skin: early life adversity and glucocorticoid receptor epigenetic regulation. Curr. Genom. 2018;19:653–664. doi: 10.2174/1389202919666171228164350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan P.O., Sasaki A., D'Alessio A.C., Dymov S., Labonte B., Szyf M. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckay M.S., Zakzanis K.K. The impact of treatment on HPA axis activity in unipolar major depression. J. Psychiatr. Res. 2010;44:183–192. doi: 10.1016/j.jpsychires.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Miller R., Plessow F., Rauh M., Gro M., Kirschbaum C. Comparison of salivary cortisol as measured by different immunoassays and tandem mass spectrometry. Psychoneuroendocrinology. 2013;38:50–57. doi: 10.1016/j.psyneuen.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Mitra R., Sapolsky R.M. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc. Natl. Acad. Sci. Unit. States Am. 2008;105:5573–5578. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad-Zadeh L., Moses L., Swaltney-Brant S. Serotonin: a review. J. Vet. Pharmacol. Therapeut. 2008;31:187–199. doi: 10.1111/j.1365-2885.2008.00944.x. [DOI] [PubMed] [Google Scholar]
- Na K.-S., Chang H.S., Won E., Han K.-M., Choi S., Tae W.S. Association between glucocorticoid receptor methylation and hippocampal subfields in major depressive disorder. PloS One. 2014;9 doi: 10.1371/journal.pone.0085425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantharat M., Wanitchanon T., Amesbutr M., Tammachote R. Glucocorticoid receptor gene (NR3C1) promoter is hypermethylated in Thai females with major depressive disorder. Genet. Mol. Res. 2015;14:19071–19079. doi: 10.4238/2015.December.29.15. [DOI] [PubMed] [Google Scholar]
- Newell-price J., Clark A.J.L., King P. DNA methylation and silencing of gene expression. Trends Endocrinol. Metabol. 2000;11:142–148. doi: 10.1016/s1043-2760(00)00248-4. [DOI] [PubMed] [Google Scholar]
- Oberlander T.F., Weinberg J., Papsdorf M., Grunau R., Misri S., Devlin A.M. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- Okada S., Morinobu S., Fuchikami M., Segawa M., Inoue T., Kusumi I. The potential of SLC6A4 gene methylation analysis for the diagnosis and treatment of major depression. J. Psychiatr. Res. 2014;53:47–53. doi: 10.1016/j.jpsychires.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I., Wong C.C.Y., Danese A., Pariante C.M., Papadopoulos A.S., Mill J., Arseneault L. Increased serotonin transporter gene (SERT) DNA methylation is associated with bullying victimization and blunted cortisol response to stress in childhood: a longitudinal study of discordant monozygotic twins. Psychol. Med. 2012;43:1813–1823. doi: 10.1017/S0033291712002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma-Gudiel H., Fananas L. An integrative review of methylation at the serotonin transporter gene and its dialogue with environmental risk factors , psychopathology and 5-HTTLPR. Neurosci. Biobehav. Rev. 2017;72:190–209. doi: 10.1016/j.neubiorev.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Palma-gudiel H., Córdova-palomera A., Carlos J. Glucocorticoid receptor gene (NR3C1) methylation processes as mediators of early adversity in stress-related disorders causality: a critical review. Neurosci. Biobehav. Rev. 2015;55:520–535. doi: 10.1016/j.neubiorev.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Pariante C.M., Lightman S.L. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Perroud N., Paoloni-Giacobino A., Prada P., Olié E., Salzmann A., Nicastro R. Increased methylation of glucocorticoid receptor gene (NR3C1) in adults with a history of childhood maltreatment: a link with the severity and type of trauma. Transl. Psychiatry. 2011;1:e59. doi: 10.1038/tp.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibert R.A., Sandhu H., Hollenbeck N., Gunter T., Adams W., Madan A. The relationship of 5HTT (SLC6A4) methylation and genotype on mRNA expression and liability to major depression and alcohol dependence in subjects from the Iowa Adoption Studies. Am J Med Genet B Neuropsychiatr Genet. 2008;147B doi: 10.1002/ajmg.b.30657. 543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner J.C., Kirschbaum C., Meinlschmid G., Hellhammer D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Roy B., Shelton R.C., Dwivedi Y. DNA methylation and expression of stress related genes in PBMC of MDD patients with and without serious suicidal ideation. J. Psychiatr. Res. 2017;89:115–124. doi: 10.1016/j.jpsychires.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith R., Hamilton M., Morley S., Humayan A.D.H., Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton pleasure scale. Br. J. Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Stetler C., Miller G. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom. Med. 2011;73:114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- Sullivan P.F., Neale M.C., Kendler K.S. 2000. Genetic Epidemiology of Major Depression: Review and Meta-Analysis; pp. 1552–1562. [DOI] [PubMed] [Google Scholar]
- Sulon J., Demey-Ponsart L., Beauduin P., Sodoyez J. Radioimmunoassay of corticosterone, cortisol and cortisone: their application to human cord and maternal plasma. J. Steroid Biochem. 1978;9:671–676. doi: 10.1016/0022-4731(78)90180-2. [DOI] [PubMed] [Google Scholar]
- Tyrka A.R., Price L.H., Marsit C., Walters O.C., Carpenter L.L. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: preliminary findings in healthy adults. PloS One. 2012;7 doi: 10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangeel E., Van Den Eede F., Hompes T., Izzi B., Del Favero J., Moorkens G. Chronic fatigue syndrome and DNA hypomethylation of the glucocorticoid receptor gene promoter 1F region: associations with hypothalamic-pituitary-adrenal Axis hypofunction and childhood trauma. Psychosom. Med. 2015;77:853–862. doi: 10.1097/PSY.0000000000000224. [DOI] [PubMed] [Google Scholar]
- Van Der Knaap LJ, Van Oort FV, Verhulst F.C., Oldehinkel A.J., Riese H. Methylation of NR3C1 and SLC6A4 and internalizing problems. The TRAILS study. J. Affect. Disord. 2015;180:97–103. doi: 10.1016/j.jad.2015.03.056. [DOI] [PubMed] [Google Scholar]
- Vangeel E.B., Kempke S., Bakusic J., Godderis L., Luyten P., Van Heddegem L. Glucocorticoid receptor DNA methylation and childhood trauma in chronic fatigue syndrome patients. J. Psychosom. Res. 2018;104:55–60. doi: 10.1016/j.jpsychores.2017.11.011. [DOI] [PubMed] [Google Scholar]
- Vrieze E., Pizzagalli D., Demyttenaere K., Hompes T., Sienaert P., de Boer P. Reduced reward learning predicts outcome in major depressive disorder. Biol. Psychiatr. 2013;73:639–645. doi: 10.1016/j.biopsych.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze E., Demyttenaere K., Bruffaerts R., Dirk H., Pizzagalli D., Sienaert P. Dimensions in major depressive disorder and their relevance for treatment outcome. J. Affect. Disord. 2014;155:35–41. doi: 10.1016/j.jad.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wankerl M., Miller R., Kirschbaum C., Hennig J., Stalder T., Alexander N. Effects of genetic and early environmental risk factors for depression on serotonin transporter expression and methylation profiles. Transl. Psychiatry. 2014;4:1–9. doi: 10.1038/tp.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weaver I.C.G., Cervoni N., Champagne F.A., D'Alessio A.C., Sharma S., Seckl J.R. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weaver I.C.G., Cervoni N., Champagne F.A., Alessio A.C.D., Sharma S., Seckl J.R. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Webb L.M., Phillips K.E., Ho M.C., Veldic M., Blacker C.J. The relationship between DNA methylation and antidepressant medications: a systematic review. Int. J. Mol. Sci. 2020;21:60–70. doi: 10.3390/ijms21030826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R., Daskalakis N.P., Desarnaud F., Makotkine I., Lehrner A.L., Koch E. Epigenetic biomarkers as predictors and correlates of symptom improvement following psychotherapy in combat veterans with PTSD. Front psychiatry. 2013;4:118. doi: 10.3389/fpsyt.2013.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R., Flory J.D., Bierer L.M., Henn-Haase C., Lehrner A., Desarnaud F. Lower methylation of glucocorticoid receptor gene promoter 1F in peripheral blood of veterans with posttraumatic stress disorder. Biol. Psychiatr. 2015;77:356–364. doi: 10.1016/j.biopsych.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Zhao J, Goldberg J, Bremner JD, Vaccarino V Association between promoter methylation of serotonin transporter gene and depressive symptoms: a monozygotic twin study. Psychosom. Med. 75: 523–529. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.