Abstract
Exposure to adversity during early life can have profound influences on brain function and behavior later in life. The peripubertal period is emerging as an important time-window of susceptibility to stress, with substantial evidence documenting long-term consequences in the emotional and social domains. However, little is known about how stress during this period impacts subsequent cognitive functioning. Here, we assessed potential long-term effects of peripubertal stress on spatial learning and memory using the water maze task. In addition, we interrogated whether individual differences in stress-induced behavioral and endocrine changes are related to the degree of adaptation of the corticosterone response to repeated stressor exposure during the peripubertal period. We found that, when tested at adulthood, peripubertally stressed animals displayed a slower learning rate. Strikingly, the level of spatial orientation in the water maze completed on the last training day was predicted by the degree of adaptation of the recovery -and not the peak-of the corticosterone response to stressor exposure (i.e., plasma levels at 60 min post-stressor) across the peripubertal stress period. In addition, peripubertal stress led to changes in emotional and glucocorticoid reactivity to novelty exposure, as well as in the expression levels of the plasticity molecule PSA-NCAM in the hippocampus. Importantly, by assessing the same endpoints in another peripubertally stressed cohort tested during adolescence, we show that the observed effects at adulthood are the result of a delayed programming manifested at adulthood and not protracted effects of stress. Altogether, our results support the view that the degree of stress-induced adaptation of the hypothalamus-pituitary-adrenal axis responsiveness at the important transitional period of puberty relates to the long-term programming of cognition, behavior and endocrine reactivity.
Keywords: Stress, Peripubertal stress, Water maze, PSA-NCAM, Dentate gyrus, Corticosterone
1. Introduction
Exposure to adversity during early life can have profound influences on brain function, behavior and cognition at adulthood (Albrecht et al., 2017; Bolton et al., 2017; Sterlemann et al., 2010; Suri et al., 2013), and the precise developmental timing when stress occurs seems to be critical in determining the precise consequences (Gee and Casey, 2015; Lupien et al., 2009). In addition to the recognized impact of neonatal (Bonapersona et al., 2019; Heim and Nemeroff, 2001; Molet et al., 2014; Veenema, 2009) and childhood/juvenile (Albrecht et al., 2017) stress, the peripubertal period is emerging as a time-window of high vulnerability to the programming of emotional (Cordero et al., 2012; Latsko et al., 2016; Márquez et al., 2013; Sheth et al., 2017) and social (Márquez et al., 2013; Poirier et al., 2014; Tzanoulinou et al., 2014a, 2014b) effects of stress (for a review, see (Tzanoulinou and Sandi, 2017). However, despite the well-known modulatory power of stress on cognition (Lupien et al., 2009; Sandi, 2013), little is known about the impact of peripubertal stress on later life cognitive functioning. A few studies in which stressors were applied during the period expanding from peripuberty till young adulthood have reported enduring learning and memory impairments specifically for the spatial domain (Isgor et al., 2004; Sterlemann et al., 2010). Therefore, whether the peripubertal period per se is susceptible to long-term programming effects of stress on spatial learning, while plausible, remains unclear.
The peripubertal period, involving time-windows right before and after puberty, comprises drastic hormonal, neurobiological and behavioral changes (Andersen and Teicher, 2008; Blakemore, 2008; Casey et al., 2010; Paus et al., 2008; Romeo et al., 2016; Spear, 2000; Tzanoulinou and Sandi, 2016). In particular, this period involves marked changes in the responsivity of the hypothalamic-pituitary-adrenal (HPA) axis to stressful experiences (McCormick et al., 2017; Romeo et al., 2016), and this transition can be modified by experiences (Gunnar et al., 2019), particularly stressful ones (Kumsta et al., 2017; Márquez et al., 2013; McCormick et al., 2017; Romeo et al., 2016). Strikingly, individual differences in the adaptation of the glucocorticoid response to repeated stress exposure during the peripubertal period in rats were found to predict subsequent changes in emotional and social phenotypes observed during adolescence (Papilloud et al., 2019) and adulthood (Walker et al, 2017, 2018). In addition, genetic selection in rats for the degree of corticosterone adaptation during peripubertal stress (Walker and Sandi, 2018) underscored genetic line-related differences in spatial learning and memory performance (Huzard et al., 2020). Accordingly, given the strong modulatory capacity of glucocorticoids on brain function and cognition (de Quervain et al., 2017; Sandi, 2011), including spatial learning (Akirav et al., 2004; Conboy et al., 2010; Sandi et al., 1997), we hypothesize that long-term programming of peripubertal stress on spatial learning would depend on the individual degree of glucocorticoid adaptation to repeated stress.
When considering the glucocorticoid adaptation to repeated stress, it is important to distinguish between the peak and the recovery phases, as they serve different adaptive functions (Romeo et al., 2016). While peak glucocorticoid levels facilitate physiological processes to deal with immediate challenges (de Kloet et al., 2008; Myers et al., 2014), the recovery phase (i.e., returning to baseline) is key to protect the organism from maladaptive overactivation and to prepare it for eventual new challenges (Karatsoreos and McEwen, 2011). Importantly, the peripubertal period has been reported to set a change in HPA responsivity in both humans and rats, including changes not only in the peak but also in the recovery phases (McCormick et al., 2017). We have previously reported a strong link between the magnitude of adaptation of the peak corticosterone response to repeated stressors given during the peripubertal period in rats and subsequent changes in emotional and social behaviors (Papilloud et al., 2018; Walker et al, 2017, 2018). However, in the context of the current study on spatial learning, we hypothesize that it will be the adaptation of the recovery phase of corticosterone responsiveness that predicts spatial learning. This hypothesis is based on several premises. First, on the crucial roles of the hippocampus in both, spatial learning (Bird and Burgess, 2008) and in providing negative feedback to the HPA axis (Herman and Mueller, 2006; Jacobson and Sapolsky, 1991; Kovács and Makara, 1988) and, thus, impacting on the corticosterone recovery phase. Second, on the high density of corticosteroid receptors present in the hippocampus (De Kloet, 1991) and their involvement in the HPA axis negative feedback (Reul et al., 1990). Finally, high glucocorticoid levels are known to promote plastic changes in hippocampal structure and function (de Kloet et al., 2018; McEwen et al., 2016), including changes in the expression levels of key plasticity molecules, such as PSA-NCAM (Montaron et al., 2003; Nacher et al., 2004). Importantly, PSA-NCAM -a key post-translational modification of the neural cell adhesion molecule (NCAM)- is critically involved in hippocampal plasticity (Kiss and Muller, 2001) and spatial memory (Bisaz et al., 2009) and modulated by stress (Sandi, 2004).
Therefore, we set this study in rats to assess potential long-term effects of peripubertal stress in spatial learning and memory in the water maze at adulthood, and to investigate whether individual differences in stress-induced changes are related to the adaptation of the corticosterone response (peak vs recovery) to repeated stressor exposure during the peripubertal period. In order to have broader information on the behavioral phenotype for data interpretation, we tested animals in emotional reactivity tasks as well. We also measured plasma corticosterone responses to novelty stress shortly before water maze training to assess both how this response relates to peripubertal corticosterone adaptation and whether it is associated with water maze performance. To understand whether any observed effects at adulthood are the result of a delayed programming or already present shortly after peripubertal stress exposure, we performed a second experiment in which animals were tested during late adolescence. Finally, we assessed levels of the learning and plasticity-related molecule PSA-NCAM in the dentate gyrus (DG) of the hippocampus and, in addition, as a control region, in the medial amygdala (MeA).
2. Materials and methods
2.1. Animals
Experimental subjects were the male offspring of Wistar Han rats purchased from Charles River Laboratories, France, and bred in our animal facility (n = 70). All animals were kept in constant conditions of humidity and temperature (22 ± 1 °C) with a 12-h light-dark cycle (lights on at 7:00 a.m.). Food and water were available ad libitum. All the procedures described were conducted in conformity with Swiss National Institutional Guidelines on Animal Experimentation, and approved by a license issued from the Swiss Cantonal Veterinary Office Committee for Animal Experimentation.
2.2. Experimental design
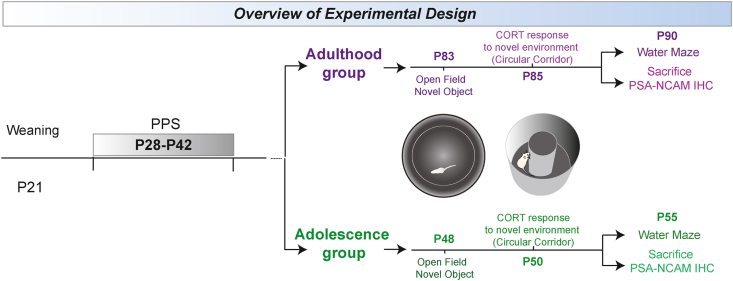
At weaning (P21), male rats from different litters were distributed into different home cages in groups of two non-siblings, and each cage was randomly assigned to control (CTRL, n = 34) or peripubertal stress (STRESS, n = 36) conditions. Animals from the STRESS group underwent the peripubertal stress protocol (PPS) starting at P28 (Márquez et al., 2013), and CTRL animals were briefly handled and returned to their home cage. Behavior and hormonal characterizations later in life of the experimental groups were performed at adolescence (P48+) and adulthood (P83+) in independent groups of animals (Fig. 1) (Adolescence CTRL n = 18; Adolescence STRESS n = 18; Adulthood CTRL n = 16; Adulthood STRESS n = 18). Before behavioral testing, animals were handled for 3 consecutive days to acclimatize to the experimenter and general conditions. Animals were tested in an Open Field and Novel Object test and two days later, their stress response was assessed after a novelty challenge by measuring corticosterone plasmatic levels (see below). Then, after five days, animals of each experimental group were further divided into two groups, one which would undergo behavioral evaluation of learning and memory in the Morris Water maze (Adolescence CTRL n = 10; Adolescence STRESS n = 10; Adulthood CTRL n = 8; Adulthood STRESS n = 10) and a second one that would be used to study basal levels of polysialylated-neural cell adhesion molecule (PSA-NCAM) in specific brain regions by immunohistochemistry in either adolescence or adulthood (Adolescence CTRL n = 8; Adolescence STRESS n = 8; Adulthood CTRL n = 8; Adulthood STRESS n = 8; with one adult animal being excluded from one PSA-NCAM measurement due to poor IHC signal due to quality of the tissue). Animals were sacrificed in basal conditions by transcardial perfusion under anesthesia, brains rapidly removed, post fixed in PFA 4% for 4 h and maintained in PBS until further processing for PSA-NCAM immunohistochemistry.
Fig. 1.
Overview of experimental design to assess the long-term effects of peripubertal stress (PPS) in different moments of development. Rats were weaned at P21 and were either exposed to the PPS protocol from P28 to P42, or assigned to the Control group. The stressors used were exposure to an elevated platform and to a predator odor (TMT) (for more details, please see materials and methods). Control rats were briefly handled on the days of the PPS and then returned to their home cages. Subsequently, control (CTRL) and peripubertally stressed (STRESS) rats were split in two age groups: the adulthood group, and as a control, the adolescence group, depending on when they underwent further tests. All animals were subjected to an open field and novel object exploration tests. Subsequently, their corticosterone reactivity was evaluated after exposure to a novel environment (i.e. exposure to a circular corridor). They were then further split into a group that performed the water maze and a group that was assessed for PSA-NCAM expression levels in the dentate gyrus and medial amygdala.
2.3. Peripubertal stress protocol
Peripubertal Stress Protocol (PPS) was performed as previously described (Márquez et al., 2013; Veenit et al., 2014). Specifically, the stress protocol consisted of presenting two different fear-inducing stressors (each one lasting 25 min): (1) exposure to the synthetic fox odor trimethylthiazoline (9 μl) (Phero Tech Inc., Delta, BC, Canada) released through a small cloth, in a plastic box (38 cm length, 27.5 cm width and 31 cm height) placed under a bright light (210–250 lx); and (2) exposure to an elevated platform (12 × 12 cm, elevated 95 cm from the ground) under direct bright light (470–500 lx). The stressors were applied subchronically during the peripubertal period (a total of 7 days across postnatal day P28 to P42, i.e., on P28–P30, P34, P36, P40 and P42), during the light phase, and according to a variable schedule, where the order and timing of the stressors were changed on different days (Fig. 2A). On some stress days, only one stressor was presented, while on other days, the two stressors were given consecutively. Following each stress session, animals were returned to their home-cages where a transparent Plexiglas wall with holes separated each animal for 15 min before rejoining their cage mates. On the first and last day of Peripubertal stress, blood samples were collected at different time points only to STRESS animals, in order to study the adaptation dynamics to the stress protocol (see below). The control animals were handled on the days that their experimental counterparts were exposed to stress. Animals in the same cage were always assigned to the same experimental group (either CTRL or STRESS).
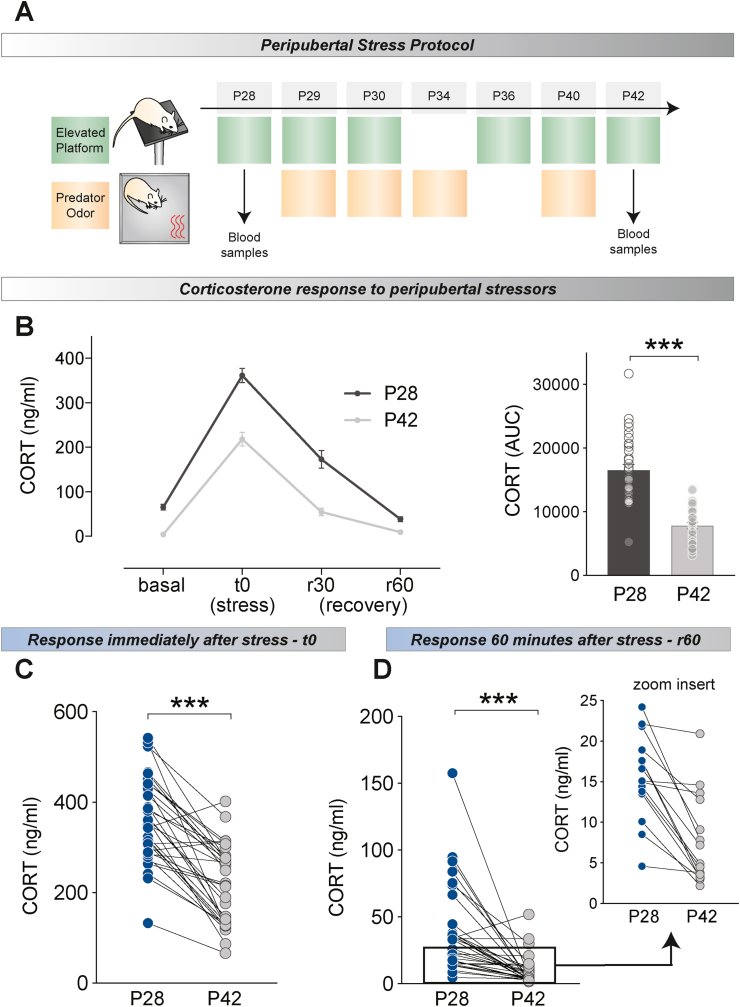
Fig. 2.
Corticosterone (CORT) response during PPS. A. Overview of PPS protocol. Blood samples were obtained on P28 and P42 from STRESS rats at different time points to assess HPA axis reactivity and adaptation to the stressors. B. Left: samples were taken at baseline conditions (i.e. before stressor exposure-basal), immediately following the stressor (stress, t0), as well as, 30 min and 60 min after the end of the exposure to stress (recovery (r) 30 and r60 respectively). Reduced CORT response was observed on P42 as compared to P28. Right: This reduced response is reflected in the CORT area under the curve (AUC) levels that was calculated by considering all four time-points (basal, stress, rec 30 and rec 60). P42 CORT levels correspond to smaller AUC overall. C. Individual differences in the ability to adapt to peak (t0) CORT response in P28 and P42 is shown for all animals. Overall, responses to the elevated platform where reduced in P42 compared to the first exposure in P28, however, this decrease was variable among animals. D. Variability of adaptation in the recovery period after stress comparing P28 and P42 is shown for all animals. Although CORT levels were low at this time point (60 min after the end of stress exposure) marked individual differences were still observed in the ability to adapt to the subchronic stress. Results are expressed as the mean ± S.E.M. ***p < 0.001.
2.4. Open field and novel object reactivity tests
Rats’ exploration levels were assessed in the open field test as previously described (Salehi et al., 2010). They were individually placed in the center of the open field arena (a circular open arena with a diameter of 100 cm) and their behavior while freely exploring was monitored for 10 min using a video camera mounted on the ceiling above the center of the arena. For analysis, the floor was divided into three virtual concentric parts, with a center zone in the middle of the arena (20 cm diameter), an interior zone (60-cm diameter), and an exterior zone made up of the remaining area along the sidewalls. Different parameters were evaluated with the video tracking system: distance moved (centimeters) and time spent (seconds) in each zone. Immediately after the open field test, rats were submitted to the novel object reactivity (NOR) test. For this purpose, a small, white plastic bottle was placed into the center of the open field while the rat was inside. Rats were then given 5 min to freely explore the novel object. The time spent exploring (touching) the novel object was recorded manually from the video recordings. Moreover, different parameters were evaluated with the video tracking system: time spent (seconds) in the center (where the novel object was placed) and the periphery of the compartment, number and latency of entries to the center, total distance moved (centimeters) in the center and in the whole compartment.
2.5. Water maze
In order to test spatial learning and memory, a round black Plexiglas tank with a diameter of 2 m and a height of 45 cm was used. The pool was filled with water each day and the temperature was maintained at 25 °C ± 1 °C during the experiment. A circular platform was submerged 1.3 cm below the water surface. The water maze was surrounded by clearly discernible visual cues to facilitate spatial orientation during the training phase. The experiment was divided in two phases: training and probe trial. The training phase lasted from Day 1 to Day 3 and it involved 4 × 90-s trials/day/rat with a 30 s inter-trial interval. The platform remained constantly at the quadrant assigned as the target quadrant. The starting point for each trial was pseudo-randomly chosen. In order to assess the spatial memory of the animals, a probe trial was performed 24 h after the last training session (Day 4). During this phase, the platform was removed and rats were allowed to swim freely for 90 s. The distance that the animals swam to find the platform was used as an indication of learning. The percentage of the time spent in the quadrant that contained the platform during training (target quadrant) versus the adjacent quadrant was used as an index of spatial memory.
2.6. Corticosterone responsiveness
Individual responsiveness to PPS was evaluated in the STRESS group by measurement of plasmatic corticosterone (CORT) levels during the first and last day of PPS exposure. Blood samples were obtained by tail-nick protocol (100 μl for peripubertal animals) within 2 min while gently holding the animals with a cloth and, then, animals were returned to their home cage. The tail-nick procedure allows for the collection of blood samples at different time points from the same animal (Márquez et al., 2004), which enables the study of hormonal dynamics. Samples were obtained in basal conditions, immediately following the termination of the elevated platform stress and 30 and 60 min after the elevated platform stress. Based on these CORT measurements, two adaptation indices were then calculated: time 0 (t0) and recovery 60 (r60). The t0 index reflected the change of the CORT response, immediately after exposure to the stressor, between the last (P42) and the first (P28) day of the protocol (CORT P42 immediately after stress * 100/CORT P28 immediately after stress), and thus, expressed a proxy for the adaptation of the initial response to the stressor after subchronic stress. In a similar way, the recovery 60 (r60) index, was calculated to assess recovery adaptation to basal corticosterone levels after exposure to stress (CORT P42 60 min after termination of stress exposure * 100/CORT P28 60 min after termination of stress exposure). Two animals, one from the adolescent group and one from the adulthood group were excluded from all analyses as the values for these variables were exceeding 3 Standard Deviations from the mean.
Later in life, the long-term effects of PPS on corticosterone reactivity to a mild stressor were evaluated in independent groups of CTRL and STRESS animals at either adolescence or adulthood period. Immediately after 30 min exposure to a novel environment (a circular corridor made of plastic; 35 cm high, 25 cm diameter) blood samples were obtained by tail-nick (250 μl). Two additional blood samples were obtained during the recovery period, 30 and 60 min after the end of circular corridor exposure. Baseline samples were collected in a previous day, in order not to interfere with behavior. Animals from the same home-cage were simultaneously tested in adjacent containers. The containers were cleaned with 1% acetic acid and dried properly before placing the animals.
Blood samples were collected into ice-cold heparin capillary tubes (Sarsted, Switzerland) and kept at 4° during the experiment. Plasma was obtained after blood centrifugation at 10,000 rpm for 25 min and stored at −20 °C until analyses. Plasma corticosterone levels were measured by enzymatic immunoassay kit (Correlate-EIA from Assay Designs Inc., USA) according to supplier's recommendations. The area under the curve of the corticosterone levels was calculated using GraphPad Prism (version 7), which computes the area under the curve using the trapezoid rule.
2.7. PSA-NCAM immunohistochemistry
For the PSA-NCAM immunohistochemistry experiment rats were anesthetized with a lethal dose of pentobarbital (Esconarkon, Streuli Pharma AG, 150 mg/kg body weight, solution provided by the EPFL veterinarian) and perfused via the ascending aorta with ice-cold 0.9% saline, followed by 4% paraformaldehyde in phosphate-buffered saline (pH = 7.5). After perfusion-fixation, the brains were removed from the skull, post-fixed in the same solution for 4 h, and stored in 4 °C PBS until further processing. Subseries of 50 μm thick sections from each group of animals were processed free floating for immunohistochemistry using the avidin-biotin-peroxidase (ABC) method (Hsu et al., 1981). Sections were incubated with 10% H2O2 in phosphate buffered saline (PBS) for 10 min to block endogenous peroxidase activity. They were then treated for 1 h with 10% normal donkey serum (NDS) (Jackson ImmunoResearch Laboratories) in PBS with 0.2% Triton-X100 (Sigma-Aldrich) and incubated for 60 h at 4 °C in the primary antibody anti-PSA-NCAM, generated in mouse, (DSHB, 1:1500) with PBS containing 0.2% Triton-X-100. Then, sections were incubated for 2 h at RT with the biotinylated secondary antibody: donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, 1:200), followed by an avidin-biotin-peroxidase complex (ABC; Vector Laboratories) for 1 h in PBS. Color development was achieved by incubating with 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma-Aldrich) and 0.033% H2O2 for 4 min. Finally, sections were mounted on slides, dried for 1 day at room temperature, dehydrated with ascending alcohols and rinsed in xylene. Sections were coverslipped using Eukitt mounting medium (PANREAC). All sections passed through all procedures simultaneously in order to minimize any difference from the immunohistochemical staining itself. To avoid any bias in the analysis, all slides were coded prior to analysis and remained so until the experiment was completed. Sections were examined with an Olympus CX41 microscope under bright-field illumination, homogeneously illuminated and digitalized using a CCD camera. Photographs of the different areas were taken at 20 Å~ magnification. Grey levels were converted to optical densities (OD) using Image J software (NIH). Means were determined for each experimental group and data were analyzed with appropriate statistical tests.
2.8. Statistical analyses
During behavioral testing animals were tracked automatically with EthoVision 3.0/3.1 (Noldus, Wageningen, the Netherlands). The results were analyzed using the SPSS 17 statistical package and the graphs and correlation matrices were made using GraphPad Prism 7. The data were analyzed with analysis of variance (ANOVA) with repeated measures, Student's t –tests or paired samples t -tests as considered appropriate. The data was checked for distribution with the Shapiro-Wilk test and when the normality was violated, non-parametric tests were applied (i.e. Mann-Whitney and Wilcoxon signed rank tests). All t-tests and paired samples t-tests were performed two-tailed, with the exception of the Mann-Whitney for Fig. 3I and J, where we specifically hypothesized a blunted CORT response (i.e., one-tailed prediction) extrapolating from previous findings (Veenit et al., 2013). Regarding the repeated measures ANOVA, when Mauchly's test of sphericity was significant, thus sphericity could not be assumed, the Greenhouse-Geisser correction was used and reported. All results represent the mean + the standard error of the mean (SEM) and the significance was set at p < 0.05.
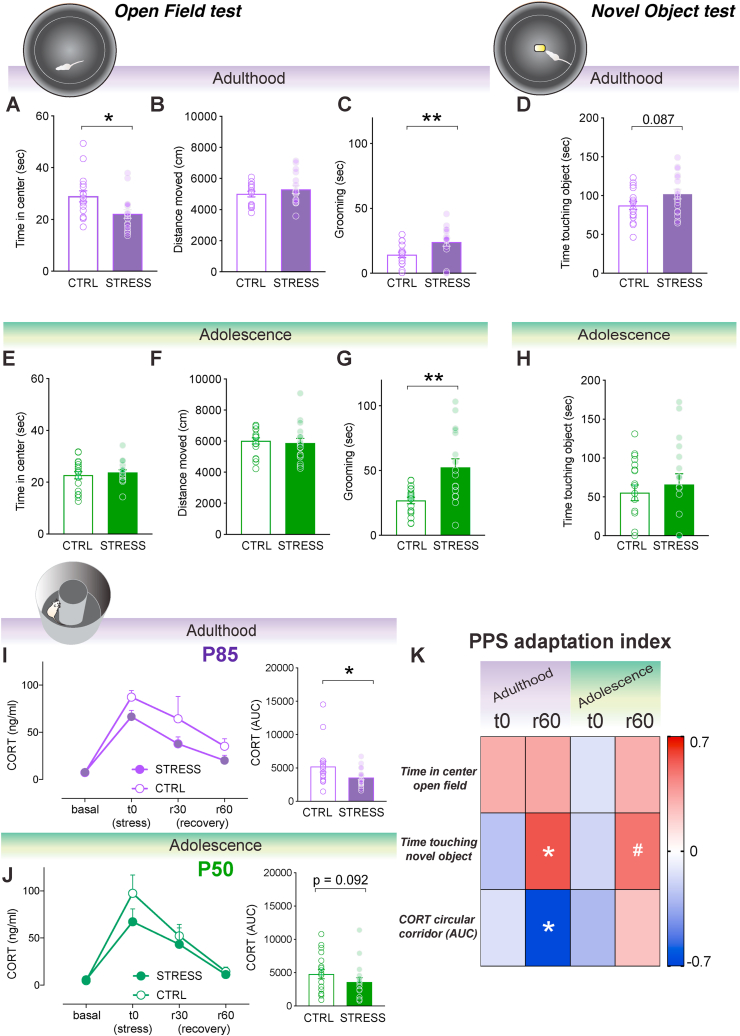
Fig. 3.
Open Field, Novel Object tests and corticosterone reactivity to circular corridor for adult and adolescent CTRL and STRESS rats. Adult STRESS rats spent less time in the central area of the Open field test (A) but no differences in general locomotor activity were observed (B). Moreover, self-grooming levels observed in the open field were also higher in STRESS animals (C). Regarding exploration, STRESS animals tended to explore more the object in the novel object test (D). None of these differences were observed in adolescence (E, F, H) except for the increase of grooming levels during open field exposure (G). CORT response was also examined upon exposure to a novel environment (i.e. circular corridor) in either CTRL or STRESS rats in adulthood. I. STRESS rats exhibited lower CORT response upon exposure to a novel environment (i.e. circular corridor) compared to CTRL animals. Temporal corticosterone dynamics are presented in the left, and AUC is shown in the right.J. The same tendency was observed in adolescence; however, it did not reach statistical significance. K. Correlation matrix between the adaptation index after peripubertal stress considering peak (t0) or recovery times (r60) and main behavioral and endocrine measurements. Animals that adapted the less during the recovery to peripubertal stress were the more impaired in the novel object test and with a more marked blunted CORT response to stress during adulthood. Results are expressed as the mean ± S.E.M. *p < 0.05, **p < 0.01, #p < 0.10.
3. Results
3.1. Marked individual differences in the corticosterone adaptation to repeated stressor exposure during the peripubertal period
In order to determine whether adaptation to the PPS protocol (peak vs recovery) could predict long term reprogramming effects of stress, we first characterized the corticosterone (CORT) response dynamics during stress exposure. Rats were exposed to threatening challenges (i.e., elevated platform, predator odor) at scattered days (i.e., P28, P29, P30, P34, P36, P40 and P42) within the peripubertal period (Fig. 2A) and blood samples collected following exposure to the same stressor, elevated platform, on the first (P28) and last (P42) days of the protocol, and at four time points: immediately before the stressor (basal), immediately after the elevated platform exposure (stress; t0) and in order to assess the recovery of the response, 30 and 60 min following the stressor (rec30 and rec60 respectively). In both days, exposure to the elevated platform induced a robust corticosterone release that was recovered to basal levels 1 h after stress termination (Fig. 2B; left). A general habituation of the CORT response from P28 to P42 was observed, with CORT levels being reduced at P42, as indicated by a decreased AUC measurement (Fig. 2B; right: t (32) = 9.398, p < 0.001). Importantly, inspection of these results indicates that rats displayed marked individual differences in their corticosterone adaptation to peripubertal stress, both as in their peak responses (Fig. 2C) and in the rec 60 time-point (Fig. 2D). Thus, while the majority of animals showed a decreased CORT response at P42 with varying levels of intensity, suggesting a good degree of adaptation, a subset of rats did not adapt at all (Fig. 2C–D). As expected, the same pattern of CORT response was obtained when animals - ascribed to the two testing conditions later in life - were analyzed separately for validation purposes (Fig. S1A-D; adulthood: A – left; Wilcoxon signed rank paired test – basal: p < 0.001, stress: p = 0.001, rec 30: p < 0.001, rec 60: p < 0.001, A – right; t (16) = 7.817, p < 0.001), adolescence: C – left; Wilcoxon signed rank paired test – basal: p < 0.001, stress: p = 0.001, rec 30: p = 0.004, rec 60: p = 0.002, C – right; t (15) = 5.830, p < 0.001, B and D; animals plotted individually for t0 and rec60 time points for adulthood and adolescence respectively. Animals showing low adaptation can be observed in both age groups).
3.2. Peripubertal stress leads to delayed programming effects on anxiety-like behavior
Before assessing for potential programming effects of peripubertal stress in spatial learning, we tested animals for their locomotor and exploratory behaviors in the Open Field and Novel Object tests, as behavioral changes in these tests may help interpreting potential differences in the water maze. When tested at adulthood, STRESS rats showed a decrease in the time spent in the center of the Open Field (Fig. 3A; Mann-Whitney – p = 0.014), no differences in total distance moved (Fig. 2B; t (31) = −0.940, p = 0.354), but an increase in self-grooming behavior (Fig. 3C; Mann-Whitney test, p = 0.007). In addition, STRESS rats showed a trend towards increased time exploring and touching the object in the novel object test (Fig. 3D; t (31) = −1.769, p = 0.087). Altogether, these results indicate a phenotype characterized by increased anxiety-like behaviors with no change in locomotion.
In order to ascertain whether these behavioral changes emerged at adulthood or were already present at earlier time points, we tested a second cohort of animals during adolescence (P48+; Fig. 1). At this time point, STRESS animals did not show changes in the time spent in the center (Fig. 3E; t (33) = −0.606, p = 0.548) or distance moved (Fig. 2F; t (33) = 0.366. p = 0.717) in the open field. However, as when tested at adulthood, STRESS animals tested at adolescence showed increased self-grooming behavior (Fig. 3G; t (20.434) = −3.584, p = 0.002). In the novel object test (Fig. 3D; t (31) = −1.769, p = 0.087), they did not differ from CTRL in time exploring the object (Fig.3H; t (33) = −0.626, p = 0.535).
Therefore, these data indicate an interesting age-dependent effect on exploratory behaviors. Specifically, long-term programming effects of peripubertal stress on anxiety-like behaviors are observed at adulthood, and in a much lesser extent (i.e., self-grooming) at adolescence. Locomotion is not changed at any of the testing times.
3.3. Peripubertal stress induces CORT hypo-reactivity in adulthood
We then sought to ascertaining if exposure to PPS would affect corticosterone reactivity to challenges later in life. Indeed, adult STRESS rats showed blunted CORT reactivity (Fig. 3I - left: significant main effect of time: F (1.4, 43.68) = 11.434, p < 0.001, trend for a main effect of stress: F (1, 31) = 3.861, p = 0.058, nonsignificant stress × time interaction: F (1.4, 43.68) = 0.152, p = 0.783, 3I - right: significant Mann-Whitney one tail test, p = 0.0265) following exposure to a novel environment (i.e., circular corridor, devoid of the anxiogenic center of the arena). This effect was particularly obvious at adulthood, as a mild reduction in CORT activation observed when STRESS animals were tested during adolescence was not significant (Fig. 3J - left; significant main effect of time: F (1.5, 49.05) = 14.565, p < 0.001, nonsignificant main effect of stress: F (1, 33) = 1.547, p = 0.222, nonsignificant stress × time interaction: F (1.5, 49.05) = 0.615, p = 0.498, 3J - right: nonsignificant Mann-Whitney one tail test, p = 0.092).
Then, we aimed to understand whether the degree to which animals adapt their corticosterone responses to repeated stressors during the peripubertal period (i.e., from P28 to P42) relates to subsequent behavioral and/or hormonal responses. To this end, we first computed two adaptation indices for time 0 (t0) and recovery 60 (r60) (see Methods for details). Then, we calculated correlations between these indices and key behavioral parameters and corticosterone reactivity (AUC) to emotional challenges (i.e., the tests reported above). As shown in Fig. 3K, it was specifically the adaptation of CORT recovery (rec60 index) during peripubertal stress that correlated with both, time touching the object in the Novel object test (Fig. 3K; r = 0.558, p = 0.020) and, negatively, with corticosterone reactivity (r = - 0.670, p = 0.003) in animals tested at adulthood. Thus, the lesser the adaptation of corticosterone recovery during PPS stress exposure, the higher the time exploring the novel object, and the lower the CORT responsiveness to a mild stressor (i.e. novel environment). A similar trend, although not significant, was observed for the correlation between time touching the novel object and rec60 index for the data from adolescence testing (Fig. 3K; r = 0.481, p = 0.059). Strikingly, no correlation was observed between the studied parameters and the PPS CORT adaptation index for t0 (i.e, peak CORT stress responses).
3.4. Peripubertal stress leads to delayed programming effects on spatial learning
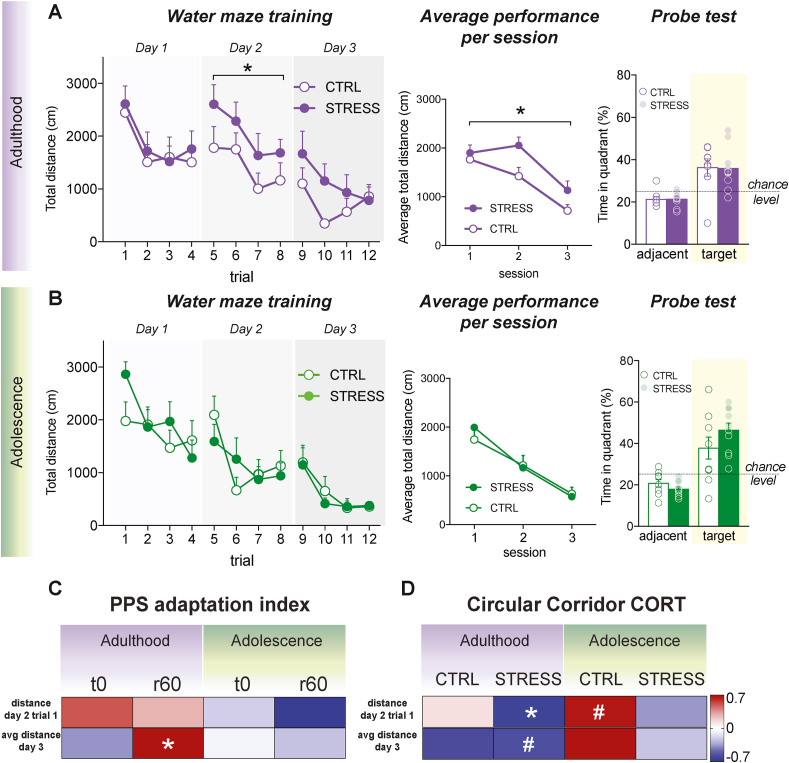
We then addressed our main question; whether peripubertal stress can have delayed effects on spatial learning and memory, and to what extent any observed effect would be related to the degree of CORT adaptation to repeated stressor exposure during peripuberty. To this end, animals were trained and tested to find a hidden platform in the water maze. Adult STRESS rats showed increased total distance swam to find the hidden platform on day 2 (Fig. 4A; left - day 1- significant main effect of time: F (3, 48) = 4.192, p = 0.010, nonsignificant main effect of stress: F (1, 16) = 0.295, p = 0.595, nonsignificant time × stress interaction: F (3, 48) = 0.109, p = 0.955, day 2 – significant main effect of time: F (3, 48) = 3.231, p = 0.030, significant main effect of stress: F (1, 16) = 5.110, p = 0.038, nonsignificant time × stress interaction: F (3, 48) = 0.085, p = 0.968, day 3 - nonsignificant main effect of time: F (3, 48) = 2.182, p = 0.102, nonsignificant main effect of stress: F (1, 16) = 2.923, p = 0.107, nonsignificant time × stress interaction: F (3, 48) = 0.797, p = 0.502) and increased distance moved when the average performance per session was considered (Fig. 4B; middle – significant main effect of time: F (2, 32) = 17.083, p < 0.001, significant main effect of stress: F (1, 16) = 5.713, p = 0.029, nonsignificant time × stress interaction: F (2, 32) = 1.049, p = 0.362). No differences between STRESS and control rats were observed during the probe trial (Fig. 4B; right - adjacent: t (16) = - 0.068, p = 0.947, target: t (16) = 0.068, p = 0.946).
Fig. 4.
Water maze training and probe trial for adult and adolescent rats, as well as correlations of water maze parameters with CORT. A. Left: Adult STRESS rats traveled more distance before finding the platform on the second day of training, compared to CTRL rats. Middle: Compared to CTRL, STRESS rats tested during adulthood showed increased distance in the water maze when the average performance per session was considered. Right: Both CTRL and STRESS adolescent groups exhibited intact memory of the position of the platform during the probe trial, when the platform was absent. No differences were found between the groups. B. Left: No differences were observed between adolescent CTRL and STRESS rats during training in the water maze. Middle: No differences between the groups were observed for the average performance per session. Right: Both CTRL and STRESS adolescent groups exhibited intact memory of the position of the platform during the probe trial, when the platform was absent. No differences were found between the groups. C. Correlation matrix showing correlation coefficients between peak (t0) and recovery (r60) adaptation indexes to peripubertal stress and performance in a in the first long term memory test (trial 1 of the second day of testing) and in the last day of training. Those animals that adapted the less to peripubertal stress (r60) were the more affected while adults and performed the worse in the Morris water maze. D. Correlation matrix between circular corridor CORT response and performance in the water maze, indicating that only in STRESS animals, a more blunted CORT response to the novel environment (lower CORT levels) correlated with impairments in the Morris water maze. Results are expressed as the mean ± S.E.M. Correlation coefficients (r values) in correlation matrix are color-coded. *p < 0.05, #p < 0.10
In order to inquire whether the observed PPS stress effects on spatial learning at adulthood were protracted or delayed, we tested the second cohort of animals in the water maze during adolescence. However, at this time point, no effect of PPS stress was observed (Fig. 4B; left – no effect in distance moved: day 1- significant main effect of time: F (3, 54) = 3.306, p = 0.027, nonsignificant main effect of stress: F (1, 18) = 0.832, p = 0.374, nonsignificant time × stress interaction: F (3, 54) = 1.455, p = 0.237, day 2 - significant main effect of time: F (3, 54) = 5.379, p = 0.003, nonsignificant main effect of stress: F (1, 18) = 0.032, p = 0.860, nonsignificant time × stress interaction: F (3, 54) = 1.476, p = 0.231, day 3 – significant main effect of time: F (1.76, 31.67) = 8.329, p = 0.002, nonsignificant main effect of stress: F (1, 18) = 0.108, p = 0.746, nonsignificant time × stress interaction: F (1.76, 31.67) = 0.217, p = 0.778) nor regarding the average performance per session (Fig. 4B; middle – significant main effect of time: F (2, 36) = 44.392, p < 0.001, nonsignificant main effect of stress: F (1, 18) = 0.058, p = 0.812, nonsignificant time × stress interaction: F (2, 36) = 0.871, p = 0.427), nor in the probe test (Fig. 4B; right - adjacent: t (18) = 1.360, p = 0.191, target: t (18) = −1.361, p = 0.190).
We then inquired whether individual differences in CORT adaptation during PPS exposure (i.e., t0 and rec60 indices) related to differences in key parameters of water maze performance. To this end, we selected average performance (i.e., distance to the platform) on the last training day, as an index for the maximal acquisition level obtained and distance to find the platform on the first trial of day 2, as a first long-term memory index. As shown in Fig. 4C, rec60 was again the parameter that showed a positive correlation with day 3 performance (Fig. 4C; r = 0.648, p = 0.043); i.e., the lesser the adaptation of CORT recovery following peripubertal stressors, the worse the maximal training performance in the water maze. This was not observed when the animals were tested in adolescence (Fig. 4C). Moreover, no correlations were found between water maze performance and PPS peak adaptation index (t0 index) at any of the age groups (Fig. 4C).
Furthermore, in order to better understand possible links between CORT responsiveness during the testing period and variation in spatial learning performance, we examined the relationship between CORT response upon exposure to the circular corridor (see Fig. 3I and J) and water maze parameters. Interestingly, CORT reactivity to the circular corridor correlated with the first long-term memory test (i.e., distance to find the platform on the first trial of day 2) in STRESS animals tested at adulthood; i.e., the lower the CORT the poorer their performance in the water maze (Fig. 4D; r = −0.641, p = 0.046). In other words, those animals that displayed more blunted corticosterone response during adulthood after a novelty challenge as a consequence of peripubertal stress exposure were the ones showing worst long-term memory in the water maze. A similar trend was observed for STRESS animals’ CORT responsiveness at adulthood and average performance on the last training day (Fig. 4D; r = −0.581, p = 0.078).
Altogether, these results suggest that peripubertal stress has delayed detrimental effects on spatial learning that become evident when the assessment happens during adulthood, and that those animals that show impaired adaptation in CORT recovery to repeated stressors exposure perform poorer in a spatial learning task.
3.5. Peripubertal stress leads to changes in PSA-NCAM in the dentate gyrus
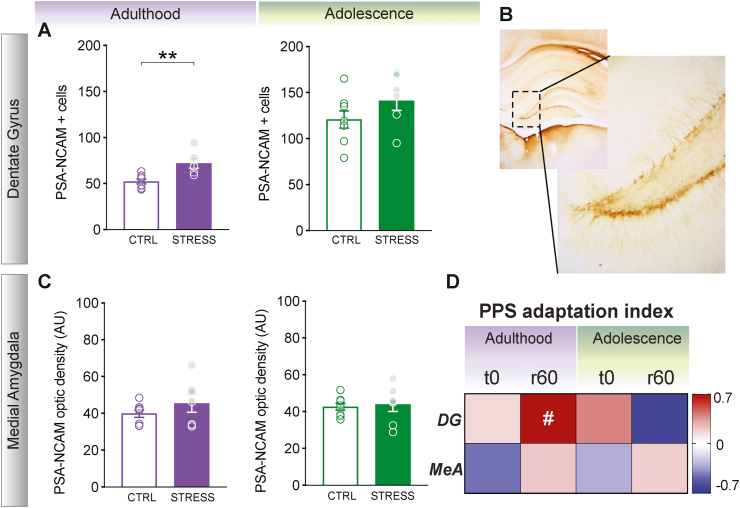
In order to gain insight into key plasticity molecules, related to learning and memory, that could be affected by peripubertal stress, two further cohorts of rats were exposed to peripubertal stress and studied at each age group (i.e., CTRL and STRESS; Adulthood and Adolescence) and assessed for the expression of PSA-NCAM in the DG of the hippocampus (Fig. 5). As shown in Fig. 5A, there was an increase of PSA-NCAM in adult rats stressed during peripuberty (Fig. 5A – left; t (12) = −3.675, p = 0.003). However, no significant differences were apparent in PSA-NCAM expression when the rats were assessed during adolescence (Fig. 5A – right; t (13) = −1.458, p = 0.169). In order to study specificity of our findings, we quantified PSA-NCAM expression in the medial amygdala. However, no differences were found between STRESS and CTRL rats regardless of the developmental age, suggesting that the PSA-NCAM alterations observed in the dentate gyrus at adulthood were not only age-dependent, but also brain region-specific (Fig. 5C – left – adulthood; t (8.04) = −1.059, p = 0.321, right – adolescence; t (13) = - 0.291, p = 0.776).
Fig. 5.
PSA-NCAM levels for adult and adolescent CTRL and STRESS rats in the Dentate Gyrus (DG) and Medial Amygdala (MeA). A. Left: Adult STRESS rats showed increased number of PSA-NCAM positive cells compared to adult CTRL rats. Right: These differences were not observed when assessed during adolescence. B. Photomicrograph of the DG area where PSA-NCAM positive cells were quantified. C. No difference was observed between CTRL and STRESS tested at adulthood (left) nor adolescence (right) in PSA-NCAM optic density in the MeA. D. Correlation matrix showing correlation coefficients between peak (t0) and recovery (r60) adaptation indexes to peripubertal stress and PSA-NCAM levels in the dentate gyrus and medial amygdala. Those animals that adapted the less to peripubertal stress (r60 index) were the ones that tended to display higher levels of DG PSA-NCAM during adulthood. Results are expressed as the mean ± S.E.M. Correlation coefficients (r values) in correlation matrix are color-coded. **p < 0.01, #p < 0.10
Interestingly, there was a trend for those animals whose CORT responsiveness would adapt suboptimally at r60 during peripubertal stress to display higher DG PSA levels in adulthood (r = 0.735 p = 0.096) (Fig. 5D). No correlations of CORT responsiveness were observed with the MeA for any of the age groups or time points.
4. Discussion
Here, we show that exposure to stressors across the peripubertal period in rats leads to cognitive, behavioral and endocrine changes at adulthood. Specifically, peripubertal stress led to impaired spatial learning, increased anxiety-like behavior, and blunted corticosterone responsiveness to novelty challenges. These effects are delayed in nature, as they were not displayed by animals tested during adolescence (i.e., shortly after peripubertal stress exposure). Strikingly, individual differences in the degree of adaptation of the recovery -and not the peak-of the corticosterone response to stressor exposure (i.e., plasma levels at 60 min post-stressor) across the peripubertal stress period (i.e., from P28 to P42) predicted the level of spatial orientation in the water maze completed on the last training day, as well as the exploratory behavior shown by animals at adulthood. In addition, this corticosterone stress adaption recovery index (rec60) was inversely related to the corticosterone responsiveness to novelty at adulthood. These findings contribute to further our understanding on the link between HPA axis adaptation to early life stressors at the important transitional period of puberty and the long-term programming of behavior and cognition.
Thus, a main finding of our study is the identification of peripuberty as a stress-sensitivity period for the modulation of adult spatial learning abilities. Previous studies had underscored the early postnatal period as a time-window in which stress exposure makes individuals particularly prone to show spatial learning impairments at long-term life stages (Brunson et al., 2005; Oomen et al., 2010). However, previous studies comprising stressor exposure across several weeks from juvenility to adulthood in which spatial learning and memory impairments were reported (Isgor et al., 2004; Sterlemann et al., 2010) did not allow disentangling the putative impact of peripubertal stress per se. In addition, we show here that the impact is not immediate (i.e., not shown during adolescence) but, similarly to the report by Isgor et al. (2004), it only emerges when testing takes place several weeks after the end of the stress protocol, at adulthood. Further evidence for this delayed phenomenon stems from studies in rats involving prepubertal stress (i.e., from P28 to P30) and showing impaired water maze at adulthood only following a second stressful challenge at adulthood that, on its own, does not affect spatial learning performance (Avital and Richter-Levin, 2005). In this connection, we previously reported that the same peripubertal stress protocol as the one applied here leads as well to attention deficits in adulthood (Tzanoulinou et al., 2016), but whether these deficits are observed already during adolescence remains to be tested. Altogether, these findings support the view that the long-term cognitive impact of peripubertal stress requires an incubation period during which stress-targeted mechanisms interact with ongoing maturational and neurodevelopmental trajectories to produce phenotypic changes at later life stages.
Spatial learning highly depends on the functioning of the hippocampus (Moser et al., 1995), a brain region that undergoes profound structural and functional changes in adolescence (McCormick and Mathews, 2010). Interestingly, efficient spatial orientation strategies in the water maze task appear around P42 (Schenk, 1985), coinciding with the last day of our peripubertal stress protocol. Our own data on the expression levels of the plasticity molecule PSA-NCAM in the dentate gyrus show a down-regulation from adolescence to adulthood; this age-dependent regulation was not observed in the medial amygdala, a brain region not involved in spatial learning. Thus, our data agrees with an age-dependent pattern of PSA-NCAM down-regulation taking place in the brain during the postnatal period (Angata and Fukuda, 2003; Rutishauser, 2008) and remaining present later in life in brain areas that maintain neurogenic potential or heightened plasticity, such as the hippocampus (Angata and Fukuda, 2003), where it has been causally involved in memory consolidation (Doyle et al., 1992; López-Fernández et al., 2007; Sandi et al., 2003; Venero et al., 2006). Importantly, we found that peripubertal stress leads to increased PSA-NCAM levels specifically in the dentate gyrus, that was particularly evident in the group of animals examined at adulthood, in agreement with similar findings following exposure to pre-pubertal/juvenile stress (Tsoory et al., 2008). In addition, Tsoory et al. (2008) reported that animals stressed during pre-puberty (P28 to P30) displayed impairments in emotional behavior when exposed to stressful situations later in adulthood, such as the two-way shuttle avoidance. Our results expand this previous observation, and link the ability to adapt to peripubertal stress with corticosterone reactivity to stressors later in life, to cognitive performance and DG PSA-NCAM levels. In the future, it would be interesting to evaluate whether these differences in behavior also translate to changes in corticosterone dynamics after exposure to more severe procedures than the one we used in our study, such as chronic stress or severe acute stress (as the shuttle avoidance used in Tsoory et al., 2008). It might well be that effects of early life stress on HPA axis responsivity might be dependent on the stress intensity, and should be addressed in future studies. Our results reflect the effectiveness of peripubertal stress to disrupt the maturation of the hippocampal learning system. PSA can promote neuronal and synaptic plasticity through mechanisms involving its de-adhesive properties, as well as by interacting with extracellular matrix molecules and glutamate receptors (Varbanov and Dityatev, 2017). However, while PSA-NCAM expression in the dentate gyrus transiently increases around 12 h following training in the water maze (Murphy et al., 1996; Sandi et al., 2003), this increase is only observed in bad -but not good-learners, that require increased effort to complete the task (Sandi et al., 2004), and it decays as animals progressively master the task (Murphy et al., 1996). Moreover, chronic stress at adulthood leads to increased hippocampal PSA-NCAM expression (Pham et al., 2003; Sandi et al., 2001) and impairs spatial learning in the water maze (Sandi, 2004; Venero et al., 2002). Therefore, the facilitation of learning and plasticity processes by PSA-NCAM seems to require an activity-dependent process triggering a transient increase in its expression. Heightened basal elevation of PSA-NCAM appears to be deleterious to information processing, which aligns with our findings in the current study. Furthermore, we should also note that we found a trend for dentate gyrus PSA-NCAM levels to correlate with the adaptation of the corticosterone recovery levels across the peripubertal stress protocol (rec60 index; i.e., the lower the adaptation, the higher PSA-NCAM levels). Although until further replication these findings should be taken with caution given the reduced sample size, they point towards a potential role of glucocorticoids on the regulation of hippocampal PSA-NCAM expression by peripubertal stress. Indeed, a complex regulation of hippocampal PSA-NCAM by glucocorticoids has been revealed (Nacher et al., 2004; Rodríguez et al., 1998), involving, in particular, glucocorticoid receptor actions (Montaron et al., 2003).
Importantly, the peripubertal period entails a transition in HPA responsivity to stressors at both, peak and recovery phases (McCormick et al., 2017). Strikingly, we found that individual differences in the spatial orientation levels achieved in the last training day were also related to the peripubertal rec60 corticosterone adaptation index. Thus, those animals that showed a poorer adaptation of the corticosterone stress recovery at puberty were the ones that attained poorer performance levels. Importantly, as hypothesized, it was the corticosterone recovery, and not the peak, index that related to water maze performance. The ability to down-regulate the HPA axis response to stress (and thus, corticosterone levels) following stress exposure through negative feedback is essential to protect the organism from maladaptive overactivation (Karatsoreos and McEwen, 2011) and also important for optimal secretion of corticosterone in basal (unstressed) conditions (Gjerstad et al., 2018). Therefore, our rec60 index seems to have captured individuals’ ability to adapt to repeated life stressors and serves as a predictive index of adult life cognitive, behavioral, and endocrine disturbances. These findings align well with the important role of the hippocampus in providing negative feedback to the HPA axis (Herman and Mueller, 2006; Jacobson and Sapolsky, 1991; Kovács and Makara, 1988) and the involvement of hippocampal glucocorticoid receptors in HPA axis negative feedback (De Kloet, 1991; Reul et al., 1990).
In addition, the first index of long-term memory performance (i.e., distance moved to find the platform on the first trial of training day 2) was related to corticosterone reactivity at adulthood. Specifically, animals that showed poorer retention levels on the first trial following training day 1 were the ones that showed blunted corticosterone reactivity when exposed as adults to a novelty challenge. These observations align well with the well-known contribution of training-triggered corticosterone levels for memory function in general (de Kloet et al., 2018; de Quervain et al., 2017; Sandi, 2011) and, specifically, for the consolidation of spatial information (Akirav et al., 2004; Conboy et al., 2010; Huzard et al., 2020; Quirarte et al., 1997; Sandi et al., 1997).
The incubation period reported here for spatial learning effects of peripubertal stress to emerge at adulthood appears to be specific for the cognitive domain. Indeed, a different process seems to be engaged in the development of anxiety-like behaviors. While, as in previous studies (Cordero et al., 2016; Tzanoulinou et al., 2014a), we observe here increased anxiety-like behavior when peripubertally stressed rats were tested at adulthood, decreased anxiety-like behaviors were reported when tested during late adolescence (Toledo-Rodriguez and Sandi, 2011). Furthermore, in the social domain, peripubertal stress leads to increased adult aggression (Márquez et al., 2013) in a protracted manner, as rats that showed aberrant play fighting during adolescence were those that developed a more aggressive phenotype at adulthood (Papilloud et al., 2018). In addition, and in line with its physiological contribution to deal with immediate challenges (de Kloet et al., 2008; Myers et al., 2014), it is the magnitude of adaptation of the peak corticosterone response to peripubertal stress that predicts alterations in emotional and social behaviors (Papilloud et al., 2018; Walker et al, 2017, 2018). Along the same lines, we demonstrated here, that the ability to adapt during recovery periods, i.e. once stress exposure has ended, is a key predicting factor for special learning impairments.
In summary, our study identifies the peripubertal period as a time-window at which stress can lead to long-term changes in HPA axis reactivity that are related to difficulties in spatial learning abilities later in life. These findings pave the way for further studies to identify mechanisms of both vulnerability and resilience to early trauma. Furthermore, our data suggest that the reprograming effects of early stress might need a period of incubation which could be compensated in young and more plastic brains, but would fail to adapt during adulthood. Accordingly, following early detection of stress-vulnerable individuals, there may be a window of opportunity for therapeutic approaches to act during adolescence deflecting the course trajectory towards psychopathology and cognitive impairments.
CRediT authorship contribution statement
S. Tzanoulinou: Formal analysis, Visualization, Writing - original draft, Writing - review & editing. E. Gantelet: Investigation, Methodology. C. Sandi: Conceptualization, Writing - review & editing, Funding acquisition. C. Márquez: Conceptualization, Supervision, Investigation, Methodology, Formal analysis, Visualization, Writing - original draft, Writing - review & editing, Funding acquisition.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgements
We thank Angélique Voucher, Coralie Siegmund, Marjorie Clerc and Aliénor Sonnay for excellent technical assistance.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ynstr.2020.100282.
Funding
This work was supported by the NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation, USA, under the grant number 26478 to C.M., the Ministerio de Ciencia e Innovación, Spain, under the grants RTI2018-097843-B-100 to C.M. and the “Severo Ochoa” Program for Centers of Excellence in R&D (SEV-2013-0317 and SEV-2017-0723) and by the Swiss National Science Foundation, Switzerland (NCCR Synapsy No. 158776 and 185897) to C.S.; C.M. was further supported by the Ministerio de Ciencia e Innovación with a Ramon y Cajal contract (RYC-2014-16450).
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Akirav I., Kozenicky M., Tal D., Sandi C., Venero C., Richter-Levin G. A facilitative role for corticosterone in the acquisition of a spatial task under moderate stress. Learn. Mem. 2004;11:188–195. doi: 10.1101/lm.61704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht A., Müller I., Ardi Z., Çalışkan G., Gruber D., Ivens S. Neurobiological consequences of juvenile stress: a GABAergic perspective on risk and resilience. Neurosci. Biobehav. Rev. 2017;74:21–43. doi: 10.1016/j.neubiorev.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Andersen S.L., Teicher M.H. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Angata K., Fukuda M. Polysialyltransferases: major players in polysialic acid synthesis on the neural cell adhesion molecule. Biochimie. 2003;85:195–206. doi: 10.1016/s0300-9084(03)00051-8. [DOI] [PubMed] [Google Scholar]
- Avital A., Richter-Levin G. Exposure to juvenile stress exacerbates the behavioural consequences of exposure to stress in the adult rat. Int. J. Neuropsychopharmacol. 2005;8:163–173. doi: 10.1017/S1461145704004808. [DOI] [PubMed] [Google Scholar]
- Bird C.M., Burgess N. The hippocampus and memory: insights from spatial processing. Nat. Rev. Neurosci. 2008;9:182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- Bisaz R., Conboy L., Sandi C. Learning under stress: a role for the neural cell adhesion molecule NCAM. Neurobiol. Learn. Mem. 2009;91:333–342. doi: 10.1016/j.nlm.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J. The social brain in adolescence. Nat. Rev. Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Bolton J.L., Molet J., Ivy A., Baram T.Z. New insights into early-life stress and behavioral outcomes. Curr. Opin. Behav. Sci. 2017;14:133–139. doi: 10.1016/j.cobeha.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonapersona V., Kentrop J., Van Lissa C.J., van der Veen R., Joëls M., Sarabdjitsingh R.A. The behavioral phenotype of early life adversity: a 3-level meta-analysis of rodent studies. Neurosci. Biobehav. Rev. 2019;102:299–307. doi: 10.1016/j.neubiorev.2019.04.021. [DOI] [PubMed] [Google Scholar]
- Brunson K.L., Kramár E., Lin B., Chen Y., Colgin L.L., Yanagihara T.K. Mechanisms of late-onset cognitive decline after early-life stress. J. Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Levita L., Libby V., Pattwell S.S., Ruberry E.J. The storm and stress of adolescence: insights from human imaging and mouse genetics. Dev. Psychobiol. 2010;52:225–235. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy L., Bisaz R., Markram K., Sandi C. Role of NCAM in emotion and learning. Adv. Exp. Med. Biol. 2010;663:271–296. doi: 10.1007/978-1-4419-1170-4_18. [DOI] [PubMed] [Google Scholar]
- Cordero M.I., Just N., Poirier G.L., Sandi C. Effects of paternal and peripubertal stress on aggression, anxiety, and metabolic alterations in the lateral septum. Eur. Neuropsychopharmacol. 2016;26:357–367. doi: 10.1016/j.euroneuro.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Cordero M.I., Poirier G.L., Márquez C., Veenit V., Fontana X., Salehi B. Evidence for biological roots in the transgenerational transmission of intimate partner violence. Transl. Psychiatry. 2012;2:e106. doi: 10.1038/tp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet E.R. Brain corticosteroid receptor balance and homeostatic control. Front. Neuroendocrinol. 1991;12(2):95–164. [Google Scholar]
- de Kloet E.R., Karst H., Joëls M. Corticosteroid hormones in the central stress response: quick-and-slow. Front. Neuroendocrinol. 2008;29:268–272. doi: 10.1016/j.yfrne.2007.10.002. [DOI] [PubMed] [Google Scholar]
- de Kloet E.R., Meijer O.C., de Nicola A.F., de Rijk R.H., Joëls M. Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Front. Neuroendocrinol. 2018;49:124–145. doi: 10.1016/j.yfrne.2018.02.003. [DOI] [PubMed] [Google Scholar]
- de Quervain D., Schwabe L., Roozendaal B. Stress, glucocorticoids and memory: implications for treating fear-related disorders. Nat. Rev. Neurosci. 2017;18:7–19. doi: 10.1038/nrn.2016.155. [DOI] [PubMed] [Google Scholar]
- Doyle E., Nolan P.M., Bell R., Regan C.M. Hippocampal NCAM180 transiently increases sialylation during the acquisition and consolidation of a passive avoidance response in the adult rat. J. Neurosci. Res. 1992;31:513–523. doi: 10.1002/jnr.490310315. [DOI] [PubMed] [Google Scholar]
- Gee D.G., Casey B.J. The impact of developmental timing for stress and recovery. Neurobiol. Stress. 2015;1:184–194. doi: 10.1016/j.ynstr.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerstad J.K., Lightman S.L., Spiga F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress. 2018;21:403–416. doi: 10.1080/10253890.2018.1470238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M.R., DePasquale C.E., Reid B.M., Donzella B., Miller B.S. Pubertal stress recalibration reverses the effects of early life stress in postinstitutionalized children. Proc. Natl. Acad. Sci. U.S.A. 2019;116:23984–23988. doi: 10.1073/pnas.1909699116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C., Nemeroff C.B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatr. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Herman J.P., Mueller N.K. Role of the ventral subiculum in stress integration. Behav. Brain Res. 2006;174:215–224. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Huzard D., Vouros A., Monari S., Astori S., Vasilaki E., Sandi C. Constitutive differences in glucocorticoid responsiveness are related to divergent spatial information processing abilities. Stress. 2020;23:37–49. doi: 10.1080/10253890.2019.1625885. [DOI] [PubMed] [Google Scholar]
- Isgor C., Kabbaj M., Akil H., Watson S.J. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Jacobson L., Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr. Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Karatsoreos I.N., McEwen B.S. Psychobiological allostasis: resistance, resilience and vulnerability. Trends Cognit. Sci. 2011;15:576–584. doi: 10.1016/j.tics.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Kiss J.Z., Muller D. Contribution of the neural cell adhesion molecule to neuronal and synaptic plasticity. Rev. Neurosci. 2001;12:297–310. doi: 10.1515/revneuro.2001.12.4.297. [DOI] [PubMed] [Google Scholar]
- Kovács K.J., Makara G.B. Corticosterone and dexamethasone act at different brain sites to inhibit adrenalectomy-induced adrenocorticotropin hypersecretion. Brain Res. 1988;474:205–210. doi: 10.1016/0006-8993(88)90435-0. [DOI] [PubMed] [Google Scholar]
- Kumsta R., Schlotz W., Golm D., Moser D., Kennedy M., Knights N. HPA axis dysregulation in adult adoptees twenty years after severe institutional deprivation in childhood. Psychoneuroendocrinology. 2017;86:196–202. doi: 10.1016/j.psyneuen.2017.09.021. [DOI] [PubMed] [Google Scholar]
- Latsko M.S., Farnbauch L.A., Gilman T.L., Lynch J.F., Jasnow A.M. Corticosterone may interact with peripubertal development to shape adult resistance to social defeat. Horm. Behav. 2016;82:38–45. doi: 10.1016/j.yhbeh.2016.04.009. [DOI] [PubMed] [Google Scholar]
- López-Fernández M.A., Montaron M.-F., Varea E., Rougon G., Venero C., Abrous D.N., Sandi C. Upregulation of polysialylated neural cell adhesion molecule in the dorsal hippocampus after contextual fear conditioning is involved in long-term memory formation. J. Neurosci. 2007;27:4552–4561. doi: 10.1523/JNEUROSCI.0396-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Márquez C., Nadal R., Armario A. The hypothalamic-pituitary-adrenal and glucose responses to daily repeated immobilisation stress in rats: individual differences. Neuroscience. 2004;123:601–612. doi: 10.1016/j.neuroscience.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Márquez C., Poirier G.L., Cordero M.I., Larsen M.H., Groner A., Marquis J., Magistretti P.J., Trono D., Sandi C. Peripuberty stress leads to abnormal aggression, altered amygdala and orbitofrontal reactivity and increased prefrontal MAOA gene expression. Transl. Psychiatry. 2013;3:e216. doi: 10.1038/tp.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C.M., Green M.R., Simone J.J. Translational relevance of rodent models of hypothalamic-pituitary-adrenal function and stressors in adolescence. Neurobiol. Stress. 2017;6:31–43. doi: 10.1016/j.ynstr.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C.M., Mathews I.Z. Adolescent development, hypothalamic-pituitary-adrenal function, and programming of adult learning and memory. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2010;34:756–765. doi: 10.1016/j.pnpbp.2009.09.019. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Nasca C., Gray J.D. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41:3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet J., Maras P.M., Avishai-Eliner S., Baram T.Z. Naturalistic rodent models of chronic early-life stress. Dev. Psychobiol. 2014;56:1675–1688. doi: 10.1002/dev.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaron M.F., Piazza P.V., Aurousseau C., Urani A., Le Moal M., Abrous D.N. Implication of corticosteroid receptors in the regulation of hippocampal structural plasticity. Eur. J. Neurosci. 2003;18:3105–3111. doi: 10.1111/j.1460-9568.2003.03048.x. [DOI] [PubMed] [Google Scholar]
- Moser M.B., Moser E.I., Forrest E., Andersen P., Morris R.G. Spatial learning with a minislab in the dorsal hippocampus. Proc. Natl. Acad. Sci. U.S.A. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K.J., O'Connell A.W., Regan C.M. Repetitive and transient increases in hippocampal neural cell adhesion molecule polysialylation state following multitrial spatial training. J. Neurochem. 1996;67:1268–1274. doi: 10.1046/j.1471-4159.1996.67031268.x. [DOI] [PubMed] [Google Scholar]
- Myers B., McKlveen J.M., Herman J.P. Glucocorticoid actions on synapses, circuits, and behavior: implications for the energetics of stress. Front. Neuroendocrinol. 2014;35:180–196. doi: 10.1016/j.yfrne.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacher J., Gómez-Climent M.Á., McEwen B. Chronic non-invasive glucocorticoid administration decreases polysialylated neural cell adhesion molecule expression in the adult rat dentate gyrus. Neurosci. Lett. 2004;370:40–44. doi: 10.1016/j.neulet.2004.07.062. [DOI] [PubMed] [Google Scholar]
- Oomen C.A., Soeters H., Audureau N., Vermunt L., van Hasselt F.N., Manders E.M.M. Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. J. Neurosci. 2010 doi: 10.1523/JNEUROSCI.0247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papilloud A., Guillot de Suduiraut I., Zanoletti O., Grosse J., Sandi C. Peripubertal stress increases play fighting at adolescence and modulates nucleus accumbens CB1 receptor expression and mitochondrial function in the amygdala. Transl. Psychiatry. 2018;8 doi: 10.1038/s41398-018-0215-6. 156–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papilloud A., Veenit V., Tzanoulinou S., Riccio O., Zanoletti O., Guillot de Suduiraut I. Peripubertal stress-induced heightened aggression: modulation of the glucocorticoid receptor in the central amygdala and normalization by mifepristone treatment. Neuropsychopharmacology. 2019;44:674–682. doi: 10.1038/s41386-018-0110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham K., Nacher J., Hof P.R., McEwen B.S. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur. J. Neurosci. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Poirier G.L., Imamura N., Zanoletti O., Sandi C. Social deficits induced by peripubertal stress in rats are reversed by resveratrol. J. Psychiatr. Res. 2014;57:157–164. doi: 10.1016/j.jpsychires.2014.05.017. [DOI] [PubMed] [Google Scholar]
- Quirarte G.L., Roozendaal B., McGaugh J.L. Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14048–14053. doi: 10.1073/pnas.94.25.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul J.M., Sutanto W., van Eekelen J.A., Rothuizen J., de Kloet E.R. Central action of adrenal steroids during stress and adaptation. Adv. Exp. Med. Biol. 1990;274:243–256. doi: 10.1007/978-1-4684-5799-5_15. [DOI] [PubMed] [Google Scholar]
- Rodríguez J.J., Montaron M.F., Petry K.G., Aurousseau C., Marinelli M., Premier S., Rougon G., Le Moal M., Abrous D.N. Complex regulation of the expression of the polysialylated form of the neuronal cell adhesion molecule by glucocorticoids in the rat hippocampus. Eur. J. Neurosci. 1998;10:2994–3006. doi: 10.1046/j.1460-9568.1998.00316.x. [DOI] [PubMed] [Google Scholar]
- Romeo R.D., Patel R., Pham L., So V.M. Adolescence and the ontogeny of the hormonal stress response in male and female rats and mice. Neurosci. Biobehav. Rev. 2016;70:206–216. doi: 10.1016/j.neubiorev.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat. Rev. Neurosci. 2008;9:26–35. doi: 10.1038/nrn2285. [DOI] [PubMed] [Google Scholar]
- Salehi B., Cordero M.I., Sandi C. Learning under stress: the inverted-U-shape function revisited. Learn. Mem. 2010;17:522–530. doi: 10.1101/lm.1914110. [DOI] [PubMed] [Google Scholar]
- Sandi C. vol. 4. Wiley Interdiscip Rev Cogn Sci; 2013. pp. 245–261. (Stress and Cognition). [DOI] [PubMed] [Google Scholar]
- Sandi C. Glucocorticoids act on glutamatergic pathways to affect memory processes. Trends Neurosci. 2011;34:165–176. doi: 10.1016/j.tins.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Sandi C. Stress, cognitive impairment and cell adhesion molecules. Nat. Rev. Neurosci. 2004;5:917–930. doi: 10.1038/nrn1555. [DOI] [PubMed] [Google Scholar]
- Sandi C., Cordero M.I., Merino J.J., Kruyt N.D., Regan C.M., Murphy K.J. Neurobiological and endocrine correlates of individual differences in spatial learning ability. Learn. Mem. 2004;11:244–252. doi: 10.1101/lm.73904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C., Loscertales M., Guaza C. Experience‐dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur. J. Neurosci. 1997;9:637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Sandi C., Merino J.J., Cordero M.I., Kruyt N.D., Murphy K.J., Regan C.M. Modulation of hippocampal NCAM polysialylation and spatial memory consolidation by fear conditioning. Biol. Psychiatr. 2003;54:599–607. doi: 10.1016/s0006-3223(03)00182-3. [DOI] [PubMed] [Google Scholar]
- Sandi C., Merino J.J., Cordero M.I., Touyarot K., Venero C. Effects of chronic stress on contextual fear conditioning and the hippocampal expression of the neural cell adhesion molecule, its polysialylation, and L1. Neuroscience. 2001;102:329–339. doi: 10.1016/s0306-4522(00)00484-x. [DOI] [PubMed] [Google Scholar]
- Schenk F. Development of place navigation in rats from weaning to puberty. Behav. Neural. Biol. 1985;43:69–85. doi: 10.1016/s0163-1047(85)91510-9. [DOI] [PubMed] [Google Scholar]
- Sheth C., McGlade E., Yurgelun-Todd D. Chronic stress in adolescents and its neurobiological and psychopathological consequences: an RDoC perspective. Chronic Stress. 2017;1 doi: 10.1177/2470547017715645. (Thousand Oaks) 247054701771564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Sterlemann V., Rammes G., Wolf M., Liebl C., Ganea K., Müller M.B. Chronic social stress during adolescence induces cognitive impairment in aged mice. Hippocampus. 2010;20:540–549. doi: 10.1002/hipo.20655. [DOI] [PubMed] [Google Scholar]
- Suri D., Veenit V., Sarkar A., Thiagarajan D., Kumar A., Nestler E.J. Early stress evokes age-dependent biphasic changes in hippocampal neurogenesis, BDNF expression, and cognition. Biol. Psychiatr. 2013;73:658–666. doi: 10.1016/j.biopsych.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Rodriguez M., Sandi C. Stress during adolescence increases novelty seeking and risk-taking behavior in male and female rats. Front. Behav. Neurosci. 2011;5:17. doi: 10.3389/fnbeh.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoory M., Guterman A., Richter-Levin G. Exposure to stressors during juvenility disrupts development-related alterations in the PSA-NCAM to NCAM expression ratio: potential relevance for mood and anxiety disorders. Neuropsychopharmacology. 2008;33:378–393. doi: 10.1038/sj.npp.1301397. [DOI] [PubMed] [Google Scholar]
- Tzanoulinou S., García-Mompó C., Castillo-Gómez E., Veenit V., Nacher J., Sandi C. Long-term behavioral programming induced by peripuberty stress in rats is accompanied by GABAergic-related alterations in the Amygdala. PloS One. 2014;9 doi: 10.1371/journal.pone.0094666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzanoulinou S., García-Mompó C., Riccio O., Grosse J., Zanoletti O., Dedousis P. Neuroligin-2 expression in the prefrontal cortex is involved in attention deficits induced by peripubertal stress. Neuropsychopharmacology. 2016;41:751–761. doi: 10.1038/npp.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzanoulinou S., Riccio O., de Boer M.W., Sandi C. Peripubertal stress-induced behavioral changes are associated with altered expression of genes involved in excitation and inhibition in the amygdala. Transl. Psychiatry. 2014;4 doi: 10.1038/tp.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzanoulinou S., Sandi C. The programming of the social brain by stress during childhood and adolescence: from rodents to humans. Curr. Top. Behav. Neurosci. 2017;30:411–429. doi: 10.1007/7854_2015_430. [DOI] [PubMed] [Google Scholar]
- Tzanoulinou S., Sandi C. The programming of the social brain by stress during childhood and adolescence: from rodents to humans. Curr. Top. Behav. Neurosci. 2016:1–19. doi: 10.1007/7854_2015_430. [DOI] [PubMed] [Google Scholar]
- Varbanov H., Dityatev A. Regulation of extrasynaptic signaling by polysialylated NCAM: impact for synaptic plasticity and cognitive functions. Mol. Cell. Neurosci. 2017;81:12–21. doi: 10.1016/j.mcn.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Veenema A.H. Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: what can we learn from animal models? Front. Neuroendocrinol. 2009;30:497–518. doi: 10.1016/j.yfrne.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Veenit V., Cordero M.I., Tzanoulinou S., Sandi C. Increased corticosterone in peripubertal rats leads to long-lasting alterations in social exploration and aggression. Front. Behav. Neurosci. 2013;7:26. doi: 10.3389/fnbeh.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenit V., Riccio O., Sandi C. CRHR1 links peripuberty stress with deficits in social and stress-coping behaviors. J. Psychiatr. Res. 2014;53:1–7. doi: 10.1016/j.jpsychires.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Venero C., Herrero A.I., Touyarot K., Cambon K., López-Fernández M.A., Berezin V. Hippocampal up-regulation of NCAM expression and polysialylation plays a key role on spatial memory. Eur. J. Neurosci. 2006;23:1585–1595. doi: 10.1111/j.1460-9568.2006.04663.x. [DOI] [PubMed] [Google Scholar]
- Venero C., Tilling T., Hermans-Borgmeyer I., Schmidt R., Schachner M., Sandi C. Chronic stress induces opposite changes in the mRNA expression of the cell adhesion molecules NCAM and L1. Neuroscience. 2002;115:1211–1219. doi: 10.1016/s0306-4522(02)00543-2. [DOI] [PubMed] [Google Scholar]
- Walker S.E., Sandi C. Long-term programing of psychopathology-like behaviors in male rats by peripubertal stress depends on individual's glucocorticoid responsiveness to stress. Stress. 2018;21:433–442. doi: 10.1080/10253890.2018.1435639. [DOI] [PubMed] [Google Scholar]
- Walker S.E., Wood T.C., Cash D., Mesquita M., Williams S.C.R., Sandi C. Alterations in brain microstructure in rats that develop abnormal aggression following peripubertal stress. Eur. J. Neurosci. 2018;48:1818–1832. doi: 10.1111/ejn.14061. [DOI] [PubMed] [Google Scholar]
- Walker S.E., Zanoletti O., Guillot de Suduiraut I., Sandi C. Constitutive differences in glucocorticoid responsiveness to stress are related to variation in aggression and anxiety-related behaviors. Psychoneuroendocrinology. 2017;84:1–10. doi: 10.1016/j.psyneuen.2017.06.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.