Abstract
The role of stress in the etiology of depression has been largely reported. In this line, exogenous glucocorticoids are employed to mimic the influence of stress on the development of depression. The N/OFQ-NOP receptor system has been implicated in the modulation of stress and emotional behaviors. In fact, the blockade of NOP receptors induces antidepressant effects and increases resilience to acute stress. This study investigated the effects of the NOP receptor blockade on dexamethasone-treated mice exposed to acute and prolonged swimming stress. Swiss and NOP(+/+) and NOP(−/−) mice were treated with dexamethasone, and the protective effects of the NOP antagonist SB-612111 (10 mg/kg, ip) or imipramine (20 mg/kg, ip) were investigated in three swimming sessions. The re-exposure to swim stress increased immobility time in Swiss and NOP(+/+), but not in NOP(−/−) mice. Acute and repeated dexamethasone administration induced a further increase in the immobility time, and facilitated body weight loss in Swiss mice. Single administration of SB-612111, but not imipramine, prevented swimming stress- and dexamethasone-induced increase in the immobility time. Repeated administrations of SB-612111 prevented the deleterious effects of 5 days of dexamethasone treatment. Imipramine also partially prevented the effects of repeated glucocorticoid administration on the immobility time, but did not affect the body weight loss. NOP(−/−) mice were more resistant than NOP(+/+) mice to inescapable swimming stress, but not dexamethasone-induced increase in the immobility time and body weight loss. In conclusion, the blockade of the NOP receptor facilitates an active stress copying response and attenuates body weight loss due to repeated stress.
Keywords: Nociceptin/orphanin FQ, NOP receptor, SB-612111, Dexamethasone, Forced swimming test, Mouse
Abbreviations: ACTH, adrenocorticotropic hormone; CRF, corticotrophin releasing factor; GR, glucocorticoid receptor; HPA, hypothalamus-pituitary-adrenal axis; LPS, lipopolysaccharide; MR, mineralocorticoid receptor; N/OFQ, nociceptin/orphanin FQ; NOP, nociceptin/orphanin FQ peptide receptor; POMC, opiomelanocortin; SPF, specific pathogen-free
1. Introduction
Stress is a nonspecific response of the organism to any factor, somatic or mental, that compromises the individual's homeostasis (Selye, 1976). The responses to stress may include changes in behavior and physiological functions, which are related to decision making, emotions, learning and memory as well as hormonal and autonomic responses (Joëls and Baram, 2009). Additionally, responses to the same stressors are strikingly different across individuals. In fact, a subject may develop an active coping strategy to a given stressor, showing intentional efforts to minimize physical, psychological or social damage of a stressor associated with real or perceived factors (Russo et al., 2012); this strategy is associated with a resilient phenotype. By contrast, another individual subjected to the same stressor may develop a passive coping strategy that is characterized by avoidance and helpless behavior, and it is related to vulnerability to stress (Southwick et al., 2005; Wood and Bhatnagar, 2015).
Specific brain regions controlling the autonomic nervous system and the hypothalamus-pituitary-adrenal axis (HPA) are sensible to stressors (Ulrich-Lai and Herman, 2009). The activation of sympathetic nervous system is the immediate physiological answer with rapid changes in target organs due to the release of adrenaline and noradrenaline, resulting in a short response duration (Ulrich-Lai and Herman, 2009; Koolhaas et al., 2011). The HPA axis activation starts with the stimulation of neurons in paraventricular nucleus of the hypothalamus, resulting in the secretion of corticotrophin releasing factor (CRF). The pituitary is then stimulated, releasing the adrenocorticotropic hormone (ACTH), which in turn stimulates the secretion of glucocorticoids by the adrenal gland, then regulating the HPA axis through negative feedback (Sapolsky, 2002).
The glucocorticoids are physiologically secreted in a pulsatile and circadian fashion, but stressors are also able to trigger the corticosteroids release that is superimposed on these rhythms (Young et al., 2004). It is widely known that glucocorticoids act through both mineralocorticoid and glucocorticoid receptors (MRs and GRs, respectively). These steroid hormones have high affinity for MRs and, consequently, when circulating hormone levels are low such receptors are mostly occupied (De Kloet et al., 2005). In contrast, glucocorticoids affinity for GRs is ten-fold lower, thus under basal conditions, GRs are only partially occupied. However, the GRs become progressively occupied and stimulated as glucocorticoid levels increase, for example, after stress (Pavlides et al., 1995).
Several studies have provided strong evidence about a relationship between stress and depression, as supported by the observation of HPA axis overactivity, elevated cortisol levels and disrupted cortisol rhythmicity in depressed patients (Ströhle and Holsboer, 2003; De Kloet et al., 2005). Moreover, in the Cushing's syndrome, which is a clinical condition caused by prolonged exposure to high circulating levels of cortisol and/or other glucocorticoids (Barbot et al., 2020), high prevalence of depressive disorders is also reported in these patients (Lin et al., 2020). Focused on the investigation of the physiological mechanisms underlying the relationship between stress and depression, some neurotransmitter systems take place, which include the nociceptin/orphanin FQ (N/OFQ) and its receptor NOP. This peptidergic system has been implicated in the modulation of stress responses and emotional states, including mood and anxiety disorders (Gavioli and Calo’, 2013; Witkin et al., 2014; Gavioli et al., 2019).
N/OFQ is a neuropeptide acting as the endogenous ligand for the NOP receptor, a member of the G protein-coupled receptor family (Meunier et al., 1995; Reinscheid et al., 1995). A growing body of evidence suggests antidepressant effects as a consequence of the NOP receptor blockade. For instance, it is reported that: (a) NOP antagonists are able to induce antidepressant-like effects in rodents as observed in the forced swimming and tail suspension tests (Redrobe et al., 2002; Gavioli et al., 2003, 2004; Rizzi et al., 2007; Witkin et al., 2016; Post et al., 2016); (b) NOP blockade exerted by antagonists is also able to prevent depressive-like behaviors resulted from the administration of E. coli lipopolysaccharide (LPS; Medeiros et al., 2015), chronic mild stress (Vitale et al., 2009, 2017), and uncontrollable electric footshocks (Holanda et al., 2016, 2018); and (c) NOP receptor knockout mice and rats display antidepressant-like phenotype in behavioral despair tests (Gavioli et al., 2003, 2004; Rizzi et al., 2011). Furthermore, a placebo, randomized, double-blind clinical trial showed a trend to reduction of symptoms in depressive patients treated during 8 weeks with BTRX-246040 (Post et al., 2016), a highly potent and selective NOP receptor antagonist (Ferrari et al., 2020). Recently, it has been shown that N/OFQ-NOP receptor system modulates susceptibility/resilience to stress. In fact, Holanda and collaborators (2019) showed that NOP ligands may modulate mouse susceptibility to the development of the helpless phenotype.
Based on previous literature findings and considering the relevance of studying the modulatory role of N/OFQ-NOP receptor system under repeated stressful conditions, this study aimed to investigate the preventive effects of the pharmacological and genetic blockade of the NOP receptor on acute and prolonged exposure to the synthetic glucocorticoid dexamethasone in mice subjected to repeated forced swimming stress.
2. Material and methods
2.1. Animals
All behavioral investigations were conducted according to the ARRIVES guidelines (Kilkenny et al., 2010) and all procedures adopted were humane as possible and complied with the European Communities Council directives (2010/63/E), Italian regulations (D.Lgs, 26/2014), and Brazilian law (n° 11,794/2008). All experimental protocols were approved by Animal Welfare Body of the University of Ferrara, by the Italian Ministry of Health (License 120/2014), and by the Ethic Committees for Animal Use of Federal University of Rio Grande do Norte (Protocol N° 202.069/2019). Male Swiss mice (8–12 weeks old) and male NOP(−/−) and NOP(+/+) mice (8–12 weeks old) were used in this study. Details about the generation of mutant mice have been published previously (Bertorelli et al., 2002; Nishi et al., 1997). In the laboratories at University of Ferrara, NOP(−/−) and NOP(+/+) mice have been backcrossed on the CD-1 strain. NOP(+/+) and NOP(−/−) littermates were obtained by mating NOP(±) mice. The procedure for mouse genotyping was described in details by Holanda et al. (2019). NOP(+/+) and NOP(−/−) mice were housed in a specific pathogen-free (SPF) animal facility, in 425 × 266 × 155 mm polycarbonate cages (Tecniplast, VA, Italy), 3–5 mice/cage, under standard conditions (22 °C, 55% humidity, 12 h light-dark cycle, lights on at 7.00 a.m.) with food (4RF, Mucedola, MI, Italy) and water ad libitum. A mouse red house (Tecniplast, VA, Italy) and nesting materials were present in each cage. Swiss mice were housed in a standard animal facility, maximum 10 mice/polypropylene cage (410 × 340 × 160 mm), under standard conditions (22 ± 2 °C, 12 h light-dark cycle, lights on at 6.00 a.m.) with food (Nuvilab CR, Colombo, PR, Brazil) and water ad libitum. In this study a total number of 62 male Swiss mice and 38 male NOP(+/+) and 36 NOP(−/−) mice were used in the experiments. Animals were used for only one experimental series as described below. Mouse body weight was assessed in the mornings on day 1 and 5 prior to the behavioral tests. The variation of mouse body weight was assessed using the following formula: % mouse body weight = [(body weight_Day 5 - body weight_Day 1)/body weight_Day 1]x100.
2.2. Drugs and treatments
Dexamethasone (Sigma-Aldrich, St. Louis, MO), the NOP antagonist SB-612111 (Tocris Bioscience, Bristol, UK) and the antidepressant imipramine (Novartis Biociências S.A., Sao Paulo, Brazil) were used in this study. Imipramine is a tricyclic antidepressant drug that acts by blocking the reuptake of 5-HT and noradrenaline. Additionally, imipramine has been previously used to reverse the depressogenic-like effects of dexamethasone (Wróbel et al., 2014). Concerning the SB-612111, it is a selective NOP antagonist, which is systemically available (Rizzi et al., 2007). Dexamethasone was solubilized in 100% ethanol and it was stored at −20 °C (stock solution: 2 mg/ml), and administered subcutaneously (sc). The doses of dexamethasone (0.3, 0.07 and 0.01 mg/kg) were selected based on previous studies aimed to investigate the effects of the glucocorticoid in the forced swimming test (Wróbel et al., 2014). A stored aliquot of dexamethasone was freshly solubilized before experiments in saline (NaCl 0.9%) in a final concentration not exceeding 1.5% ethanol (control group was treated with 1.5% ethanol). Stock solutions of SB-612111 were prepared in 100% dimethylsulfoxide (DMSO), stored at −20 °C and dissolved in saline just before the experiments; the final concentration of DMSO did not exceed 1% (control group was treated with 1% DMSO) and administered intraperitoneally (ip) at 10 mg/kg. Previous studies showed the antidepressant-like effects of SB-612111 (10 mg/kg) in mice exposed to distinct stressful conditions (Rizzi et al., 2007; Medeiros et al., 2015; Holanda et al., 2016). Additionally, when administered prior to acute inescapable stress, SB-612111 (10 mg/kg) facilitated an active stress copying strategy in mice (Holanda et al., 2019). Imipramine (20 mg/kg, ip) was solubilized in saline (control group was treated with saline). The dose of imipramine was selected based on a dose-response study developed in mice exposed to the forced swimming test (Kulkarni and Dhir, 2007). All drugs were injected in a volume of 10 ml/kg. Mice were randomly assigned to the groups. Control groups were treated with the same vehicle, route and volume of administration as drug-treated groups. Administrations of dexamethasone (were given 60 min before the forced swimming sessions. Additionally, single and repeated administrations of imipramine and SB-612111 were daily given 15 min before dexamethasone injections.
2.3. Forced swimming stress
The forced swimming stress was performed with modifications as previously described by Porsolt et al. (1977) for rats. The protocol uses repeated swimming sessions, which naturally evokes an increase in the immobility time. The increase in the time that animals spent immobile after re-exposure to swim stress reflects the switch between an active strategy toward a passive response to stress (De Kloet and Molendijk, 2016). Mice were placed individually in glass cylinders (height 18 cm; diameter 17 cm) containing 12 cm of water at 24 ± 1 °C, for three forced swimming sessions: day 1 (training session) - a 15-min session; day 2 (test session) - a 5-min session; day 5 (retest session) - a 5-min session. The immobility time (i.e., the time spent floating in the water without struggling) was manually recorded in seconds by an experienced observer blind to the treatments and genotype conditions. The immobility time registered in each session was relative to the first 5-min of forced swimming. All behavioral tests were performed at the light phase between 7:00 a.m. and 12:00 p.m.
2.4. Experimental design
2.4.1. Effects of single dexamethasone administration prior to swimming training session
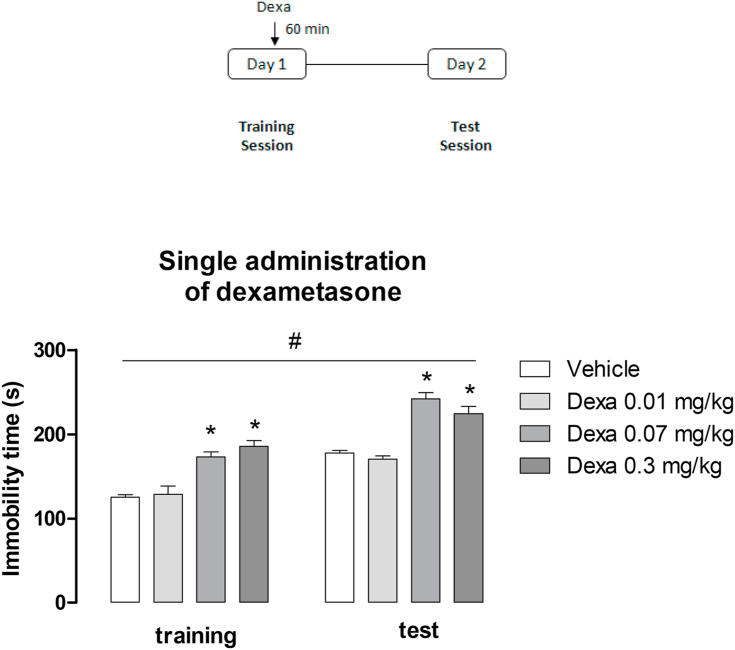
In order to evaluate the effect of a single administration of dexamethasone prior to forced swimming stress, Swiss mice were injected with dexamethasone (0.01, 0.07 and 0.3 mg/kg; sc) or vehicle 60 min before the training session (Fig. 1). Twenty-four hours later mice were re-exposed to a swimming test session. Time spent immobile was registered during the first 5 min of both swimming sessions.
Fig. 1.
- Effects of a single administration of dexamethasone (Dexa, 0.01–0.3 mg/kg, sc) before the training session in mice exposed to forced swimming stress. Data are presented as mean ± SEM of the immobility time in the training and test sessions of 6 mice/group. *P < 0.05 vs. vehicle; #P < 0.05 vs. training session, Repeated measure two-way ANOVA followed by Tukey's multiple comparisons test.
2.4.2. Effects of single imipramine or SB-612111 administration in acutely dexamethasone-treated mice prior to swimming training session
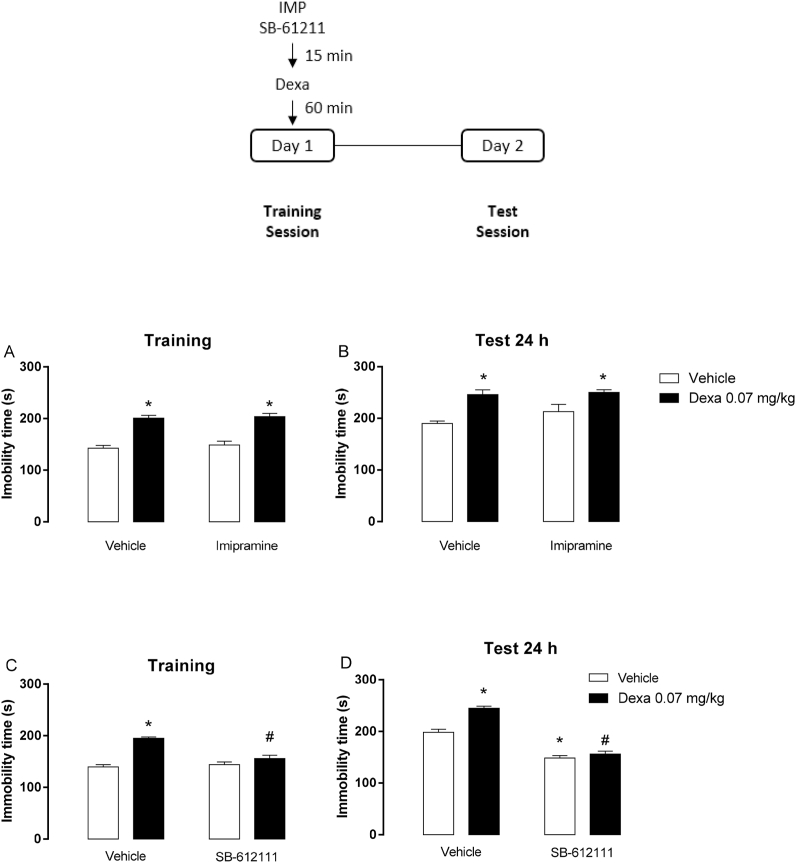
The acute effects of SB-612111 (10 mg/kg, ip) or imipramine (20 mg/kg, ip) before a single dexamethasone (0.07 mg/kg, sc) administration in mice exposed to the forced swimming sessions were investigated. The dose of dexamethasone used was selected based on the behavioral effects observed in the previous dose-response experiment. Two separate series of experiments were performed in this step: 1) animals were treated with imipramine or vehicle 15 min prior to dexamethasone injection; 2) animals were treated with SB-612111 or vehicle 15 min before dexamethasone injection. In both experimental series, 60 min after the treatment with the glucocorticoid, mice were exposed to a swimming training session, and 24 h later, they were subjected to the test session. The immobility time was registered during the first 5 min of each swimming session (Fig. 2).
Fig. 2.
- Effects of the administration of imipramine (20 mg/kg, ip; A,B) or SB-612111 (10 mg/kg, ip; C,D) before a single dexamethasone (Dexa, 0.07 mg/kg, sc) injection in mice exposed to forced swimming sessions. Data are presented as mean ± SEM of the immobility time in the training (A,C) and test (B,D) sessions of 4 (vehicle), 5 (Dexa, SB), 6 (Dexa + SB) or 7 (A,B) mice/group. *P < 0.05 vs. vehicle; #P < 0.05 vs. dexamethasone, two-way ANOVA followed by Tukey's multiple comparisons test.
2.4.3. Effects of subchronic imipramine or SB-612111 administration in repeatedly dexamethasone-treated mice exposed to swimming sessions
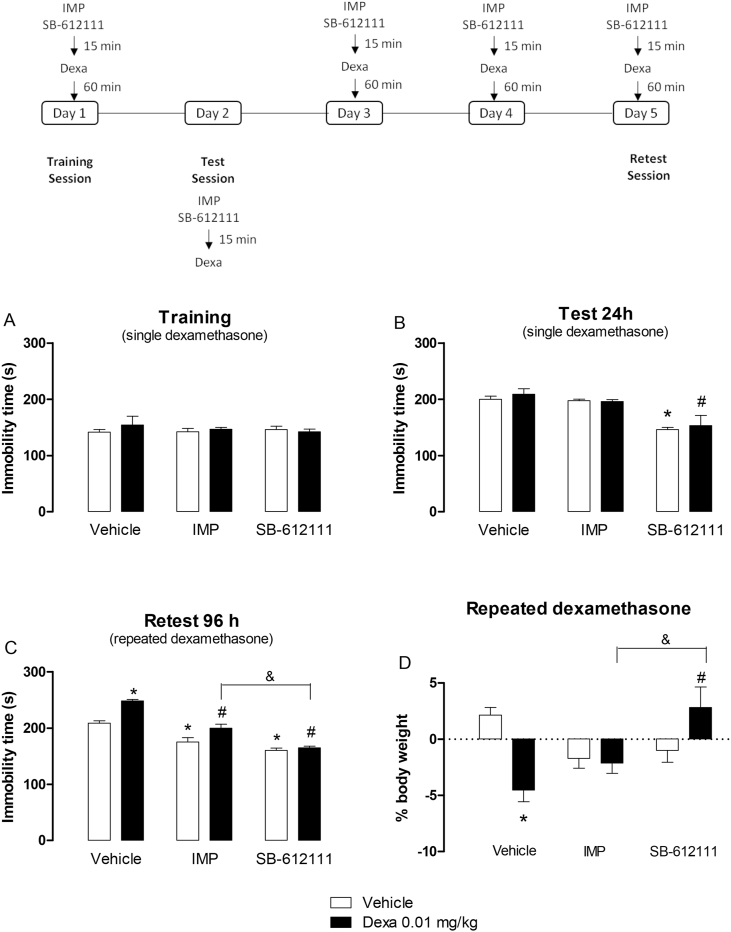
This experimental series investigated the effects of subchronic (5 days) administrations of SB-612111 (10 mg/kg/day, ip), imipramine (20 mg/kg/day, ip) or vehicle before repeated dexamethasone (0.01 mg/kg, sc) injections. A pilot series of experiments indicated that dexamethasone 0.01 mg/kg was able to induce behavioral alterations only after repeated treatment (for details see: Wróbel et al., 2014). On day 1, dexamethasone was injected 60 min before the training session. On day 2, mice were firstly exposed to the forced swimming session, and then they were treated with dexamethasone. On days 3 and 4, dexamethasone was administered sc as previously described, and mice returned to the animal facility. On day 5, mice were again treated with dexamethasone and 60 min later, they were re-exposed to the forced swimming stress (retest session). SB-612111 (10 mg/kg, ip), imipramine (20 mg/kg, ip) or vehicle were injected daily 15 min before dexamethasone administration (Fig. 3). Immobility time was registered during the first 5 min of the training, test and retest swimming sessions.
Fig. 3.
- Effects of the acute (A,B) and repeated (C,D) administrations (5 days) of imipramine (20 mg/kg, ip, IMP) or SB-612111 (10 mg/kg, ip) prior to dexamethasone (Dexa, 0.01 mg/kg, sc)-injection in the immobility time assessed in forced swimming sessions and mouse body weight. Data are presented as mean ± SEM of 7 mice/group. *P < 0.05 vs. its respective vehicle; #P < 0.05 vs. vehicle/Dexa; &P < 0.05 vs. IMP/Dexa, two-way ANOVA followed by Tukey's multiple comparisons test.
2.4.4. Effects of acute and repeated administrations of dexamethasone in NOP(−/−) mice exposed to swimming sessions
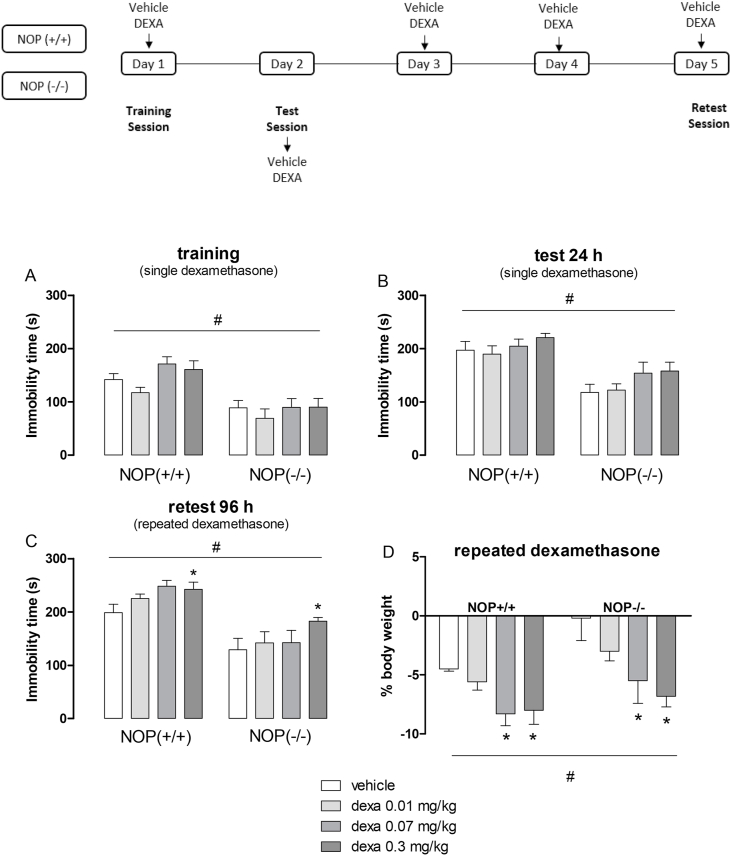
The effects of a single or repeated administrations (for 5 days) of dexamethasone (0.01–0.3 mg/kg, sc) on NOP(−/−) and NOP(+/+) mice exposed to forced swimming sessions were evaluated. On day 1, dexamethasone was injected 60 min before the training session. On day 2, mice were firstly exposed to the forced swimming session, thereafter the animals were treated with dexamethasone. On days 3 and 4, mice were treated with dexamethasone as described above. On day 5, mice were again treated with dexamethasone and 60 min later, they were re-exposed to the forced swimming stress (retest session) (Fig. 4). Immobility time was counted during the first 5-min of each swimming sessions.
Fig. 4.
- Effects of acute (A,B) and repeated (C,D) administrations of dexamethasone (Dexa, 0.01–0.3 mg/kg, sc) on the immobility time of NOP(+/+) and NOP(−/−) mice exposed to forced swimming sessions and mouse body weight. Data are presented as mean ± SEM of 8 (NOP(−/−) Dexa 0.3), 9 (NOP(+/+) vehicle, Dexa 0.01, Dexa 0.3; NOP(−/−) Dexa 0.01, Dexa 0.07), 10 (NOP(−/−) vehicle), or 11 (NOP(+/+) Dexa 0.07) mice/group (A,B) and 5 (NOP(+/+) Dexa 0.01; NOP(−/−) Dexa 0.07), 6 (NOP(+/+) vehicle, Dexa 0.3; NOP(−/−) Dexa 0.01, Dexa 0.3), or 7 (NOP(+/+) Dexa 0.07; NOP(−/−) vehicle) mice/group (C,D). *P < 0.05 vs. vehicle; #P < 0.05 vs. NOP(+/+) mice. Two-way ANOVA followed by Tukey's post hoc test.
2.5. Data analysis
Determination of the animal number to be used in each experimental series was carried out to ensure that the minimal number was used. Sample sizes were calculated performing the power analysis with the G*Power software 3.0.10. Specifically, for some experimental series, the number of animals to be used has been calculated to avoid type I errors with an alpha level <0.05 and type II errors at a statistical power of 0.8. Generally, for Swiss mice, taking account the effect size of 4.0 (based on Holanda et al., 2019) and two-way ANOVA tests, a sample size of six animals/group was calculated. For NOP receptor knockout mice, considering the effect size of 1.8 (based on Gavioli et al., 2003) and ANOVA tests, a sample size of nine mice/group was estimated. However, lower size samples were accepted in experimental series without previous literature support. Data are presented as mean ± SEM of n animals. Data sets were initially checked for normality and homogeneity of variance before using parametric statistical tests. Differences on the immobility time between swimming sessions and dexamethasone treatment or mouse genotype were detected using repeated measures two-way ANOVA (with one between variable – treatment or genotype condition, and one within variable – swimming sessions) followed by Tukey's post hoc test. Differences on the immobility time and % mouse body weight of distinct treatment groups were evaluated through the two-way ANOVA (independent factors: dexamethasone and drug treatments or mouse genotype) followed by Tukey's post hoc test. Differences were considered statistically significant when P < 0.05. For the statistical analysis, the GraphPad Prism software version 6.0 (Graph Pad Software Inc., San Diego, USA) was used.
3. Results
3.1. Effects of single dexamethasone administration prior to swimming training session
The effects of a single administration of dexamethasone (0.01, 0.07 and 0.3 mg/kg, sc) before swimming training session are showed in Fig. 1. The re-exposition to swimming sessions significantly increased the immobility time of mice (Fig. 1; session effect: F (1,40) = 123.75, P < 0.05). The administration of dexamethasone augmented immobility time (Fig. 1; treatment effect: F (3,40) = 50.35, P < 0.05). No significant interaction between factors (i.e., dexamethasone and re-exposition to swimming stress) was observed (P > 0.05). Post-hoc analysis indicated that the higher doses of the glucocorticoid (0.07 and 0.3 mg/kg) significantly increased the immobility time in Swiss mice.
3.2. Effects of single imipramine or SB-612111 administration in acutely dexamethasone-treated mice prior to swimming training session
In two distinct series of experiment, we evaluated the effects of a single administration of imipramine (20 mg/kg, ip) or SB-612111 (10 mg/kg, ip) before dexamethasone (0.07 mg/kg, sc) injection in the immobility time assessed in the swimming training session (Fig. 2). Two-way ANOVA revealed a dexamethasone effect in the training (Fig. 2A; F (1,24) = 82,52, P < 0.05) and test (Fig. 2B: F (1,24) = 25,88, P < 0.05) sessions. Neither imipramine treatment nor an interaction between factors was significant. Post-hoc analysis showed that dexamethasone increased per se the immobility time in both training and test sessions compared to vehicle (Fig. 2A and B; P < 0.05). Notably, the systemic administration of imipramine, given before the training session, did not change the immobility time during both swimming sessions.
Concerning the effects of the NOP antagonist, two-way ANOVA revealed an interaction between the independent factors (NOP antagonist and dexamethasone) in the training (Fig. 2C; F (1,16) = 16.97, P < 0.05) and test (Fig. 2D; F (1,16) = 13.93, P < 0.05) sessions. Dexamethasone increased per se the immobility time in both swimming sessions (Fig. 2C and D; P < 0.05). The systemic administration of SB-612111 per se prevented the increase in the immobility time during the test session (Fig. 2D; P < 0.05). However, one single administration of SB-612111 before the training session blocked dexamethasone-induced increase in the immobility time in both swimming sessions (Fig. 2C and D; P < 0.05).
3.3. Effects of subchronic imipramine or SB-612111 administration in repeatedly dexamethasone-treated mice exposed to swimming sessions
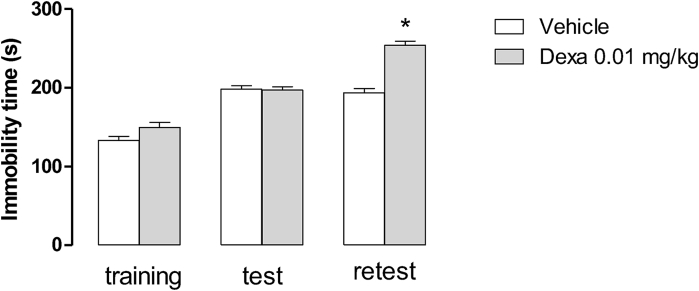
In a pilot set of experiments, the effects of repeated administration of dexamethasone (0.01 mg/kg, sc, for 5 consecutive days) were assessed in Swiss mice subjected to swimming stress. At this dose, dexamethasone further increased the immobility time only in the retest session (Fig. 1S, P < 0.05) while it was inactive during the training and test sessions (Fig. 1S, P > 0.05).
The effects of the tricyclic antidepressant imipramine (20 mg/kg, ip, 5 days) or the NOP antagonist SB-612111 (10 mg/kg, ip, 5 days) prior to repeated dexamethasone injections were investigated in this study (Fig. 3A–D). No significant effects for dexamethasone, imipramine or SB-612111 treatments were observed in the training session (Fig. 3A; P > 0.05). Two-way ANOVA revealed a significant effect for the treatment with imipramine or SB-612111 in the test session (Fig. 3B, F(2,36) = 22.26, P < 0.05). It is noteworthy to mention that the acute administration of SB-612111 (on day 1) also prevented the increase in the immobility time induced by an inescapable stressful situation in the test session assessed on day 2 (Fig. 3B, P < 0,05). In the retest session, a significant effect for the treatment with imipramine, NOP antagonist (Fig. 3C; F (2,36) = 84.59, P < 0.05), dexamethasone (Fig. 3C; F (1,36) = 29.73, P < 0.05), and an interaction between independent factors (drug treatments and dexamethasone; Fig. 3C; F (2,36) = 5.79, P < 0.05) was observed in the immobility time. Dexamethasone (0.01 mg/kg) increased immobility time exclusively in the retest session after 5 consecutive days of treatment (Fig. 3C, P > 0.05). Repeated administrations of imipramine or SB-612111 prevented dexamethasone and vehicle-induced increase in the immobility time in the retest session (Fig. 3C, P < 0.05 for both). The effects of imipramine and SB-612111 on vehicle group are related to the actions of these compounds against the increase in the immobility time induced by repeated forced swimming sessions. Interesting, SB-612111 also significantly reduced immobility time in dexamethasone-treated mice compared to imipramine (Fig. 3C, P < 0.05).
Regarding the mouse body weight, two-way ANOVA revealed an interaction effect between factors (i.e., treatment with imipramine or SB-612111 and dexamethasone) (Fig. 3D; F (2,36) = 10.98, P < 0.05). Repeated administrations of dexamethasone, but not imipramine or SB-612111, induced a statistically significant reduction of mouse body weight compared to vehicle-treated animals (Fig. 3D). Additionally, the treatment with SB-612111, but not imipramine, prevented dexamethasone-induced body weight loss in Swiss mice (Fig. 3D). The body weight (g) of mice pretreated with imipramine (IMP 20 mg/kg, ip) or SB-612111 (10 mg/kg, ip) 15 min before the administration of dexamethasone (0.01 mg/kg, sc) during 5 days from the start (day 1) and at the end (day 5) of the experimental series is detailed in Table 1S.
3.4. Effects of acute and repeated administrations of dexamethasone in NOP(−/−) mice exposed to swimming sessions
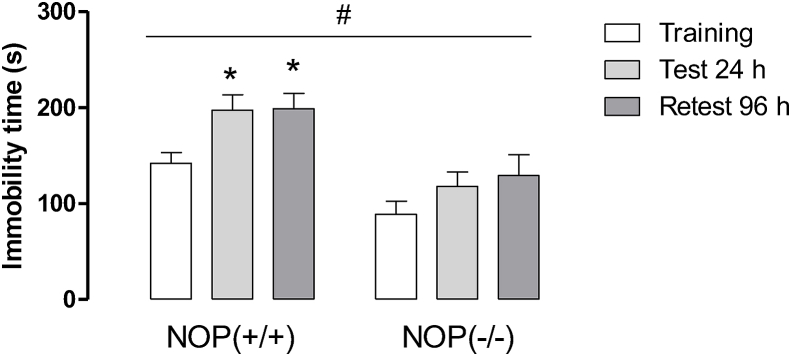
First of all, the behavioral phenotype of NOP(−/−) mice was investigated after repeated exposure to forced swimming sessions (Fig. 2S). Repeated measures two-way ANOVA revealed the effects of genotype and time as source of variation (Fig. 2S, time factor: F (2,45) = 5.52, P < 0.05; phenotype factor: F (1,45) = 27.11, P < 0.05). Knockout mice for the NOP receptor displayed a significant reduction in the immobility time compared to wild-type animals. Additionally, the re-exposition to swimming sessions increased immobility time in NOP(+/+) mice, but not in NOP(−/−) mice.
Fig. 4 illustrates the effects of acute and repeated administrations of dexamethasone in NOP(+/+) and NOP(−/−) mice. Two-way ANOVA indicated genotype as source of variation in the training (Fig. 4A, genotype factor: F (1,66) = 38.03, P < 0.05), test (Fig. 4B, genotype factor: F (1,66) = 37.48, P < 0.05) and retest (Fig. 4C; genotype factor: F (1,40) = 46.03, P < 0.05) sessions, and an effect for dexamethasone treatment only in the retest session (Fig. 4C; treatment factor: F (3,40) = 3.02, P < 0.05). In all swimming sessions, NOP(−/−) mice displayed a significant reduction in the immobility time compared with NOP(+/+) mice. Additionally, unlike Swiss mice, a single administration of dexamethasone, at the doses used in our study, did not affect the immobility time of NOP(+/+) and NOP(−/−) mice. However, repeated injections of the glucocorticoid increased immobility time in NOP(+/+) and NOP(−/−) mice at the highest dose tested (i.e., 0.3 mg/kg). Concerning mouse body weight, two-way ANOVA revealed genotype (Fig. 4D; F (1,40) = 46.11, P < 0.05) and dexamethasone treatment (Fig. 4D; F (3,40) = 3.19, P < 0.05) as source of variation. As compared to wild-type animals, the genetic blockade of NOP receptor protects mice from body weight loss due to the swimming stress. Moreover, repeated administrations of dexamethasone at higher doses (0.07 and 0.3 mg/kg) significantly reduced mouse body weight compared to vehicle of both genotypes. The body weight of NOP(+/+) and NOP(−/−) mice from the start (Day 1) and at the end (Day 5) of the experimental series is summarized in Table 2S.
4. Discussion
Previous study showed a clear relationship between the N/OFQ-NOP receptor system and resilience to stress (Holanda et al., 2019). In order to extend available information about the effects of the blockade of NOP receptor signaling under exposition to stressful stimuli, we evaluated herein the consequences of pharmacological (selective antagonist) or genetic blockade (knockout mice) of NOP receptors on dexamethasone-treated mice exposed to swimming stress. Then, we demonstrated that blockade of the NOP receptor signaling facilitates the acquisition of an active behavioral response due to repeated swimming stress and exogenous dexamethasone.
In this study, re-exposition to swim stress increased the time that mice spent immobile in the water. Porsolt et al. (1977) proposed two swimming sessions for the screening of antidepressant drugs in rats: a first 15-min training session and a second 5-min test after one day. It is widely showed that rodents develop immobility much faster during the second session. The frequency/duration of immobility increases with the time spent in the water (Lino-de-Oliveira et al., 2005) and over several re-test sessions with a parallel decrease in the latency to immobility (Mezadri et al., 2011). This increase in immobility time across re-exposures might reflect the switch between an active strategy toward a passive response to stress, and it allows us to examine the mechanistic underpinning of coping with inescapable stressors (Molendijk and De Kloet, 2019). Additionally, the repeated swimming session protocol has been previously employed in mice for investigating the antidepressant effects of NOP ligands (Gavioli et al., 2003, 2004; Asth et al., 2016; Holanda et al. 2018, 2019).
Our findings showed that the increase in the immobility time due to the re-exposition to swim sessions was: i) prevented by the administration of the NOP antagonist SB-612111 after single or repeated administrations, and ii) no longer evident in NOP(−/−) mice. Holanda et al. (2019) showed that the administration of NOP antagonist SB-612111 before inescapable stress reduced the development of helpless mice, and slowed up the acquisition of an immobile posture when mice were forced to swim. Similar findings have also been reported for NOP(−/−) mice and rats in the swimming stress (Gavioli et al., 2003; Rizzi et al., 2011) and for NOP(−/−) mice subjected to inescapable footshock stress (Holanda et al., 2019). Altogether, our findings and the aforementioned studies convincingly support the hypothesis that pharmacological and genetic blockade of NOP receptors facilitate the acquisition of an active copying strategy, which is related to a resilient phenotype (Russo et al., 2012).
The administration of dexamethasone was employed in this study to mimic a stressful stimulus which is able to evoke depressive-like behaviors in mice (Wróbel et al., 2014). Dexamethasone is more potent, longer lasting, and more selective for the GR than the endogenous hormone corticosterone (Czock et al., 2005) since natural corticosteroids have higher affinity for MR than GR at low plasma levels (De Kloet et al., 2005). As endogenous corticosteroid levels increase, for instance, after stress, GR receptors become more occupied/stimulated by these hormones (Pavlides et al., 1995). It is described that an imbalance in MR x GR activation may induce HPA axis dysregulation, leading to impaired behavioral adaptation which in turn enhances the susceptibility to stress-related mental disorders (De Kloet, 2014). In this sense, dexamethasone facilitates an imbalance of MR x GR activation, thus promptly contributing to the endocrine scenario of HPA axis dysregulation reported in depressive patients (Baes et al., 2014; Juruena et al., 2015).
We showed that a single administration of dexamethasone, in a dose-dependent manner, further increased the immobility time in mice. In addition, repeated administrations of lower doses of dexamethasone, which were inactive as acute treatment, further increased the mouse immobility time in the retest session. These results are in line with literature findings demonstrating that dexamethasone increased immobility time in mice in a dose- and time-depended manner (Wróbel et al., 2014). It is important to mention that NOP(+/+) mice, which were backcrossed in the CD-1 strain, naturally display lower propensity to immobility time increase in response to forced swimming in comparison with Swiss mice. Moreover, repeated administrations of dexamethasone, only at the higher dose tested (i.e., 0.3 mg/kg), increased the time that NOP(+/+) mice spent immobile in the water. Previous findings from our research group have shown CD-1 mice more resistant to stress than Swiss mice (Holanda et al., 2019). Thus, in the learned helplessness model, a 2 consecutive inescapable footshock sessions were required for 50% of CD-1 mice display a helpless phenotype, while only one session was enough for Swiss mice (Holanda et al., 2019). The strain-dependent resistance to stress reinforces the importance to test the depressogenic-like effects of dexamethasone in dose-response studies.
Our findings showed that single and repeated administrations of the NOP antagonist SB-612111, prior to dexamethasone, inhibited the glucocorticoid effects in the training, test and retest swimming sessions. However, the treatment with imipramine for 5 days significantly prevented the effects of dexamethasone only in the retest session. We hypothesized that such effect is possibly due to the elevated mean half-life of imipramine (i.e., t1/2 = 19 h), which may contribute to cumulative effects after repeated administrations. No information about the pharmacokinetic profile of SB-612111 is still available in the literature, but an antidepressant with a t1/2 similar to SB-612111 would be ideal to a fair comparison of effects. Dexamethasone also facilitated body weight loss while a protective effect was observed after repeated administrations of SB-612111, but not imipramine. A significant positive correlation between weight loss and plasma and cerebrospinal fluid cortisol levels were also observed in humans (Casper et al., 1987), thus reinforcing the relevance of measuring the body weight in dexamethasone-treated mice. Previously, SB-6121111 did not significantly affect food intake in feeding freely and in food-deprived mice (Rizzi et al., 2007), but when injected in Long Evans rats eating a high fat/high sugar diet, SB-612111, dose-dependently reduced food intake and body weight gain (Witkin et al., 2014). Collectively, the present study demonstrates for the first time the beneficial effects of a NOP antagonist to protect from behavioral and physiological responses to repeated stress, and brings new evidence about the effectiveness of NOP antagonists compared to conventional antidepressants in promoting the development of a resilient phenotype.
Evidence from the literature showed that knockout mice for the NOP receptor under immunological stress promptly restored circulating corticosterone plasma levels to baseline when compared to NOP(+/+) mice (Mallimo et al., 2010). In this study, NOP(−/−) mice were more resistant than wild-type animals to the effects of repeated inescapable swimming stress. In contrast to the effects of SB-612111 that promoted a temporary blockade of the NOP receptor in an adult animal, the genetic blockade of NOP receptor did not protect animals from the development of dexamethasone-induced increase in the immobility time. It is relevant to mention that NOP(+/+) mice displayed a similar sensitivity to dexamethasone as NOP(−/−) mice (i.e., only at the highest dose increased immobility time). A possible explanation for these differences in response to dexamethasone can be due to the fact that NOP(−/−) mice developed under the depletion of the NOP receptor signaling, thus adaptive processes took place to allow these individuals surviving.
The increased resilience to depression due to the blockade of the NOP receptor can be explained considering the close relationship between N/OFQ – NOP receptor system and the modulation of adaptive responses to stress. Firstly, the N/OFQ-NOP receptor system is expressed in the HPA axis and limbic structures involved in the modulation of emotions and stress responses (Boom et al., 1999; Mollereau and Mouledous, 2000) as well as stressful situations facilitate N/OFQ release (Devine et al., 2003; Nativio et al., 2012). Furthermore, an increase in the NOP mRNA was also observed in the amygdala and periventricular nucleus of hypothalamus under acute stress (Green et al., 2009; Ciccocioppo et al., 2014). In humans, positron emission tomography studies conducted with a NOP receptor radiotracer have demonstrated that binding to NOP receptors is increased in women with severe posttraumatic stress disorder symptoms after sexual violence (Narendran et al., 2019) and following an acute elevation in plasma cortisol levels (Flanigan et al., 2020). Therefore, human and animals studies suggest that there is an increase in NOP receptor signaling in response to stressful events. In agreement with this hypothesis, alcohol preferring rats in which NOP receptor is upregulated show a hyperactive stress response and depressive-like phenotype (Ciccocioppo et al., 1999; Economidou et al., 2008). Further studies are required to fully understand the possible alterations in the peptide expression and its receptor under chronic stress.
One of the consequences of the stress-induced increase in the NOP receptor signaling is the further activation of the HPA axis. A growing body of evidence showed that central injections of N/OFQ in naïve mice increase corticosterone and ACTH plasma levels (Devine et al., 2001; Fernandez et al., 2004; Broccardo et al., 2005; Vitale et al., 2006; Filippetti et al., 2007; Green et al., 2007), CRF mRNA in paraventricular hypothalamic nucleus and pro-opiomelanocortin (POMC) mRNA in the anterior pituitary (Leggett et al., 2006). On the other hand, administration of NOP antagonists did not change glucocorticoid and ACTH plasma levels or CRF and POMC expression levels in naïve rats (Leggett et al., 2006; Vitale et al., 2009; Zhang et al., 2015). However, the blockade of the NOP receptor exerted by specific antagonists restored circulating corticosterone plasma levels to baseline in stressed rodents (Leggett et al., 2009; Vitale et al., 2009, 2017; Delaney et al., 2012; Zhang et al., 2015). It is plausible to assume that stress, via glucocorticoids, increases the N/OFQ (Devine et al., 2003; Nativio et al., 2012) and NOP receptor expression (Green et al., 2009; Ciccocioppo et al., 2014) in rodents, leading to an enhancement of the endogenous N/OFQ system. This could also partially explain the beneficial effects of the NOP antagonist SB-612111 in preventing the dexamethasone- and swimming stress-induced depressive-like behaviors in mice. Therefore, glucocorticoids might play a role as mediators of the effect of stress in the expression of N/OFQ peptide and its receptor, as previously suggested by Nativio et al. (2012). This hypothesis is in part supported by the existence of several glucocorticoid-responding elements on the ppN/OFQ human gene (Xie et al., 1999). Studies aimed to investigate the presence of glucocorticoid-responding elements in the NOP receptor gene are still needed.
Contrasting to the pro-stress effects of the N/OFQ system herein presented, a large amount of evidence supports anti-stress properties of N/OFQ. This current idea comes from the ability of N/OFQ to act as a functional antagonist for the CRF. It has been shown that N/OFQ within the bed nucleus of stria terminalis and the central amygdala prevents the anxiogenic (Rodi et al., 2008; Uchiyama et al., 2008; Ciccocioppo et al., 2014; Filaferro et al., 2014) and anorectic effects of CRF and stress (Ciccocioppo et al., 2001; Ciccocioppo et al., 2003). Furthermore, ex vivo electrophysiological recording from brain slices showed that the treatment with N/OFQ avoids the CRF ability to stimulate GABAergic neurotransmission in the central amygdala (Cruz et al., 2012; Ciccocioppo et al., 2014). In summary, the contrasting actions of N/OFQ on stress (i.e., anti-stress x pro-stress profile) can be explained based on the effects of N/OFQ in activating: i) the HPA axis, which mimics a stress-like response, and ii) the extrahypothalamic sites, where NOP receptor activation evokes anti-stress properties (at least under acute stress). It is also possible to suppose that the N/OFQ-NOP receptor system is part of the stress-coping mechanisms associated with physiological responses to stress (for a review see: Witkin et al., 2014).
Locomotor activity and memory are two important confound variables for the forced swimming task. Despite the pharmacological intervention in systems (i.e., glucocorticoid and N/OFQ) that in some extension influence memory and locomotor processes, it is unlike that these factors are biasing our findings. Firstly, it is very well established that prolonged use of glucocorticoid (in higher doses) induces impairment in learning and memory process, mainly in declarative and working memory (for review: Brown, 2009). In this study, acute or repeated administration of dexamethasone in a dose-dependent manner facilitated, instead of impaired, acquisition and retention of the immobility posture in the swimming stress. These facilitatory effects of dexamethasone (and other glucocorticoids) on memory are relevant to investigate the mechanisms of stress coping strategies (De Kloet and Molendijk, 2016). Additionally, NOP antagonists did not affect the acquisition of a given task, as assessed in the Morris Water Maze (Redrobe et al., 2000; Kuzmin et al., 2009), and inhibitory avoidance (Liu et al., 2007). By contrast, NOP(−/−) mice displayed a promnestic profile, by showing improvement of acquisition in spatial memory tasks (Manabe et al., 1998; Nagai et al., 2007), and in retention of inhibitory avoidance (Manabe et al., 1998) and contextual memory (Mamiya et al., 2003), which could theoretically be a confound variable to the present study. Nevertheless, both NOP(−/−) mice and the treatment with SB-612111 counteracted the stress stimuli-induced increase in the immobility time. Ultimately, these actions could be interpreted as impairment, not improvement, on the memory acquisition of the most natural response (i.e., increase in the immobility time) when mice were forced to swim. Finally, as showed previously, neither the blockade of NOP receptor nor dexamethasone influences locomotor activity and muscle strength of rodents (Gavioli et al., 2007; Rizzi et al., 2007, 2011; Arcuri et al., 2016; De Souza et al., 2019; Silva et al., 2019). Altogether, the reduction in the immobility time induced by pharmacological and genetic blockade of the NOP receptor are not possibly influenced by confound variables such as memory and locomotion. In conclusion, our study clearly demonstrates that pharmacological and genetic blockade of NOP receptors acts on stress coping responses disturbing the switch between active to passive strategies induced by dexamethasone and swimming stress. In fact, acute and subchronic treatment with the NOP antagonist SB-612111 attenuated the consequences to dexamethasone treatment and repeated swimming stress in mice. Similarly, NOP(−/−) mice seem to be protected from the swimming stress-induced increase in the immobility time and body weight loss. Finally, this study provides further evidence about the potential therapeutic effects of NOP antagonists as a preemptive treatment in patients with severe risk factors for depression.
CRediT authorship contribution statement
Victor A.D. Holanda: Conceptualization, Data curation, Formal analysis, Methodology, Writing - review & editing. Matheus C. Oliveira: Data curation, Methodology. Edilson D. Da Silva Junior: Methodology, Writing - review & editing. Girolamo Calo': Methodology, Writing - review & editing. Chiara Ruzza: Methodology, Writing - review & editing. Elaine C. Gavioli: Conceptualization, Data curation, Formal analysis, Methodology, Writing - review & editing.
Declaration of competing interest
None to declare.
Acknowledgments
This work was supported by funds from the Brazilian National Council Research (CNPq; Grant No. 303072/2019-9, and No. 401837/2016-5 to ECG), from the Italian Ministry of Research (PRIN 2015WX8Y5B grant to GC), and from the University of Ferrara (FAR grant to GC). VADH was recipient of PhD scholarship from CAPES Foundation - Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (Finance Code 001).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2020.100255.
Appendix A.
Fig. 1S - Effects of single (training and test sessions) and repeated (retest sessions) administrations of dexamethasone (Dexa, 0.01 mg/kg, sc) before the swimming sessions in mice. Data are presented as mean ± SEM of 5 mice/group. *P < 0.05 vs. vehicle, Repeated measure two-way ANOVA followed by Tukey's multiple comparisons test.
Fig. 2S – Behavioral phenotype of NOP(+/+) and NOP(−/−) mice exposed to repeated forced swimming sessions. Data are presented as mean ± SEM of the immobility time in the training and test sessions of 9 NOP(+/+) or 10 NOP(−/−) mice/group and retest session of 6 NOP(+/+) or 7 NOP(−/−) mice/group. *P < 0.05 vs. training; #P < 0.05 vs. NOP(−/−) mice. Repeated measure two-way ANOVA followed by Tukey's multiple comparisons test.
Table 1S.
Body weight (g) of mice pretreated with imipramine (IMP; 20 mg/kg, ip) or SB-612111 (Dexa; 10 mg/kg, ip) 15 min before the administration of dexamethasone (0.01 mg/kg, sc) during 5 days from the start (day 1) and at the end (day 5) of the experimental series.
| Drug treatment | Day 1 Body weight (g) |
Day 5 Body weight (g) |
Sample size |
|---|---|---|---|
| vehicle + vehicle | 40.7 ± 1.1 | 41.6 ± 1.0 | 7 |
| vehicle + IMP 20 mg/kg | 39.0 ± 2.0 | 38.3 ± 1.8 | 7 |
| vehicle + SB-612111 10 mg/kg | 40.6 ± 2.8 | 40.1 ± 2.8 | 7 |
| Dexa 0.01 mg/kg + vehicle | 38.1 ± 2.1 | 36.4 ± 2.1 | 7 |
| Dexa 0.01 mg/kg + IMP 20 mg/kg | 42.3 ± 2.4 | 41.4 ± 2.5 | 7 |
| Dexa 0.01 mg/kg + SB-612111 10 mg/kg | 40.1 ± 2.9 | 41.1 ± 2.7 | 7 |
Data are presented as mean ± SEM.
Table 2S.
Body weight (g) of NOP(+/+) and NOP (−/−) mice from the start (day 1) and at the end (day 5) of the experimental series.
| Genotype and drug treatment | Day 1 Body weight (g) |
Day 5 Body weight (g) |
Sample size |
|---|---|---|---|
| NOP(+/+) vehicle | 48.3 ± 1.8 | 46.2 ± 1.7 | 6 |
| NOP(+/+) Dexa 0.01 mg/kg | 46.4 ± 2.7 | 43.8 ± 2.4 | 5 |
| NOP(+/+) Dexa 0.07 mg/kg | 49.0 ± 1.6 | 45.0 ± 1.2 | 7 |
| NOP(+/+) Dexa 0.3 mg/kg | 47.2 ± 2.0 | 43.5 ± 2.3 | 6 |
| NOP(−/−) vehicle | 43.9 ± 1.0 | 43.7 ± 0.7 | 7 |
| NOP(−/−) Dexa 0.01 mg/kg | 40.8 ± 1.9 | 39.7 ± 2.1 | 6 |
| NOP(−/−) Dexa 0.07 mg/kg | 42.0 ± 1.9 | 39.7 ± 1.9 | 6 |
| NOP(−/−) Dexa 0.3 mg/kg | 44.2 ± 1.8 | 41.2 ± 1.7 | 6 |
Data are presented as mean ± SEM.
Appendix B. Supplementary data
The following is the Supplementary data to this article:
References
- Arcuri L., Viaro R., Bido S., Longo F., Calcagno M., Fernagut P.O., Zaveri N.T., Calò G., Bezard E., Morari M. Genetic and pharmacological evidence that endogenous nociceptin/orphanin FQ contributes to dopamine cell loss in Parkinson's disease. Neurobiol. Dis. 2016;89:55–64. doi: 10.1016/j.nbd.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asth L., Ruzza C., Malfacini D., Medeiros I., Guerrini R., Zaveri N.T., Gavioli E.C., Calo G. Beta-arrestin 2 rather than G protein efficacy determines the anxiolytic-versus antidepressant-like effects of nociceptin/orphanin FQ receptor ligands. Neuropharmacology. 2016;105:434–442. doi: 10.1016/j.neuropharm.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baes C.V., Martins C.M., Tofoli S.M., Juruena M.F. Early life stress in depressive patients: HPA Axis response to GR and MR agonist. Front. Psychiatr. 2014;5:2. doi: 10.3389/fpsyt.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbot M., Zilio M., Scaroni C. Cushing's syndrome: overview of clinical presentation, diagnostic tools and complications. Best Pract. Res. Clin. Endocrinol. Metabol. 2020;34(2):101380. doi: 10.1016/j.beem.2020.101380. [DOI] [PubMed] [Google Scholar]
- Bertorelli R., Bastia E., Citterio F., Corradini L., Forlani A., Ongini E. Lack of the nociceptin receptor does not affect acute or chronic nociception in mice. Peptides. 2002;23(9):1589‐1596. doi: 10.1016/s0196-9781(02)00102-x. [DOI] [PubMed] [Google Scholar]
- Boom A., Mollereau C., Meunier J.C., Vassart G., Parmentier M., Vanderhaeghen J.J., Schiffmann S.N. Distribution of the nociceptin and nocistatin precursor transcript in the mouse central nervous system. Neuroscience. 1999;91(3):991–1007. doi: 10.1016/s0306-4522(98)00683-6. [DOI] [PubMed] [Google Scholar]
- Broccardo M., Scaccianoce S., Del Bianco P., Agostini S., Petrella C., Improta G. Nociceptin/orphanin FQ-induced delay in gastric emptying: role of central corticotropin-releasing factor and glucocorticoid receptors. Neuro Gastroenterol. Motil. 2005;17(6):871‐877. doi: 10.1111/j.1365-2982.2005.00717.x. [DOI] [PubMed] [Google Scholar]
- Brown E.S. Effects of glucocorticoids on mood, memory, and the hippocampus. Treatment and preventive therapy. Ann. N. Y. Acad. Sci. 2009;1179:41–55. doi: 10.1111/j.1749-6632.2009.04981.x. [DOI] [PubMed] [Google Scholar]
- Casper R.C., Swann A.C., Stokes P.E., Chang S., Katz M.M., Garver D. Weight loss, cortisol levels, and dexamethasone suppression in major depressive disorder. Acta Psychiatr. Scand. 1987;75(3):243–250. doi: 10.1111/j.1600-0447.1987.tb02784.x. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R., Martin-Fardon R., Weiss F., Mass i M. Nociceptin/orphanin FQ inhibits stress- and CRF-induced anorexia in rats. Neuroreport. 2001;12(6):1145–1149. doi: 10.1097/00001756-200105080-00019. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R., de Guglielmo G., Hansson A.C., Ubaldi M., Kallupi M., Cruz M.T., Oleata C.S., Heilig M., Roberto M. Restraint stress alters nociceptin/orphanin FQ and CRF systems in the rat central amygdala: significance for anxiety-like behaviors. J. Neurosci. 2014;34(2):363–372. doi: 10.1523/JNEUROSCI.2400-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R., Panocka I., Froldi R., Colombo G., Gessa G.L., Massi M. Antidepressant-like effect of ethanol revealed in the forced swimming test in Sardinian alcohol-preferring rats. Psychopharmacology (Berlin) 1999;144(2):151–157. doi: 10.1007/s002130050988. [DOI] [PubMed] [Google Scholar]
- Cruz M.T., Herman M.A., Kallupi M., Roberto M. Nociceptin/orphanin FQ blockade of corticotropin-releasing factor-induced gamma-aminobutyric acid release in central amygdala is enhanced after chronic ethanol exposure. Biol. Psychiatr. 2012;71:666–676. doi: 10.1016/j.biopsych.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czock D., Keller F., Rasche F.M., Häussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin. Pharmacokinet. 2005;44(1):61‐98. doi: 10.2165/00003088-200544010-00003. [DOI] [PubMed] [Google Scholar]
- De Kloet E.R. From receptor balance to rational glucocorticoid therapy. Endocrinology. 2014;155(8):2754–2769. doi: 10.1210/en.2014-1048. [DOI] [PubMed] [Google Scholar]
- De Kloet E.R., Joëls M., Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- De Kloet E.R., Molendijk M.L. Neural plasticity; 2016. Coping with the Forced Swim Stressor: towards Understanding an Adaptive Mechanism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza I.B.M.B., Costa L.R.F., Tiago P.R.F., Cagni F.C., Lima R.H., Silva Junior E.D., Gavioli E.C. Venlafaxine and nortriptyline reverse acute dexamethasone-induced depressive-like behaviors in male and female mice. Exp. Clin. Psychopharmacol. 2019;27(5):433–442. doi: 10.1037/pha0000263. [DOI] [PubMed] [Google Scholar]
- Delaney G., Dawe K.L., Hogan R., Hunjan T., Roper J., Hazell G., Lolait S.J., Fulford A.J. Role of nociceptin/orphanin FQ and NOP receptors in the response to acute and repeated restraint stress in rats. J. Neuroendocrinol. 2012;24(12):1527–1541. doi: 10.1111/j.1365-2826.2012.02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine D.P., Hoversten M.T., Ueda Y., Akil H. Nociceptin/orphanin FQ content is decreased in forebrain neurones during acute stress. J. Neuroendocrinol. 2003;15(1):69–74. doi: 10.1046/j.1365-2826.2003.00868.x. [DOI] [PubMed] [Google Scholar]
- Devine D.P., Watson S.J., Akil H. Nociceptin/orphanin FQ regulates neuroendocrine function of the limbic–hypothalamic–pituitary–adrenal axis. Neuroscience. 2001;102(3):541–553. doi: 10.1016/s0306-4522(00)00517-0. [DOI] [PubMed] [Google Scholar]
- Economidou D., Hansson A.C., Weiss F., Terasmaa A., Sommer W.H., Cippitelli A., Fedeli A., Martin-Fardon R., Massi M., Ciccocioppo R., Heilig M. Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol. Psychiatr. 2008;64(3):211–218. doi: 10.1016/j.biopsych.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez F., Misilmeri M.A., Felger J.C., Devine D.P. Nociceptin/orphanin FQ increases anxiety-related behavior and circulating levels of corticosterone during neophobic tests of anxiety. Neuropsychopharmacology. 2004;29:59–71. doi: 10.1038/sj.npp.1300308. [DOI] [PubMed] [Google Scholar]
- Ferrari F., Rizzo S., Ruzza C., Calo G. Detailed in vitro pharmacological characterization of the clinically viable nociceptin/orphanin FQ peptide receptor antagonist BTRX-246040. J. Pharmacol. Exp. Therapeut. 2020;373(1):34–43. doi: 10.1124/jpet.119.262865. [DOI] [PubMed] [Google Scholar]
- Filaferro M., Ruggieri V., Novi C., Calò G., Cifani C., Micioni Di Bonaventura M.V., Sandrini M., Vitale G. Functional antagonism between nociceptin/orphanin FQ and corticotropin-releasing factor in rat anxiety-related behaviors: involvement of the serotonergic system. Neuropeptides. 2014;48(4):189–197. doi: 10.1016/j.npep.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Filippetti R., Kloting I., Massi M., Cifani C., Polidori C. Involvement of cocaine-amphetamine regulated transcript in the differential feeding responses to nociceptin/orphanin FQ in dark agouti and Wistar Ottawa Karlsburg W rats. Peptides. 2007;28(10):1966–1973. doi: 10.1016/j.peptides.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Flanigan M., Tollefson S., Himes M.L., Jordan R., Roach K., Stoughton C., Lopresti B., Mason N.S., Ciccocioppo R., Narendran R. Acute elevations in cortisol increase the in vivo binding of [11C]NOP-1A to nociceptin receptors: a novel imaging paradigm to study the interaction between stress- and antistress-regulating neuropeptides. Biol. Psychiatr. 2020;87(6):570–576. doi: 10.1016/j.biopsych.2019.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavioli E.C., Calo G. Nociceptin/orphanin FQ receptor antagonists as innovative antidepressant drugs. Pharmacol. Ther. 2013;140(1):10–25. doi: 10.1016/j.pharmthera.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Gavioli E.C., Holanda V.A.D., Ruzza C. NOP ligands for the treatment of anxiety and mood disorders. Handb. Exp. Pharmacol. 2019;254:233–257. doi: 10.1007/164_2018_188. [DOI] [PubMed] [Google Scholar]
- Gavioli E.C., Marzola G., Guerrini R., Bertorelli R., Zucchini S., De Lima T.C. Blockade of nociceptin/orphanin FQ-NOP receptor signalling produces antidepressant-like effects: pharmacological and genetic evidences from the mouse forced swimming test. Eur. J. Neurosci. 2003;17(9):1987–1990. doi: 10.1046/j.1460-9568.2003.02603.x. [DOI] [PubMed] [Google Scholar]
- Gavioli E.C., Rizzi A., Marzola G., Zucchini S., Regoli D., Calo G. Altered anxiety-related behavior in nociceptin/orphanin FQ receptor gene knockout mice. Peptides. 2007;28(6):1229–1239. doi: 10.1016/j.peptides.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Gavioli E.C., Vaughan C.W., Marzola G., Guerrini R., Mitchell V.A., Zucchini S. Antidepressant-like effects of the nociceptin/orphanin FQ receptor antagonist UFP-101: new evidence from rats and mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004;369(6):547–553. doi: 10.1007/s00210-004-0939-0. [DOI] [PubMed] [Google Scholar]
- Green M.K., Barbieri E.V., Brown B.D., Chen K.W., Devine D.P. Roles of the bed nucleus of stria terminalis and of the amygdala in N/OFQ-mediated anxiety and HPA axis activation. Neuropeptides. 2007;41(6):399–410. doi: 10.1016/j.npep.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Green M.K., Devine D.P. Nociceptin/orphanin FQ and NOP receptor gene regulation after acute or repeated social defeat stress. Neuropeptides. 2009;43(6):507–514. doi: 10.1016/j.npep.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holanda V.A.D., Pacifico S., Azevedo Neto J., Finetti L., Lobão-Soares B., Calo G., Gavioli E.C., Ruzza C. Modulation of the NOP receptor signaling affects resilience to acute stress. J. Psychopharmacol. 2019;33(12):1540–1549. doi: 10.1177/0269881119864942. [DOI] [PubMed] [Google Scholar]
- Holanda V.A.D., Santos W.B., Asth L., Guerrini R., Calo G., Ruzza C., Gavioli E.C. NOP agonists prevent the antidepressant-like effects of nortriptyline and fluoxetine but not R-ketamine. Psychopharmacology (Berlin) 2018;235(11):3093–3102. doi: 10.1007/s00213-018-5004-7. [DOI] [PubMed] [Google Scholar]
- Holanda V.A., Medeiros I.U., Asth L., Guerrini R., Calo G., Gavioli E.C. Antidepressant activity of nociceptin/orphanin FQ receptor antagonists in the mouse learned helplessness. Psychopharmacology (Berlin) 2016;233(13) doi: 10.1007/s00213-016-4310-1. 2525-32. [DOI] [PubMed] [Google Scholar]
- Joëls M., Baram T.Z. The neuro-symphony of stress. Nat. Rev. Neurosci. 2009;10(6):459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juruena M.F., Werne Baes C.V., Menezes I.C., Graeff F.G. Early life stress in depressive patients: role of glucocorticoid and mineralocorticoid receptors and of hypothalamic-pituitary-adrenal axis activity. Curr. Pharmacogenomics. 2015;21(11):1369–1378. doi: 10.2174/1381612821666150105125500. [DOI] [PubMed] [Google Scholar]
- Kilkenny C., Browne W., Cuthill I.C., Emerson M., Altman D.G. NC3Rs Reporting Guidelines Working Group. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br. J. Pharmacol. 2010;160(7):1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas J.M., Bartolomucci A., Buwalda B., de Boer S.F., Flügge G., Korte S.M., Meerlo P., Murison R., Olivier B., Palanza P., Richter-Levin G., Sgoifo A., Steimer T., Stiedl O., van Dijk G., Wöhr M., Fuchs E. Stress revisited: a critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011;35(5):1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Kulkarni S.K., Dhir A. Effect of various classes of antidepressants in behavioral paradigms of despair. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2007;31(6):1248–1254. doi: 10.1016/j.pnpbp.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Kuzmin A., Madjid N., Johansson B., Terenius L., Ogren S.O. The nociceptin system and hippocampal cognition in mice: a pharmacological and genetic analysis. Brain Res. 2009;1305(Suppl. l):S7–S19. doi: 10.1016/j.brainres.2009.09.075. [DOI] [PubMed] [Google Scholar]
- Leggett J.D., Dawe K.L., Jessop D.S., Fulford A.J. Endogenous nociceptin/orphanin FQ system involvement in hypothalamic-pituitary-adrenal axis responses: relevance to models of inflammation. J. Neuroendocrinol. 2009;21(11):888–897. doi: 10.1111/j.1365-2826.2009.01912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett J.D., Harbuz M.S., Jessop D.S., Fulford A.J. The nociceptin receptor antagonist [Nphe1,Arg14,Lys15]nociceptin/orphanin FQ-NH2 blocks the stimulatory effects of nociceptin/orphanin FQ on the HPA axis in rats. Neuroscience. 2006;141(4):2051–2057. doi: 10.1016/j.neuroscience.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Lin T.Y., Hanna J., Ishak W.W. Psychiatric symptoms in Cushing's syndrome: a systematic review. Innov Clin Neurosci. 2020;17(1–3):30–35. [PMC free article] [PubMed] [Google Scholar]
- Lino-de-Oliveira C., De Lima T.C., de Pádua-Carobrez A. Structure of the rat behaviour in the forced swimming test. Behav. Brain Res. 2005;158(2):243–250. doi: 10.1016/j.bbr.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Liu E.H., Lee T.L., Nishiuchi Y., Kimura T., Tachibana S. Nocistatin and its derivatives antagonize the impairment of short-term acquisition induced by nociceptin. Neurosci. Lett. 2007;416(2):155–159. doi: 10.1016/j.neulet.2007.01.066. [DOI] [PubMed] [Google Scholar]
- Mallimo E.M., Ansonoff M.A., Pintar J.E., Kusnecov A.W. Role of opioid receptor like-1 receptor in modulation of endocrine, immunological, and behavioral responses to the T-cell superantigen staphylococcal enterotoxin A. J. Neuroimmunol. 2010;218(1–2):48–56. doi: 10.1016/j.jneuroim.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamiya T., Yamada K., Miyamoto Y., König N., Watanabe Y., Noda Y., Nabeshima T. Neuronal mechanism of nociceptin-induced modulation of learning and memory: involvement of N-methyl-D-aspartate receptors. Mol. Psychiatr. 2003;8(8):752–765. doi: 10.1038/sj.mp.4001313. [DOI] [PubMed] [Google Scholar]
- Manabe T., Noda Y., Mamiya T., Katagiri H., Houtani T., Nishi M., Noda T., Takahashi T., Sugimoto T., Nabeshima T., Takeshima H. Facilitation of long-term potentiation and memory in mice lacking nociceptin receptors. Nature. 1998;394(6693):577–581. doi: 10.1038/29073. [DOI] [PubMed] [Google Scholar]
- Medeiros I.U., Ruzza C., Asth L., Guerrini R., Romão P.R.T., Gavioli E.C., Calo G. Blockade of Nociceptin/Orphanin FQ receptor signaling reversesLPS-induced depressive-like behavior in mice. Peptides. 2015;15:S0196. doi: 10.1016/j.peptides.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Meunier J.C., Mollereau C., Toll L., Suaudeau C., Moisand C., Alvinerie P. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377(6549):532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mezadri T.J., Batista G.M., Portes A.C., Marino-Neto J., Lino-de-Oliveira C. Repeated rat-forced swim test: reducing the number of animals to evaluate gradual effects of antidepressants. J. Neurosci. Methods. 2011;195(2):200–205. doi: 10.1016/j.jneumeth.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Molendijk M.L., De Kloet E.R. Coping with the forced swim stressor: current state-of-the-art. Behav. Brain Res. 2019;364:1–10. doi: 10.1016/j.bbr.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Mollereau C., Mouledous L. Tissue distribution of the opioid receptor-like (ORL1) receptor. Peptides. 2000;21(7):907–917. doi: 10.1016/s0196-9781(00)00227-8. [DOI] [PubMed] [Google Scholar]
- Nagai J., Kurokawa M., Takeshima H., Kieffer B.L., Ueda H. Circadian-dependent learning and memory enhancement in nociceptin receptor-deficient mice with a novel KUROBOX apparatus using stress-free positive cue task. J. Pharmacol. Exp. Therapeut. 2007;321(1):195–201. doi: 10.1124/jpet.106.115121. [DOI] [PubMed] [Google Scholar]
- Narendran R., Tollefson S., Fasenmyer K., Paris J., Himes M.L., Lopresti B., Ciccocioppo R., Scott M.N. Decreased nociceptin receptors are related to resilience and recovery in college women who have experienced sexual violence: therapeutic implications for posttraumatic stress disorder. Biol. Psychiatr. 2019;85(12):1056–1064. doi: 10.1016/j.biopsych.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nativio P., Pascale E., Maffei A., Scaccianoce S., Passarelli F. Effect of stress on hippocampal nociceptin expression in the rat. Stress. 2012;15(4):378–384. doi: 10.3109/10253890.2011.627071. [DOI] [PubMed] [Google Scholar]
- Nishi M., Houtani T., Noda Y., Mamiya T., Sato K., Doi T., Kuno J., Takeshima H., Nukada T., Nabeshima T., Yamashita T., Noda T., Sugimoto T. Unrestrained nociceptive response and disregulation of hearing ability in mice lacking the nociceptin/orphaninFQ receptor. EMBO J. 1997;16:1858–1864. doi: 10.1093/emboj/16.8.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C., Watanabe Y., Magariños A.M., McEwen B.S. Opposing roles of type, I and type II adrenal steroid receptors in hippocampal long-term potentiation. Neuroscience. 1995;68(2):387–394. doi: 10.1016/0306-4522(95)00151-8. [DOI] [PubMed] [Google Scholar]
- Porsolt R.D., Le Pichon M., Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Post A., Smart T.S., Krikke-Workel J., Dawson G.R., Harmer C.J., Browning M., Jackson K., Kakar R., Mohs R., Statnick M., Wafford K., McCarthy A., Barth V., Witkin J.M. A selective nociceptin receptor antagonist to treat depression: evidence from preclinical and clinical studies. Neuropsychopharmacology. 2016;41(10):2624. doi: 10.1038/npp.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redrobe J.P., Calo G., Guerrini R., Regoli D., Quirion R. [Nphe(1)]-Nociceptin (1-13)-NH(2), a nociceptin receptor antagonist, reverses nociceptin-induced spatial memory impairments in the Morris water maze task in rats. Br. J. Pharmacol. 2000;131(7):1379–1384. doi: 10.1038/sj.bjp.0703724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redrobe J.P., Calo G., Regoli D., Quirion R. Nociceptin receptor antagonists display antidepressantlike properties in the mouse forced swimming test. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2002;365(2):164–167. doi: 10.1007/s00210-001-0511-0. [DOI] [PubMed] [Google Scholar]
- Reinscheid R.K., Nothacker H.P., Bourson A., Ardati A., Henningsen R.A., Bunzow J.R. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270(5237):792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Rizzi A., Gavioli E.C., Marzola G., Spagnolo B., Zucchini S., Ciccocioppo R., Trapella C., Regoli D., Calo G. Pharmacological characterization of the nociceptin/orphanin FQ receptor antagonist SB-612111 [(-)-cis-1-methyl-7-[[4-(2,6- dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol]: in vivo studies. J. Pharmacol. Exp. Therapeut. 2007;321(3):968–974. doi: 10.1124/jpet.106.116780. [DOI] [PubMed] [Google Scholar]
- Rizzi A., Molinari S., Marti M., Marzola G., Calo G. Nociceptin/orphanin FQ receptor knockout rats: in vitro and in vivo studies. Neuropharmacology. 2011;60(4):572–579. doi: 10.1016/j.neuropharm.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Rodi D., Zucchini S., Simonato M., Cifani C., Massi M., Polidori C. Functional antagonism between nociceptin/orphanin FQ (N/OFQ) and corticotropin-releasing factor (CRF) in the rat brain: evidence for involvement of the bed nucleus of the stria terminalis. Psychopharmacology (Berlin) 2008;196:523–531. doi: 10.1007/s00213-007-0985-7. [DOI] [PubMed] [Google Scholar]
- Russo S.J., Murrough J.W., Han M.H., Charney D.S., Nestler E.J. Neurobiology of resilience. Nat. Neurosci. 2012;15(11):1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R.M. Chickens, eggs and hippocampal atrophy. Nat. Neurosci. 2002;5(11):1111–1113. doi: 10.1038/nn1102-1111. [DOI] [PubMed] [Google Scholar]
- Selye H. The stress concept. Can. Med. Assoc. J. 1976;115:718. [PMC free article] [PubMed] [Google Scholar]
- Silva E.F., Silva A.I., Asth L., Souza L.S., Zaveri N.T., Guerrini R., Calo G., Ruzza C., Gavioli E.C. Nociceptin/orphanin FQ receptor agonists increase aggressiveness in the mouse resident-intruder test. Behav. Brain Res. 2019;356:120–126. doi: 10.1016/j.bbr.2018.08.019. [DOI] [PubMed] [Google Scholar]
- Southwick S.M., Vythilingam M., Charney D.S. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu. Rev. Clin. Psychol. 2005;1:255–291. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- Ströhle A., Holsboer F. Stress responsive neurohormones in depression and anxiety. Pharmacopsychiatry. 2003;36(Suppl. 3):S207–S214. doi: 10.1055/s-2003-45132. [DOI] [PubMed] [Google Scholar]
- Uchiyama H., Toda A., Hiranita T., Watanabe S., Eyanagi R. Role of amygdaloid nuclei in the anxiolytic-like effect of nociceptin/orphanin FQ in rats. Neurosci. Lett. 2008;431(1):66–70. doi: 10.1016/j.neulet.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai Y.M., Herman J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale G., Arletti R., Ruggieri V., Cifani C., Massi M. Anxiolytic-like effects of nociceptin/orphanin FQ in the elevated plus maze and in the conditioned defensive burying test in rats. Peptides. 2006;27(9):2193–2200. doi: 10.1016/j.peptides.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Vitale G., Filaferro M., Micioni Di Bonaventura M.V., Ruggieri V., Cifani C., Guerrini R., Simonato M., Zucchini S. Effects of [Nphe1, Arg14, Lys15] N/OFQ-NH2 (UFP-101), a potent NOP receptor antagonist, on molecular, cellular and behavioural alterations associated with chronic mild stress. J. Psychopharmacol. 2017;31(6):691–703. doi: 10.1177/0269881117691456. [DOI] [PubMed] [Google Scholar]
- Vitale G., Ruggieri V., Filaferro M., Frigeri C., Alboni S., Tascedda F. Chronic treatmentwith the selective NOP receptor antagonist [Nphe 1, Arg 14,Lys 15]N/OFQ-NH 2 (UFP-101) reverses the behavioural and biochemical effects of unpredictable chronic mild stress in rats. Psychopharmacology (Berlin) 2009;207(2):173–189. doi: 10.1007/s00213-009-1646-9. [DOI] [PubMed] [Google Scholar]
- Witkin J.M., Statnick M.A., Rorick-Kehn L.M., Pintar J.E., Ansonoff M., Chen Y., Tucker R.C., Ciccocioppo R. The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence. Pharmacol. Ther. 2014;141(3):283–299. doi: 10.1016/j.pharmthera.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin J.M., Rorick-Kehn L.M., Benvenga M.J., Adams B.L., Gleason S.D., Knitowski K.M., Li X., Chaney S., Falcone J.F., Smith J.W., Foss J., Lloyd K., Catlow J.T., McKinzie D.L., Svensson K.A., Barth V.N., Toledo M.A., Diaz N., Lafuente C., Jiménez A., Benito A., Pedregal C., Martínez-Grau M.A., Post A., Ansonoff M.A., Pintar J.E., Statnick M.A. Preclinical findings predicting efficacy and side-effect profile of LY2940094, an antagonist of nociceptin receptors. Pharmacology Research & Perspectives. 2016;4(6) doi: 10.1002/prp2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S.K., Bhatnagar S. Resilience to the effects of social stress: evidence from clinical and preclinical studies on the role of coping strategies. Neurobiol Stress. 2015;1:164–173. doi: 10.1016/j.ynstr.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wróbel A., Serefko A., Wlaź P., Poleszak E. The depressogenic-like effect of acute and chronic treatment with dexamethasone and its influence on the activity of antidepressant drugs in the forced swim test in adult mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2014;54:243–248. doi: 10.1016/j.pnpbp.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Xie G.X., Ito E., Maruyama K., Suzuki Y., Sugano S., Sharma M. The promoter region of human prepro-nociceptin gene and its regulation by cyclic AMP and steroid hormones. Gene. 1999;238(2):427–436. doi: 10.1016/s0378-1119(99)00350-9. [DOI] [PubMed] [Google Scholar]
- Young E.A., Abelson J., Lightman S.L. Cortisol pulsatility and its role in stress regulation and health. Front. Neuroendocrinol. 2004;25:69–76. doi: 10.1016/j.yfrne.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Simpson-Durand C.D., Standifer K.M. Nociceptin/orphanin FQ peptide receptor antagonist JTC-801 reverses pain and anxiety symptoms in a rat model of post-traumatic stress disorder. Br. J. Pharmacol. 2015;172(2):571–582. doi: 10.1111/bph.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.