Abstract
Angiosarcoma (AS) is a rare aggressive sarcoma with differentiation toward blood or lymphatic endothelium. There are few epidemiological data available on AS. To address this limitation, we investigated the epidemiological and clinical features of angiosarcoma diagnosed in a French administrative area (the Doubs department) from 1979 to 2016. A retrospective cohort study was conducted using the Doubs cancer registry database. A total of 45 patients with invasive AS were diagnosed between 1979 and 2016 in the Doubs department. Among the 45 AS, 51% were either cutaneous AS (27%), including head and neck and extremities, or breast AS (24%) as compared to visceral AS (42%). Eleven patients had metastasis at diagnosis (26%). Age-standardized incidence rate was 0.15 per 100,000 persons-years (95%CI, 0.10–0.20) for the entire study period (1979–2016) and 0.26 (95%CI, 0.15–0.42) for the last decade (2007–2016). Crude survival at 1, 3, 5 years after diagnosis was 44%, 21%, and 12%, respectively. Our population-based study provides updated data on the incidence and overall survival of AS in a French population-based cancer registry.
Keywords: angiosarcoma, incidence, survival
Introduction
Angiosarcoma (AS) is a rare aggressive sarcoma with differentiation toward blood or lymphatic endothelium. The tumor can arise at any anatomic location including, most commonly, the scalp, the breast and the extremities. AS can present everywhere in the body, either as a sporadic tumor (cutaneous or visceral AS) or as a secondary tumor including three main variants: lesions on the face and scalp of elderly patients (Wilson-Jones angiosarcoma), and two forms of secondary angiosarcoma, one localized in areas of chronic lymphedema, particularly in the arms of women who undergo radical mastectomy (Stewart-Treves syndrome) and another that develops over areas of irradiated skin, particularly in the pectoral area of women who undergo radiotherapy after breast cancer.1
There are few epidemiological data available on AS. To address this limitation, we wanted to investigate the epidemiological and clinical features of angiosarcoma diagnosed in a French administrative area (the Doubs department) from 1979 to 2016.
Methods
Patients selection
A retrospective cohort study was conducted using the database of the Doubs cancer registry, a French population-based cancer registry which provides continuous and exhaustive recording of all cancer cases diagnosed in patients living in the Doubs Department, a well-defined administrative area located in eastern France (505,557 inhabitants according to the INSEE population census of 2003). Cancer registry population-based studies provide non-biased evidence, since they include all cases of cancer diagnosed in the inhabitants of a given geographical area, regardless of where they receive care. There is therefore no selection bias, as may occur in hospital-based patient series. Moreover, in France, the quality and completeness of registry data are assessed every 5 years by the public health authority’s Registry Evaluation Committee. Cancer registry routinely collected cancer cases from different sources including histopathology laboratories, oncology departments, multidisciplinary meetings, and computerized hospital discharge databases.
Inclusion criteria were all primary invasive angiosarcoma (coded M-91203 according to the International Classification of Diseases for Oncology, 3rd edition),2 diagnosed between 1 January 1979 and 31 December 2016, in patients resident in the Doubs Department. Patients with Kaposi sarcoma were excluded. Data collected from medical records were: demographic characteristics (age, sex), tumor characteristics at diagnosis (date of diagnosis, topography, histology, metastatic status at diagnosis), vital status, date of death, and last available follow-up date.
Statistical analysis
Quantitative variables were expressed as median, mean, standard error, range and interquartile range (IQR); qualitative variables as number and percentage.
Incidence of AS was described according to sex, two age groups (< and ⩾ 70 years), angiosarcoma type (i.e. cutaneous, breast, visceral, soft-tissue, and unknown) and metastatic status.
Crude (CIR) and age-standardized incidence rates (ASIR) were expressed per 100,000 person-years, with their 95% confidence intervals (95%CI). ASIR were estimated using direct standardization and were calculated using the world standard population. Crude and standardized incidence rates were calculated for the whole period 1979–2016 and for the more recent 10 years period 2007–2016.
To compare Survival time between sex, age groups, angiosarcoma type and metastasis status, Student test and the Wilcoxon test were used.
Survival analyses were performed on all AS diagnosed between January 1, 1979 and December 31, 2016 and followed-up until June 30, 2018 (i.e. patients alive at that date had their survival time censored). The probability of overall survival (OS) was estimated using the Kaplan-Meier method. Survival curves between groups were compared using the Log-Rank test. All statistical tests were two-sided, with a significance level at 0.05. Analyses were performed using STATA Version 15.1 (Stata Corporation, College Station, TX, USA).
Results
Study population
A total of 45 patients with invasive AS were diagnosed between 1979 and 2016 in the Doubs department (Table 1). The male-to-female ratio was 0.61 for overall AS and 0.72 for cutaneous AS, but ranging from 0.2 for limb AS to 2.0 for cutaneous head and neck AS. The median age at diagnosis was 69 years (range: 27–87).
Table 1.
Angiosarcoma (AS) survival according to clinical characteristics.
| N (%) | Median (months) | Q1–Q3 | Min–Max | Mean (months) | SE | p | |
|---|---|---|---|---|---|---|---|
| All patients | 45 | 10 | 3–29 | 0–271 | 26.2 | 7.4 | |
| Sex | 0.55 | ||||||
| Men | 17 (38) | 8 | 3–30 | 1–271 | 31.9 | 16.1 | |
| Women | 28 (62) | 11 | 2–25 | 0–188 | 22.7 | 7.0 | |
| Age | |||||||
| <50 years | 5 (11) | 17 | 7–98 | 4–271 | 79.3 | 51.0 | 0.005 |
| ⩾50 years | 45 (89) | 9 | 2–25 | 0–188 | 19.5 | 5.1 | |
| <70 years | 23 (51) | 18 | 4–44 | 0–271 | 41.3 | 13.7 | 0.02 |
| ⩾70 years | 22 (49) | 6 | 1–16 | 1–47 | 10.3 | 2.6 | |
| Angiosarcoma types | 0.06 | ||||||
| Cutaneous and breast AS | 23 (55) | 18 | 10–40 | 0.5–271 | 39.2 | 13.3 | |
| Visceral AS | 19 (45) | 4 | 1–17 | 0–98 | 14.4 | 5.6 | |
| Distribution | 0.32 | ||||||
| Cutaneous AS | 12 (29) | 18 | 10–32 | 1–271 | 50.9 | 24.8 | |
| Head and neck | 6 (14) | 18 | 10–29 | 10–34 | 20.0 | 4.1 | |
| Other | 6 (14) | 15 | 1–188 | 0–271 | 81.9 | 48.1 | |
| Breast AS | 11 (26) | 18 | 8–47 | 3–66 | 26.3 | 6.6 | |
| Metastasis | 0.07 | ||||||
| No | 31 (74) | 16 | 4–30 | 1–271 | 28.5 | 8.9 | |
| Yes | 11 (26) | 1 | 0–5 | 0–44 | 5.9 | 3.9 | |
| Initial treatment | |||||||
| Surgery | 15 | ||||||
| Chemotherapy | 4 | ||||||
| Surgery and radiotherapy | 20 | ||||||
| Surgery and chemotherapy | 3 | ||||||
| Chemotherapy and surgery | 2 | ||||||
| Palliative care | 1 |
Q1: 25%; Q3: 75%; SE: standard error.
Among the 45 AS, 55% were either cutaneous AS (29%) including head and neck and extremities, or breast AS (27%) as compared to visceral AS (45%). Eleven patients out of 42 for whom information were known had metastasis at diagnosis (26%) (Table 1).
Most patients in our study had secondary AS (24/45 = 53%) including 9 (38%) ultraviolet radiation-associated cutaneous AS, 10 (42%) radiotherapy-induced AS and 5 (21%) Stewart Treves syndrome compared to primary AS (21/45 = 47%). All the breast AS, except one, were related to prior radiotherapy.
Incidence
World age-standardized incidence rate (ASIR) was 0.15 per 100,000 persons-years (95%CI, 0.10–0.20) for the entire study period (1979–2016) and 0.26 (95%CI, 0.15–0.42) for the last decade (2007–2016) (Table 2).
Table 2.
Angiosarcoma incidence rates (crude and world standardized).
*38 years; **10 years; CIR: crude incidence rate; ASIR: age-standardized incidence rate per 100,000 person-years; 95%CI: 95% confidence interval.
Survival
The survival analysis included 45 cases diagnosed between 1979 and 2016 (Table 1). For the entire study period, the median of overall survival time was 10 months (range: 3–29). Five years after diagnosis, 91% of patients had died, and one patient was lost to follow-up. Survival time was significantly different by age group, regardless of the threshold (50 or 70 years) (Table 1, p = 0.005 and 0.02, respectively).
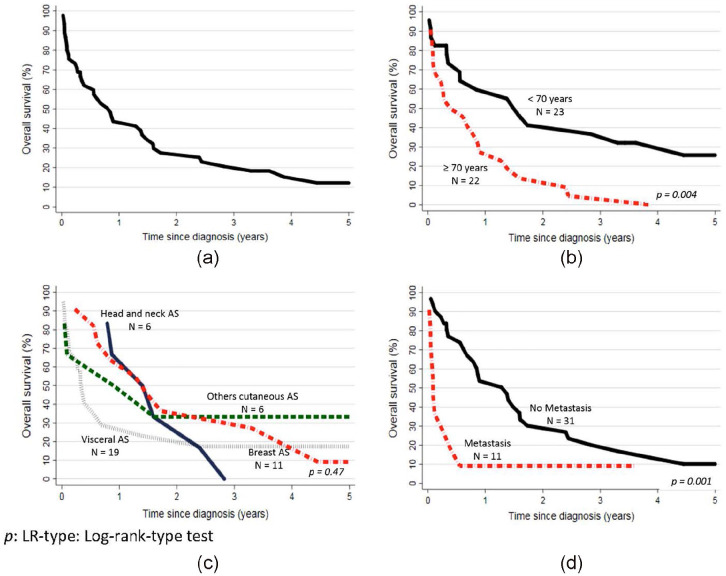
Crude survival probability at 1, 3, 5 years after diagnosis were 44%, 21%, and 12%, respectively (Figure 1(a)). Survival probability curves were not statistically different between male and female (p = 0.94). Patients ⩾70 years had significantly lower survival probability (Figure 1(b), p = 0.004). During the 1979–2016 period, visceral AS demonstrated a poorer survival probability curves as compared to cutaneous and breast AS (Figure 1(c), p = 0.06). Patients with metastasis at diagnosis had statistically different survival probability curves (Figure 1(d), p = 0.001).
Figure 1.
Kaplan-Meier survival curve of angiosarcoma (AS) patients: (a) n = 45; 1979–2016, (b) by age (< and ⩾70 years), (c) by AS type, and (d) by metastatic status.
Discussion
The exact overall incidence of sarcomas is unknown and the incidence of the different histological and molecular subtypes has not been determined precisely. The goal of this retrospective study was to describe a population-based cancer registry series of consecutive angiosarcoma (AS) diagnosed between 1979 and 2016 in a define geographic area. AS are one of the most rare subtypes (1%–2%) of adult soft tissue sarcomas.1,3
In Europe, AS annual incidence is approximately 0.31 cases per 100,000 people per year,4,5 very close to our rate of 0.26 per 100,000 for the 2007–2016 period.
Angiosarcomas have an overall 5-year survival rate of about 40%6 decreasing to 15% in metastatic patients.1 In a recent study3 from the National Cancer Institute’s Surveillance, Epidemiology and End Results database including 4537 US patients diagnosed with primary AS from 1973 to 2014, the median overall survival was 82.1 months (95% CI: 76.5–87.7) and the overall 1-, 2-, and 5-year survival rates were 55.2 ± 0.7, 41.0 ± 0.7, and 26.3 ± 0.7%, respectively.
In a large Dutch study7 based on the Netherlands Cancer registry of 479 AS, the authors observed a lower median OS of 13 months (95% CI, 10–16 months) and a 5-year survival rate of 22%.
Overall survival in our cohort was relatively poor with a median of 10 months and a 3- and 5-year OS of 21% and 12%, respectively.
The discrepancies between OS may be explained by the different distribution of AS between cohorts. Prognostic factors vary between studies, probably due to differences between clinical factors included in the analyses.
Factors including age ⩾ to 70 years, male gender, unmarried status, pre-existing lymphedema, primary site of AS (cutaneous versus visceral), tumor size ⩾5 cm, tumor depth, distant metastases at diagnosis and non-surgery were reported to be independently associated with poor survival on prognosis.3,7,8
In a previous prospective French study9 including 658 patients with soft tissue sarcomas, 28 AS were identified. Among them, radiation-induced AS represented 18% out of all cases and only one case of Stewart-Treves syndrome was identified. In our series, 46% were primary, 42% were visceral, and 24% were metastatic as compared to 35%, 14%, and 17%, respectively in the Dutch series.7
When considering cutaneous AS,10 5-year OS was even higher, superior to 90% for patients with dermatofibrosarcoma protuberans, leiomyosarcoma or malignant fibrous histiocytoma. As previously expected,3,6–8 visceral AS, distant metastases at diagnosis and age ⩾ to 70 years were predictive of worse OS in our cohort (Figure 1). In contrast, a French retrospective multicenter study (June 1980 to October 2009) of 107 patients with localized AS revealed higher 1-year and 5-year OS rates at 74.8% (95% CI: 70.7–78.9) and 39.8% (95% CI: 30.6–43.2), respectively.5
The limitations of this study include potential selection bias due to its retrospective nature and the small number of patients. However, this study covers a very large period (38 years) and is derived from a population-based cancer registry.
In conclusion, our population-based study provides updated data on the incidence and OS of AS in a French population-based cancer registry.
Acknowledgments
The authors thank all those who contributed to the inclusion of cancer patients in the registries, in particular pathologists, health information technology staff in public and private healthcare institutions, medical staff of health insurance companies, chest physicians and oncologists, as well as medical practitioners. French cancer registries are supported by the Institut National du Cancer (INCa) and Santé Publique France (SpF).
Footnotes
Author contributions: MC, AG, DP, EP, LC, ASW, ASD, CN: conception and design, acquisition of data; revising critically the manuscript; final approval of the version to be published. FA: Substantial contributions to analysis and interpretation of data; drafting the manuscript; final approval of the version to be published.
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: The director of the cancer registry (ASW) agreed to use the registry data and validated the results.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iD: François Aubin  https://orcid.org/0000-0002-1421-4996
https://orcid.org/0000-0002-1421-4996
References
- 1. Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol 2010; 11(10): 983–991. [DOI] [PubMed] [Google Scholar]
- 2. Fritz A, Percy C, Jack A, et al. International classification of diseases for oncology. 3rd ed. Geneva: World Health Organization, 2000. [Google Scholar]
- 3. Zhang C, Xu G, Liu Z, et al. Epidemiology, tumor characteristics and survival in patients with angiosarcoma in the United States: a population-based study of 4537 cases. JPN J Clin Oncol 2019; 49(12): 1092–1099. [DOI] [PubMed] [Google Scholar]
- 4. Ducimetiere F, Lurkin A, Ranchere-Vince D, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS one 2011; 6(8): e20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lindet C, Neuville A, Penel N, et al. Localised angiosarcomas: the identification of prognostic factors and analysis of treatment impact. A retrospective analysis from the French Sarcoma Group (GSF/GETO). Eur J Cancer 2013; 49(2): 369–376. [DOI] [PubMed] [Google Scholar]
- 6. Fayette J, Martin E, Piperno-Neumann S, et al. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol 2007; 18(12): 2030–2036. [DOI] [PubMed] [Google Scholar]
- 7. Weidema ME, Flucke UE, van der Graaf WTA, et al. Prognostic factors in a large nationwide cohort of histologically confirmed primary and secondary angiosarcomas. Cancers (Basel) 2019; 11(11): 1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Basse C, Italiano A, Penel N, et al. Sarcomas in patients over 90: natural history and treatment-A nationwide study over 6 years. Int J Cancer 2019; 145(8): 2135–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Penel N, Grosjean J, Robin YM, et al. Frequency of certain established risk factors in soft tissue sarcomas in adults: a prospective descriptive study of 658 cases. Sarcoma 2008; 2008: 459386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rouhani P, Fletcher CD, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer 2008; 113(3): 616–627. [DOI] [PubMed] [Google Scholar]