Abstract
Objective:
This study aims to compare recurrence patterns and outcomes of biologically effective dose (BED10, α/β = 10) of 60–70 Gy with those of a BED10 >70 Gy for locally advanced pancreatic cancer (LAPC).
Methods:
Patients from three centers with a biopsy and a radiographically proven LAPC were retrospectively included and data were prospectively collected from June 2012 to June 2019. Radiotherapy was delivered by stereotactic body radiation therapy. Recurrences were categorized as in-field, marginal, and outside-the-field recurrence. Patients in two groups were required to receive abdominal enhanced contrast CT or MRI every 2–3 months and CA19-9 examinations every month during follow-up. Treatment-related toxicities were evaluated every month. Overall survival (OS) and progression-free survival (PFS) were estimated using the Kaplan–Meier method.
Results:
After propensity score matching, there were 486 patients in each group. The median prescription dose of the two groups was 37 Gy/5–8 f (range: 36–40.8 Gy/5–8 f) and 42 Gy/5–8 f (range: 40–49.6 Gy/5–8 f), respectively. The median OS of patients with a BED10 >70 Gy and a BED10 60–70 Gy was 20.3 months (95% CI: 19.1–21.5 months) and 18.2 months (95% CI: 17.8–18.6 months) respectively (p < 0.001). The median PFS of the two cohorts was 15.4 months (95% CI: 14.2–16.6 months) and 13.3 months (95% CI: 12.9–13.7 months) respectively (p < 0.001). A higher incidence of in-field and marginal recurrence was found in patients with BED10 of 60–70 Gy (in-field: 97/486 versus 72/486, p = 0.034; marginal: 109/486 versus 84/486, p = 0.044). However, more patients with BED10 >70 Gy had grade 2 or 3 acute (87/486 versus 64/486, p = 0.042) and late gastrointestinal toxicities (77/486 versus 55/486, p = 0.039) than those with BED10 of 60–70 Gy.
Conclusion:
BED10 >70 Gy was found to have the best survival benefits along with a higher incidence of acute and late gastrointestinal toxicities. Therefore, a higher dose may be required in the case of patients’ good tolerance.
Keywords: dose escalation, outcome, pancreatic cancer, patterns of failure, radiotherapy
Introduction
Pancreatic cancer remains one of the most lethal malignancies and is the fourth leading cause of cancer death with a dismal survival in the US, where its mortality increases slightly.1 Similar findings were also identified in China2 as well as the rest of the globe,3 which indicates that the mortality may surpass that of breast cancer in Western countries in the future.4
A recent study showed that about one-third of patients died from local progression rather than distant metastases, which underlined the importance of local control.5 Additionally, less than 20% of patients with resectable pancreatic cancer could receive upfront surgical resection at the initial diagnosis, while chemoradiotherapy may be taken as the first treatment option for most patients with locally advanced pancreatic cancer.6,7 Therefore, radiation therapy may be pivotal in preventing local progression.
Radiation dose escalation has been proven to be beneficial for local control and thus improving survival in prostate and head and neck cancers.8,9 In our previous studies, it was clarified that biologically effective dose (BED10, α/β = 10) ⩾60 Gy was the predictor of superior overall survival (OS) and better tumor response.10–13 Meanwhile, BED10 >70 Gy was demonstrated to be correlated with better survival outcomes.14 As a result, it was vital to determine a proper dose that may provide improved prognosis. Furthermore, patterns of local failure of dose escalation may imply the correlation between tumor local control and radiation doses. However, no studies have compared benefits and adverse effects between variable high doses. Hence, a comprehensive understanding about the outcomes and recurrence patterns of dose escalation could provide evidence for dose prescriptions. The aim of our study was to investigate the local failure patterns and outcomes of patients with BED10 of 60–70 Gy and over 70 Gy.
Methods
Patient selection
The study was approved by the institutional review boards of Changhai Hospital affiliated to Navy Medical University, Affiliated Tumor Hospital of Guangxi Medical University and General Hospital of Eastern Theater Command with the approval number of 2012-CH-068, 2012-GXZH-066 and 2012-NCGH-088, respectively. Patients included in the study were from these centers. Before treatment, patients were required to receive personal interviews with physicians for a detailed explanation of the whole study and related treatments. In addition, written informed consent about the whole treatment and potential adverse effects and benefits was required prior to the patients’ participation in the study, stating their willingness to be treated according to the study. Consecutive patients’ data were prospectively collected from different centers from June 2012 to June 2019. Biopsies with fine-needle aspiration guided by endoscopic ultrasound were required before treatment. Contrast computed tomography (CT) and magnetic resonance imaging (MRI) were routinely performed before and after stereotactic body radiation therapy (SBRT) for staging. Patients with a biopsy and a radiographically proven locally advanced pancreatic cancer and receiving BED10 ⩾60 Gy were included in the study. Patients without completion of consequent chemotherapy (4–6 cycles) were excluded. As a result, recurrence patterns and outcomes were compared between patients with a BED10 of 60–70 Gy and BED10 >70 Gy.
Delivery of radiotherapy
The protocol was similar to our previous studies.10,12,15 SBRT was delivered via CyberKnife® (Accuray Incorporated, Sunnyvale, CA). Three fiducials within or adjacent to the tumor were preferable. Synchrony™ Respiratory Tracking System (Accuray Incorporated, Sunnyvale, CA) was used. Gross tumor volume (GTV) was defined as the gross disease identified in the imaging examinations. The planning target volume (PTV) was generated based on 2–5 mm margin expansions from GTV. Doses were prescribed to the 75–80% isodose covering 90% of the PTV. Dose constraints of organs at risk referred to the American Association of Physicists in Medicine guidelines in TG-101.16 The delineation of targets and organs at risk were reviewed by a radiation oncologist and a radiologist. Triphasic CT datasets were acquired to delineate the tumor.
Chemotherapy
All patients received chemotherapy 2–3 weeks after the completion of SBRT. The regimen was gemcitabine and S-1. It has been clarified that similar survival outcomes and mild toxicities were found with S-1 compared with gemcitabine.17–19 Gemcitabine (1000 mg/m2) was performed on day 1, 8 and 15 during a 4-week cycle, which lasted for 4–6 cycles. S-1 was orally given at a dose of 80 mg/m2 for 28 days followed by a 14-day rest, which also lasted for 4–6 cycles.
Data collection
The upper limit of normal CA19-9 is usually considered as 37 U/mL.20 Additionally, it was clarified that the decreased CA19-9 levels of ⩾50% was correlated with an improved survival.21 Therefore, CA19-9 response was defined as a decrease of CA19-9 level by 50% from the pre-SBRT level of ⩾74 U/mL. Patients were required to undergo CA19-9 examinations with each follow-up. The nadir value of CA19-9 after treatment was used for the estimation of CA19-9 decrease. Hence, CA19-9 response was stratified as follows: CA19-9 levels ⩾74 U/mL with response versus CA19-9 levels ⩾74 U/mL with no response (including CA19-9 levels within the normal range before treatment while increasing after treatment) versus CA19-9 levels <74 U/mL all along before and after treatment.15 Furthermore, it was shown that a better response was found in patients with a baseline CA19-9 level of <200 U/mL after neoadjuvant therapy.22 Therefore, baseline CA19-9 level was stratified as: <200 U/mL versus ⩾200 U/mL in our study. The change in GTV (ΔGTV) was defined as the volume of primary recurrence minus that of the primary lesion before SBRT.
Identification of recurrences was the same as that in our previous study,15 and was performed by a radiologist specialized in pancreatic cancer. Recurrences were identified by contrast CT and functional imaging, including contrast MR and/or positron emission tomography–computed tomography (PET-CT) and CA19-923 but usually without biopsy.24 Evidence of any progression inferior to the diaphragm and superior to the bottom of the L3 vertebra excluding hepatic or gastric metastases24 by RECIST criteria25 was considered as a loco-regional recurrence. Besides, recurrences at the hepatic hilum were also defined as local failure.15 Distant metastasis included malignant ascites or radiographically new lesions in other organs.
The primary outcome was OS. The secondary outcomes were recurrence patterns, progression-free survival (PFS) and radiation-induced toxicities.
Recurrence mapping
Recurrences were plotted on a template CT scan of a healthy person with Multiplan software (Accuray Incorporated, Sunnyvale, CA), creating a 3-dimensional map of local failures, which were plotted in relation to the celiac axis, superior mesenteric artery, scaling for individual abdominal width. Because the recurrence map was based on estimations of each recurrence location, there might be uncertainty between the estimation and the actual location of local failure. In order to minimize the impact, two radiologists will reach consensus in the tumor recurrences’ identification and plotting if any disagreement exists. Moreover, local recurrences were categorized as in-field or marginal recurrence if more than 80% or 20–80% of the recurrence volume was located in the prescription dose line, while outside-the-field recurrence was defined as any new lesions outside the prescription dose line or if less than 20% of the recurrence volume was located inside the prescription dose line.26,27
Evaluations of toxicity
Radiation-induced acute toxicity was evaluated by the “acute radiation morbidity scoring criteria” from the Radiation Therapy Oncology Group, while late toxicity was determined by the “late radiation morbidity scoring schema” from the Radiation Therapy Oncology Group/European Organization for Research on the Treatment of Cancer.28
Follow-up
Patients in two groups were required to receive abdominal enhanced contrast CT or MRI every 2–3 months and CA19-9 examinations every month during follow-up. Treatment-related toxicities were evaluated every month by physicians blinded to the study. Any other examinations, such as PET-CT, prompted by new-onset symptoms or at the physician’s discretions were also used to record events.
Propensity score matching
A logistic regression model was built with all potential factors that may influence the outcomes. Finally, 1:1 propensity score matching was performed to evaluate the impact of a BED10 of 60–70 Gy versus that over 70 Gy on the OS.
Statistical analysis
Patient characteristics were presented with descriptive statistics. The primary objective of our study was to identify recurrence patterns. The secondary objectives were outcomes including OS and PFS and treatment-related toxicity. A Student t-test or a Mann–Whitney U test was used for analysis in the case of normally or non-normally distributed continuous covariates. Categorical variables were compared using the χ2 test. OS and PFS were estimated and compared with the Kaplan–Meier and log-rank methods, respectively. Factors with a p-value < 0.05 in the univariate Cox regression analysis were entered as candidate variables into the multivariate Cox proportional hazard regression analysis for identification of predictors correlating with OS and PFS. All p-values < 0.05 were considered as statistically significant. Statistical analyses were performed with IBM SPSS version 22.0 (SPSS Inc., Armonk, NY) and SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Patient characteristics
Before propensity score matching, 527 and 493 patients received a BED10 of 60–70 Gy and a BED10 >70 Gy, respectively. The median follow-up was 20.2 months (range: 4.6–51.7 months). The median prescription dose of the two groups was 37 Gy/5–8 f (range: 36–40.8 Gy/5–8 f) and 42 Gy/5–8 f (range: 40–49.6 Gy/5–8 f), respectively. The median BED10 of the two groups was 64.38 Gy/5–8 f (range: 60.264–69.42 Gy/5–8 f) and 74.62 Gy/5–8 f (range: 71.4–88.32 Gy/5–8 f), respectively. Details are shown in Table 1.
Table 1.
Patient characteristics before and after propensity score matching.
| Characteristics | Before propensity score matching |
After propensity score matching |
||||
|---|---|---|---|---|---|---|
| BED10 of 60–70 Gy | BED10 >70 Gy | p-value | BED10 of 60–70 Gy | BED10 >70 Gy | p-value | |
| No. of patients | 527 | 493 | 486 | 486 | ||
| Age, years | 66 (40–90) | 65 (32–90) | 0.168 | 65 (40–89) | 65 (32–90) | 0.128 |
| Gender | ||||||
| Male | 307 (58.3%) | 287 (58.2%) | 0.990 | 277 (57.0%) | 285 (58.6%) | 0.603 |
| Female | 220 (41.7%) | 206 (41.8%) | 209 (43.0%) | 201 (42.4%) | ||
| Tumor diameter (cm) | 4.0 (1.7–8.6) | 4.4 (1.5–8.7) | 0.075 | 4.1 (1.7–8.5) | 4.5 (1.5–8.7) | 0.060 |
| ECOG | 1 (0–2) | 1 (0–2) | 0.019 | 1 (0–2) | 1 (0–2) | 1.000 |
| CA19-9 | ||||||
| <200 U/ml | 215 (40.8%) | 173 (35.1%) | 0.061 | 192 (39.5%) | 169 (34.8%) | 0.127 |
| ⩾200 U/ml | 312 (59.2%) | 320 (64.9%) | 294 (60.5%) | 317 (65.2%) | ||
| Tumor location | ||||||
| Pancreatic head | 347 (65.8%) | 305 (61.9%) | 0.186 | 326 (67.1%) | 302 (62.1%) | 0.107 |
| Pancreatic body or tail | 180 (34.2%) | 188 (38.1%) | 160 (32.9%) | 184 (37.9%) | ||
| GTV (cc) | 108.87 (26.09–209.97) | 111.23 (20.09–204.88) | 0.522 | 108.73 (26.09–209.97) | 111.04 (20.09–204.88) | 0.637 |
BED, biologically effective dose; ECOG, Eastern Cooperative Oncology Group; GTV, gross tumor volume
Propensity score matching
According to the results above, Eastern Cooperative Oncology Group (ECOG) was included as the independent factor for propensity score matching. Therefore, 486 patients were included in each group (Table 1). No significance was found in baseline characteristics between these two groups. The median BED10 of the two groups was 64.38 Gy (range: 60–69.42 Gy) and 74.62 Gy (range: 71.4–88.32 Gy), respectively.
Outcomes of two cohorts
The median OS of patients with a BED10 >70 Gy and a BED10 of 60–70 Gy was 20.3 months (95% CI: 19.1–21.5 months) and 18.2 months (95% CI: 17.8–18.6 months), respectively (p < 0.001). The median PFS of the two cohorts was 15.4 months (95% CI: 14.2–16.6 months) and 13.3 months (95% CI: 12.9–13.7 months), respectively (p < 0.001) (Figure 1). The OS rates of patients with a BED10 >70 Gy and a BED10 of 60–70 Gy at 2 years was 36.8% versus 24.5% and at 3 years of 18.7% versus 8.8%. The PFS rates of the two cohorts at 2 years were 22.2% versus 8.6%.
Figure 1.
(A) Overall survival and (B) progression-free survival curves of the biologically effective dose (BED10, α/β = 10) >70 Gy and the BED10 of 60–70 Gy.
Moreover, it was demonstrated that tumor diameter (<4.0 cm as reference, ⩾4.0 cm HR: 1.29, 95% CI: 1.12–1.48, p < 0.001), Eastern Cooperative Oncology Group (ECOG) (0 point as reference, 1 point HR: 1.50, 95% CI: 1.29–1.76; 2 points HR: 2.54, 95% CI: 2.02–3.20), p < 0.001), CA19-9 level (<200 U/mL as reference, ⩾200U/mL HR: 1.39, 95% CI: 1.16–1.66, p < 0.001), CA19-9 response (CA19-9 levels ⩾74 U/mL with response as reference, CA19-9 levels <74 U/mL all along HR: 1.45, 95%CI: 1.14–1.84; CA19-9 levels ⩾74 U/mL with no response HR: 1.94, 95% CI: 1.66–2.28, p < 0.001) and recurrence patterns (primary recurrence as reference, primary recurrence + recurrence at the hepatic hilum HR: 7.42, 95% CI: 5.34–10.30, p < 0.001) correlated with OS after multivariate analysis. Similarly, ECOG, CA19-9 level, CA19-9 response and recurrence patterns were predictors of PFS (Supplemental Tables 1 and 2).
Sub-group analysis
It was demonstrated that survival benefits could be found in patients of all ages, genders and baseline CA19-9 levels (Table 2). However, regarding ECOG, better outcomes could only be found in patients with ECOG of 0 and 1 point receiving a BED10 >70 Gy (BED10 of 60–70 Gy as reference; 0 point: HR: 0.46, 95% CI: 0.36–0.60, p < 0.001; 1 point: HR: 0.83, 95% CI: 0.69–0.99, p = 0.042), while patients with ECOG of 2 points may not achieve benefits after irradiation with a BED10 >70 Gy (BED10 of 60–70 Gy as reference; HR: 0.88, 95% CI: 0.60–1.28, p = 0.508). Similarly, no favorable survival was found in patients with a BED10 >70 Gy in the case of tumor diameter ⩾4 cm (BED10 of 60–70 Gy as reference; HR: 0.84, 95% CI: 0.70–1.00, p = 0.049). Nevertheless, a BED10 >70 Gy was shown to favor patients with tumor diameter <4 cm (BED10 of 60–70 Gy as reference; HR: 0.51, 95% CI: 0.41–0.64, p < 0.001).
Table 2.
Sub-group analysis of benefits of BED10 >70 Gy.
| Variable | Cox regression analysis (BED10 of 60–70 Gy as reference) |
||
|---|---|---|---|
| HR (95% CI) | p-value | ||
| Age | <65 | 0.71 (0.58–0.87) | 0.001 |
| ⩾65 | 0.66 (0.54–0.80) | <0.001 | |
| Gender | Female | 0.68 (0.55–0.84) | <0.001 |
| Male | 0.69 (0.58–0.82) | <0.001 | |
| Tumor diameter | <4.0 cm | 0.51 (0.41–0.64) | <0.001 |
| ⩾4.0 cm | 0.84 (0.70–1.00) | 0.049 | |
| ECOG | 0 | 0.46 (0.36–0.60) | <0.001 |
| 1 | 0.83 (0.69–0.99) | 0.042 | |
| 2 | 0.88 (0.60–1.28) | 0.508 | |
| CA19-9 level | <200 U/ml | 0.52 (0.41–0.65) | <0.001 |
| ⩾200 U/ml | 0.80 (0.67–0.94) | 0.008 | |
BED, biologically effective dose; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio
Patterns of local failure
Local failure was found in 309 (63.6%) and 280 (57.6%) patients with a BED10 of 60–70 Gy and over 70 Gy respectively, while 177 (36.4%) and 206 (42.4%) patients in the corresponding two cohorts experienced both local recurrences and distant metastases. A larger median ΔGTV was found in patients receiving a BED10 of 60–70 Gy (63.37 cc, range: 20.15–108.79 cc) compared with ΔGTV in patients with a BED10 over 70 Gy (55.97 cc, range: 7.15–99.72 cc) (p < 0.001). More patients with a BED10 of 60–70 Gy had recurrences at the hepatic hilum than those with a BED10 >70 Gy (37/486 versus 21/486, p = 0.03) in addition to primary recurrences. Furthermore, a higher incidence of both in-field and marginal recurrence was found in patients with a BED10 of 60–70 Gy (in-field: BED10 of 60–70 Gy: 97/486 versus BED10 >70 Gy: 72/486, p = 0.034; marginal: BED10 of 60–70 Gy: 109/486 versus BED10 >70 Gy: 84/486, p = 0.044) (Table 3 and Figure 2). Additionally, there are more patients with simultaneous liver and lung metastases found in the group of patients receiving a BED10 of 60–70 Gy than the group of patients receiving a BED10 >70 Gy. However, there is no statistical difference between these two groups (BED10 of 60–70 Gy: 9/486 versus BED10 >70 Gy: 2/486, p = 0.064).
Table 3.
Patterns of local failure between BED10 of 60–70 Gy and BED10 >70 Gy.
| Patterns of failure | BED10 of 60–70 Gy (n) | BED10 >70 Gy (n) | p-value |
|---|---|---|---|
| In-field recurrence alone | 97 | 72 | 0.034 |
| Marginal recurrence alone | 109 | 84 | 0.044 |
| Outside-the-field recurrence alone | 103 | 124 | 0.111 |
| In-field plus distant recurrences | 86 | 101 | 0.222 |
| Outside-the-field plus distant recurrences | 91 | 105 | 0.263 |
BED, biologically effective dose
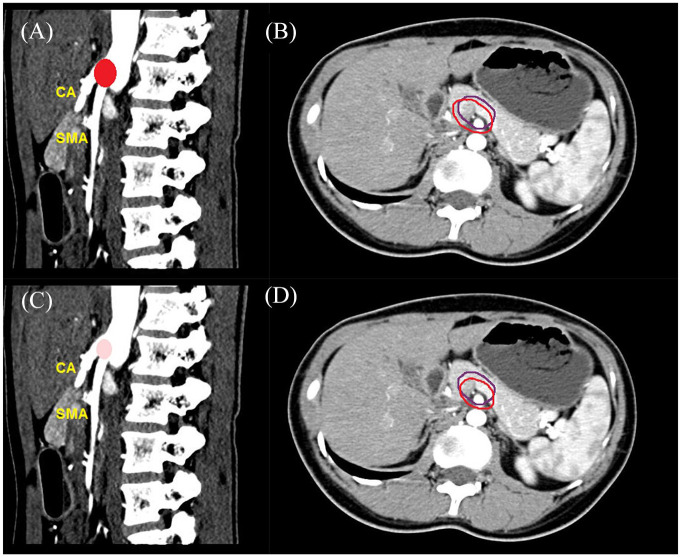
Figure 2.
Patterns of local failure of a BED10 >70 Gy and a BED10 of 60–70 Gy plotted in a healthy person in contrast CT. (A) Primary recurrences in a BED10 of 60–70 Gy; (B) In-field and marginal recurrence alone in a BED10 of 60–70 Gy; (C) Primary recurrences in a BED10 >70 Gy; and (D) In-field and marginal recurrence alone in a BED10 >70 Gy. The projected red areas represented the primary recurrences and the deep color indicated a high frequency of local failure in A and C. A larger area in A compared with that in C indicated a larger recurrent lesion volume in a BED10 of 60–70 Gy. The area encompassed by the purple and red line represented projected gross tumor volume before SBRT and primary recurrence volume after SBRT in B and D.
BED, biologically effective dose; CT, computed tomography; SBRT, stereotactic body radiation therapy
Toxicity
Compared with a BED10 of 60–70 Gy, more patients with a BED10 >70 Gy suffered from grade 2 or 3 acute and late gastrointestinal toxicities (acute gastrointestinal toxicity: BED10 >70 Gy: 87/486 versus BED10 of 60–70 Gy: 64/486, p = 0.042; late gastrointestinal toxicity: BED10 >70 Gy: 77/486 versus BED10 of 60–70 Gy: 55/486, p = 0.039). Meanwhile, patients with ECOG of 2 points experienced a higher incidence of grade 2 or 3 acute (BED10 >70 Gy: 15/62 versus BED10 of 60–70 Gy: 6/62, p = 0.031) and late gastrointestinal toxicities (BED10 >70 Gy: 12/62 versus BED10 of 60–70 Gy: 3/62, p = 0.025) in the group of patients with a BED10 >70 Gy. Furthermore, more patients with tumor diameter ⩾4 cm receiving a BED10 >70 Gy experienced grade 2 or 3 acute gastrointestinal toxicities (BED10 >70 Gy: 56/288 versus BED10 of 60–70 Gy: 31/264, p = 0.015).
Discussion
Our study showed favorable outcomes including superior survival and better local control along with a higher incidence of radiation-induced gastrointestinal toxicities in the group with a BED10 >70 Gy.
In early previous studies about SBRT, it has been shown that a high BED, including 25 Gy/f,29 24–36 Gy/3f,30,31 22–30 Gy/1–3f32 or 45 Gy/3f,33 may contribute to the improved local control or even OS, but with high incidences of grade 3 or more toxicity ranging from 6% to 41.6%. Therefore, the increase of the number of fractions and decrease of fraction size were commonly adopted in sequential studies, which also demonstrated that higher BED might be the predictive factor of superior OS or better local control.10,11,14 Nevertheless, a recent meta-analysis has clarified that BED10 >70 Gy did not correlate with the improvement of 1-year local control rate.34 As a result, an understanding of the correlation between higher BED and OS was necessary. To the best of our knowledge, this is the first study to compare outcomes and recurrence patterns of high BED with different ranges.
It was clarified that favorable OS and PFS were found in patients with a BED10 >70 Gy, which was consistent with previous studies.10,11,14 The median OS and 2-year OS rates in our study were in line with the ones in Krishnan et al.14 (median OS: 20.3 months versus 17.8 months, 2-year OS rate: 36.8% versus 36.0%, 3-year OS rate: 18.7% versus 31.0%). However, there were controversial results from the meta-analysis;34 the median OS of two studies with the most weight employing BED10 >70 Gy was 12.5 months and 10.3 months, which was inferior to our study. In contrast, the median OS with the most weight employing a BED10 of 60–70 Gy from two studies were found to be 13.9 months and 15.0 months, respectively. The controversial result could be attributed to the different radiosurgery platforms and chemotherapy regimens. Furthermore, the heterogeneity between studies was not evaluated and the primary endpoint was local control rather than OS. Hence, the interpretations of the results may not negatively impact clinical practice with dose escalation as a therapeutic paradigm for locally advanced pancreatic cancer.
Furthermore, the upper limit of the dose for BED10 >70 Gy was not determined. In the case of dose escalation of SBRT for pancreatic cancer, no consensus has been reached about a cut-off dose, beyond which survival benefits may be counteracted by severe adverse effects or other potential unfavorable factors. In ASTRO clinical practice guideline for pancreatic cancer, 33–40 Gy/5f was recommended for locally advanced pancreatic cancer.35 Other studies have only investigated the feasibility and safety of higher doses. One study of doses of 51 Gy to PTV and simultaneous integrated boost to GTV with a median cumulative dose of 66 Gy reported that 10 of 28 patients presented acute toxicities >grade 2, and the median OS and time to local recurrence was 19 and 13 months, respectively.36 Another study demonstrated that total dose ⩾61 Gy was predictive of higher OS and PFS.37 Further, a comprehensive review has clarified that appropriate patient selection, technical considerations with treatment delivery, advanced image guidance, and respiratory management techniques are required to safely deliver higher radiation doses.38 Hence, the upper limit dose may be dependent of highly accurate delivery of radiotherapy without compromise of dose constraints of organs at risk, which warrants randomized clinical trials.
Nevertheless, dose escalation of SBRT may not provide better prognosis for patients with ECOG of 2 points and tumor diameter ⩾4 cm in our study. This may be ascribed to the benefits of higher doses counteracted by radiation-induced toxicities, especially in patients with poor medical conditions. This was confirmed by the higher incidences of acute and late gastrointestinal toxicities in patients with ECOG of 2 points. Moreover, given the shorter distance between the tumor and the gastrointestinal tract, if the tumor diameter was more than 4 cm, more doses would be delivered to the normal tissues thus increasing the risk of toxicities. Hence, for patients with large tumor sizes and poor medical conditions, higher doses may not be recommended. Furthermore, our study showed that patients with a BED10 >70 Gy had higher incidence of acute and late grade 2 or 3 gastrointestinal toxicities, though favorable survival was found. Therefore, a higher dose should be prescribed at the discretion of physicians without compromise of dose constraints of organs at risk.
Concerning recurrence patterns, a higher incidence of both in-field and marginal recurrence was found in the group with a BED10 of 60–70 Gy. Additionally, more patients with a BED10 of 60–70 Gy experienced recurrences at the hepatic hilum and a larger ΔGTV. This was consistent with our previous studies with similar failure patterns in higher doses.15 Hence, it may be indicated that higher doses were beneficial for better tumor local control, which might be pivotal in survival improvement.
There are several limitations in this study. First, though propensity score matching was used to minimize the selection bias, any potential bias or confounding factors may be not eliminated completely due to the retrospective nature of the study. Therefore, generalization of results should be cautious. Second, although patients in two groups received BED10 of 60–70 Gy or BED10 >70 Gy, the dose per fraction and number of fractions varied in three centers. Third, we only compared a BED10 of 60–70 Gy with a BED10 >70 Gy. Practically, it was not allowed to prescribe a higher dose to pancreatic cancer due to the gastrointestinal tracts abutting to the pancreas. Additionally, there was no upper limit in the case of a BED10 >70 Gy in our study as there are no studies demonstrating the cut-off dose until now, and we need to further explore the dose escalation scheme. Fourth, chemoradiotherapy was taken as the first treatment option regarding locally advanced pancreatic cancer; variable chemotherapy regimens could affect prognosis. The regimen applied in our study may be more prevalent in Asia; a favorable outcome obtained from BED10 >70 Gy should be further explored in other regimens.
In conclusion, a higher radiation dose could be prescribed with limited dose toxicity as well as dose constraints of organs at risk being met. The findings might be taken as treatment guidance for patients with locally advanced pancreatic cancer by SBRT. Further prospective studies are required to confirm the results in variable chemotherapy regimens.
Supplemental Material
Supplemental material, sj-pdf-1-tam-10.1177_1758835920977155 for Failure patterns and outcomes of dose escalation of stereotactic body radiotherapy for locally advanced pancreatic cancer: a multicenter cohort study by Xiaofei Zhu, Yangsen Cao, Tingshi Su, Xixu Zhu, Xiaoping Ju, Xianzhi Zhao, Lingong Jiang, Yusheng Ye, Fei Cao, Shuiwang Qing and Huojun Zhang in Therapeutic Advances in Medical Oncology
Acknowledgments
We appreciated Dr. Jiuhong Chen and Huijun Chen, Dr. Xiaofei Zhu’s fiancé, for their precise comments and LinkDoc for their constructive advice in patient follow-up. We are also grateful for Amaya Lanson Hirigoyenberry and Lucien Lanson Hirigoyenberry’s comments on revisions of the manuscript.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded in part by the Special Project of Ministry of Science and Technology (2017YFC0113104).
ORCID iD: Xiaofei Zhu  https://orcid.org/0000-0001-5769-9308
https://orcid.org/0000-0001-5769-9308
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Xiaofei Zhu, Department of Radiation Oncology, Changhai Hospital Affiliated to Navy Medical University, Shanghai, China.
Yangsen Cao, Department of Radiation Oncology, Changhai Hospital Affiliated to Navy Medical University, Shanghai, China.
Tingshi Su, Department of Radiation Oncology, Affiliated Tumor Hospital of Guangxi Medical University, Nanning, Guangxi Zhuang Autonomous Region, China.
Xixu Zhu, Department of Radiation Oncology, General Hospital of Eastern Theater Command, Nanjing, Jiangsu, China.
Xiaoping Ju, Department of Radiation Oncology, Changhai Hospital Affiliated to Navy Medical University, Shanghai, China.
Xianzhi Zhao, Department of Radiation Oncology, Changhai Hospital Affiliated to Navy Medical University, Shanghai, China.
Lingong Jiang, Department of Radiation Oncology, Changhai Hospital Affiliated to Navy Medical University, Shanghai, China.
Yusheng Ye, Department of Radiation Oncology, Changhai Hospital Affiliated to Navy Medical University, Shanghai, China.
Fei Cao, Department of Radiation Oncology, Changhai Hospital Affiliated to Navy Medical University, Shanghai, China.
Shuiwang Qing, Department of Radiation Oncology, Changhai Hospital Affiliated to Navy Medical University, Shanghai, China.
Huojun Zhang, Department of Radiation Oncology, Changhai Hospital Affiliated to Navy Medical University, Shanghai, China.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69: 7–34. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–132. [DOI] [PubMed] [Google Scholar]
- 3. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 4. Ferlay J, Partensky C, Bray F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol 2016; 55: 1158–1160. [DOI] [PubMed] [Google Scholar]
- 5. Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009; 27: 1806–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the national cancer database. J Am Coll Surg 1999; 189: 1–7. [DOI] [PubMed] [Google Scholar]
- 7. Myrehaug S, Sahgal A, Russo SM, et al. Stereotactic body radiotherapy for pancreatic cancer: recent progress and future directions. Expert Rev Anticancer Ther 2016; 16: 523–530. [DOI] [PubMed] [Google Scholar]
- 8. Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys 2002; 53: 1097–1105. [DOI] [PubMed] [Google Scholar]
- 9. Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet 2006; 368: 843–854. [DOI] [PubMed] [Google Scholar]
- 10. Zhu X, Li F, Ju X, et al. Prediction of overall survival after re-irradiation with stereotactic body radiation therapy for pancreatic cancer with a novel prognostic model (the SCAD score). Radiother Oncol 2018; 129: 313–318. [DOI] [PubMed] [Google Scholar]
- 11. Zhu X, Li F, Ju X, et al. Prognostic role of stereotactic body radiation therapy for elderly patients with advanced and medically inoperable pancreatic cancer. Cancer Med 2017; 6: 2263–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu X, Li F, Liu W, et al. Stereotactic body radiation therapy plus induction or adjuvant chemotherapy for early stage but medically inoperable pancreatic cancer: a propensity score-matched analysis of a prospectively collected database. Cancer Manag Res 2018; 10: 1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu X, Shi D, Li F, et al. Prospective analysis of different combined regimens of stereotactic body radiation therapy and chemotherapy for locally advanced pancreatic cancer. Cancer Med 2018; 7: 2913–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krishnan S, Chadha AS, Suh Y, et al. Focal radiation therapy dose escalation improves overall survival in locally advanced pancreatic cancer patients receiving induction chemotherapy and consolidative chemoradiation. Int J Radiat Oncol Biol Phys 2016; 94: 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu X, Ju X, Cao Y, et al. Patterns of local failure after stereotactic body radiation therapy and sequential chemotherapy as initial treatment for pancreatic cancer: implications of target volume design. Int J Radiat Oncol Biol Phys 2019; 104: 101–110. [DOI] [PubMed] [Google Scholar]
- 16. Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys 2010; 37: 4078–4101. [DOI] [PubMed] [Google Scholar]
- 17. Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 2013; 31: 1640–1648. [DOI] [PubMed] [Google Scholar]
- 18. Sudo K, Yamaguchi T, Nakamura K, et al. Phase II study of S-1 in patients with gemcitabine-resistant advanced pancreatic cancer. Cancer Chemother Pharmacol 2011; 67: 249–254. [DOI] [PubMed] [Google Scholar]
- 19. Morizane C, Okusaka T, Furuse J, et al. A phase II study of S-1 in gemcitabine-refractory metastatic pancreatic cancer. Cancer Chemother Pharmacol 2009; 63: 313–319. [DOI] [PubMed] [Google Scholar]
- 20. Ferrone CR, Finkelstein DM, Thayer SP, et al. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol 2006; 24: 2897–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol 2011; 29: 4548–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cloyd JM, Wang H, Egger ME, et al. Association of clinical factors with a major pathologic response following preoperative therapy for pancreatic ductal adenocarcinoma. JAMA Surg 2017; 152: 1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zins M, Matos C, Cassinotto C. Pancreatic adenocarcinoma staging in the era of preoperative chemotherapy and radiation therapy. Radiology 2018; 287: 374–390. [DOI] [PubMed] [Google Scholar]
- 24. Dholakia AS, Kumar R, Raman SP, et al. Mapping patterns of local recurrence after pancreaticoduodenectomy for pancreatic adenocarcinoma: a new approach to adjuvant radiation field design. Int J Radiat Oncol Biol Phys 2013; 87: 1007–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 26. Minniti G, Amelio D, Amichetti M, et al. Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother Oncol 2010; 97: 377–381. [DOI] [PubMed] [Google Scholar]
- 27. Lee SW, Fraass BA, Marsh LH, et al. Patterns of failure following high-dose 3-D conformal radiotherapy for high-grade astrocytomas: a quantitative dosimetric study. Int J Radiat Oncol Biol Phys 1999; 43: 79–88. [DOI] [PubMed] [Google Scholar]
- 28. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995; 31: 1341–1346. [DOI] [PubMed] [Google Scholar]
- 29. Schellenberg D, Goodman KA, Lee F, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2008; 72: 678–686. [DOI] [PubMed] [Google Scholar]
- 30. Mahadevan A, Miksad R, Goldstein M, et al. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys 2011; 81: e615–e622. [DOI] [PubMed] [Google Scholar]
- 31. Mahadevan A, Jain S, Goldstein M, et al. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2010; 78: 735–742. [DOI] [PubMed] [Google Scholar]
- 32. Goyal K, Einstein D, Ibarra RA, et al. Stereotactic body radiation therapy for nonresectable tumors of the pancreas. J Surg Res 2012; 174: 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoyer M, Roed H, Sengelov L, et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol 2005; 76: 48–53. [DOI] [PubMed] [Google Scholar]
- 34. Zaorsky NG, Lehrer EJ, Handorf E, et al. Dose escalation in stereotactic body radiation therapy for pancreatic cancer: a meta-analysis. Am J Clin Oncol 2019; 42: 46–55. [DOI] [PubMed] [Google Scholar]
- 35. Palta M, Godfrey D, Goodman KA, et al. Radiation therapy for pancreatic cancer: executive summary of an ASTRO Clinical Practice Guideline. Pract Radiat Oncol 2019; 9: 322–332. [DOI] [PubMed] [Google Scholar]
- 36. Zschaeck S, BluÈmke B, Wust P, et al. Dose-escalated radiotherapy for unresectable or locally recurrent pancreatic cancer: dose volume analysis, toxicity and outcome of 28 consecutive patients. PLoS One 2017; 12: e0186341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chung SY, Chang JS, Lee BM, et al. Dose escalation in locally advanced pancreatic cancer patients receiving chemoradiotherapy. Radiother Oncol 2017; 123: 438–445. [DOI] [PubMed] [Google Scholar]
- 38. Koay EJ, Hanania AN, Hall WA, et al. Dose-escalated radiation therapy for pancreatic cancer: a simultaneous integrated boost approach. Pract Radiat Oncol. Epub ahead of print 13 February 2020. DOI: 10.1016/j.prro.2020.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tam-10.1177_1758835920977155 for Failure patterns and outcomes of dose escalation of stereotactic body radiotherapy for locally advanced pancreatic cancer: a multicenter cohort study by Xiaofei Zhu, Yangsen Cao, Tingshi Su, Xixu Zhu, Xiaoping Ju, Xianzhi Zhao, Lingong Jiang, Yusheng Ye, Fei Cao, Shuiwang Qing and Huojun Zhang in Therapeutic Advances in Medical Oncology