Abstract
Lasmiditan, a highly selective 5-hydroxytryptamine receptor 1F (5-HT1F) agonist, is the first drug in its class and is lacking triptan-like vasoactive properties. The US Food and Drug Administration (FDA) has recently approved lasmiditan for the acute treatment of migraine in adults based on positive results of two pivotal phase III trials, which showed a significant difference to placebo in the proportion of patients achieving total migraine freedom within 2 h. More patients with lasmiditan achieved headache freedom and, in addition, freedom from the most bothersome symptom, that is, photophobia, than with placebo. Treatment-related side effects seem to be related to the rapid penetration of the drug into the brain and include dizziness, paresthesia and drowsiness, mostly of mild to moderate intensity. Interim results from an ongoing long-term phase III trial suggest a decrease in the frequency of adverse events after multiple lasmiditan use. Lasmiditan is a promising acute anti-migraine therapy, in particular for patients with cardiovascular risk factors, contraindications, or unwanted side effects to triptans.
Keywords: CGRP, cluster headache, ditan, dizziness, 5-HT1F, lasmiditan, migraine
Introduction
According to the Global Burden of Disease Study, migraine is the second leading cause of years lived with disability among non-fatal diseases.1 Migraine affects people in the most productive years of their lives and is more prevalent in women (18%) than in men (6%).2 The disease is therefore a significant public health topic. Most patients use unspecific pain medications such as non-steroidal anti-inflammatory drugs (NSAIDs; for example, ibuprofen or acetylsalicylic acid) for acute migraine headache relief.3 In patients who do not benefit from unspecific drugs, triptans or dihydroergotamine (DHE) are widely used.3
Both triptans and DHE have been developed when vasodilation was considered the primary nociceptive stimulus in migraine pathophysiology. They induce vasoconstriction by binding to the serotonin 5-HT1B receptor type on smooth muscle cells in the coronary and cerebral arteries.4 Hence, neither triptans nor ergots are suitable for patients with cardio- or cerebrovascular diseases. Diseases such as hemiplegic migraine or migraine with complicated aura also do not allow the intake of a triptan.5
Among the risk factors for cardiovascular disease, obesity is on the rise, also affecting a younger population.6 Together with other risk factors; for example, hypercholesterinemia or diabetes, these changes in the general population may also lead to significantly more migraine patients with cardiovascular disease, who are in need for acute anti-migraine drugs without vasoactive properties.
Moreover, a significant percentage of patients do not benefit from oral triptans and numerous others do not tolerate typical triptan side effects such as muscle pain/stiffness, paraesthesia, neck or chest tightness.7 In line with this, in the American Migraine Prevalence and Prevention (AMPP) study, more than 40% of people with episodic migraine reported at least one unmet therapeutic need in a large US population sample.8
Based on these observations, acute migraine treatment is all but optimal at this stage and it is obvious that we need novel drugs. The treatment of acute migraine pain usually happens in an outpatient setting and only a minority of patients come to the emergency room for acute care. Therefore, new drugs should come primarily in an oral formulation.
Two types of drug classes for acute migraine therapy are novel: the small molecule calcitonin gene-related peptide (CGRP) antagonists, known as gepants, and the selective serotonin 5-HT1F receptor agonist lasmiditan as the first drug of the class of ditans. The US Food and Drug Administration (FDA) approved ubrogepant and rimegepant (gepants), and lasmiditan in 2019. While CGRP plays a role in blood vessel tone and vascular reactivity, lasmiditan is devoid of any interaction with blood vessels.9 Other differences may relate to the unique ability of lasmiditan to penetrate the blood–brain barrier rapidly due to its lipophilic nature.9 In light of the treatment limitations of triptans and the independent increased cardiovascular risk of migraine patients,10 it is an important step into the future to have an acute migraine drug available devoid of vascular action. Lasmiditan may also tell us whether drugs that have, at least in part, a central mode of action have a higher efficacy rate to abort acute migraine than drugs with a predominant mode of action outside the CNS and low blood–brain barrier penetration.
Chemistry and developmental history
Lasmiditan (2,4,6-trifluor-N-6-[(1-methyl-piperidin-4-yl)carbonyl]pyridin-2-yl-benzamid) is a highly selective 5-HT1F receptor agonist.11 The 5-HT1F receptor is expressed on the presynaptic surface of central and peripheral trigeminal sensory neurons, and its activation does not lead to vasoconstriction.12 These properties render the receptor an ideal target for new migraine acute treatments. In the 1990s, the first selective 5-HT1F receptor agonists (LY344864 and LY334370) showed in animal models their ability to inhibit dura protein extravasation without causing vasoconstriction.12 However, the developmental programme ended due to toxicity issues in animals.12 Unlike triptans and the aforementioned precursor substances, lasmiditan presents a pyridinoylpiperidine scaffold, which is unique for an acute migraine medication and replaces the typical indol structure of triptans.13 Because of its central binding site, lasmiditan strengthens our revised understanding of migraine, which for many years was considered an acute trigeminal-vascular pain syndrome that can be aborted exclusively by the constriction of cranial blood vessels. Our current understanding of migraine pathophysiology considers vasodilation rather as an epiphenomenon during migraine attacks.14
Preclinical studies
Lasmiditan was studied in several preclinical models with predictive value for anti-migraine efficacy.13 In vitro studies using radioligand-binding techniques showed a 470-fold higher selectivity of lasmiditan for the 5-HT1F receptor than the 5-HT1B and 5-HT1D receptors (Ki 2.21 nM versus 1043 nM/1357 nM),13 meaning that lasmiditan has no affinity at the 5-HT1B/1D receptor in clinically relevant doses. In line with the lack of binding to this 5-HT1B receptor subtype, lasmiditan does not cause vasoconstriction in experimental in vitro and animal studies.13
Calcitonin gene-related peptide (CGRP) is a transmitter of critical importance in migraine pathophysiology.15 The release of this neuropeptide is a crucial component in the development of acute migraine headache. Triptans bind to presynaptic 5-HT1B/D/F receptors in vivo and in vitro and thereby reduce CGRP release.16 This mechanism seems to be a key component for the abortion of acute migraine, but vasoconstriction may also contribute.
The efficacy of lasmiditan to block CGRP release via the 5-HT1F receptor has been studied in vitro and in animal models in samples of dura mater, trigeminal ganglion and trigeminal nucleus caudalis of rodents.17 Lasmiditan blocked the release of CGRP in all tissues in a magnitude comparable to the blocking activity of sumatriptan in the identical setting.17 In vivo, lasmiditan infusion inhibits neurogenic dural vasodilation induced through i.v. capsaicin and electrical trigeminal ganglion stimulation, at lower doses compared to sumatriptan.17 However, lasmiditan was not able to attenuate non-neurogenic vasodilation in dura mater in response to exogenous CGRP infusion, which implies a presynaptic mechanism of action; that is, inhibition of endogenous CGRP release. Unfortunately, a lasmiditan dose–response curve in this assay has not been published. Yet, these results indicate that the activation of the 5-HT1F receptor alone is sufficient to block CGRP release.
Another assay for predictive drug testing in migraine uses the leakage of plasma protein from venous blood vessels into dura mater tissue.13 Experimental stimulation of the trigeminal ganglion by the application of electrical, chemical, or immunological impulses leads to an ipsilateral response of enhanced protein leakage in dura mater. Administration (i.v./i.p.) of a non-selective 5-HT1B/D/F agonist (sumatriptan/rizatriptan) or the selective 5-HT1D receptor agonist (alniditan) prior to stimulation reduced ipsilateral meningeal plasma protein extravasation in experimental animal studies and so does the highly selective 5-HT1F receptor agonist lasmiditan.13 In another series of experiments, lasmiditan reduced stimulus-induced ipsilateral expression of the proto-oncogene c-fos in trigeminal nucleus caudalis neurons.13 The potential of drugs to block the activation of these second order neurons is also thought to predict the anti-migraine potential of novel substances. Oral lasmiditan doses of 3 µg/kg or higher reduced the number of stimulus induced c-fos signals in neurons by 50%.13 A lower dose of lasmiditan had a lower effect, indicating a dose-dependent effect.
Lasmiditan behaves in all these aforementioned experimental migraine assays very similarly to triptans, indicating efficacy in acute migraine treatment. There is no clear efficacy benefit for lasmiditan over triptans in these preclinical models. In summary, preclinical studies show that the specific activation of the 5-HT1F receptor by lasmiditan leads to the blockade of trigeminally mediated responses, such as CGRP release, which can also be achieved by non-selective 5-HT agonists (e.g. triptans).
The observation that lasmiditan can stimulate mitochondrial biogenesis is of interest for the pathophysiology of migraine.18 Mitochondrial dysfunction has been proposed to play a critical role and substances interfering with cell energy metabolism such as riboflavin are efficacious in migraine prevention.19 It is not yet clear whether the effect on mitochondria has any meaning for long-term acute migraine therapy, but this effect of lasmiditan will stimulate future research on brain metabolism in migraine.20
Phase I clinical trials
Five phase I lasmiditan trials have been conducted between 2003 and 2015, the first one with an intravenous formulation, the others with oral solutions or tablets. The oral bioavailability of lasmiditan is approximately 40%, and oral doses of 50–400 mg lasmiditan reach Tmax after 1.5–2 h, independent on gender.21 No peer-reviewed publications exist for these studies.
A phase I study assessed the effects of lasmiditan on cardiovascular parameters in 44 healthy subjects receiving propranolol.22 In combination with propranolol (80 mg BID), lasmiditan tablets decreased the heart rate shortly after dosing while increasing arterial blood pressure when compared to propranolol alone. While arterial blood pressure values returned to pre-dose levels within 3 h, the heart rate remained significantly lower over a 12-hour time period post-dose. The mechanism of this phenomenon is unclear, but the observation indicates at least a minimal cardiovascular activity of lasmiditan.
A phase I randomized, crossover study assessed the abuse potential of lasmiditan (100, 200, 400 mg) in adult recreational polydrug users in comparison to placebo and alprazolam 2 mg as positive control.23 This study was based on the side effect profile of lasmiditan in a few cases who experienced euphoric mood changes and abnormal feelings. The latter indicates a risk for substance abuse. The primary endpoint was the maximal effect score of the Drug-Liking Visual Analog Scale. Drug-liking scores for a high dose of 400 mg of lasmiditan, which are not used for therapy, were not significantly different from alprazolam but the drug-liking scores at the therapeutic doses (100 and 200 mg) were significantly different from alprazolam, but not as low as placebo. Therefore, the potential for abuse of lasmiditan appears to be low. In light of the development of medication overuse headache (MOH) in patients with high-frequency episodic migraine (EM) and chronic migraine (CM), this observation is of importance. However, the question of whether lasmiditan exerts higher rates of MOH than triptans remains to be determined.
Phase II clinical trials
Two phase II, randomized, multicenter, placebo controlled, double-blind studies were published in 2011 and 2012.24,25 The first study used an intravenous formulation (COL MIG-201) and was a dose-finding, proof of concept study.24 A total of 130 subjects between 18 and 65 years with moderate to severe migraines, with at least a 1-year history of migraine, and between one and eight migraine attacks per month were given adjusted doses of i.v. lasmiditan or placebo. The trial did not allow the enrolment of patients with prophylactic medications.
Participants were allocated to either placebo (n = 42) or lasmiditan (n = 88) in intravenous doses ranging from 2.5 to 45 mg. The study design allowed up and down titration of the study drug in an adaptive-treatment design, depending on efficacy and adverse events of small cohorts. The drug was infused over 20 min and subjects were monitored for 4 h after infusion for electrocardiogram (ECG), vital signs, adverse events, headache, and other migraine symptoms. The primary endpoint was headache response (improvement from moderate or severe to mild or none) after 2 h of initiation of study dose.
A dose–response relationship was detected with increasing doses of lasmiditan leading to better response rates. The 2-hour headache response with escalating doses of intravenous lasmiditan was statistically significantly superior for the 10 mg (54%), 20 mg (64%), 30 mg (69%), and 45 mg (75%) doses when compared to placebo (45%, p = 0.01). The onset of pain relief occurred after 20–40 min. The design of the study does not allow the detection of a statistical difference of a specific lasmiditan dose.
There were no serious adverse events reported. The most common side effects were paresthesia and dizziness, with no clear dose-related response.
The second phase II trial (COL MIG-202) used a rapid disintegrating tablet to evaluate the safety and efficacy of oral lasmiditan in acute migraine.25 This randomized, double-blind, placebo controlled, dose ranging study, was conducted in healthy patients between 18 and 65 years with a history of one to eight migraine attacks per month. Previous prophylactic drugs were discontinued at least 2 weeks before screening. Patients were randomly allocated to either oral lasmiditan (50, 100, 200, or 400 mg) or placebo in a 1:1:1:1:1 ratio. Of the 378 participants included in the study, 297 received lasmiditan.
The percentage of patients who were pain free at 2 h was significant with the 200 mg (19%, p = 0.032) and 400 mg doses (28%, p = 0.0007), but was not statistically significant at the lower oral doses of 50 mg (14%, p = 0.18) and 100 mg (14%, p = 0.19) when compared to placebo (7.4%).
The percentage of headache responders after 2 h was statistically significant when compared to placebo (26%) for the 50 mg (43%, p = 0.022), 100 mg (64%, p = 0.0001), 200 mg (51%, p = 0.0018), and 400 mg (65%, p = 0.0001) doses of lasmiditan. Patients reported an improvement of associated migraine symptoms such as nausea, vomiting, phono and photophobia after 2 h and the strongest effect was seem for phono and photophobia with the 100 and 400 mg doses.
Phase III clinical trials
Lasmiditan has been studied in three phase III clinical trials.26–28 The two pivotal double-blind, placebo controlled, randomized trials have been completed and the results are published. A third phase III trial is an open-label, long-term safety study that is still ongoing but not recruiting anymore. The interim results have also been published.28
SAMURAI (COL MIG-301)
SAMURAI is a prospective randomized, double-blind, placebo controlled, parallel group study, analyzing the efficacy of two doses (100 mg and 200 mg) of lasmiditan versus placebo on a single attack of migraine (with or without aura) within 4 h of onset.26
Inclusion criteria were a history of disabling migraine for at least 1 year, defined as Migraine Disability Assessment Score (MIDAS) total score of >11, a history of three to eight migraine attacks per month, and migraine onset before 50 years. Subjects had to be >18 years of age with no upper limit, fulfilling the International Headache Society diagnostic criteria for migraine with or without aura. Exclusion criteria included a history of chronic migraine within the past 12 months, other forms of secondary headache or medication-overuse headache, known coronary artery disease, clinically significant arrhythmia, or uncontrolled hypertension. Preventive medication was allowed if stable on the dose for the previous 3 months.
The primary endpoint was headache freedom at 2 h post 200 mg dose. Secondary endpoints were headache freedom at 2 h post 100 mg dose and freedom of the most bothersome symptom at 2 h. The most bothersome symptom is a novel endpoint. During a migraine attack (prior to dosing) patients indicated the presence of nausea, phonophobia, or photophobia and identified which was the most bothersome symptom.
The study enrolled 2231 patients, of whom 1856 (83%) used the first dose of the study drug. A total of 1805 patients (97%) completed the study. Data were collected with electronic diaries. Patients were randomly allocated (1:1:1) to a first dose of lasmiditan 100 mg, 200 mg, or placebo. For rescue or recurrence of headache, the study protocol allowed a second dose of the study drug between 2 and 24 h after the first dose. Patients were also randomly allocated to a second dose of lasmiditan (2:1) or placebo (all patients in the placebo group received placebo as the second dose).
The study population consisted mainly of women (84%), white people (75%), with a mean age of 42 years. Over 75% of patients had at least one cardiovascular risk factor in addition to the diagnosis of migraine. Cardiovascular risk factors included: current smoker, hypertension, hyperlipidemia, history of diabetes, and over 40 years of age. Patients had a long history of migraine, with a mean duration of 19 years (SD 13 years), and had experienced an average of 5 ± 1.9 migraines per month in the previous 3 months. In the study, 32% of patients reported the existence of migraine aura. Photophobia was the most commonly reported symptom (54%) followed by nausea (24%) and phonophobia (22%).
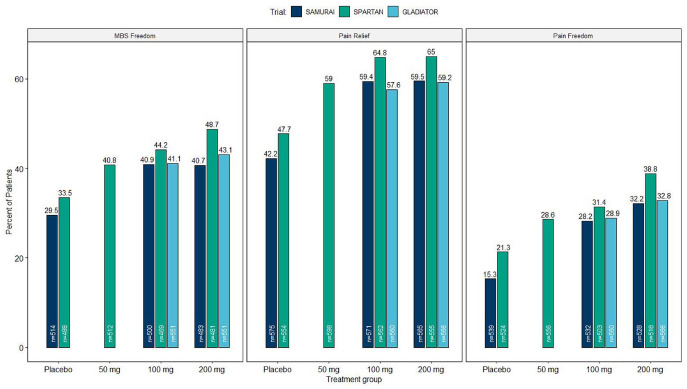
The percentages of patients achieving headache freedom at 2 h after the first dose were statistically significant (p < 0.001) when compared to placebo (15.3%) for 200 mg (32.2%) and 100 mg (28.2%) doses of lasmiditan. Superiority over placebo (p < 0.05) was noted after 1 h for lasmiditan 200 mg and after 1.5 h for 100 mg of lasmiditan. Similarly, the percentages of patients experiencing the absence of the most bothersome symptom were higher for lasmiditan 200 mg (40.7%) and lasmiditan 100 mg (40.9%) than placebo (29.5%, p < 0.001). The analysis revealed a difference at 0.5 h after dosing.
SPARTAN (COL MIG-303)
The second pivotal study has a similar design, as well as identical primary and secondary outcomes as SAMURAI.27 In this study, three doses of lasmiditan (50, 100, and 200 mg) were compared to placebo in the acute treatment of a single attack of migraine. The study ended on 30 June 2017, with 3005 patients enrolled. A total of 2583 patients received at least one dose of the study drug. In contrast to SAMURAI, SPARTAN did not exclude patients with known coronary artery disease, clinically significant arrhythmia, or uncontrolled hypertension.
The percentage of patients who were pain free 2 h after administration of 50 mg (28.6%), 100 mg (31.4%), and 200 mg (38.8%) of lasmiditan was statistically significantly different (p < 0.005) when compared to placebo (21.3%). Improvements of the most bothersome symptom 2 h post-treatment were statistically significant when compared to placebo (33.5%) with 50 mg (40.8%, p = 0.003), 100 mg (44.2%, p < 0.001), and 200 mg (48.7%, p < 0.001) doses of lasmiditan.
Although the primary endpoint was set at the 2-hour mark, which is around the Tmax for oral doses, effects of lasmiditan became significant earlier. In a post-hoc analysis of both trials significantly higher rates were seen for freedom from the most bothersome symptom (100 mg, 11.1%; 200 mg, 13.0%; placebo, 7.9%), and pain relief (100 mg, 17.5%; 200 mg, 19.1%; placebo, 13.4%) as early as 30 min.29
In a post-hoc analysis of both pivotal studies (SAMURAI and SPARTAN), it was analyzed whether the response to lasmiditan differed according to prior triptan response.30 Patients were asked to rate themselves as good, poor, or non-responders to prior treatments. Only patients who had used triptans within the past 3 months were analyzed. Patients taking lasmiditan (100 mg and 200 mg) experienced higher rates of headache pain freedom at 2 h versus placebo regardless of prior response to triptans. In participants randomly assigned to lasmiditan 100 mg, the percentage of patients who were pain free at 2 h was significantly better in triptan poor/non-responders (33.3%) versus good responders (24.0%, p < 0.05). Similar results were obtained for most bothersome symptom (MBS) freedom at 2 h. Higher placebo response rates in good responders than in poor/non-responders may explain the differences. The response in participants randomly allocated to lasmiditan 200 mg was similar in triptan poor/non-responders versus good responders. Therefore, lasmiditan offers a possible alternative migraine therapy option regardless of prior response to triptans.
In the two pivotal trials (SAMURAI and SPARTAN) 698 of 3981 patients (17.5%) used migraine preventive treatments. In a post-hoc analysis, the efficacy and safety of lasmiditan in patients using a concomitant migraine preventative were not significantly different compared to patients not using preventive medication.31 The average baseline monthly attack frequency in the past 3 months did not differ significantly between these groups.
In both trials (SAMURAI and SPARTAN) a second dose of the study drug was allowed between 2 and 24 h after the first dose for rescue or recurrence of headache. The proportion of patients taking a second dose was lower with lasmiditan than with placebo and decreased with a higher lasmiditan dose.32 A second dose of lasmiditan showed some evidence of efficacy versus placebo when taken for headache recurrence for freedom of the most bothersome symptom (71% versus 41%, p = 0.02) and pain relief (77% versus 52%, p = 0.03), but in pain freedom (50% versus 32%, p > 0.05) there was no significant difference.32 There was also no clear benefit of a second dose of lasmiditan as a rescue treatment.
Sustained responses to lasmiditan were found after 1 and 2 days for several efficacy endpoints in a further post-hoc analysis of both pivotal trials (SAMURAI and SPARTAN).33 The rate of sustained pain freedom was significantly higher at 24 h (200 mg: 21.2%; 100 mg: 16.9%; 50 mg: 17.4%; placebo: 10.3% (all p < 0.01)) and at 48 h (200 mg: 18.4%; 100 mg: 15.2%; 50 mg: 14.9%; placebo: 9.6% (all p < 0.05)).
GLADIATOR (COL MIG-305)
Gladiator is an on-going open-label phase III trial to evaluate the safety and efficacy of long-term intermittent use of lasmiditan 100 and 200 mg for the acute treatment of migraine. Eligible patients from SAMURAI and SPARTAN were enrolled and will be treated over a 1-year period for up to eight migraine attacks per month. Patients were randomly allocated to either lasmiditan 100 mg or 200 mg regardless of their treatment assignment in the feeder studies, which may pose a limitation. Also, not every attack has to be treated with the study drug.
The primary endpoints are the proportion of patients who experienced adverse events and the proportion of migraine attacks associated with adverse events. The secondary endpoint aims to evaluate the proportion of attacks treated with study drug, which respond at 2 h post-treatment, for each 3-month period.
Interim results were recently published including a total of 1978 patients who received at least one dose of the study drug and with a total of 19,058 treated migraine attacks.28 Across all treated attacks, patients reached pain freedom at 2 h post-treatment in 26.9% of the attacks with lasmiditan 100 mg and 32.4% of the attacks treated with lasmiditan 200 mg. For both doses, efficacy measures were generally consistent over study quarters and treated attacks. Study analysis did not reveal treatment-related serious adverse events and treatment-related cardiovascular adverse events due to vasoconstriction. The migraine-related disability, measured with the MIDAS, was significantly lower at 3, 6, 9, and 12 months for both dose groups34 (Figure 1).
Figure 1.
Shows the percentage of patients in clinical phase III trials of lasmiditan who achieved pain freedom, freedom of the most bothersome symptom (MBS), and pain relief at 2 h post-dose.
Clinical safety and tolerability
The safety profile of lasmiditan was published in phase II and III clinical trials. In general, lasmiditan was well tolerated and safe. In the phase II clinical trial (COL MIG-202) most of the adverse events were mild or moderate in intensity.25 The most frequently reported treatment-emergent adverse events were associated with the central nervous system or the vestibular system. Dizziness was the most frequently reported severe adverse event, increasing with dose 50 mg (24.3%), 100 mg (35.4%), 200 mg (35.4%), 200 mg (54.2%). Other side effects include paresthesia, somnolence, fatigue, and nausea. Phase II clinical trials did report chest pain as an adverse event.
In phase III clinical trials (SAMURAI and SPARTAN) the incidence of adverse events was higher in the lasmiditan 200 and 100 mg groups compared with the placebo group. The majority of adverse events were mild or moderate in intensity, and none of the patients discontinued the study due to the adverse event. Dizziness was the most frequently reported adverse event.26 In a post-hoc analysis of SPARTAN and SAMURAI, the onset and duration of dizziness was similar across all treatment groups (50, 100, 200 mg).35 Generally, dizziness onset occurred approximately 30–40 min after dosing, lasted 1.5–2 h, and was of mild to moderate severity. The presence of dizziness did not appear to have a negative influence on the drug’s effect on daily activity, the patient global impression of change, freedom from pain, or freedom from the most bothersome symptom.35
The occurrence of dizziness increased with a higher drug dose. In participants who received lasmiditan as their first study drug, a lower body mass index was a risk factor for dizziness. Dizziness is distinct from vertigo, which had a frequency of 0.6% in the treatment groups compared to <0.1% in the placebo group in the two pivotal trials. The study design may have influenced the frequency of vertigo, which prompted investigators with a follow-up assessment when patients mentioned this symptom. The question was whether the participant had experienced a sensation of rotation or movement. If this was not the case, dizziness was suggested as the alternative adverse event (AE). It is not clear whether it is clinically relevant to distinguish between vertigo and dizziness in this particular case. The dose-dependent occurrence of dizziness and the presence of 5-HT1F receptors on the cerebellum and vestibular nuclei suggest a central cause of this side effect.36
The interim results from the GLADIATOR trial showed treatment-emergent adverse events similar to those in the single-attack studies and included dizziness (18.6%), somnolence (8.5%), and paresthesia (6.8%).28 Interestingly, in this trial, the frequency of treatment-emergent adverse events generally decreased with subsequent attacks. Table 1 shows the frequency of adverse events in the phase III trials.
Table 1.
Frequency of adverse events in the clinical phase III trials of lasmiditan.
| SAMURAI (COL MIG-301)27 |
SPARTAN (COL MIG-303)29 |
GLADIATOR28 (interim results) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Randomization | Placebo (n = 617) | 100 mg (n = 630) | 200 mg (n = 609) | Placebo (n = 645) | 50 mg (n = 654) | 100 mg (n = 635) | 200 mg (n = 649) | 100 mg (n = 963) | 200 mg (n = 1015) |
| Frequent adverse events | |||||||||
| Dizziness | 3.4% | 13% | 16% | 2.5% | 8.6% | 18.1% | 18% | 15.8% | 21.3% |
| Paresthesia | 2.1% | 5.7% | 7.9% | 0.9% | 2.8% | 6% | 6.6% | 5.3% | 8.3% |
| Somnolence | 2.3% | 5.7% | 5.4% | 2.0% | 5.4% | 4.6% | 6.5% | 7.8% | 9.3% |
| Fatigue | 0.3% | 4.1% | 3.1% | 0.9% | 2.8% | 4.1% | 4.8% | 4.7% | 6.2% |
| Nausea | 1.9% | 3.0% | 5.3% | 1.2% | 2.8% | 3.3% | 2.8% | 4.2% | 5.2% |
| Lethargy | 0.3% | 1.9% | 2.5% | 0.2% | 1.2% | 1.3% | 2.2% | 2% | 1.3% |
| Vertigo | 0% | 1% | 0.3% | 0.2% | 0.3% | 0.8% | 0.6% | 1% | 2.4% |
Because lasmiditan penetrates the blood–brain barrier easily and the most common side effects are central nervous system (CNS)-related events (e.g. dizziness, drowsiness, and fatigue), driving studies were necessary to evaluate a possible impairment of driving skills after substance intake. Two crossover studies revealed an impaired simulated driving performance at 1.5 h post-dose, but no clinically meaningful driving impairment was observed at 8, 12, or 24 h after the administration.37 This led the FDA to the recommendation that patients should be advised not to drive or operate machinery for at least 8 h after taking lasmiditan.
Among cardiovascular symptoms, palpitations were the most frequently reported adverse event in a few patients: 0.7% for 200 mg, 0.3% for 100 mg and 0% in placebo in SAMURAI. SPARTAN reported similar findings. In SAMURAI 77.9% of patients had at least one cardiovascular risk factor at baseline, although patients with known coronary artery disease, clinically significant arrhythmia, or uncontrolled hypertension could not participate due to exclusion criteria. SPARTAN, however, included patients with pre-existing cardiovascular diseases.
In a pooled post-hoc analysis of SPARTAN and SAMURAI, there was a low frequency of likely cardiovascular treatment-emergent adverse events overall (lasmiditan 0.9%; placebo 0.4%).38 There was also no statistical difference in the frequency of probable cardiovascular treatment-emergent adverse events relating to the presence or absence of cardiovascular risk factors.
In both studies, there were no reports of chest pain or serious cardiovascular side effects.
Thus, lasmiditan seems to be well tolerated among patients with cardiovascular risk factors.
Future directions
A series of clinical trials established the efficacy and tolerability of lasmiditan. Most trials did not allow the participation of patients with migraine preventive medications. Therefore, we have limited information on the efficacy of lasmiditan in patients who are on migraine prevention with another drug that interferes with the CGRP system such as a monoclonal CGRP receptor or CGRP antibody (monoclonal antibody). This topic parallels the discussion on the efficacy of small molecule CGRP receptor antagonists (e.g. ubrogepant, rimegepant) in patients using a mAb. While clinical observations support the combination, such a randomized clinical trial to establish the efficacy of lasmiditan or a gepant in patients with a CGRP monoclonal antibody is missing. In addition to the efficacy measures, safety and tolerability are of interest with the aforementioned combinations.
A potential indication for lasmiditan consists of the use of lasmiditan for cluster headache (CH) prevention. The idea is based on the release of CGRP in acute CH attacks and the existence of cardiovascular risk factors in this patient population.39 Patients with cluster headache present with an increased risk of cardiovascular disease.40 Drugs such as triptans that abort CGRP release block acute CH attacks.39 If a novel drug that blocks CGRP release is devoid of vasoconstriction, a daily use seems feasible. Very low doses of lasmiditan may be of interest for CH prevention in light of the car driving limitations with doses used for migraine therapy.
With a short attack duration between 15 and 180 min, a fast-acting acute medication is needed for acute and rapid relief in these patients and an oral application is probably too slow (Tmax after 1.5–2 h), although patients reported significant benefits as early as 30 min in migraine trials.21,29 Faster acting applications of lasmiditan such as an intranasal spray could be useful for acute CH therapy.
Conclusion
In October 2019, the FDA approved lasmiditan oral tablets for the acute treatment of migraine based on the two pivotal phase III clinical trials. The FDA recommends a dose of 50 mg, 100 mg or 200 mg lasmiditan for first-time treatment. The maximum dose should not exceed 200 mg in 24 h. Based on the results from the two pivotal trials the FDA does not recommended a second dose for the same migraine attack. The first effects of lasmiditan are seen as early as 30 min after intake, while Tmax is reached after around 2 h. Pain-free rates of lasmiditan seem to be above gepant pain-free rates, while CNS adverse events are more common than gepant central nervous system side effects. This needs to be stated with caution as a head to head trial between lasmiditan and gepant for the acute treatment of migraine does not exist.
Lasmiditan has a distinct adverse event profile when compared to triptans or gepant, with dizziness as the predominant side effect. Other common adverse events are paresthesia, drowsiness, fatigue, and nausea. Interim results from the ongoing GLADIATOR trial suggest that treatment-associated adverse events of lasmiditan decrease with subsequent attacks, which would be very beneficial for patients. In contrast, the efficacy of lasmiditan is not reduced by multiple attack use. The risk of lasmiditan overuse will also become clear over time.
Interestingly, a post-hoc analysis of both pivotal studies (SAMURAI and SPARTAN) suggests that the response to lasmiditan is independent of self-reported prior triptan response.30 The issue herewith relates to self-reported prior triptan response. This is a most subjective parameter, which cannot be argued. It is almost impossible to have an objective measure of non-response, partial or full response. Real-world data will determine whether a response to lasmiditan is also seen in distinct patient populations including patients with a 100% or insufficient triptan response. An identical response of lasmiditan in triptan ‘super’ responders will shed further light on the importance of a vascular component of triptans in order to abort migraine headaches.
Currently, the drug seems to be a new therapeutic option for patients with contraindications for triptan use due to cardiovascular risk factors or patients with unwanted side effects, and thereby expands the arsenal of acute anti-migraine therapies.
Finally, the clinical availability of a specific 5-HT1F receptor agonist will provide insights into the relevance of this sub-receptor; for example, in the development of medication overuse headache. We might also learn more about the pathophysiology of vertigo and dizziness based on the profile of lasmiditan.
One might ask why we need another medication for acute migraine therapy. Primarily we have another therapeutic option for patients whose attacks are ‘difficult to treat’ as of today. We are fully aware that this term needs a clear definition, but we are also very much aware that these patients exist. We are also convinced that novel highly specific substances shed new light on the pathophysiology of migraine and thereby help to understand the disease better. New findings stipulate new questions.
Footnotes
Conflict of interest: UR has received honoraria for the participation in advisory boards, speaker fees from Pharm Allergan, Amgen, Autonomic Technologies, ElectroCore, Eli Lilly, Medscape, Novartis, STREAMedUP, and Teva. UR received research support from Novartis and BMBF. JM has received honoraria for the participation in advisory boards from Novartis. BR has received research funding and honoraria from Novartis Pharma, TEVA, Hormosan, and Pharm Allergan. LN received honoraria for the participation in advisory boards and speaker fees for oral presentations from Novartis Pharma, Eli Lilly, Pharm Allergan, Desitin, Hormosan, and TEVA. MS received honoraria for the participation in advisory boards and speaker fees for oral presentations from Allergan, Eli Lilly, Novartis, and Teva. LN and UR have research support from Novartis and BMBF (Innovation fund).
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Uwe Reuter  https://orcid.org/0000-0002-8527-0725
https://orcid.org/0000-0002-8527-0725
Contributor Information
Jasper Mecklenburg, Department of Neurology, Charité Universitätsmedizin Berlin, Berlin, Germany.
Bianca Raffaelli, Department of Neurology, Charité Universitätsmedizin Berlin, Berlin, Germany.
Lars Neeb, Department of Neurology, Charité Universitätsmedizin Berlin, Berlin, Germany.
Margarita Sanchez del Rio, Neurology Department, Clinica Universidad de Navarra, Madrid, Spain.
Uwe Reuter, Department of Neurology, Charité Universitätsmedizin Berlin, Charitéplatz 1, Berlin 10117, Germany.
References
- 1. GBD 2015. Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin 2019; 37: 631–649. [DOI] [PubMed] [Google Scholar]
- 3. Becker WJ. Acute migraine treatment in adults. Headache 2015; 55: 778–793. [DOI] [PubMed] [Google Scholar]
- 4. Ong JJY, De Felice M. Migraine treatment: current acute medications and their potential mechanisms of action. Neurotherapeutics 2018; 15: 274–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mathew PG, Klein BC. Getting to the heart of the matter: migraine, triptans, DHE, ditans, CGRP antibodies, first/second-generation gepants, and cardiovascular risk. Headache 2019; 59: 1421–1426. [DOI] [PubMed] [Google Scholar]
- 6. Nittari G, Scuri S, Petrelli F, et al. Fighting obesity in children from European World Health Organization member states. Epidemiological data, medical-social aspects, and prevention programs. Clin Ter 2019; 170: e223–e230. [DOI] [PubMed] [Google Scholar]
- 7. Gallagher RM, Cutrer FM. Migraine: diagnosis, management, and new treatment options. Am J Manag Care 2002; 8: S58–S73. [PubMed] [Google Scholar]
- 8. Lipton RB, Buse DC, Serrano D, et al. Examination of unmet treatment needs among persons with episodic migraine: results of the American Migraine Prevalence and Prevention (AMPP) study. Headache 2013; 53: 1300–1311. [DOI] [PubMed] [Google Scholar]
- 9. Rubio-Beltrán E, Labastida-Ramírez A, Haanes KA, et al. Characterization of binding, functional activity, and contractile responses of the selective 5-HT1F receptor agonist lasmiditan. Br J Pharmacol 2019; 176: 4681–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurth T, Rohmann JL, Shapiro RE. Migraine and risk of cardiovascular disease. BMJ 2018; 360: k275. [DOI] [PubMed] [Google Scholar]
- 11. Lamb YN. Lasmiditan: first approval. Drugs 2019; 79: 1989–1996. [DOI] [PubMed] [Google Scholar]
- 12. Mitsikostas DD, Tfelt-Hansen P. Targeting to 5-HT1F receptor subtype for migraine treatment: lessons from the past, implications for the future. Cent Nerv Syst Agents Med Chem 2012; 12: 241–249. [DOI] [PubMed] [Google Scholar]
- 13. Nelson DL, Phebus LA, Johnson KW, et al. Preclinical pharmacological profile of the selective 5-HT1F receptor agonist lasmiditan. Cephalalgia 2010; 30: 1159–1169. [DOI] [PubMed] [Google Scholar]
- 14. Cutrer FM, Charles A. The neurogenic basis of migraine. Headache 2008; 48: 1411–1414. [DOI] [PubMed] [Google Scholar]
- 15. Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 1990; 28: 183–187. [DOI] [PubMed] [Google Scholar]
- 16. Benemei S, Cortese F, Labastida-Ramírez A, et al. Triptans and CGRP blockade – impact on the cranial vasculature. J Headache Pain 2017; 18: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Labastida-Ramirez A, Rubio-Beltran E, Haanes KA, et al. Lasmiditan inhibits calcitonin gene-related peptide release in the rodent trigeminovascular system. Pain 2020; 161: 1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dupre TV, Jenkins DP, Muise-Helmericks RC, et al. The 5-hydroxytryptamine receptor 1F stimulates mitochondrial biogenesis and angiogenesis in endothelial cells. Biochem Pharmacol 2019; 169: 113644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sparaco M, Feleppa M, Lipton RB, et al. Mitochondrial dysfunction and migraine: evidence and hypotheses. Cephalalgia 2006; 26: 361–372. [DOI] [PubMed] [Google Scholar]
- 20. Clemow DB, Johnson KW, Hochstetler HM, et al. Lasmiditan mechanism of action – review of a selective 5-HT1F agonist. J Headache Pain 2020; 21: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pilgrim A, Dussault B, Rupniak N, et al. COL-144, an orally bioavailable selective 5-HT1F receptor agonist for acute migraine therapy. Cephalalgia 2009; 29: 24–25. [Google Scholar]
- 22. Tsai M, Case M, Ardayfio P, et al. Effects of lasmiditan on cardiovascular parameters and pharmacokinetics in healthy subjects receiving oral doses of propranolol. Clin Pharmacol Drug Dev. Epub ahead of print 16 January 2020. DOI: 10.1002/cpdd.768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilbraham D, Berg PH, Tsai M, et al. Abuse potential of lasmiditan: a phase 1 randomized, placebo- and alprazolam-controlled crossover study. J Clin Pharmacol 2020; 60: 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferrari MD, Farkkila M, Reuter U, et al. Acute treatment of migraine with the selective 5-HT1F receptor agonist lasmiditan – a randomised proof-of-concept trial. Cephalalgia 2010; 30: 1170–1178. [DOI] [PubMed] [Google Scholar]
- 25. Farkkila M, Diener HC, Geraud G, et al. Efficacy and tolerability of lasmiditan, an oral 5-HT1F receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol 2012; 11: 405–413. [DOI] [PubMed] [Google Scholar]
- 26. Kuca B, Silberstein SD, Wietecha L, et al. Lasmiditan is an effective acute treatment for migraine: a phase 3 randomized study. Neurology 2018; 91: e2222–e2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goadsby PJ, Wietecha LA, Dennehy EB, et al. Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine. Brain 2019; 142: 1894–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brandes JL, Klise S, Krege JH, et al. Interim results of a prospective, randomized, open-label, phase 3 study of the long-term safety and efficacy of lasmiditan for acute treatment of migraine (the GLADIATOR study). Cephalalgia 2019; 39: 1343–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ashina M, Vasudeva R, Jin L, et al. Onset of efficacy following oral treatment with lasmiditan for the acute treatment of migraine: integrated results from 2 randomized double-blind placebo-controlled phase 3 clinical studies. Headache 2019; 59: 1788–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Knievel K, Buchanan AS, Lombard L, et al. Lasmiditan for the acute treatment of migraine: subgroup analyses by prior response to triptans. Cephalalgia 2020; 40: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Loo LS, Ailani J, Schim J, et al. Efficacy and safety of lasmiditan in patients using concomitant migraine preventive medications: findings from SAMURAI and SPARTAN, two randomized phase 3 trials. J Headache Pain 2019; 20: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Loo LS, Plato BM, Turner IM, et al. Effect of a rescue or recurrence dose of lasmiditan on efficacy and safety in the acute treatment of migraine: findings from the phase 3 trials (SAMURAI and SPARTAN). BMC Neurol 2019; 19: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Doty EG, Krege JH, Jin L, et al. Sustained responses to lasmiditan: results from post-hoc analyses of two phase 3 randomized clinical trials for acute treatment of migraine. Cephalalgia 2019; 39: 1569–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lipton RB, Lombard L, Ruff DD, et al. Trajectory of migraine-related disability following long-term treatment with lasmiditan: results of the GLADIATOR study. J Headache Pain 2020; 21: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tepper SJ, Krege JH, Lombard L, et al. Characterization of dizziness after lasmiditan usage: findings from the SAMURAI and SPARTAN acute migraine treatment randomized trials. Headache 2019; 59: 1052–1062. [DOI] [PubMed] [Google Scholar]
- 36. Ahn SK, Khalmuratova R, Jeon SY, et al. Colocalization of 5-HT1F receptor and calcitonin gene-related peptide in rat vestibular nuclei. Neurosci Lett 2009; 465: 151–156. [DOI] [PubMed] [Google Scholar]
- 37. Pearlman EM, Wilbraham D, Dennehy EB, et al. Effects of lasmiditan on simulated driving performance: results of two randomized, blinded, crossover studies with placebo and active controls. Hum Psychopharmacol 2020; 35: e2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shapiro RE, Hochstetler HM, Dennehy EB, et al. Lasmiditan for acute treatment of migraine in patients with cardiovascular risk factors: post-hoc analysis of pooled results from 2 randomized, double-blind, placebo-controlled, phase 3 trials. J Headache Pain 2019; 20: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goadsby PJ, Edvinsson L. Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain 1994; 117: 427–434. [DOI] [PubMed] [Google Scholar]
- 40. Lasaosa SS, Diago EB, Calzada JN, et al. Cardiovascular risk factors in cluster headache. Pain Med 2017; 18: 1161–1167. [DOI] [PubMed] [Google Scholar]