Abstract
Background:
Spirometry is a primary tool for early chronic obstructive pulmonary disease (COPD) detection in patients with risk factors, for example, cigarette smoking. The aim of this study was to evaluate the strategy of an active screening for COPD among smokers admitted to the pulmonary and cardiology department.
Methods:
This prospective study was conducted between February and March 2019. All hospitalized smokers aged 40 years and older completed an original questionnaire and had spirometry measurement with a bronchial reversibility test (if applicable) performed by medical students using a portable spirometer.
Results:
One hundred and eighty-eight patients were eligible to participate in the study. Seventy (37%) subjects refused to participate. Eventually, 116 (62%) patients were included in the final analysis and 94 (81%) performed spirometry correctly. In total, 32 (34 %) patients were found to have COPD. Nine (28%) of these patients were newly diagnosed, 89% of them had mild-to-moderate airway obstruction. Patients with newly diagnosed COPD were significantly younger [age 63 (56–64) versus 69 (64–78) years], had a longer smoking-free period [17 (13–20) versus 9 (2–12) years], had fewer symptoms and had a better lung function compared with patients with a previous diagnosis of COPD (p < 0.05 for all comparisons).
Conclusion:
The proposed diagnostic strategy can be successfully used to improve COPD detection in the inpatient setting. The majority of the newly diagnosed COPD patients had mild-to-moderate airway obstruction. Patients who should be particularly screened for COPD include ex-smokers with less pronounced respiratory symptoms.
Keywords: chronic obstructive pulmonary disease, fixed airway obstruction, misdiagnosis, portable spirometry, screening
Introduction
Although chronic obstructive pulmonary disease (COPD) affects approximately one-fourth of the population of smokers aged 40 years and older,1 most of the affected subjects are unaware of having the disease.2,3 This is, at least partially, related to the fact that even 80% of undiagnosed subjects have mild-to-moderate disease.2,3 In particular, early COPD, which is often mildly symptomatic,4 can hinder patients from seeking medical help and receiving treatment. Underdiagnosis and undertreatment of COPD is critically important as the disease is progressive and leads to continuous lung function decline. It is hypothesized that the annual loss of forced expiratory volume in the first second (FEV1) is more pronounced in earlier stages of the disease.5,6 Moreover, early COPD does not exclude the possibility of an exacerbation, which is an important cause of hospital admission and death.7 Therefore, diagnosing and treating patients with COPD, especially in early stages, can lead to improvement in their health status.8
The gold standard for COPD diagnosis and monitoring is spirometry. Although it is an easy and low-cost method for basic lung function assessment, spirometry is frequently underused both in general practice and hospital settings, leading to underdiagnosis or overdiagnosis of COPD,9–11 especially in patients with dyspnea related to cardiovascular diseases.12 In a study by Spero et al., only 8.4% of patients admitted to hospital with a diagnosis of COPD had a spirometry performed at discharge and in more than 30% of these patients spirometry did not confirm the diagnosis. We believe that accessibility to spirometry can be improved by the use of portable devices. It has been shown that portable spirometers linked to a mobile phone could be used as an alternative to laboratory spirometry and performed in the office or at bedside prior to discharge.13–17 In this study, we propose an active screening strategy for hospitalized smokers to improve the diagnosis of COPD, especially when there is limited accessibility to laboratory spirometry. Briefly, all hospitalized patients with risk factors for COPD (smokers aged ⩾40 years) admitted to the pulmonary and the cardiology departments were questioned on their symptoms and comorbidities and underwent spirometry with the use of a portable spirometer. The choice of these two departments was based on the common risk factor of respiratory and cardiovascular diseases, that is, cigarette smoking. In this study we aimed to evaluate the feasibility of the proposed strategy to assess the prevalence of COPD and to identify under- and overdiagnosed patients with COPD among smokers aged 40 years and older admitted to the pulmonary and the cardiology departments.
Patients and methods
General study design
This was a prospective, cross-sectional study performed at the Central Teaching Hospital of the Medical University of Warsaw, Poland. All patients admitted to the pulmonary and the cardiology departments between February and March 2019 were screened for eligibility to participate in the study. Patient recruitment was performed by trained medical students who were guided on the study algorithm (Figure 1) by an interactive electronic questionnaire (KoBoToolbox, Harvard Humanitarian Initiative, Cambridge, MA, USA) and were working under the supervision of two pulmonologists. The questionnaire consisted of 45 questions on demographic data, smoking history, respiratory symptoms, comorbidities, medications, contraindications to perform spirometry and World Health Organization (WHO) performance status (see Supplemental Material). Patients who met the inclusion criteria (see below) underwent spirometry with a portable spirometer. In both departments, spirometry was carried out by the same group of students. Patients who were hospitalized because of acute medical conditions had spirometry after stabilization, whereas patients who were stable and admitted to perform planned procedures had spirometry on admission. The study project was approved by the Institutional Review Board of the Medical University of Warsaw, Warsaw, Poland (KB/232/2018) and was performed in accordance with the principles stated in the Declaration of Helsinki. All enrolled patients gave their written informed consent to participate in the study. All data were anonymized and treated with confidentiality according to Good Clinical Practice guidelines.
Figure 1.
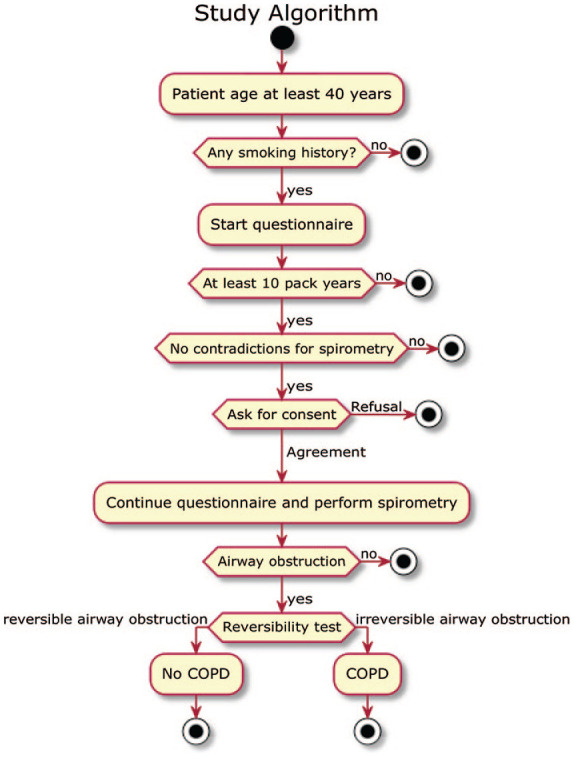
Study algorithm.
COPD, chronic obstructive pulmonary disease.
Study participants
The two major inclusion criteria were age ⩾40 years and smoking history of at least 10 pack-years. Exclusion criteria comprised contraindications to perform spirometry and refusal or inability to give a written informed consent to participate in the study.
Spirometry and definitions
A portable AioCare® spirometer (HealthUp, Poland) with a wireless connection (via Bluetooth) to a dedicated software running on mobile phone operating systems was used to perform spirometry. The AioCare® spirometer meets all performance criteria described in international standards.18 The device measures all commonly used spirometry parameters including forced vital capacity (FVC), FEV1 and peak expiratory flow. The measurements were performed in a sitting position with a nose clip clamping the nostrils. In this study, spirometry was defined as a complete spirometry test consisting of at least three maneuvers with the measurement of FVC. If a maneuver was not in line with American Thoracic Society/European Respiratory Society (ATS/ERS) spirometry quality criteria, automatic feedback was provided to the medical students responsible for conducting the test. Spirometry quality assessment and interpretation of the results was performed by two pulmonologists according to the ATS/ERS guidelines.19,20 In patients with baseline airway obstruction [FEV1/FVC < lower limit of normal (LLN) according to the Global Lung Initiative reference values], a reversibility test with 400 µg salbutamol via a pressurized metered-dose inhaler with a spacer was performed. In patients who received a short- or a long-acting bronchodilator within 6 or 24 h, respectively, before the test was performed, the spirometry was considered a post-bronchodilator examination and a reversibility test was not conducted. COPD diagnosis was made when the post-bronchodilator FEV1/FVC was below the LLN after the exclusion of other reasons for fixed airway obstruction.21 The severity of airway obstruction was graded according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations.22 COPD overdiagnosis was considered when the study participant reported a diagnosis of COPD, but there was no fixed airway obstruction (post-bronchodilator FEV1/FVC ⩾ LLN). COPD underdiagnosis was defined as the presence of irreversible airway obstruction in a patient without an earlier diagnosis of COPD.23
Statistical analysis
Estimation of the sample size was based on the prevalence of COPD in the Polish population aged ⩾40 years and among smokers, estimated to be 9.3%3 and 22.95%,1 respectively. We assumed that the COPD prevalence in our study would be 25% lower than that found in the above cited literature data. Assuming the power of 80% and the significance level of 5%, the sample size was estimated as 92 subjects (46 in the pulmonary and 46 in the cardiology department).
Continuous data are expressed as medians and interquartile ranges (25th to 75th percentiles) and categorical data are presented as numbers and percentages. Statistical analysis was performed using Statistica 13.3 (StatSoft Inc., Tulsa, OK, USA). The differences between continuous variables in two groups were tested using the non-parametric Mann–Whitney U-test. The categorical variables were compared using chi-squared test or Fisher’s exact test. The statistical significance was accepted at a p-value less than 0.05.
Results
Patient characteristics
Four hundred and eighty-five patients were screened and 188 were eligible to participate in the study. Seventy (37%) subjects refused to participate in the study. The data on patient inclusion are presented in Figure 2. Ultimately, 116/188 (62%) patients (51 and 65 in the pulmonary and in the cardiology department, respectively) were included in the analysis. The characteristics of the study group are presented in Table 1. There were no differences in age, body-mass index, smoking history or WHO performance status between patients from the two departments. The cardiology group was characterized by a higher proportion of men compared with the pulmonary group (p = 0.03). In the pulmonary group, more subjects had had spirometry in the past and had a shorter time interval since the last spirometry compared with the cardiology group (p = 0.005). In the whole cohort, 92% patients had cardiovascular diseases and 22% had diabetes. Overall, 94 (81%) spirometry measurements were performed correctly and were used for analysis.
Figure 2.
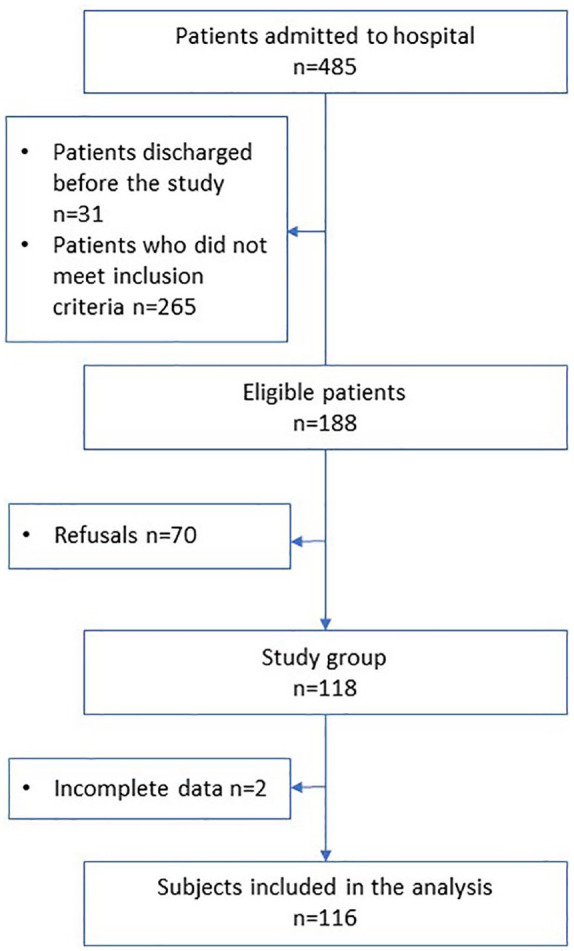
Flow diagram of the subjects screened and included in the full cohort.
Table 1.
Characteristics of the study population.
| All N = 116 |
Pulmonary department n = 51 |
Cardiology department n = 65 |
p | |
|---|---|---|---|---|
| Age, years | 66 (59–73) | 66 (59–73) | 66 (59–73) | 0.76 |
| Male gender | 79 (68%) | 29 (57%) | 50 (77%) | 0.03 |
| BMI, kg/m2) | 27.4 (24.2–30.8) | 27.3 (23.4–32.0) | 27.4 (24.8–29.7) | 0.75 |
| Current smoker/ex-smoker | 35 (30%)/81 (70%) | 18 (35%)/33 (65%) | 17 (26%)/48 (74%) | 0.31 |
| Pack-years | 30 (20–40) | 30 (20–40) | 30 (20–40) | 0.58 |
| Years free from smoking | 13 (4–25) | 12 (3–22) | 14.5 (4–25) | 0.60 |
| Spirometry in the past | 87 (75%) | 45 (88%) | 42 (65%) | 0.005 |
| Months since the last spirometry | 9 (1–60) | 1 (0–4) | 48 (12–168) | <0.001 |
| Performance status according to the WHO | ||||
| Grade 0 | 54 (46%) | 22 (43%) | 32 (49%) | 0.33 |
| Grade 1 | 43 (37%) | 18 (35%) | 25 (38%) | |
| Grade 2 | 16 (14%) | 9 (18%) | 7 (11%) | |
| Grade 3 | 1 (1%) | 0 (0%) | 1 (2%) | |
| Grade 4 | 2 (2%) | 2 (4%) | 0 (0%) | |
| Previous diagnosis of obstructive lung disease | ||||
| COPD | 30 (26%) | 21 (41%) | 9 (14%) | <0.001 |
| Asthma | 10 (9%) | 6 (12%) | 4 (6%) | |
| None | 76 (65%) | 24 (47%) | 52 (80%) | |
| Comorbidities | ||||
| Cardiovascular diseases | 102/111 (92%) | 39/46 (85%) | 63/65 (97%) | 0.031 |
| Diabetes | 24/111 (22%) | 7/46 (15%) | 17/65 (26%) | 0.241 |
Data are presented as median (interquartile range) or n (%).
BMI, body mass index; COPD, chronic obstructive pulmonary disease; WHO, World Health Organization.
Prevalence of COPD and detection of new cases of COPD
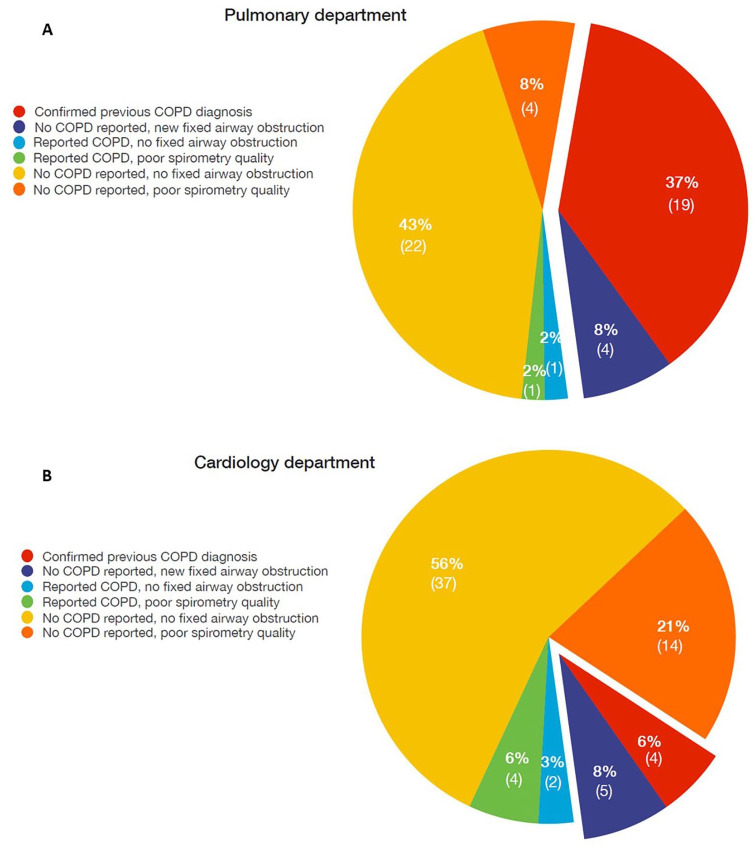
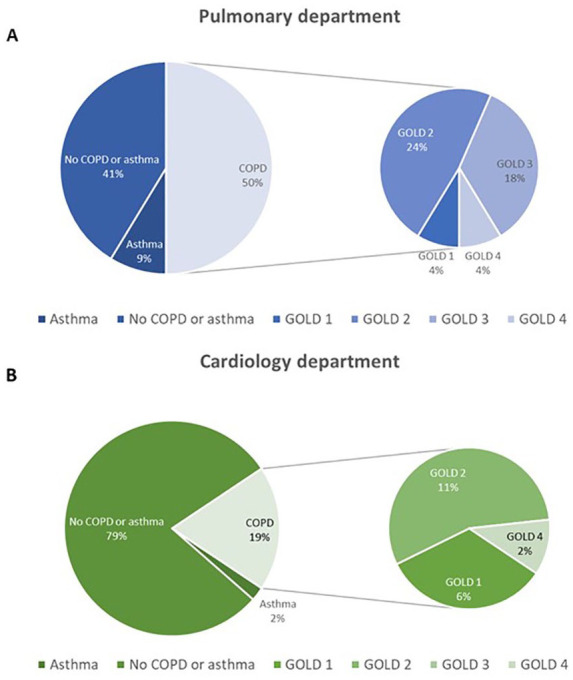
Fixed airway obstruction consistent with the diagnosis of COPD was found in 32 (34%) patients: 23/46 in the pulmonary and 9/48 in the cardiology department, respectively, p = 0.019 (Figure 3). Of those, nine (28%) (four in the pulmonary and five in the cardiology department, respectively) subjects were newly diagnosed with COPD. The proportion of newly diagnosed COPD patients to non-COPD patients was numerically higher in the pulmonary than in the cardiology department but the difference was not statistically significant (17% versus 10%, respectively, p = 0.72)).
Figure 3.
Results of screening for chronic obstructive pulmonary disease (COPD) in the pulmonary (A) and the cardiology (B) departments.
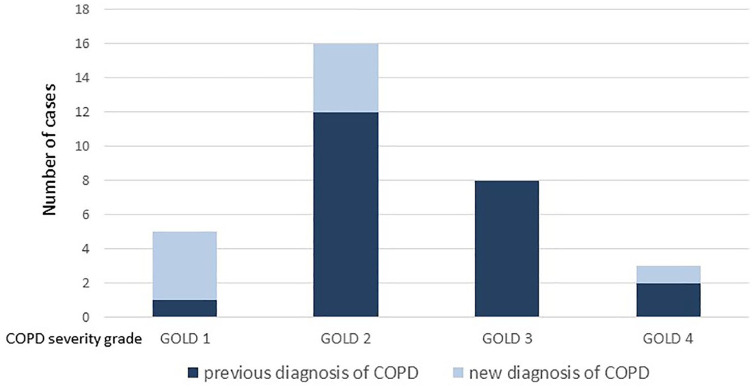
Five of the nine newly diagnosed COPD patients had had spirometry in the past (three within the previous year, two more than 1 year prior to the study). Patients with newly diagnosed COPD were significantly younger [age 63 (56–64) versus 69 (64–78) years], had a longer smoking-free period [17 (13–20) versus 9 (2–12) years], had fewer symptoms and had better lung function compared with patients with earlier COPD diagnosis (Table 2) (p < 0.05 for all comparisons). Figure 4 presents the prevalence of COPD and airway obstruction severity according to GOLD in the pulmonary and in the cardiology department. The underdiagnosis rate in patients with different airway obstruction severity grades was as follows: 80% for mild, 25% for moderate, 0% for severe and 33% for very severe (Figure 5). In 3/30 (10%) patients (one in the pulmonary and two in the cardiology department) who had reported an earlier COPD diagnosis, there was no fixed airway obstruction and the diagnosis of COPD was excluded. Two of these patients had no airway obstruction in the post-bronchodilator spirometry and one had a normal spirometry without prior bronchodilator intake. All of the overdiagnosed patients had had a spirometry performed in the past.
Table 2.
Comparison of patients with a previous versus new COPD diagnosis.
| Previous diagnosis of COPD n = 23 |
New COPD diagnosis n = 9 |
p | |
|---|---|---|---|
| Age, years | 69 (64–78) | 63 (56–64) | 0.005 |
| Male gender | 11 (48%) | 7 (78%) | 0.23 |
| BMI, kg/m2 | 24.7 (21.8–29.7) | 25.8 (24.7–28.3) | 0.52 |
| Pack-years | 40 (30–50) | 31 (20–46) | 0.32 |
| Years free from smoking | 9 (2–12) | 17 (13–20) | 0.03 |
| WHO | 1 (0–2) | 0 (0–1) | 0.06 |
| mMRC | 2 (1–3) | 1 (0–1.5) | 0.03 |
| CAT score | 20 (14–23) | 6.5 (5.5–9) | 0.001 |
| Cough | 2 (0–3) | 2 (1–3) | 0.78 |
| Phlegm | 2 (1–3) | 1 (0.5–2) | 0.14 |
| Chest tightness | 0.5 (0–2) | 0 (0–1) | 0.26 |
| Breathlessness | 4 (2–5) | 0 (0–1) | 0.004 |
| Activities | 3 (2–4) | 0 (0–1) | 0.003 |
| Confidence | 2.5 (0–4) | 0 (0–1) | 0.01 |
| Sleep | 2.5 (0–4) | 0 (0–1) | 0.046 |
| Energy | 3 (3–3) | 1 (1–2) | 0.002 |
| % predicted FEV1 | 51 (40–64) | 72.1 (55.9–85) | 0.01 |
| % predicted FVC | 79 (67–94) | 93.9 (77–102.6) | 0.14 |
| GOLD 1/2/3/4, % | 4/52/35/9 | 44/44/0/12 | 0.02 |
| GOLD A/B/C/D, % | 9/36/9/46 | 50/13/0/37 | 0.08 |
Data are presented as median (interquartile range) or n (%).
BMI, body mass index; CAT, COPD Assessment Test scale; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; mMRC, modified Medical Research Council scale; WHO, World Health Organization.
Figure 4.
Prevalence of chronic obstructive pulmonary disease (COPD) and airway obstruction severity according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) in the pulmonary (A) and the cardiology (B) departments.
Figure 5.
Number of patients with new and previous diagnosis of chronic obstructive pulmonary disease (COPD) according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) airway obstruction severity grade.
Acceptability of spirometry examinations in the pulmonary and the cardiology departments and in patients with different performance status
A larger proportion of patients hospitalized in the pulmonary department could perform a spirometry meeting the quality standards compared with the patients treated in the cardiology department: 46 (90%) versus 48 (74%) respectively, p = 0.014. The subjects who performed a quality spirometry were significantly younger and had had spirometry more recently compared with those who did not (Table 3). There were no differences in the smoking history or the WHO performance status between the groups.
Table 3.
Comparison of patients who performed technically correct spirometry and those who did not.
| Patients who performed technically correct spirometry n = 94 |
Patients who did not perform technically correct spirometry n = 22 |
p | |
|---|---|---|---|
| Age, years | 65 (59–71) | 77 (67–83) | <0.001 |
| Male gender | 64 (67%) | 15 (71%) | 0.80 |
| BMI, kg/m2 | 27.0 (24.3–31.2) | 27.7 (24.0–30.5) | 0.94 |
| Current smokers | 29 (31%) | 6 (29%) | 1.00 |
| Pack-years | 30 (20–40) | 30 (20–52.5) | 0.79 |
| Years free from smoking | 12 (3–25) | 15 (7–30) | 0.22 |
| Previous diagnosis of an obstructive lung disease | 34 (36%) | 6 (27%) | 0.47 |
| Spirometry in the past | 74 (78%) | 13 (62%) | 0.16 |
| Months since the last spirometry | 3.5 (0–48) | 36 (24–84) | 0.04 |
| Performance status according to the WHO | 0 (0–1) | 1 (0–1) | 0.13 |
Data are presented as median (interquartile range) or n (%).
BMI, body mass index; WHO, World Health Organization.
We found no difference in the percentage of quality examinations between patients with good (WHO 0–1) and poor (2–4) WHO performance status, 81% versus 80%, p = 0.76. As there were only one and two patients with WHO performance status 3 and 4, respectively, we could not reliably assess and compare the ability to perform spirometry in those patients. Out of these three patients, only one performed spirometry which met the quality standards. The other two patients failed to perform any correct maneuver.
Discussion
The present study showed that active COPD screening in the inpatient setting is feasible. We found that the medical students’ involvement allowed to achieve spirometry of sufficient quality and to detect irreversible airway obstruction, which is crucial for COPD diagnosis. Patients with newly diagnosed COPD have mainly mild-to-moderate airway obstruction. Of note, although our study was addressed to hospitalized patients with COPD risk factors, a significant proportion of these patients were unwilling to perform spirometry. Our results demonstrated that COPD screening should be considered not only for active smokers but also for subjects who have a long smoking-free period and report mild or no respiratory symptoms.
To our knowledge, this is one of the few studies in which all patients with COPD risk factors visiting a health center were included in the COPD detection program and had spirometry with reversibility test performed. There are still no system solutions which would reduce the COPD underdiagnosis and overdiagnosis rate. It has been shown that active screening for COPD allowed to detect new cases in 22% patients, whereas in the standard care strategy only in 3% of patients could a new diagnosis of COPD be established.24 Some authors have suggested implementing questionnaires which would help to select at-risk patients for spirometry examination.25,26 A proposal of an active COPD case-finding strategy for primary care with the use of portable spirometers was presented in a study by Kim et al.27 The authors found that the strategy was feasible in a primary clinical setting and should also include asymptomatic smokers aged ⩾40 years. In another study, a targeted spirometry screening program was implemented within the presurgical clinic and included smokers with respiratory symptoms and patients with a history of COPD or asthma.28 Although in both of these studies the reversibility test, which is crucial for diagnosis of COPD,22 was not performed, it was shown that about 25% of smokers had newly diagnosed airflow limitation.27,28
We identified COPD in 34% of the evaluated patients. The prevalence of COPD in our study was higher than previously reported (22.1–24.3%) in the Polish population with risk factors.1,29 Such a high prevalence of COPD in our study could be related to several factors. Almost half of the study participants were hospitalized in the pulmonary department, where the prevalence of COPD was significantly higher compared with the cardiology department. Moreover, smokers requiring hospitalization are a specific group of patients, probably in a worse health condition than healthy smokers. It has been shown that the COPD population has a significant rate of comorbidity.30 COPD, especially in more severe stages, is characterized by systemic inflammation and the concurrence of COPD and cardiovascular diseases was shown to be independent of smoking history.31 Of note, it has been shown that congestive heart failure might cause airway obstruction in patients without COPD and, therefore, poses a diagnostic challenge.32–34 Brenner et al. have demonstrated that in up to 50% of patients, airway obstruction resolved after 6 months of treatment for heart failure.32 In our study, we found that 10% of the patients who had declared having COPD did not have fixed airway obstruction. Overdiagnosis of COPD should be considered as a serious issue as it is associated with unnecessary and ineffective treatment and might delay the correct diagnosis. On the other hand, patients without fixed airway obstruction could still be at risk of developing COPD in the future and should be, similarly to COPD patients, educated on the harmful effects of smoking, advised on smoking cessation to avoid or delay lung function decline and be regularly followed up to assess lung function.
It is estimated that even 67–81%2,3,23 of COPD patients are undiagnosed. In our study, the underdiagnosis rate was considerably lower, as 28% of COPD subjects were newly diagnosed. We may assume that a number of patients remained undiagnosed, as a significant proportion of subjects who are at risk of COPD declined participation in the study. Interestingly, the proportion of newly diagnosed to all non-COPD patients was similar in both the pulmonary and cardiology departments. Patients with newly diagnosed COPD had better lung function and reported fewer respiratory symptoms (in particular, less pronounced dyspnea) than those with an earlier diagnosis of COPD, which is consistent with findings from other studies.35 We also found that newly diagnosed COPD patients had a longer smoking-free period compared with patients with an earlier diagnosis of COPD. All these factors could have contributed to the fact that some of the underdiagnosed patients had not been identified earlier. Patients with mild COPD are often unidentified, as the symptoms often do not interfere with their daily activity36 and are accepted as a consequence of smoking and aging and, therefore, these patients do not seek medical advice.37 In our study, 80% of patients with mild COPD were previously undiagnosed. Therefore, we believe it is important to actively search for patients with COPD risk factors and make the COPD diagnosis early to prevent further lung function deterioration and exacerbations and to maintain good quality of life.
The high COPD under- and overdiagnosis rate in the general population could be associated with the poor accessibility to spirometry and the reluctance of smokers to undergo screening. In a study performed outside a healthcare setting aimed to offer spirometry to smoking pedestrians, only 20% agreed to participate.38 In the present study, the patients’ willingness to undergo bedside COPD screening was substantially higher, as 62% of eligible subjects participated in the study. It is likely that hospitalized patients are more willing to participate as they already have other significant comorbidities and a greater health awareness and spirometry is an additional test performed during their hospitalization.
Spirometry of an adequate quality is critical for diagnosing airway obstruction. In our study 19% of participants were unable to perform spirometry of an adequate quality. It cannot be excluded that some of those patients could also suffer from COPD and, therefore, should have spirometry repeated. In our study, patients with poor quality spirometry were older than those who performed spirometry with good quality standards. Based on other reports, the quality of spirometry in elderly patients was found to be similar to that in younger adults.39 The inability to perform the test could be associated with the deterioration of cognitive function, which, however, was not assessed in this study. On the other hand, it has been shown that the quality of spirometry depends mainly on the skills of the person conducting the examination.40 In our study, spirometry was conducted by trained medical students with little experience and this could have impacted the poor quality of some examinations. It has been shown that repeating spirometry by the person conducting the examination resulted in improvement of the quality of spirometry.41 However, the fact that medical students obtained 81% of spirometry examinations of an adequate quality suggests that portable spirometers can be successfully used by operators with very limited experience and we believe that the device should be considered for the outpatient setting to improve detection of COPD. Moreover, we believe that the standardization of COPD screening during medical students’ education may increase the early detection of COPD patients.
We are aware of several limitations in our study. The study group was relatively small; however, it must be emphasized that all patients admitted to the two departments during the study period were included in the analysis and the number of participants was based on the sample size estimation. The study was conducted only in the pulmonary and cardiology departments. The choice of the departments, however, was based on the fact that cigarette smoking is a major risk factor for respiratory and cardiovascular diseases and we aimed to compare the prevalence of undiagnosed COPD in these two departments. Furthermore, in this study, we did not compare the effect of portable spirometry on COPD detection with standard laboratory spirometry. However, it must be emphasized that the device we used was validated,18 whereas the access to laboratory spirometry can be limited and our target to examine all eligible patients would not be met. We are aware that some of the patients, especially those with airway diseases, for example, asthma, might have suffered from an exacerbation. Therefore, lung function of these patients at the time of spirometry could be deteriorated and could potentially cause COPD overdiagnosis. Moreover, in some patients taking inhaled medication, we did not perform pre-bronchodilator spirometry and, therefore, we could not assess whether these patients had pre-bronchodilator obstruction and whether they were at risk of developing COPD. Also, we did not perform any additional investigations, for example, chest computed tomography scan, exercise tests to assess whether patients with newly diagnosed COPD presented with signs of early disease. Finally, we have no information on the reasons for refusal to participate in the study.
In conclusion, COPD detection strategy with the use of a portable spirometer is feasible in the inpatient setting and should be also considered in the outpatient setting as it allows to detect patients with COPD under- or overdiagnosis. Patients with newly diagnosed COPD have mainly mild-to-moderate airway obstruction. However, a significant proportion of patients with COPD risk factors were unwilling to perform spirometry.
Footnotes
Author contributions: KG and PK conceived the concept of the study. KM, PJ, LK, GO and RK contributed to the design of the research. MK created the electronic questionnaire and integrated the collected data. OZ, MZ, KW, US performed spirometry examinations and collected the data. KM, PK, PJ and KG analyzed the data. KM and KG prepared the first draft of the manuscript. All authors edited and approved the final version of the manuscript.
Conflict of interest statement: KM, PJ, MK, OZ, MZ, KW, US, GO declare no conflict of interest. PK reports personal fees from Polpharma and Chiesi outside the submitted work. LK is the inventor, founder and shareholder of the AioCare portable spirometry system. RK reports fees for lectures and travel expenses from Boehringer Ingelheim, Chiesi, AstraZeneca and Polpharma, outside the submitted work. KG reports fees for lectures and travel expenses from Boehringer Ingelheim, Chiesi, AstraZeneca, Polpharma and Roche, outside the submitted work. The authors alone are responsible for the content and writing of the paper.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Katarzyna Gorska  https://orcid.org/0000-0003-4686-910X
https://orcid.org/0000-0003-4686-910X
Contributor Information
Katarzyna Mycroft, Department of Internal Medicine, Pulmonary Diseases and Allergy, Medical University of Warsaw, Warsaw, Poland.
Piotr Korczynski, Department of Internal Medicine, Pulmonary Diseases and Allergy, Medical University of Warsaw, Warsaw, Poland.
Piotr Jankowski, Department of Internal Medicine, Pulmonary Diseases and Allergy, Medical University of Warsaw, Warsaw, Poland.
Mikolaj Kutka, Students’ Research Group “Alveolus”, Medical University of Warsaw, Warsaw, Poland.
Olga Zelazna, Students’ Research Group “Alveolus”, Medical University of Warsaw, Warsaw, Poland.
Marcin Zagaja, Students’ Research Group “Alveolus”, Medical University of Warsaw, Warsaw, Poland.
Kornelia Wozniczko, Students’ Research Group “Alveolus”, Medical University of Warsaw, Warsaw, Poland.
Urszula Szafranska, Students’ Research Group “Alveolus”, Medical University of Warsaw, Warsaw, Poland.
Lukasz Koltowski, 1st Department of Cardiology, Medical University of Warsaw, Warsaw, Poland.
Grzegorz Opolski, 1st Department of Cardiology, Medical University of Warsaw, Warsaw, Poland.
Rafal Krenke, Department of Internal Medicine, Pulmonary Diseases and Allergy, Medical University of Warsaw, Warsaw, Poland.
Katarzyna Gorska, Department of Internal Medicine, Pulmonary Diseases and Allergy, Medical University of Warsaw, Banacha 1a, Warsaw, 02-097, Poland.
References
- 1. Zieliński J, Bednarek M; The Know the Age of Your Lung Study Group. Early detection of COPD in a high-risk population using spirometric screening. Chest 2001; 119: 731–736. [DOI] [PubMed] [Google Scholar]
- 2. Hill K, Goldstein RS, Guyatt GH, et al. Prevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary care. CMAJ 2010; 182: 673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bednarek M, Maciejewski J, Wozniak M, et al. Prevalence, severity and underdiagnosis of COPD in the primary care setting. Thorax 2008; 63: 402–407. [DOI] [PubMed] [Google Scholar]
- 4. Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010; 11: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vestbo J, Edwards LD, Scanlon PD, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med 2011; 365: 1184–1192. [DOI] [PubMed] [Google Scholar]
- 6. Bridevaux PO, Gerbase MW, Probst-Hensch NM, et al. T. Long-term decline in lung function, utilisation of care and quality of life in modified GOLD stage 1 COPD. Thorax 2008; 63: 768–774. [DOI] [PubMed] [Google Scholar]
- 7. Wedzicha JA, Brill SE, Allinson JP, et al. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med 2013; 11: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singh D, D’Urzo AD, Donohue JF, et al. Weighing the evidence for pharmacological treatment interventions in mild COPD; a narrative perspective. Respir Res 2019; 20: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heffler E, Crimi C, Mancuso S, et al. Misdiagnosis of asthma and COPD and underuse of spirometry in primary care unselected patients. Respir Med 2018; 142: 48–52. [DOI] [PubMed] [Google Scholar]
- 10. Fu SN, Yu WC, Wong CK, et al. Prevalence of undiagnosed airflow obstruction among people with a history of smoking in a primary care setting. Int J Chron Obstruct Pulmon Dis 2016; 11: 2391–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nishi SP, Wang Y, Kuo YF, et al. Spirometry use among older adults with chronic obstructive pulmonary disease: 1999-2008. Ann Am Thorac Soc 2013; 10: 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spero K, Bayasi G, Beaudry L, et al. Overdiagnosis of COPD in hospitalized patients. Int J Chron Obstruct Pulmon Dis 2017; 12: 2417–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bambra G, Jalota L, Kapoor C, et al. Office spirometry correlates with laboratory spirometry in patients with symptomatic asthma and COPD. Clin Respir J 2017; 11: 805–811. [DOI] [PubMed] [Google Scholar]
- 14. Kerwin EM, Hickey L, Small CJ. Relationship between handheld and clinic-based spirometry measurements in asthma patients receiving beclomethasone. Respir Med 2019; 151: 35–42. [DOI] [PubMed] [Google Scholar]
- 15. Puri V, Zoole JB, Musick J, et al. Handheld office-based spirometry versus laboratory spirometry in low-risk patients undergoing lung resection. Innovations 2011; 6: 257–261. [DOI] [PubMed] [Google Scholar]
- 16. Hudson JL, Bell JM, Crabtree TD, et al. Office-based spirometry: a new model of care in preoperative assessment for low-risk lung resections. Ann Thorac Surg 2018; 105: 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tran D, Lim M, Vogrin S, et al. Point of care portable spirometry in the diagnosis and treatment of inpatients with chronic obstructive pulmonary disease. Lung 2020; 198: 143–150. [DOI] [PubMed] [Google Scholar]
- 18. Aiocare. Accuracy validation report, https://aiocare.com/wp-content/uploads/2020/05/Device_may2020_report_V1.pdf (accessed 29 May 2020).
- 19. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 20. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005; 26: 948–968. [DOI] [PubMed] [Google Scholar]
- 21. Sliwiński P, Górecka D, Jassem E, et al. Zalecenia Polskiego Towarzystwa Chorób Płuc dotyczą;ce rozpoznawania i leczenia przewlekłej obturacyjnej choroby płuc [Polish respiratory society guidelines for chronic obstructive pulmonary disease]. Pneumonol Alergol Pol 2014; 82: 227–263. [DOI] [PubMed] [Google Scholar]
- 22. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: updated 2020, http://www.goldcopd.org (accessed 17 May 2020).
- 23. Diab N, Gershon AS, Sin DD, et al. Underdiagnosis and overdiagnosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2018; 198: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 24. Bertens LC, Reitsma JB, van Mourik Y, et al. COPD detected with screening: impact on patient management and prognosis. Eur Respir J 2014;44:1571–1578. [DOI] [PubMed] [Google Scholar]
- 25. Haroon S, Adab P, Riley RD, et al. Predicting risk of undiagnosed COPD: development and validation of the TargetCOPD score. Eur Respir J 2017; 49: 1602191. [DOI] [PubMed] [Google Scholar]
- 26. López Varela MV, Montes de Oca M, Rey A, et al. Development of a simple screening tool for opportunistic COPD case finding in primary care in Latin America: the PUMA study. Respirology 2016; 21: 1227–1234. [DOI] [PubMed] [Google Scholar]
- 27. Kim JK, Lee CM, Park JY, et al. Active case finding strategy for chronic obstructive pulmonary disease with handheld spirometry. Medicine 2016; 95: e5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robitaille C, Dajczman E, Hirsch AM, et al. Implementation of a targeted screening program to detect airflow obstruction suggestive of chronic obstructive pulmonary disease within a presurgical screening clinic. Can Respir J 2015; 22: 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nizankowska-Mogilnicka E, Mejza F, Buist AS, et al. Prevalence of COPD and tobacco smoking in Malopolska region–results from the BOLD study in Poland. Pol Arch Med Wewn 2007; 117: 402–410. [PubMed] [Google Scholar]
- 30. Rubinsztajn R, Przybylowski T, Grabicki M, et al. Comorbidities in chronic obstructive pulmonary disease: Results of a national multicenter research project. Adv Clin Exp Med 2019; 28: 319–324. [DOI] [PubMed] [Google Scholar]
- 31. Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J 2009; 33: 1165–1185. [DOI] [PubMed] [Google Scholar]
- 32. Brenner S, Güder G, Berliner D, et al. Airway obstruction in systolic heart failure–COPD or congestion? Int J Cardiol 2013; 168: 1910–1916. [DOI] [PubMed] [Google Scholar]
- 33. Hawkins NM, Virani S, Ceconi C. Heart failure and chronic obstructive pulmonary disease: the challenges facing physicians and health services. Eur Heart J 2013; 34: 2795–2803. [DOI] [PubMed] [Google Scholar]
- 34. Cˇelutkienė J, Balcˇiu-nas M, Kablucˇko D, et al. Challenges of treating acute heart failure in patients with chronic obstructive pulmonary disease. Card Fail Rev 2017; 3: 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnson KM, Bryan S, Ghanbarian S, et al. Characterizing undiagnosed chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respir Res 2018; 19: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boros PW, Lubinński W. Health state and the quality of life in patients with chronic obstructive pulmonary disease in Poland: a study using the EuroQoL-5D questionnaire. Pol Arch Med Wewn 2012; 122: 73–81. [DOI] [PubMed] [Google Scholar]
- 37. Rossi A, Butorac-Petanjek B, Chilosi M, et al. Chronic obstructive pulmonary disease with mild airflow limitation: current knowledge and proposal for future research - a consensus document from six scientific societies. Int J Chron Obstruct Pulmon Dis 2017; 12: 2593–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Korczynski P, Gorska K, Jankowski P, et al. Public spirometry campaign in chronic obstructive pulmonary disease screening - hope or hype? Adv Respir Med 2017; 85: 143–150. [DOI] [PubMed] [Google Scholar]
- 39. Haynes JM. Pulmonary function test quality in the elderly: a comparison with younger adults. Respir Care 2014; 59: 16–21. [DOI] [PubMed] [Google Scholar]
- 40. Upton MN, Ferrell C, Bidwell C, et al. Improving the quality of spirometry in an epidemiological study: the Renfrew-Paisley (Midspan) family study. Public Health 2000; 114: 353–360. [PubMed] [Google Scholar]
- 41. Pérez-Padilla R, Vázquez-García JC, Márquez MN, et al. ; PLATINO Group. Spirometry quality-control strategies in a multinational study of the prevalence of chronic obstructive pulmonary disease. Respir Care 2008; 53: 1019–1026. [PubMed] [Google Scholar]