Abstract
Aim: Coronavirus disease 2019 antibody testing often relies on venous blood collection, which is labor-intensive, inconvenient and expensive compared with finger-stick capillary dried blood spot (DBS) collection. The purpose of our work was to determine if two commercially available anti-severe acute respiratory syndrome coronavirus 2 enzyme-linked immunosorbent assays for IgG antibodies against spike S1 subunit and nucleocapsid proteins could be validated for use with DBS. Materials & methods: Kit supplied reagents were used to extract DBS, and in-house DBS calibrators were included on every run. Results: Positive/negative concordance between DBS and serum was 100/99.3% for the spike S1 subunit assay and 100/98% for the nucleocapsid assay. Conclusion: Validation of the DBS Coronavirus disease 2019 IgG antibody assays demonstrated that serum and DBS can produce equivalent results with minimal kit modifications.
Keywords: : antibody, COVID-19, DBS, dried blood spot, ELISA, filter paper, IgG, pandemic, SARS-CoV-2
When Coronavirus disease 2019 (COVID-19) was first identified in Hubei Provence China in late 2019 [1,2], it triggered a worldwide effort to produce diagnostic tests used to detect SARS-CoV-2 infections. In the United States, the US FDA issued emergency use of in vitro diagnostics assays for detection of COVID-19 after the US Department of Health and Human Services declared COVID-19 a pandemic in February 2020 [3]. The FDA allowed for Emergency Use Authorization (EUA) of diagnostic devices during the duration of the pandemic, and has regulatory responsibility over medical devices, manufacturer-supplied assays and laboratory developed tests related to COVID-19 [4]. The FDA EUA process is rigorous and labor-intensive to ensure consumer confidence, and it is imperative to extensively validate methods to be submitted for EUA during the COVID-19 pandemic.
Utilization of dried blood spots (DBS) as an alternative to serum, plasma or whole blood samples for laboratory analysis has been used for well over a half-century, beginning with Dr. Robert Guthrie’s work detecting metabolomic disorders in newborns [5]. Nearly 4 million neonates are tested annually in the USA using capillary blood collected on FDA-cleared Whatman 903 filter paper from a lancet puncture on the heel [6]. Capillary blood collection from the finger using a lancet is typically preferred over a venous blood collection [7–12] and has shown to be more cost-effective [13,14]. The US Department of Transportation and US Postal Service consider DBS to be nonregulated and exempt materials for shipping if they are properly packaged [15], making it easy to return DBS specimens from a clinic or home directly to a high complexity clinical laboratory for testing. Prior studies have successfully used DBS to detect antibodies against Epstein–Barr [16], Rabies [17], Hepatitis B and C [18–20], HIV [18,21,22], Herpes Simplex [23] and Ebola [24] viruses.
Immunoglobulin G, M and A (IgG, IgM and IgA) antibodies produced during an COVID-19 immune response are present in nearly all COVID-19 patients at varying titers and duration [25–27]. IgM antibodies typically appear within days after infection, followed by longer-lasting IgA and IgG antibodies at 1–4 weeks from the start of symptoms [25–28]. IgG antibodies are the predominant COVID-19 immune response, lasting greater than 4 months in most individuals infected by SARS-CoV-2 [29]. Two relevant SARS-CoV-2 structural proteins that induce a host immune response are the spike protein, which binds to a host cell receptor, and the nucleocapsid protein that encapsulates the viral RNA. The SARS-CoV-2 spike protein is composed of the S1 subunit containing the receptor-binding domain (RBD), and S2 subunit. The use of spike S1 subunit rather than the RBD or spike S2 subunit antigens for detection of COVID-19 antibodies is ideal as the spike S1 subunit can bind non-RBD and RBD antibodies while maintaining high SARS-CoV-2 specificity [30].
COVID-19 IgG antibody testing is important not only for determining if a COVID-19 infection occurred but will also be important for evaluating and differentiating endogenous vaccine-stimulated antibody response. Research has shown that IgG antibodies against the SARS-CoV-2 spike protein are associated with viral-neutralizing activity [31,32], making the spike protein an ideal target for SARS-CoV-2 vaccines [33]. Using an IgG antibody assay against the SARS-CoV-2 spike S1 subunit can detect a relevant vaccine response while an assay against the nucleocapsid protein can help identify a native infection.
As the COVID-19 pandemic progresses, there continues to be a need for a sampling alternative to expensive and labor-intensive venous blood collection routinely used for antibody testing. We report on the validation of DBS as an alternative to serum or plasma for use in two commercially available COVID-19 IgG antibody enzyme-linked immunosorbent assays (ELISAs).
Materials & methods
Study protocol
We recruited 177 subjects for our study, with criteria for participation including the presence or absence of flu-like symptoms up to 4 months prior to collection. The study group consisted of 43 subjects with prior PCR testing for COVID-19 infection (PCR+: n = 22; PCR-: n = 21) and 134 subjects without prior PCR testing. There were no gender, race or language restrictions, and all participants were 18 years or older. All participants completed a questionnaire detailing COVID-19 exposure history and symptomology and signed a consent form. Our study was approved by the institutional review board of the National University of Natural Medicine (IRB number: RB42420).
ZRT Laboratory sent healthcare providers responsible for study subject enrollment a kit containing materials to complete the serum and DBS collection. Blood was drawn by venipuncture (antecubital vein) in a serum-separating vacutainer tube (Becton Dickinson, BD Vacutainer SST 8.5 ml). Blood was allowed to clot for 30–60 min before centrifugation at 1500 g to yield serum. Serum was withdrawn from the tube using a closed polypropylene bulb pipette and transferred to a polypropylene screw top tube. DBS were collected with healthcare professional assistance, immediately after the blood draw, using a lancet (Becton Dickinson, BD Microtainer contact-activated Lancet, 1.5-mm wide blade) to produce capillary finger-stick blood. Single capillary blood drops were deposited by wiping away the first drop of blood with gauze and gently milking the finger without squeezing, allowing gravity to assist with collection. As blood drops form, they are gently touched to the filter paper, filling up to 12, but no less than one premarked 1.5-cm circles on a Whatman 903 filter card (Eastern Business Forms, ZRT 12 Spot 903 Card, Lot 181). Blood spots were dried at room temperature for at least 4 h. After thorough drying, filter cards containing the DBS were placed in a resealable bag (Uline, Uline 5 × 5′′ 4 Mil resealable bags) with desiccant (Multisorb, MiniPax 7 g). Serum and DBS were stored at 2°C until mailed to ZRT Laboratory on an ice pack (Uline, Cold Pack S-9902) with 24-h delivery (UPS, next day delivery). Once the samples arrived at ZRT Laboratory, they were barcoded and stored at -80°C until use.
Anti-SARS-CoV-2 IgG antibody ELISAs
Two commercial anti-SARS-CoV-2 IgG antibody ELISAs from EUROIMMUN and Epitope Diagnostics were used for serum analysis and modified for DBS use. The EUROIMMUN anti-SARS-CoV-2 ELISA (IgG) has a documented 90.0% sensitivity/100% specificity and EUA from the FDA. The Epitope Diagnostics Novel Coronavirus COVID-19 IgG ELISA kit, KT-1032, has a documented 98.4% sensitivity/99.8% specificity and has applied for EUA from the FDA. The EUROIMMUN assay detects IgG antibodies against the S1 subunit of the SARS-CoV-2 spike protein containing the RBD, while the Epitope Diagnostics assay detects IgG antibodies against the SARS-CoV-2 nucleocapsid protein. All reagents necessary for analysis of serum and DBS were included in the manufacturers’ kits.
Once manufacturer-supplied assays are modified to work with an alternate sample type not listed in their instructions, such as DBS, they must be renamed and independently validated. For the purpose of this paper, we will identify the DBS-modified EUROIMMUN IgG antibody assay as the ZRT COVID-19 IgG S1 Spike Antibody Test (ZRT DBS IgG S1) and the DBS-modified Epitope Diagnostics antibody assay as the ZRT COVID-19 IgG Nucleocapsid Antibody Test (ZRT DBS IgG NCP).
DBS punching & extraction
The EUROIMMUN and Epitope Diagnostic IgG antibody ELISAs require a 1:101 dilution of serum, equivalent to 10-μl serum per 1-ml sample diluent. A single 3.2-mm punch contains around 3.4 μl of whole blood, or approximately 1.7 μl serum [34,35], therefore two 3.2-mm punches in a total extraction volume of 300 μl are approximately equivalent of 11.4 μl serum per 1-ml extraction solution. Two 3.2-mm punches were taken from a single DBS or two separate DBSs, using a DBS puncher (Perkin Elmer, DBS Puncher® Instrument) that directly drops the punches into a 96-well fritted block (Nunc, 96-Well filter plate). The fritted 96-well block was placed on a deep 96-well block (Labcon, 96-Well 2.2 ml) and 150 μl of EUROIMMUN sample buffer from the EUROIMMUN kit was added to each well with DBS punches. EUROIMMUN’s sample buffer was selected over Epitope Diagnostics sample buffer because EUROIMMUN ELISA currently has FDA EUA. The combination blocks were rotated on an orbital shaker at 700 rpm for 1 h. The combined block setup was centrifuged at 1500 g for 5 min. EUROIMMUN sample buffer was added a second time to each well at the same volume, rotated at 700 rpm for 1 h and centrifuged at 1500 g for 5 min. The fritted extraction block was set aside and the deep 96-well block with extracted DBS sample was kept for analysis.
ZRT DBS IgG S1 & NCP assay protocol
All manufacturer supplied reagents and ELISA plates were brought to room temperature 1 h before use. DBS extract (100 μl) was added to both the EUROIMMUN and Epitope Diagnostic 96-well plates as if it were 1:101 diluted serum according to manufacturer kit instructions. All remaining steps followed manufacturer kit instructions apart from assay calibration and negative/indeterminate/positive cutoffs. Liquid positive and negative controls supplied in the EUROIMMUN and Epitope Diagnostic IgG antibody kits were run neat for additional quality control. Liquid-handling robotics (Tecan, Evo 200) was used for all pipetting, and automated plate washers (BioTek, 405 TS Washer) were used for all washing steps. Plates were read at 450 nm on a microplate reader (Perkin Elmer, Wallac 1420 Victor 2 Multi-Label Microplate Reader).
ZRT DBS IgG S1 & NCP assay calibration
Whole blood from three positive and three negative IgG antibody study subjects was used to create DBS calibrators to be run on every assay. Expected values were established by running calibrators on seven separate runs and determining an average absorbance. Once established, experimental calibrator absorbance was compared with expected absorbance using simple linear regression forced through zero to create a correction factor determined by the slope of the line. Assay calibration helps to account for assay absorbance variation due to lot changes and slight differences in run conditions such as temperature and incubation time.
EUROIMMUN & epitope diagnostics IgG antibody assay serum protocols
Serum samples were analyzed, and results determined, according to the EUROIMMUN and Epitope Diagnostics kit instructions.
ZRT DBS IgG S1 & NCP assay validation
DBS sample preparation
Additional venous whole blood was collected from select study subjects in EDTA-coated vacutainer tubes (Becton Dickinson, BD Vacutainer K2EDTA). DBS were created by pipetting 65-μl spots of whole blood on Whatman 903 filter paper, enough to fill the preprinted 1.5-cm circles, for assay validation and calibration, except where indicated otherwise.
Intra-assay precision
DBS from four positive and two negative IgG antibody study subjects were extracted eight-times and run as individual samples batched together on the ZRT DBS IgG S1 and NCP assays. Intra-assay precision was determined from calibrated sample absorbance by evaluating the percent coefficient of variation (%CV), a measure of the precision and repeatability of an assay, of the sample replicates.
Interassay precision & month-long stability at different temperatures
DBS from four positive and three negative IgG antibody study subjects were run on the ZRT DBS IgG S1 and NCP assays on days 1, 2, 3, 4, 5, 6, 7, 14 and 30 while stored at temperatures of -80°C, -20°C, 2°C, 22°C, 38°C and 55°C. Samples were kept in a resealable bag with desiccant during the duration of the stability testing. Interassay precision and month-long stability were determined from calibrated sample absorbance by looking at the %CV of the seven samples over 30 days at each temperature.
Hematocrit effect
To determine the effect of hematocrit on DBS results, whole blood collected from four positive and two negative IgG antibody study subjects was centrifuged at 1500 g to separate plasma (0% hematocrit) from red blood cells (100% hematocrit). Plasma was then mixed with red blood cells to replicate 20, 30, 40, 50, 60 and 70% hematocrit equivalents, and then pipetted at 65 μl on Whatman 903 filter paper. Hematocrit adjusted DBS were run on the ZRT DBS IgG S1 and NCP assays and absorbance compared against 50% hematocrit.
Interference
To assess liquid interference on DBS samples eight potential interfering substances (albumin, urea, biotin, NaOH, NaCl, ascorbic acid, EDTA and glucose) ranging from 1 to 100 mg/ml were added to extracted DBSs from one positive and one negative IgG antibody study subject. EUROIMMUN’s kit insert provided documented interference limits of 10 mg/ml hemoglobin, 20 mg/ml triglycerides and 0.4 mg/ml bilirubin while the Epitope Diagnostic kit did not include interference information. Interference solutions were made up using EUROIMMUN sample buffer with increasing amounts of NaOH added if there were absorption issues. Samples with interference were made up by adding one part interference to nine parts extracted DBS sample. Samples without interference were made up by adding one part EUROIMMUN sample buffer to nine parts extracted DBS sample. This was done to keep the matrix as similar as possible. Interference solutions were further diluted 1:10 to check for interference at 1/10 the concentration. All samples were run on the ZRT COVID-19 IgG S1 and NCP assays. Acceptable interference concentrations were established.
Hook effect/serial dilution
DBS from a single study subject that was strongly positive for IgG antibodies was extracted, serially diluted 1:1 using EUROIMMUN sample buffer from neat to 1/1024, and run on the ZRT DBS IgG S1 and NCP assays. Sample absorbance was observed for any sign of a hook effect caused by decreased immune complexes at high antibody titers.
DBS drying & shipping conditions
DBS from two positive and one negative IgG antibody study subject were stored under 24 unique drying and shipping conditions for 7 days. Conditions include 4-h sample drying time followed by storage in open air, a resealable bag and a resealable bag with desiccant. Conditions also include no dry time (wet sample) followed by storage in open air, a resealable bag and a resealable bag with desiccant. Each drying condition was tested in environments replicating a home freezer (-19°C, 40% humidity), a home refrigerator (5°C, 66% humidity), room temperature (22°C, 30% humidity), and a hot and humid environment (29°C, 99% humidity). Humidity in the hot and humid environment was created using a humidifier (Evergreen and Humidifier/Fogger) connected to an incubator (VWR, model 1510E). Temperature and humidity (Vaisala, DL2000) were monitored. After 7 days, samples were run on the ZRT DBS IgG S1 and NCP assays. Percent recovery of IgG antibodies under each condition was compared with a 4-h sample drying time followed by storage in a resealable bag with desiccant.
DBS size
Whole blood from four positive and two negative IgG antibody negative study subjects was pipetted on filter paper to create DBS of 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60 and 65 μl, and were run on the ZRT DBS IgG S1 and NCP assays and absorbance compared.
DBS punch location
DBS from four positive and two negative IgG antibody study subjects were sampled from the edge, inner and center of a 65-μl spot and were run on the ZRT DBS IgG S1 and NCP assays and absorbance compared.
DBS dry time
Whole blood from two positive and two negative IgG antibody subject samples was pipetted at 65 μl on all collection circles of separate Whatman 903 filter cards, which were weighed before application for blank subtraction. Drying conditions were 22°C, 30% humidity and indoors away from ventilation. Blood spots were weighed after whole blood application on filter paper, and every 30 min for 5 h as they dried. DBS samples were then placed in a resealable bag with desiccant, and the weight was determined at 24 and 48 h. Weights were averaged for each time point and plotted against dry time.
Whatman 903 lot comparison
Four lots of Whatman 903 filter paper (W151, W162, W171 and W181) were examined for any possible influence on assay performance. DBS from two positive and two negative IgG antibody study subjects on four lots of Whatman 903 filter paper along with blanks were run on the ZRT DBS IgG S1 and NCP assays and absorbance compared.
Serum versus DBS raw absorbance comparison
Raw assay absorbance was compared for all 177 study subject samples using ZRT’s DBS IgG S1 and NCP assays and EUROIMMUN and Epitope Diagnostics IgG antibody assays using serum according to manufacturers’ instructions. It is important to note that absorbances are not calibrated on the serum or DBS assays due to differences in calibration procedures.
Negative/indeterminate/positive antibody detection cutoffs
Absorbance was plotted from the lowest to highest for all 177 study subjects using ZRT DBS IgG S1 and NCP assay results. Negative, indeterminate and positive cutoff absorbance values were determined by visual inspection, which was later verified using PCR-confirmed results.
Positive/negative concordance
Positive and negative concordance were determined by comparing ZRT DBS IgG S1 and NCP assay results (candidate test) to EUROIMMUN and Epitope Diagnostics IgG antibody assay serum results (comparative test) for all 177 study subjects using a 2 × 2 contingency table. Indeterminate results were counted as positive for DBS and serum. Percent positive concordance was determined using the equation: percent positive concordance = positive results concordance/(positive results concordance + positive results disagreement). Percent negative concordance was determined using the equation: percent negative concordance = negative results concordance/(negative results concordance + negative results disagreement).
Sensitivity/specificity (clinical agreement)
Sensitivity and specificity were determined by comparing ZRT DBS IgG S1 and NCP assay results (candidate test) from PCR-confirmed COVID-19 positive (n = 22) and negative (n = 21) study subjects to disease status (diagnostic accuracy criteria) using a 2 × 2 contingency table. Indeterminate results were counted as positive. Sensitivity was determined using the equation: sensitivity = true positive/(true positive + false negative). Specificity was determined using the equation: specificity = true negative/(true negative + false positive).
Results
Study subjects
All study subjects (n = 177) provided enough DBS with spots of uniform shape with no overlapping or smearing, and with paper completely soaked through that allowed for at least three independent extractions, with some study subjects completely filling all 12 1.5-cm circles of the DBS card allowing for 50+ independent extractions if needed. All DBS samples were received as instructed in a resealable bag with desiccant.
Study subject information included age, gender, symptom timing and symptom frequency for PCR-confirmed COVID-19 cases (n = 22, 9 males, 13 females, average age 46-year old) (Table 1). One PCR-confirmed COVID-19 subject did not show symptoms, while two PCR-confirmed COVID-19 subjects could not recall how long symptoms lasted. The most common symptoms for confirmed COVID-19 cases were fatigue (20/22), headache (18/22), chills (17/22), dry cough (15/22), fever >100.4°F (15/22), trouble breathing (13/22), diarrhea (12/22) and loss of taste (11/22).
Table 1. . Study subject data for PCR confirmed COVID-19 cases.
| Patient | Symptom timing | Symptom frequency | IgG antibody testing result | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Gender | Symptom length (days) | Symptoms to study collection (days) | Loss of taste | Loss of smell | Dry cough | Wet cough | Diarrhea | Trouble breathing | Fatigue | Fever (>100.4°F) | Chills | Headache | Pneumonia | Sore throat | Hospitalized | ZRT DBS S1 | ZRT DBS NCP |
| 28 | F | 6 | 43 | X | X | X | X | X | X | X | Positive | Indeterminate | ||||||
| 37 | M | 7 | 60 | X | X | X | X | X | X | X | Positive | Positive | ||||||
| 33 | M | 10 | 65 | X | X | X | X | X | X | X | X | Positive | Positive | |||||
| 73 | M | 25 | 28 | X | X | X | X | X | Positive | Positive | ||||||||
| 63 | M | Unknown | 54 | X | X | X | X | X | X | X | X | Positive | Positive | |||||
| 24 | M | 36 | 72 | X | X | X | X | X | X | X | Positive | Positive | ||||||
| 50 | F | 13 | 28 | X | X | X | X | X | X | X | Positive | Positive | ||||||
| 55 | M | 14 | 53 | X | X | X | Negative | Negative | ||||||||||
| 55 | F | Unknown | 59 | X | X | X | X | X | X | X | X | X | Positive | Positive | ||||
| 38 | F | 14 | 31 | X | X | X | X | X | X | X | X | X | Positive | Positive | ||||
| 50 | M | 20 | 67 | X | X | X | X | X | X | X | X | X | X | X | Positive | Positive | ||
| 50 | F | 8 | 43 | X | X | X | X | X | Positive | Positive | ||||||||
| 27 | F | 6 | 78 | X | X | X | X | Positive | Positive | |||||||||
| 62 | F | 16 | 82 | X | X | X | X | X | X | X | X | X | X | X | Positive | Positive | ||
| 43 | F | 28 | 58 | X | X | X | X | Positive | Positive | |||||||||
| 40 | M | 14 | 90 | X | X | X | X | X | X | X | X | X | Positive | Positive | ||||
| 41 | F | 16 | 90 | X | X | X | X | X | X | X | X | X | X | Positive | Positive | |||
| 60 | M | 15 | 87 | X | X | X | X | X | Positive | Positive | ||||||||
| 26 | F | 15 | 22 | X | X | X | X | X | X | X | Positive | Negative | ||||||
| 31 | F | No symptoms | Positive | Positive | ||||||||||||||
| 64 | F | 38 | 118 | X | X | X | X | X | X | X | Negative | Negative | ||||||
| 58 | F | 11 | 94 | X | X | X | X | X | X | X | Positive | Positive | ||||||
Intra-assay & interassay precision/month-long stability
Six intra-assay samples were run in replicates of eight, with %CVs between 1.1–4.2% and 2.1–5.2% for the ZRT DBS IgG S1 and NCP assays, respectively. Seven interassay samples stored at -80°C, -20°C, 2°C, 22°C, 38°C and 55°C were run nine-times over a period of 1 month (Figure 1), with %CVs between 4.4–8.1%, 2.3–11.5%, 3.1–8.9%, 5.0–13.7%, 3.9–16.8% and 4.6–32.0% for the ZRT DBS IgG S1 assay and 5.2–17.3%, 3.0–17.9%, 4.0–15.0%, 3.0–17.7%, 3.9–20.4% and 10.1–24.1% for the ZRT DBS IgG NCP assay, respectively. A %CV below 20% is considered acceptable for intra- and interassay precision. The ZRT DBS IgG S1 and NCP assays demonstrated DBS sample stability across all temperature ranges, except for DBS samples stored at 55°C, for 30 days. The CDC and WHO indicate that high temperatures and direct sunlight can influence the quality of DBS [15,36], and a visual inspection of extracted DBS stored at 55°C showed incomplete extraction with sample remaining on the filter paper by day 14 (Figure 2). While it is unlikely that samples will remain at 55°C for an extended period during transportation, any samples visually displaying partial extraction should be rejected.
Figure 1. Interassay precision and month long stability.
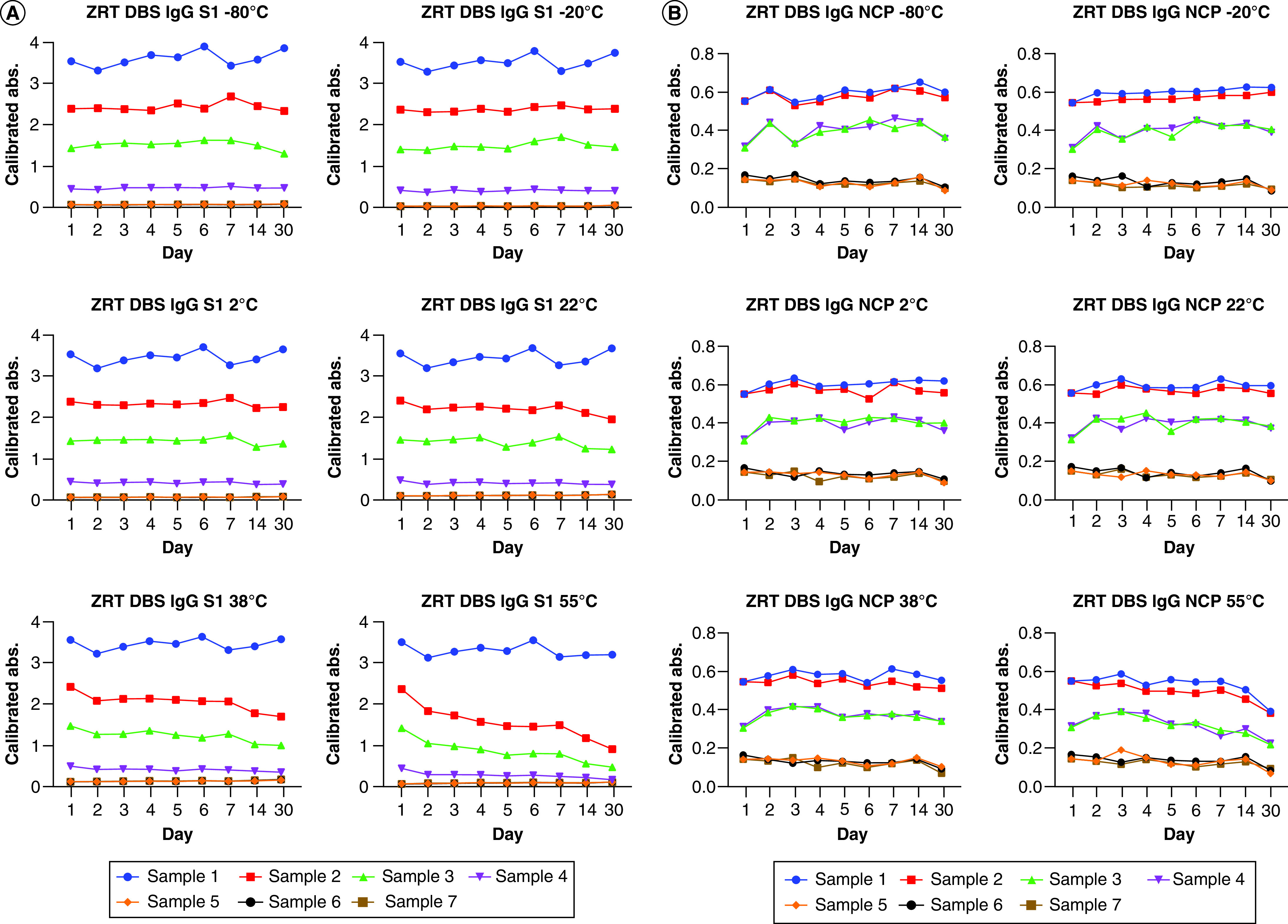
Interassay precision and month-long stability of seven study subject DBSs stored at -80°C to 55°C over 30 days run on the (A) ZRT DBS IgG S1 and (B) ZRT DBS IgG NCP assays.
DBS: Dried blood spot.
Figure 2. Extracted dried blood spots in the fritted filter block at day 14 of interassay precision/stability testing with marked samples representing DBS from seven study subjects stored at 55°C showing partial extraction.

DBS: Dried blood spot.
Hematocrit effect
Six contrived DBS samples with hematocrits ranging from 20 to 70% were compared with a baseline hematocrit of 50%, with absorbance recoveries of 89.9–125% (mean: 102%) and 76.1–144.5% (mean: 105%) for the ZRT DBS IgG S1 and NCP assays, respectively. Hematocrit values above 60% and below 20% are rare, with normal ranges of 40–54% for men and 37–47% for women [37]. The absorbance recoveries when comparing 30% and 40% hematocrit to 50% hematocrit were 92.6–104.3% (mean: 98.4%) and 85.1–111.3% (mean: 103.0%) for the ZRT DBS IgG S1 and NCP assays, respectively. A comparison between assay absorbance and percent hematocrit can be seen in Supplementary Figure 3. We expect minimal result variation due to hematocrit, especially in the range of 30–50% hematocrit.
Six contrived DBS samples with hematocrits ranging from 20 to 70% were compared with a baseline hematocrit of 50%, with absorbance recoveries of 89.9–125% (mean: 102%) and 76.1–144.5% (mean: 105%) for the ZRT DBS IgG S1 and NCP assays, respectively. Hematocrit values above 60% and below 20% are rare, with normal ranges of 40–54% for men and 37–47% for women [37]. The absorbance recoveries when comparing 30% and 40% hematocrit to 50% hematocrit were 92.6–104.3% (mean: 98.4%) and 85.1–111.3% (mean: 103.0%) for the ZRT DBS IgG S1 and NCP assays, respectively. A comparison between assay absorbance and percent hematocrit can be seen in Supplementary Figure 3. We expect minimal result variation due to hematocrit, especially in the range of 30–50% hematocrit.
Interference
Interferences at different concentrations were spiked into two study subject DBS samples post-extraction. Acceptable inference based on 80–120% recovery was observed at 100 mg/ml of albumin, biotin, and NaCl, and 10 mg/ml of NaOH, ascorbic acid, EDTA and glucose.
Hook effect/serial dilution
To check for hook effect, our highest DBS IgG antibody titer study subject sample was serially diluted 1:1 from neat to 1/1024th (Supplementary Figure 4). No hook effect was observed.
DBS drying & shipping conditions
Drying and shipping conditions were tested using three DBS study subject samples split between 24 unique environmental and storage conditions for 7 days. Percent recovery of absorbance was compared with a standard 4-h sample drying time followed by storage in a resealable bag with desiccant, and ranged from 65.2 to 115.8% (mean: 99.2%) and 74.1 to 105.7% (mean: 95.7%) for the ZRT DBS IgG S1 and NCP assays, respectively (Table 2). All recoveries were within the acceptability limit of 80–120% recovery except for samples stored in a hot and humid environment (29°C, 99% humidity), without a bag or desiccant. We believe that the low recovery of IgG antibodies from hot and humid exposed DBS samples was due to poor recovery during extraction. Although considered unlikely, any samples visually displaying partial extraction should be rejected, as previously displayed in Figure 2.
Table 2. . Percent recovery of dried blood spot IgG antibodies from three study subjects under simulated drying and shipping conditions at 7 days run on the (A) ZRT DBS IgG S1 and (B) ZRT DBS IgG NCP assays compared with 4 h drying at 22°C and 30% humidity, then storage in a resealable bag with desiccant at -19°C and 40% humidity†.
| (A) ZRT DBS IgG S1 | ||||
|---|---|---|---|---|
| Sample 1 | -19°C, 40% humidity (% recovery) | 5°C, 66% humidity (% recovery) | 22°C, 30% humidity (% recovery) | 29°C, 99% humidity (% recovery) |
| 4 h dry, no bag | 107.4 | 103.0 | 95.9 | 74.8 |
| 4 h dry, then in resealable bag | 108.9 | 114.4 | 101.4 | 84.8 |
| 4 h dry, then in resealable bag with desiccant | 100.0† | 103.2 | 97.2 | 92.2 |
| Wet, No Bag | 115.8 | 113.0 | 102.2 | 93.2 |
| Wet, then in resealable bag | 109.5 | 106.2 | 94.9 | 90.6 |
| Wet, then in resealable bag with desiccant | 111.5 | 107.2 | 102.1 | 89.7 |
| Sample 2 | -19°C, 40% humidity (% recovery) | 5°C, 66% humidity (% recovery) | 22°C, 30% humidity (% recovery) | 29°C, 99% humidity (% recovery) |
| 4 h dry, no bag | 106.0 | 103.7 | 95.3 | 65.2 |
| 4 h dry, then in resealable bag | 104.0 | 105.3 | 99.4 | 76.9 |
| 4 h dry, then in resealable bag with desiccant | 100.0† | 101.6 | 97.3 | 84.9 |
| Wet, no bag | 114.1 | 107.9 | 90.0 | 80.9 |
| Wet, then in resealable bag | 111.0 | 101.6 | 89.0 | 71.9 |
| Wet, then in resealable bag with desiccant | 110.4 | 114.1 | 95.0 | 86.2 |
| Sample 3 | -19°C, 40% humidity (% recovery) | 5°C, 66% humidity (% recovery) | 22°C, 30% humidity (% recovery) | 29°C, 99% humidity (% recovery) |
| 4 h dry, no bag | 103.2 | 106.1 | 105.6 | 92.3 |
| 4 h dry, then in resealable bag | 103.8 | 105.7 | 108.9 | 105.4 |
| 4 h dry, then in resealable bag with desiccant | 100.0† | 100.1 | 105.8 | 104.6 |
| Wet, no bag | 109.7 | 95.2 | 104.3 | 95.0 |
| Wet, then in resealable bag | 111.4 | 85.7 | 94.7 | 88.8 |
| Wet, then in resealable bag with desiccant | 102.7 | 93.8 | 94.2 | 92.2 |
| (B) ZRT DBS IgG NCP | ||||
| Sample 1 | -19°C, 40% humidity (% recovery) | 5°C, 66% humidity (% recovery) | 22°C, 30% humidity (% recovery) | 29°C, 99% humidity (% recovery) |
| 4 h dry, no bag | 97.8 | 97.2 | 93.7 | 84.6 |
| 4 h dry, then in resealable bag | 101.3 | 98.9 | 94.1 | 93.2 |
| 4 h dry, then in resealable bag with desiccant | 100.0† | 102.3 | 94.7 | 91.1 |
| Wet, no bag | 105.7 | 96.6 | 92.8 | 90.0 |
| Wet, then in resealable bag | 94.8 | 96.6 | 87.3 | 82.6 |
| Wet, then in resealable bag with desiccant | 102.3 | 96.7 | 94.7 | 89.5 |
| Sample 2 | -19°C, 40% humidity (% recovery) | 5°C, 66% humidity (% recovery) | 22°C, 30% humidity (% recovery) | 29°C, 99% humidity (% recovery) |
| 4 h dry, no bag | 104.3 | 97.6 | 95.9 | 74.1 |
| 4 h dry, then in resealable bag | 99.9 | 92.0 | 99.4 | 85.7 |
| 4 h dry, then in resealable bag with desiccant | 100.0† | 98.8 | 90.1 | 84.9 |
| Wet, no bag | 104.9 | 91.6 | 99.5 | 93.7 |
| Wet, then in resealable bag | 102.4 | 98.0 | 90.8 | 81.1 |
| Wet, then in resealable bag with desiccant | 104.9 | 101.4 | 97.9 | 94.0 |
| Sample 3 | -19°C, 40% humidity (% recovery) | 5°C, 66% humidity (% recovery) | 22°C, 30% humidity (% recovery) | 29°C, 99% humidity (% recovery) |
| 4 h dry, no bag | 91.5 | 100.8 | 96.9 | 97.4 |
| 4 h dry, then in resealable bag | 95.2 | 98.4 | 100.4 | 101.6 |
| 4 h dry, then in resealable bag with desiccant | 100.0† | 94.6 | 83.1 | 98.7 |
| Wet, no bag | 100.1 | 94.5 | 102.7 | 92.1 |
| Wet, then in resealable bag | 105.7 | 82.7 | 95.6 | 94.8 |
| Wet, then in resealable bag with desiccant | 102.9 | 98.2 | 98.8 | 98.9 |
Humidity.
DBS: Dried blood spot.
DBS size & punch location
To determine if DBS size and punch location influence results, six study subject whole blood samples were used to make 12 blood spots ranging from 10 to 65 μl. There were no significant differences in absorbance observed in DBS ranging in size from 10 to 65 μl, or in DBS punches taken from the edge, inside and center of a 65-μl spot (Supplementary Figure 5). A wide variety of blood spot sizes and shapes can be expected from finger-stick collection, and there should be little concern over DBS volume or punch location if a single blood drop is applied to each collection circle on the filter card.
DBS dry time
To test DBS dry time under ambient indoor conditions, four study subject whole blood samples were applied to all 12 collection circles of separate DBS cards. The DBS cards were weighed at different time points while drying, then placed in a resealable bag with desiccant to replicate shipping conditions, then weighed at 24 and 48 h (Supplementary Figure 6). It took a minimum of 2 h to dry the sample, with further moisture removed from the blood spot and/or filter card with the use of a resealable bag and desiccant. We recommend that capillary blood applied to filter paper dry for at least 4 h before being placed in a resealable bag with desiccant to ensure the DBS are completely dry.
Whatman 903 lot comparison
Four lots of Whatman 903 filter paper were used to test filter paper blank absorbance and DBS study subject result consistency. There were no significant absorbance differences between lots of Whatman 903 filter paper (Supplementary Figure 7). We do not expect any concern about future filter paper lots, as Whatman 903 filter paper is an IVD Class II medical device that must meet strict criteria for sample absorbance and recovery for its use in the CDC Newborn Screening Quality Assurance Program.
Serum versus DBS raw absorbance comparison
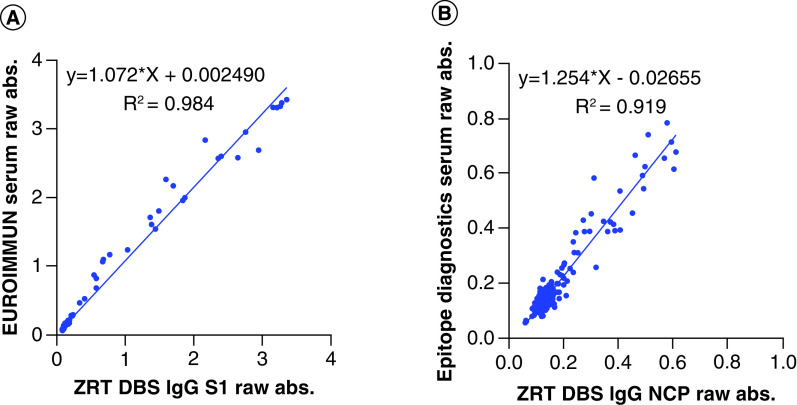
Raw absorbance of serum from study subjects run according to EUROIMMUN and Epitope Diagnostics kit instructions and matching DBS run using the ZRT DBS IgG S1 and NCP assays were compared. Linear regression analysis showed significant correlation between the ZRT DBS IgG S1 and EUROIMMUN serum assays with a regression equation of y = 1.072x + 0.002 and a coefficient of determination of R2 = 0.984. Linear regression analysis showed acceptable correlation between the ZRT DBS IgG NCP and Epitope Diagnostics serum assays with a regression equation of y = 1.254x + 0.027 and a coefficient of determination of R2 = 0.919 (Figure 3).
Figure 3. Serum versus dried blood spot raw absorbance comparison.
Raw assay absorbance comparison between the (A) ZRT DBS IgG S1 and the EUROIMMUN serum IgG antibody assays and the (B) ZRT DBS IgG NCP and Epitope Diagnostics serum IgG antibody assays (n = 177).
DBS: Dried blood spot.
Negative/indeterminate/positive antibody detection cutoffs
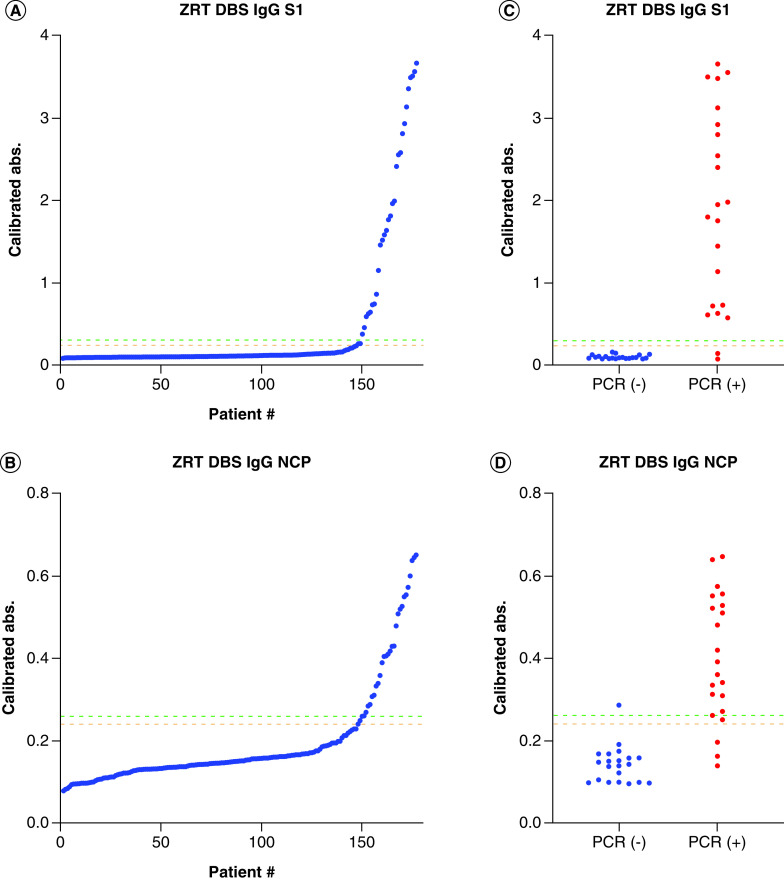
To determine seropositivity cutoffs, absorbances from study subject DBS samples run on the ZRT DBS IgG S1 and NCP assays were plotted from the lowest to highest. We visually determined what we considered to be negative, indeterminate and positive results, and applied the cutoffs to PCR-confirmed study subject results for verification (Figure 4). We selected negative, indeterminate and positive absorbance cutoffs of <0.24, 0.24–0.3 and >0.3 for the ZRT DBS IgG S1 assay and <0.24, 0.24–0.26 and >0.26 for the ZRT DBS IgG NCP assay to be used at our laboratory only.
Figure 4. Negative/indeterminate/positive antibody detection cutoffs.
Absorbance from all study subject DBSs run on the (A) ZRT DBS IgG S1 and (B) ZRT DBS IgG NCP assays, organized from the lowest to highest, to visually determine seropositivity cutoffs of negative, indeterminate and positive. Seropositivity cutoffs were verified using PCR-confirmed results on the (C) ZRT DBS IgG S1 and (D) ZRT DBS IgG NCP assays (n = 43).
DBS: Dried blood spot; PCR: Polymerase chain reaction.
Positive/negative concordance
Positive and negative concordance between ZRT DBS IgG S1 and NCP assay results (candidate test) and the EUROIMMUN and Epitope Diagnostics IgG antibody assay serum results (comparative test) for all study subjects (n = 177) were determined. Positive and negative concordance was 100 and 99.3% between the ZRT DBS IgG S1 and EUROIMMUN serum assays, and 100 and 98% between the ZRT DBS IgG NCP and Epitope Diagnostics serum assays, respectively (Table 3). This shows statistically significant agreement between results produced using serum according to the manufacturer’s kit instructions and DBS using the ZRT DBS IgG S1 and NCP assays.
Table 3. Positive and negative concordance between the (A) ZRT DBS IgG S1 and the EUROIMMUN serum IgG antibody assays and the (B) ZRT DBS IgG NCP and Epitope Diagnostics serum IgG antibody assays.
| (A) | ||||
|---|---|---|---|---|
| EUROIMMUN serum | ||||
| Positive concordance = 100% | ||||
| Negative concordance = 99.3% | Positive | Negative | Total | |
| ZRT DBS IgG S1 spike | Positive | 28 | 1 | 29 |
| Negative | 0 | 148 | 148 | |
| Total | 28 | 149 | N = 177 | |
| (B) | ||||
| Epitope Diagnostics serum | ||||
| Positive concordance = 100% | ||||
| Negative concordance = 98% | Positive | Negative | Total | |
| ZRT DBS IgG NCP | Positive | 27 | 3 | 30 |
| Negative | 0 | 147 | 147 | |
| Total | 27 | 150 | N = 177 | |
DBS: Dried blood spot.
Sensitivity/specificity (clinical agreement)
Sensitivity and specificity were determined by comparting ZRT DBS IgG S1 and NCP assay results (candidate test) to disease status (diagnostic accuracy criteria) using PCR-confirmed samples (n = 43). Sensitivity and specificity were 91.7 and 100% for the ZRT DBS IgG S1 assay, and 87.5 and 95.2% for the ZRT DBS IgG NCP assay, respectively (Table 4).
Table 4. Sensitivity and specificity of the (A) ZRT DBS IgG S1 and (B) ZRT DBS IgG NCP assays determined using PCR-confirmed study subject dried blood spots.
| (A) | ||||
|---|---|---|---|---|
| PCR confirmed results | ||||
| Sensitivity = 90.9% | ||||
| Specificity = 100% | Positive | Negative | Total | |
| ZRT DBS IgG S1 | Positive | 20 | 0 | 20 |
| Negative | 2 | 21 | 23 | |
| Total | 22 | 21 | N = 43 | |
| (B) | ||||
| PCR confirmed results | ||||
| Sensitivity = 86.4% | ||||
| Specificity = 95.2% | Positive | Negative | Total | |
| ZRT DBS IgG NCP | Positive | 19 | 1 | 20 |
| Negative | 3 | 20 | 23 | |
| Total | 22 | 21 | N = 43 | |
DBS: Dried blood spot.
Discussion/conclusion
Validation of the ZRT DBS IgG S1 and NCP assays demonstrated that DBS can produce equivalent results to serum with minimal modifications to manufacturer’s kit instructions. The use of DBS for COVID-19 antibody testing increases collection options when resources are limited while minimizing sample hazards for postal workers and laboratory staff. Unlike liquid blood samples that are categorized as category B infectious substances requiring UN 3373 labeling and packaging requirements, DBS are considered a nonregulated exempt specimen by the US Department of Transportation [38] and require fewer safeguards during sample transportation and processing. A completed DBS collection cannot leak or spill, and the drying process significantly reduces or eliminates pathogen infection risk [39]. Unlike collection of clear nasal or oral fluids for PCR or antigen testing that have no outright visible signs of proper collection, it is visually apparent if a DBS sample is acceptable for testing if the blood spot is in a fully saturated uniform circle, without irregular shape or color caused by overlapping spots, finger contact with the filter paper, or anemic blood [36]. The use of two 3.2-mm DBS punches, equivalent to 6.8 μl of whole blood, significantly reduces sample requirements while a small extraction volume of 300 μl significantly reduces reagent use while still generating enough extraction volume for both assays and liquid handling robotics. Throughput of ZRT DBS IgG S1 and NCP assays is estimated to be 1000 DBS samples per day by a single laboratory technician with little difference in overall process time compared with liquid serum/plasma samples.
One limitation when determining the validity of the ZRT DBS IgG S1 and NCP assays was the amount of time that had elapsed between COVID-19 symptoms and DBS/serum sample collection. COVID-19 IgG antibody titers peak around the third week after symptoms manifest and begin to slowly disappear over months [40]. There is evidence that those who are immunocompromised or asymptomatic may not seroconvert or only have a detectable antibody response for a short period of time [41,42]. Patients hospitalized due to COVID-19 with moderate or severe symptoms have shown a more pronounced and long-lived antibody response than those with mild or no symptoms [41,43,44]. Unlike commercial assay manufacturers that often rely on COVID-19 hospitalized patients with strict sample collection windows to determine the sensitivity and specificity of their assay, our study focused on a wide variety of participants of all ages with large variations in symptoms, outcomes and collection times. While we believe that this better represents real-world testing scenarios, it inherently leads to slightly lower calculated sensitivity and specificity. For this reason, we believe positive and negative concordance between serum run according to the manufacturer’s kit instructions and matching DBS run using the ZRT DBS S1 and NCP assays is a better measure of assay performance if the manufacturer’s assay demonstrates high sensitivity and specificity.
Another limitation of our study is that we relied on self-reporting of PCR results and did not request the specific PCR test information, or the sample collection method used. Attempts to retroactively gather this information largely failed because most participants received results over the phone. PCR false negatives are typically a result of poorly collected or stored samples, improper timing of collection or poor assay sensitivity and specificity [45]. The ideal PCR testing window is within 5 days of the start of symptoms [46], and PCR samples collected after symptoms have passed can jeopardize the diagnosis of COVID-19.
Determination of COVID-19 IgG antibodies is an essential tool f47or understanding and managing the COVID-19 pandemic. The use of DBS collection for antibody testing allows for simple and remote collection and shipment back to the lab, long-term sample stability, reduced storage requirements and minimal reagent use. While it is not always possible to confirm true positive or negative antibody results, the use of two IgG antibody assays against different viral proteins with unique epitopes to the SARS-CoV-2 virus can help increase confidence in results. Additional analytes detected in DBS such as Vitamin D [47], adrenal and sex hormones [48], and cardiometabolic markers [49] relevant to COVID-19 outcome and survival [50,51] can be combined with DBS COVID-19 antibody testing using the same sample. It is unclear when the COVID-19 pandemic will end, but it is essential to continue developing and improving on diagnostic tests to help provide information on the course of the disease, preparation for population-wide vaccinations and the possibility that COVID-19 becomes endemic.
Future perspective
The response to the COVID-19 pandemic by assay manufacturers, government health agencies and laboratory professionals to provide high-quality diagnostic testing to detect SARS-CoV-2 infection has been unprecedented. Many new challenges such as limited laboratory supplies and reagents and the urgent need for at-home sample collection have significantly increased interest in finger-stick capillary DBS for COVID-19 antibody testing. With appropriate method modifications and validation, additional capillary finger-stick sample collection devices using volumetric absorptive microsampling or plasma separation paper can be used in a similar way to DBS. While the COVID-19 pandemic may be a once in a century event, there is little doubt that, as the human population grows, the risk of a similar viral pandemic occurring increases. The use of DBS increases access to testing in developed and undeveloped countries, reduces costs associated with sample collection and helps store and preserve samples for future use, making DBS an essential tool for current and future pandemics.
Summary points.
Background
The US Department of Health and Human Services declared Coronavirus Disease 2019 (COVID-19) a pandemic in February 2020.
The US FDA allowed for Emergency Use Authorization (EUA) of diagnostic devices during the duration of the pandemic.
Methods to be submitted for EUA must be rigorously validated.
-
Dried blood spots (DBS) are an ideal sample type for COVID-19 IgG antibody testing.
Small sample volume and reagent requirement.
Easy to collect/ship/process/store.
Potential for at-home collection.
Sample is visible and quality can be graded.
Methods/results
- We successfully modified and validated two commercially available anti-SARS-CoV-2 IgG antibody enzyme-linked immunosorbent assays for use with DBS instead of serum or plasma.
- Minimal method modifications from manufacturers’ kit instructions.
- Added in-house DBS calibrators to adjust for interassay variation.
- Strong correlation between serum and DBS.
Discussion/conclusion
Study subject samples used for assay validation replicate real-world testing scenarios.
-
The use of two COVID-19 IgG antibody assays against spike S1 subunit and nucleocapsid SARS-CoV-2 proteins has multiple benefits.
Monitoring vaccine response.
Detection of native COVID-19 infections.
Confirmation testing.
DBS is an essential tool for current and future viral pandemics.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.4155/bio-2020-0289
Acknowledgments
The authors thank MN Groves and DR Burger for their work editing the manuscript, and R Bradley for coordinating institutional review board submission and amendments. The authors would also like to acknowledge Kenneth Conor Willard (laboratory technician at ZRT Laboratory) for Tecan script writing.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
Our study was approved by the institutional review board of the National University of Natural Medicine (IRB Number: RB42420). The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest
- 1.Zhou P, Yang XL, Wang XG. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579(7798), 270–273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W. et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 382(8), 727–733 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services. Determination of public health emergency. (2020). https://www.federalregister.gov/documents/2020/02/07/2020-02496/determination-of-public-health-emergency
- 4.US Department of Health and Human Services. Policy for Coronavirus Disease-2019 tests during the public health emergency (Revised): immediately in effect Guidance for Clinical Laboratories, Commercial Manufacturers, and Food and Drug Administration Staff. (2020). https://www.fda.gov/media/135659/download
- 5.Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics 32, 338–343 (1963). [PubMed] [Google Scholar]
- 6.Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: final data for 2015. Natl Vital. Stat. Rep. 66(1), 1 (2017). [PubMed] [Google Scholar]
- 7.Boons CCLM, Timmers L, Janssen JJWM, Swart EL, Hugtenburg JG, Hendrikse NH. Feasibility of and patients’ perspective on nilotinib dried blood spot self-sampling. Eur. J. Clin. Pharmacol. 75(6), 825–829 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Jager NG, Rosing H, Linn SC, Schellens JH, Beijnen JH. Dried blood spot self-sampling at home for the individualization of tamoxifen treatment: a feasibility study. Ther. Drug Monit. 37(6), 833–836 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Kromdijk W, Mulder JW, Smit PM, Ter Heine R, Beijnen JH, Huitema AD. Therapeutic drug monitoring of antiretroviral drugs at home using dried blood spots: a proof-of-concept study. Antivir. Ther. 18(6), 821–825 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Leichtle AB, Ceglarek U, Witzigmann H, Gäbel G, Thiery J, Fiedler GM. Potential of dried blood self-sampling for cyclosporine c(2) monitoring in transplant outpatients. J. Transplant. 2010, 201918 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall JM, Fowler CF, Barrett F, Humphry RW, Van Drimmelen M, MacRury SM. HbA1c determination from HemaSpot™ blood collection devices: comparison of home prepared dried blood spots with standard venous blood analysis. Diabet. Med. 37(9), 1463–1470 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatti P, Kampa D, Alexander BH. et al. Blood spots as an alternative to whole blood collection and the effect of a small monetary incentive to increase participation in genetic association studies. BMC Med. Res. Methodol. 9, 76 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amsterdam PV, Waldrop C. The application of dried blood spot sampling in global clinical trials. Bioanalysis. 2(11), 1783–1786 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Martial LC, Aarnoutse RE, Schreuder MF, Henriet SS, Brüggemann RJ, Joore MA. Cost evaluation of dried blood spot home sampling as compared to conventional sampling for therapeutic drug monitoring in children. PLoS ONE 11(12), e0167433 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control. Shipping guidelines for dried-blood spot specimens. (2017). https://www.cdc.gov/labstandards/pdf/nsqap/Bloodspot_Transportation_Guidelines.pdf
- 16.Eick G, Urlacher SS, McDade TW, Kowal P, Snodgrass JJ. Validation of an optimized ELISA for quantitative assessment of Epstein–Barr virus antibodies from dried blood spots. Biodemography Soc. Biol. 62(2), 222–233 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doornekamp L, Embregts CW, Aron GI. et al. Dried blood spot cards: a reliable sampling method to detect human antibodies against rabies virus. PLoS Negl. Trop. Dis. 14(10), e0008784 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mössner BK, Staugaard B, Jensen J, Lillevang ST, Christensen PB, Holm DK. Dried blood spots, valid screening for viral hepatitis and human immunodeficiency virus in real-life. World J. Gastroenterol. 22(33), 7604 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto C, Nagashima S, Isomura M. et al. Evaluation of the efficiency of dried blood spot-based measurement of hepatitis B and hepatitis C virus seromarkers. Sci. Rep. 10(1), 3857 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh R, Hazarika NK. Detection of anti-hepatitis C virus and hepatitis C virus RNA in dried blood spot specimens using Whatman No. 1 filter paper. Indian J. Med. Microbiol. 36(2), 230–235 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Stefic K, Guinard J, Peytavin G. et al. Screening for Human Immunodeficiency Virus infection by use of a fourth-generation antigen/antibody assay and dried blood spots: in-depth analysis of sensitivity and performance assessment in a cross-sectional study. J. Clin. Microbiol. 58(1), e01645–19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang N, Pahalawatta V, Frank A. et al. HIV-1 viral load measurement in venous blood and fingerprick blood using Abbott RealTime HIV-1 DBS assay. J. Clin. Virol. 92, 56–61 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Nsobya SL, Hewett PC, Kalibala S, Mensch BS. Performance of Kalon herpes simplex virus 2 assay using dried blood spots among young women in Uganda. Afr. J. Lab. Med. 5(1), 429 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarkar S, Singh MP, Ratho RK. Dried blood spot for Ebola testing in developing countries. Lancet Infect. Dis. 15(9), 1005 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Borremans B, Gamble A, Prager KC. et al. Quantifying antibody kinetics and RNA detection during early-phase SARS-CoV-2 infection by time since symptom onset. Elife. 9, e60122 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Seroconversion times for IgG antibodies against spike S1 subunit and nucleocapsid SARS-CoV-2 proteins.
- 26.Long QX, Liu BZ, Deng HJ. et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 26(6), 845–848 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Egger M, Bundschuh C, Wiesinger K. et al. Comparison of the Elecsys® Anti-SARS-CoV-2 immunoassay with the EDI™ enzyme linked immunosorbent assays for the detection of SARS-CoV-2 antibodies in human plasma. Clin. Chim. Acta 509, 18–21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jääskeläinen AJ, Kekäläinen E, Kallio-Kokko H. et al. Evaluation of commercial and automated SARS-CoV-2 IgG and IgA ELISAs using coronavirus disease (COVID-19) patient samples. Euro. Surveill. 25(18), 2000603 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gudbjartsson DF, Norddahl GL, Melsted P. et al. Humoral immune response to SARS-CoV-2 in Iceland. N. Engl. J. Med. 383(18), 1724–1734 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • IgG antibody titers against spike S1 subunit and nucleocapsid SARS-CoV-2 proteins beyond 100 days postinfection.
- 30.Tian Y, Lian C, Chen Y. et al. Sensitivity and specificity of SARS-CoV-2 S1 subunit in COVID-19 serology assays. Cell Discov. 6, 75 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Benefit of using spike S1 subunit antigen over receptor-binding domain for assays detecting COVID-19 antibodies.
- 31.Casadevall A, Pirofski LA. The convalescent sera option for containing COVID-19. J. Clin. Invest. 130(4), 1545–1548 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suthar MS, Zimmerman M, Kauffman R. et al. Rapid generation of neutralizing antibody responses in COVID-19 patients. medRxiv (Preprint). (2020). Update in: Cell Rep. Med. 1(3), 100040 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 20(10), 615–632 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • The spike protein is the target for nearly all COVID-19 vaccines in development.
- 34.Adam BW, Alexander JR, Smith SJ. et al. Recoveries of phenylalanine from two sets of dried-blood-spot reference materials: prediction from hematocrit, spot volume, and paper matrix. Clin. Chem. 46(1), 126–128 (2000). [PubMed] [Google Scholar]
- 35.Mei JV, Alexander JR, Adam BW, Hannon WH. Use of filter paper for the collection and analysis of human whole blood specimens. J. Nutr. 131(5), S1631–S1636 (2001). [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. Blood collection and handling – Dried blood Spot (DBS). (2005) https://www.who.int/diagnostics_laboratory/documents/guidance/pm_module14.pdf
- 37.Chernecky CC, Berger BJ (Eds). H. Hematocrit (Hct) – blood. : Laboratory Tests and Diagnostic Procedures (6th Edition). Elsevier Saunders, St Louis, MO, USA, 620–621 (2013). [Google Scholar]
- 38.Postal Service 39 CFR Part 111. New mailing standards for Division 6.2 Infectious Substances. Federal Register 71(211), 64121–64125 (2006). https://www.govinfo.gov/content/pkg/FR-2006-11-01/pdf/E6-18062.pdf [Google Scholar]
- 39.Parker SP, Cubitt WD. The use of the dried blood spot sample in epidemiological studies. J. Clin. Pathol. 52(9), 633–639 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iyer AS, Jones FK, Nodoushani A. et al. Dynamics and significance of the antibody response to SARS-CoV-2 infection. medRxiv (Preprint). (2020). [Google Scholar]; • Details waning COVID-19 IgG antibody response over time.
- 41.Long QX, Liu BZ, Deng HJ. et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 26(6), 845–848 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Yongchen Z, Shen H, Wang X. et al. Different longitudinal patterns of nucleic acid and serology testing results based on disease severity of COVID-19 patients. Emerg. Microbes Infect. 9(1), 833–836 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma H, Zeng W, He H. et al. Serum IgA, IgM, and IgG responses in COVID-19. Cell. Mol. Immunol. 17(7), 773–775 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu WT, Howell JC, Ozturk T. et al. Antibody profiles according to mild or severe SARS-CoV-2 infection, Atlanta, Georgia, USA, 2020. Emerg. Infect. Dis. 26(12), (2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang YW, Schmitz JE, Persing DH, Stratton CW. Laboratory diagnosis of COVID-19: current issues and challenges. J. Clin. Microbiol. 58(6), e00512–e00520 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller TE, Garcia Beltran WF, Bard AZ. et al. Clinical sensitivity and interpretation of PCR and serological COVID-19 diagnostics for patients presenting to the hospital. FASEB J. (2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newman MS, Brandon TR, Groves MN. et al. Aliquid chromatography/tandem mass spectrometry method for determination of 25-hydroxy vitamin D2 and 25-hydroxy vitamin D3 in dried blood spots: a potential adjunct to diabetes and cardiometabolic risk screening. J. Diabetes Sci. Technol. 3(1), 156–162 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edelman A, Stouffer R, Zava DT, Jensen JT. et al. A comparison of blood spot vs. plasma analysis of gonadotropin and ovarian steroid hormone levels in reproductive-age women. Fertil. Steril.. 88(5), 1404–1407 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kapur S, Groves MN, Zava DT,Kapur S. et al. Postprandial insulin and triglycerides after different breakfast meal challenges: use of finger stick capillary dried blood spots to study postprandial dysmetabolism. J. Diabetes Sci. Technol. 4(2), 236–243 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williamson EJ, Walker AJ, Bhaskaran K. et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 584(7821), 430–436 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw. Open. 3(9), e2019722 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]