Over the last two decades, halide perovskites (HPs) have been identified as one of the most promising materials in photovoltaic and light-emitting devices.1,2 This has led to major breakthroughs in materials science3,4 but has also brought about a general misunderstanding and misuse of the term “perovskite”. In this Viewpoint, we will address the definition of a perovskite, with a main focus on the subgroup of perovskites that consist of heavier halides (Cl, Br, and I), both fully inorganic and hybrid organic–inorganic ones, as well as the many variants that can be found within this subgroup. In doing so, we will clarify what defines, in our opinion, a halide perovskite and discuss the commonly misnamed nonperovskite crystal structures.
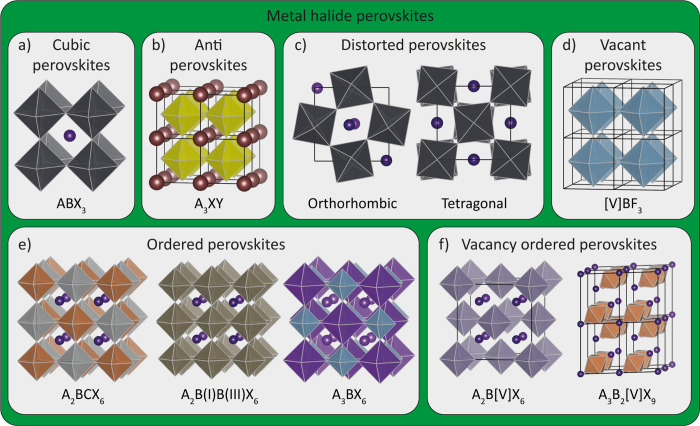
A perovskite crystal lattice is defined as a network of corner-sharing BX6 octahedra that crystallize with a general ABX3 (or equivalent) stoichiometry, as is shown in Figures 1 and 2a.5 Deviations from this ABX3 stoichiometry can be obtained when the A and B cation sites become partially or fully vacant (vacancy-ordered perovskites), or when they are replaced by a combination of other cations (with different valences but with an overall neutral charge balance), forming double or quadruple perovskites. The aristotype (the highest symmetric structure of a group of crystal structures)6 perovskite belongs to the Pm3̅m cubic space group, with SrTiO3 (tausonite) usually considered the prime example. The vast majority of perovskites though have a reduced symmetry (hettotypes, i.e. structures that are similar to the aristotype, but with a lower symmetry)6 due to lattice distortions, distorted octahedra, ordered cations, vacancies, or the presence of organic cations or inorganic clusters.
Figure 1.
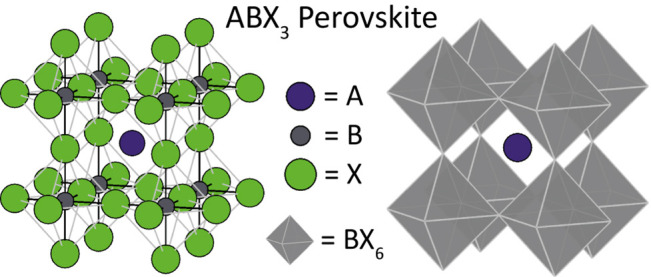
Standard depiction of the aristotype cubic perovskite. Shown in display styles evidencing either all the atoms (left) or only the BX6 octahedral network and A atoms (right).
Figure 2.
Overview of different halide perovskites. (a) Standard ABX3 cubic halide perovskites. (b) Antiperovskites, with A being a monovalent metal (like Li+ or Ag+), X a halide, and Y a chalcogenide. (c) Common orthorombic and tetragonal disordered perovskites, arising from the tilting of the octahedra. (d) Vacant BX3 perovskites, like AlF3. (e) Ordered perovskites, where two M(II) metals are replaced by a M(I) and M(III) metal. (f) Vacancy-ordered perovskites, where a part of the B-site cations are replaced with a M(III) or M(IV) and vacancies.
The term “perovskite” was first coined by Gustav Rose in 1839 for the CaTiO3 mineral, who named it after the Russian nobleman and mineralogist Count Lev Alekseyevich von Perovski. Later, in 1926, it was first used as a general term for the crystal structure group by Victor Goldschmidt.3 In nature, perovskites are primarily found as oxides, with the majority being silicates (such as bridgmanite minerals),7 but they also exist as fluorides, chlorides, hydroxides, arsenides, and intermetallic compounds.5 While the number of natural perovskite minerals is limited, synthetic perovskites span across the whole periodic table in terms of elemental composition, and they can exist in many complex formulas such as metallic perovskites (e.g., MgCNi3),8 hybrid organic–inorganic perovskites (e.g., [CH3NH3]PbI3 and [CH3NH3]Mn(HCOO)3),9 metal-free perovskites (e.g., [DABCO][NH4]I3 with MDABCO = N-methyl-N′-diazabicyclo[2.2.2]octonium),10 and even noble gas-based perovskites (KSrXeNaO6).11 This indicates that the perovskite is perhaps the most adaptable type of crystal lattice.
Inorganic Metal Halide Perovskites. The subgroup of perovskites (and ternary metal halides in general) that will be discussed in this Viewpoint have halides as their X anions. In the case of standard perovskites (that is, those with an ABX3 stoichiometry) the B cations are divalent (like Pb2+, or Sn2+) and the A cations are large monovalent alkali metals (most commonly cesium) or small organic cations like methylammonium (MA) or formamidinium (FA). Because of the single charge of the anions in HPs, the A and B cations are limited to M(I) and M(II), respectively (not considering ordered or vacant perovskites).
While this Viewpoint will mainly concentrate on heavy metal-based halides, which generally have bandgaps in the visible spectrum and NIR (therefore they are of more interest for use in photovoltaic and light emitting devices), there is a vast group of first row transition metal fluoride perovskites, which can be interesting for lithium intercalation or as a host material for up- or down-conversion emission.12−14 One of these fluoride perovskites, KMgF3 (parascandolaite),15 is also one of the few HPs that can be found in nature, and it crystallizes in a perfect cubic perovskite structure (Pm3̅m). Therefore, KMgF3 is a more suitable aristotype for HPs than SrTiO3.5 Fluoride perovskites can also crystallize in a so-called “inverse perovskite” (which is not to be confused with antiperovskite), in which the A cation has a higher oxidation state than the B cation, like in the case of BaLiF3.16 This occurs only when the A cations (e.g., Ba2+ and Sr2+) are very large and the B cations (e.g., Li+) are very small. Moreover, these small B cations can only form perovskites with F– and H– ions. Finally, halide antiperovskites also exist, in which the A and B sites are occupied by anions (halide and chalcogenide respectively) and the X sites are occupied by a monovalent cation (as is shown in Figure 2b).17,18 Halide antiperovskites like Li3OBr and Ag3SI will not be discussed in this Viewpoint, as the halide is not part of the BX framework, but they certainly do classify as perovskites.
Fully inorganic, heavy metal HPs do not crystallize in the pure cubic perovskite crystal lattice. Rather, they exhibit a distorted lattice, resulting in hettotype structures. These distortions occur as a result of the size difference between the A, B, and X cations. Consequently, the individual octahedra tilt, which generally results in either orthorhombic (commonly referred to as the “GdFeO3-type”)19 or tetragonal crystal structures (Figure 2c).19 Although these distortions are responsible for the lower crystal structure symmetry, the overall perovskite framework is preserved; therefore, these materials are classified as hettotypes of the perovskite group. In fact, even the original perovskite (CaTiO3) exhibits a small distortion and does not actually crystallize in a “perfect” cubic perovskite structure.5
Several variants of the perovskite structure, and thus of ABX3 stoichiometry, can be obtained by partially or fully replacing (ordering) or even removing A and/or B cations. Although the latter (i.e., fully removing A cations) is more frequently found in metal pnictides (e.g., skutterudites, CoAs3),20 the A cation site in HPs can be fully vacant, resulting in the so-called “A-site vacant BX3” crystal structure, as is shown in Figure 2d. This type of structure is observed only in fluorides, like AlF3, FeF3 CoF3, and MnF3.5 Another variant of the ABX3 perovskite is in the form of ordered perovskites (Figure 2e). Here, the B cations are heterovalently replaced by a combination of two (or more) cations that are located at specific crystallographic sites.21 In the case of HPs, this results in an A2BCX6 elpasolite structure (which is also sometimes termed “A2BB′X6”), with its respective aristotype being the K2NaAlF6 mineral. Because of the double occupancy of the B-site cation, these perovskites are often simply called “double perovskites”, and they have been frequently studied as lead-free photovoltaic materials, albeit with limited success,21 or for broadband light emission.22 For heavy metal halides, the double perovskite subgroup includes materials such as Cs2AgInCl6, Cs2AgBiBr6, and MA2AgSbI6, with a crystal structure that belongs to the Fm3̅m space group.21 It is important to note that these are not the same as mixed A, B, or X site perovskites (for example, (Cs:MA:FA)(Pb:Sn)(Br:I)3), in which the mixed cations occupying the respective A/B/X sites have the same valence, meaning that they do not occupy specific crystallographic sites. Furthermore, ordered perovskites have fixed stoichiometries, which is not the case for mixed perovskites. A variant of the double perovskite can also be obtained with a single metal in both the (I) and (III) oxidation states. These double perovskites include materials such as CsTlX3 and CsAuX3 (even though the latter has strongly distorted octahedra).23,24 Finally, a small group of fluoride ordered perovskites crystallize in the A3BX6 cryolite (Na3AlF6) phase, where half of the B sites are occupied by the same cations that occupy the A sites (that is, the formula is the same as that of an ordered perovskite when it is written as A2ABX6).5 While the ordering of cations in HPs occurs only with B site cations, oxide perovskites can also form quadruple perovskites when both the A and B sites are occupied by two differently charged cations: these materials are generally described as having an AA′BB′O6 formula, like KSrXeNaO6, but the CaCu3Fe2Re2O12 compound also falls under this scheme.5,11,25 In the case of oxide perovskites, there are also examples of ordered perovskites that consist of either alternating layers or columns of different metal octahedra. One example of this type of ordered perovskites is Sr2La2CuTi3O12, which is formed by three layers of TiO6 octahedra alternated with a layer of CuO6 octahedra. These structures have not yet been reported for HPs, but any advances in this direction will certainly lead to interesting new halide materials.26
The final subgroup of halide perovskites are vacancy-ordered perovskites (Figure 2f).27 These are similar to ordered perovskites, but the B-site cation is partially replaced with a vacancy. Among the most studied vacancy-ordered perovskites are those belonging to the Cs2BX6 group, in which half of the B-site cations are occupied by M(IV) cations and the other half by vacancies. Because these are crystallographically identical to ordered perovskites (A2B[V]X6, in which [V] is a vacancy), they share the same Fm3̅m space group. Vacancy-ordered perovskites in the M(IV) group include Cs2SnI6, Cs2PdBr6, Cs2TeI6, and Cs2TiBr6.27 Vacancy-ordered perovskites can also form with trivalent metals like Sb3+ and Bi3+. In this case, the B sites are not occupied by M(III) and by a vacancy in equal ratios; instead, they are occupied at a ratio of 2:1, resulting in an A3B2X9 stoichiometry (A3B2[V]X9) such as Cs3Sb2I9.28 In these ordered perovskites, the vacancies are ordered along the [111] planes, giving rise to a two-dimensional (2D) layer of BX6 octahedra. By mixing these vacancy-ordered perovskites with Cd2+, the fraction of vacancies can be further lowered to 25%, leading to a Cs4CdSb2Cl12 (A4BC2[V]X12) vacancy-ordered triple perovskite.29 Because of the high concentration of vacancies, vacancy-ordered perovskites often exhibit low conductivity and thus have limited applications.27 It is important to note that not all A3B2X9 halides crystallize in this vacancy-ordered perovskite phase: for example, Cs3Bi2I9 crystallizes in a nonperovskite phase (as will be discussed below).
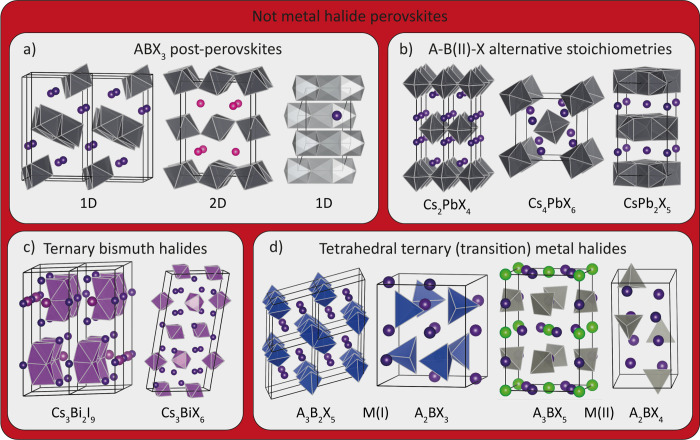
Nonperovskite Inorganic Metal Halides. Not all ternary metal halides can form a stable perovskite structure. In general, the HP framework collapses if the A-site cation is either too small or too large for the metal halide network19 (the reader should refer to the Goldsmith tolerance factor, which is a figure of merit that indicates whether ternary metal halides can form geometrically stable perovskites or not).12,30,31 As is shown in Figure 3a, ABX3 metal halides that cannot form geometrically stable perovskites crystallize in either 1D or 2D edge-sharing octahedral lattices (like CsPbI3 or RbPbBr3, in which the A-site cation would be too small to stabilize the hypothetical perovskite structure) or a 1D face-sharing crystal lattice (like FAPbI3, in which the A-site cation would be too large for the perovskite structure). The structural stability of ternary metal halide perovskites depends not only on the size of the ions but also on temperature and pressure:19 ternary metal halides that do not form stable perovskites under ambient conditions may do so at high temperatures or pressures; therefore, they are often called “post-perovskites”. In the case of LHPs, the two most studied post-perovskites are CsPbI3 and FAPbI3, both of which are post-perovskites under ambient conditions, but they form a perovskite phase when they are annealed at around 200–300 °C.19
Figure 3.
Overview of ternary metal halides not crystallizing in a perovskite structure. (a) Post-perovskites formed by ABX3 ternary metal halides that would form stable perovskites at higher temperatures/pressures. (b) Ternary M(II) halides with nonperovskite stoichiometries, including inorganic Ruddlesden–Popper metal halide (A2BX4), A4BX6, and AB2X5 phases. (c) Ternary bismuth halides not crystallizing in vacancy-ordered perovskites, including the isolated dimer structure (Cs3Bi2I9) and isolated octahedra (Cs3BiX6). (d) Several ternary M(I) and M(II) (mainly transition metal and F– and Cl–) phases based on MX4 tetrahedra.
Inorganic ternary M(II) halides (like those based on Sn2+ and Pb2+ions) can also crystallize in several other stoichiometries (as shown in Figure 3b) with phases that can be very different from perovskites. One of these phases, the A4BX6 one (such as Cs4PbBr6), has often been called a “0D perovskite”.31,32 Although this phase consists of individual disconnected octahedra, similar to the A2BX6 vacancy-ordered perovskites, the BX6 octahedra are no longer in the crystallographic positions that correspond to those of a perovskite. Moreover, the A-site cations now occupy two different crystallographic sites.33 Therefore, an A4BX6 phase cannot be considered a perovskite. One ternary phase which is often called a “2D perovskite” is the AB2X5 phase (the most known example of which is CsPb2Br5). CsPb2Br5 is generally described as a stack of layers of connected [B2X5]+ clusters, separated by layers of Cs+ ions.34 A close inspection of this structure (Figure 3b, right structure) reveals that it does not even contain MX6 octahedra; therefore, it falls short of being considered a perovskite. Although thus not perovskites, these materials recently gained interest for their bright and narrow green photoluminescence (Cs4PbBr6 and CsPb2Br5) and for their use as a remote thermography material (Cs4SnBr6).31,32,35
Heavy metal (Bi and Sb) based A3B2X9 compounds usually crystallize in a vacancy-ordered perovskite structure (as mentioned above). However, ternary bismuth iodides with large A-site cations, like Cs3Bi2I9, MA3Bi2I9, and FA3Bi2I9, crystallize in a different phase.36 In this A3B2X9 phase, [Bi2I9]3+ dimers are formed by two face-sharing BiI6 octahedra, similar to Cs3Cr2Cl9.37 These dimers are separated by Cs+ ions, resulting in a hexagonal 0D ternary metal halide, as is shown in Figure 3c. Thus, this phase does not much resemble a perovskite. Finally, Cs–Bi–X compounds can also crystallize in the Cs3BiX6 phase featuring single BX6 octahedra which are no longer in the crystallographic positions that correspond to those of a perovskite. Therefore, they are not a A3BX6 cryolite, as described above. Consequently, they are not (double) perovkites.38
In addition to the tolerance factor, another important parameter that dictates the stability of perovskites is the octahedral factor,12 as it relates to the stability of the BX6 octahedron. For instance, small cations that are coordinated by large anions (such as first row d metals and large anions like Br– and I–) generally prefer tetrahedral coordination. Therefore, HPs containing first row transition metals are primarily limited to fluorides, and there are only a few examples of HPs based on chlorides.12 For example, ternary compounds like A3Cu2X5 and ACu2X3,39 or compounds like A2BX4 and A3BX5 in which B is a first row transition metal (such as Zn2+, Ni2+, Cu2+, and Mn2+)40 all consist of tetrahedral BX4 clusters surrounded by A+ cations. Therefore, they cannot be classified as perovskites (see Figure 3d). However, they can be interesting nontoxic broad emitters (or at least less toxic than Pb-based ones).40
Inorganic Layered Metal Halides. Among the various ternary metal halides, Ruddlesden–Popper (RP) phases (and layered metal halides in general, which will be discussed below) present a structurally interesting case. In the RP phase, which has an A2BX4 stoichiometry, a single 2D layer of corner-sharing M(II) halide octahedra is separated by a layer of A atoms (Figure 3b).41 Even though oxide RP phases can be synthesized using a wide variety of metals (such as Sr2RuO4, La2NiO4, and LaSrCoO4),42 inorganic RP halide phases have, thus far, been limited to Cs2PbI2Cl2 and Cs2SnI2Cl2.43 In an inorganic halide RP phase, the separation into layers is driven by the size difference between the two halides.41 Although (in oxides) these RP phases are often characterized as perovskites (“Ruddlesden–Popper perovskites”), it is our opinion that they should be classified only as RPs, and not as perovskites. We justify this opinion on the basis of the following two points: (i) These phases already have a common name (i.e., “Ruddlesden–Popper”). (ii) Starting from a perovskite structure, one cannot derive such phases without having to severely dismantle the perovskite lattice, at least locally. Furthermore, RP halides exhibit electronic and optical properties that are markedly different from their perovskite counterparts.41 The same reasoning applies to other phases consisting of stacks of layers made of corner-sharing octahedra, like Dion–Jacobson phases and the Aurivillius phase (both of which have been reported only for oxides to date),44 as well as hybrid organic–inorganic layered metal halides, which will be discussed next.
Hybrid Organic–Inorganic Metal Halides. Hybrid organic–inorganic metal halides have gained an increasing amount of attention over the past decade.19 These are often also referred to as “hybrid perovskites” (Figure 4).9,45 In these materials, the A site is occupied by an organic cation, most commonly an alkyl ammonium cation. These types of materials are not organometallic compounds,46,47 as they do not contain any carbon–metal bonds, therefore they should not be called “organometallics” or “organolead” perovskites, but rather “hybrid organic–inorganic perovskites” (HOIPs).9 In the case of HOIPs, the A cations can consist of only small (pseudo spherical) MA and FA cations and the larger B cations, like Pb2+ and Sn2+,9 as is shown in Figure 4a. Here, it must be noted that not all of the hybrid organic–inorganic Pb and Sn halides form perovskites,19 as some (like FAPbI3) are stable only at high temperatures, thus forming “post-perovskites” at ambient conditions.19 Small transition metal fluorides can also form stable HOIPs with NH4+, like NH4MnF3 and NH4ZnF3.48 Because of the anisotropy in the inorganic cations, the HOIPs have a lower symmetry than their inorganic counterparts, even in their pure cubic crystal structure.19 As the size of the A cation increases, layered compounds are formed, and these compounds usually crystallize in an A2BX4 (or ABX4 in the case of cations with two ammonium groups) stoichiometry, in which layers of corner-sharing octahedra are separated by a layer of interlocking organic hydrocarbon chains (Figure 4c).45,49 Following the same line of reasoning as in the RP case above, these materials should not be considered perovskites either. In our opinion, these compounds should be simply classified as “layered hybrid organic−inorganic metal halides”.
Figure 4.
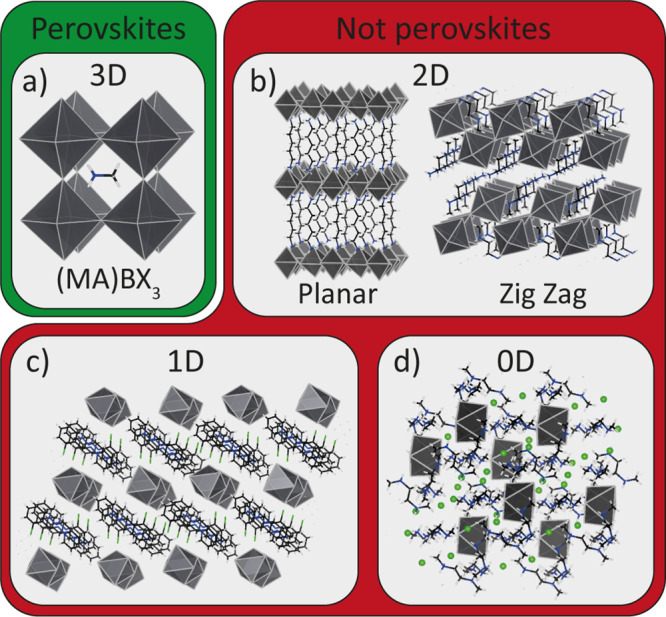
Overview of layered and hybrid organic–inorganic metal halides. (a) Hybrid organic–inorganic perovskite structure, with the A cation being a small organic cation like methylammonium. (b) Layered (2D) hybrid organic–inorganic metal halides, consisting of planar or zigzag corner-sharing octahedra. (c) 1D metal halides, consisting of chains of facet or corner-sharing octahedra. (d) 0D hybrid organic–inorganic metal halides, consisting of isolated metal halide octahedra.
One subclass of these layered hybrid organic–inorganic metal halides that has gained considerable interest because of their better moisture stability and higher degree of optoelectronic fine-tuning than conventional lead halide perovskites is the one containing several consecutive layers of 3D connected metal halides alternated with a single layer of organic cations (often called “2D perovskites” or “layered perovskites”).19 These compounds can be synthesized by using a mixture of a small cation that can form a perovskite (e.g., Cs+ or MA) and a large organic cation that can form only layered metal halides. These layered materials have a general [L]An–1BnX3n+1 formula, in which L is the large organic cation (sometimes referred to as a “ligand”) and n is the number of layers of 3D connected octahedra.45,46,49 In this case, the line between a layered metal halide (n = 1) and a perovskite (n ≈ ∞) remains a topic of debate and, to a certain extent, a matter of opinion. Finally, hybrid organic–inorganic metal halides have been reported using bulky organic cations or organic cations consisting of multiple ammonium groups.46 These compounds often are either formed by single chains of metal halide octahedra (Figure 4c) or by completely isolated metal halide clusters (Figure 4D), and they are sometimes referred to as “1D” or “0D organic perovskites”.46 These types of compounds have very little in common with the perovskite crystal structure, therefore they should not be identified as perovskites, but rather as “0D” or ‘1D hybrid organic–inorganic metal halides’.
Antiperovskite with Halide B Sites. Although the aforementioned AB2X5 and A3BX5 compositions do not consist of octahedrally coordinated metal cations (and are therefore strictly not perovskites), they can qualify as inverse antiperovskites, because they have XB6 octahedra and small X or BX clusters occupying the A site. They are not the same as antiperovskites, in which the halides generally occupy only the A site, and are not part of the perovskite framework. In the case of an A3BX5 phase, using Cs3ZnBr5 as an example, the Br– anion can be interpreted as being octahedrally coordinated by Cs+ cations, resulting in a [BrCs3]2+ cubic framework.50 Within this framework, the [ZnBr4]2– clusters occupy the A sites, as is shown in Figure 5a. This is also similar to a double antiperovskite Na6FCl(SO4)2 (sulphohalite, a natural mineral), in which the A site is occupied by a SO42– cluster and the perovskite framework consists of FNa6 and ClNa6 octahedra, as well as Ba3(FeS4)Br, which has a [FeS4]−5 A-site cluster.5,51 Small inorganic clusters in A sites can also be found in a standard perovskite structure, like Tl4SnTe3, which is a chalcogenide perovskite (consisting of SnTe6 octahedra) with an A site that is occupied by a tetrahedral Tl44+ cluster.52 Similarly, the AB2X5 phase (for example, CsPb2Br5, as is shown in Figure 5b) can also be considered an antiperovskite consisting of octahedrally coordinated halide anions. Here, [BrPb2Cs]4+ octahedra form a perovskite framework, with the A cation site being occupied by a cluster of 4 Br– ions. This is analogous to the TlSn2I5 case.53 The possibility for small inorganic clusters to occupy the A site of a standard perovskite was recently demonstrated with the vacancy-ordered halide perovskite structure of Cs3Cu4In2Cl13, in which 25% of the A sites are occupied by [Cu4Cl]3+ clusters and half of the B sides are vacant (a structure that can be more appropriately described as ([Cu4Cl]Cs3In2[V]2Cl12), is shown in Figure 5c.54 These cases of inverse antiperovskites, together with the inverse perovskites like BaLiF3, demonstrate that the A-site cations in perovskites are not simply limited to alkali metals (or small organic molecules), but they can also consist of small inorganic clusters and metal cations with different charges, a consideration that should inspire the discovery of new halide perovskites.
Figure 5.
Antiperovskites of A3BX5 and AB2X5 phases. (a) Cs3ZnBr5 and (b) CsPb2Br5 with (left) polyhedra centered on the halides and (right) top view shown along the c-axis. (c) Cs3Cu4In2Cl13 vacancy-ordered perovskite, consisting of InCl63– octahedra and Cu4Cl3+ tetrahedra occupying the A sites.
Conclusions and Recommendations. Although there are many different types of HPs, the recent and rapid advancements in the field has led to a confusion on the correct way of naming all the different compounds that have been reported. In our opinion, if a compound is to be considered a “perovskite”, it must have an ABX3 (or equivalent) stoichiometry consisting of a cubic network of corner-sharing BX octahedra, with the following exceptions: (i) the A and B sites can be partially or fully vacant or ordered; (ii) the A cations can consist of small organic molecules or inorganic clusters; (iii) the formation of anti-halide perovskites, with a halide occupying either the A or B site. 1D and 0D hybrid organic–inorganic metal halides do not satisfy these conditions; therefore, they should not be considered as perovskites. Although this is a somewhat controversial subject, layered Ruddlesden–Popper (and similar) phases, as well as 2D hybrid organic–inorganic metal halides, do not fit these criteria either. Thus, it is our opinion that they should not be referred to as perovskites. As a final comment, we stress the importance of stating whether a perovskite is vacant, ordered, anti, or a combination of these. This not only identifies the deviations from a typical perovskite stoichiometry but also, in most cases, explains the completely different properties of the materials compared to their perovskite equivalent.
Acknowledgments
We thank Emma De Cecco for proofreading the viewpoint. The research leading to these results has received funding from the seventh European Community Framework Programme under Grant Agreement No. 614897 (ERC Consolidator Grant “TRANS-NANO”).
Author Present Address
† Q.A.A.: Department of Chemistry and Applied Biosciences, ETH Zürich, Vladimir Prelog Weg 1, CH-8093 Zürich, Switzerland.
The views expressed in this Viewpoint are those of the authors and not necessarily the views of the ACS.
The authors declare no competing financial interest.
References
- Correa-Baena J.-P.; Saliba M.; Buonassisi T.; Grätzel M.; Abate A.; Tress W.; Hagfeldt A. Promises and Challenges of Perovskite Solar Cells. Science 2017, 358, 739–744. 10.1126/science.aam6323. [DOI] [PubMed] [Google Scholar]
- Lin K.; Xing J.; Quan L. N.; de Arquer F. P. G.; Gong X.; Lu J.; Xie L.; Zhao W.; Zhang D.; Yan C.; et al. Perovskite Light-Emitting Diodes with External Quantum Efficiency Exceeding 20 Per Cent. Nature 2018, 562, 245–248. 10.1038/s41586-018-0575-3. [DOI] [PubMed] [Google Scholar]
- Akkerman Q. A.; Rainò G.; Kovalenko M. V.; Manna L. Genesis, Challenges and Opportunities for Colloidal Lead Halide Perovskite Nanocrystals. Nat. Mater. 2018, 17, 394–405. 10.1038/s41563-018-0018-4. [DOI] [PubMed] [Google Scholar]
- Best Research-Cell Efficiency Chart. https://www.nrel.gov/pv/cell-efficiency.html.
- Mitchell R. H.; Welch M. D.; Chakhmouradian A. R. Nomenclature of the Perovskite Supergroup: A Hierarchical System of Classification Based on Crystal Structure and Composition. Mineral. Mag. 2017, 81, 411–461. 10.1180/minmag.2016.080.156. [DOI] [Google Scholar]
- Megaw H. D.Crystal Structures: A Working Approach; Saunders, 1973. [Google Scholar]
- Hirose K.; Sinmyo R.; Hernlund J. Perovskite in Earth’s Deep Interior. Science 2017, 358, 734–738. 10.1126/science.aam8561. [DOI] [PubMed] [Google Scholar]
- He T.; Huang Q.; Ramirez A. P.; Wang Y.; Regan K. A.; Rogado N.; Hayward M. A.; Haas M. K.; Slusky J. S.; Inumara K.; et al. Superconductivity in the Non-Oxide Perovskite MgCNi3. Nature 2001, 411, 54–56. 10.1038/35075014. [DOI] [PubMed] [Google Scholar]
- Li W.; Wang Z.; Deschler F.; Gao S.; Friend R. H.; Cheetham A. K. Chemically Diverse and Multifunctional Hybrid Organic–Inorganic Perovskites. Nat. Rev. Mater. 2017, 2, 16099. 10.1038/natrevmats.2016.99. [DOI] [Google Scholar]
- Ye H.-Y.; Tang Y.-Y.; Li P.-F.; Liao W.-Q.; Gao J.-X.; Hua X.-N.; Cai H.; Shi P.-P.; You Y.-M.; Xiong R.-G. Metal-Free Three-Dimensional Perovskite Ferroelectrics. Science 2018, 361, 151–155. 10.1126/science.aas9330. [DOI] [PubMed] [Google Scholar]
- Britvin S. N.; Kashtanov S. A.; Krzhizhanovskaya M. G.; Gurinov A. A.; Glumov O. V.; Strekopytov S.; Kretser Y. L.; Zaitsev A. N.; Chukanov N. V.; Krivovichev S. V. Perovskites with the Framework-Forming Xenon. Angew. Chem., Int. Ed. 2015, 54, 14340–14344. 10.1002/anie.201506690. [DOI] [PubMed] [Google Scholar]
- Li C.; Lu X.; Ding W.; Feng L.; Gao Y.; Guo Z. Formability of ABX3 (X = F, Cl, Br, I) Halide Perovskites. Acta Crystallogr., Sect. B: Struct. Sci. 2008, 64, 702–707. 10.1107/S0108768108032734. [DOI] [PubMed] [Google Scholar]
- Yi T.; Chen W.; Cheng L.; Bayliss R. D.; Lin F.; Plews M. R.; Nordlund D.; Doeff M. M.; Persson K. A.; Cabana J. Investigating the Intercalation Chemistry of Alkali Ions in Fluoride Perovskites. Chem. Mater. 2017, 29, 1561–1568. 10.1021/acs.chemmater.6b04181. [DOI] [Google Scholar]
- Zhang Y.; Lin J. D.; Vijayaragavan V.; Bhakoo K. K.; Tan T. T. Y. Tuning Sub-10 Nm Single-Phase NaMnF3 Nanocrystals as Ultrasensitive Hosts for Pure Intense Fluorescence and Excellent T1 Magnetic Resonance Imaging. Chem. Commun. 2012, 48, 10322–10324. 10.1039/c2cc34858f. [DOI] [PubMed] [Google Scholar]
- Demartin F.; Campostrini I.; Castellano C.; Russo M. Parascandolaite, KMgF3, a New Perovskite-Type Fluoride from Vesuvius. Phys. Chem. Miner. 2014, 41, 403–407. 10.1007/s00269-014-0668-y. [DOI] [Google Scholar]
- Wiedemann D.; Meutzner F.; Fabelo O.; Ganschow S. The Inverse Perovskite BaLiF3: Single-Crystal Neutron Diffraction and Analyses of Potential Ion Pathways. Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater. 2018, 74, 643–650. 10.1107/S2052520618014579. [DOI] [Google Scholar]
- Emly A.; Kioupakis E.; Van der Ven A. Phase Stability and Transport Mechanisms in Antiperovskite Li3OCl and Li3OBr Superionic Conductors. Chem. Mater. 2013, 25, 4663–4670. 10.1021/cm4016222. [DOI] [Google Scholar]
- Hoshino S.; Sakuma T.; Fujii Y. A Structural Phase Transition in Superionic Conductor Ag3SI. J. Phys. Soc. Jpn. 1979, 47, 1252–1259. 10.1143/JPSJ.47.1252. [DOI] [Google Scholar]
- Stoumpos C. C.; Kanatzidis M. G. The Renaissance of Halide Perovskites and Their Evolution as Emerging Semiconductors. Acc. Chem. Res. 2015, 48, 2791–2802. 10.1021/acs.accounts.5b00229. [DOI] [PubMed] [Google Scholar]
- Rull-Bravo M.; Moure A.; Fernández J. F.; Martín-González M. Skutterudites as Thermoelectric Materials: Revisited. RSC Adv. 2015, 5, 41653–41667. 10.1039/C5RA03942H. [DOI] [Google Scholar]
- Volonakis G.; Filip M. R.; Haghighirad A. A.; Sakai N.; Wenger B.; Snaith H. J.; Giustino F. Lead-Free Halide Double Perovskites Via Heterovalent Substitution of Noble Metals. J. Phys. Chem. Lett. 2016, 7, 1254–1259. 10.1021/acs.jpclett.6b00376. [DOI] [PubMed] [Google Scholar]
- Luo J.; Wang X.; Li S.; Liu J.; Guo Y.; Niu G.; Yao L.; Fu Y.; Gao L.; Dong Q.; et al. Efficient and Stable Emission of Warm-White Light from Lead-Free Halide Double Perovskites. Nature 2018, 563, 541–545. 10.1038/s41586-018-0691-0. [DOI] [PubMed] [Google Scholar]
- Retuerto M.; Emge T.; Hadermann J.; Stephens P. W.; Li M. R.; Yin Z. P.; Croft M.; Ignatov A.; Zhang S. J.; Yuan Z.; et al. Synthesis and Properties of Charge-Ordered Thallium Halide Perovskites, CsTl+0.5Tl3+0.5X3 (X = F or Cl): Theoretical Precursors for Superconductivity?. Chem. Mater. 2013, 25, 4071–4079. 10.1021/cm402423x. [DOI] [Google Scholar]
- Wang S.; Hirai S.; Shapiro M. C.; Riggs S. C.; Geballe T. H.; Mao W. L.; Fisher I. R. Pressure-Induced Symmetry Breaking in Tetragonal CsAuI3. Phys. Rev. B: Condens. Matter Mater. Phys. 2013, 87, 054104. 10.1103/PhysRevB.87.054104. [DOI] [Google Scholar]
- Chen W.-t.; Mizumaki M.; Seki H.; Senn M. S.; Saito T.; Kan D.; Attfield J. P.; Shimakawa Y. A Half-Metallic a- and B-Site-Ordered Quadruple Perovskite Oxide CaCu3Fe2Re2O12 with Large Magnetization and a High Transition Temperature. Nat. Commun. 2014, 5, 3909. 10.1038/ncomms4909. [DOI] [PubMed] [Google Scholar]
- King G.; Woodward P. M. Cation Ordering in Perovskites. J. Mater. Chem. 2010, 20, 5785–5796. 10.1039/b926757c. [DOI] [Google Scholar]
- Maughan A. E.; Ganose A. M.; Scanlon D. O.; Neilson J. R. Perspectives and Design Principles of Vacancy-Ordered Double Perovskite Halide Semiconductors. Chem. Mater. 2019, 31, 1184–1195. 10.1021/acs.chemmater.8b05036. [DOI] [Google Scholar]
- Saparov B.; Hong F.; Sun J.-P.; Duan H.-S.; Meng W.; Cameron S.; Hill I. G.; Yan Y.; Mitzi D. B. Thin-Film Preparation and Characterization of Cs3Sb2I9: A Lead-Free Layered Perovskite Semiconductor. Chem. Mater. 2015, 27, 5622–5632. 10.1021/acs.chemmater.5b01989. [DOI] [Google Scholar]
- Lin Y.-P.; Hu S.; Xia B.; Fan K.-Q.; Gong L.-K.; Kong J.-T.; Huang X.-Y.; Xiao Z.; Du K.-Z. Material Design and Optoelectronic Properties of Three-Dimensional Quadruple Perovskite Halides. J. Phys. Chem. Lett. 2019, 10, 5219–5225. 10.1021/acs.jpclett.9b01757. [DOI] [PubMed] [Google Scholar]
- Bartel C. J.; Sutton C.; Goldsmith B. R.; Ouyang R.; Musgrave C. B.; Ghiringhelli L. M.; Scheffler M. New Tolerance Factor to Predict the Stability of Perovskite Oxides and Halides. Sci. Adv. 2019, 5, eaav0693. 10.1126/sciadv.aav0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkerman Q. A.; Abdelhady A. L.; Manna L. Zero-Dimensional Cesium Lead Halides: History, Properties, and Challenges. J. Phys. Chem. Lett. 2018, 9, 2326–2337. 10.1021/acs.jpclett.8b00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almutlaq J.; Yin J.; Mohammed O. F.; Bakr O. M. The Benefit and Challenges of Zero-Dimensional Perovskites. J. Phys. Chem. Lett. 2018, 9, 4131–4138. 10.1021/acs.jpclett.8b00532. [DOI] [PubMed] [Google Scholar]
- Hu M.; Ge C.; Yu J.; Feng J. Mechanical and Optical Properties of Cs4BX6 (B = Pb, Sn; X = Cl, Br, I) Zero-Dimension Perovskites. J. Phys. Chem. C 2017, 121, 27053–27058. 10.1021/acs.jpcc.7b10629. [DOI] [Google Scholar]
- Dursun I.; De Bastiani M.; Turedi B.; Alamer B.; Shkurenko A.; Yin J.; El-Zohry A. M.; Gereige I.; AlSaggaf A.; Mohammed O. F.; et al. CsPb2Br5 Single Crystals: Synthesis and Characterization. ChemSusChem 2017, 10, 3746–3749. 10.1002/cssc.201701131. [DOI] [PubMed] [Google Scholar]
- Yakunin S.; Benin B. M.; Shynkarenko Y.; Nazarenko O.; Bodnarchuk M. I.; Dirin D. N.; Hofer C.; Cattaneo S.; Kovalenko M. V. High-Resolution Remote Thermometry and Thermography Using Luminescent Low-Dimensional Tin-Halide Perovskites. Nat. Mater. 2019, 18, 846–852. 10.1038/s41563-019-0416-2. [DOI] [PubMed] [Google Scholar]
- Akkerman Q. A.; Martínez-Sarti L.; Goldoni L.; Imran M.; Baranov D.; Bolink H. J.; Palazon F.; Manna L. Molecular Iodine for a General Synthesis of Binary and Ternary Inorganic and Hybrid Organic–Inorganic Iodide Nanocrystals. Chem. Mater. 2018, 30, 6915–6921. 10.1021/acs.chemmater.8b03295. [DOI] [Google Scholar]
- Oliver L. The Crystal Structure of Cesium Bismuth Iodide, Cs3Bi2I9. Acta Chem. Scand. 1968, 22, 2943–2952. [Google Scholar]
- Creutz S. E.; Liu H.; Kaiser M. E.; Li X.; Gamelin D. R. Structural Diversity in Cesium Bismuth Halide Nanocrystals. Chem. Mater. 2019, 31, 4685–4697. 10.1021/acs.chemmater.9b00640. [DOI] [Google Scholar]
- Luo J.; Hu M.; Niu G.; Tang J. Lead-Free Halide Perovskites and Perovskite Variants as Phosphors toward Light-Emitting Applications. ACS Appl. Mater. Interfaces 2019, 11, 31575–31584. 10.1021/acsami.9b08407. [DOI] [PubMed] [Google Scholar]
- Morad V.; Cherniukh I.; Pöttschacher L.; Shynkarenko Y.; Yakunin S.; Kovalenko M. V. Manganese(II) in Tetrahedral Halide Environment: Factors Governing Bright Green Luminescence. Chem. Mater. 2019, 31, 10161–10169. 10.1021/acs.chemmater.9b03782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkerman Q. A.; Bladt E.; Petralanda U.; Dang Z.; Sartori E.; Baranov D.; Abdelhady A. L.; Infante I.; Bals S.; Manna L. Fully Inorganic Ruddlesden–Popper Double Cl–I and Triple Cl–Br–I Lead Halide Perovskite Nanocrystals. Chem. Mater. 2019, 31, 2182–2190. 10.1021/acs.chemmater.9b00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran P. V.; Puggioni D.; Rondinelli J. M. Crystal-Chemistry Guidelines for Noncentrosymmetric A2BO4 Ruddlesden–Popper Oxides. Inorg. Chem. 2014, 53, 336–348. 10.1021/ic402283c. [DOI] [PubMed] [Google Scholar]
- Xu Z.; Chen M.; Liu S. F. Layer-Dependent Ultrahigh-Mobility Transport Properties in All-Inorganic Two-Dimensional Cs2PbI2Cl2 and Cs2SnI2Cl2 Perovskites. J. Phys. Chem. C 2019, 123, 27978–27985. 10.1021/acs.jpcc.9b09512. [DOI] [Google Scholar]
- Schaak R. E.; Mallouk T. E. Perovskites by Design: A Toolbox of Solid-State Reactions. Chem. Mater. 2002, 14, 1455–1471. 10.1021/cm010689m. [DOI] [Google Scholar]
- Grancini G.; Nazeeruddin M. K. Dimensional Tailoring of Hybrid Perovskites for Photovoltaics. Nat. Rev. Mater. 2019, 4, 4–22. 10.1038/s41578-018-0065-0. [DOI] [Google Scholar]
- Lin H.; Zhou C.; Tian Y.; Siegrist T.; Ma B. Low-Dimensional Organometal Halide Perovskites. ACS Energy Lett. 2018, 3, 54–62. 10.1021/acsenergylett.7b00926. [DOI] [Google Scholar]
- Kojima A.; Teshima K.; Shirai Y.; Miyasaka T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. 10.1021/ja809598r. [DOI] [PubMed] [Google Scholar]
- Bartolome J; Palacio F; Calleja J M; Rueda F A.; Cardona M; Migoni R Spectroscopic Study of NH4ZnF3 and NH4MnF3 Perovskites. J. Phys. C: Solid State Phys. 1985, 18, 6083–6098. 10.1088/0022-3719/18/32/020. [DOI] [Google Scholar]
- Smith M. D.; Connor B. A.; Karunadasa H. I. Tuning the Luminescence of Layered Halide Perovskites. Chem. Rev. 2019, 119, 3104–3139. 10.1021/acs.chemrev.8b00477. [DOI] [PubMed] [Google Scholar]
- Kanishcheva A. S.; Zaitseva I. Y.; Kovalyova I. S.; Mikhailov Y. N. Synthesis and Crystal Structure of Cs3ZnBr5. Russ. J. Inorg. Chem. 2010, 55, 1882–1887. 10.1134/S0036023610120119. [DOI] [Google Scholar]
- Zhang X.; Liu K.; He J.-Q.; Wu H.; Huang Q.-Z.; Lin J.-H.; Lu Z.-Y.; Huang F.-Q. Antiperovskite Chalco-Halides Ba3(FeS4)Cl, Ba3(FeS4)Br and Ba3(FeSe4)Br with Spin Super-Super Exchange. Sci. Rep. 2015, 5, 15910. 10.1038/srep15910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpino K. E.; Wallace D. C.; Nie Y. F.; Birol T.; King P. D. C.; Chatterjee S.; Uchida M.; Koohpayeh S. M.; Wen J. J.; Page K.; et al. Evidence for Topologically Protected Surface States and a Superconducting Phase in [Tl4](Tl1-XSnx)Te3 Using Photoemission, Specific Heat, and Magnetization Measurements, and Density Functional Theory. Phys. Rev. Lett. 2014, 112, 017002. 10.1103/PhysRevLett.112.017002. [DOI] [PubMed] [Google Scholar]
- Lin W.; Stoumpos C. C.; Liu Z.; Das S.; Kontsevoi O. Y.; He Y.; Malliakas C. D.; Chen H.; Wessels B. W.; Kanatzidis M. G. TlSn2I5, a Robust Halide Antiperovskite Semiconductor for Γ-Ray Detection at Room Temperature. ACS Photonics 2017, 4, 1805–1813. 10.1021/acsphotonics.7b00388. [DOI] [Google Scholar]
- Kaiukov R.; Almeida G.; Marras S.; Dang Z.; Baranov D.; Petralanda U.; Infante I.; Mugnaioli E.; Griesi A.; De Trizio L.; et al. Cs3Cu4In2Cl13 Nanocrystals: A Perovskite-Related Structure with Inorganic Clusters at A Sites. Inorg. Chem. 2020, 59, 548–554. 10.1021/acs.inorgchem.9b02834. [DOI] [PMC free article] [PubMed] [Google Scholar]