Abstract
Reciprocity can explain cooperative behaviour among non-kin, where individuals help others depending on their experience in previous interactions. Norway rats (Rattus norvegicus) cooperate reciprocally according to direct and generalized reciprocity. In a sequence of four consecutive experiments, we show that odour cues from a cooperating conspecific are sufficient to induce the altruistic help of rats in a food-exchange task. When rats were enabled to help a non-cooperative partner while receiving olfactory information from a rat helping a conspecific in a different room, they helped their non-cooperative partner as if it was a cooperative one. We further show that the cues inducing altruistic behaviour are released during the act of cooperation and do not depend on the identity of the cue provider. Remarkably, olfactory cues seem to be more important for cooperation decisions than experiencing a cooperative act per se. This suggests that rats may signal their cooperation propensity to social partners, which increases their chances to receive help in return.
Keywords: reciprocity, Norway rat, olfactory signalling, cooperation, altruism, mammals
1. Introduction
Cooperation among unrelated individuals is widespread in nature [1–3]. It can evolve by mutualism, enforcement or reciprocity [4,5]. Here, we define cooperation in a descriptive, general sense as simultaneous or consecutive acting together of two or more individuals, without implying fitness costs and benefits to either partner [5,6]. Reciprocity is defined as a helpful act apparently benefitting a receiver at immediate costs to the actor, which increases the probability to receive a helpful act in return [7]. If cooperative behaviour is exchanged reciprocally, the decision to help a social partner depends on previously experienced help. In reciprocal cooperation, individuals may apply one of three decision rules: help anyone if helped by someone (generalized reciprocity), help someone who has helped you (direct reciprocity) or help someone who has helped others (indirect reciprocity) [5]. Whether and which reciprocity rules animals apply can be studied by exposing them to a sequential iterated prisoner's dilemma (IPD) game, in which in each round individuals can decide to help a social partner to the latter's benefit, but at its own cost [8].
Many animals have been shown to consistently reciprocate help [5,9,10] and numerous models unveiled the mechanisms underlying evolutionary stability of such behaviour [8,11–14]. By contrast, the proximate mechanisms responsible for reciprocal cooperation are largely unclear [9,15–17], which has caused scepticism regarding the prevalence of reciprocal cooperation in nature [17,18]. There is an ongoing debate about whether complex cognition is required for reciprocal cooperation, and to which extent individuals communicate with each other in social dilemmas [9,16,19–21]. Experimental results in rats have shown that individuals communicate their need for help to social partners by behaviours such as reaching towards a potential reward and emitting ultrasonic calls [22–24]. However, do individuals also signal their helping effort in order to increase their partner's propensity to pay back help in the future? If this were the case, cooperators could influence the tendency of partners to return a provided favour.
Female wild-type Norway rats apply direct and generalized reciprocity rules in the sequential IPD [22,25,26], and they exhibit reciprocal cooperation despite the energetic costs involved in producing food rewards for a social partner [23]. Here, we asked whether and how individuals transmit information about their cooperative behaviour to a social partner. Further, we investigated whether receivers of such information modify their cooperation propensity accordingly.
Olfaction is a major sensory modality used in rodent communication. By olfactory cues rats individually recognize conspecifics [27,28], gain information about a social partner's state, age, sex and relatedness [27,29], and determine whether conspecifics are stressed or anxious [30,31], or in need of help [23,32]. Odours are important for social interactions in rats, not least because of their nocturnal and fossorial way of life [33]. As the social behaviour of rats depends so strongly on olfactory cues, we hypothesized that the acquisition of olfactory information may be crucial for their ability to reciprocate help. In addition, previous experiments revealed that visual information transferred between social partners is not required when they reciprocate help [34]. We therefore scrutinized the importance of olfactory information exchange for reciprocal cooperation of Norway rats in a sequence of four consecutive experiments. We predicted that the olfactory information originating from a cooperating conspecific would be instrumental and sufficient for releasing helpful behaviour in wild-type Norway rats tested in a sequential IPD.
2. Material and methods
(a). Experimental setup
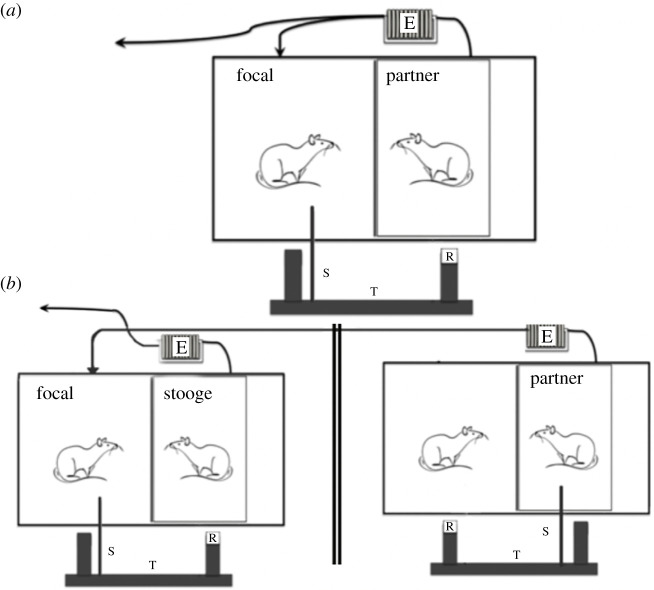
To test for reciprocal cooperation, we used wild-type Norway rats that were capable of producing food for a partner (see below for details on the apparatus and the familiarization procedure). In addition, we manipulated the availability of olfactory information by controlling the airflow between social partners. We performed a series of four consecutive experiments to stepwise clarify which information rats consider when deciding to provision a social partner with food (experimental procedures for each experiment are described in detail below). In all experiments, the experimental cage was divided into halves by a wire mesh. A transparent Plexiglas box was placed into the compartment of the focal rat's partner. This box was connected at its backside to an exhauster sucking out air from the box. At the front side of the box, there was an opening for the platform and the pulling stick. The airflow created by the exhauster prevented the focal individual, which was placed in the neighbouring compartment, to smell the partner in the box. The sucked-out air was either blown into another room or into the compartment of the focal rat, depending on the experimental treatment (figure 1).
Figure 1.
Experimental setup. The focal rat had access to a wooden stick (S) in the test phase of the experiment and could thereby move a tray (T) towards the partner or stooge that could then reach a food reward (R). The partner rat and stooge were put in a sealed Plexiglas box that was connected to an exhauster (E). (a) Set-up for experiments 1 and 2, where olfactory information was either provided or discarded from the partner in the neighbouring compartment. (b) Set-up for experiments 3 and 4, where the focal rat was provided with olfactory information from a partner rat that either helped a social partner or not in a different room, while the focal rat experienced the behaviour, but no olfactory information, of a stooge in the neighbouring compartment of its cage that never provided help.
The exhauster was an Intex10 electric air pump with a maximal working pressure of 0.83 bar, which was connected to the Plexiglas box with a plastic tubing system (with a diameter of approx. 1 cm). Rat sounds were not transmitted through the tubing system due to the overlaying pump noise; neither audible nor ultrasonic calls (using a bat detector: CDB 103 R3-bat detector, Ciel Electronique, Saint-André-de-la-Roche, France) could be detected when the pump was running.
Every experiment was divided into two phases, the experience and the test phase. In the experience phase, focal rats experienced the behaviour and/or olfactory information from a partner/stooge (depending on the experiment, see below). After a 1 min acclimatization time, cooperators pulled approximately 10 times within the 7 min of the experience phase. In the non-cooperator treatment, non-cooperators had access to the stick but the platform was blocked in order to prevent non-cooperators from accidentally pulling the platform. Depending on the experiment, focal rats were sometimes exposed to several experience phases with different partners before the roles were reversed in the test phase (see the description of each experiment below for details). In the test phase, the focal individual had access to the pulling stick and could provide food to a partner or stooge in the neighbouring compartment. Again, the rats had 1 min of acclimatization time before their behaviour was recorded in the 7 min test phase. The frequency of pulls and the latency to the first pull were recorded for the focal individual [23,25,35]. For the experiments one and two, a different batch of rats was used than for the subsequent experiments. There was no possibility of physical contact between rats in the experiment, and no injuries occurred. In all experiments, focal rats, partners and stooges were unrelated and unfamiliar to each other. Stooges and partner rats were always trained cooperators or non-cooperators, and these specially trained rats were never used as focal individuals.
(b). Study subjects and holding conditions
We used individually marked, outbred female wild-type Norway rats (source: Animal Physiology Department, University of Groningen, Netherlands), which on average weighed 300 g. All rats experienced handling by humans from an early age and thus were not stressed by handling or by the observer. The rats were housed in groups of five littermates in cages (80 × 50 × 37.5 cm) provided with nesting material and hiding opportunities. Pellets and water were provided ad libitum, thus the animals were not food-deprived for any of the experiments. The rats experienced a 12 : 12 h light/dark cycle and all experiments were conducted in the dark phase under red light conditions, as rats are primarily nocturnal [33].
(c). Familiarization with the experimental paradigm
Before any rats received experience with the experimental apparatus, six individuals of the rat colony were chosen randomly to become non-cooperative partners. These rats were familiarized with the experimental set-up but never learnt to pull the stick by which food could be produced. All other rats in the colony learnt to pull a stick attached to a tray to bring in an oat flake for their own consumption. After this initial training step, the rats were never directly rewarded for pulling the stick any more. In the second step, the rats learnt to provide access to a treat for a littermate by pulling the stick (see figure 1 for a sketch of the cage, stick and moving tray). The roles of two rats were exchanged after each trial, and thus the rats learnt that after providing help their partner had the chance to return the favour. Throughout the training period, the intervals between these exchanges were gradually increased. Six individuals out of 12 reliably pulling rats were randomly assigned to become ‘cooperators’. Their role was to provide cooperative experience to focal rats in the subsequent experiments (see below). Cooperators were trained to repeatedly pull for a partner during the 7 min experimental period. All focal subjects learnt over 18 sessions how to pull food for a partner (see [22,36] for a detailed description of the procedure), and they were habituated to the experimental setup (including the pulling apparatus and an exhauster, see above). The experimental rats did not show any signs of stress or anxiety.
(d). Experimental procedure
(i). Is olfactory information important for cooperation decisions (experiment 1)?
To test for the importance of olfactory information in cooperation decisions every focal rat was tested in four different treatments in a full factorial design, either experiencing a cooperative or a non-cooperative partner, and either with or without olfactory information, plus a control treatment without a partner being present (electronic supplementary material, figure S1). This experiment was performed twice in random order, once using a direct and once using a generalized reciprocity paradigm (cf. [25]). In the direct reciprocity paradigm, we tested whether a rat increases cooperative behaviour towards an individual that was helpful to them, whereas in the generalized reciprocity paradigm we tested whether rats generally increase their cooperation propensity if they experienced cooperative behaviour from any other conspecific. The sequence of treatments and the identity of the partner rats were randomized.
In the direct reciprocity paradigm, the focal rat (n = 23) was put into the test compartment and the partner (cooperator or non-cooperator) was placed into the box in the neighbouring compartment. In the morning of each experimental day, the focal rats experienced either a cooperative or a non-cooperative partner for 7 min. Four hours later, focal rats had the opportunity to pull during 7 min for the same partner they had experienced in the morning. In addition to these four treatments, the control treatment served to test for the importance of a social context (i.e. the presence of a social partner). After experiencing a cooperative partner, the focal rats had the opportunity to pull for an empty cage for 7 min. Every focal rat was tested in a random order in each of the five situations on five consecutive days (electronic supplementary material, figure S1).
In the generalized reciprocity paradigm, the focal rat (n = 21) experienced three different cooperating or non-cooperative partners (depending on the treatment) for 7 min on three consecutive days. On the fourth day, the focal rat could pull for a cooperator or a non-cooperator (again depending on the treatment), which had not been met in the experience phase (electronic supplementary material, figure S1).
(ii). Is olfactory information important for cooperation decisions when receiving or when providing help (experiment 2)?
To investigate whether cooperative behaviour is triggered by the transmission of olfactory information either during the experience or during the test phase of the experimental interaction, in experiment 2 focal rats got access to the smell of their partner in one of these phases only. If the cooperation propensity of focal rats was enhanced when receiving olfactory information only in the experience phase, this would indicate that cooperative partners may have communicated their helping effort. On the other hand, if the cooperation propensity of focal rats was enhanced when receiving information only in the test phase, this would indicate that partners may communicate a demand for help.
Every focal rat (n = 23) was tested in four different treatments in a full factorial design, either experiencing a cooperator or a non-cooperator, and with olfactory information present either only in the experience phase or only in the test phase, depending on the treatment (electronic supplementary material, figure S2). For this experiment, we used the generalized reciprocity paradigm, where the decision to help is independent of individual recognition of the partner. In the cooperator treatment, the rats experienced three different cooperative partners for 7 min each on three consecutive days. In the non-cooperator treatment, the focal rats were paired with three different non-cooperative partners in the same way. On the fourth day, the rats were tested with an unknown partner rat, either a cooperator or a non-cooperator, depending on the treatment. Olfactory information was blocked either in the experience phase or in the test phase, whereas it was available in the respective other phase, again depending on the treatment.
(iii). Does the mere smell of cooperation provide sufficient information to induce helpful behaviour (experiment 3)?
In this experiment we disentangle the olfactory information received from a social partner from information about its behaviour to test whether the smell associated with helpful behaviour is sufficient to trigger cooperation in the receiver of that odour, thereby suggesting the existence of a ‘smell of cooperation’. For that purpose, the focal rat received olfactory information from an individual acting in another room, while it encountered a partner in the neighbouring compartment (stooge) from which it did not receive any olfactory information (electronic supplementary material, figure S3). In all treatments of this experiment, a non-cooperating partner (referred to as ‘stooge’) was put into the neighbouring compartment of the experimental cage to standardize the visually experienced behaviour for the focal rat. Thereby, the focal individual never experienced help by the partner in its neighbouring compartment. While always experiencing a non-cooperative partner, the focal rat was provided with the smell from a cooperative or a non-cooperative conspecific, acting in another room. This conspecific was faced with a cage mate, and observed and handled by a second experimenter in an adjacent room. While this conspecific was either cooperating or non-cooperating with its cage mate, its odour was blown into the focal rat's compartment in the neighbouring room via an exhauster as described above. To investigate the importance of the olfactory information in both phases of the experiment, the experience and the test phase, two experiments (3a and 3b) were conducted (see electronic supplementary material, figure S3).
In the first experiment (3a), we manipulated the olfactory information during the experience phase only. Every focal individual (n = 24) was tested in two situations with one experimental trial per day conducted in random order. After 1 min of habituation, the focal individual experienced a non-cooperative partner in the neighbouring cage compartment for 7 min. At the same time, the focal rat received olfactory information of an unknown partner from an adjacent room that either cooperated or did not cooperate, depending on the treatment. After this experience, the airflow was disconnected so that the focal individual received no olfactory information from any individual anymore, while it got access to the stick in order to be able to pull for the stooge in the neighbouring cage compartment.
In the second experiment (3b), we manipulated the olfactory information during the test phase only, and there was no experience phase in this experiment. Every focal individual (n = 24) was again tested in two treatments with one experimental treatment per day in random order. After 1 min of habituation, the focal individual got access to the pulling stick and had the opportunity to pull for an unknown partner, which was again a trained non-cooperator to standardize the behavioural experience for focal rats. During this test phase, the focal rat received olfactory information from a conspecific that either cooperated or did not cooperate in an adjacent room.
For both experiments (3a and 3b), the experimenters recording the focal rats' behaviour were blind to the experimental treatments (with or without odour transmission) but informed about the type of experiment (3a or 3b).
(iv). Does individual identity or the performance of help (cooperating versus non-cooperating) trigger cooperative responses (experiment 4)?
In order to unravel whether the smell of certain individuals or the smell of an individual while performing a cooperative act has caused focal rats to reciprocate help in the previous experiments, we performed another experiment. The experimental procedure was the same as in the previous experiment as the focal rat (n = 24) always experienced a non-cooperative stooge. However, the focal rat experienced the smell of the same partner rat in two different situations—the same partner individual was either helping a cage mate or not to obtain food in a different room. In the ‘cooperative situation’, the ‘odour provider’ had access to the pulling stick to provision a cage mate with food, whereas in the ‘neutral situation’ it had no access to the pulling stick and was, therefore, not helping its cage mate (see electronic supplementary material, figure S4). The experimenters recording the focal rats' behaviour were again blind to the experimental treatments.
(e). Statistical analysis
The statistical analysis was performed using R 3.0.1 (http://cran.r-project.org). To analyse the latencies to the first pull, Cox proportional hazard regression models [37] were fitted using the survival package [38] with the respective treatment and the interaction between the partner's role (cooperator or non-cooperator) and the smell treatment as fixed factors. To account for repeated measurements, a random effect for the focal rats' identity was included in the model. The model assumptions were tested visually, and the proportional hazard assumption was additionally tested using the cox.zph function [38]. To compare the pulling frequencies between two treatments, pairwise Wilcoxon signed-rank tests were performed. When comparing more than two treatments, general linear mixed models (GLMM) were performed with the package lme4 [39] for the pulling frequency where the olfactory treatment, cooperator/non-cooperator treatment, and their interaction were included in the model. Given discrete values and zero inflation of our data, we performed Poisson GLMMs. We ran separate GLMMs for direct and generalized reciprocity in experiment 1, and for different experiments. The individual identity of the focal animals was included as a random factor to account for repeated measures. When there was a significant interaction of the treatments, we performed post hoc multiple comparisons using Tukey contrasts. The model assumptions were tested and if models were overdispersed, an observation-level random effect was included to deal with overdispersion.
3. Results
(a). Is olfactory information important for cooperation decisions (experiment 1)?
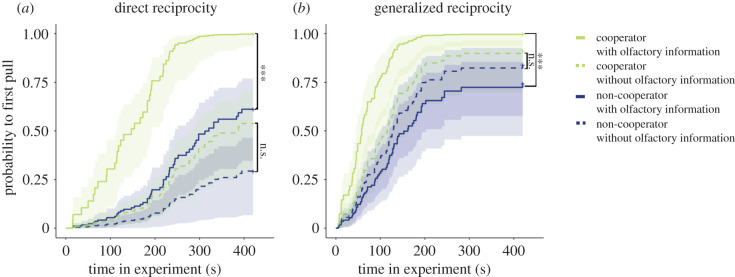
In the direct reciprocity paradigm, focal rats donated food more often and earlier when olfactory information from their partner in the adjacent compartment was available in both the experience and test phases than when this information was blocked, irrespective of the partner's previous helpfulness (frequency: GLMM, n = 23, p < 0.001; latency: Cox. prop. haz., n = 21, p < 0.001; figure 2a; electronic supplementary material, tables S1 and S2). In addition, focal rats pulled earlier for a social partner that had helped them before than for a formerly non-cooperative partner when they had access to olfactory information of their partner (latency: Cox. prop. haz. with post hoc Tukey, n = 23, p < 0.001; figure 2a; electronic supplementary material, table S2b). By contrast, when no olfactory information was provided, there was no significant difference in the latencies to pull for the partner between the previously cooperative and non-cooperative partners (latency: Cox. prop. haz., n = 23, p > 0.05; electronic supplementary material, table S2a,b). In addition, focal rats tended to provide food more often for previously cooperative than for previously non-cooperative partners (frequency: GLMM, n = 23, p = 0.08) in the direct reciprocity paradigm.
Figure 2.
Is olfactory information important for cooperative decisions (experiment 1)? Kaplan–Meier curves according to the experimental treatments, showing that rats provided food earlier to cooperators (light green) than to non-cooperator (dark blue) when olfactory information was present (solid lines) compared to when no olfactory information was available (dotted lines); shading represents the 95 confidence intervals. (a) Kaplan–Meier estimator for direct reciprocity and (b) for generalized reciprocity. Three asterisks indicate a significant difference (p < 0.001), whereas n.s. indicates a lack of a significant difference (non-significant). See electronic supplementary material, table S7 for all statistical comparisons. (Online version in colour.)
In the generalized reciprocity paradigm, the latency to pull for a new, unknown partner was shorter when another rat had provided help to the focal rat before, but only when olfactory information was available to the focal rat (latency: Cox. prop. haz., n = 21, p = 0.006; figure 2b; electronic supplementary material, table S3a,b). By contrast, when no olfactory information was available the latencies to the first pulling for the novel partner did not differ in dependence of having received help or not from a different partner before (latency: Cox. prop. haz., n = 21, p > 0.05; electronic supplementary material, table S3a,b). No difference between these two situations was detected in the number of pulls (electronic supplementary material, table S2). Interestingly, the slopes of the Kaplan–Meier curves were steeper for the generalized reciprocity paradigm compared to the direct reciprocity setup, when comparing the interaction of treatment × reciprocity (latency: Cox. prop. haz., n = 23, p < 0.001; figure 2). Thus, the probability to pull for a partner increased faster over time in the generalized reciprocity paradigm.
To test whether the social context is important for pulling the stick, we tested focal rats with an empty partner compartment after they had received the same cooperative experience with olfactory information present as described above. Only six out of 23 rats pulled at all for an empty partner compartment, and in these cases, the latency to the first pull was significantly longer than when they pulled with a partner present (latency: Wilcoxon signed-rank test, n = 23, p = 0.003). However, post hoc tests revealed that both the latency to pull and the pulling frequency for an empty cage differed significantly only from the cooperator treatment in which olfactory information was available (electronic supplementary material, figure S5 and table S7). The empty cage control was not significantly different from all other treatments.
In summary, the data of experiment 1 show that olfactory information from their partner is crucial when Norway rats decide to return received help; olfactory cues are apparently also important when rats decide to help an unfamiliar conspecific after receiving help from a different social partner (generalized reciprocity).
(b). Is olfactory information important for cooperation decisions when receiving or when providing help (experiment 2)?
We next investigated in which phase olfactory information is required so that rats are able to respond appropriately to a partner's cooperation. There was no difference in the number of provided food items or in the latency to the first pull when olfactory information was provided in the experience phase and blocked in the test phase, compared to when olfactory information was blocked in the experience phase and available in the test phase (electronic supplementary material, table S4a). However, focal rats pulled earlier (latency: Cox. prop. haz., n = 23, p = 0.003; electronic supplementary material, table S4b) and more often (frequency: GLMM, n = 23, p = 0.007; electronic supplementary material, table S4b) for cooperators than for non-cooperators when olfactory information was present in at least one of the two phases.
Experiment 2 thus shows that in at least one of the two stages of the experiment (i.e. the experience or the test phase) focal rats need olfactory information when deciding to help unfamiliar conspecifics after having received help or not.
(c). Does the mere smell of cooperation provide sufficient information to induce helpful behaviour (experiment 3)?
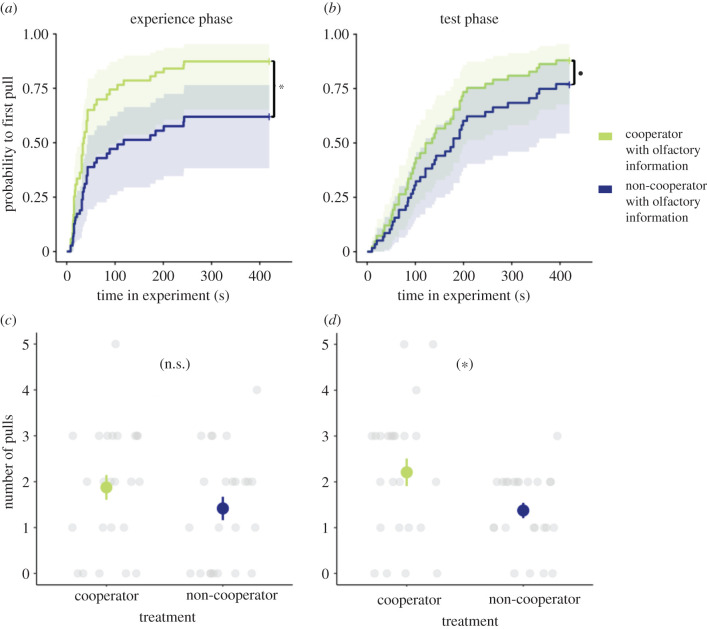
In this experiment, the focal individual received the smell of an individual from a different room (i.e. a chemically perceived social partner), while olfactory information from the conspecific (stooge) in the neighbouring compartment was blocked (electronic supplementary material, figure S3). When focal rats received odour cues of a rat that was providing help to a different conspecific in an adjacent room, focal rats showed a higher propensity to help their uncooperative stooge to get food than when they experienced odour cues from a rat that did not help their respective partner in the other room. This effect emerged either in pulling latencies or frequencies in both situations, when odour cues were manipulated in the experience phase (latency: Cox. prop. haz., n = 23, p = 0.013; figure 3a; electronic supplementary material, table S5a; frequency: Wilcoxon signed-rank test, n = 23, p = 0.196; figure 3c; electronic supplementary material, table S5a), and when odour cues were manipulated in the test phase of the experiment (latency: Cox. prop. haz., n = 23, p = 0.057; figure 3b; electronic supplementary material, table S5; frequency: Wilcoxon signed-rank test, n = 23, p = 0.022; figure 3d; electronic supplementary material, table S5).
Figure 3.
Does the smell of cooperation suffice to induce cooperative behaviour (experiment 3)? Focal rats provided food to a stooge earlier (a, b) and more often (c, d) while having access to smell of a cooperative individual (light green lines and symbols) than they did to a stooge while receiving olfactory information of a non-cooperative individual (dark blue lines and symbols). This applies in the experience phase (a, c) and in the test phase (b,d). Asterisks indicate a significant difference (p < 0.05), n.s. indicates a lack of a significant difference, a dot indicates a non-significant trend (p = 0.057). (a,b) Kaplan–Meier curves with 95% confident intervals. (c,d) mean numbers of pulls ± one standard error and the raw data points. For better visibility, overlapping data points were randomly offset horizontally. See electronic supplementary material, table S5 for statistical comparisons. (Online version in colour.)
When comparing figures 3a,b, it seems that the response of the focal rats was somewhat delayed when they received the olfactory information from the remote partner in the different room only during the test phase (experiment 3b), as compared with the situation when this information was available during the experience phase (experiment 3a). This delay might be explained by the fact that when olfactory information was available already before the test (because it was provided during the experience phase; condition 3a), the rats had more time to process this information than when it was only provided in the test phase itself (condition 3b).
The results of experiment three reveal that the decision of focal rats to help a social partner is affected by olfactory cues received from a cooperative or uncooperative conspecific, even if they do not receive any other cues or experience cooperative behaviour themselves. A ‘smell of cooperation’ thus seems to enhance altruistic food provisioning in rats.
(d). Does individual identity or the performance of help trigger cooperative responses (experiment 4)?
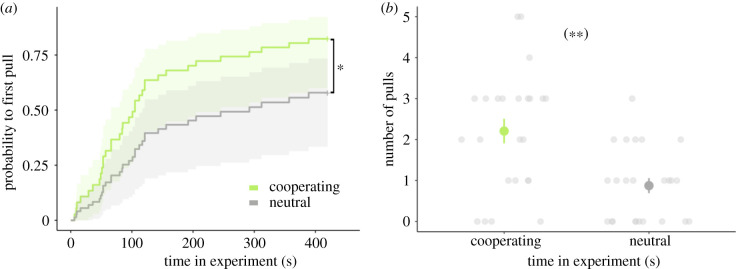
Focal rats provided more food to a non-cooperative stooge (frequency: Wilcoxon signed-rank test, n = 23, p = 0.003; figure 4b; electronic supplementary material, table S6) and did so earlier (latency: Wilcoxon signed-rank test, n = 23, p = 0.003; figure 4a; electronic supplementary material, table S6) when they smelled an individual helping a partner to get food in a different room compared to a situation where the same individual was not helpful. Hence the results of this experiment show that the smell of the cooperative action itself and not of an individual attribute (such as ‘cooperator’ versus ‘non-cooperator’ individuals) triggers a cooperative response in rats.
Figure 4.
Does the performance of cooperation trigger cooperative responses (experiment 4)? Focal rats pulled earlier (a) and more often (b) for a stooge if receiving the smell from a cooperating conspecific (light green) acting in a different room than if receiving the smell of the same individual when it was not cooperating (dark grey). (a) Kaplan–Meier curves with 95% confident intervals. (b) Raw data and the mean number of pulls ± one standard error. For better visibility, overlapping raw data points were randomly offset horizontally. One asterisk indicates a significant difference at p < 0.05, two asterisks at p < 0.01. See electronic supplementary material, table S6 for statistical comparisons. (Online version in colour.)
4. Discussion
Our experiments show that the propensity of rats to help a social partner increases if they receive odour cues from a helpful act of a conspecific, even if they have not received help themselves. The scent of a social partner's cooperative behaviour was crucial for inducing cooperation in both the direct and generalized reciprocity paradigms. Remarkably, the scent of a cooperative act triggered an altruistic response of focal subjects towards a stooge even if the odour cue came from a conspecific being helpful to someone else in a different room. Thus, olfactory information about cooperative behaviour seems to yield the essential information for a rat's decision to donate food to a conspecific. This information is obviously independent of the individual identity of a social partner.
Interestingly, rats provided similar levels of help to conspecifics as they did for an empty cage if olfactory information from social partners was blocked. Only when the smell was available from a partner providing help, rats increased their propensity to help a conspecific compared to a situation in which the cage compartment receiving the food was empty. This might suggest that if no olfactory information is available from a helpful social partner, rats may pull the stick for other reasons than returning perceived aid.
In our experiment, olfactory cues conveyed information either about a partner's cooperative behaviour in the experience phase of the interaction, or about the need of a partner in the test phase. Our results show that even if olfactory information was absent in one of these two phases, the information conveyed in the other phase was sufficient to increase the motivation to donate food to a partner. This suggests that both the ‘smell of cooperation’ and the ‘smell of demand’ can trigger altruistic help. Rats studied in a similar sequential IPD setup were shown to solicit a partner's attention by a cascade of visual and auditory cues, which increased the help level of solicited subjects [24]. Our results indicate that such solicitations may also involve chemical cues. This corroborates recent results of an experiment in which merely the olfactory information about the hunger state of a social partner located in another room decided about the helpfulness of focal rats to a stooge [32]. There might be other sensory cues that rats are using before deciding to provide help to a conspecific. In humans, a recent study showed that acoustic patterns can inform about the cooperative propensity of social partners [40], and rats have been shown to solicit cooperative behaviour through ultrasonic calls [24,41]. However, in all our experiments, we could not detect any ultrasonic calls and visual information was blocked when the ‘olfactory cue provider’ was acting in a different room.
Importantly, our results indicate that one and the same individual transmits different olfactory cues when cooperating compared to when not cooperating. This is particularly noteworthy because previous results of experiments with a similar setup showed that there is no difference in the general activity between rats that pull a stick for a conspecific and those that do not [22]. Based on these results it seems unlikely that physical activity explains the behavioural response of focal rats. In our experiment, we have not directly assessed the physical activity of the animals. However, we assessed whether the pulling effort (number of pulls) of the rat providing odour cues to the focal rat in the experience phase, as a measure of activity, correlated with the number of pulls of the focal subject during the test phase. This was not the case (see electronic supplementary material, figure S6). Thus, this proxy of activity did not suggest an influence on the decision of focal rats to help a conspecific. The ‘smell of cooperation’ hence seems to be a more likely trigger of cooperative behaviour of rats in this paradigm than a more general ‘smell of activity’. Nevertheless, this result suggests that the odour of a cooperating rat releases a qualitative response that is not adjusted to the quantity of helpful acts of the odour provider.
Our results suggest that the essential social information focal rats have used before deciding to help a conspecific originated from the actual act of cooperating. This implies that rats may not only signal their need to potential help providers [23,24,32], but also their own cooperative behaviour. Such cues can generate a helpful response in receivers of cooperation if their pay-offs are correlated due to the repeated nature of the sequential IPD, causing alignment of fitness interests [5,14,29,42,43] and enabling animals to trade different commodities among each other [44,45].
A lack of divergence in fitness interests due to correlated pay-offs is one potential reason why signals that communicate cooperative investment may be reliable. In effect, this situation resembles a synergistic mutualism game [46], which has two Nash equilibria and induces communication to prevent players from falling into the trap of mutual defection [16]. Furthermore, the odour cues might be costly to produce and therefore reflect a reliable signal of quality, which may influence cooperation decisions [47]. Alternatively, the odour cues produced by a cooperative act might be an inevitable by-product of the behaviour, just like molecules unavoidably released by predators that can inform potential prey [48]. In natural populations, such uncheatable cues could be a by-product of performing a helpful action, such as some prosocial acts seem to relate to oxytocin levels. In chimpanzees, for example, urinary oxytocin levels are increased after food sharing tasks [49]. Rats communicate a lot via smell and such changes in hormone levels might release molecules that are detectable in the odour of a rat and act as a reliable cue for the performance of cooperative behaviour.
Future research needs to clarify which of these possibilities pertains, and which odour components mediate the crucial information modifying cooperation propensity. Identifying these chemical components is a challenging task that will help to clarify the mechanisms underlying the intriguing response to behaviour witnessed by odour cues alone. It is also an interesting target for future studies to elucidate the time period for which the influence of olfactory information persists in the context of reciprocal cooperation; theoretical models predict that increasing time delays between received information and the possibility to respond can reduce the effect on the cooperation propensity of social partners [50]. Nevertheless, experimental evidence in wild-type Norway rats showed that cooperative food providers received more allogrooming bouts from their previous partners than uncooperative subjects even after 6 days of separation between the partners [51], and reciprocal food exchange between social partners occurred after a break of 3 days [26]. This suggests long-term memory of rats regarding cooperative experience with a social partner.
In conclusion, our study shows that in rats chemical cues originating from the cooperative behaviour of a conspecific are essential and more important than direct behavioural cues for the decision to perform altruistic helping behaviour towards a conspecific. Thus, in rats and perhaps also in other animals, the transmission of olfactory information may be crucial for the regulation of complex and demanding cognitive tasks such as helping a social partner. The information exchange between social partners is an intriguing subject for future studies of the mechanisms involved in reciprocal cooperation among animals.
Supplementary Material
Acknowledgements
We thank Evi Zwygart for help with animal care, Vassilissa Dolivo for discussion and help in the training of the rats and Valentina Balzarini for providing drawings.
Ethics
All rats were housed and treated according to the animal welfare regulations of Switzerland; the Swiss federal veterinary office authorized the experimental procedure under licence BE25/14.
Data accessibility
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.h18931zjd [52].
Authors' contributions
M.T. and M.K.S. conceived and supervised the project. N.G. and M.K.S. performed the experiment and analysed the data. N.G., M.K.S. and M.T. wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
Funding was provided by SNF–grant nos. 421 310030B_138660 and 31003A_156152 to M.T.
References
- 1.Dugatkin L. 2002. Cooperation in animals: an evolutionary overview. Biol. Philos. 17, 459–476. ( 10.1023/a:1020573415343) [DOI] [Google Scholar]
- 2.Clutton-Brock T. 2009. Cooperation between non-kin in animal societies. Nature 462, 51–57. ( 10.1038/nature08366) [DOI] [PubMed] [Google Scholar]
- 3.Riehl C. 2013. Evolutionary routes to non-kin cooperative breeding in birds. Proc. R. Soc. B 280, 20132245 ( 10.1098/rspb.2013.2245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehmann L, Keller L. 2006. The evolution of cooperation and altruism – a general framework and a classification of models. J. Evol. Biol. 19, 1365–1376. ( 10.1111/j.1420-9101.2006.01119.x) [DOI] [PubMed] [Google Scholar]
- 5.Taborsky M, Frommen JG, Riehl C. 2016. Correlated pay-offs are key to cooperation. Phil. Trans. R. Soc. B 371, 20150084 ( 10.1098/rstb.2015.0084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taborsky M. 2007. Cooperation built the Tower of Babel. Behav. Process. 76, 95–99. ( 10.1016/j.beproc.2007.01.013) [DOI] [PubMed] [Google Scholar]
- 7.Trivers RL. 1971. The evolution of reciprocal altruism. Quart. Rev. Biol. 46, 35–57. ( 10.1086/406755) [DOI] [Google Scholar]
- 8.Axelrod R, Hamilton W. 1981. The evolution of cooperation. Science 211, 1390–1396. ( 10.1126/science.7466396) [DOI] [PubMed] [Google Scholar]
- 9.Schweinfurth MK, Call J. 2019. Reciprocity: different behavioural strategies, cognitive mechanisms and psychological processes. Learn. Behav. 47, 284–301. ( 10.3758/s13420-019-00394-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schweinfurth MK, Call J. 2019. Revisiting the possibility of reciprocal help in non-human primates. Neurosci. Biobehav. Rev. 104, 73–86. ( 10.1016/j.neubiorev.2019.06.026) [DOI] [PubMed] [Google Scholar]
- 11.McNamara JM, Barta Z, Houston AI. 2004. Variation in behaviour promotes cooperation in the Prisoner's Dilemma game. Nature 428, 745–748. ( 10.1038/nature02432) [DOI] [PubMed] [Google Scholar]
- 12.Nowak MA, Sigmund K. 2005. Evolution of indirect reciprocity. Nature 437, 1291–1298. ( 10.1038/nature04131) [DOI] [PubMed] [Google Scholar]
- 13.Barta Z, McNamara JM, Huszár DB, Taborsky M. 2011. Cooperation among non-relatives evolves by state-dependent generalized reciprocity. Proc. R. Soc. B 278, 843–848. ( 10.1098/rspb.2010.1634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Doorn G, Taborsky M. 2012. The evolution of generalized reciprocity on social interaction networks. Evolution 66, 651–664. ( 10.1111/j.1558-5646.2011.01479.x) [DOI] [PubMed] [Google Scholar]
- 15.Stevens JR, Hauser MD. 2004. Why be nice? Psychological constraints on the evolution of cooperation. Trends Cogn. Sci. 8, 60–65. ( 10.1016/j.tics.2003.12.003) [DOI] [PubMed] [Google Scholar]
- 16.Noë R. 2006. Cooperation experiments: coordination through communication versus acting apart together. Anim. Behav. 71, 1–18. ( 10.1016/j.anbehav.2005.03.037) [DOI] [Google Scholar]
- 17.Schino G, Aureli F. 2010. A few misunderstandings about reciprocal altruism. Commun. Integr. Biol. 3, 561–563. ( 10.4161/cib.3.6.12977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter G. 2014. The reciprocity controversy. Anim. Behav. Cogn. 1, 368 ( 10.12966/abc.08.11.2014) [DOI] [Google Scholar]
- 19.Stevens JR, Cushman FA, Hauser MD. 2005. Evolving the psychological mechanisms for cooperation. Annu. Rev. Ecol. Evol. Syst. 36, 499–518. ( 10.1146/annurev.ecolsys.36.113004.083814) [DOI] [Google Scholar]
- 20.Balliet D. 2009. Communication and cooperation in social dilemmas: a meta-analytic review. J. Confl. Resolut. 54, 39–57. ( 10.1177/0022002709352443) [DOI] [Google Scholar]
- 21.Dolivo V, Rutte C, Taborsky M. 2016. Ultimate and proximate mechanisms of reciprocal altruism in rats. Learn. Behav. 44, 223–226. ( 10.3758/s13420-016-0236-z) [DOI] [PubMed] [Google Scholar]
- 22.Rutte C, Taborsky M. 2007. Generalized reciprocity in rats. PLoS Biol. 5, e196 ( 10.1371/journal.pbio.0050196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneeberger K, Dietz M, Taborsky M. 2012. Reciprocal cooperation between unrelated rats depends on cost to donor and benefit to recipient. BMC Evol. Biol. 12, 41 ( 10.1186/1471-2148-12-41) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schweinfurth MK, Taborsky M. 2018. Norway rats (Rattus norvegicus) communicate need, which elicits donation of food. J. Comp. Psychol. 132, 119–129. ( 10.1037/com0000102) [DOI] [PubMed] [Google Scholar]
- 25.Rutte C, Taborsky M. 2008. The influence of social experience on cooperative behaviour of rats (Rattus norvegicus): direct vs generalised reciprocity. Behav. Ecol. Sociobiol. 62, 499–505. ( 10.1007/s00265-007-0474-3) [DOI] [Google Scholar]
- 26.Schweinfurth MK, Taborsky M. 2020. Rats play tit-for-tat instead of integrating social experience over multiple interactions. Proc R. Soc. B 287, 20192423 ( 10.1098/rspb.2019.2423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown RE. 1979. Mammalian social odors: a critical review. Adv. Stud. Behav. 10, 103–162. ( 10.1016/s0065-3454(08)60094-7) [DOI] [Google Scholar]
- 28.Gheusi G, Goodall G, Dantzer R. 1997. Individually distinctive odours represent individual conspecifics in rats. Anim. Behav. 53, 935–944. ( 10.1006/anbe.1996.0314) [DOI] [Google Scholar]
- 29.Schweinfurth MK, Taborsky M. 2018. Relatedness decreases and reciprocity increases cooperation in Norway rats. Proc. R. Soc. B 285, 20180035 ( 10.1098/rspb.2018.0035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisenberg J, Kleiman D. 1972. Olfactory communication in mammals. Annu. Rev. Ecol. Syst. 3, 1–32. ( 10.1146/annurev.es.03.110172.000245) [DOI] [Google Scholar]
- 31.Galef BG, Wigmore SW. 1983. Transfer of information concerning distant foods: a laboratory investigation of the ‘information-centre’ hypothesis. Anim. Behav. 31, 748–758. ( 10.1016/s0003-3472(83)80232-2) [DOI] [Google Scholar]
- 32.Schneeberger K, Röder G, Taborsky M. 2020. The smell of hunger: Norway rats provision social partners based on odour cues of need. PLoS Biol. 18, e3000628 ( 10.1371/journal.pbio.3000628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calhoun JB. 1963. The ecology and sociology of the Norway rat Bethesda, MD: Department of Health, Education and Welfare. [Google Scholar]
- 34.Dolivo V, Taborsky M. 2015. Cooperation among Norway rats: the importance of visual cues for reciprocal cooperation, and the role of coercion. Ethology 121, 1071–1080. ( 10.1111/eth.12421) [DOI] [Google Scholar]
- 35.Schweinfurth MK, Taborsky M. 2017. The transfer of alternative tasks in reciprocal cooperation. Anim. Behav. 131, 35–41. ( 10.1016/j.anbehav.2017.07.007) [DOI] [Google Scholar]
- 36.Dolivo V, Taborsky M. 2015. Norway rats reciprocate help according to the quality of help they received. Biol. Lett. 11, 20140959 ( 10.1098/rsbl.2014.0959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox D. 1972. Regression models and life-tables. J. R. Stat. Soc. Ser. B (Methodol.) 34, 187–202. ( 10.1111/j.2517-6161.1972.tb00899.x) [DOI] [Google Scholar]
- 38.Therneau T.2015. Survival: a package for survival analysis in S. Version 2.38. https://CRAN.R-project.org/package=survival .
- 39.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 40.Tognetti A, Durand V, Barkat-Defradas M, Hopfensitz A. 2019. Does he sound cooperative? Acoustic correlates of cooperativeness. Br. J. Psychol. 111, 823–839. ( 10.1111/bjop.12437) [DOI] [PubMed] [Google Scholar]
- 41.Łopuch S, Popik P. 2011. Cooperative behavior of laboratory rats (Rattus norvegicus) in an instrumental task. J. Comp. Psychol. 125, 250–253. ( 10.1037/a0021532) [DOI] [PubMed] [Google Scholar]
- 42.Rankin DJ, Taborsky M. 2009. Assortment and the evolution of generalized reciprocity. Evolution 63, 1913–1922. ( 10.1111/j.1558-5646.2009.00656.x) [DOI] [PubMed] [Google Scholar]
- 43.Carter GG, Farine DR, Crisp RJ, Vrtilek JK, Ripperger SP, Page RA. 2020. Development of new food-sharing relationships in vampire bats. Curr. Biol. 30, 1275–1279. ( 10.1016/j.cub.2020.01.055) [DOI] [PubMed] [Google Scholar]
- 44.Gfrerer N, Taborsky M. 2018. Working dogs transfer different tasks in reciprocal cooperation. Biol. Lett. 14, 20170460 ( 10.1098/rsbl.2017.0460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schweinfurth MK, Taborsky M. 2018. Reciprocal trading of different commodities in Norway rats. Curr. Biol. 28, 594–599. ( 10.1016/j.cub.2017.12.058) [DOI] [PubMed] [Google Scholar]
- 46.Smith MJ. 1984. Game theory and the evolution of behaviour. Behav. Brain Sci. 7, 95–101. ( 10.1017/s0140525(00026327) [DOI] [Google Scholar]
- 47.Grafen A. 1990. Biological signals as handicaps. J. Theor. Biol. 144, 517–546. ( 10.1016/s0022-5193(05)80088-8) [DOI] [PubMed] [Google Scholar]
- 48.Ferrari MC, Messier F, Chivers DP. 2006. The nose knows: minnows determine predator proximity and density through detection of predator odours. Anim. Behav. 72, 927–932. ( 10.1016/j.anbehav.2006.03.001) [DOI] [Google Scholar]
- 49.Wittig RM, Crockford C, Deschner T, Langergraber KE, Ziegler TE, Zuberbühler K. 2014. Food sharing is linked to urinary oxytocin levels and bonding in related and unrelated wild chimpanzees. Proc. R. Soc. B 281, 20133096 ( 10.1098/rspb.2013.3096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Doorn SG, Riebli T, Taborsky M. 2014. Coaction versus reciprocity in continuous-time models of cooperation. J. Theor. Biol. 356, 1–10. ( 10.1016/j.jtbi.2014.03.019) [DOI] [PubMed] [Google Scholar]
- 51.Stieger B, Schweinfurth MK, Taborsky M. 2017. Reciprocal allogrooming among unrelated Norway rats (Rattus norvegicus) is affected by previously received cooperative, affiliative and aggressive behaviours. Behav. Ecol. Sociobiol. 71, 182 ( 10.1007/s00265-017-2406-1). [DOI] [Google Scholar]
- 52.Gerber N, Schweinfurth MK, Taborsky M. 2020. Data from: The smell of cooperation: rats increase helpful behaviour when receiving odour cues of a conspecific performing a cooperative task. Dryad Digital Repository. ( 10.5061/dryad.h18931zjd) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.h18931zjd [52].