Abstract
Hepatocellular carcinoma (hcc) is one of the most common cancers in the world. It has a high mortality rate, especially when localized treatments fail. For about a decade, the only systemic treatment shown to improve survival was sorafenib. Recently, lenvatinib was found to be noninferior to sorafenib for overall survival, and combination atezolizumab–bevacizumab improved survival compared with sorafenib. Similarly, in the post-sorafenib setting, a number of recent positive clinical trials have been reported, and they indicate that regorafenib, cabozantinib, and ramucirumab are effective and safe in the second-line setting.
With so many new options available, including immunotherapy, it is challenging to define the best sequence of systemic treatment for patients with hcc. In the present review, we introduce the current data for second-line systemic treatment and beyond in hcc. A treatment algorithm is also suggested, based on the best available evidence and expert opinion.
Keywords: Hepatocellular carcinoma, treatment sequencing, second-line management, third-line management
INTRODUCTION
Hepatocellular carcinoma (hcc) is one of the most common and deadly cancers worldwide1. In Canada, about 3100 new cases and 1450 deaths are expected in 2020, but the incidence is increasing2. Hepatocellular carcinoma frequently develops in a background of chronic liver disease, most commonly chronic hepatitis B or C, alcoholic cirrhosis, and non-alcoholic steatohepatitis3,4. The severity of the patient’s underlying liver dysfunction must be considered when deciding on a management plan for hcc.
Sorafenib, an oral tyrosine kinase inhibitor (tki), was the only systemic treatment option shown to improve survival in advanced hcc, and it remained the standard first-line treatment for a decade5,6. A number of other systemic treatments failed to show any significant survival benefit when compared with sorafenib in clinical trials7–11. In 2018, lenvatinib was shown to be noninferior to sorafenib for overall survival (os) and was approved in Canada as a first-line treatment option for advanced hcc12. Recently, the IMbrave150 clinical trial demonstrated a significant improvement in os, progression-free survival (pfs), and response rate with combination atezolizumab–bevacizumab compared with sorafenib13. Combination atezolizumab–bevacizumab is now a new standard of care in the first-line treatment of advanced hcc.
In addition to those recent advances in the first-line systemic treatment of hcc, several positive studies in the second-line setting have also shown survival benefits for new treatments compared with placebo. Here, we review the current evidence for second-line treatment and beyond in hcc. The data available as of 1 July 2020 are reviewed, and a recommended sequencing strategy is discussed.
DISCUSSION
TKIs
Tyrosine kinases have an important role in the signalling cascade for cells, being involved in the activation of several molecular processes related to cell growth, differentiation, and apoptosis14. Hepatocellular carcinoma is a hypervascularized tumour, and angiogenesis has been shown to play an essential role in its pathogenesis15–18. The tkis were the first class of drugs to be associated with an improvement in os for patients with advanced hcc5. Since the initial approval of sorafenib for hcc in 2008, several other tkis have been developed and have been associated with improvements in survival in second-line treatment. Those drugs include regorafenib, cabozantinib, and apatinib. Table I shows the molecular targets that distinguish them. Table II summarizes the positive second-line phase iii trials for those tkis in patients with hcc.
TABLE I.
Molecular targets of second-line tyrosine kinase inhibitors (TKIs) in hepatocellular carcinoma
| TKI | VEGFR | PDGFR | RAF | FGFR | KIT | RET | TIE-2 | MET | AXL | SRC |
|---|---|---|---|---|---|---|---|---|---|---|
| Regorafenib | X | X | X | X | X | X | X | |||
| Cabozantinib | X | X | X | X | X | X | ||||
| Apatinib | X | X | X | X | X |
TABLE II.
Positive phase III trials of anti-angiogenic agents in the second- and third-line treatment of hepatocellular carcinoma
| Study name | Phase | Patients (n) | Arms | Child–Pugh score | OS (months) | PFS (months) | ORR (%) | Discontinuation rate because of drug-related AEs (%) | Treatment-related AE | |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Any grade | Grade 3 or 4 | |||||||||
| RESORCE | III | 573 | Regorafenib | A | 10.6 | 3.9 | 11 | 10 | 93 | 50 |
| vs. placebo | 7.8 | 1.5 | 4 | 4 | 52 | 17 | ||||
|
| ||||||||||
| CELESTIAL | III | 707 | Cabozantinib | A | 10.2 | 5.2 | 4 | 16 | 99 (any AE) | 68 (any AE) |
| vs. placebo | 8.0 | 1.9 | <1 | 3 | 92 (any AE) | 36 (any AE) | ||||
|
| ||||||||||
| AHELP | III | 393 | Apatinib | A–B7 | 8.7 | 4.5 | 10.1 | 12.5 | 97 | 77 |
| vs. placebo | 6.8 | 1.9 | 1.5 | 0 | 71 | 19 | ||||
|
| ||||||||||
| REACH-2 | III | 292 | Ramucirumab | A | 8.5 | 2.8 | 5 | 11 | 11 | NR |
| vs. placebo | 7.3 | 1.6 | 1 | 3 | 5 | NR | ||||
OS = overall survival; PFS = progression free survival; ORR = objective response rate; AEs = adverse events; NR = not reported.
Regorafenib
Regorafenib was the first tki to be approved for the second-line treatment of hcc. Although it has some structural similarities with sorafenib, regorafenib was shown to have a different molecular target profile, with stronger action in the vascular endothelial growth factor (vegf) pathway and inhibition of various targets involved in oncogenesis (Table I)19.
The resorce trial was a phase iii trial that randomized 573 patients with hcc who progressed after first-line sorafenib to regorafenib or to placebo20. Patients eligible for the study had Child–Pugh A liver function, had progressed on sorafenib, and had previously tolerated sorafenib (received sorafenib ≥400 mg daily for at least 20 of 28 days before discontinuation). The starting dose of regorafenib was 160 mg once daily for 3 weeks on and 1 week off. Regorafenib was associated with a significant improvement in os compared with placebo [10.6 months vs. 7.8 months; hazard ratio (hr): 0.63; 95% confidence interval (ci): 0.50 to 0.79; one-sided p < 0.0001], pfs (3.1 months vs. 1.5 months; hr: 0.46; 95% ci: 0.37 to 0.56; one-sided p < 0.0001), and response rate (11% vs. 4%, p = 0.0047). The disease control rate (dcr) was also improved with regorafenib (65% vs. 36%, p < 0.0001). Those response assessments were based on the modified Response Evaluation Criteria in Solid Tumors (recist). Median time on regorafenib was 7.8 months, and the most common reason for discontinuation was disease progression (60%).
With respect to safety, 93% of patients who received regorafenib experienced at least 1 treatment-related adverse event. The most common grade 3 or 4 adverse events for regorafenib were hypertension (15%), hand–foot syndrome (13%), fatigue (9%), and diarrhea (12%). Drug-related adverse events resulted in treatment discontinuation in 10% of patients receiving regorafenib compared with 4% receiving placebo. No clinically meaningful differences in terms of quality of life were noted between the treatment arms.
Regorafenib is also indicated for the treatment of metastatic colorectal cancer, and the recommended starting dose is also 160 mg daily for 21 days of a 28-day schedule. Because of concerns about the tolerability of the full recommended dose, the phase ii redos trial examined a dose-escalation strategy for regorafenib in patients with metastatic colorectal cancer, finding that, compared with the standard starting dose, the escalation strategy provided similar efficacy and a more favourable toxicity profile21. In the dose-escalation group, patients received 80 mg daily to begin, with weekly increments of 40 mg until 160 mg daily was reached, if the drug was well tolerated. Although that strategy was not specifically studied in the hcc setting, given the number of patients who required dose reductions in the resorce trial, it might be reasonable to start regorafenib at a lower dose and to escalate it as tolerated.
A subsequent post hoc analysis of the resorce trial evaluated the sequence of sorafenib and regorafenib in terms of survival22. In that study, median survival from the start of sorafenib to death was 26 months for patients who received second-line regorafenib and 19 months for those who received placebo in the second line. The survival benefit with regorafenib was independent of the last dose of sorafenib (800 mg or <800 mg daily) and of time to progression after prior sorafenib. When taking regorafenib, patients who had previously been taking a lower dose of sorafenib (<800 mg daily) experienced higher rates of fatigue (50% vs. 36%), anorexia (40% vs. 25%), and grade 3 hand–foot syndrome (17% vs. 10%) than did patients who had tolerated sorafenib at 800 mg daily—indicating that patients who can tolerate sorafenib only at lower doses are at higher risk of certain adverse events with regorafenib and might benefit from closer follow-up or a dose escalation strategy when starting treatment.
Cabozantinib
Cabozantinib is another oral tki with multiple targets, including vegf receptor, Met, and axl (Table I). Overexpression of Met has a negative effect on prognosis and was shown to be associated with sorafenib resistance23–25.
In the celestial phase iii trial, 707 patients with advanced hcc who had previously received sorafenib were randomized 2:1 to receive either cabozantinib or placebo26. Other eligibility criteria included progression on at least 1 prior treatment, and patients could have previously received up to 2 systemic treatments. Median os was improved with cabozantinib (10.2 months) compared with placebo (8.0 months; hr: 0.76; 95% ci: 0.63 to 0.92; p = 0.005). The magnitude of the survival benefit was even higher for the subgroup of patients treated with cabozantinib in the second-line setting after previous treatment with sorafenib alone (11.3 months with cabozantinib vs. 7.2 months with placebo; hr: 0.70; 95% ci: 0.55 to 0.88). Progression-free survival in this subgroup also favoured cabozantinib compared with placebo (median pfs: 5.5 months vs. 1.9 months; hr: 0.40; 95% ci: 0.32 to 0.50). The objective response rate (orr) according to recist 1.1 was 4% with cabozantinib compared with 0.4% with placebo (p = 0.009). The dcr was 64% compared with 33%. With respect to safety, grade 3 or 4 adverse events were reported in 68% of patients who received cabozantinib and in 37% of those who received placebo. The most common grades 3 and 4 adverse events in the cabozantinib arm were hand–foot syndrome (17%), hypertension (16%), increased aspartate aminotransferase (12%), fatigue (10%), and diarrhea (10%). Dose reductions were required in 62% of patients receiving cabozantinib, and the rate of discontinuation because of toxicity was 16%.
Apatinib
Apatinib is another novel oral tki that selectively inhibits vegf receptor 2. It was previously shown to be effective at improving survival in Chinese patients with metastatic gastric carcinoma who progressed on 2 or more lines of chemotherapy27 and was recently studied for the treatment of advanced hcc in China.
At the 2020 American Society of Clinical Oncology annual meeting, the results of the ahelp study, a phase iii randomized trial of apatinib compared with placebo in Chinese patients with hcc, were presented28. The study randomized 393 patients with hcc who had progressed on, or who were intolerant to, at least 1 line of systemic therapy (sorafenib or systemic chemotherapy) in a 2:1 ratio to apatinib 750 mg daily or to placebo. Patients with Child–Pugh A or B7 liver function were included. The patients who received apatinib experienced improved os (8.7 months vs. 6.8 months with placebo; hr: 0.785; 95% ci: 0.617 to 0.998; p = 0.0476) and pfs (4.5 months vs. 1.9 months with placebo; hr: 0.471; 95% ci: 0.369 to 0.601; p < 0.0001). The orr per recist 1.1 was 10.7% compared with 1.5% with placebo (p < 0.001). The dcr was 61.3% with apatinib compared with 28.8% with placebo. Grade 3 or 4 adverse events occurred in 77.4% of the patients receiving apatinib and in 19.2% of the patients receiving placebo. The most common grades 3 and 4 adverse events in the apatinib arm were hypertension (27.6%), hand–foot syndrome (17.9%), and decreased platelet count (13%). It should be noted that more than 85% of the study population had hepatitis B and only 40% received first-line sorafenib (many others received chemotherapy). That population differs significantly from patients with hcc in Western countries.
Monoclonal Antibodies
Ramucirumab
Ramucirumab is an intravenously administered recombinant monoclonal antibody with high affinity for vegf receptor 2.
In the phase iii reach trial, 565 patients who had previously received sorafenib were randomized to intravenous (iv) ramucirumab 8 mg/kg every 2 weeks or to placebo29. In the intention-to-treat population, ramucirumab, compared with placebo, failed to show a significant improvement in os (median os: 9.2 months vs. 7.6 months; hr: 0.87; 95% ci: 0.72 to 1.05; p = 0.14). A subgroup analysis suggested a potential os benefit for patients with serum alpha-fetoprotein (afp) 400 ng/mL or greater at diagnosis (median survival: 7.8 months vs. 4.2 months; hr: 0.67; 95% ci: 0.51 to 0.90; p = 0.006). Subsequently, the reach-2 trial was conducted specifically to investigate the benefit of ramucirumab in that patient population30. The reach-2 trial included 292 patients with hcc and serum afp 400 ng/mL or greater who had previously received first-line sorafenib. They were randomized to ramucirumab or to placebo. The patients who received ramucirumab experienced a significant improvement in median os (8.5 months vs. 7.3 months; hr: 0.71; 95% ci: 0.53 to 0.95; p = 0.020). The os benefit of 1.2 months was numerically less than the 3.6 months suggested in the subgroup analysis of the original reach trial. Median pfs was 2.8 months for ramucirumab compared with 1.6 months for placebo (hr: 0.45; 95% ci: 0.34 to 0.60; p < 0.001). The response rate with ramucirumab was only 5% per recist 1.1, which was not statistically significant when compared with placebo (1%, p = 0.1697). The dcr was 59.9% compared with 38.9% in the placebo group (p = 0.0006). With respect to safety, the discontinuation rate for treatment-related adverse events associated with ramucirumab was 11%. The most common adverse events were fatigue (27%), peripheral edema (25%), hypertension (25%), bleeding (24%, mostly epistaxis), and decreased appetite (23%).
A combined analysis from the reach and reach-2 trials included a total of 542 patients with serum afp 400 ng/mL or greater (250 from reach and 292 from reach-2). The pooled analysis showed that median os in patients treated with ramucirumab, compared with placebo, was 8.1 months compared with 5.0 months (hr: 0.69; 95% ci: 0.5 to 0.84; p = 0.0002), with response rates of 5.4% and 0.9% (p = 0.004) and dcrs of 56.3% and 37.2% (p < 0.0001) respectively31. The pooled population was also used to analyze patient-reported outcomes with ramucirumab. Based on the Functional Assessment of Cancer Therapy–Hepatobiliary Symptom Index 8 (FACIT.org, Ponte Vedra, FL, U.S.A.) at baseline, every 6 weeks, and at the end of treatment, ramucirumab was associated with a consistent trend toward a benefit in disease-related symptoms32.
Immunotherapy
In recent years, immunotherapy with checkpoint inhibitors has been studied for the management of many different types of cancer. Theoretically, such treatments are likely to be effective for hcc given that hcc is usually associated with chronic liver disease and a high level of immune-cell infiltrate. Some analyses suggest that approximately 25% of hccs have high inflammatory scores, with significant levels of lymphocyte infiltration33,34. Although cellular response activity is increased, that response can be dysfunctional, being associated with immune tolerance and resulting in worse prognosis35. Additionally, in patients with hepatitis B–related hcc, high levels of PD-L1 in tumour cells were associated with a poor prognosis and a higher chance of recurrence after surgery36,37. Considering those characteristics, several studies were conducted to evaluate the role of checkpoint inhibitors in the treatment of hcc. Table III summarizes the phase ii and iii trials of immunotherapy treatments in the second- and third-line treatment of hcc.
TABLE III.
Phase II and III trials of immunotherapy in the second- and third-line treatment of hepatocellular carcinoma
| Study name | Phase | Arms | Child–Pugh score | OS (months) | PFS (months) | ORR (%) | Discontinuation rate because of drug-related AEs (%) | Treatment-related AEs (%) | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Any grade | Grade 3 or 4 | ||||||||
| CheckMate 040 | I/II | Single-arm nivolumab | |||||||
| ♦ Dose escalation phase | A–B7 | — | — | 15 | 2 | 83 | 25 | ||
| ♦ Dose expansion phase | A | 20 | 4 | NR | 19 | ||||
|
| |||||||||
| CheckMate 040 | I/II | Nivolumab–ipilimumab | A | — | — | 31 | 14 | NR | 37 |
|
| |||||||||
| KEYNOTE-240 | III | Pembrolizumab | A–B7 | 13.9 | 3 | 18 | 30 | 61 | 19 |
| vs. placebo | 10.6a | 2.8a | 4 | 16 | 48 | 7 | |||
|
| |||||||||
| Study 22 | II | Tremelimumab 300 mg + durvalumab | — | 18.7 | — | 24 | 10.8 | 82 | 35 |
| Tremelimumab 75 mg + durvalumab | 11.3 | 9.5 | 6.1 | 69 | 23 | ||||
| Single-agent durvalumab | 13.6 | 10.6 | 7.9 | 60 | 18 | ||||
| Single-agent tremelimumab | 15.1 | 7.2 | 13 | 84 | 43 | ||||
Not statistically significant.
OS = overall survival; PFS = progression free survival; ORR = objective response rate; AEs = adverse events; NR = not reported.
Nivolumab
Nivolumab is an anti–PD-1 human monoclonal antibody, administered intravenously, that actives the T cell immune response against tumour cells. The phase i/ii CheckMate 040 study showed that nivolumab is active in the treatment of hcc38. Patients included in the study had advanced hcc, had previously received at least 1 line of systemic therapy, and had previously received or refused sorafenib treatment. Patients eligible for the dose-escalation phase had Child–Pugh A or B7 liver function, but only patients with Child–Pugh A liver function were included in the dose-expansion phase. Of 262 patients treated, 48 in the dose-escalation phase received nivolumab 0.1–10 mg/kg every 2 weeks, and 214 in the dose-expansion phase received nivolumab 3 mg/kg.
In the dose-escalation phase, the orr was 15% (3 complete responses, 4 partial responses), and the dcr was 58%. Median os was 15.0 months. During that phase, nivolumab showed a manageable safety profile. Grade 3 or 4 treatment-related adverse events affected 25% of patients, and 6% experienced treatment-related serious events including pemphigoid, adrenal insufficiency, and liver injury. Those events did not appear to be related to dose, and no maximum tolerated dose was reached.
Based on those results, the dose of 3 mg/kg was selected for the dose-expansion phase. In that phase, 68% of patients had previously received sorafenib. The orr was 20% (3 complete responses, 39 partial responses). The 6-month os rate was 83%, and the 9-month os rate was 74%. In the study, baseline tumour cell PD-L1 status was not associated with tumour response. Also, the benefits of nivolumab were observed in patients who were sorafenib-naïve and sorafenib-experienced alike39.
Patients with Child–Pugh B liver function in the study experienced a response rate of 10% and a dcr of 55%. Median os was 7.6 months and was higher in patients who were sorafenib-naïve (9.8 months) than in those who were sorafenib-experienced (7.3 months). Tolerability was similar to that in the patients having Child–Pugh A liver function40.
Nivolumab–Ipilimumab
Ipilimumab is a monoclonal antibody that targets ctla-4. The combination of ipilimumab and nivolumab was also evaluated as part of the phase i/ii CheckMate 040 trial41. Patients previously treated with sorafenib (n = 148) were randomized to one of three arms:
■ Arm A—nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks (4 doses), followed by nivolumab 240 mg every 2 weeks
■ Arm B—nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks (4 doses), followed by nivolumab 240 mg every 2 weeks
■ Arm C—nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks
Patients were required to have Child–Pugh A liver function. The orr was 31% overall, and 7 patients experienced a complete response. The median duration of response was 17 months. In arm A, the response rate was 32%; it was 31% in arm B and 31% in arm C. The dcr was highest for patients in arm A, at 54% compared with 43% for arm B and 49% for arm C. The median os in arm A was 23 months; it was 12 months for arm B and 13 months for arm C. With respect to safety, 37% of patients experienced grade 3 or 4 treatment-related adverse events, with only 5% discontinuing therapy because of grades 3 and 4 treatment-related toxicity. The most common adverse events (any grade) were rash (35%), adrenal insufficiency (18%), hypothyroidism or thyroiditis (22%), colitis (10%), pneumonitis (10%), and infusion reactions (8%).
Pembrolizumab
Pembrolizumab is another anti–PD-1 monoclonal antibody that has been evaluated in the second-line treatment of patients with advanced hcc. In the phase ii keynote-224 trial, 104 patients who had progressed on or were intolerant to sorafenib were treated with pembrolizumab 200 mg every 3 weeks42. The response rate was 17%, and the dcr was 62%. Treatment-related adverse events occurred in 73% of patients, and grade 3 toxicity occurred in 24%.
In the phase iii keynote-240 trial, pembrolizumab was compared with placebo in patients with advanced hcc who had previously received sorafenib43. In the study, the median os was 13.9 months in the pembrolizumab arm and 10.6 months in the placebo arm (hr: 0.78; 95% ci: 0.61 to 0.99; p = 0.024), and the median pfs was 3.0 months compared with 2.8 months (hr: 0.72; 95% ci: 0.57 to 0.90; p = 0.002). Although the numerical improvement in median os appeared to be clinically meaningful, it did not reach statistical significance per specified criteria. The orr was, however, higher for pembrolizumab (18.3% vs. 4.4%, p = 0.00007). With respect to toxicity, grade 3 or 4 adverse events occurred in 53% of the patients receiving pembrolizumab and in 46% of those receiving placebo. Treatment-related adverse events occurred in 19% and 8% of the patients respectively. The most common adverse events reported with pembrolizumab were increased levels of aspartate aminotransferase (23%) and of bilirubin (19%), fatigue (19%), and pruritus (18%).
Tremelimumab–Durvalumab
The activity of durvalumab (an anti–PD-L1 antibody) and tremelimumab (an anti–ctla-4 antibody) in patients with hcc was assessed in a phase ii study, and the results were recently presented at the 2020 American Society of Clinical Oncology annual meeting44. Patients from Study 22 who had previously received or refused sorafenib were randomized to one of these regimens:
■ Arm A—tremelimumab 300 mg (1 dose) plus durvalumab 1500 mg every 4 weeks
■ Arm B—tremelimumab 75 mg (4 doses) plus durvalumab 1500 mg every 4 weeks
■ Arm C—single-agent durvalumab 1500 mg every 4 weeks
■ Arm D—single-agent tremelimumab 750 mg every 4 weeks (7 doses, then every 12 weeks thereafter)
Patients in arm A who received tremelimumab 300 mg (1 dose) plus durvalumab appeared to experience superior outcomes, including a higher orr (arm A, 24.0%; arm B, 9.5%; arm C, 10.6%; arm D, 7.2%) and a higher median os (arm A, 18.7 months; arm B, 11.3 months; arm C, 13.6 months; arm D, 15.1 months). Treatment-related severe adverse events occurred in 16% of patients on arm A, 15% on arm B, 11% on arm C, and 25% on arm D.
Based on the results of Study 22, a single priming dose of tremelimumab 300 mg plus durvalumab every 4 weeks showed promising efficacy and safety in the second-line treatment of patients with hcc. The efficacy and safety of durvalumab with or without tremelimumab, compared with sorafenib, in the first-line treatment of hcc is being studied in the phase iii himalaya trial. That study has completed accrual, and results are expected to be presented in early 2021.
SUMMARY
The management of advanced hcc is now rapidly evolving after a decade in which sorafenib was the only treatment offering a survival benefit. Lenvatinib and atezolizumab–bevacizumab are now also first-line treatment options.
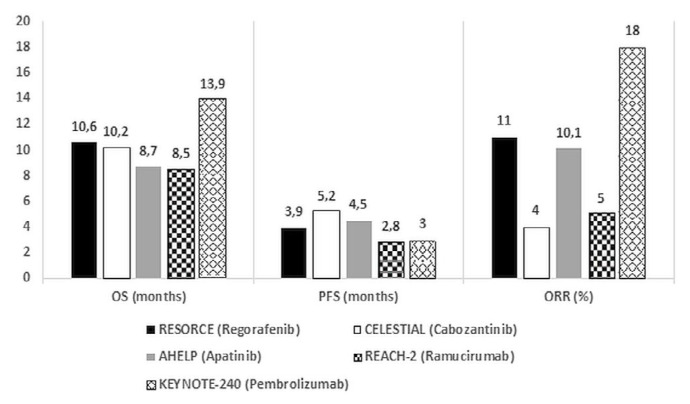
Figure 1 summarizes the efficacy endpoints for positive phase iii trials in second-line hcc.
FIGURE 1.
Comparison of efficacy endpoints in positive second-line phase III trials.
Based on the trial evidence reviewed and the clinically and statistically significant survival benefits observed, it is clear that regorafenib and cabozantinib should be considered standard second-line options for patients with hcc who have previously been treated with sorafenib. Regorafenib has been approved by Health Canada for this indication and is funded in most provinces. Regorafenib might be the preferred second-line treatment in patients who responded to and tolerated sorafenib well, given that those factors were specific inclusion criteria for the resorce trial.
Cabozantinib has also been approved by Health Canada, but funding by the provinces is currently pending. Cabozantinib might be preferred in patients who did not tolerate sorafenib well or if a tki is required in the third-line setting, given that these patients were included in the celestial trial.
Ramucirumab can be considered a treatment option in the second-line treatment of patients with hcc and serum afp of 400ng/mL or greater, although this is an iv treatment with a small-magnitude survival benefit. Ramucirumab is not approved by Health Canada for this indication and is not funded by the provinces.
Apatinib could be considered in patients with hepatitis B–related hcc who have received chemotherapy in the first line, but it is not available in Canada.
Currently, there is no evidence that any immunotherapy treatment improves survival in the second-line setting, and therefore none are funded anywhere in Canada. However, nivolumab was approved by Health Canada based on the CheckMate 040 study results. The subsequent phase iii CheckMate 459 trial, which compared nivolumab with sorafenib in the first-line treatment of advanced hcc, failed to show a statistically significant survival benefit45. Despite the Health Canada approval, no province currently funds nivolumab for the second-line treatment of hcc.
The main challenge in the systemic treatment of hcc in the second line and beyond is that there is a paucity of evidence about how to treat patients if they have received lenvatinib or atezolizumab–bevacizumab in the first-line setting. Theoretically, patients who received either of those treatments followed by sorafenib would have been eligible for the celestial trial, and phase iii trial evidence supporting third-line cabozantinib in that scenario therefore exists. However, no available trial evidence supports the use of sorafenib as a second-line treatment.
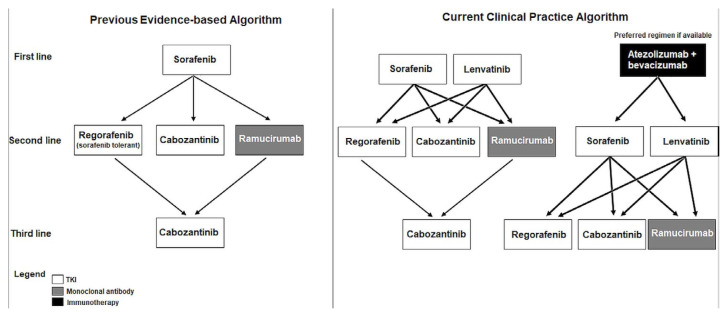
Given the lack of clinical trial evidence to inform optimal treatment sequencing for advanced hcc in the face of the new first-line treatment options, oncologists in Canada will likely rely on expert opinion, while taking into account provincial funding decisions. Figure 2 illustrates a number of the potential treatment sequencing strategies.
FIGURE 2.
Possible treatment sequencing in hepatocellular carcinoma.
It is likely that atezolizumab–bevacizumab will be the new standard of care in first-line treatment. In that case, a reasonable second-line treatment would be a tki. It could also be argued that a first-line tki should be used after immunotherapy, which would include either of lenvatinib or sorafenib. Lenvatinib stands out as the tki with the highest reported response rate and longest pfs. Third-line treatment in that scenario would consist of a second-line tki such as cabozantinib or regorafenib. If lenvatinib were to be used as a first-line treatment, then a second-line tki could be considered upon progression and would most likely be funded by the provinces. Although a checkpoint inhibitor such as nivolumab or pembrolizumab could theoretically be beneficial for some patients in that setting, it is unlikely to be publicly funded.
Another challenge in second-line treatment and beyond is that most patients with hcc have concomitant chronic liver disease, which can also be life-threatening. With time, liver function can deteriorate, and because most trials include only patients classified Child–Pugh A, patients who remain well with Child–Pugh B liver function might be left without any evidence-based treatment options. For patients classified Child–Pugh B, evidence for the safety and efficacy of treatments is based mostly on observational and retrospective studies. In a recent Canadian study, for example, only 13.1% of real-world patients would have been eligible for the resorce, celestial, and reach-2 trials after progression on sorafenib. If patients with Child–Pugh B7 liver function and an Eastern Cooperative Oncology Group performance status of 2 could have been included, the number of patients eligible for those second-line treatment trials would increase to 31.7%46. Using performance status, given its subjectivity, to strictly enforce public funding for hcc treatments is difficult. However, it is anticipated that well patients with Child–Pugh B liver function will continue to be denied publicly funded second-line systemic treatment in Canada because of their exclusion from the positive phase iii clinical trials.
Systemic treatment of hcc is evolving rapidly, and determining the optimal sequencing strategy for second-line and beyond is challenging now that first-line sorafenib is no longer a standard. In the future, results from additional clinical trials and real-world studies will hopefully have helped to inform treatment sequencing decisions in hcc.
Despite the challenges, it is exciting to see that there are now many systemic treatment options to help significantly prolong survival for patients with incurable hcc.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: VCT has received honoraria for work on advisory boards for Bristol Myers Squibb, Eisai, Ipsen, and Roche, and research funding to his institution from Bayer, Eisai, and Ipsen. CPA has no conflicts to disclose.
REFERENCES
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: globocan sources and methods. Int J Cancer. 2019;144:1941–53. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Brenner DR, Weir HK, Demers AA, et al. Projected estimates of cancer in Canada in 2020. CMAJ. 2020;192:E199–205. doi: 10.1503/cmaj.191292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singal AG, El-Serag HB. Hepatocellular carcinoma from epidemiology to prevention: translating knowledge into practice. Clin Gastroenterol Hepatol. 2015;13:2140–51. doi: 10.1016/j.cgh.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomes MA, Priolli DG, Tralhão JG, Botelho MF. Hepatocellular carcinoma: epidemiology, biology, diagnosis, and therapies. Rev Assoc Med Bras (1992) 2013;59:514–24. doi: 10.1016/j.ramb.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, et al. on behalf of the sharp Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia–Pacific region with advanced hepatocellular carcinoma: a phase iii randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 7.Abou-Alfa GK, Niedzwieski D, Knox JJ, et al. Phase iii randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (hcc): calgb 80802 (Alliance) [abstract 192] J Clin Oncol. 2016;34 [Available online at: https://ascopubs.org/doi/abs/10.1200/jco.2016.34.4_suppl.192; cited 14 October 2020] [Google Scholar]
- 8.Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase iii trial. J Clin Oncol. 2013;31:4067–75. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 9.Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase iii brisk-fl study. J Clin Oncol. 2013;31:3517–24. doi: 10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- 10.Siegel AB, Cohen EI, Ocean A, et al. Phase ii trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26:2992–8. doi: 10.1200/JCO.2007.15.9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu AX, Rosmorduc O, Jeffery Evans TR, et al. search: a phase iii, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33:559–66. doi: 10.1200/JCO.2013.53.7746. [DOI] [PubMed] [Google Scholar]
- 12.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–73. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 13.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 14.Paul MK, Mukhopadhyay AK. Tyrosine kinase—role and significance in cancer. Int J Med Sci. 2004;1:101–15. doi: 10.7150/ijms.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito Y, Sasaki Y, Horimoto M, et al. Activation of mitogen-activated protein kinases/extracellular signal-regulated kinases in human hepatocellular carcinoma. Hepatology. 1998;27:951–8. doi: 10.1002/hep.510270409. [DOI] [PubMed] [Google Scholar]
- 16.Villanueva A, Newell P, Chiang DY, Friedman SL, Josep M, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007;27:55–76. doi: 10.1055/s-2006-960171. [DOI] [PubMed] [Google Scholar]
- 17.Calvisi DF, Ladu S, Gorden A, et al. Ubiquitous activation of Ras and jak/stat pathways in human hcc. Gastroenterology. 2006;130:1117–28. doi: 10.1053/j.gastro.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Semela D, Dufour JF. Angiogenesis and hepatocellular carcinoma. J Hepatol. 2004;41:864–80. doi: 10.1016/j.jhep.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase ii safety study. Eur J Cancer. 2013;49:3412–19. doi: 10.1016/j.ejca.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 20.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (resorce): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 21.Bekaii-Saab TS, Ou FS, Ahn DH, et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (redos): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol. 2019;20:1070–82. doi: 10.1016/S1470-2045(19)30272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for hcc: additional analyses from the phase iii resorce trial. J Hepatol. 2018;69:353–8. doi: 10.1016/j.jhep.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Rimassa L, Assenat E, Peck-Radosavljevic M, et al. Tivantinib for second-line treatment of met-high, advanced hepatocellular carcinoma (metiv-hcc): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018;19:682–93. doi: 10.1016/S1470-2045(18)30146-3. [DOI] [PubMed] [Google Scholar]
- 24.Firtina Karagonlar Z, Koc D, Iscan E, Erdal E, Atabey N. Elevated hepatocyte growth factor expression as an autocrine c-Met activation mechanism in acquired resistance to sorafenib in hepatocellular carcinoma cells. Cancer Sci. 2016;107:407–16. doi: 10.1111/cas.12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiang Q, Chen W, Ren M, et al. Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of vegfr2 and Met. Clin Cancer Res. 2014;20:2959–70. doi: 10.1158/1078-0432.CCR-13-2620. [DOI] [PubMed] [Google Scholar]
- 26.Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase iii trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34:1448–54. doi: 10.1200/JCO.2015.63.5995. [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Qin S, Gu S, et al. Apatinib as second-line therapy in Chinese patients with advanced hepatocellular carcinoma: a randomized, placebo-controlled, double-blind, phase iii study [abstract 4507] J Clin Oncol. 2020;38 [Available online at: https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.4507; cited 14 October 2020] [Google Scholar]
- 29.Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (reach): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859–70. doi: 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- 30.Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (reach-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–96. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 31.Zhu A, Finn R, Galle PR, et al. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma (hcc) and elevated alpha-fetoprotein (afp) following first-line sorafenib: pooled efficacy and safety across two global randomized phase 3 studies (reach-2 and reach) [abstract LBA-001] Ann Oncol. 2018;29(suppl 5):v122–23. doi: 10.1093/annonc/mdy208. [DOI] [Google Scholar]
- 32.Zhu AX, Finn RS, Galle PR, et al. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma (hcc) and elevated alpha-fetoprotein (afp) following first-line sorafenib: patient reported outcome results across two phase iii studies (reach-2 and reach) [abstract 622PD] Ann Oncol. 2018;29(suppl 8):viii208. doi: 10.1093/annonc/mdy282.006. [DOI] [Google Scholar]
- 33.Sia D, Jiao Y, Martinez-Quetglas I, et al. Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology. 2017;153:812–26. doi: 10.1053/j.gastro.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169:1327–41.e23. doi: 10.1016/j.cell.2017.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu J, Xu D, Liu Z, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–39. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 36.Han X, Gu YK, Li SL, et al. Pre-treatment serum levels of soluble programmed cell death–ligand 1 predict prognosis in patients with hepatitis B–related hepatocellular carcinoma. J Cancer Res Clin Oncol. 2019;145:303–12. doi: 10.1007/s00432-018-2758-6. [DOI] [PubMed] [Google Scholar]
- 37.Shi F, Shi M, Zeng Z, et al. PD-1 and PD-L1 upregulation promotes CD8+ T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128:887–96. doi: 10.1002/ijc.25397. [DOI] [PubMed] [Google Scholar]
- 38.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crocenzi TS, El-Khoueiry AB, Yau TC, et al. Nivolumab (nivo) in sorafenib (sor)–naive and–experienced pts with advanced hepatocellular carcinoma (hcc): CheckMate 040 study [abstract 4013] J Clin Oncol. 2017;35 doi: 10.1200/JCO.2017.35.15_suppl.4013. [Available online at: https://ascopubs.org/doi/abs/10.1200/JCO.2017.35.15_suppl.4013 ; cited 29 October 2020] [DOI] [Google Scholar]
- 40.Kudo M, Matilla A, Santoro A, et al. CheckMate-040: nivolumab (nivo) in patients (pts) with advanced hepatocellular carcinoma (ahcc) and Child–Pugh B (cpb) status [abstract 327] J Clin Oncol. 2019;37 [Available online at: https://ascopubs.org/doi/abs/10.1200/JCO.2019.37.4_suppl.327; cited 14 October 2020] [Google Scholar]
- 41.Yau T, Kang YK, Kim TY, et al. Nivolumab (nivo) + ipilimumab (ipi) combination therapy in patients (pts) with advanced hepatocellular carcinoma (ahcc): results from CheckMate 040 [abstract 4012] J Clin Oncol. 2019;37 [Available online at: https://ascopubs.org/doi/abs/10.1200/JCO.2019.37.15_suppl4012. ; cited 14 October 2020] [Google Scholar]
- 42.Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (keynote-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–52. doi: 10.1016/S1470-2045(18)30351-6. [Erratum in: Lancet Oncol 2018;19:940–52] [DOI] [PubMed] [Google Scholar]
- 43.Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in keynote-240: a randomized, double-blind, phase iii trial. J Clin Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 44.Kelley RK, Sangro B, Harris WP, et al. Efficacy, tolerability, and biologic activity of a novel regimen of tremelimumab (t) in combination with durvalumab (d) for patients (pts) with advanced hepatocellular carcinoma (ahcc) [abstract 4508] J Clin Oncol. 2020;38 doi: 10.1200/JCO.19.00827. [Available online at: https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.4508; cited 29 October 2020] [DOI] [Google Scholar]
- 45.Yau T, Park JW, Finn RS, et al. CheckMate 459: a randomized, multi-center phase iii study of nivolumab (nivo) vs sorafenib (sor) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (ahcc) [abstract LBA38_PR] Ann Oncol. 2019;30(suppl 5):v874–5. doi: 10.1093/annonc/mdz394.029. [Available online at: https://www.annalsofoncology.org/article/S0923-7534(19)60389-3/fulltext ; cited 29 October 2020] [DOI] [Google Scholar]
- 46.Fung AS, Tam VC, Meyers DE, et al. Real world eligibility for cabozantinib (c), regorafenib (reg), and ramucirumab (ram) in hepatocellular carcinoma (hcc) patients after sorafenib (s) [abstract 422] J Clin Oncol. 2019;37 [Available online at: https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.4508; cited 14 October 2020] [Google Scholar]