Abstract
The multikinase inhibitor sorafenib was the only approved systemic therapy in advanced hepatocellular carcinoma (hcc) for about a decade. In recent years, the number of approved agents has increased significantly as a result of a number of positive phase iii clinical trials. Lenvatinib as a first-line treatment, and regorafenib, cabozantinib, and ramucirumab in the second-line setting are now approved by the U.S. Food and Drug Administration (fda) and the European Medicines Agency.
In phase ii studies, immunotherapy with nivolumab and monotherapy using pembrolizumab yielded impressive results for overall survival in therapy-naïve and pretreated patients, leading to the accelerated approval by the fda of nivolumab and pembrolizumab for second-line treatment. However, phase iii trials of nivolumab in the first line and pembrolizumab in the second line as single agents failed to reach statistical significance, although clinical benefit for a subset of patients with long durations of response could be demonstrated. Despite that setback, immunotherapy for hcc is a promising therapeutic approach, and the combination of immunotherapy with other treatment modalities such as monoclonal antibodies, tyrosine kinase inhibitors, or local therapies has the potential to increase the overall response rate and survival.
Recently, the results of a phase iii trial of combination atezolizumab–bevacizumab compared with sorafenib showed a highly significant survival benefit and median overall survival that was not reached in the immunotherapy arm, making the combination the preferred standard of care in first-line therapy.
Despite the impressive results and generally good toxicity profile of immunotherapy, patients who respond to therapy constitute only a subset of the overall population, and response rates are still limited.
This review focuses on the currently reported results and ongoing clinical trials of checkpoint inhibitor–based immunotherapy in hcc.
Keywords: Hepatocellular carcinoma, immunotherapy, checkpoint inhibitors, nivolumab, pembrolizumab, durvalumab, tremelimumab, ipilimumab
INTRODUCTION
Hepatocellular carcinoma (hcc) is an aggressive tumour, usually arising in the context of liver cirrhosis. The incidence of hcc has been rising worldwide during the last 20 years because of the increased number of patients with known risk factors such as chronic viral hepatitis B (hbv) or hepatitis C (hcv) and non-alcoholic steatohepatitis1. With approximately 840,000 cases worldwide and 781,000 deaths in the year 2018, hcc was the 5th most common cancer and 2nd most frequent cause of cancer mortality2.
Curative treatment options in hcc are liver resection, liver transplantation, and local ablation. Unfortunately, most cases are diagnosed at an intermediate or advanced stage, not amenable to potentially curative treatments3, making systemic therapy an important part of the therapeutic armamentarium.
For about a decade, the only systemic therapy with a proven survival benefit (10.7 months vs. 7.9 months in the phase iii sharp trial) was the multikinase inhibitor sorafenib4, which was approved for the first-line treatment of hcc in the United States and the European Union. After a number of failed phase iii trials evaluating other kinase inhibitors, the noninferiority of lenvatinib compared with sorafenib was demonstrated in the phase iii reflect trial, and lenvatinib was added to the treatment guidelines of the European Association for the Study of the Liver and the European Society for Medical Oncology5.
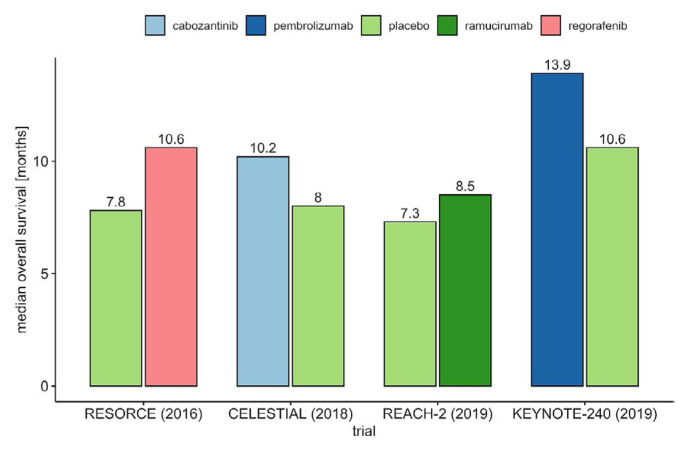
In the second line, after progression on or intolerance to sorafenib, the multikinase inhibitor regorafenib, tested in the resorce trial, was the first agent to show a survival benefit (10.6 months vs. 7.8 months for placebo)6. Other approved second-line options are cabozantinib, an inhibitor of vascular endothelial growth factor (vegf) receptors and the met and tam kinases (10.2 months vs. 8.0 months for placebo, celestial trial)7, and ramucirumab, a monoclonal antibody that targets vegf receptor 2 in patients with elevated alpha-fetoprotein at 400 ng/mL or greater (8.5 months vs. 7.3 months, reach-2 trial)8.
Despite the increased number of systemic therapeutic options, prognosis is still limited: about 12–14 months in first-line therapy and 8–11 months in second-line therapy with the currently approved kinase inhibitors. The survival benefit with tyrosine kinase inhibitor (tki) therapy is further limited in patients with impaired liver function; because of the risk of further deterioration of liver function, treatment should be limited to patients with compensated cirrhosis9. The common side effects of tki therapy—such as diarrhea, asthenia, weight loss, and hand–foot skin reaction—can severely limit patient compliance, the tolerated dose, and quality of life. Those limitations of the available therapeutic approaches demonstrate a medical need for more effective systemic therapy in hcc that will further improve on overall survival (os) while preserving quality of life.
After an impressive clinical benefit was demonstrated and checkpoint inhibitors were approved in melanoma, the use of those drugs in other tumour entities, including hcc, was extensively investigated10. Here, we review the currently available evidence for immunotherapy in hcc.
DISCUSSION
PD-1, PD-L1, and CTLA-4 Inhibitors
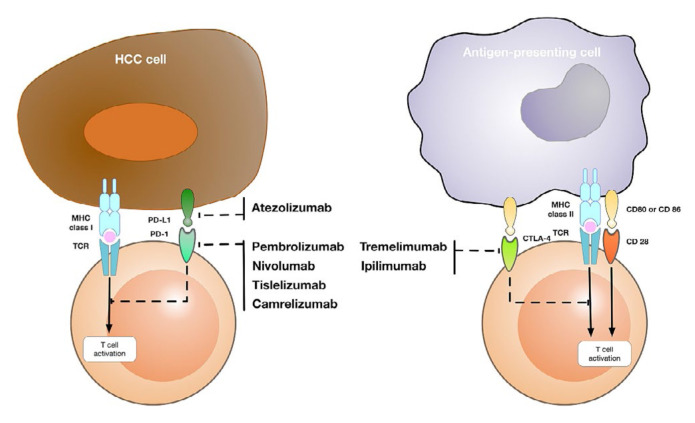
Clinical trials of immune checkpoint inhibitors (icis) have used mainly monoclonal antibodies inhibiting PD-1, PD-L1, or ctla-4 (Figure 1). Currently PD-1/-L1 and ctla-4 blockade are successfully used in routine clinical practice, and immunotherapy has become a new promising method for inhibiting hcc tumour progression, recurrence, and metastasis. Table I presents a list of currently approved icis in hcc.
FIGURE 1.
Targets and available checkpoint inhibitors in hepatocellular carcinoma (HCC). MHC = major histocompatibility complex; TCR = T cell receptor.
TABLE I.
Current approval status of immunotherapy in hepatocellular carcinoma
| Agent | Type | Line | U.S. FDA | EMA |
|---|---|---|---|---|
| Nivolumab | Anti–PD-1 | 2 | Yes (Sep 2017) | No |
| Pembrolizumab | Anti–PD-1 | 2 | Yes (Nov 2018) | No |
| Atezolizumab | Anti–PD-L1 | 1 | Yes (May 2020)a | Yes (Nov 2020) |
| Ipilimumab | Anti–CTLA-4 | 2 | Yes (Mar 2020)b | No |
In combination with bevacizumab.
In combination with nivolumab.
FDA = Food and Drug Administration; EMA = European Medicines Agency.
The subsections that follow discuss the checkpoint inhibitors that are currently approved or in clinical development. The reported results of clinical trials with icis are presented in Table II, and ongoing trials are presented in Table III.
TABLE II.
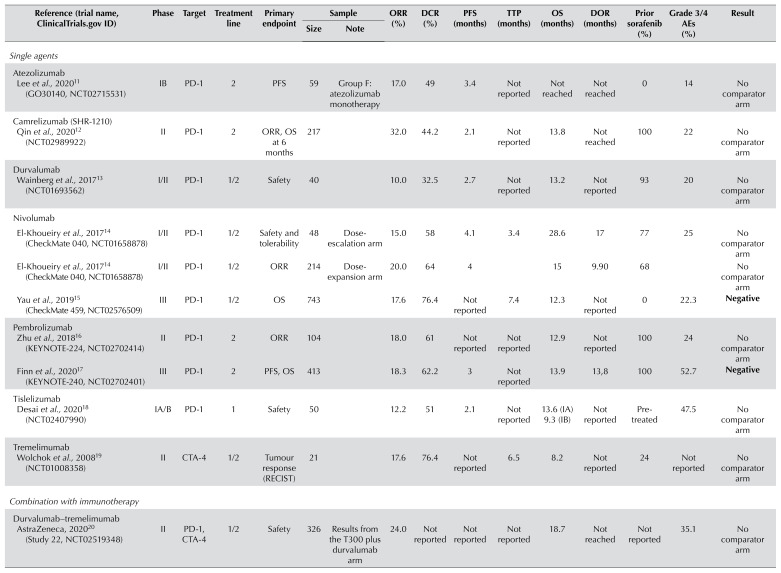
Clinical trials with reported results for immunotherapy in hepatocellular carcinoma
| Reference (trial name, ClinicalTrials.gov ID) | Phase | Target | Treatment line | Primary endpoint | Sample | ORR (%) | DCR (%) | PFS (months) | TTP (months) | OS (months) | DOR (months) | Prior sorafenib (%) | Grade 3/4 AEs (%) | Result | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Size | Note | ||||||||||||||
| Single agents | |||||||||||||||
|
| |||||||||||||||
| Atezolizumab | |||||||||||||||
| Lee et al., 202011 (GO30140, NCT02715531) | IB | PD-1 | 2 | PFS | 59 | Group F: atezolizumab monotherapy | 17.0 | 49 | 3.4 | Not reported | Not reached | Not reached | 0 | 14 | No comparator arm |
|
| |||||||||||||||
| Camrelizumab (SHR-1210) | |||||||||||||||
| Qin et al., 202012 (NCT02989922) | II | PD-1 | 2 | ORR, OS at 6 months | 217 | 32.0 | 44.2 | 2.1 | Not reported | 13.8 | Not reached | 100 | 22 | No comparator arm | |
|
| |||||||||||||||
| Durvalumab | |||||||||||||||
| Wainberg et al., 201713 (NCT01693562) | I/II | PD-1 | 1/2 | Safety | 40 | 10.0 | 32.5 | 2.7 | Not reported | 13.2 | Not reported | 93 | 20 | No comparator arm | |
|
| |||||||||||||||
| Nivolumab | |||||||||||||||
| El-Khoueiry et al., 201714 (CheckMate 040, NCT01658878) | I/II | PD-1 | 1/2 | Safety and tolerability | 48 | Dose-escalation arm | 15.0 | 58 | 4.1 | 3.4 | 28.6 | 17 | 77 | 25 | No comparator arm |
| El-Khoueiry et al., 201714 (CheckMate 040, NCT01658878) | I/II | PD-1 | 1/2 | ORR | 214 | Dose-expansion arm | 20.0 | 64 | 4 | 15 | 9.90 | 68 | No comparator arm | ||
| Yau et al., 201915 (CheckMate 459, NCT02576509) | III | PD-1 | 1/2 | OS | 743 | 17.6 | 76.4 | Not reported | 7.4 | 12.3 | Not reported | 0 | 22.3 | Negative | |
|
| |||||||||||||||
| Pembrolizumab | |||||||||||||||
| Zhu et al., 201816 (KEYNOTE-224, NCT02702414) | II | PD-1 | 2 | ORR | 104 | 18.0 | 61 | Not reported | Not reported | 12.9 | Not reported | 100 | 24 | No comparator arm | |
| Finn et al., 202017 (KEYNOTE-240, NCT02702401) | III | PD-1 | 2 | PFS, OS | 413 | 18.3 | 62.2 | 3 | Not reported | 13.9 | 13,8 | 100 | 52.7 | Negative | |
|
| |||||||||||||||
| Tislelizumab | |||||||||||||||
| Desai et al., 202018 (NCT02407990) | IA/B | PD-1 | 1 | Safety | 50 | 12.2 | 51 | 2.1 | Not reported | 13.6 (IA) 9.3 (IB) | Not reported | Pre-treated | 47.5 | No comparator arm | |
|
| |||||||||||||||
| Tremelimumab | |||||||||||||||
| Wolchok et al., 200819 (NCT01008358) | II | CTA-4 | 1/2 | Tumour response (RECIST) | 21 | 17.6 | 76.4 | Not reported | 6.5 | 8.2 | Not reported | 24 | Not reported | No comparator arm | |
|
| |||||||||||||||
| Combination with immunotherapy | |||||||||||||||
|
| |||||||||||||||
| Durvalumab–tremelimumab | |||||||||||||||
| AstraZeneca, 202020 (Study 22, NCT02519348) | II | PD-1, CTA-4 | 1/2 | Safety | 326 | Results from the T300 plus durvalumab arm | 24.0 | Not reported | Not reported | Not reported | 18.7 | Not reached | Not reported | 35.1 | No comparator arm |
|
| |||||||||||||||
| Nivolumab–ipilimumab | |||||||||||||||
| Yau et al., 201921 (CheckMate 040, NCT01658878) | I/II | PD-1, CTA-4 | 2 | Safety, ORR | 148 | OS results from the nivo1/ipi3 arm | 31.0 | 49 | Not reported | Not reported | 23 | 17 | 100 | 37 | No comparator arm |
| Combination with TKI or anti-VEGF | |||||||||||||||
|
| |||||||||||||||
| Atezolizumab–bevacizumab | |||||||||||||||
| Finn et al., 202022 (IMbrave 150, NCT03434379) | III | PD-1 VEGF | 1 | OS, PFS | 336 | 27.3 | 73.6 | 6.8 | Not reported | Not reached | Not reported | 0 | 56.5 | Positive | |
| Lee et al., 202011 (GO30140, NCT02715531) | IB | PD-1, VEGF | 1 | ORR | 104 | Group A | 36.0 | 71 | 7.3 | Not reported | 17.1 | Not reached | 0 | 53 | No comparator arm |
| Lee et al., 202011 (GO30140, NCT02715531) | IB | PD-1 VEGF | 1 | PFS | 60 | Group F | 20.0 | 67 | 5.6 | Not reported | Not reached | Not reported | 0 | 37 | No comparator arm |
|
| |||||||||||||||
| Avelumab–axitinib | |||||||||||||||
| Kudo et al., 201923 (VEGF Liver 100, NCT03289533) | I | PD-1 VEGF | 1 | Safety | 22 | 13.6 | 68.2 | 5.5 | Not reported | Not reported | Not reported | 0 | Not reported | No comparator arm | |
|
| |||||||||||||||
| Camrelizumab (SHR-1210)–apanitinib | |||||||||||||||
| Xu et al., 201924 (NCT02942329) | IA/B | PD-1, tyrosine kinases | 1/2 | OS rate | 18 | 50.0 | 93.8 | 5.8 | Not reported | Not reached | Not reached | 83 | 60.6 | No comparator arm | |
|
| |||||||||||||||
| Durvalumab–ramucirumab | |||||||||||||||
| Bang et al., 201925 (NCT02572687) | IB | PD-1, VEGF | 2 | Safety | 28 | 11.0 | 61 | 4.4 | Not reported | Not reported | Not reported | Pre-treated | Not reported | No comparator arm | |
|
| |||||||||||||||
| Pembrolizumab–lenvatinib | |||||||||||||||
| Finn et al., 202026 (NCT03006926) | IB | PD-1, tyrosine kinases | 1 | DLT, ORR, and DOR | 104 | 46.0 | 86 | 9.3 | Not reported | 22 | 8.6 | 4 | 67 | No comparator arm | |
|
| |||||||||||||||
| Combination with local therapy | |||||||||||||||
|
| |||||||||||||||
| Tremelimumab and subtotal ablation | |||||||||||||||
| Duffy et al., 201727 (NCT01853618) | I/II | CTA-4 | 1 | Safety | 32 | 26.3 | Not reported | 7.4 | 12.3 | Not reported | 66 | Not reported | No comparator arm | ||
ORR = objective response rate; DCR = disease control rate; PFS = progression-free survival; TTP = time to progression; OS = overall survival; DOR = duration of response; aes = adverse events; RECIST = Response Evaluation Criteria in Solid Tumors; TKI = tyrosine kinase inhibitor; VEGF = vascular endothelial growth factor; DLT = dose-limiting toxicity.
TABLE III.
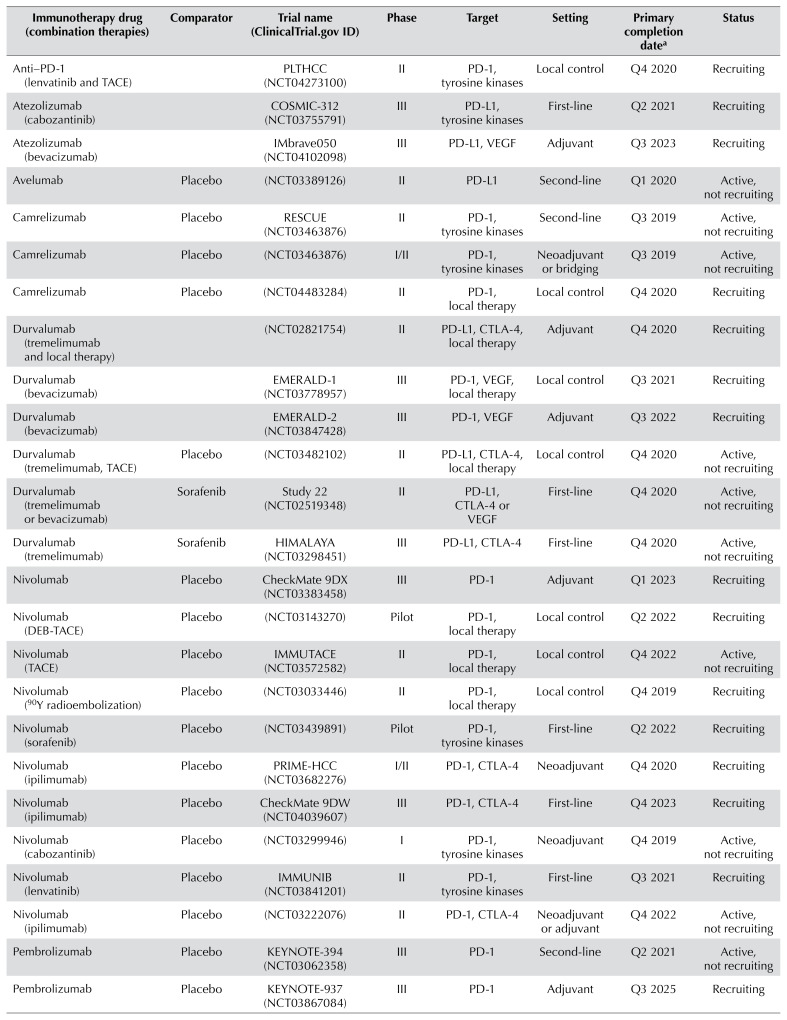
Selection of ongoing clinical trials of immunotherapy in hepatocellular carcinoma
| Immunotherapy drug (combination therapies) | Comparator | Trial name (ClinicalTrials.gov ID) | Phase | Target | Setting | Primary completion datea | Status |
|---|---|---|---|---|---|---|---|
| Anti–PD-1 (lenvatinib and TACE) | PLTHCC (NCT04273100) | II | PD-1, tyrosine kinases | Local control | Q4 2020 | Recruiting | |
| Atezolizumab (cabozantinib) | COSMIC-312 (NCT03755791) | III | PD-L1, tyrosine kinases | First-line | Q2 2021 | Recruiting | |
| Atezolizumab (bevacizumab) | IMbrave050 (NCT04102098) | III | PD-L1, VEGF | Adjuvant | Q3 2023 | Recruiting | |
| Avelumab | Placebo | (NCT03389126) | II | PD-L1 | Second-line | Q1 2020 | Active, not recruiting |
| Camrelizumab | Placebo | RESCUE (NCT03463876) | II | PD-1, tyrosine kinases | Second-line | Q3 2019 | Active, not recruiting |
| Camrelizumab | Placebo | (NCT03463876) | I/II | PD-1, tyrosine kinases | Neoadjuvant or bridging | Q3 2019 | Active, not recruiting |
| Camrelizumab | Placebo | (NCT04483284) | II | PD-1, local therapy | Local control | Q4 2020 | Recruiting |
| Durvalumab (tremelimumab and local therapy) | (NCT02821754) | II | PD-L1, CTLA-4, local therapy | Adjuvant | Q4 2020 | Recruiting | |
| Durvalumab (bevacizumab) | EMERALD-1 (NCT03778957) | III | PD-1, VEGF, local therapy | Local control | Q3 2021 | Recruiting | |
| Durvalumab (bevacizumab) | EMERALD-2 (NCT03847428) | III | PD-1, VEGF | Adjuvant | Q3 2022 | Recruiting | |
| Durvalumab (tremelimumab, TACE) | Placebo | (NCT03482102) | II | PD-L1, CTLA-4, local therapy | Local control | Q4 2020 | Active, not recruiting |
| Durvalumab (tremelimumab or bevacizumab) | Sorafenib | Study 22 (NCT02519348) | II | PD-L1, CTLA-4 or VEGF | First-line | Q4 2020 | Active, not recruiting |
| Durvalumab (tremelimumab) | Sorafenib | HIMALAYA (NCT03298451) | III | PD-L1, CTLA-4 | First-line | Q4 2020 | Active, not recruiting |
| Nivolumab | Placebo | CheckMate 9DX (NCT03383458) | III | PD-1 | Adjuvant | Q1 2023 | Recruiting |
| Nivolumab (DEB-TACE) | Placebo | (NCT03143270) | Pilot | PD-1, local therapy | Local control | Q2 2022 | Recruiting |
| Nivolumab (TACE) | Placebo | IMMUTACE (NCT03572582) | II | PD-1, local therapy | Local control | Q4 2022 | Active, not recruiting |
| Nivolumab (90Y radioembolization) | Placebo | (NCT03033446) | II | PD-1, local therapy | Local control | Q4 2019 | Recruiting |
| Nivolumab (sorafenib) | Placebo | (NCT03439891) | Pilot | PD-1, tyrosine kinases | First-line | Q2 2022 | Recruiting |
| Nivolumab (ipilimumab) | Placebo | PRIME-HCC (NCT03682276) | I/II | PD-1, CTLA-4 | Neoadjuvant | Q4 2020 | Recruiting |
| Nivolumab (ipilimumab) | Placebo | CheckMate 9DW (NCT04039607) | III | PD-1, CTLA-4 | First-line | Q4 2023 | Recruiting |
| Nivolumab (cabozantinib) | Placebo | (NCT03299946) | I | PD-1, tyrosine kinases | Neoadjuvant | Q4 2019 | Active, not recruiting |
| Nivolumab (lenvatinib) | Placebo | IMMUNIB (NCT03841201) | II | PD-1, tyrosine kinases | First-line | Q3 2021 | Recruiting |
| Nivolumab (ipilimumab) | Placebo | (NCT03222076) | II | PD-1, CTLA-4 | Neoadjuvant or adjuvant | Q4 2022 | Active, not recruiting |
| Pembrolizumab | Placebo | KEYNOTE-394 (NCT03062358) | III | PD-1 | Second-line | Q2 2021 | Active, not recruiting |
| Pembrolizumab | Placebo | KEYNOTE-937 (NCT03867084) | III | PD-1 | Adjuvant | Q3 2025 | Recruiting |
| Pembrolizumab (lenvatinib) | Placebo | LEAP-002 (NCT03713593) | III | PD-1, tyrosine kinases | First-line | Q2 2022 | Active, not recruiting |
| Pembrolizumab (TACE) | Placebo | PETAL (NCT03397654) | IB | PD-1 | Local control | Q2 2020 | Recruiting |
| Pembrolizumab | Placebo | (NCT03419481) | II | PD-1 | First-line | Q3 2020 | Recruiting |
| Pembrolizumab (elbasvir/grazoprevir) | Placebo | (NCT02940496) | I/II | PD-1, HCV therapy | Second-line | Q4 2021 | Recruiting |
| Pembrolizumab (local therapy) | Placebo | IMMULAB (NCT03753659) | II | PD-1, local therapy | Local control | Q3 2022 | Recruiting |
| Tislelizumab | Placebo | RATIONALE-208 (NCT03419897) | II | PD-1 | Second-line | Q2 2021 | Active, not recruiting |
| Tislelizumab | Sorafenib | RATIONALE-301 (NCT03412773) | III | PD-1 | Second-line | Q2 2021 | Active, not recruiting |
Actual or estimated.
TACE = transarterial chemoembolization; VEGF = vascular endothelial growth factor; DEB-TACE = drug-eluting bead TACE.
Nivolumab
Nivolumab is a fully human immunoglobulin G4 monoclonal antibody that blocks PD-1. It was first tested in a noncomparative prospective phase i/ii study (CheckMate 040, NCT01658878 at https://ClinicalTrials.gov/) in patients who were either treatment-naïve (n = 80) or pretreated with sorafenib (n = 182). The study had two phases: a dose-escalation phase (groups of participants received nivolumab 0.1–10 mg/kg every 2 weeks) and a dose-expansion phase (all participants received nivolumab 3 mg/kg every 2 weeks)14. Inclusion criteria were well-preserved liver function (Child–Pugh score: ≤7), antiviral therapy in case of hbv infection, and histologically confirmed hcc. Primary endpoints were safety, tolerability, and the objective response rate (orr). The orr and the disease control rate (dcr) were 20% and 64% respectively, with 2 patients experiencing a complete response (cr). At 9 months, the os was 94%, and the median os duration was 28.6 months in patients naïve to sorafenib and 15 months in patients already treated with sorafenib. The trial reported an acceptable safety profile and durable responses of 9.9 months in patients who achieved disease control14. Based on those data, nivolumab was approved by the U.S. Food and Drug Administration (fda) in September 2017 for use in hcc after sorafenib treatment28.
Based on the CheckMate 040 trial, the CheckMate 459 phase iii study (see NCT02576509 at https://ClinicalTrials.gov/) compared first-line treatment with nivolumab or with sorafenib in 743 patients with advanced hcc, using os as the primary endpoint. Additional endpoints were the orr and progression-free survival (pfs). Patients were randomized 1:1 to receive intravenous (iv) nivolumab 240 mg every 2 weeks (n = 371) or oral sorafenib 400 mg twice daily (n = 372). Initial results were presented at the congress of the European Society for Medical Oncology in June 201929. The difference in os (16.4 months for nivolumab and 14.7 months for sorafenib) failed to meet statistical significance [hazard ratio (hr): 0.85; 95% confidence interval (ci): 0.72 to 1.02; p = 0.0752]. The orr was 15% for nivolumab and 7% for sorafenib, with 14 (4%) and 5 (1%) patients respectively achieving a cr. Grade 3 or 4 adverse events (aes) were observed in 22% and 49% of the patients respectively15. Although the difference was not statistically significant per the pre-specified protocol, the improvements in os, orr, and the cr rate were deemed clinically meaningful. Long-term survival data at a minimum follow-up of 33.6 months were presented at the 2020 virtual World Congress on Gastrointestinal Cancer of the European Society for Medical Oncology30. The 33-month os rates for nivolumab and sorafenib were 29% (95% ci: 25% to 34%) and 21% (95% ci: 17% to 25%) respectively. The os benefit was more pronounced in patients with chronic hbv and hcv, at 16.1 months compared with 10.4 months (hr: 0.79; 95% ci: 0.59 to 1.07) for hbv and 17.5 months compared with 12.7 months (hr: 0.72; 95% ci: 0.51 to 1.02) for hcv. An important finding was the slower deterioration of liver function with nivolumab therapy as evidenced by albumin–bilirubin levels and Child–Pugh scores.
Nivolumab monotherapy is currently being evaluated in the adjuvant phase iii trial CheckMate 9DX (see NCT03383458 at https://ClinicalTrials.gov/) in patients with hcc who are at high risk of recurrence after curative hepatic resection or ablation. Patients are being randomized 1:1 to receive either iv nivolumab 480 mg every 4 weeks for up to a year, or placebo. The primary endpoint is recurrence-free survival31.
Pembrolizumab
Pembrolizumab is another humanized anti–PD-1 antibody. It was investigated in the phase ii keynote-224 trial (see NCT02702414 at https://ClinicalTrials.com/) as second-line treatment after failure of or intolerance to an initial tki16. The study recruited 104 patients for a dose-limiting toxicity phase and an expansion phase. Inclusion criteria were an Eastern Cooperative Oncology Group 1–2 performance status, previous sorafenib treatment, and well-preserved liver function classed Child–Pugh A. Patients received a fixed dose of pembrolizumab 240 mg every 3 weeks for 2 years or until disease progression. An orr of 18%, with 1 complete response, and a dcr of 61% were recorded. The os was 12.9 months, and treatment-related adverse events occurred in 24% (grade 3) and 1% (grade 4) of patients. Immune-mediated hepatitis was reported in 3 patients, but no viral flares occurred. Most aes were changes in laboratory results. The trial led to the accelerated approval by the fda, in November 2018, of pembrolizumab for patients pretreated with sorafenib32.
Building on the results of the keynote-224 study, the phase iii keynote-240 trial (see NCT02702401 at https://ClinicalTrials.gov/) was initiated17. Altogether, 413 patients were randomized in a 2:1 ratio to pembrolizumab (a 200 mg fixed dose every 3 weeks for up to 35 cycles) or to placebo in this double-blind trial conducted in 27 countries. Median os was 13.9 months for pembrolizumab compared with 10.6 months for placebo (hr: 0.781; 95% ci: 0.611 to 0.998; p = 0.0238), and the pfs was 3.0 months (95% ci: 2.8 months to 4.1 months) compared with 2.8 months (95% ci: 2.5 months to 4.1 months). The orr was 18.3% for pembrolizumab compared with 4.4% in the placebo arm. The cr, progressive disease, and dcr rates were 2.2%, 16.2%, and 62.2% in the treatment arm and 0%, 4.4%, and 53.3% in the placebo arm. Again, no hepatitis flares were recorded. Although pembrolizumab reduced the risk of death by 22%, the trial again failed to meet the pre-specified os endpoint of p = 0.0174, despite demonstrating the same benefit as in the phase ii trial and a clinical benefit of durable responses for patients who achieved a response to treatment. The increasing availability of other approved agents for second-line therapy (resulting in post-study treatment) and an imbalance of macrovascular invasion in the treatment arm might have contributed to a better-than-anticipated os in the placebo arm.
Despite this negative trial, the clinical evaluation of pembrolizumab for the treatment of hcc is ongoing. Recently, early data relating to combined pembrolizumab–lenvatinib were reported. Those results are discussed later in this article, in the Combination Strategies for Immune Therapy section.
An ongoing trial of pembrolizumab monotherapy in the palliative setting is the phase iii keynote-394 trial (see NCT03062358 at https://ClinicalTrials.gov/) using the regimen and inclusion criteria of keynote-240 in Asian patients33.
The keynote-937 trial (NCT03867084) investigates a different oncologic setting, comparing placebo with pembrolizumab (a fixed dose of 200 mg on day 1 of each 21-day cycle for up to 17 cycles) as adjuvant therapy after a complete radiologic response following surgical resection or local ablation34.
A phase ii trial comparing pembrolizumab with placebo in hbv-related hcc (NCT03419481) will focus on changes in the immune environment by comparing serial changes incytokine profiles and tumour-infiltrating lymphocytes in tumour samples.
In another small trial in hcv-associated hcc, pembrolizumab is being combined with antiviral therapy (elbasvir/grazoprevir, NCT02940496).
Tislelizumab
Tislelizumab (BGB-A317) is a humanized immunoglobulin G4 monoclonal antibody with high affinity and binding specificity for PD-1. It is differentiated from the currently approved PD-1 antibodies by an engineered Fc region, which is believed to minimize potentially negative interactions with other immune cells18.
In a dose-finding phase ia/ib study (see NCT02407990 at https://ClinicalTrials.gov/), tislelizumab was evaluated in range of doses, with a 200 mg fixed dose every 3 weeks being chosen for further evaluation. In pretreated patients, the orr and dcr were 12.2% (95% ci: 4.6% to 24.8%) and 51.0% (95% ci: 36.3% to 65.6%) respectively, with the most common treatment-emergent aes being decreased appetite, rash, decreased weight, and cough. In 1 patient, a grade 5 ae of acute hepatitis occurred18.
A global randomized phase iii trial (rationale-301, NCT03412773) comparing tislelizumab with sorafenib in patients with hcc who have no received prior systemic therapy is currently recruiting. The primary endpoint is os35.
Camrelizumab
Camrelizumab (SHR-1210) is a humanized monoclonal anti–PD-1 antibody. A phase ii trial in Chinese patients with hcc given camrelizumab 3 mg/kg every 2 weeks (n = 109) or every 3 weeks (n = 108) reported an orr of 14.7%, an os probability of 74.4% at 6 months, and a median os duration of 13.8 months12.
Further results are discussed in the Combination Strategies for Immune Therapy section.
Atezolizumab
Atezolizumab is a fully humanized immunoglobulin G1 isotype monoclonal antibody against PD-L1.
Within the GO30140 trial (see NCT02715531 at https://ClinicalTrials.gov/), 59 patients who had not previously received systemic therapy were treated with atezolizumab 1200 mg monotherapy every 3 weeks. Median pfs was 3.4 months (95% ci: 1.9 months to 5.2 months), significantly shorter than in the comparator arm of combined atezolizumab–bevacizumab. No grade 3 or 4 treatment-related aes were observed in the monotherapy arm11. Combined atezolizumab–bevacizumab is discussed in the ICI and Anti-VEGF subsection.
Durvalumab
Durvalumab is a fully human immunoglobulin G1κ monoclonal antibody that blocks the interaction of PD-L1 with PD-1.
A phase i/ii trial of durvalumab monotherapy (see NCT01693562 at https://ClinicalTrials.gov/) in 40 patients with hcc mostly pretreated with sorafenib gave iv durvalumab 10 mg/kg every 2 weeks for 12 months or until disease progression. The resulting orr was 10.3%, and median survival was 13.2 months, with grade 3 and 4 events (mostly elevated aspartate aminotransferase and alanine aminotransferase)13.
Tremelimumab
Tremelimumab is a fully human immunoglobulin G2 monoclonal antibody directed against ctla-4. Anti–ctla-4 antibodies outcompete the binding of the CD28 co-stimulatory receptor to CD80 and CD86 with higher avidity, thus releasing a natural “brake” signal for T cell activation36.
Tremelimumab is the first checkpoint inhibitor that was tested in patients with hcc. In a phase ii trial (see NCT01008358 at https://ClinicalTrials.gov/), 21 patients with hcc and hcv were treated with tremelimumab 5 mg/kg on day 1 of every 90-day cycle for up to 4 cycles. Of the 17 assessable patients, 18% experienced a partial response, and the dcr was 76%. Although 45% of patients experienced a grade 3 or higher rise in transaminases after the first dose, that rise was transient and not associated with a decline in liver function. Interestingly, a significant drop in viral load was observed37.
Ipilimumab
Ipilimumab is another fully human monoclonal antibody targeting ctla-419.
Ipilimumab was evaluated in combination with nivolumab for the treatment of hcc. Results are discussed in the Combination Strategies for Immune Therapy section that follows.
Combination Strategies for Immune Therapy
ICIs and TKIs
The combination of icis with targeted agents is expected to exert synergistic effects. In addition to a direct effect of tkis on tumour cells, an indirect contribution affecting immune cells is posited38. Given that several antiangiogenic agents have already demonstrated efficacy in treating hcc, those agents are being evaluated in combination with icis in clinical trials39.
Sorafenib is a multikinase inhibitor of Raf-1 and B-Raf; the vascular endothelial growth factor receptors 1, 2, and 3; and platelet-derived growth factor receptor β40. Since the demonstration of a significant survival benefit in the practice-changing sharp trial (see NCT00105443 at https://ClinicalTrials.gov/), sorafenib is the first approved systemic therapy for advanced hcc4.
Combined nivolumab–sorafenib is currently being tested in a small pilot trial (NCT03439891)41.
Lenvatinib is multikinase inhibitor of vascular endothelial growth factor receptors 1–3, fibroblast growth factor receptors 1–4, platelet-derived growth factor receptor α, ret, and kit. In the phase iii reflect study (NCT01761266), lenvatinib was proved to be noninferior to sorafenib in the first-line treatment of unresectable hcc, being associated with a median survival of 13.6 months (95% ci: 12.1 months to 14.9 months) compared with 12.3 months for sorafenib (95% ci: 10.4 months to 13.9 months; hr: 0.92; 95% ci: 0.79 to 1.06). The orr was statistically significantly improved with lenvatinib treatment (24.1% vs. 9.2% with sorafenib), as was the dcr (75.5% vs. 60.5%)42.
Lenvatinib and pembrolizumab were tested in a phase ib trial (keynote-524/Study 116, NCT03006926) in patients with unresectable hcc who received oral lenvatinib daily (12 mg if ≥60 kg, 8 mg if <60 kg) and iv pembrolizumab (200 mg on day 1 of a 21-day cycle)26. In the 104 patients who were enrolled, median os was 22 months, with an orr of 46.0% (95% ci: 36.0% to 56.3%) and a dcr of 86%. Grade 3 or 4 treatment-related aes occurred in 67% of the patients. On trial, 3 deaths occurred (1 acute respiratory failure, 1 liver insufficiency, and 1 intestinal perforation) that could be attributed to the drugs; bleeding complications were not reported26.
Based on those promising efficacy data, a double-blind randomized phase iii trial of lenvatinib–pembrolizumab compared with lenvatinib alone is ongoing (leap-002, NCT03713593), and the fda has granted breakthrough therapy designation to that combination, although it is not yet approved43.
The anti–PD-1-antibody camrelizumab was tested in combination with apatinib (a selective vascular endothelial growth factor receptor 2 tki). In that small phase ia/ib trial in 18 Chinese patients with advanced hcc and chronic hbv who did or did not have prior sorafenib exposure, an orr of 44% was observed, and median os was not reached (NCT02942329). The treatment was well tolerated, and toxicity was manageable24. A phase ii single-arm open-label trial for patients with advanced hcc for whom sorafenib failed or was intolerable has been initiated (rescue, NCT03463876). The primary endpoint is orr, with secondary endpoints duration of response (dor), dcr, and time to objective response.
The cosmic-312 trial (NCT03755791) is an ongoing phase iii trial of combined atezolizumab–cabozantinib for patients who are therapy-naïve. In 3 arms, the combination is being compared with single-agent sorafenib and single-agent cabozantinib. Primary endpoints are pfs and os.
ICIs and Anti-VEGF Therapy
Hepatocellular carcinoma regularly displays increased vascularity and overexpresses vegf, leading to disease development and progression. In addition, vegf mediates immunosuppression within the tumour and its microenvironment44. Both factors make hcc targetable with anti-vegf therapy45.
Ramucirumab is a fully human monoclonal immunoglobulin G1 antibody against vegf receptor 2. In a small phase ib study (see NCT02572687 at https://ClinicalTrials.gov/) in patients pretreated with sorafenib, ramucirumab in combination with durvalumab was associated with an orr of 11% and a dcr of 61%25.
Bevacizumab is a humanized monoclonal antibody that inhibits the interaction of vegf A with the vegf receptors on the surface of endothelial cells. In a phase ii trial, bevacizumab was evaluated as a single agent in advanced hcc, with 43 patients being treated with bevacizumab 5 mg/kg or 10 mg/kg every 2 weeks46. Treatment toxicity was generally low: 37% patients experienced grades 3–4 toxicities, with 3 cases of hemorrhage. Because of the approval of sorafenib, the study was stopped, and bevacizumab was not evaluated in a phase iii study.
The open-label phase ib study GO30140 (NCT02715531) examined combined iv atezolizumab 1200 mg and iv bevacizumab 15 mg/kg every 3 weeks in patients with unresectable carcinoma not amenable to curative treatment who had received no previous systemic treatment. Because liver cirrhosis is present in most patients with hcc, leading to compromised coagulation, bleeding is of special concern with an anti-vegf therapy. Patients with untreated or incompletely treated high-risk varices were excluded from participating.
Patients were treated in two groups:
■ Group A: all patients received iv atezolizumab 1200 mg and iv bevacizumab 15 mg/kg every 3 weeks
■ Group F: patients were randomly assigned (1:1) to receive iv atezolizumab 1200 mg plus iv bevacizumab 15 mg/kg every 3 weeks or atezolizumab alone
The orr in group A was 36% (95% ci: 26% to 46%), and the pfs in group F (combination treatment) was 5.6 months (95% ci: 3.6 months to 7.4 months).
The most common grade 3 or 4 treatment-related aes were hypertension (13% group A, 5% group F) and proteinuria (7% and 3%). In group A, 3 treatment-related deaths occurred (1 abnormal hepatic function, 1 hepatic cirrhosis, and 1 pneumonitis). Bleeding complications were not increased compared with those observed in previous anti-vegf therapy trials11.
Combined atezolizumab–bevacizumab compared with sorafenib was further evaluated in a 2:1 ratio (n = 336 atezolizumab–bevacizumab, n = 165 sorafenib) in the global phase iii IMbrave150 trial (NCT03434379) in a first-line setting of unresectable hcc. Inclusion criteria were an Eastern Cooperative Oncology Group 0–1 performance status, well-preserved liver function (Child–Pugh score: ≤6), no history of autoimmune disease, and untreated or incompletely treated esophageal or gastric varices. Disease causes were predominantly hbv and hcv, with non-viral causes constituting 30% of the atezolizumab–bevacizumab arm and 32% of the sorafenib arm. Macrovascular invasion was frequent at 38% and 43%, and 82% and 81% were staged as Barcelona Clinic Liver Cancer C22. Median pfs was 6.8 months (95% ci: 5.7 months to 8.3 months) in the combination group and 4.3 months (95% ci: 4.0 months to 5.6 months) in the sorafenib group. The hr for disease progression or death was 0.59 (95% ci: 0.47 to 0.76; p < 0.001). Median os was not reached in the combination arm. Of patients in the atezolizumab–bevacizumab group, 56.5% experienced grade 3 or 4 adverse events, but high-grade toxic effects apart from hypertension were infrequent22. Bleeding complications were observed in 7% of the atezolizumab–bevacizumab group and in 4.5% of the sorafenib group; such events were not a limiting toxicity risk. Combined atezolizumab–bevacizumab is already approved by the fda, and approval by the European Medicines Agency is expected. With that approval, atezolizumab–bevacizumab is expected to become the most widely used first-line therapy in advanced hcc.
ICI and ICI
A couple of trials have explored combination therapy using two icis.
Nivolumab–Ipilimumab
Initial results for combined nivolumab–ipilimumab were reported from the single-arm phase i/ii CheckMate 040 trial (see NCT01658878 at https://ClinicalTrials.gov/)21. Patients previously treated with sorafenib in advanced hcc were randomized to 3 treatment arms:
■ Arm 1: nivolumab 1 mg/kg and ipilimumab 3 mg/kg every 3 weeks (4 doses), followed by nivolumab 240 mg every 2 weeks
■ Arm 2: nivolumab 3 mg/kg and ipilimumab 1 mg/kg every 3 weeks (4 doses), followed by nivolumab 240 mg every 2 weeks
■ Arm 3: nivolumab 3 mg/kg every 2 weeks and ipilimumab 1 mg/kg every 6 weeks
The primary endpoints were safety and tolerability, and secondary endpoints included orr, dor, dcr, and os.
In arm 1, the orr was 31%, with 7 patients reaching a cr; os was 23 months. The combination was well tolerated, with 37% grade 3 or 4 treatment-related aes (mostly pruritus and rash). In 5% of patients, the aes led to treatment discontinuation. The orr was more than double the rate seen with nivolumab monotherapy (31% vs. 14%).
Based on the results of that phase i/ii trial, which demonstrated a median os of 22.8 months in arm 1, combined nivolumab–ipilimumab received accelerated approved by the U.S. fda.
Currently, a phase iii trial of nivolumab (1 mg/kg every 3 weeks) plus ipilimumab (3 mg/kg for 4 doses) compared with the current standard tki agents (sorafenib or lenvatinib) in the first-line setting is recruiting participants (CheckMate 9DW, NCT04039607). The primary endpoint is os, and secondary endpoints are orr, dor, and time to symptom deterioration.
In the United Kingdom, nivolumab–ipilimumab is currently being evaluated in a 2-phase design in patients ineligible for liver transplantation and planned for resection (prime-hcc, NCT03682276). The primary endpoints are delay to surgery, safety, and tolerability; secondary outcomes are orr and pathologic response rate47.
Durvalumab–Tremelimumab
In the phase ii Study 22 trial (NCT02519348), durvalumab and tremelimumab were tested each as monotherapy, and durvalumab was tested in combination with tremelimumab or bevacizumab. Primary outcomes were safety and evaluation of dose-limiting toxicities.
Results were presented at the 2020 American Society of Clinical Oncology Virtual Scientific Program. All arms had an acceptable safety profile. The best os, at 18.7 months, was associated with the combination of a single priming dose of tremelimumab 300 mg combined with durvalumab 1500 mg and continuation of durvalumab 1500 mg every 4 weeks. Grade 3 or 4 aes were seen in 35.1% of patients, and the orr was 24.0%20.
Combination durvalumab–tremelimumab is currently being evaluated in a phase iii trial (himalaya, NCT03298451) as a first-line treatment in patients with advanced hcc. In a 4-arm design, durvalumab monotherapy and combination durvalumab–tremelimumab are being compared with sorafenib treatment. The primary endpoint is os, and secondary endpoints are time to progression, pfs, orr, dcr, and dor48.
ICIs and Local Therapy
The combination of ablative therapies (with their potential to result in shedding of tumour-associated antigens) with immunotherapy (capable of augmenting the immune response) might act synergistically27.
In a phase i/ii trial (NCT01853618), the combination of iv tremelimumab (at 2 dose levels—3.5 mg/kg and 10 mg/kg—every 4 weeks for 6 doses, followed by 3-monthly infusions) with an ablation procedure was explored. On day 35, patients underwent subtotal radiofrequency ablation or chemoablation. For the 19 patients who could be evaluated, median os was 12.3 months (95% ci: 9.3 months to 15.4 months), and an abscopal partial response effect outside the area of local treatment was achieved in 25%27.
The currently running phase ib petal trial (NCT03397654) is testing the use of pembrolizumab after transarterial chemoembolization (tace) in a small single-arm multicentre study, with primary outcomes of safety and tolerability.
The phase ii plthcc trial (NCT04273100) evaluated tace in combination with the anti–PD-1 antibody lenvatinib, with a primary endpoint of orr. An even broader range of local ablation modalities (radiofrequency ablation, microwave ablation, brachytherapy, or tace) is being tested in the phase ii immulab study (NCT03753659), again with orr as the primary outcome measure.
Combined checkpoint inhibition using durvalumab–tremelimumab combined with tace, cryotherapy, or radiofrequency ablation with respect to pfs is being tested in the NCT02821754 phase II trial. Preliminary results showed a 20% orr and a dcr of 60%49.
The phase iii emerald-1 trial (NCT03778957) is examining tace in combination with durvalumab–bevacizumab. The primary endpoint is pfs.
Adjuvant and Neoadjuvant Therapy with ICI
Given the high recurrence rate and lack of therapeutic options with proven benefit in the adjuvant setting after the negative results of a phase iii trial evaluating sorafenib (storm, NCT00692770), adjuvant therapy remains an unmet medical need, and immunotherapy is under active evaluation50,51.
Combination nivolumab–cabozantinib is being evaluated in a phase i trial (NCT03299946) with the primary endpoints being number of aes and number of patients who proceed to surgery52.
In addition, the efficacy and safety of durvalumab alone or in combination with bevacizumab after curative resection or ablation with a high risk of recurrence is being evaluated in the phase iii double-blind placebo-controlled emerald-2 trial (NCT03847428). The primary endpoint is recurrence-free survival.
Another phase iii trial is the IMbrav050 study (NCT04102098) which is testing combination atezolizumab–bevacizumab, with a primary endpoint of pfs.
A perioperative concept using neoadjuvant and adjuvant administration of nivolumab–ipilimumab or placebo in patients with resectable hcc is in phase ii evaluation (NCT03222076). The trial is no longer recruiting; results are awaited.
SUMMARY
Despite an increasing number of approved drugs, systemic therapy of hcc is still a challenge. Because of underlying liver cirrhosis in most cases, treatment has to be adapted to a patient’s liver functional reserve and performance status.
Therapy with the tkis sorafenib, lenvatinib, regorafenib, and cabozantinib has been associated with improved survival in patients with well-preserved liver function, os being more than 24 months with sequential treatment in some cases53. Ramucirumab is a therapeutic option for patients with serum alpha-fetoprotein 400 ng/mL or more, representing a subset of cases with a limited prognosis.
The impressive results of the phase ii trials for nivolumab in the first line14 and pembrolizumab in the second line led to accelerated approval of those drugs by the fda and to high expectations for the results of the phase iii studies. Unfortunately, CheckMate 45915 and the keynote-240 trial17 both failed to reach statistical significance. Each trial demonstrated longer os with ici therapy and durable responses in a number of patients. There could be a couple of reasons for the failure of those trials.
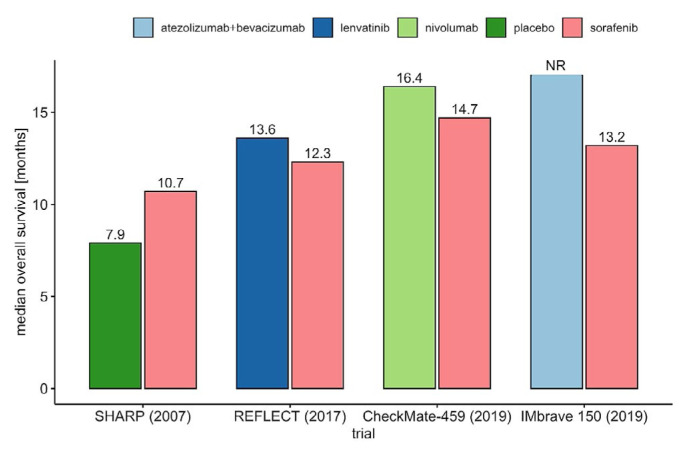
Patients in CheckMate 459 experienced longer os (16.4 months vs. 14.7 months; hr: 0.85). The excellent survival in both arms is probably attributable to the subsequent therapy that patients received (49% for nivolumab and 53% for sorafenib, with 20% of patients treated with sorafenib receiving subsequent immunotherapy), which contributed to the negative result. The effect of improved survival in the comparison arm of clinical trials as more options of subsequent therapies become available is depicted in Figure 2 for first-line trials and Figure 3 for second-line trials.
FIGURE 2.
Positive phase III first-line trials evaluating tyrosine kinase inhibitor therapy and phase III trials evaluating checkpoint immunotherapy. NR = not reached.
FIGURE 3.
Positive phase III second-line trials evaluating tyrosine kinase inhibitor therapy and phase III trials evaluating checkpoint immunotherapy
The statistical design of the keynote-240 trial, with its dual endpoint and 2 interim analyses, required high stringency for a positive outcome. With a 22% decrease in death, a clinically meaningful result was achieved, and the median dor was 13.8 months. Survival in the sorafenib control arm was again very long, at 10.6 months, attributable to the exclusion of macrovascular invasion, better management of patients, and the availability, after the study, of second-line treatments, including immunotherapy, that were not available at study start54. While failing statistical significance, both trials demonstrated clinical benefit.
Although single-agent immunotherapy could not demonstrate superiority to the standard of care, combination atezolizumab–bevacizumab in the IMbrave150 trial was associated with an unprecedented survival benefit, the median os not being reached in the verum arm22. The atezolizumab–bevacizumab combination is expected to become the new standard of care in first-line therapy of hcc. The selection of patients remains crucial to address safety and efficacy concerns: alternative therapies should be preferred in the presence of prior organ transplantation or uncontrolled autoimmune disease, given the risk of organ rejection and high-grade flares. In inflammatory bowel disease (even in a clinically stable clinical situation), the risk for gastrointestinal aes such as high-grade diarrhea and perforations is increased55. And although no excessive bleeding risk was reported in the study, long-term experience will demonstrate the necessary management (such as ligation therapy for esophageal varices and patients at risk for bleeding events).
An important aspect of tki therapy is the management of aes. Diarrhea, hand–foot skin reaction, worsening of liver function, weight loss, and fatigue can severely impair patient quality of life and could lead to a need for dose interruption or modification.
Immunotherapy with icis challenges patients and physicians with a novel spectrum of side effects. Although aes with immunotherapy can be life-threatening, they are usually mild and do not affect quality of life in a serious way. An analysis of health-related quality of life in the CheckMate 459 trial (comparing nivolumab with sorafenib) demonstrated a longer time to first deterioration and time to definitive deterioration. A greater proportion of patients receiving nivolumab did not experience an increased burden of side effects (50%–67.7% vs. 26.8%–45% with sorafenib)56. Immunotherapy has the potential to stabilize or even improve quality of life for patients with hcc and might be beneficial in a sequence with tkis to counteract loss of appetite, weight loss, and fatigue.
The orrs for tki therapies are generally limited, reaching 24.1% with lenvatinib in the reflect trial42. Because of those low response rates, tkis are rarely used in a neoadjuvant setting. The orr was 33% in the IMbrave150 trial and 46% if evaluated by the modified Response Evaluation Criteria in Solid Tumors in the early trial of pembrolizumab with lenvatinib26. An increasingly better tumour response will open new options for patients achieving tumour size reductions, leading to secondary resectability and possibly liver transplantation.
Another important medical need is an effective adjuvant therapy after resection and ablation to reduce the high number of recurrences. The storm trial (NCT00692770) was not able to demonstrate a benefit of adjuvant sorafenib treatment50, but immunotherapy might be effective in that setting while having a limited impact on quality of life during treatment. That concept is currently being evaluated in a number of trials that are using single-agent immunotherapy or combinations with anti-vegf receptor therapy.
The positive results of IMbrave150 for systemic therapy in hcc has added another important weapon in the fight for longer and better survival. But despite the enthusiasm, questions remain. A significant percentage of tumours are not responsive to immunotherapy, and so far, no biomarkers to predict response have been uncovered. An immunologic classification of patients applicable in a real-world setting would help to guide treatment decisions. And to reach optimal survival results, cohort studies and clinical trials will be needed to identify the best therapeutic sequence of the available agents. Despite those challenges, immunotherapy has already become and will continue to be a mainstay of systemic therapy in hcc.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: AW has received lecture fees from Bayer, Leo Pharma, and Eisai, and has received advisory board fees and travel support from Bayer, Bristol Myers Squib, and Wako Diagnostics. PRG has received consulting fees from AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, Eli Lilly, Hoffmann–La Roche, Ipsen Biopharmaceuticals, Merck Sharp and Dohme, and Sirtex Medical.
REFERENCES
- 1.Lai SW. Risk factors for hepatocellular carcinoma. Cancer. 2019;125:482. doi: 10.1002/cncr.31802. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in globocan 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Galle PR, Tovoli F, Foerster F, Wörns MA, Cucchetti A, Bolondi L. The treatment of intermediate stage tumours beyond tace: from surgery to systemic therapy. J Hepatol. 2017;67:173–83. doi: 10.1016/j.jhep.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(suppl 4):iv238–55. doi: 10.1093/annonc/mdy308. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Qin S, Merle P, et al. on behalf of the resorce investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (resorce): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 7.Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (reach-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–96. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 9.Marrero JA, Kudo M, Venook AP, et al. Observational registry of sorafenib use in clinical practice across Child–Pugh subgroups: the gideon study. J Hepatol. 2016;65:1140–7. doi: 10.1016/j.jhep.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Ghavimi S, Apfel T, Azimi H, Persaud A, Pyrsopoulos NT. Management and treatment of hepatocellular carcinoma with immunotherapy: a review of current and future options. J Clin Translat Hepatol. 2020;8:168–76. doi: 10.14218/JCTH.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee MS, Ryoo BY, Hsu CH, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21:808–20. doi: 10.1016/S1470-2045(20)30156-X. [DOI] [PubMed] [Google Scholar]
- 12.Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21:571–80. doi: 10.1016/S1470-2045(20)30011-5. [DOI] [PubMed] [Google Scholar]
- 13.Wainberg ZA, Segal NH, Jaeger D, et al. Safety and clinical activity of durvalumab monotherapy in patients with hepatocellular carcinoma (hcc) [abstract 4071] J Clin Oncol. 2017;35 [Available online at: https://ascopubs.org/doi/10.1200/JCO.2017.35.15_suppl.4071; cited 20 October 2020] [Google Scholar]
- 14.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yau T, Park JW, Finn RS, et al. CheckMate 459: a randomized, multi-center phase iii study of nivolumab (nivo) vs sorafenib (sor) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (ahcc) [abstract LBA38_PR] Ann Oncol. 2019;30(suppl 5):v874–5. doi: 10.1093/annonc/mdz394.029. [DOI] [Google Scholar]
- 16.Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (keynote-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–52. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 17.Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in keynote-240: a randomized, double-blind, phase iii trial. J Clin Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 18.Desai J, Deva S, Lee JS, et al. Phase ia/ib study of single-agent tislelizumab, an investigational anti–PD-1 antibody, in solid tumors. J Immunother Cancer. 2020;8:e000453. doi: 10.1136/jitc-2019-000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolchok JD, Saenger Y. The mechanism of anti–ctla-4 activity and the negative regulation of T-cell activation. Oncologist. 2008;13(suppl 4):2–9. doi: 10.1634/theoncologist.13-S4-2. [DOI] [PubMed] [Google Scholar]
- 20.AstraZeneca. Imfinzi plus tremelimumab demonstrated promising clinical activity and tolerability in patients with advanced liver cancer [online news article] Wilmington, DE: AstraZeneca; 2020. [Available at: https://www.astrazeneca.com/media-centre/press-releases/2020/imfinzi-plus-tremelimumab-demonstrated-promising-clinical-activity-and-tolerability-in-patients-with-advanced-liver-cancer.html; cited 27 September 2020] [Google Scholar]
- 21.Yau T, Kang YK, Kim TY, et al. Nivolumab (nivo) + ipilimumab (ipi) combination therapy in patients (pts) with advanced hepatocellular carcinoma (ahcc): results from CheckMate 040 [abstract 4012] J Clin Oncol. 2019;37 [Available online at: https://ascopubs.org/doi/abs/10.1200/JCO.2019.37.15_suppl.4012; cited 20 October 2020] [Google Scholar]
- 22.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 23.Kudo M, Motomura K, Wada Y, et al. First-line avelumab + axitinib in patients with advanced hepatocellular carcinoma: results from a phase 1b trial (VEGF Liver 100). [abstract 4072] J Clin Oncol. 2019;37 doi: 10.1200/JCO.2019.37.15_suppl.4072. [Available online at: https://meetinglibrary.asco.org/record/173301/abstract; cited 10 November 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu J, Zhang Y, Jia R, et al. Anti–PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res. 2019;25:515–23. doi: 10.1158/1078-0432.CCR-18-2484. [DOI] [PubMed] [Google Scholar]
- 25.Bang YJ, Golan T, Lin CC, et al. Ramucirumab (Ram) and durvalumab (Durva) treatment of metastatic non–small cell lung cancer (nsclc), gastric/gastroesophageal junction (g/gej) adenocarcinoma, and hepatocellular carcinoma (hcc) following progression on systemic treatment(s) [abstract 2528] J Clin Oncol. 2019;37 [Available online at: https://meetinglibrary.asco.org/record/172780/abstract; cited 10 November 2020] [Google Scholar]
- 26.Finn RS, Ikeda M, Zhu AX, et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38:2960–70. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duffy AG, Ulahannan SV, Makorova-Rusher O, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–51. doi: 10.1016/j.jhep.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.United States Department of Health and Human Services Food and Drug Administration. FDA grants accelerated approval to nivolumab for HCC previously treated with sorafenib [Web resource] Silver Spring, MD: Oncology Center of Excellence; 2017. [Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-hcc-previously-treated-sorafenib; cited 22 September 2020] [Google Scholar]
- 29.European Society for Medical Oncology (esmo) Clinical benefit with first-line immunotherapy in advanced hepatocellular carcinoma [online media release] Lugano, Switzerland: EMSO; 2019. [Available at: https://www.esmo.org/newsroom/press-office/esmo-congress-hepatocellular-carcinoma-cancer-checkmate459-yau; cited 22 September 2020] [Google Scholar]
- 30.Sangro B, Park J, Finn R, et al. CheckMate 459: long-term (minimum follow-up 33.6 months) survival outcomes with nivolumab versus sorafenib as first-line treatment in patients with advanced hepatocellular carcinoma [abstract LBA-3] Ann Oncol. 2020;31(suppl 3):S241–2. doi: 10.1016/j.annonc.2020.04.078. [DOI] [Google Scholar]
- 31.Jimenez Exposito MJ, Akce M, Montero Alvarez JL, et al. CA209-9DX: phase iii, randomized, double-blind study of adjuvant nivolumab vs placebo for patients with hepatocellular carcinoma (hcc) at high risk of recurrence after curative resection or ablation [abstract 209TiP] Ann Oncol. 2018;29(suppl 8):viii267–8.. doi: 10.1093/annonc/mdy282.166. [DOI] [Google Scholar]
- 32.United States Department of Health and Human Services Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for hepatocellular carcinoma [Web resource] Silver Spring, MD: Oncology Center of Excellence; 2018. [Available at: https://www.fda.gov/drugs/fda-grants-accelerated-approval-pembrolizumab-hepatocellular-carcinoma ; cited 22 September 2020] [Google Scholar]
- 33.Xu W, Liu K, Chen M, et al. Immunotherapy for hepatocellular carcinoma: recent advances and future perspectives. Ther Adv Med Oncol. 2019;11 doi: 10.1177/1758835919862692. 175883591986269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu A, Kudo M, Vogel A, et al. Phase 3 keynote-937: adjuvant pembrolizumab versus placebo in patients with hepatocellular carcinoma and complete radiologic response after surgical resection or local ablation [abstract CT284] Cancer Res. 2020;80 doi: 10.1158/0008-5472.CAN-19-2388. [Available online at: http://cancerres.aacrjournals.org/lookup/doi/10.1158/1538-7445.AM2020-CT284; cited 22 September 2020] [DOI] [Google Scholar]
- 35.Qin S, Finn RS, Kudo M, et al. rationale 301 study: tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Future Oncol. 2019;15:1811–22. doi: 10.2217/fon-2019-0097. [DOI] [PubMed] [Google Scholar]
- 36.Rudd CE. The reverse stop-signal model for ctla4 function. Nat Rev Immunol. 2008;8:153–60. doi: 10.1038/nri2253. [DOI] [PubMed] [Google Scholar]
- 37.Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of ctla-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81–8. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 38.Hou J, Zhang H, Sun B, Karin M. The immunobiology of hepatocellular carcinoma in humans and mice: basic concepts and therapeutic implications. J Hepatol. 2020;72:167–82. doi: 10.1016/j.jhep.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 39.Seliger B. Combinatorial approaches with checkpoint inhibitors to enhance antitumour immunity. Front Immunol. 2019;10:999. doi: 10.3389/fimmu.2019.00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilhelm SM, Carter C, Tang LY, et al. BAY 43-9006 exhibits broad spectrum oral antitumour activity and targets the Raf/mek/erk pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 41.Keenan B, Griffith MJ, Bauer K, et al. Phase ii multicenter pilot study of safety, efficacy, and immune cell profiling in advanced hepatocellular carcinoma (hcc) on combination of sorafenib (sor) plus nivolumab (nivo) [abstract TPS464] J Clin Oncol. 2019;37 doi: 10.1200/JCO.2019.37.15_suppl.6529. [Available online at: https://ascopubs.org/doi/abs/10.1200/JCO.2019.37.4_suppl.TPS464; cited 20 October 2020] [DOI] [Google Scholar]
- 42.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–73. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 43.Global Eisai. Eisai and Merck receive complete response letter for Lenvima (lenvatinib) plus Keytruda (pembrolizumab) combination as first-line treatment for unresectable hepatocellular carcinoma [online medial release] Tokyo, Japan: Eisai Global; 2020. [Available at: https://www.eisai.com/news/2020/news202039.html; cited 22 September 2020] [Google Scholar]
- 44.Voron T, Colussi O, Marcheteau E, et al. vegf-A modulates expression of inhibitory checkpoints on CD8++ T cells in tumors. J Exp Med. 2015;212:139–48. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morse MA, Sun W, Kim R, et al. The role of angiogenesis in hepatocellular carcinoma. Clin Cancer Res. 2019;25:912–20. doi: 10.1158/1078-0432.CCR-18-1254. [DOI] [PubMed] [Google Scholar]
- 46.Boige V, Malka D, Bourredjem A, et al. Efficacy, safety, and biomarkers of single agent bevacizumab therapy in patients with advanced hepatocellular carcinoma. Oncologist. 2012;17:1063–72. doi: 10.1634/theoncologist.2011-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okusaka T, Ikeda M. Immunotherapy for hepatocellular carcinoma: current status and future perspectives. ESMO Open. 2018;3(suppl 1):e000455. doi: 10.1136/esmoopen-2018-000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghassan K, Abou-Alfa GK, Chan SL, et al. A randomized, multicenter phase 3 study of durvalumab (d) and tremelimumab (t) as first-line treatment in patients with unresectable hepatocellular carcinoma (hcc): himalaya study [abstract TP4144] J Clin Oncol. 2018;36 [Available online at: https://ascopubs.org/doi/abs/10.1200/JCO.2018.36.15_suppl.TPS4144; cited 20 October 2020] [Google Scholar]
- 49.Charalampos S, Floudas CS, Xie C, et al. Combined immune checkpoint inhibition (ici) with tremelimumab and durvalumab in patients with advanced hepatocellular carcinoma (hcc) or biliary tract carcinomas (btc) [abstract 336] J Clin Oncol. 2019;37 [Available online at: https://ascopubs.org/doi/abs/10.1200/JCO.2019.37.4_suppl.336 ; cited 20 October 2020] [Google Scholar]
- 50.Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (storm): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–54. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 51.Brown ZJ, Greten TF, Heinrich B. Adjuvant treatment of hepatocellular carcinoma: prospect of immunotherapy. Hepatology. 2019;70:1437–42. doi: 10.1002/hep.30633. [DOI] [PubMed] [Google Scholar]
- 52.Popovic A, Sugar E, Ferguson A, et al. Feasibility of neoadjuvant cabozantinib plus nivolumab followed by definitive resection for patients with locally advanced hepatocellular carcinoma: a phase ib trial ( NCT03299946) [abstract CT207] Cancer Res. 2019;79(suppl) [Available online at: https://cancerres.aacrjournals.org/content/79/13_Supplement/CT207; cited 8 October 2020] [Google Scholar]
- 53.Alsina A, Kudo M, Vogel A, et al. Effects of subsequent systemic anticancer medication following first-line lenvatinib: a post hoc responder analysis from the phase 3 reflect study in unresectable hepatocellular carcinoma. Liver Cancer. 2020;9:93–104. doi: 10.1159/000504624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ternyila D. Expert discusses impact of survival outcomes in KEYNOTE-240 trial for advanced HCC [online new article] Plainsboro, NJ: Targeted Oncology; 2019. Available at: https://www.targetedonc.com/view/expert-discusses-impact-of-survival-outcomes-in-keynote240-trial-for-advanced-hcc ; cited 22 September 2020] [Google Scholar]
- 55.Pinter M, Scheiner B, Peck-Radosavljevic M. Immunotherapy for advanced hepatocellular carcinoma: a focus on special subgroups. Gut. 2020 doi: 10.1136/gutjnl-2020-321702. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Edeline J, Yau T, Park JW, et al. CheckMate 459: health-related quality of life (hrqol) in a randomized, multicenter phase iii study of nivolumab (nivo) versus sorafenib (sor) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (ahcc) [abstract 483] J Clin Oncol. 2020;38 [Available online at: https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.4_suppl.483; cited 20 October 2020] [Google Scholar]