Abstract
Aim
Nutrition affects the growth and neurodevelopmental outcomes of preterm infants, yet controversies exist about the optimal enteral feeding regime. The objective of this study was to compare enteral feeding guidelines in Canadian neonatal intensive care units (NICUs).
Method
The research team identified key enteral feeding practices of interest. Canadian Neonatal Network site investigators at 30 Level 3 NICUs were contacted to obtain a copy of their 2016 to 2017 feeding guidelines for infants who weighed less than 1,500 g at birth. Each guideline was reviewed to compare recommendations around the selected feeding practices.
Results
Five of the 30 NICUs did not have a feeding guideline. The other 25 NICUs used 22 different enteral feeding guidelines. The guidelines in 40% of those NICUs recommend commencing minimal enteral nutrition (MEN) within 24 hours of birth and maintaining that same feeding volume for 24 to 96 hours. In 40% of NICUs, the guideline recommended that MEN be initiated at a volume of 5 to 10 mL/kg/day for infants born at <1,000 g. Guidelines in all 25 NICUs recommend the use of bovine-based human milk fortifier (HMF), and in 56% of NICUs, it is recommended that HMF be initiated at a total fluid intake of 100 mL/kg/day. Guidelines in only 16% of NICUs recommended routine gastric residual checks. Donor milk and probiotics are used in 76% and 72% of the 25 NICUs, respectively.
Conclusion
This study revealed substantial variability in recommended feeding practices for very low birth weight infants, underscoring the need to establish a national feeding guideline for this vulnerable group.
Keywords: Enteral feeding, Human milk fortifier, Preterm, Very low birth weight
Nutritional management is an integral component of care in the neonatal intensive care unit (NICU). During critical periods of early life, it can positively influence growth parameters and reduce the incidence of neonatal morbidity in very low birth weight (VLBW) infants (birth weight [BW] <1,500 g) (1). Enteral feeding is the preferred route of nutrition delivery because it stimulates the development of gastrointestinal mucosa, intestinal motility, enzyme synthesis, and hormonal release and decreases the risk of sepsis related to bacterial translocation (2). Current evidence supports standardization of feeding guidelines to increase timely achievement of nutritional milestones, improve growth and shorten length of hospitalization, and to decrease the risk of mortality, necrotising enterocolitis (NEC), late-onset sepsis, and chronic lung disease in vulnerable newborns (i.e., those born prematurely and/or with a low BW) (3–8). However, initiation and advancement of enteral feeding in these infants remains a challenge because of acute illnesses in the early neonatal period and functional immaturity of the gastrointestinal system, which can lead to feeding intolerance (9).
Due to existing studies’ small sample sizes and heterogeneous populations and methodologies, there remains a lack of consensus on the optimal feeding strategies for preterm infants, especially in terms of volume and fortification. This is reflected in studies that have demonstrated significant inter-NICU variation in feeding practices and growth outcomes internationally (10–12).
Establishing a standardized nutritional management guideline across Canada that integrates experiences from multiple NICUs will enable effective evaluation of nutritional management strategies in large cohorts, thus providing the potential to improve overall outcomes. As a first step towards this goal, the objective of this study was to examine and compare enteral feeding guidelines for VLBW infants in all of Canada’s Level 3 NICUs, which provide the highest level of medical care for neonates. The findings will set the stage for future knowledge translation activities to establish a national feeding guideline for VLBW neonates.
METHODS
Several members of the research team (PSS, KD) identified key enteral feeding practices of interest, which included i) whether recommendations for minimal enteral nutrition (MEN) initiation and advancement were stratified by BW; ii) MEN initiation volumes, timing, and duration; iii) daily feeding advancement rates; iv) human milk fortifier (HMF) type and amount and timing of initiation; v) use of protein supplement in addition to HMF, vi) use of routine gastric residual checks (i.e., prior to every enteral feeding); vii) policy on withholding feeds during pharmacological patent ductus closure; viii) use of donor milk; ix) use of probiotics; and x) contraindications to enteral feeds.
In March 2016, an e-mail was sent to the Canadian Neonatal Network (CNN) site investigators in all 30 of the country’s Level 3 NICUs to request a copy of their current enteral feeding guideline for VLBW infants. Each guideline was reviewed to abstract information on the feeding practices of interest into an Excel file. The data that were abstracted for each site were then forwarded to the relevant CNN site investigator, who was asked to verify the content. If a guideline did not contain any recommendations on the use of donor milk and/or probiotics, the CNN site investigators were asked during the data verification process to indicate whether those were used in their NICUs.
All analyses were done in Microsoft Excel. Most comparisons were performed directly on the abstracted data (i.e., no calculations were involved). In order to gain a better understanding of how the different enteral feeding guidelines might affect feeding progression, however, the recommendations on MEN initiation, duration and progression were used to estimate the median time required for an infant born at 800, 1,100, and 1,400 g to reach 120 mL/kg/day at each site, the volume at which central lines and TPN are ordinarily discontinued.
The study was approved by the Queen’s University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board.
RESULTS
Responses were received from the CNN site investigators at all 30 Level 3 NICUs. Five units (17%) did not have a feeding guideline. The remaining 25 NICUs used 22 different feeding guidelines for VLBW infants. Unless otherwise indicated, all subsequent numbers and percentages pertain to these 25 sites.
The guidelines in only one NICU (4%) recommend initiating and advancing MEN feeds in the same manner for all VLBW infants. In the other NICUs, the recommendations are stratified by BW categories, the latter of which ranged in number from two (n=4 [16%]) to six (n=5 [20%]). Stratification by three BW categories was most common (n=8 [32%]).
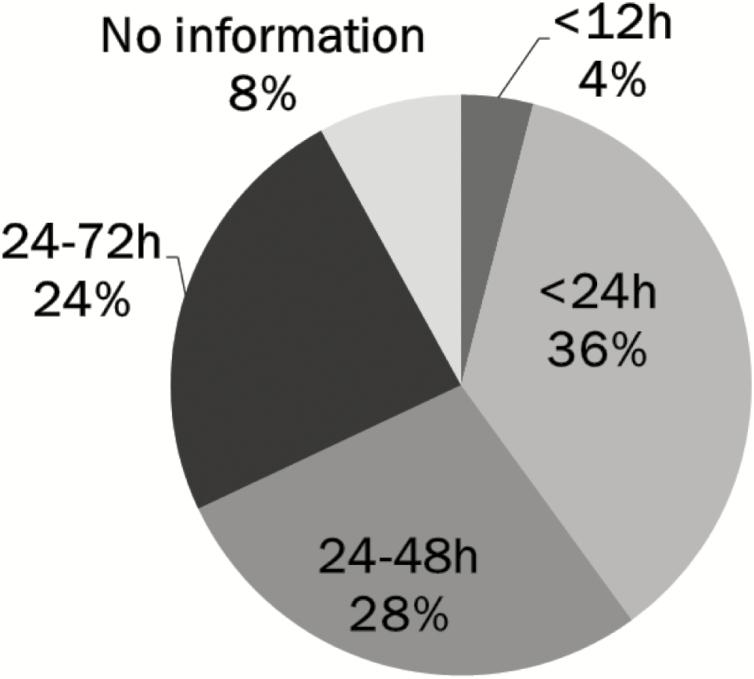
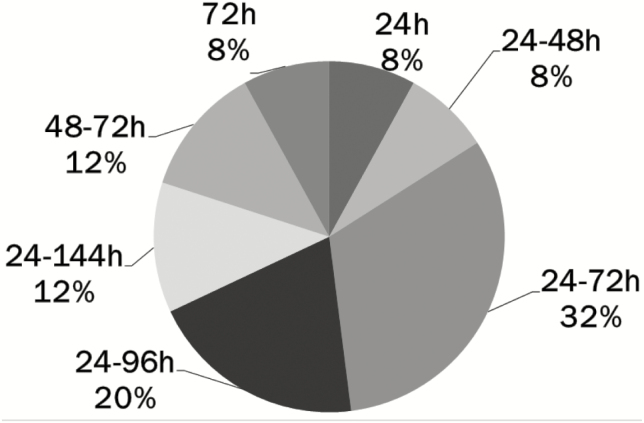
The guidelines in NICUs across the country have variable recommendations for MEN volumes based on different sets of weight stratification, ranging from as low as 3 to 5 mL/kg/day to as high as 20 mL/kg/day. The recommended age at initiation of MEN varies from <12 hours to 24 to 72 hours of age, with <24 hours being most common (n=9 [36%]; see Figure 1). Similarly, the recommended duration of MEN prior to feeding advancement also varies (Figure 2), with the guidelines in two NICUs (8%) recommending that MEN be maintained for 24 hours or less. By comparison, in slightly over half the units (n=13 [52%]) the recommended duration ranges from 24 to 96 hours.
Figure 1.
Age at initiation of minimal enteral nutrition in feeding guidelines for very low birth weight infants in 25 Canadian NICUsa. aIncludes all Canadian level 3 NICUs with a feeding guideline for very low birth weight infants. MEN Minimal enteral nutrition; h Hours.
Figure 2.
Duration of minimal enteral nutrition prior to advancement of feeds in feeding guidelines for very low birth weight infants in 25 Canadian NICUsa. aIncludes all Canadian level 3 NICUs with a feeding guideline for very low birth weight infants. MEN Minimal enteral nutrition; h Hours.
The estimated median time for 800, 1,100, and 1,400 g infant to reach 120 mL/kg/day was, respectively, 9 days (range 3 to 14 days, interquartile range [IQR] 5 days), 7 days (range 3 to 11 days, IQR 2 days), and 6 days (range 3 to 8 days, IQR 1 day).
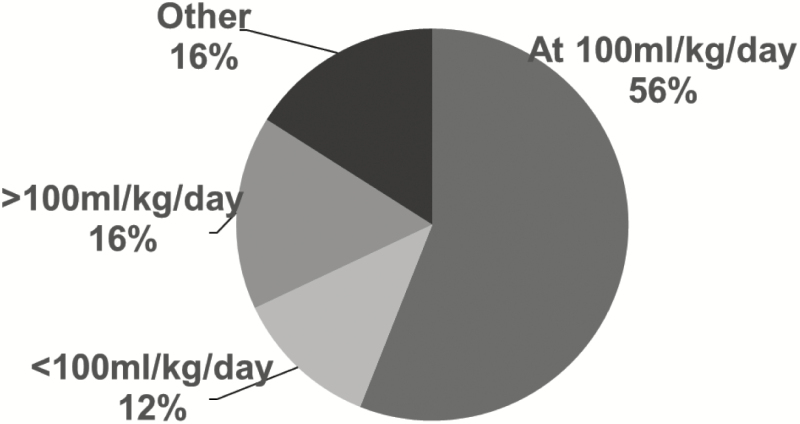
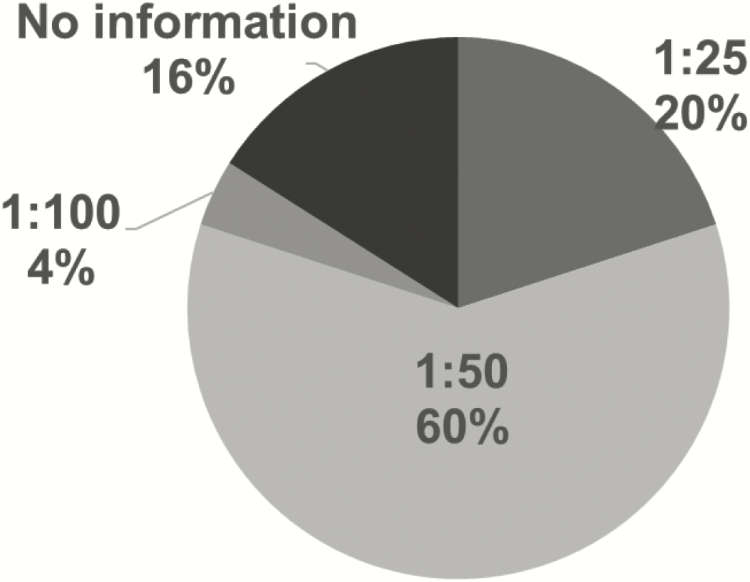
All the guidelines recommend bovine-based HMF. However, the timing of use varies: in 14 NICUs (56%) the guidelines recommend initiating HMF at a total feeding intake of 100 mL/kg/day (Figure 3) and in 15 NICUs (60%) at one package of HMF in 50 mL feeds (Figure 4). Guidelines in 16 NICUs (64%) recommend the use of routine protein supplementation in addition to HMF.
Figure 3.
Volume of feeds at time of human milk fortifier initiation in feeding guidelines for very low birth weight infants in 25 Canadian NICUsa. aIncludes all Canadian level 3 NICUs with a feeding guideline for very low birth weight infants. HMF Human milk fortifier.
Figure 4.
Volumea of human milk fortifier at initiation in feeding guidelines for very low birth weight infants at 25 Canadian NICUsb. aVolume is HMF volume:feed volume. bIncludes all Canadian Level 3 NICUs with a feeding guideline for very low birth weight infants. HMF Human milk fortifier.
Routine gastric residual checks are recommended in only about one-quarter of NICUs (n=7 [28%]). Guidelines in only 3 (12%) recommend holding enteral feeds during pharmacological patent ductus arteriosus closure, but in 13 units (52%) feeding advancement is not recommended during this process. A large majority of units offer donor milk (n=19 [76%]) and order probiotics for all infants <1,500 g (n=18 [72%]), with a large variability in inclusion criteria for the use of both (data not shown). For example, the gestational age at which probiotics are used ranges from < 31 weeks to as high as < 35 weeks with no mention of BW in some units. Those that mention their use by BW all report < 1,500 g as the inclusion criterion. Twelve units (48%) use both donor milk and probiotics. Guidelines in three-quarters of NICUs (n=19 [76%]) consider hemodynamic instability of any cause, suspected NEC, and surgical abdomen as absolute contraindications to enteral feeds. In three NICUs (12%), birth asphyxia is an additional contraindication and in four NICUs (16%), the guidelines recommend holding feeds during blood transfusions. Guidelines in six NICUs (24%) do not list any contraindications to enteral feeds.
DISCUSSION
This study highlights the diversity of recommended feeding practices in Level 3 NICUs across Canada. Previous studies looking at international and US data also reported significant variation in feeding practice, although a 2013 survey showed this was not the case in South Africa (10–12). An international survey published in 2012 by Klingenberg et al. (11) revealed that MEN initiation occurred within 24 hours of life in 35%, 43%, and 71% of tertiary NICUs for infants <25, 25 to 27, and 28 to 31 weeks’ gestational age, respectively. Twenty-nine of the 124 responding units in the survey were Canadian and only 17%, 28%, and 65% initiated MEN within 24 hours of life for the three gestational age groups listed above (11). Our data show that in Canadian Level 3 NICUs since 2012 there has been a move towards early feeding initiation: current guidelines in nine NICUs (36%) recommend starting MEN within 24 hours of life for infants born at <1,000 g, as opposed to the 17% and 28% reported for infants of <25 and 25 to 27 weeks’ gestational age, respectively, in the 2012 survey. However, there remains wide variability in the recommended timing, volume, and number of BW categories used for initiating MEN. In addition, recommended differences in the duration of MEN prior to increasing feeds continue to exist despite evidence from a Cochrane review that showed there is no increased risk of NEC or mortality with the introduction of feeding volumes higher than 10 to 20 mL/kg/day at 1 to 2 days compared to progressive enteral feeding defined as advancing above 24 mL/kg/day initiated at 5 days of age or later (13).
Because of the nutritional demands of the preterm infant, advancement of feeds is an important aspect of the feeding strategy in NICUs, with the goal being to simulate third trimester intrauterine growth velocity. A published guideline from one Canadian NICU recommends increasing nutritional feeds in infants with BW of <1,000 g by 15 to 20 mL/kg/day (9). However, a Cochrane review compared daily feeding intake increments of 15 to 20 mL/kg/day versus 30 to 35 mL/kg/day and concluded that the more rapid advancement did not increase the risk of NEC, mortality, or interruption of feeds (14). Our study indicated that while the feeding advancement rate is stratified by BW (like MEN volume), guidelines in most NICUs recommend similar rates for all infants with BW <1,000 g. The estimated minimum time to reach 120 mL/kg/day for an 800 g infant in NICUs across Canada is quite variable, with a median of 9 days (IQR 5.5 days). However, the ‘minimum time to reach 120 mL/kg/day’ in our study is based on an infant with no contraindications to starting or advancing feeds, which is often not the clinical reality. While a Cochrane review did not find any trophic feeding related adverse effects in the context of birth asphyxia, respiratory distress, sepsis, hypotension, glucose disturbances, ventilation, or umbilical lines, the guidelines in three-quarters of Canadian Level 3 NICUs recommend withholding feeds in cases of hemodynamic instability and suspected NEC or surgical abdomen (15).
HMF adds protein content to nutritional feeds to meet the high protein and energy demands of rapidly growing preterm infants. Often clinicians are reluctant to fortify feeds early, as it increases the osmolality of human milk and is thought to delay gastric emptying (16,17). However, despite increases in gastric residuals, with the addition of HMF, there is no difference in the number of hours feeding was withheld or in the time to reach full feeds as compared with babies who had not had HMF added (17). Two studies which compared early versus delayed introduction of HMF, one a prospective randomized controlled trial (at 40 mL/kg/day versus 100 mL/kg/day) (18) and the other a retrospective study (at first feed versus 50 to 100 mL/kg/day) (19), showed no difference in either adverse outcomes such as NEC or desired outcomes such as weight gain. The international survey by Klingenberg et al. (11) found large variations in the timing of initiation and volume of HMF at initiation, with about half of all NICUs only starting HMF once feeds reached 150 mL/kg/day. Similarly, we identified significant variation in both recommended timing of initiation and recommended volume of HMF at initiation within Canada.
There is a lack of evidence showing that gastric residual volume (GRV) is a predictor of NEC (20). In addition, GRV did not predict the ability to reach full feeds (21), and routine checking of GRV only led to delayed target feeding volumes (22). Our study shows that guidelines in most Canadian NICUs no longer recommend this practice.
Both prospective (23) and retrospective (24) studies have demonstrated that feeding during pharmacological closure of PDA is not associated with adverse outcomes, including time to reach full feeds, NEC incidence, or gastric residuals. Our results indicate that most Canadian units (88%) no longer hold feeds during pharmacological closure of PDA, but 52% hold further advancement of feeds.
Use of human donor milk is preferred over preterm formula because of the former’s association with a reduced incidence of NEC (25,26). While the majority of Canadian NICUs report using donor milk, there is still room for improvement given that approximately 25% of units in the study did not have donor milk available. In addition to donor milk, both a 2014 Cochrane review and a Canadian study (27,28) supported the use of multiple strain probiotics for the prevention of NEC in preterm infants, results which were echoed in a recent meta-analysis (29). It is hoped that this accumulating evidence will further increase the use of probiotics in Canadian NICUs.
One limitation of this study is the lack of information on how closely health care providers in the NICU adhere to their site’s feeding guidelines. This information could possibly have been captured by surveying individual providers working within each NICU. However, the response rate for any such survey would likely have been far less than 100%. We also did not evaluate whether the different feeding guidelines are associated with infant growth and outcomes such as NEC, the incidence of which is known to vary across Canadian NICUs (30).
CONCLUSION
Our study reveals a marked lack of consistency in recommended enteral feeding practices across Canadian Level 3 NICUs. Most NICUs have developed their own guidelines, while five units do not have any feeding guideline. These findings underscore the need to create a national evidence-based nutrition management guideline for VLBW infants, which would include elements such as the volume and duration of MEN prior to the advancement of feeds, the speed of advancement, the timing of HMF introduction and inclusion criteria for donor milk and probiotics. It is hoped that by using CNN outcome data and individual unit practices as identified through CNN’s Evidence-based Practice for Improving Outcomes (EPIQ) project (31), national guidelines based on best practice to optimize patient outcomes will be standardized with the aim of standardizing feeding protocols and optimizing patient outcomes.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge all site investigators of the Canadian Neonatal Network (CNN) for their participation in this survey. We thank the staff at the Maternal-Infant Care (MiCare) Research Centre at Mount Sinai Hospital, Toronto, ON for organizational support of CNN. In addition, we thank Sarah Hutchinson, PhD, from MiCare for editorial assistance in the preparation of this manuscript. MiCare is supported by a team grant from the Canadian Institutes of Health Research (CTP 87518), the Ontario Ministry of Health, and support from participating hospitals.
Funding: There are no funders to report for this submission.
Potential Conflicts of Interest: All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Stefanescu BM, Gillam-Krakauer M, Stefanescu AR, Markham M, Kosinski JL. Very low birth weight infant care: Adherence to a new nutrition protocol improves growth outcomes and reduces infectious risk. Early Hum Dev 2016;94:25–30. [DOI] [PubMed] [Google Scholar]
- 2. Adamkin AH, Radmacher PG, Lewis S, Fanaroff AA, Wilson-Costello D, Kliegman RM. Nutrition and selected disorders of the gastrointestinal tract. In: Fanaroff AA, Klaus MH Klaus and Fanaroff’s Care of the High-Risk Neonate, 6th Edition. 6th ed. Philadelphia: Elsevier Saunders, 2013. [Google Scholar]
- 3. Gephart SM, Hanson C, Wetzel CM, et al. NEC-zero recommendations from scoping review of evidence to prevent and foster timely recognition of necrotizing enterocolitis. Matern Health Neonatol Perinatol 2017;3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jadcherla SR, Dail J, Malkar MB, McClead R, Kelleher K, Nelin L. Impact of process optimization and quality improvement measures on neonatal feeding outcomes at an all-referral neonatal intensive care unit. JPEN J Parenter Enteral Nutr 2016;40(5):646–55. [DOI] [PubMed] [Google Scholar]
- 5. Kish MZ. Improving preterm infant outcomes: Implementing an evidence-based oral feeding advancement protocol in the neonatal intensive care unit. Adv Neonatal Care 2014;14(5):346–53. [DOI] [PubMed] [Google Scholar]
- 6. McCallie KR, Lee HC, Mayer O, Cohen RS, Hintz SR, Rhine WD. Improved outcomes with a standardized feeding protocol for very low birth weight infants. J Perinatol 2011;31 (Suppl 1):S61–7. [DOI] [PubMed] [Google Scholar]
- 7. Patole SK, de Klerk N. Impact of standardised feeding regimens on incidence of neonatal necrotising enterocolitis: A systematic review and meta-analysis of observational studies. Arch Dis Child Fetal Neonatal Ed 2005;90:F147–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rønnestad A, Abrahamsen TG, Medbø S, et al. Late-onset septicemia in a Norwegian national cohort of extremely premature infants receiving very early full human milk feeding. Pediatrics 2005;115(3):e269–76. [DOI] [PubMed] [Google Scholar]
- 9. Dutta S, Singh B, Chessell L, et al. Guidelines for feeding very low birth weight infants. Nutrients 2015;7(1):423–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blackwell MT, Eichenwald EC, McAlmon K et al. Interneonatal intensive care unit variation in growth rates and feeding practices in healthy moderately premature infants. J Perinatol 2005;25(7):478–85. [DOI] [PubMed] [Google Scholar]
- 11. Klingenberg C, Embleton ND, Jacobs SE, O’Connell LA, Kuschel CA. Enteral feeding practices in very preterm infants: An international survey. Arch Dis Child Fetal Neonatal Ed 2012;97:F56–61. [DOI] [PubMed] [Google Scholar]
- 12. Raban MS, Joolay Y, Horn AR, Harrison MC. Enteral feeding practices in preterm infants in South Africa. SAJCH 2013;7:8–12. [Google Scholar]
- 13. Morgan J, Young L, McGuire W. Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev 2014;(12). Art. No.: CD001970. doi:10.1002/14651858.CD001970.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oddie SJ, Young L, McGuire W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev 2017;8(8):CD001241. doi:10.1002/14651858.CD001241.pub7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morgan J, Bombell S, McGuire W. Early trophic feeding versus enteral fasting for very preterm or very low birth weight infants. Cochrane Database Syst Rev 2013;(3). Art. No.: CD000504. doi:10.1002/14651858.CD000504.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ewer AK, Yu VY. Gastric emptying in pre-term infants: The effect of breast milk fortifier. Acta Paediatr 1996;85(9):1112–5. [DOI] [PubMed] [Google Scholar]
- 17. Moody GJ, Schanler RJ, Lau C, Shulman RJ. Feeding tolerance in premature infants fed fortified human milk. J Pediatr Gastroenterol Nutr 2000;30(4):408–12. [DOI] [PubMed] [Google Scholar]
- 18. Sullivan S, Schanler RJ, Kim JH, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr 2010;156(4):562–7.e1. [DOI] [PubMed] [Google Scholar]
- 19. Tillman S, Brandon DH, Silva SG. Evaluation of human milk fortification from the time of the first feeding: Effects on infants of less than 31 weeks gestational age. J Perinatol 2012;32(7):525–31. [DOI] [PubMed] [Google Scholar]
- 20. Bertino E, Giuliani F, Prandi G, Coscia A, Martano C, Fabris C. Necrotizing enterocolitis: Risk factor analysis and role of gastric residuals in very low birth weight infants. J Pediatr Gastroenterol Nutr 2009;48(4):437–42. [DOI] [PubMed] [Google Scholar]
- 21. Shulman RJ, Ou CN, Smith EO. Evaluation of potential factors predicting attainment of full gavage feedings in preterm infants. Neonatology 2011;99(1):38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Torrazza RM, Parker LA, Li Y, Talaga E, Shuster J, Neu J. The value of routine evaluation of gastric residuals in very low birth weight infants. J Perinatol 2015;35(1):57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clyman R, Wickremasinghe A, Jhaveri N, et al. ; Ductus Arteriosus Feed or Fast with Indomethacin or Ibuprofen (DAFFII) Investigators Enteral feeding during indomethacin and ibuprofen treatment of a patent ductus arteriosus. J Pediatr 2013;163(2):406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bellander M, Ley D, Polberger S, Hellström-Westas L. Tolerance to early human milk feeding is not compromised by indomethacin in preterm infants with persistent ductus arteriosus. Acta Paediatr 2003;92(9):1074–8. [DOI] [PubMed] [Google Scholar]
- 25. Cacho NT, Parker LA, Neu J. Necrotizing enterocolitis and human milk feeding: A systematic review. Clin Perinatol 2017;44(1):49–67. [DOI] [PubMed] [Google Scholar]
- 26. O’Connor DL, Gibbins S, Kiss A, et al. ; GTA DoMINO Feeding Group Effect of supplemental donor human milk compared with preterm formula on neurodevelopment of very low-birth-weight infants at 18 months: A randomized clinical trial. JAMA 2016;316(18):1897–905. [DOI] [PubMed] [Google Scholar]
- 27. AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Evid Based Child Health 2014;9(3):584–671. [DOI] [PubMed] [Google Scholar]
- 28. Janvier A, Malo J, Barrington KJ. Cohort study of probiotics in a North American neonatal intensive care unit. J Pediatr 2014;164(5):980–5. [DOI] [PubMed] [Google Scholar]
- 29. Chang HY, Chen JH, Chang JH, Lin HC, Lin CY, Peng CC. Multiple strains probiotics appear to be the most effective probiotics in the prevention of necrotizing enterocolitis and mortality: An updated meta-analysis. Plos One 2017;12(2):e0171579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shah PS, Yoon EW, Chan P, Members of the Annual Report Review Committee The Canadian Neonatal Network 2016 Annual Report [Internet]. Canadian Neonatal Network; [cited October 9, 2018]. <http://www.canadianneonatalnetwork.org/Portal/LinkClick.aspx?fileticket=PJSDwNECsMI%3d&tabid=39> [Google Scholar]
- 31. Shah PS, Dunn M, Aziz K, et al. Sustained quality improvement in outcomes of preterm neonates with a gestational age less than 29 weeks: Results from the evidence-based practice for improving quality phase 3 1. Can J Physiol Pharmacol 2019;97(3):213–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.