Abstract
Luteolin is a natural flavonoid possessing certain beneficial pharmacological properties, including anti-oxidant, anti-inflammatory, anti-microbial and anti-cancer properties. The majority of types of gastric cancer with chronic gastritis are caused by infection with Helicobacter pylori (H. pylori). The present study evaluated the effect of luteolin on a number of selected factors that are potentially involved in gastric cancer development. The study was performed using gastric cancer CRL-1739 cells treated with 30 µM luteolin and H. pylori alone or combined. ELISA and reverse transcription PCR were used to assess the expression levels of MUC1, GalNAcα-R (Tn antigen) and NeuAcα2-3Galβ1-3GalNAc-R (sT antigen), ADAM-17, IL-8, IL-10 and NF-κB. H. pylori and luteolin independently and in combination significantly reduced the expression levels of the extracellular domain of MUC1 in gastric cancer cells compared with the untreated control cells. ADAM-17 expression was reduced by treatment with the pathogen and luteolin. Additionally, both factors reduced sT antigen expression. Treatment with 30 ≤M luteolin significantly induced IL-8 expression at the mRNA and protein level, and the mRNA expression levels of IL-10 and NF-κB compared with the control. Both H. pylori and luteolin induced IL-8 protein expression. The present preliminary results suggest that luteolin may be used to treat patients with gastric cancer.
Keywords: gastric cancer, Helicobacter pylori, interleukin, luteolin, MUC1, NF-κB
Introduction
Luteolin (3',4',5,7-tetrahydroxyflavone; Fig. 1), is a natural, dietary flavonoid commonly present at high concentrations in several types of fruits, vegetables and medicinal herbs (1,2). Flavonoids are polyphenols that serve an important role in defending plant cells against microorganisms, insects and UV irradiation (3). A number of previous studies have demonstrated that luteolin possesses beneficial pharmacological properties, including anti-oxidant, anti-inflammatory, anti-microbial and anti-cancer properties (4,5). Some of these properties could be functionally associated with each other. For example, the anti-inflammatory effects of luteolin may also be associated to its anti-cancer properties (6).
Figure 1.
Structure of luteolin.
Infection with Helicobacter pylori (H. pylori), a bacterial carcinogen, is the greatest risk factor for development of gastric cancer, which is the fifth most common malignant disease and the third most common cause of cancer-associated mortality worldwide (7). It has been reported that ~75% of global cases of gastric cancer and 5.5% of malignancies worldwide may be attributed to H. pylori-induced inflammation and injury (8,9). However, the mechanisms that regulate cancer development in response to this organism are not well defined.
Altered glycosylation is considered to be one of the most common characteristic features of cancer, and is the most common post-translational modification of proteins occurring during neoplastic transformation (10). MUC1 mucin, transmembrane glycoprotein is expressed on the apical surfaces of most epithelia, including the stomach, and is the primary O-glycosylated protein of epithelial tissues. Generally, MUC1 is composed of two subunits, a long N-terminal extracellular domain and a short C-terminal tail, which remain associated through hydrogen bonds (11). The extracellular domain can be released from the cell surface by an additional proteolytic cleavage event, which has been implicated in the pathogenesis of inflammatory conditions and cancer (12,13). Release of the N-terminal domain can be induced via activation of specific enzymes, including the matrix metalloprotease a disintegrase and metalloprotease (ADAM) metallopeptidase domain 17 (ADAM-17) (14,15).
In cancer, the O-glycan chains attached to glycoproteins are often truncated and they commonly contain Tn (GalNAcα1-O-Ser/Thr) and T (Galβ1-3GalNAcα1-O-Ser/Thr) antigens and their sialylated forms (sTn and sT) (16). The aforementioned antigens are often markers of poorly differentiated adenocarcinomas and mucinous carcinoma, and their increased occurrence is associated with highly proliferative tumors, metastasis, and poor clinical outcomes (11).
The majority of types of gastric cancer are end products of an inflammatory process. Chronic H. pylori infection is characterized by inflammation of the gastric mucosa and is the major cause of chronic gastritis (9,17,18). IL-8 serves an important role in the response of epithelial cells to H. pylori infection, as well as in the pathological processes which ultimately result in gastric diseases. IL-8 is a chemokine that is specific to neutrophil granulocyte chemotaxis, and has been shown to be associated with the histological severity of gastritis. IL-8 secretion is typically regulated by the transcription factor NF-κB, and H. pylori can induce IL-8 expression by activating the NF-κB signaling pathway in gastric epithelial cells (19). IL-10 represents anti-inflammatory cytokines in general; there are several studies regarding the multifunctional roles of IL-10, including both its immunosuppressive and anti-angiogenic effects, and its varied roles in the pathogenesis, progression, metastasis, and development of several types of cancer (20,21).
The primary purpose of the present study was to determine the influence of luteolin on cancer-associated MUC1 (together with Tn and sT antigens), ADAM-17, the metalloprotease involved in the release the extracellular domain of MUC1, IL-8 and IL-10, which are associated with inflammation, and NF-κB, a transcription factor regulating the expression levels of a number of human genes. All these factors are potentially involved in cancer development. The present experiments were performed on H. pylori infected, gastric cancer CRL-1739 cells.
Materials and methods
Ethical approval and consent
The research protocol used in the present study was approved by the Ethics Committee of Medical University of Białystok (Białystok, Poland) and was performed in accordance with the 2008 Declaration of Helsinki (22). Written informed consent was obtained from the patient.
Bacteria and cell culture
One laboratory H. pylori strain from the Department of Microbiology of the Medical University of Białystok (Białystok, Poland) was used in the present study. It was isolated from gastric epithelial cells of a patient with gastritis. Prior to the beginning of treatment, the scrapings were collected from the prepyloric area and the body of the stomach under endoscopic examination. Immediately afterwards, the scrapings were transferred to the transport medium Portagerm pylori (bioMerieux SA) and homogenized. Subsequently, the bacteria were cultured on Pylori Agar and Columbia Agar supplemented with 5% sheep blood (bioMerieux SA) for 7 days at 37˚C under microaerophilic conditions using a Genbag microaer (bioMerieux SA). Bacteria were identified based on the colony morphology, using the Gram method (23). Additionally, the activities of bacterial urease, catalase and oxidase were determined as described previously (24,25). To determine the H. pylori species, an ELISA test (cat. no. HpAg48; EQUIPAR) was used. Subsequently the bacteria were sub-cultured under the same conditions, suspended at 1.2x109 bacteria/ml in PBS and added to growing gastric cancer cells at a multiplicity of infection of 10 for 24 h.
Gastric adenocarcinoma cells (CRL-1739; American Type Culture Collection) were cultured in F-12 medium containing 10% heat inactivated FBS (Thermo Fisher Scientific, Inc), 100 U/ml penicillin and 100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA) at 37˚C with 5% CO2. Cells were seeded in 6-well plates. A total of 24 h prior to H. pylori treatment, the cell medium was changed to antibiotic-free F-12 medium. Subsequently, media were supplemented with 30 µM luteolin alone or with bacteria, and cultured for 24 h. The cells were washed with PBS and lysed at 4˚C using RIPA buffer (Sigma-Aldrich; Merck KGaA) supplemented with protease inhibitors (1:200; Sigma-Aldrich; Merck KGaA). Culture media and lysates were centrifuged at 1,000 x g for 5 min at 4˚C, and supernatants were aliquoted, stored at -70˚C and used for ELISA. For reverse transcription-quantitative PCR (RT-qPCR), the monolayers were washed three times with sterile 10 mM PBS, and cell membranes were disrupted using a sonicator (Sonics Vibra Cell; Sonics & Materials, Inc). Aliquots of the homogenate were used for RNA isolation. Cells not treated with either luteolin or H. pylori addition were used as a control.
Cell viability assay
Cell viability assessment was performed as previously described by Carmichael et al (26), using MTT (Sigma-Aldrich; Merck KGaA). Confluent cells were cultured for 24 h with various concentrations of luteolin (20-100 µM; Sigma-Aldrich; Merck KGaA) in six-well plates, washed in PBS and incubated for 4 h in 1 ml MTT solution (0.5 mg/ml PBS) at 37˚C with 5% CO2. Absorbance of the converted dye in living cells was measured at a wavelength of 570 nm. Cell viability of gastric cancer cells in the presence of luteolin was calculated as a percentage of the control cells.
ELISA for MUC1, and the Tn and sT antigens
To assess the expression levels of MUC1 mucin, an ELISA with an anti-MUC1 monoclonal antibody (BC2; Abcam; cat. no. ab89492) was used according to the manufacturer's protocol. A total of 50 µl cell lysates (100 µg protein/ml) or 50 µl media (1:100) were coated on microtiter plates (NUNC F96; Maxisorp; Thermo Fisher Scientific, Inc.) at room temperature overnight. Following blocking with 100 µl 1% blocking reagent for ELISA, (Roche Diagnostics) and three washes with 100 µl PBS with 0.05% Tween-20 (Sigma-Aldrich; Merck KGaA), the plates were incubated with 100 µl anti-MUC1 antibody (1:600) for 2 h and horseradish peroxidase conjugated rabbit anti-mouse IgG (Sigma-Aldrich; Merck KGaA) for 1 h at room temperature. The colored reaction was developed by incubation with 100 µl 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (Sigma-Aldrich; Merck KGaA) liquid substrate for horseradish peroxidase. Absorbance at 405 nm was measured after 30-45 min. Wells treated with BSA (Sigma-Aldrich; Merck KGaA) were used as negative controls.
To assess the expression levels of GalNAc-R (Tn antigen) and NeuAcα2-3Gal (sT antigen), an ELISA-like test with biotinylated lectins (Vector Laboratories, Inc.; VVA lectin cat. no. B-1235; MAA II lectin cat. no. B-1265) at a concentration of 5 µg/ml was performed. VVA lectin (from Vicia villosa with binding preference to GalNAc) (27) and MAA II lectin (from Maackia amurensis with binding preference to NeuAcα2-3Gal) (28) were used. A total of 50 μl medium (dilution 1:100) was used to coat microtiter plates at room temperature overnight. Blocking and washing steps were performed as mentioned above. Subsequently, the plates were incubated with 100 µl proper lectins (2 h) and 100 µl horseradish peroxidase avidin D (Vector Laboratories) for 1 h at room temperature. The colored reaction was developed as described above. All samples were analyzed in triplicate in three independent experiments.
ELISA for IL-8
IL-8 expression was quantitatively determined using a commercially available ELISA capture and detection antibody kit (BD OptEIATM Set Human IL-8; BD Biosciences; cat. no. 2654KI) according to the manufacturer's protocol. Briefly, microwells of a 96-well plate were coated with 100 µl capture antibody (1:250) in bicarbonate buffer (pH 9.5; 0.1 M) and incubated overnight at 4˚C. The plates were washed three times with 200 µl PBS with Tween-20 (0.05%) between all the steps. Unbound sites were blocked with 200 µl PBS with 10% FBS (Sigma-Aldrich; Merck KGaA). Subsequently the cell culture media (50 µl; 1:100) were added and plates were incubated for 2 h at room temperature, followed by incubation with biotinylated detection antibody (1:250), peroxidase-labeled streptavidin and tetramethylbenzidine substrate (Sigma-Aldrich; Merck KGaA). Absorbance at 450 nm was measured after 30 min and IL-8 levels were determined from a standard curve prepared with serial dilutions of purified chemokines. All samples were analyzed in triplicate or quadruplicate, in three independent tests, and standard curves were plotted for each plate.
RT-qPCR
Total RNA was isolated using Total RNA Mini Plus Concentrator (A&A Biotechnology) according to the manufacturer's protocol. The concentration and purity of RNA was determined by spectrophotometry using a Nanodrop 2000 (Thermo Fisher Scientific, Inc.). First-strand cDNA was synthesized from 1 µg total RNA using a Tetro cDNA Synthesis kit (Bioline; Meridian Bioscience). The reaction mixture (volume, 20 µl) containing 1 µl oligo(dT)18 primer, 1 µl dNTP mixture (10 mM each), 5 µl 5X RT Buffer, 1 µl RiboSafe RNase Inhibitor (10 U/µl), 1 µl Tetro Reverse Transcriptase (200 U/µl) and diethylpyrocarbonate-treated water was incubated for 30 min at 45˚C and then inactivated at 85˚C for 5 min. qPCR assay was performed using a CFX96 Real-time system (Bio-Rad Laboratories, Inc.) and a SensiFASTTM SYBR kit (Bioline; Meridian Bioscience). The reaction mixtures contained 2 µl twice diluted cDNA template, 0.8 µl of each primer (10 µmol/l), 10 µl 2X SensiFAST SYBR mix and nuclease-free water to a final volume of 20 µl. Forward and reverse primer sequences are listed in Table I. The primers were synthesized by Genomed. GAPDH was used as the housekeeping gene. The thermocycling conditions were: 95˚C for 1 min to activate the DNA polymerase, followed by 40 cycles of denaturation for 10 sec at 95˚C, annealing for 15 sec at 60˚C and extension for 20 sec at 72˚C. The reaction was then subjected to a melting protocol from 55 to 95˚C in 0.2˚C increments and 1 sec holding at each increment to assess the specificity of the amplified products. Single product formation was confirmed by melting point analysis and agarose gel electrophoresis. As a negative control, water was used instead of mRNA samples. Samples were run in triplicate and the 2-ΔΔCq method was used to calculate the relative expression (29). The relative gene expression levels were standardized to those measured for the untreated control.
Table I.
Sequences of primers used in the present study.
| Gene | Forward primer, 5'-3' | Reverse primer, 5'-3' |
|---|---|---|
| MUC1 | TGCCTTGGCTGTCTGTCAGT | GTAGGTATCCCGGGCTGGAA |
| NF-κB | TACTCTGGCGCAGAAATTAGGTC | CTGTCTCGGAGCTCGTCTATTTG |
| IL-8 | TAGCAAAATTGAGGCCAAGG | AAACCAAGGCACAGTGGAAC |
| IL-10 | TGGTGAAACCCCGTCTCTAC | CTGGAGTACAGGGGCAGTAT |
| ADAM-17 | ACCTGAAGAGCTTGTTCATCGAG | CCATGAAGTGTTCCGATAGATGTC |
| GAPDH | GTGAACCATGAGAAGTATGACAA | CATGAGTCCTTCCACGATAC |
Statistical analysis
Experimental data are presented as the mean ± standard deviation of three experimental repeats. A one-way ANOVA followed by a Duncan's post hox test was used to analyze differences between the control and specific treatment groups. Statistica version 10.0 (StatSoft, Inc.) was used to statistically analyze the data. P<0.05 was considered to indicate a statistically significant difference.
Results
Cell viability
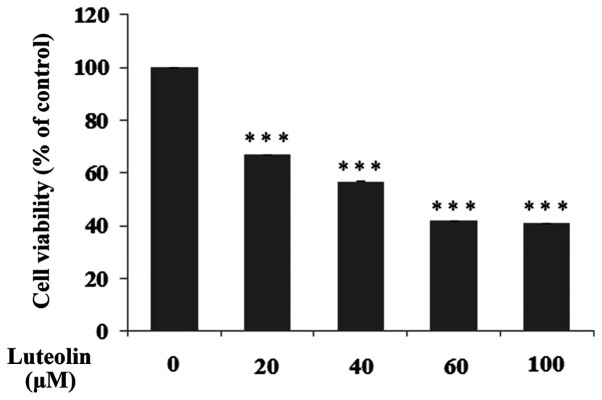
The effect of luteolin on a number of selected biochemical factors was examined in H. pylori infected gastric cancer CRL-1739 cells. In all experiments, 30 µM luteolin was used as a concentration, which was below the IC50 (Fig. 2).
Figure 2.
Viability of the gastric cancer CRL-1739 cells treated with different concentrations of luteolin (20, 40, 60 and 100 µM). Cell viability was assessed using an MTT assay and results are presented as a percentage of the control. Data are presented as the mean ± standard deviation of three experimental repeats. ***P<0.001.
Determination of MUC1
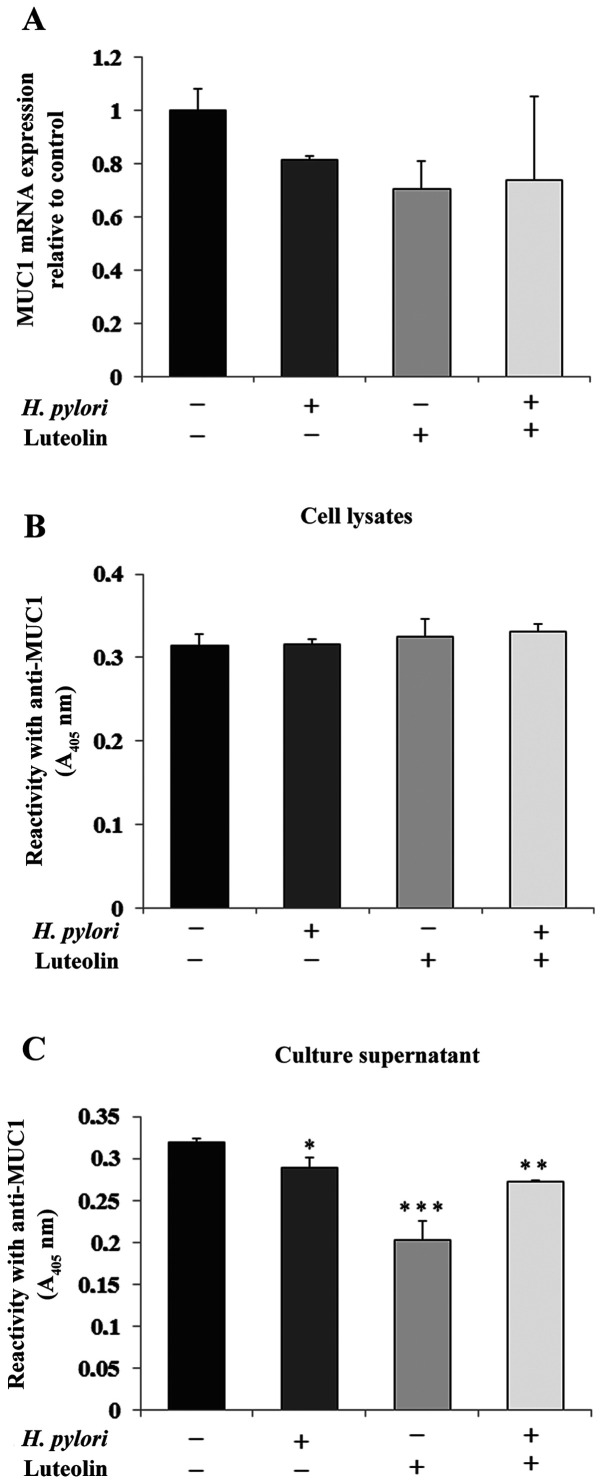
MUC1 is the primary epithelial glycoprotein present in the gastric epithelium (11). As shown in Fig. 3A, H. pylori, luteolin and H. pylori combined with luteolin did not have significant effects on MUC1 mRNA expression compared with the untreated control. There was no significant change in MUC1 expression in the cell lysates (Fig. 3B). However, the examined factors notably reduced MUC1 extracellular domain expression in culture medium (Fig. 3C).
Figure 3.
Effect of luteolin on MUC1 expression in gastric cancer CRL-1739 cells. Effect of (A) luteolin on MUC1 mRNA expression, MUC1 glycoproteins in (B) cell lysates and (C) culture supernatants. Cells were treated with 30 µM luteolin and H. pylori for 24 h. mRNA expression is expressed as the relative fold-change in MUC1 mRNA levels compared with the control, the expression of which was set as 1. For the ELISA of cell lysates, equivalent amounts of protein (5 µg/50 µl) was applied to each well. For culture supernatant, equivalent volumes (50 µl; 1:100) of sample was used. The results are presented as the absorbance at 405 nm after incubation with an anti-MUC1 monoclonal antibody. Data are presented as the mean ± standard deviation of three experimental repeats. *P<0.05, **P<0.01, ***P<0.001. MUC1, mucin 1; A, absorbance; H. pylori, Helicobacter pylori.
Determination of Tn and sT antigens
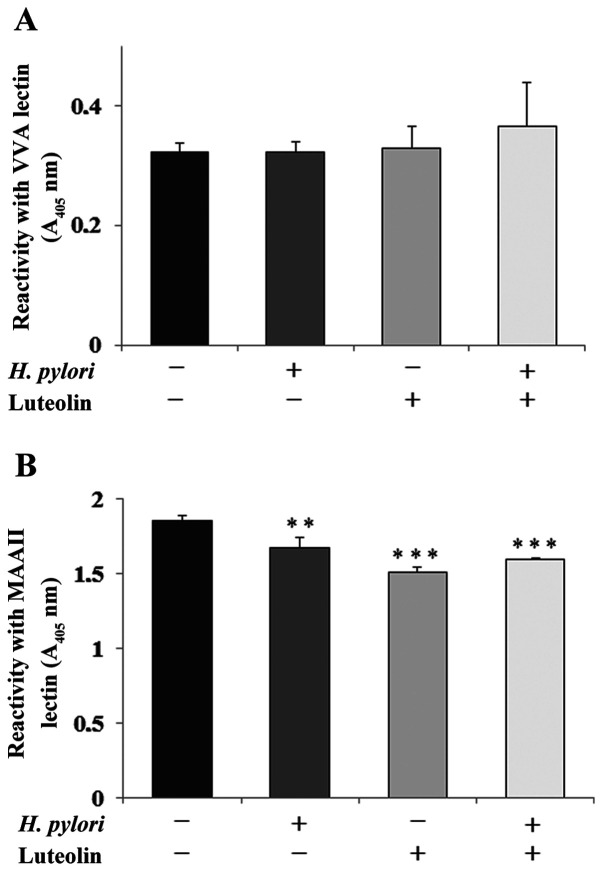
GalNAcα-R (Tn antigen) and NeuAcα2-3Galβ1-3GalNAc-R (sT antigen) are amongst the core sugar structures that are present on the extracellular domain of MUC1 mucin (11). The expression levels of Tn antigen (based on reactivity with VVA lectin) were not significantly affected by bacteria or luteolin (Fig. 4A). Sialylation of T antigen (detected by MAAII lectin) was significantly inhibited by flavonoid and H. pylori addition separately and by both agents combined, compared with the control (Fig. 4B).
Figure 4.
Effect of luteolin on Tn and sT antigen expression. Effect of luteolin on (A) Tn and (B) sT antigen expression in the culture medium of gastric cancer CRL-1739 cells examined using ELISA. The cells were subjected to 30 µM luteolin and H. pylori for 24 h. The same aliquots of culture supernatants (50 µl; 1:100) were applied. The results are presented as the absorbance at 405 nm following incubation with biotinylated VVA (with specificity to T antigen) and MAAII (with specificity to sTn antigen) lectin. Data are presented as the mean ± standard deviation of three experimental repeats. **P<0.01, ***P<0.001. VVA lectin, lectin from Vicia villosa; MAAII lectin, lectin from Maackia amurensis; A, absorbance; H. pylori, Helicobacter pylori; Tn antigen, GalNAcα-R; sT antigen, NeuAcα2-3Galβ1-3GalNAc-R.
Determination of ADAM-17 levels
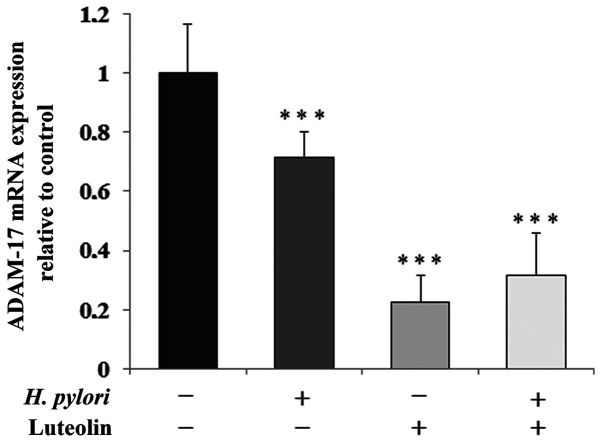
ADAM-17 is one of the sheddases that is responsible for the release of MUC1 extracellular domain. In all conditioned cultures, sheddase was markedly inhibited by the examined factors at the mRNA level (by 29, 78 and 68% for gastric cancer cells treated with H. pylori, luteolin independently and combined, respectively) compared with the untreated control (Fig. 5).
Figure 5.
Effect of luteolin on ADAM-17 mRNA expression in gastric cancer CRL-1739 cells. Cells were treated with 30 µM luteolin and H. pylori for 24 h. Results are expressed as the relative fold-change in MUC1 mRNA expression compared with the control, the expression of which was set as 1. Data are presented as the mean ± standard deviation of three experimental repeats. ***P<0.001. ADAM-17, ADAM metallopeptidase domain 17; MUC1, mucin 1; H. pylori, Helicobacter pylori.
Determination of IL-8 and IL-10
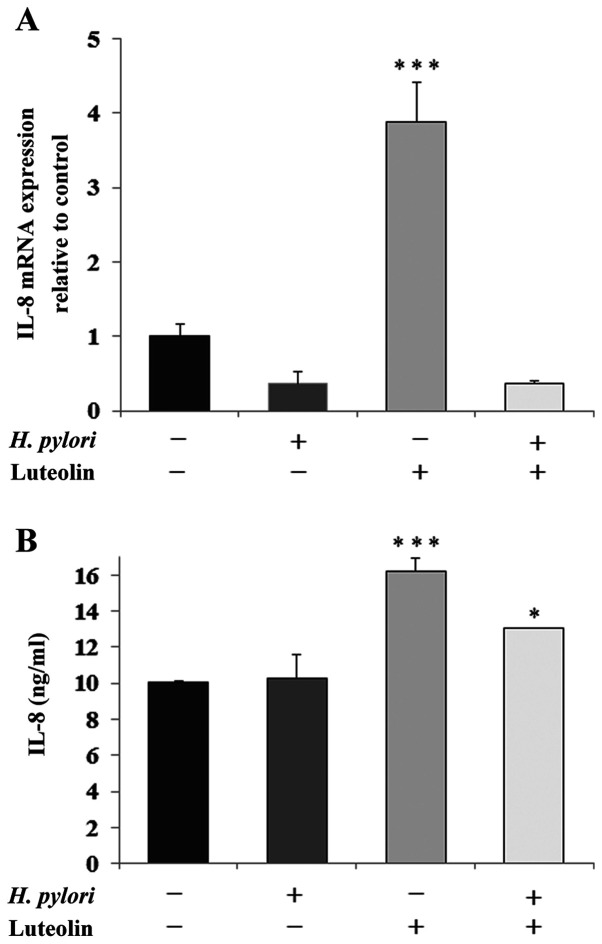
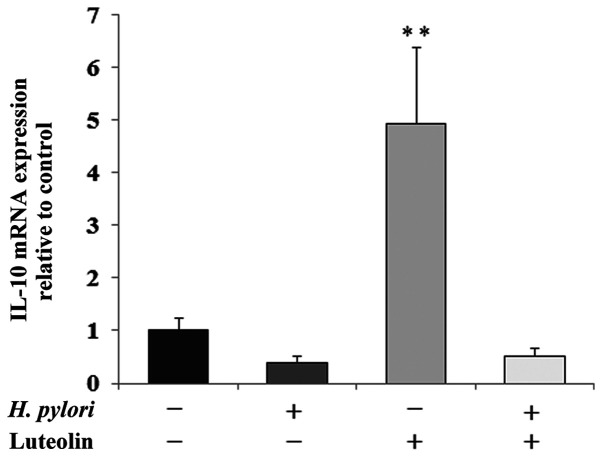
IL-8 is a proinflammatory chemokine that is considered to be an important regulatory factor in the tumor environment. Fig. 6 shows a marked stimulatory effect of luteolin on IL-8 expression at both the mRNA (Fig. 6A) and protein (Fig. 6B) levels compared with the untreated control. The protein expression levels of IL-8 were also stimulated by the simultaneous action of both examined factors compared with the control. IL-10 is an inhibitory cytokine which can help tumor cells evade the immune system to avoid destruction by cell-mediated immune mechanisms. A marked increase in IL-10 mRNA expression following luteolin treatment was observed (Fig. 7).
Figure 6.
Effect of luteolin on IL-8 expression in gastric cancer CRL-1739 cells. Effect of luteolin on (A) IL-8 mRNA and (B) IL-8 expression in cell culture supernatants were determined. Cells were treated with 30 µM luteolin and H. pylori for 24 h. For mRNA expression analysis, the results are expressed as the relative fold-change in MUC1 mRNA expression compared with the control, the expression of which was set as 1. For ELISA of culture supernatants, equivalent volumes (50 µl; 1:100) of samples were used. Results are expressed as ng of IL-8 per ml of culture medium. Data are presented as the mean ± standard deviation of three experimental repeats. *P<0.05, ***P<0.001. MUC1, mucin 1; H. pylori, Helicobacter pylori.
Figure 7.
Effect of luteolin on IL-10 mRNA expression in gastric cancer CRL-1739 cells. Cells were treated with 30 µM luteolin and H. pylori for 24 h. Results are expressed as the relative fold-change in IL-10 mRNA expression compared with the control, the expression of which was set as 1. Data are presented as the mean ± standard deviation of three experimental repeats. **P<0.01; H. pylori, Helicobacter pylori.
Determination of NF-κB expression
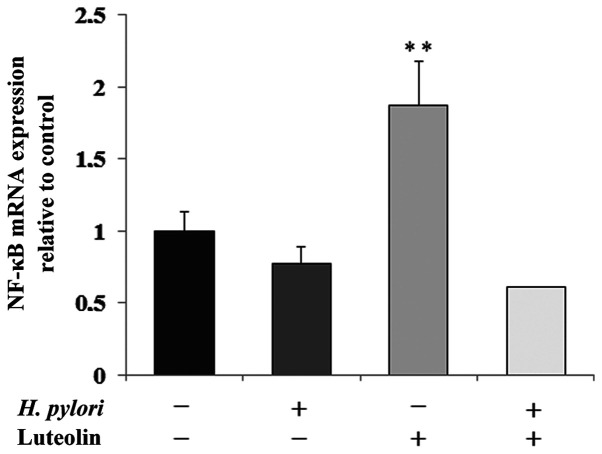
NF-κB is a transcription factor modulated by numerous stimuli, and is able to regulate the expression levels of several human genes. As shown in Fig. 8, NF-κB mRNA expression was significantly increased by luteolin treatment by 87% compared with the untreated control.
Figure 8.
Effect of luteolin on NF-κB mRNA expression in gastric cancer CRL-1739 cells. Cells were treated with 30 µM luteolin and H. pylori for 24 h. Results are presented as the relative fold-change in NF-κB mRNA expression compared with the control, expression of which was set as 1. Data are presented as the mean ± standard deviation of three experimental repeats. **P<0.01; H. pylori, Helicobacter pylori.
Discussion
Gastric cancer is one of the most common malignances worldwide, and is a serious risk to human health due to its asymptomatic course of development, nonspecific symptoms during the early stages and the high mortality rates associated with advanced stages (7,9). Furthermore, there are limited efficacious therapeutic strategies for treating advanced gastric cancer (30,31). Therefore, it remains a priority to develop novel therapeutic reagents for treatment of gastric cancer. Long-term consumption of fruit and vegetables reduces the risk of cancer (32). Therefore, identification of natural phytochemicals as potential anticancer agents may improve treatment as they are typically less toxic than chemotherapeutic agents, and thus, this approach has gained increasing attention (3,33,34).
Luteolin is a dietary flavonoid that has been reported to inhibit the development and progression of several different types of tumors (35,36). Pu et al (36) reported that luteolin reduced cell viability, induced cell cycle arrest, colony formation, proliferation and migration, and promoted apoptosis of gastric cancer MKN45 and BGC823 cells. According to Zang et al (37) luteolin inhibited gastric cancer progression via suppression of Notch 1 signaling and reversal of epithelial-mesenchymal transition in gastric cancer Hs-746T and MKN28 cells. Due to these promising results regarding the use of luteolin as an anti-cancer agent, the present study attempted to examine its effect on a number of other factors potentially involved in gastric cancer development. Since H. pylori is considered to be a causative agent of gastric cancer (8) H. pylori-infected gastric cancer cells were used, as well as the cells not treated with these bacteria.
Cancers are diverse and complex diseases based on multiple etiologies and various cell targets. A number of these targets are associated with cancer development, however, not all the relations have been identified. A possible target for cancer cells is MUC1 mucin with truncated O-glycans (11,38). The present study examined the effects of luteolin on a number of selected factors, which were all cancer-related; however not all of these appeared to be associated with cancer development or progression in the present study.
MUC1 mucin is considered to be an oncoprotein based on evidence which suggests its cancer-promoting function. MUC1 can activate anti-apoptotic proteins (Bcl-xL) (39), attenuate apoptosis execution pathways (40,41) and may contribute to metastasis (42). Furthermore, MUC1 is involved in H. pylori infection development via direct interaction with bacterial adhesins (17,43). To the best of our knowledge, there are no studies assessing the effects of luteolin on MUC1 mucin. The present study showed the inhibitory effects of bacteria and luteolin on the expression levels of the extracellular domain of MUC1 mucin, with a more potent effect being observed following luteolin treatment. Notably, this effect was not observed at the mRNA level. It has been suggested that ADAM-17 is involved in the release of MUC1 extracellular domain into the culture medium (14,15). The results of the present study appear to support this hypothesis, since ADAM-17 mRNA expression was associated increased MUC1 release into the culture medium. Additionally, ADAM-17 (also known as TNF-α-converting enzyme) has been identified to function as a signaling scissor in the tumor microenvironment, and thus contributes to tumorigenesis and tumor progression (44). Upregulation of ADAM-17 is associated with the progression of non-small cell lung cancer (45) or the promotion of breast cancer tumorigenesis by regulating cell proliferation, angiogenesis, invasion and apoptosis (46). Upregulation of ADAM-17 contributes to the progression of gastric cancer and is associated with a poor prognosis (47,48). Therefore, the enzyme is considered to be a potential target for treatment of cancer, as well as an indicator for predicting therapeutic outcomes. The present study revealed the inhibitory effect of luteolin on ADAM-17 gene expression, which appears to support the hypothesis regarding the potential anti-cancer effects of the examined flavonoids. Thus, it is hypothesized that luteolin is involved in the inhibition of degradation of ECM components by decreasing ADAM-17 expression.
In several types of cancer, O-glycan chains attached to glycoproteins, including MUC1 mucin, the primary O-glycoprotein of gastric epithelium, commonly contain short carbohydrate forms, Tn and T antigens and their sialylated derivatives. Their increased occurrence is associated with highly proliferative tumors, metastasis, and poor clinical outcomes (11,16). Loss or acquisition of glycans affects interactions of MUC1 and other cellular proteins implicated in the migratory and metastatic activities of cancer cells. Santos-Silva et al (49) showed that 53.2% of gastric carcinomas express the sT antigen, indicating that sialylation may contribute to the low frequency of cases observed with T antigen expression. The synthesis of the sT structure stops further processing and elongation of the carbohydrate chain (16). Yu et al (50) showed that the cancer-associated T antigen on MUC1 is the natural ligand of galectin-3, a galactose binding protein, which after connecting to MUC1, initiates MUC1-dependent intracellular signaling in cancer cells and facilitates adhesion of cancer cells to each other and to endothelial cells (51). Therefore, inhibition of sT structure expression by luteolin, which was observed in the present study, may be associated with its possible anti-cancer activity.
It has been stated that the interaction between H. pylori with the gastric epithelium induces the production of IL-8, which is a proinflammatory cytokine and chemotaxin for neutrophils and mononuclear cells, and this can lead to chronically activated gastritis (19). The majority of gastric cancer cases are the end products of an inflammatory process (19,20,52). A significant association between high expression levels of IL-8 in the gastric mucosa and the risk of gastric cancer has been reported previously (19,53). Furthermore, H. pylori can stimulate the secretion of IL-10, which has been recently recognized as the most potent anti-inflammatory cytokine, and also as the agent enabling cancer immune surveillance and tumor rejection (54). The results of the present study were not consistent with the aforementioned results, as H. pylori reduced IL-8 and IL-10 expression, in contrast to the previous studies. One possible explanation for this result could be that the action of the pathogen on gastric cancer cells was too short (24 h). Notably, in the present study, luteolin clearly increased expression of both cytokines at the mRNA level and IL-8 at the protein level as well. These results are not consistent with those reported in other studies which demonstrated the inhibition of IL-8 upregulation by the examined flavonoid (55,56). However, there is also at least one report regarding the stimulating effects of luteolin on IL-8 expression. Lee et al (57) showed that relatively low concentrations of luteolin (20 and 40 µM) increased the IL-8 levels, and only a high concentration of flavonoid (80 µM) was shown to decrease the expression levels of IL-8 in lung cancer cells.
It has been reported that direct contact of H. pylori and gastric cancer cells induces NF-κB activation (58). However, this result was not observed in the present study, and it was suggested that this could be due to the exposure of gastric cells to the pathogen being too short as mentioned above. Notably, luteolin induced NF-κB mRNA expression, which is not consistent with the general tendency of anti-inflammatory actions of the flavonoid via the NF-κB signaling pathway (58). At the current stage of the present study, this discrepancy cannot be explained. However, an association between the effect of luteolin on nuclear factor and IL-8 expression was revealed, in agreement with the general tendency of NF-κB to stimulate chemokine production (58).
Based on the preliminary results of the present study, it may be assumed that luteolin may be used as an adjuvant for treatment of gastric cancer. This hypothesis is particularly based on the outcomes concerning MUC1 extracellular domain, mRNA ADAM-17 and sT antigen expression. There are some discrepancies between the results of the present study and the results of previous studies, for example regarding IL-8, IL-10 and NF-κB levels. This requires further exploration in future experiments. There were also some shortcomings of the present study. The limitations include using only one cancer cell line and the lack of a normal cell line. However, it has been shown in other studies that the effects of luteolin are specific to cancer cells at the concentrations used in the present study (59). Additional experiments, such as apoptosis assays and cell cycle analysis, combined with the use of additional experimental methods, such as western blotting and immunofluorescence analysis should be performed to determine how luteolin regulates the changes in the examined factors and to identify the targets of the flavonoid. In future experiments, how H. pylori affects cells growth at different multiplicities of infection will be assessed. Other experiments where MUC1 or ADAM-17 are knocked down will also be performed.
Experimental and clinical data suggest that pharmacological regulation of various factors participating in cancer development exerts several beneficial effects improving the outcomes of different anti-cancer therapies. Dysregulation of cancer cells results from a number of genetic alterations, which impacts the signaling network and the control of numerous cellular processes. Thus, therapeutic targeting of specific factors may also elicit opposing, often unwanted effects. Therefore, extensive investigations are required to understand the molecular signaling pathway network for the development of synergistic complex therapies for cancer treatments.
Acknowledgements
The authors would like to thank Ms. Joanna Wosek (Department of Medical Chemistry, Medical University of Białystok (Białystok, Poland) for her technical support.
Funding
The present study was supported by the Medical University of Białystok (Białystok, Poland) (grant no. N/ST/15/004/2203).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
IR designed the study, interpreted the data and drafted the manuscript. MBK performed some of the experiments, assisted in interpretation of the data and performed the statistical analysis. KL performed some of the experiments and assisted in interpretation of the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The research protocol was approved by the Ethics Committee of Medical University of Białystok (Białystok, Poland) and was performed in accordance with the 2008 Declaration of Helsinki. Written informed consent was obtained the patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they no competing interests.
References
- 1.Xiong J, Li S, Wang W, Hong Y, Tang K, Luo Q. Screening and identification of the antibacterial bioactive compounds from Lonicera japonica Thunb. leaves. Food Chem. 2013;138:327–333. doi: 10.1016/j.foodchem.2012.10.127. [DOI] [PubMed] [Google Scholar]
- 2.López-Lázaro M. Distribution and biological activities of the flavonoid luteolin. Mini Rev Med Chem. 2009;9:31–59. doi: 10.2174/138955709787001712. [DOI] [PubMed] [Google Scholar]
- 3.Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: Flavonoids and isoflavonoids. Pharmacol Ther. 2001;90:157–177. doi: 10.1016/s0163-7258(01)00137-1. [DOI] [PubMed] [Google Scholar]
- 4.Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets. 2008;8:634–646. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nepali S, Son JS, Poudel B, Lee JH, Lee YM, Kim DK. Luteolin is a bioflavonoid that attenuates adipocyte-derived inflammatory responses via suppression of nuclear factor-κB/mitogen-activated protein kinases pathway. Pharmacogn Mag. 2015;11:627–635. doi: 10.4103/0973-1296.160470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson JL, Gonzalez de Mejia E. Interactions between dietary flavonoids apigenin or luteolin and chemotherapeutic drugs to potentiate anti-proliferative effect on human pancreatic cancer cells, in vitro. Food Chem Toxicol. 2013;60:83–91. doi: 10.1016/j.fct.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 7.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 8.Amieva M, Peek RM Jr. Pathobiology of Helicobacter pylori -induced gastric cancer. Gastroenterology. 2016;150:64–78. doi: 10.1053/j.gastro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ang TL, Fock KM. Clinical epidemiology of gastric cancer. Singapore Med J. 2014;55:621–628. doi: 10.11622/smedj.2014174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christiansen MN, Chik J, Lee L, Anugraham M, Abrahams JL, Packer NH. Cell surface protein glycosylation in cancer. Proteomics. 2014;14:525–546. doi: 10.1002/pmic.201300387. [DOI] [PubMed] [Google Scholar]
- 11.Cascio S, Finn OJ. Intra- and extra-cellular events related to altered glycosylation of MUC1 promote chronic inflammation, tumor progression, innovation and metastasis. Biomolecules. 2016;6(E39) doi: 10.3390/biom6040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollingsworth MA, Swanson BJ. Mucins in cancer: Protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 13.Mukhopadhyay P, Chakraborty S, Ponnusamy MP, Lakshmanan I, Jain M, Batra SK. Mucins in the pathogenesis of breast cancer: Implications in diagnosis, prognosis and therapy. Biochim Biophys Acta. 2011;1815:224–240. doi: 10.1016/j.bbcan.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thathiah A, Blobel CP, Carson DD. Tumor necrosis factor-α converting enzyme/ADAM 17 mediates MUC1 shedding. J Biol Chem. 2003;278:3386–3394. doi: 10.1074/jbc.M208326200. [DOI] [PubMed] [Google Scholar]
- 15.Goth CK, Halim A, Khetarpal SA, Rader DJ, Clausen H, Schjoldager KT. A systematic study of modulation of ADAM-mediated ectodomain shedding by site-specific O-glycosylation. Proc Natl Acad Sci USA. 2015;112:14623–14628. doi: 10.1073/pnas.1511175112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu C, Zhao H, Wang Y, Cai H, Xiao Y, Zeng Y, Chen H. Tumor-associated antigens: Tn antigen, sTn antigen, and T antigen. HLA. 2016;88:275–286. doi: 10.1111/tan.12900. [DOI] [PubMed] [Google Scholar]
- 17.Lillehoj EP, Guang W, Ding H, Czinn SJ, Blanchard TG. Helicobacter pylori and gastric inflammation: Role of MUC1 mucin. J Pediatr Biochem. 2012;2:125–132. doi: 10.3233/JPB-2012-00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196–202. doi: 10.1016/j.canlet.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Lee KE, Khoi PN, Xia Y, Park JS, Joo YE, Kim KK, Choi SY, Jung YD. Helicobacter pylori and interleukin-8 in gastric cancer. World J Gastroenterol. 2013;19:8192–8202. doi: 10.3748/wjg.v19.i45.8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad N, Ammar A, Storr SJ, Green AR, Rakha E, Ellis IO, Martin SG. IL-6 and IL-10 are associated with good prognosis in early stage invasive breast cancer patients. Cancer Immunol Immunother. 2018;67:537–549. doi: 10.1007/s00262-017-2106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fasoulakis Z, Kolios G, Papamanolis V, Kontomanolis EN. Interleukins associated with breast cancer. Cureus. 2018;10(e.3549) doi: 10.7759/cureus.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puri KS, Suresh KR, Gogtay NJ, Thatte UM. Declaration of Helsinki, 2008: Implications for stakeholders in research. J Postgrad Med. 2009;55:131–134. doi: 10.4103/0022-3859.52846. [DOI] [PubMed] [Google Scholar]
- 23.Beveridge TJ. Use of the gram stain in microbiology. Biotech Histochem. 2001;76:111–118. [PubMed] [Google Scholar]
- 24.Uotani T, Graham DY. Diagnosis of Helicobacter pylori using the rapid urease test. Ann Transl Med. 2015;3:9–7. doi: 10.3978/j.issn.2305-5839.2014.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai Y, Zhang YL, Jin JF, Wang JD, Zhang ZS, Zhou DY. Recombinant Helicobacter pylori catalase. World J Gastroenterol. 2003;9:1119–1122. doi: 10.3748/wjg.v9.i5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987;47:936–942. [PubMed] [Google Scholar]
- 27.Puri KD, Gopalakrishnan B, Surolia A. Carbohydrate binding specificity of the Tn-antigen binding lectin from Vicia villosa seeds (VVLB4) FEBS Lett. 1992;312:208–212. doi: 10.1016/0014-5793(92)80937-c. [DOI] [PubMed] [Google Scholar]
- 28.Redelinghuys P, Antonopoulos A, Liu Y, Campanero-Rhodes MA, McKenzie E, Haslam SM, Dell A, Feizi T, Crocker PR. Early murine T-lymphocyte activation is accompanied by a switch from N-Glycolyl- to N-acetyl-neuraminic acid and generation of ligands for siglec-E. J Biol Chem. 2011;286:34522–34532. doi: 10.1074/jbc.M111.243410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Zong L, Abe M, Seto Y, Ji J. The challenge of screening for early gastric cancer in China. Lancet. 2016;388(2606) doi: 10.1016/S0140-6736(16)32226-7. [DOI] [PubMed] [Google Scholar]
- 31.Cervantes A, Roda D, Tarazona N, Roselló S, Pérez-Fidalgo JA. Current questions for the treatment of advanced gastric cancer. Cancer Treat Rev. 2013;39:60–67. doi: 10.1016/j.ctrv.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349(g4490) doi: 10.1136/bmj.g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranjan A, Ramachandran S, Gupta N, Kaushik I, Wright S, Srivastava S, Das H, Srivastava S, Prasad S, Srivastava SK. Role of phytochemicals in cancer prevention. Int J Mol Sci. 2019;20(4981) doi: 10.3390/ijms20204981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chikara S, Nagaprashantha LD, Singhal J, Horne D, Awasthi S, Singhal SS. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018;413:122–134. doi: 10.1016/j.canlet.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Ma L, Peng H, Li K, Zhao R, Li L, Yu Y, Wang X, Han Z. Luteolin exerts an anticancer effect on NCI-H460 human non-small cell lung cancer cells through the induction of Sirt1-mediated apoptosis. Mol Med Rep. 2015;12:4196–4202. doi: 10.3892/mmr.2015.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pu Y, Zhang T, Wang J, Mao Z, Duan B, Long Y, Xue F, Liu D, Liu S, Gao Z. Luteolin exerts an anticancer effect on gastric cancer cells through multiple signaling pathways and regulating miRNAs. J Cancer. 2018;9:3669–3675. doi: 10.7150/jca.27183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zang MD, Hu L, Fan ZY, Wang HX, Zhu ZL, Cao S, Wu XY, Li JF, Su LP, Li C, et al. Luteolin suppresses gastric cancer progression by reversing epithelial-mesenchymal transition via suppression of the Notch signaling pathway. J Transl Med. 2017;15(52) doi: 10.1186/s12967-017-1151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhatia R, Gautam SK, Cannon A, Thompson C, Hall BR, Aithal A, Banerjee K, Jain M, Solheim JC, Kumar S, et al. Cancer-associated mucins: Role in immune modulation and metastasis. Cancer Metastasis Rev. 2019;38:223–236. doi: 10.1007/s10555-018-09775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raina D, Kharbanda S, Kufe D. The MUC1 oncoprotein activates the anti-apoptotic phosphoinositide 3-kinase/Akt and Bcl-xL pathways in rat 3Y1 fibroblasts. J Biol Chem. 2004;279:20607–20612. doi: 10.1074/jbc.M310538200. [DOI] [PubMed] [Google Scholar]
- 40.Raina D, Ahmad R, Kumar S, Ren J, Yoshida K, Kharbanda S, Kufe D. MUC1 oncoprotein blocks nuclear targeting of c-Abl in the apoptotic response to DNA damage. EMBO J. 2006;25:3774–3783. doi: 10.1038/sj.emboj.7601263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agata N, Ahmad R, Kawano T, Raina D, Kharbanda S, Kufe D. MUC1 oncoprotein blocks death receptor-mediated apoptosis by inhibiting recruitment of caspase-8. Cancer Res. 2008;68:6136–6144. doi: 10.1158/0008-5472.CAN-08-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahn JJ, Chow JW, Horne GJ, Mah BK, Emerman JT, Hoffman P, Hugh JC. MUC1 mediates transendothelial migration in vitro by ligating endothelial cell ICAM-1. Clin Exp Metastasis. 2005;22:475–483. doi: 10.1007/s10585-005-3098-x. [DOI] [PubMed] [Google Scholar]
- 43.Lindén SK, Sheng YH, Every AL, Miles KM, Skoog EC, Florin TH, Sutton P, McGuckin MA. MUC1 limits Helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy. PLoS Pathog. 2009;5(e1000617) doi: 10.1371/journal.ppat.1000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy G. The ADAMs: Signaling scissors in the tumour microenvironment. Nat Rev Cancer. 2008;8:929–941. doi: 10.1038/nrc2459. [DOI] [PubMed] [Google Scholar]
- 45.Ni SS, Zhang J, Zhao WL, Dong XC, Wang JL. ADAM17 is overexpressed in non small cell lung cancer and its expression correlates with poor patient survival. Tumour Biol. 2013;34:1813–1818. doi: 10.1007/s13277-013-0721-3. [DOI] [PubMed] [Google Scholar]
- 46.Zhao J, Tang J. The role of ADAM17 in tumorigenesis and progression of breast cancer. Tumour Biol. 2016;37:15359–15370. doi: 10.1007/s13277-016-5418-y. [DOI] [PubMed] [Google Scholar]
- 47.Fang W, Qian J, Wu Q, Chen Y, Yu G. ADAM 17 expression is enhanced by FoxM1 and is a poor prognostic sign in gastric carcinoma. J Surg Res. 2017;220:223–233. doi: 10.1016/j.jss.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 48.Li W, Wang D, Sun X, Zhang Y, Wang L, Suo J. ADAM17 promotes lymph node metastasis in gastric cancer via activation of the Notch and Wnt signaling pathways. Int J Mol Med. 2019;43:914–926. doi: 10.3892/ijmm.2018.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santos-Silva F, Fonseca A, Caffrey T, Carvalho F, Mesquita P, Reis C, Almeida R, David L, Hollingsworth MA. Thomsen-Friedenreich antigen expression in gastric carcinomas is associated with MUC1 mucin VNTR polymorphism. Glycobiology. 2005;15:511–517. doi: 10.1093/glycob/cwi027. [DOI] [PubMed] [Google Scholar]
- 50.Yu LG, Andrews N, Zhao Q, McKean D, Williams JF, Connor LJ, Gerasimenko OV, Hilkens J, Hirabayashi J, Kasai K, et al. Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion. J Biol Chem. 2007;282:773–781. doi: 10.1074/jbc.M606862200. [DOI] [PubMed] [Google Scholar]
- 51.Mori Y, Akita K, Yashiro M, Sawada T, Hirakawa K, Murata T, Nakada H. Binding of galectin-3, a β-galactoside-binding lectin, to MUC1 protein enhances phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2) and Akt, promoting tumor cell malignancy. J Biol Chem. 2015;290:26125–26140. doi: 10.1074/jbc.M115.651489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peek RM Jr, Fiske C, Wilson KT. Role of innate immunity in Helicobacter pylori-induced gastric malignancy. Physiol Rev. 2010;90:831–858. doi: 10.1152/physrev.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamada S, Kato S, Matsuhisa T, Makonkawkeyoon L, Yoshida M, Chakrabandhu T, Lertprasertsuk N, Suttharat P, Chakrabandhu B, Nishiumi S, et al. Predominant mucosal IL-8 mRNA expression in non-cagA Thais is risk for gastric cancer. World J Gastroenterol. 2013;19:2941–2949. doi: 10.3748/wjg.v19.i19.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mumm JB, Oft M. Pegylated IL-10 induces cancer immunity: The surprising role of IL-10 as a potent inducer of IFN-γ-mediated CD8(+) T cell cytotoxicity. Bioessays. 2013;35:623–631. doi: 10.1002/bies.201300004. [DOI] [PubMed] [Google Scholar]
- 55.Kim JA, Kim DK, Kang OH, Choi YA, Park HJ, Choi SC, Kim TH, Yun KJ, Nah YH, Lee YM. Inhibitory effect of luteolin on TNF-alpha-induced IL-8 production in human colon epithelial cells. Int Immunopharmacol. 2005;5:209–217. doi: 10.1016/j.intimp.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 56.Nunes C, Almeida L, Barbosa RM, Laranjinha J. Luteolin suppresses the JAK/STAT pathway in a cellular model of intestinal inflammation. Food Funct. 2017;8:387–396. doi: 10.1039/c6fo01529h. [DOI] [PubMed] [Google Scholar]
- 57.Lee YJ, Lim T, Han MS, Lee SH, Baek SH, Nan HY, Lee C. Anticancer effect of luteolin is mediated by downregulation of TAM receptor tyrosine kinases, but not interleukin-8, in non-small cell lung cancer cells. Oncol Rep. 2017;37:1219–1226. doi: 10.3892/or.2016.5336. [DOI] [PubMed] [Google Scholar]
- 58.Sokolova O, Naumann M. NF-κB signaling in gastric cancer. Toxins (Basel) 2017;9:1–22. doi: 10.3390/toxins9040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Imran M, Rauf A, Abu-Izneid T, Nadeem M, Shariati MA, Khan IA, Imran A, Orhan IE, Rizwan M, Atif M, et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed Pharmacother. 2019;112(108612) doi: 10.1016/j.biopha.2019.108612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.