Abstract
Selenium (Se) is an essential trace element for animals and exists in nature in both inorganic and organic forms. Although organic Se is more bioavailable than inorganic Se, there are inconsistent reports on the effect of organic Se on the reproductive performance of sows. This study was conducted to investigate the effect of maternal organic Se (2-hydroxy-4-methylselenobutanoic [HMSeBA]) supplementation on reproductive performance and antioxidant capacity of sows, and the long-term effect on the growth performance and antioxidant capacity of their offspring with or without lipopolysaccharide (LPS) challenge. The experimental design used in this study was a completely randomized design; 45 Landrace × Yorkshire sows were randomly allocated to receive one of the following three diets during gestation: control diet (Control, basal diet, n = 15), sodium selenite (Na2SeO3)-supplemented diet (Na2SeO3, basal diet + 0.3 mg Se/kg Na2SeO3, n = 15), and HMSeBA-supplemented diet (HMSeBA, basal diet + 0.3 mg Se/kg HMSeBA, n = 15). On day 21 of age, male offspring from each group were injected with LPS or saline (n = 6). As compared with the control group, maternal HMSeBA supplementation increased the number of total born piglets, while decreased birth weight (P < 0.05). In the first week of lactation, maternal HMSeBA supplementation increased litter weight gain compared with the Na2SeO3 group (P < 0.05) and increased the average daily gain of piglets compared with the control group and Na2SeO3 group (P < 0.05). Meanwhile, maternal HMSeBA supplementation decreased piglet birth interval as compared with the control group and Na2SeO3 group (P < 0.05). Besides, plasma glutathione peroxidase (GSH-Px) activity was higher in the HMSeBA group on farrowing 0 min and 90 min, while malondialdehyde (MDA) concentration was lower on farrowing 0, 90, and 135 min than those in the control group (P < 0.05). In addition, maternal HMSeBA supplementation increased the concentration of selenoprotein P (SELENOP) in colostrum compared with the control group (P < 0.05). Further study revealed that the LPS-challenged HMSeBA group had higher GSH-Px and total antioxidant capacity and lower MDA in weaning piglets compared with the LPS-challenged control group (P < 0.05). Taken together, maternal HMSeBA supplementation increased the number of total born piglets, shortened the duration of farrowing, improved the antioxidant capacities of sows and their offspring, and improved the growth performance of suckling pigs at the first week of lactation. Thus, HMSeBA supplementation during gestation has the potentiality to produce more kilogram of meat.
Keywords: antioxidant capacity, hydroxy-analog of selenomethionine, reproductive performance, selenoprotein P, sow
Introduction
Oxidative stress is a key factor in early fetal loss (Hempstock et al., 2003; Jauniaux et al., 2003) and is considered to reduce female reproductive performance (Agarwal and Allamaneni, 2004; Agarwal et al., 2006). Thus, the antioxidant defense system is important for animal reproduction (Surai, 2006; Surai and Fisinin, 2015a, 2015b). In addition, maternal nutrition or oxidative stress has a long-term effect on the performance and health of the progeny (Luo et al., 2006; Pappas et al., 2008; Koletzko et al., 2012). During the gestation period of sows, oxidative stress is increased in late pregnancy and lactation and is not restored when weaning compared with 30 d of gestation (Berchieri-Ronchi et al., 2011). Meanwhile, newborn piglets suffered seriously from birth oxidative stress because of the naive antioxidant system (Yin et al., 2013). Thus, fetal development and growth processes are susceptible to oxidative stress and may continuously have a negative effect on the progeny. Our previous study has revealed that improvement in maternal, placental, and fetal antioxidant capacities by nutrients benefits the development and growth of fetus (Lin et al., 2012; Mou et al., 2018). Therefore, improvement of antioxidant capacities of sows at gestation would be of great importance for their reproductive performance and on the health and growth performance of their offspring.
Selenium (Se) is an essential trace element for sows and exists in nature in both inorganic and organic forms (Surai and Fisinin, 2016; Akahoshi et al., 2019). It was reported that the blood selenium level of sow continued to decrease during pregnancy (Chavez, 1985). However, supplementation of 0.10 mg/Kg sodium selenite in sows’ diet can improve serum selenium levels of both sows and fetuses (Mahan et al., 1974, 1975). Peters et al. (2010) compared the organic and inorganic micromineral source on sow and progeny mineral compositions over six parities and found that the dietary micromineral source and level had a minimal effect on sow body and liver mineral contents or in colostrum and pigs at birth, except Se, which was greater when the organic form was fed. In addition, a large number of studies have explored the effect of selenium yeast on the reproductive performance and selenium level of sows, which showed that sows fed organic Se (selenium yeast) have a lower number of stillborn pigs and higher colostrum and milk Se content (Mahan and Kim, 1996; Mahan, 2000; Mahan and Peters, 2004; Yoon and McMillan, 2006; Quesnel et al., 2008; Svoboda et al., 2008; Peters et al., 2010; Fortier et al., 2012; Horký et al., 2014; Chen et al., 2016) compared with sows fed sodium selenite. Moreover, selenomethionine supplementation during late gestation and lactation improves maternal antioxidation capacity and also has potential for improving the growth performance of offspring (Zhan et al., 2011; Falk et al., 2019). However, there are inconsistent reports on the effect of organic selenium on the reproductive performance of sows (Surai and Fisinin, 2016). Besides, previous reports mainly focused on the effect of organic selenium supplementation during late gestation and lactation, while the effect of organic Se supplementation during the whole gestation on reproductive performance and offspring growth performance is unknown.
An organic Se source, 2-hydroxy-4-methylselenobutanoic acid (HMSeBA), has been shown to be more bioavailable than sodium selenite or selenium yeast (Jlali et al., 2014). The bioavailable efficacy of HMSeBA in poultry, growing pigs, and weaned pigs has been demonstrated (Jlali et al., 2013, 2014; Briens et al., 2014; Couloigner et al., 2015; Tufarelli et al., 2016; Chao et al., 2019; Li et al., 2020). However, the effect of HMSeBA on the reproductive performance of sows and the growth performance and health of offspring is unclear. In the current study, the effect of HMSeBA supplementation during gestation on sow reproductive performance and the growth performance and health of their offspring was examined. We hypothesis that maternal HMSeBA supplementation during gestation could improve sow reproductive performance and the antioxidant capacities of sows and their offspring. The current study was conducted to test this hypothesis.
Materials and Methods
Animals and experimental design
The protocol of this study was approved by the Animal Care and Use Committee of Sichuan Agricultural University (Approval number: DKYB20131704) and was performed in accordance with the National Research Council’s (NRC) Guideline for the Care and Use of Laboratory Animals (NRC, 2011). The HMSeBA (Selisseo 2% Se) was provided by Adisseo France S.A.S. and Na2SeO3 was obtained from Chengdu Shuxing Feed Co. Ltd. (1% Se).
A total of 45 five-parity sows (Landrace × Yorkshire), with similar initial body weights (BWs) and backfat (BF) thickness, were used in this study (Table 1). Sows were artificially inseminated with pooled semen obtained from two littermate Duroc boars (housed on the research farm) on the day of estrus and then 12 and 24 h later. The experimental design used in this study was a completely randomized design. After artificial insemination, sows were randomly assigned to receive one of the following three diets during gestation: control diet (Control, basal diet, n = 15), Na2SeO3-supplemented diet (Na2SeO3, basal diet + 0.3 mg Se/kg Na2SeO3, n = 15), and HMSeBA-supplemented diet (HMSeBA, basal diet + 0.3 mg Se/kg HMSeBA, n = 15). The experimental diets were formulated to meet the nutrient requirements of gestating sows as recommended by NRC (2012) (Table 2), except for that of selenium. The selenium additives were added to a premix and then used for the manufacturing of the diet of sow. During gestation, all sows ingested 2.32 kg diet daily (0830 and 1430 hours) from day 0 to 90 of gestation and 2.76 kg diet daily (0830 and 1430 hours) from day 91 of gestation to parturition. Sows were moved to the farrowing pen on day 109 of gestation. In the first 24 h after farrowing, the litter sizes were adjusted to 12 ± 1 piglets per nest by cross-fostering within the same treatment.
Table 1.
Effect of maternal selenium intake during gestation on BW and P2-BF of sows1
| Treatment | Gestation stage | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | Control | Na2SeO3 | HMSeBA | Day 1 | Day 30 | Day 60 | Day 90 | Day 110 | T | S | T × S |
| BW, kg | 237.52 ± 6.67 | 236.91 ± 7.25 | 241.30 ± 6.67 | 222.69 ± 3.29d | 225.06 ± 3.75d | 235.05 ± 4.14c | 245.17 ± 4.34b | 264.90 ± 4.67a | 0.886 | <0.001 | 0.444 |
| BF, mm | 13.50 ± 0.10 | 13.41 ± 1.09 | 14.29 ± 0.10 | 13.80 ± 0.62 | 13.72 ± 0.61 | 13.48 ± 0.55 | 13.93 ± 0.64 | 13.73 ± 0.61 | 0.801 | 0.213 | 0.154 |
1Data are shown as mean values with their standard errors. Sows were regarded as the experimental units, n = 13 for Control group at each gestation stage; n = 11 for Na2SeO3 group at each gestation stage; n = 13 for HMSeBA group at each gestation stage. When significant main effects or interactive effects were observed, the means were compared using the Tukey–Kramer method with a P < 0.05 indicating significance. Therefore, a-d within a row and within a main effect, mean values without a common letter are significantly different (P < 0.05). T, treatment; S, gestation stage, T × S, interaction between treatment and gestation stag; Control, basal diet; Na2SeO3, 0.3 mg/Kg Na2SeO3; HMSeBA, 0.3 mg/Kg HMSeBA.
Table 2.
Composition and nutrient levels of the basal diet (as-fed basis)
| Item | Gestation | Lactation |
|---|---|---|
| Ingredients, % | ||
| Corn | 63.53 | 62.89 |
| Soybean meal | 14.50 | 22.13 |
| Soybean oil | 2.00 | |
| Wheat bran | 18.00 | 6.00 |
| Fish meal | 2.60 | |
| l-Lysine HCl (98%) | 0.05 | 0.27 |
| d, l-Methionine (99%) | 0.02 | 0.13 |
| l-Threonine (98.5%) | 0.05 | |
| Limestone | 1.15 | 0.98 |
| Dicalcium phosphate | 1.65 | 1.50 |
| Choline chloride (50%) | 0.15 | 0.15 |
| Sodium chloride | 0.40 | 0.40 |
| Sodium bicarbonate | 0.40 | |
| Vitamin and mineral premix | 0.501 | 0.552 |
| Total | 100.00 | 100.00 |
| Nutrient level3 | ||
| Digestible energy, Mcal/kg | 3.04 | 3.27 |
| Crude protein, % | 14.03 | 17.50 |
| Standard ideal digestible-Lysine, % | 0.56 | 0.98 |
| Total calcium, % | 0.88 | 0.90 |
| Total phosphorus, % | 0.71 | 0.70 |
1Vitamin and mineral mixture for gestation sows supplied the following amounts of vitamins/kg and minerals/kg of complete diet: 6,000 IU vitamin A; 1,500 IU vitamin D3; 80 IU vitamin E; 2.6 mg vitamin B1; 6.5 mg vitamin B2; 3.9 mg vitamin B6; 15 μg vitamin B12; 26 mg niacin; 1.3 mg folate; 120 mg iron; 20 mg copper; 120 mg zinc; 30 mg manganese; and 0.3 mg iodine. Control, 0 mg selenium/kg (analyzed value is 0.13 mg selenium/kg); Na2SeO3, 0.3 mg selenium/kg (analyzed value is 0.41 mg selenium/kg); HMSeBA, 0.30 mg selenium/kg (analyzed value is 0.46 mg selenium/kg).
2Vitamin and mineral mixture for lactation sows supplied the following amounts of vitamins/kg and minerals/kg of complete diet: 6,000 IU vitamin A; 1,200 IU vitamin D3; 50 IU vitamin E; 1.0 mg vitamin B1; 3.6 mg vitamin B2; 1.8 mg vitamin B6; 12.5 μg vitamin B12; 20 mg niacin; 12.5 mg pantothenic acid; 2.0 mg folacin; 120 mg iron; 20 mg copper; 120 mg zinc; 30 mg manganese; 0.3 mg selenium; and 0.3 mg iodine.
3Calculated according to Chinese Feed Database (2014).
After farrowing, sows were received the same standard lactation diet as recommended by NRC (2012) (Table 2) and were offered the diet three times per day (i.e., 0800, 1400, and 1730 hours), starting at 1.50 kg/d, then gradually increasing by 1 kg/d until the sixth day ad libitum. Sow’s milk was the unique source of food for the suckling piglet during lactation. All animals had free access to water throughout the experiment. The ambient temperature for gestation sows was maintained at 20 to 23 °C. Heating light and pads were provided for suckling piglets, and the temperature was maintained at 26 to 32 °C, which gradually decreased with the increase in age. All sows were housed in individual feed stalls (2.0 × 0.8 m) during gestation and were housed in farrowing pens during lactation. Daily feed intake during lactation was recorded. Male piglets were castrated on day 7 after birth, and all piglets were weaned on day 21 of lactation. The study began with 45 sows; 2 sows in the Na2SeO3 group were eliminated due to limb and hoof diseases after mating, and the final number of pregnant sows used for the analysis was 37 because the other 6 sows (2 sows each group) were found to be nonpregnant or to have spontaneously aborted; the final number of lactation sows used for analysis was 36 because 1 sow died at labor.
Escherichia coli lipopolysaccharide challenge
At 21 d of age, 12 healthy male pigs were selected from 6 litters of each treatment group, and 6 pigs per group were intraperitoneally injected with 50 μg/kg BW of lipopolysaccharide (LPS), while the other 6 pigs in each treatment were intraperitoneally injected with an equivalent amount of sterile saline. Pigs prepared for the LPS challenge raised in a separate metabolic cage (1.2 × 0.4 × 0.5 m), 4 h before the injection. The rectal temperature of weaning piglets was measured at 0, 2, and 4 h after the LPS challenge, and the blood samples were also collected at 0, 2, and 4 h after the LPS challenge. The LPS (E. coli L2880, Sigma) was dissolved in sterile saline (9 g/L) to make the LPS solution (500 mg LPS per liter of saline). The dosage of the LPS injection to pigs was described in our previous study (Chen et al., 2017).
Measurement
The fasting BW and BF of sows were measured on days 0, 30, 60, 90, and 110 of gestation, farrowing day, and day 21 of lactation. BF was measured at 65 mm to the left side of the dorsal midline at the level of the last rib (P2) using ultrasound (Renco Lean-Meater, USA). At farrowing, the total number of pigs born, birth weight, placental weight, and piglet birth interval was recorded. In addition, the uniformity of newborns was determined using the piglet birth weight distribution and the intra-litter coefficient of variation (CV). BW of each piglet was measured weekly during lactation.
Sample collection
Blood samples of sows (10 mL) were collected from the ear vein at 0, 45, 90, and 135 min of farrowing and on day 21 of lactation. Blood samples of piglets (10 mL) were collected from the precaval vein of the piglet whose BW was closest to the average BW of the litter on days 7 and 14 of lactation and day 14 postweaning. Colostrum samples (10 mL) were collected on day 0 of lactation, and milk samples (10 mL) were collected on day 21 of lactation and stored at −20 °C for further analysis (Balzani et al., 2016). Serum samples were stored at −20 °C for further analysis.
The representative feed samples were stored at −20 °C until analyzing for selenium. At various time points after injection of saline or LPS, blood samples of the pigs were collected from the precaval vein with vacuum tubes, and serum was stored at −20 °C for further analysis (Gu et al., 2017).
Analysis of serum and milk SELENOP level
The concentration of selenoprotein P (SELENOP) in serum and milk was determined using ELISA Assay kits suitable for pigs (Nanjing Institute of Jiancheng Biological Engineering, Nanjing, China) according to the manufacturer’s instructions.
Analysis of oxidant and antioxidant contents
The contents of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), catalase (CAT), total antioxidant capacity (T-AOC), and malondialdehyde (MDA) were analyzed using the respective assay kits (Nanjing Institute of Jiancheng Biological Engineering, Nanjing, China) according to the manufacturer’s instructions.
Statistical analysis
Data were analyzed using the SAS statistical package (version 9.4; SAS Inst. Inc., Cary, NC). Before statistical analysis, the original data were tested using the Grubbs’ test method and outliers were removed (Li et al., 2018), and the normality and homogeneity of variances were evaluated by Shapiro–Wilk W test and Levene’s test, respectively. Unless otherwise specified, data were analyzed using one-way analysis of variance (ANOVA) procedures followed by Tukey’s multiple range test. Data for BW and BF of gestation sows, growth performance of lactation piglets, and oxidative status of farrowing sows were analyzed using the Mixed Model procedure with repeated measures. Before analysis, the best covariance assumption structures model (simple covariance structure, compound symmetry, autoregression, first order ante-dependence covariance structure, unstructured, heterogeneour compound symmetry) was selected based on the Akaike and Bayesian information criteria values. Blood antioxidative indicators of weaning pigs after LPS challenge were analyzed using the MIXED procedure according to the following model: , in which Y is the analyzed variable; μ is the mean; α i is the effect of diets (i = 1, 2, or 3); β j is the effect of LPS (j = 1 or 2); γ k is the effect of time (k = 1, 2, or 3); (αβ)ij refers to the interaction between diets and LPS; (αγ)ik refers to the interaction between diets and time; (βγ)jk refers to the interaction between LPS and time; (αβγ)ijk refers to the interaction among diets, LPS, and time; and ε ijk represents the residual error. The ranking of birth weight and CVBW was analyzed by Chi-square tests. All data were shown as means with their SE. Differences were considered significant when P < 0.05, whereas differences with 0.10 > P ≥ 0.05 were considered to indicate a trend toward significance.
Results
BW, BF, and average daily feed intake of sows
Results showed that BW and P2-BF of sows during gestation were not affected by maternal diets (P > 0.05, Table 1). Besides, HMSeBA supplementation during gestation had no effect on the BW, BF, and average daily feed intake (ADFI) of lactation sows as compared with the control and Na2SeO3 diets (P > 0.05, Table 3).
Table 3.
Effect of maternal selenium intake during gestation on BW, P2-BF, and ADFI of lactation sows
| Treatment1 | ||||
|---|---|---|---|---|
| Item | Control | Na2SeO3 | HMSeBA | P-value |
| Litters, n | 13 | 10 | 13 | |
| BW, kg | ||||
| Day 1 of lactation | 247.92 ± 4.78 | 247.56 ± 6.70 | 256.15 ± 7.02 | 0.546 |
| Day 21 of lactation | 250.35 ± 5.55 | 257.55 ± 8.38 | 251.77 ± 6.44 | 0.740 |
| Day 1 to 21 of lactation | 0.83 ± 2.17 | 2.91 ± 3.85 | −1.87 ± 2.78 | 0.524 |
| BF thickness, mm | ||||
| Day 1 of lactation | 12.96 ± 0.86 | 12.63 ± 0.90 | 13.61 ± 1.14 | 0.782 |
| Day 21 of lactation | 12.60 ± 0.82 | 12.92 ± 1.13 | 12.81 ± 1.03 | 0.974 |
| Day 1 to 21 of lactation | −0.36 ± 0.25 | −0.11 ± 0.61 | −0.80 ± 0.33 | 0.481 |
| ADFI, kg/d | ||||
| First week of lactation | 4.58 ± 0.32 | 4.92 ± 0.39 | 4.80 ± 0.34 | 0.775 |
| Second week of lactation | 6.30 ± 0.30 | 6.42 ± 0.43 | 6.00 ± 0.27 | 0.660 |
| Third week of lactation | 6.90 ± 0.41 | 7.21 ± 0.35 | 6.55 ± 0.27 | 0.449 |
| Overall | 5.92 ± 0.27 | 6.18 ± 0.34 | 5.78 ± 0.26 | 0.634 |
1Data are shown as mean values with their standard errors. Control, basal diet; Na2SeO3, 0.3 mg/Kg Na2SeO3; HMSeBA, 0.3 mg/Kg HMSeBA.
Sow and litter performance
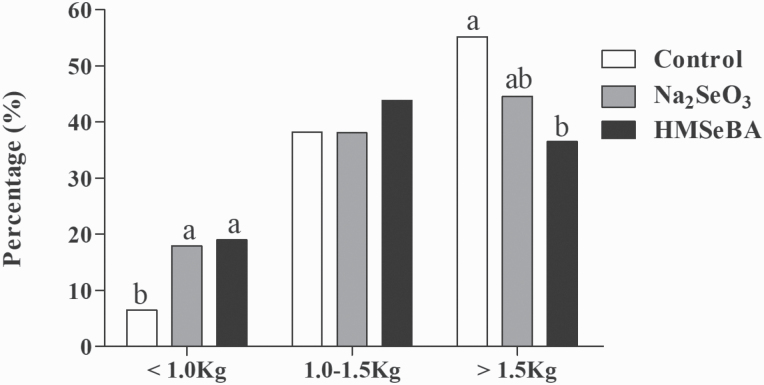
Compared with the control group, maternal HMSeBA and Na2SeO3 supplementation improved the number of total born piglets, while HMSeBA supplementation decreased birth weight (P < 0.05, Table 4). Meanwhile, the percentage of piglets with birth weight more than 1.5 kg was lower in the HMSeBA group than that in the control group, while the percentage of piglets with birth weight less than 1.0 kg was higher in the HMSeBA group than that in the control group (P < 0.05, Figure 1). Besides, maternal HMSeBA supplementation decreased the birth interval of piglets (P < 0.05, Table 4) and tended to decrease the duration of farrowing (P = 0.055, Table 4) as compared with control and Na2SeO3.
Table 4.
Effect of maternal selenium intake during gestation on reproductive performance of sows
| Treatment1 | ||||
|---|---|---|---|---|
| Item | Control | Na2SeO3 | HMSeBA | P-value |
| Litters, n | 13 | 11 | 13 | |
| Total born | 14.38 ± 0.68b | 17.00 ± 1.00a | 16.92 ± 0.75a | 0.038 |
| Birth weight, kg2 | 1.57 ± 0.03a | 1.46 ± 0.10ab | 1.34 ± 0.05b | 0.029 |
| Litter weight, kg2 | 20.73 ± 1.26 | 19.27 ± 2.02 | 20.71 ± 1.09 | 0.727 |
| CVBW, % | 21.99 ± 1.26 | 22.87 ± 1.94 | 25.22 ± 1.90 | 0.380 |
| Total placental weight for live piglets born, kg | 4.89 ± 0.33 | 4.85 ± 0.69 | 4.67 ± 0.43 | 0.939 |
| Placental efficiency for piglets born alive3 | 4.33 ± 0.24 | 4.30 ± 0.32 | 4.84 ± 0.22 | 0.260 |
| Piglet birth interval, min | 20 ± 1.09a | 19 ± 1.99a | 14 ± 1.16b | 0.013 |
| Duration of farrowing, min4 | 236 ± 18 | 252 ± 21 | 195 ± 11 | 0.055 |
1Data are shown as mean values with their standard errors. Control, basal diet; Na2SeO3, 0.3 mg/Kg Na2SeO3; HMSeBA, 0.3 mg/Kg HMSeBA.
2Litter weight at birth and average birth weight of all piglets born alive.
3Placental efficiency = live litter weight/placental weight for live piglets born.
4Duration of farrowing: defined as the time interval between the birth of first and last piglet.
a,bValues within a row with different superscript letters were significantly different (P < 0.05).
Figure 1.
Effect of selenium intake during gestation on the ranking of birth weight. Control, basal diet; Na2SeO3, 0.3 mg/Kg Na2SeO3; HMSeBA, 0.3 mg/Kg HMSeBA. a,bColumns with different superscript letters mean significant differences (P < 0.05).
During the first week of lactation, maternal HMSeBA supplementation during pregnancy increased litter weight gain compared with the Na2SeO3 group (P < 0.05, Table 5) and increased piglet average daily gain (ADG) compared with the control and Na2SeO3 groups (P < 0.05, Table 5).
Table 5.
Effect of maternal selenium intake during gestation on growth performance of lactation piglets
| Treatment1 | P-value | |||||
|---|---|---|---|---|---|---|
| Item | Control | Na2SeO3 | HMSeBA | Trt | Time | Trt × time |
| Litters, n | 13 | 10 | 13 | |||
| After cross-fostering | 12.00 ± 0.25 | 11.80 ± 0.36 | 11.77 ± 0.28 | |||
| Pigs weaned | 11.31 ± 0.41 | 10.00 ± 0.79 | 11.00 ± 0.42 | |||
| Piglet mean BW, kg | ||||||
| After cross-fostering | 1.57 ± 0.03 | 1.46 ± 0.10 | 1.42 ± 0.06 | 0.280 | <0.0001 | 0.032 |
| Day 7 of lactation | 2.61 ± 0.06 | 2.44 ± 0.08 | 2.63 ± 0.10 | |||
| Day 14 of lactation | 3.79 ± 0.12 | 3.58 ± 0.14 | 3.83 ± 0.10 | |||
| Day 21 of lactation | 5.55 ± 0.19 | 5.25 ± 0.18 | 5.35 ± 0.08 | |||
| Litter weight, kg | ||||||
| After cross-fostering | 18.76 ± 0.35 | 17.35 ± 1.52 | 16.73 ± 0.73 | 0.035 | <0.0001 | 0.019 |
| Day 7 of lactation | 31.16 ± 0.76 | 28.15 ± 1.64 | 30.54 ± 0.87 | |||
| Day 14 of lactation | 44.08 ± 1.94a | 36.30 ± 3.13b | 42.80 ± 1.23ab | |||
| Day 21 of lactation | 62.50 ± 2.79 | 52.50 ± 4.26 | 58.69 ± 2.13 | |||
| Litter weight gain, kg | ||||||
| First week of lactation | 12.40 ± 0.78ab | 10.81 ± 0.71b | 13.81 ± 0.51a | 0.832 | 0.0002 | 0.625 |
| Second week of lactation | 12.92 ± 5.21 | 8.15 ± 3.19 | 12.26 ± 1.57 | |||
| Third week of lactation | 18.42 ± 1.25 | 16.20 ± 1.26 | 15.90 ± 1.46 | |||
| Day 1 to 21 of lactation | 43.74 ± 2.72 | 35.16 ± 4.53 | 41.97 ± 2.39 | |||
| Piglet mean ADG, g/d | ||||||
| First week of lactation | 143.4 ± 9.91b | 140.9 ± 8.09b | 172.5 ± 9.37a | 0.552 | <0.0001 | 0.364 |
| Second week of lactation | 169.6 ± 12.02 | 163.0 ± 20.81 | 171.4 ± 11.29 | |||
| Third week of lactation | 250.5 ± 14.11 | 238.9 ± 14.33 | 216.9 ± 10.01 | |||
| Day 1 to 21 of lactation | 568.5 ± 26.85 | 542.7 ± 33.58 | 560.7 ± 14.38 | |||
1Data are shown as mean values with their standard errors. On the same day or week of lactation, the means were compared using the Tukey method with a P < 0.05 indicating significance. Therefore, a,b within a row mean values without a common letter are significantly different (P < 0.05). Trt, treatment; Control, basal diet; Na2SeO3, 0.3 mg/Kg Na2SeO3; HMSeBA, 0.3 mg/Kg HMSeBA.
Antioxidative and oxidative indicators in the plasma of sows at parturition
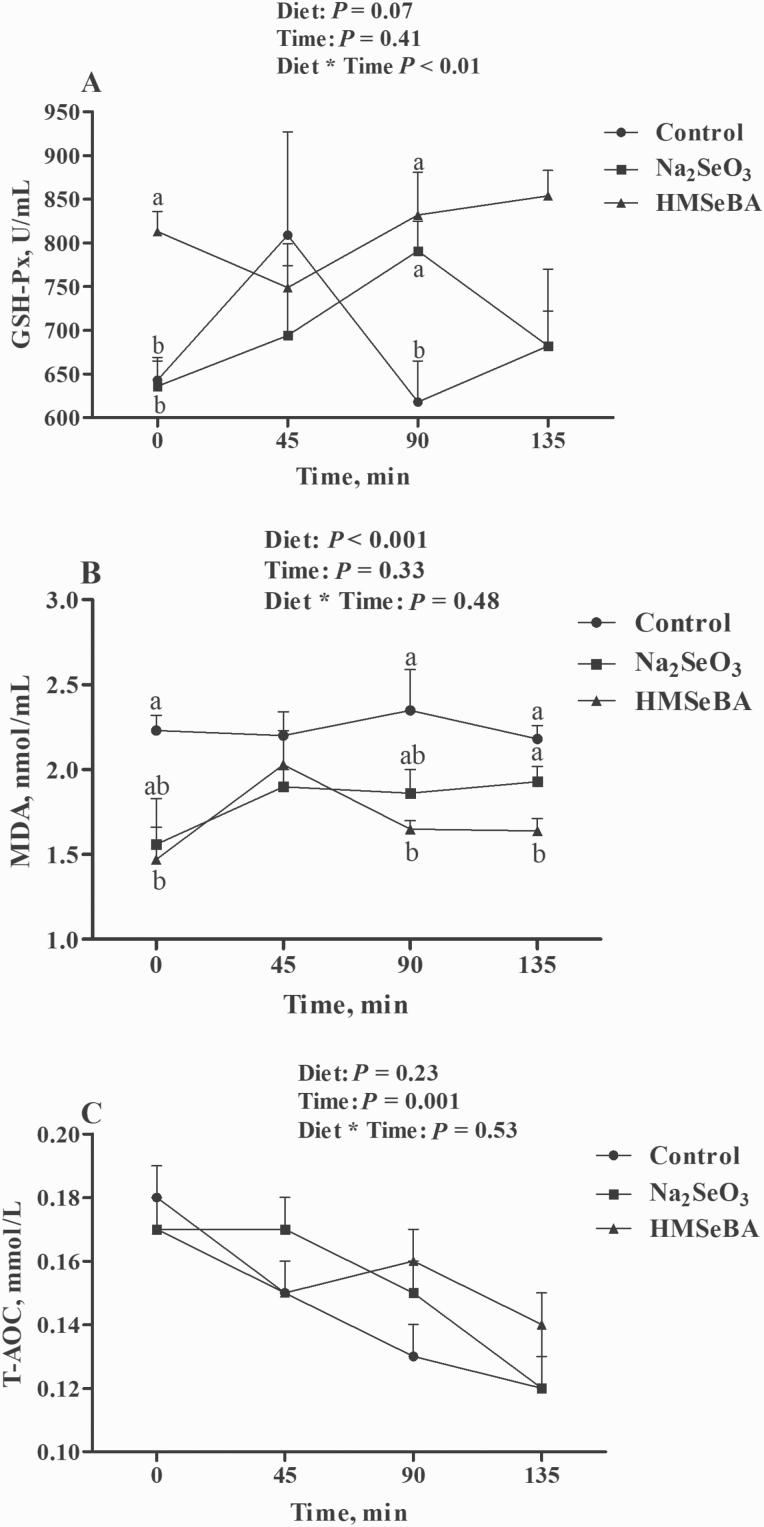
To analyze the effect of maternal diet on antioxidant status at delivery, sows’ blood samples were collected at 0, 45, 90, and 135 min of farrowing. Results showed that maternal diets tended to affect the activity of GSH-Px (P = 0.07, Figure 2) and affected the concentration of MDA (P < 0.001, Figure 2). Meanwhile, farrowing time affected the concentration of T-AOC (P < 0.01, Figure 2). The interaction between maternal diet and farrowing time affected the activity of GSH-Px (P < 0.01, Figure 2). The plasma GSH-Px activities were higher in the HMSeBA group than those in the control and Na2SeO3 groups on farrowing 0 min and higher than those in the control group on farrowing 90 min (Figure 2). In addition, when compared with the control group, maternal HMSeBA supplementation significantly decreased MDA concentration at 0 and 90 min of farrowing (Figure 2). At 135 min, maternal HMSeBA supplementation significantly decreased MDA compared with the control and Na2SeO3 groups (Figure 2). Serum T-AOC concentrations gradually decreased with the extension of farrowing time (Figure 2).
Figure 2.
Effect of maternal selenium intake during gestation on the oxidative status of farrowing sows. (A) Plasma activities of GSH-Px. (B) Plasma levels of MDA. (C) Plasma levels of T-AOC. Data were shown as mean ± SE. n = 6 for each group. At the same point in time, the means were compared using the Tukey method with a P < 0.05 indicating significance. Therefore, different superscript letters for the same point in time were significantly different (P < 0.05). Control, basal diet; Na2SeO3, 0.3 mg/Kg Na2SeO3; HMSeBA, 0.3 mg/Kg HMSeBA.
Concentration of SELENOP
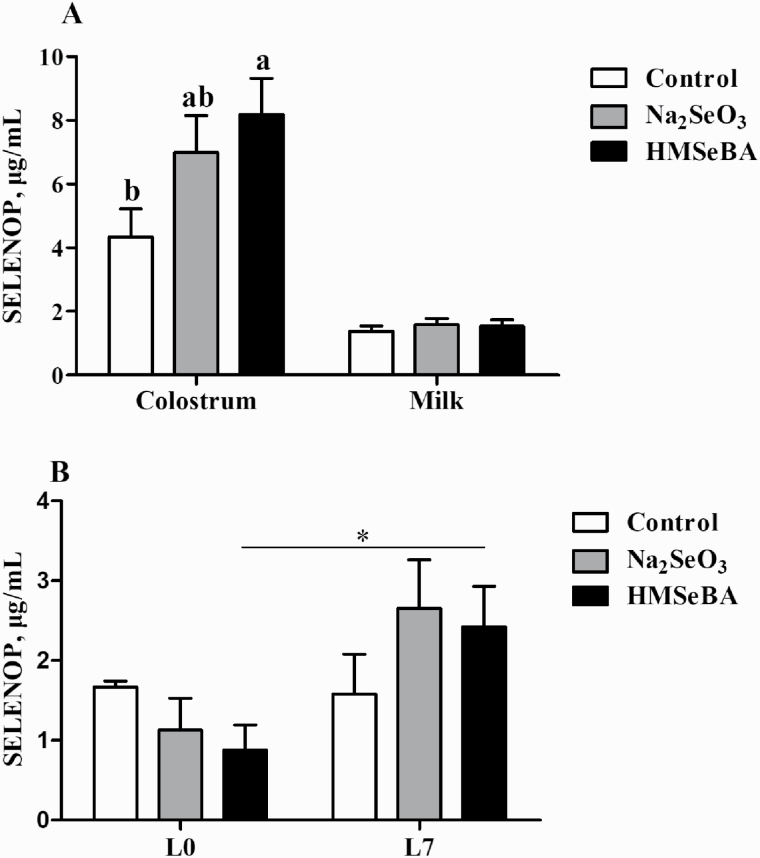
Maternal HMSeBA supplementation increased the concentration of SELENOP in colostrum compared with the control group (P < 0.05, Figure 3A) and Na2SeO3 supplementation being intermediate between the control and HMSeBA group. Moreover, only in the HMSeBA group, SELENOP was higher on day 7 of lactation than day 0 of lactation (P < 0.05, Figure 3B).
Figure 3.
Effect of maternal selenium intake during gestation on the SELENOP of colostrum, milk, and piglets. (A) Effect of maternal selenium intake during gestation on the SELENOP of colostrum and milk (n = 10 for each group). (B) Effect of maternal selenium intake during gestation on the SELENOP of newborn piglets and suckling piglets (n = 6 for each group). Control, basal diet; Na2SeO3, 0.3 mg/Kg Na2SeO3; HMSeBA, 0.3 mg/Kg HMSeBA. Control, basal diet; Na2SeO3, 0.3 mg/Kg Na2SeO3; HMSeBA, 0.3 mg/Kg HMSeBA. L0, day 0 of lactation; L7, day 7 of lactation. Data were shown as mean ± SE. a,bColumns with different superscript letters mean significant differences (P < 0.05). *P < 0.05 of the two groups.
Antioxidative and oxidative indicators of sows during lactation
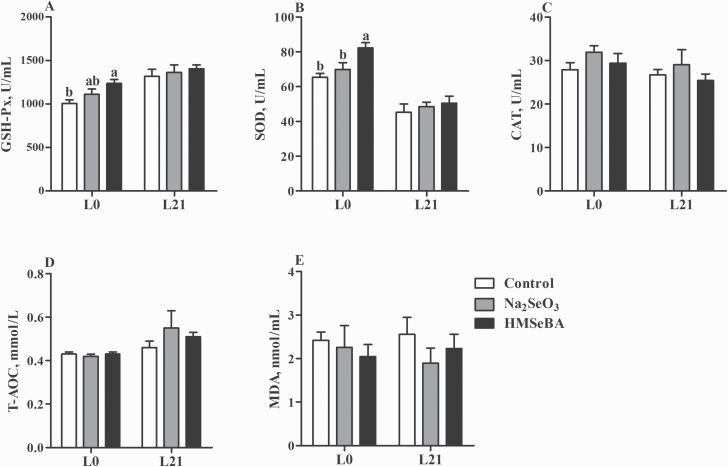
On day 0 of lactation, maternal HMSeBA supplementation increased the activity of GSH-Px compared with the control group (P < 0.05, Figure 4A) and increased the activity of SOD compared with both the control and Na2SeO3 groups (P < 0.05, Figure 4B).
Figure 4.
Effect of maternal selenium intake during gestation on the oxidative status of lactation sows. (A) Serum activities of GSH-Px. (B) Serum activities of SOD. (C) Serum activities of CAT. (D) Serum levels of T-AOC. (E) Serum levels of MDA. L0, day 0 of lactation; L21, day 21 of lactation. Data were shown as mean ± SE; n = 10 for each group. Control, basal diet; Na2SeO3, 0.3 mg/Kg Na2SeO3; HMSeBA, 0.3 mg/Kg HMSeBA. a,bColumns with different superscript letters mean significant differences (P < 0.05).
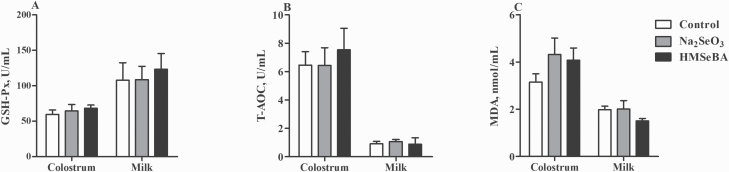
However, there was no significant difference in antioxidant indicators in colostrum and milk between the three groups (P > 0.05, Figure 5).
Figure 5.
Effect of maternal selenium intake during gestation on the oxidative status of colostrum and milk. (A) The activities of GSH-Px. (B) The activities of T-AOC. (C) The levels of MDA. Data were shown as mean ± SE. n = 10 for each group. Control, basal diet; Na2SeO3, 0.3 mg/Kg Na2SeO3; HMSeBA, 0.3 mg/Kg HMSeBA.
Antioxidative and oxidative indicators of suckling piglets
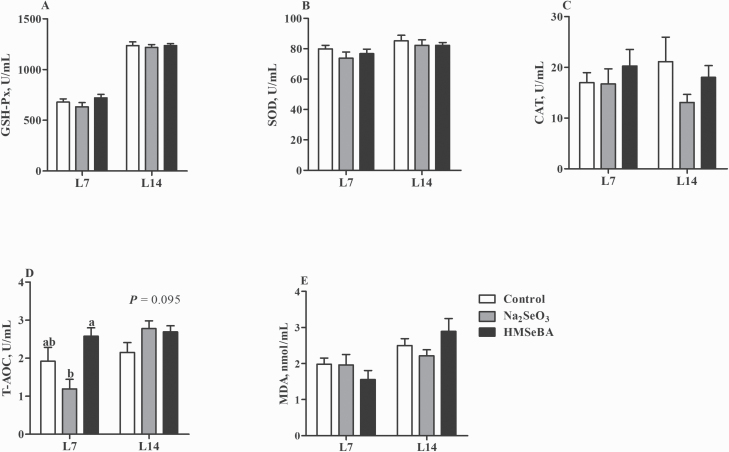
Serum T-AOC concentration of suckling piglets was higher in the HMSeBA group compared with the Na2SeO3 group on day 7 of lactation (P < 0.05, Figure 6D). Moreover, maternal HMSeBA and Na2SeO3 supplementation tended to increase T-AOC compared with the control group on day 14 of lactation (P = 0.095, Figure 6D).
Figure 6.
Effect of maternal selenium intake during gestation on the oxidative status of suckling piglets. (A) Serum activities of GSH-Px. (B) Serum activities of SOD. (C) Serum activities of CAT. (D) Serum levels of T-AOC. (E) Serum levels of MDA. L7, day 7 of lactation; L14, day 14 of lactation. Data were shown as mean ± SE. n = 10 for each group. Control, basal diet; Na2SeO3, 0.3 mg/Kg Na2SeO3; HMSeBA, 0.3 mg/Kg HMSeBA. a,bColumns with different superscript letters mean significant differences (P < 0.05).
Antioxidant and oxidative indicators of weaning piglets challenged with E. coli LPS
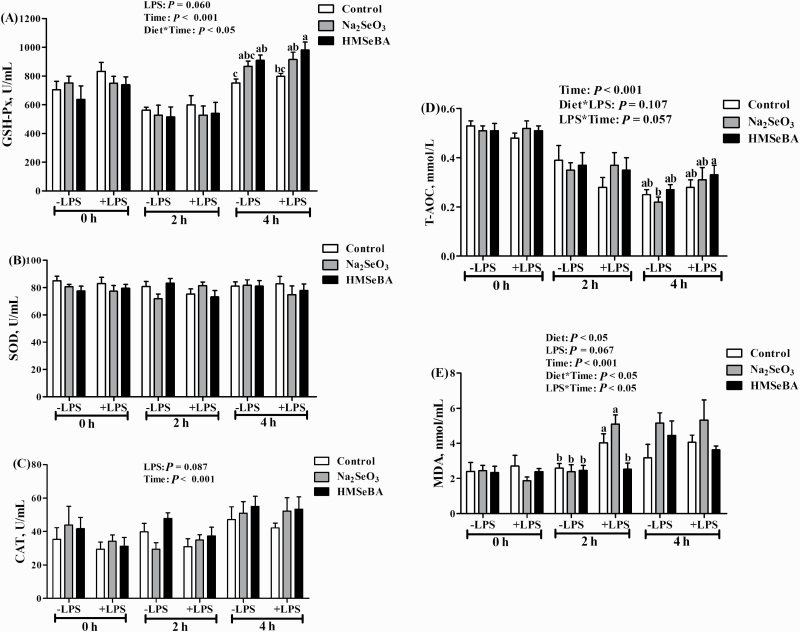
To determine the potential protective effect of maternal HMSeBA supplementation on oxidative stress of weaning piglets, the piglets were challenged with LPS on day 21 of age. The activity of GSH-Px was affected by Time (P < 0.001, Figure 7A) and Diet * Time (P < 0.05, Figure 7A) and tended to be affected by LPS (P = 0.060, Figure 7A). Four hours after the LPS challenge, LPS-challenged piglets from the HMSeBA group had a higher serum GSH-Px activity compared with both without LPS-challenged control group and with the LPS-challenged control group, while the Na2SeO3 group was intermediate. In addition, the activity of CAT was affected by the Time of LPS challenge (P < 0.001, Figure 7C) and tended to be affected by LPS in a similar way to GSH-Px activity (P = 0.087, Figure 7C). Meanwhile, the concentration of T-AOC was affected by Time (P < 0.001, Figure 7D) and tended to be affected by LPS * Time (P = 0.057, Figure 7D). Four hours after LPS challenge, T-AOC concentration in piglets from the LPS-challenged HMSeBA group was higher than those in without the LPS-challenged Na2SeO3 group. Moreover, MDA concentration was significantly affected by Diet (P < 0.05, Figure 7E), Time (P < 0.001, Figure 7E), Diet * Time (P < 0.05, Figure 7E), and LPS * Time (P < 0.05, Figure 7E) and tended to be affected by LPS (P = 0.067, Figure 7E). Two hours after LPS challenge, MDA concentration in the LPS-challenged control group and LPS-challenged Na2SeO3 group was higher than those in the LPS-challenged HMSeBA group and LPS-unchallenged control, Na2SeO3, and HMSeBA groups.
Figure 7.
Effect of maternal selenium intake during gestation on the systematic oxidative status of weaning piglets challenged with LPS. −LPS, piglets not challenged with LPS; +LPS, piglets challenged with LPS. Mean values with their standard errors, n = 6 in each group. (A) Serum activities of GSH-Px. (B) Serum activities of SOD. (C) Serum activities of CAT. (D) Serum levels of T-AOC. (E) Serum levels of MDA. Control, 0 mg/Kg Na2SeO3; Na2SeO3, 0.3 mg/Kg Na2SeO3; HMSeBA, 0.3 mg/Kg HMSeBA. a,bColumns with different superscript letters at the same point in time mean significant differences (P < 0.05).
Discussion
Selenium is an essential trace element in sows and exists in nature in both inorganic and organic forms (Surai and Fisinin, 2016; Akahoshi et al., 2019). HMSeBA is an organic Se source, which has been studied in poultry, growing pigs, and weaned pigs (Jlali et al., 2013, 2014; Briens et al., 2014; Couloigner et al., 2015; Tufarelli et al., 2016; Chao et al., 2019; Li et al., 2020), and this is the first study focused on the effect of HMSeBA supplementation in gestation diet on reproductive performance of sows and long-term effects on the growth performance and health of offspring. Consistent with our experimental hypothesis, HMSeBA supplementation during pregnancy improved sow reproductive performance and the antioxidant capacities of sows and offspring. The results of this study will provide a reference for the application of HMSeBA in sow diet.
In the current study, maternal HMSeBA intake during gestation significantly increased the number of total born piglets compared with the control group, not the Na2SeO3 supplementation. Thus, this organic form of Se (HMSeBA) induces additional improvement compared with the mineral form. Meanwhile, piglets from the HMSeBA group existed lower birth weight compared with the control group. Similar results were obtained by Mahan and Peters (2004); during the grower period and continued through four parities study, sows supplemented with 0.15 ppm of organic or inorganic Se significantly increased the number of pigs born (total, live). Besides, Chen et al. (2016) also found that sows fed organic selenium during gestation and lactation produced more born alive piglets compared with inorganic Se. Meanwhile, an increase in litter size was shown to be genetically connected with a decrease in the mean piglet birth weight (Wolf et al., 2008; Beaulieu et al., 2010). In addition, the lower the birth weight, the shorter the piglet birth intervals (van Dijk et al., 2005). Our results also showed that sows of the HMSeBA group had a shorter birth interval than the sows of the control and Na2SeO3 groups. Moreover, shortening of the expulsion period limits the occurrence of diseases in this period and positively affects the vitality and development of piglets (Alexopoulos et al., 1998). This may be more conducive to the growth and development of piglets in the HMSeBA group.
Reactive oxygen detection (ROS) overproduction during parturition may lead to oxidative stress postpartum in both mother and neonate (Mocatta et al., 2004; Castillo et al., 2005; Argüelles et al., 2006; Wullepit et al., 2012). Previous study also showed that spontaneous parturition could induce oxidative stress at the early parturient period in sows, and the intensity of oxidative stress is related to the duration of the expulsive stage (Szczubiał et al., 2013). Moreover, newborn piglets suffered from serious birth oxidative stress because of the naive antioxidant system, and the oxidative balance gradually recovered with the development of antioxidant system (Yin et al., 2013). To explore the effect of maternal selenium supplementation on the parturition oxidative stress, we analyzed the oxidative status in the sows’ blood during parturition. Our results indicated that the activity of GSH-Px was higher at 0 min of farrowing, and the MDA content was lower at 135 min of farrowing in the blood of the HMSeBA group than those in the control and Na2SeO3 groups. Although previous studies showed that maternal organic Se supplementation during late gestation and lactation improved antioxidative capacities of sows (Hu et al., 2011; Zhan et al., 2011; Chen et al., 2016; Falk et al., 2019), this is the first study focused on the effect of HMSeBA supplementation in gestation diet on the oxidative status of sows during parturition. The results showed that diets supplemented with HMSeBA, more than Na2SeO3, improved the antioxidant capacities of sows during parturition, which ultimately benefits the health of the mother and offspring.
Maternal Se can transfer to progeny through the placenta and milk (Burk et al., 2013; Hill et al., 2014), and selenoprotein P is the major selenium transport protein in milk (Hill et al., 2014). This was confirmed in the current study, as maternal HMSeBA supplementation significantly increased the content of SELENOP in colostrum, and SELENOP in colostrum is higher than milk. Moreover, in the HMSeBA group, SELENOP content is higher in suckling piglets at days 7 of lactation compared with day 0 of lactation. It is worth noting that piglets did not receive colostrum when blood was collected on day 0 of lactation. However, after eating colostrum, suckling piglets in the HMSeBA group significantly increased SELENOP content on days 7 of lactation. These results suggest that the HMSeBA group sows transmitted more SELENOP to their offspring through maternal milk. Similarly, previous study found that dietary organic Se supplement increased milk Se level compared with inorganic Se (Mahan and Peters, 2004; Quesnel et al., 2008; Falk et al., 2019), and Se in colostrum is higher than milk (Chavez, 1985). Moreover, Li et al. (2020) also reported that maternal HMSeBA supplementation during late gestation and lactation significantly increased weaning piglet plasma Se concentration compared with base diet (selenium deficient) group. Although we did not analyze the total selenium content in milk and piglet plasma, the concentration of SELENOP in milk and piglet plasma is sufficient to explain the transmission of selenium from mother to offspring. At the same time, our results also support that the HMSeBA group has the potential to transfer more selenium to offspring. SELENOP is an important Se-containing protein that plays a key role in Selenoproteins synthesis, such as GSH-Px (Short et al., 2018). In the present study, HMSeBA supplementation significantly increased the activity of GSH-Px in the serum of sows on day 0 of lactation and increased T-AOC of suckling piglets on day 7 of lactation. Oxidative stress impairs growth performance of pigs (Yuan et al., 2007; Chen et al., 2018), while enhanced antioxidant capacity can improve the growth performance of weaned pigs. In the present study, HMSeBA supplementation significantly increased the litter weight gain at the first week of lactation compared with the Na2SeO3 group and increased mean ADG of piglets during the first week of lactation compared with both the control group and Na2SeO3 group. This might be because dietary HMSeBA supplementation increased the antioxidant capacity of sows and piglets. It is worth noting that although the piglet BW and litter size were the same after cross-fostering, the HMSeBA group had a higher number of total born piglets and higher growth performance during lactation, which shows that HMSeBA has more potential to produce more kilogram of meat.
We also determined the effect of maternal HMSeBA supplementation on growth performance and oxidative stress response of LPS-challenged piglets. LPS is a cell wall component of Gram-negative bacteria, which can induce host cell inflammation (Rietschel et al., 1994). LPS-challenged animals are used as immune and oxidative stress models (Wang et al., 2011; Ginsberg et al., 2012; Al-Amin et al., 2016; Chen et al., 2017; Gu et al., 2017). The activation of TLR signaling by LPS enhanced ROS generation (Park et al., 2004, 2006; Ryan et al., 2004). Consistent with previous studies, LPS challenge significantly increased the body temperature of pigs (Wang et al., 2011; Chen et al., 2017; Gu et al., 2017). In addition, LPS challenge tended to increase the concentration of MDA in weaning pigs. Similarly, previous studies showed that LPS challenge increased the concentration of MDA (Yi et al., 2014; Al-Amin et al., 2016). In addition, antioxidants (N-acetylcysteine and quercetin) can alleviate LPS-induced ROS generation (Yi et al., 2014; Tang et al., 2019). In the present study, GSH-Px was significantly affected by the interaction between maternal diets and LPS challenge time in weaning pigs. Meanwhile, LPS-challenged pigs from the HMSeBA group had higher GSH-Px and T-AOC, while lower MDA than pigs from the control group at day 21 of age. Although selenium supplementation seems to support increased GSH-Px activity, only HMSeBA supplementation was observed to significantly increase GSH-Px activity compared with the control group. So, within the two Se sources, the organic form of the HMSeBA supplementation tended to induce an additional increase of GSH-Px activity. Thus, maternal HMSeBA supplementation might have a long-term effect on the antioxidant capacity of offspring.
In conclusion, maternal dietary HMSeBA supplementation during gestation increased litter size, shortened duration of farrowing, enhanced the antioxidant capacities of sows, and promoted the growth performance of suckling pigs at the first week of lactation as well as enhanced antioxidant capacity of offspring. Although HMSeBA increased litter size and lowered birth weight, improved growth performance during lactation did not affect the subsequent long-term growth performance. Therefore, sows HMSeBA supplement during gestation has the potentiality to produce more kilogram of meat.
Acknowledgment
The present study was supported by Adisseo France S.A.S and the HIGHER EDUCATION DISCIPLINE INNOVATION PROJECT of CHINA (D17015). B.F. was a recipient of Initial Research Funding from Sichuan Agricultural University and Thousand Talent Program from Sichuan Province.
Glossary
Abbreviations
- ADFI
average daily feed intake
- BF
backfat
- BW
body weight
- CAT
catalase
- GSH-Px
glutathione peroxidise
- HMSeBA
2-hydroxy-4-methylselenobutanoic
- LPS
lipopolysaccharide
- MDA
malondialdehyde
- SOD
superoxide dismutase
- T-AOC
total antioxidant capacity
Ethical Statement
The use of animals for this research was approved by the Institutional Animal Care and Use Committee of Sichuan Agricultural University.
Conflict of interest statement
The authors declare that they have no competing interests.
Literature Cited
- Agarwal A., and Allamaneni S. S.. . 2004. Role of free radicals in female reproductive diseases and assisted reproduction. Reprod Biomed Online. 9(3):338–347. doi: 10.1016/s1472-6483(10)62151-7 [DOI] [PubMed] [Google Scholar]
- Agarwal A., Gupta S., and Sikka S.. . 2006. The role of free radicals and antioxidants in reproduction. Curr. Opin. Obstet. Gynecol. 18:325–332. doi: 10.1097/01.gco.0000193003.58158.4e [DOI] [PubMed] [Google Scholar]
- Akahoshi N., Anan Y., Hashimoto Y., Tokoro N., Mizuno R., Hayashi S., Yamamoto S., Shimada K. I., Kamata S., and Ishii I.. . 2019. Dietary selenium deficiency or selenomethionine excess drastically alters organ selenium contents without altering the expression of most selenoproteins in mice. J. Nutr. Biochem. 69:120–129. doi: 10.1016/j.jnutbio.2019.03.020 [DOI] [PubMed] [Google Scholar]
- Al-Amin M. M., Alam T., Hasan S. M., Hasan A. T., and Quddus A. H.. . 2016. Prenatal maternal lipopolysaccharide administration leads to age- and region-specific oxidative stress in the early developmental stage in offspring. Neuroscience 318:84–93. doi: 10.1016/j.neuroscience.2016.01.002 [DOI] [PubMed] [Google Scholar]
- Alexopoulos C., Saratsis P., Samouilidis S., Saoulidis K., Brozos C., and Kyriakis S. C.. . 1998. The effect of cloprostenol alone or with oxytocin on induction of parturition, litter characteristics and subsequent fertility of the sow. Reprod. Domest. Anim. 33(2):83–88. doi: 10.1111/j.1439-0531.1998.tb01319.x [DOI] [Google Scholar]
- Argüelles S., Machado M. J., Ayala A., Machado A., and Hervías B.. . 2006. Correlation between circulating biomarkers of oxidative stress of maternal and umbilical cord blood at birth. Free Radic. Res. 40:565–570. doi: 10.1080/10715760500519834 [DOI] [PubMed] [Google Scholar]
- Balzani A., Cordell H. J., and Edwards S. A.. . 2016. Evaluation of an on-farm method to assess colostrum IgG content in sows. Animal 10:643–648. doi: 10.1017/S1751731115002451 [DOI] [PubMed] [Google Scholar]
- Beaulieu A. D., Aalhus J. L., Williams N. H., and Patience J. F.. . 2010. Impact of piglet birth weight, birth order, and litter size on subsequent growth performance, carcass quality, muscle composition, and eating quality of pork. J. Anim. Sci. 88:2767–2778. doi: 10.2527/jas.2009-2222 [DOI] [PubMed] [Google Scholar]
- Berchieri-Ronchi C. B., Kim S. W., Zhao Y., Correa C. R., Yeum K. J., and Ferreira A. L.. . 2011. Oxidative stress status of highly prolific sows during gestation and lactation. Animal 5:1774–1779. doi: 10.1017/S1751731111000772 [DOI] [PubMed] [Google Scholar]
- Briens M., Mercier Y., Rouffineau F., Mercerand F., and Geraert P. A.. . 2014. 2-Hydroxy-4-methylselenobutanoic acid induces additional tissue selenium enrichment in broiler chickens compared with other selenium sources. Poult. Sci. 93:85–93. doi: 10.3382/ps.2013-03182 [DOI] [PubMed] [Google Scholar]
- Burk R. F., Olson G. E., Hill K. E., Winfrey V. P., Motley A. K., and Kurokawa S.. . 2013. Maternal-fetal transfer of selenium in the mouse. FASEB J. 27:3249–3256. doi: 10.1096/fj.13-231852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo C., Hernandez J., Bravo A., Lopez-Alonso M., Pereira V., and Benedito J. L.. . 2005. Oxidative status during late pregnancy and early lactation in dairy cows. Vet. J. 169:286–292. doi: 10.1016/j.tvjl.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Chao Y., Yu B., He J., Huang Z., Mao X., Luo J., Luo Y., Zheng P., Yu J., and Chen D.. . 2019. Effects of different levels of dietary hydroxy-analogue of selenomethionine on growth performance, selenium deposition and antioxidant status of weaned piglets. Arch. Anim. Nutr. 73:374–383. doi: 10.1080/1745039X.2019.1641368 [DOI] [PubMed] [Google Scholar]
- Chavez E. R. 1985. Nutritional significance of selenium supplementation in a semi-purified diet fed during gestation and lactation to first-litter gilts and their piglets. Can. J. Anim. Sci. 65(2):497–506. doi: 10.4141/cjas85-057 [DOI] [Google Scholar]
- Chen J., Han J. H., Guan W. T., Chen F., Wang C. X., Zhang Y. Z., Lv Y. T., and Lin G.. . 2016. Selenium and vitamin E in sow diets: II. Effect on selenium status and antioxidant status of the progeny. Anim. Feed Sci. Technol. 221:101–110. doi: 10.1016/j.anifeedsci.2016.08.021 [DOI] [Google Scholar]
- Chen J. L., Li Y., Yu B., Chen D. W., Mao X. B., Zheng P., Luo J. Q., and He J.. . 2018. Dietary chlorogenic acid improves growth performance of weaned pigs through maintaining antioxidant capacity and intestinal digestion and absorption function. J. Anim. Sci. 96(3):1108–1118. doi: 10.1093/jas/skx078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. L., Mou D. L., Hu L., Zhen J., Che L. Q., Fang Z. F., Xu S. Y., Lin Y., Feng B., Li J., . et al. 2017. Effects of maternal low-energy diet during gestation on intestinal morphology, disaccharidase activity, and immune response to lipopolysaccharide challenge in pig offspring. Nutrients 9(10):1115. doi: 10.3390/nu9101115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Feed Database. 2014. Table of Feed Composition and Nutritive Value in China. 25th edition. China Feed, 21:29–42. [Google Scholar]
- Couloigner F., Jlali M., Briens M., Rouffineau F., Geraert P. A., and Mercier Y.. . 2015. Selenium deposition kinetics of different selenium sources in muscle and feathers of broilers. Poult. Sci. 94:2708–2714. doi: 10.3382/ps/pev282 [DOI] [PubMed] [Google Scholar]
- van Dijk A. J., van Rens B. T., van der Lende T., and Taverne M. A.. . 2005. Factors affecting duration of the expulsive stage of parturition and piglet birth intervals in sows with uncomplicated, spontaneous farrowings. Theriogenology 64:1573–1590. doi: 10.1016/j.theriogenology.2005.03.017 [DOI] [PubMed] [Google Scholar]
- Falk M., Lebed P., Bernhoft A., Framstad T., Kristoffersen A. B., Salbu B., and Oropeza-Moe M.. . 2019. Effects of sodium selenite and l-selenomethionine on feed intake, clinically relevant blood parameters and selenium species in plasma, colostrum and milk from high-yielding sows. J. Trace Elem. Med. Biol. 52:176–185. doi: 10.1016/j.jtemb.2018.12.009 [DOI] [PubMed] [Google Scholar]
- Fortier M. E., Audet I., Giguère A., Laforest J. P., Bilodeau J. F., Quesnel H., and Matte J. J.. . 2012. Effect of dietary organic and inorganic selenium on antioxidant status, embryo development, and reproductive performance in hyperovulatory first-parity gilts. J. Anim. Sci. 90:231–240. doi: 10.2527/jas.2010-3340 [DOI] [PubMed] [Google Scholar]
- Ginsberg Y., Lotan P., Khatib N., Awad N., Errison S., Weiner Z., Maravi N., Ross M. G., Itskovitz-Eldor J., and Beloosesky R.. . 2012. Maternal lipopolysaccharide alters the newborn oxidative stress and C-reactive protein levels in response to an inflammatory stress. J. Dev. Orig. Health Dis. 3:358–363. doi: 10.1017/S204017441200027X [DOI] [PubMed] [Google Scholar]
- Gu Y., Song Y., Yin H., Lin S., Zhang X., Che L., Lin Y., Xu S., Feng B., Wu D., . et al. 2017. Dietary supplementation with tributyrin prevented weaned pigs from growth retardation and lethal infection via modulation of inflammatory cytokines production, ileal expression, and intestinal acetate fermentation. J. Anim. Sci. 95:226–238. doi: 10.2527/jas.2016.0911 [DOI] [PubMed] [Google Scholar]
- Hempstock J., Jauniaux E., Greenwold N., and Burton G. J.. . 2003. The contribution of placental oxidative stress to early pregnancy failure. Hum. Pathol. 34:1265–1275. doi: 10.1016/j.humpath.2003.08.006 [DOI] [PubMed] [Google Scholar]
- Hill K. E., Motley A. K., Winfrey V. P., and Burk R. F.. . 2014. Selenoprotein P is the major selenium transport protein in mouse milk. PLoS One 9:e103486. doi: 10.1371/journal.pone.0103486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horký P. 2014. Influence of increased dietary selenium on glutathione peroxidase activity and glutathione concentration in erythrocytes of lactating sows. Ann. Anim. Sci. 14(4):869–882. doi: 10.2478/aoas-2014-0056 [DOI] [Google Scholar]
- Hu H., Wang M., Zhan X., Li X., and Zhao R.. . 2011. Effect of different selenium sources on productive performance, serum and milk Se concentrations, and antioxidant status of sows. Trace Elem. Res. 142(3):471–480. doi: 10.1007/s12011-010-8803-1 [DOI] [PubMed] [Google Scholar]
- Jauniaux E., Greenwold N., Hempstock J., and Burton G. J.. . 2003. Comparison of ultrasonographic and Doppler mapping of the intervillous circulation in normal and abnormal early pregnancies. Fertil. Steril. 79:100–106. doi: 10.1016/s0015-0282(02)04568-5 [DOI] [PubMed] [Google Scholar]
- Jlali M., Briens M., Rouffineau F., Geraert P. A., and Mercier Y.. . 2014. Evaluation of the efficacy of 2-hydroxy-4-methylselenobutanoic acid on growth performance and tissue selenium retention in growing pigs. J. Anim. Sci. 92:182–188. doi: 10.2527/jas.2013-6783 [DOI] [PubMed] [Google Scholar]
- Jlali M., Briens M., Rouffineau F., Mercerand F., Geraert P. A., and Mercier Y.. . 2013. Effect of 2-hydroxy-4-methylselenobutanoic acid as a dietary selenium supplement to improve the selenium concentration of table eggs. J. Anim. Sci. 91:1745–1752. doi: 10.2527/jas.2012-5825 [DOI] [PubMed] [Google Scholar]
- Koletzko B., Brands B., Poston L., Godfrey K., and Demmelmair H.; Early Nutrition Project 2012. Early nutrition programming of long-term health. Proc. Nutr. Soc. 71:371–378. doi: 10.1017/S0029665112000596 [DOI] [PubMed] [Google Scholar]
- Li N. Y., Sun Z. J., Ansari A. R., Cui L., Hu Y. F., Li Z. W., Briens M., Kai L., Sun L. H., Karrow N. A., . et al. 2020. Impact of maternal selenium supplementation from late gestation and lactation on piglet immune function. Biol. Trace Elem. Res. 194:159–167. doi: 10.1007/s12011-019-01754-y [DOI] [PubMed] [Google Scholar]
- Li S., Zheng J., Deng K., Chen L., Zhao X. L., Jiang X., Fang Z., Che L., Xu S., Feng B., . et al. 2018. Supplementation with organic acids showing different effects on growth performance, gut morphology, and microbiota of weaned pigs fed with highly or less digestible diets. J. Anim. Sci. 96:3302–3318. doi: 10.1093/jas/sky197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Han X. F., Fang Z. F., Che L. Q., Wu D., Wu X. Q., and Wu C. M.. . 2012. The beneficial effect of fiber supplementation in high- or low-fat diets on fetal development and antioxidant defense capacity in the rat. Eur. J. Nutr. 51:19–27. doi: 10.1007/s00394-011-0185-4 [DOI] [PubMed] [Google Scholar]
- Luo Z. C., Fraser W. D., Julien P., Deal C. L., Audibert F., Smith G. N., Xiong X., and Walker M.. . 2006. Tracing the origins of “fetal origins” of adult diseases: programming by oxidative stress? Med. Hypotheses. 66:38–44. doi: 10.1016/j.mehy.2005.08.020 [DOI] [PubMed] [Google Scholar]
- Mahan D. C. 2000. Effect of organic and inorganic selenium sources and levels on sow colostrum and milk selenium content. J. Anim. Sci. 78:100–105. doi: 10.2527/2000.781100x [DOI] [PubMed] [Google Scholar]
- Mahan D. C., and Kim Y. Y.. . 1996. Effect of inorganic or organic selenium at two dietary levels on reproductive performance and tissue selenium concentrations in first-parity gilts and their progeny. J. Anim. Sci. 74:2711–2718. doi: 10.2527/1996.74112711x [DOI] [PubMed] [Google Scholar]
- Mahan D. C., Moxon A. L., and Cline J. H.. . 1975. Efficacy of supplemental selenium in reproductive diets on sow and progeny serum and tissue selenium values. J. Anim. Sci. 40:624–631. doi: 10.2527/jas1975.404624x [DOI] [PubMed] [Google Scholar]
- Mahan D. C., Penhale L. H., Cline J. H., Moxon A. L., Fetter A. W., and Yarrington J. T.. . 1974. Efficacy of supplemental selenium in reproductive diets on sow and progeny performance. J. Anim. Sci. 39:536–543. doi: 10.2527/jas1974.393536x [DOI] [PubMed] [Google Scholar]
- Mahan D. C., and Peters J. C.. . 2004. Long-term effects of dietary organic and inorganic selenium sources and levels on reproducing sows and their progeny. J. Anim. Sci. 82:1343–1358. doi: 10.2527/2004.8251343x [DOI] [PubMed] [Google Scholar]
- Mocatta T. J., Winterbourn C. C., Inder T. E., and Darlow B. A.. . 2004. The effect of gestational age and labour on markers of lipid and protein oxidation in cord plasma. Free Radic. Res. 38:185–191. doi: 10.1080/10715760310001646048 [DOI] [PubMed] [Google Scholar]
- Mou D., Wang J., Liu H., Chen Y., Che L., Fang Z., Xu S., Lin Y., Feng B., Li J., . et al. 2018. Maternal methyl donor supplementation during gestation counteracts bisphenol A-induced oxidative stress in sows and offspring. Nutrition 45:76–84. doi: 10.1016/j.nut.2017.03.012 [DOI] [PubMed] [Google Scholar]
- National Research Council. 2011. Guide for the Care and Use of Laboratory Animals. Eighth Edition. The National Academy Press, Washington, DC. doi: 10.17226/12910 [DOI] [Google Scholar]
- National Research Council. 2012. Nutrient requirements of swine. 11th Edition. National Academies Press, Washington DC. [Google Scholar]
- Pappas A. C., Zoidis E., Surai P. F., and Zervas G.. . 2008. Selenoproteins and maternal nutrition. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 151:361–372. doi: 10.1016/j.cbpb.2008.08.009 [DOI] [PubMed] [Google Scholar]
- Park H. S., Chun J. N., Jung H. Y., Choi C., and Bae Y. S.. . 2006. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc. Res. 72:447–455. doi: 10.1016/j.cardiores.2006.09.012 [DOI] [PubMed] [Google Scholar]
- Park H. S., Jung H. Y., Park E. Y., Kim J., Lee W. J., and Bae Y. S.. . 2004. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J. Immunol. 173:3589–3593. doi: 10.4049/jimmunol.173.6.3589 [DOI] [PubMed] [Google Scholar]
- Peters J. C., Mahan D. C., Wiseman T. G., and Fastinger N. D.. . 2010. Effect of dietary organic and inorganic micromineral source and level on sow body, liver, colostrum, mature milk, and progeny mineral compositions over six parities. J. Anim. Sci. 88:626–637. doi: 10.2527/jas.2009-1782 [DOI] [PubMed] [Google Scholar]
- Quesnel H., Renaudin A., Le Floc’h N., Jondreville C., Pere M. C., Taylor-Pickard J. A., and Le Dividich J.. . 2008. Effect of organic and inorganic selenium sources in sow diets on colostrum production and piglet response to a poor sanitary environment after weaning. Animal 2(6):859–866. doi: 10.1017/S1751731108001869 [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Kirikae T., Schade F. U., Mamat U., Schmidt G., Loppnow H., Ulmer A. J., Zähringer U., Seydel U., and Di Padova F.. . 1994. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 8:217–225. doi: 10.1096/fasebj.8.2.8119492 [DOI] [PubMed] [Google Scholar]
- Ryan K. A., Smith M. F. Jr, Sanders M. K., and Ernst P. B.. . 2004. Reactive oxygen and nitrogen species differentially regulate Toll-like receptor 4-mediated activation of NF-kappa B and interleukin-8 expression. Infect. Immun. 72:2123–2130. doi: 10.1128/iai.72.4.2123-2130.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. P., Pilat J. M., and Williams C. S.. . 2018. Roles for selenium and selenoprotein P in the development, progression, and prevention of intestinal disease. Free Radic. Biol. Med. 127:26–35. doi: 10.1016/j.freeradbiomed.2018.05.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surai P. F. 2006. Selenium in nutrition and health. Nottingham (UK): Nottingham University Press. [Google Scholar]
- Surai P. F., and Fisinin V. I.. . 2015a. Selenium in pig nutrition and reproduction: boars and semen quality–a review. Asian-Australas. J. Anim. Sci. 28:730–746. doi: 10.5713/ajas.14.0593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surai P. F., and Fisinin V. I.. . 2015b. Antioxidant-prooxidant balance in the intestine: applications in chick placement and pig weaning. J. Vet. Sci. Med. 3:1–16. doi: 10.13188/2325-4645.1000011 [DOI] [Google Scholar]
- Surai P. F., and Fisinin V. I.. . 2016. Selenium in sow nutrition. Anim. Feed Sci. Technol. 211:18–30. doi: 10.1016/j.anifeedsci.2015.11.006 [DOI] [Google Scholar]
- Svoboda M., Ficek R., and Drabek J.. . 2008. Efficacy of organic selenium from Se-enriched yeast on selenium transfer from sows to piglets. Acta Vet. Brno. 77(4):515–521. doi: 10.2754/avb200877040515 [DOI] [Google Scholar]
- Szczubiał M., Dąbrowski R., Bochniarz M., and Komar M.. . 2013. The influence of the duration of the expulsive stage of parturition on the occurrence of postpartum oxidative stress in sows with uncomplicated, spontaneous farrowings. Theriogenology 80(7):706–711. doi: 10.1016/j.theriogenology.2013.05.015 [DOI] [PubMed] [Google Scholar]
- Tang J., Diao P., Shu X., Li L., and Xiong L.. . 2019. Quercetin and quercitrin attenuates the inflammatory response and oxidative stress in LPS-induced RAW264.7 cells: in vitro assessment and a theoretical model. Biomed Res. Int. 2019:7039802. doi: 10.1155/2019/7039802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufarelli V., Ceci E., and Laudadio V.. . 2016. 2-Hydroxy-4-methylselenobutanoic acid as new organic selenium dietary supplement to produce selenium-enriched eggs. Biol. Trace Elem. Res. 171:453–458. doi: 10.1007/s12011-015-0548-4 [DOI] [PubMed] [Google Scholar]
- Wang J. P., Yoo J. S., Jang H. D., Lee J. H., Cho J. H., and Kim I. H.. . 2011. Effect of dietary fermented garlic by Weissella koreensis powder on growth performance, blood characteristics, and immune response of growing pigs challenged with Escherichia coli lipopolysaccharide. J Anim Sci. 89(7):2123–2131. doi: 10.2527/jas.2010-3186 [DOI] [PubMed] [Google Scholar]
- Wolf J., Žáková E., and Groeneveld E.. . 2008. Within-litter variation of birth weight in hyperprolific Czech Large White sows and its relation to litter size traits, stillborn piglets and losses until weaning. Livest. Sci. 115(2–3):195–205. doi: 10.1016/j.livsci.2007.07.009 [DOI] [Google Scholar]
- Wullepit N., Hostens M., Ginneberge C., Fievez V., Opsomer G., Fremaut D., and De Smet S.. . 2012. Influence of a marine algae supplementation on the oxidative status of plasma in dairy cows during the periparturient period. Prev. Vet. Med. 103:298–303. doi: 10.1016/j.prevetmed.2011.09.007 [DOI] [PubMed] [Google Scholar]
- Yi D., Hou Y., Wang L., Ding B., Yang Z., Li J., Long M., Liu Y., and Wu G.. . 2014. Dietary N-acetylcysteine supplementation alleviates liver injury in lipopolysaccharide-challenged piglets. Br. J. Nutr. 111(1):46–54. doi: 10.1017/S0007114513002171 [DOI] [PubMed] [Google Scholar]
- Yin J., Ren W., Liu G., Duan J., Yang G., Wu L., Li T., and Yin Y.. . 2013. Birth oxidative stress and the development of an antioxidant system in newborn piglets. Free Radic. Res. 47:1027–1035. doi: 10.3109/10715762.2013.848277 [DOI] [PubMed] [Google Scholar]
- Yoon I., and McMillan E.. . 2006. Comparative effects of organic and inorganic selenium on selenium transfer from sows to nursing pigs. J. Anim. Sci. 84:1729–1733. doi: 10.2527/jas.2005-311 [DOI] [PubMed] [Google Scholar]
- Yuan S. B., Chen D. W., Zhang K. Y., and Yu B.. . 2007. Effects of oxidative stress on growth performance, nutrient digestibilities and activities of antioxidative enzymes of weanling pigs. Asian-Australas. J. Anim. Sci. 20:1600–1605. doi: 10.5713/ajas.2007.1600 [DOI] [Google Scholar]
- Zhan X., Qie Y., Wang M., Li X., and Zhao R.. . 2011. Selenomethionine: an effective selenium source for sow to improve Se distribution, antioxidant status, and growth performance of pig offspring. Biol. Trace Elem. Res. 142:481–491. doi: 10.1007/s12011-010-8817-8 [DOI] [PubMed] [Google Scholar]