Abstract
Individuals with spinal cord injury (SCI) have a significantly increased risk for cognitive impairment that is associated with cerebrovascular remodeling and endothelial dysfunction. The sub-acute stage following high thoracic SCI is characterized by increased fibrosis and stiffness of cerebral arteries. However, a more prolonged duration after SCI exacerbates cerebrovascular injury by damaging endothelium. Endothelial dysfunction is associated with reduced expression of transient receptor potential cation channel 4 that mediates the production of nitric oxide and epoxyeicosatrienoic acids following shear stress and the response to carbachol and other endothelium-dependent vasodilators. Reduced expression of CD31 in cerebral arteries also suggests the loss of endothelial cell integrity following chronic SCI. Repetitively transient hypertension and intermittent hypotension contribute to cerebrovascular endothelial dysfunction in the animals with a sub-acute stage of high thoracic SCI. The increase in vascular remodeling and endothelial dysfunction ultimately reduce cerebral blood flow, which promotes cerebral hypoperfusion and cognitive dysfunction in the chronic phase of SCI. In conclusion, the duration and magnitude of fluctuations in blood pressure after SCI play a vital role in the onset and progress of cerebrovascular dysfunction, which promotes the development of cognitive impairment.
Keywords: Spinal cord injury, Cerebrovasculature, Cognitive impairment, Vascular remodeling, Endothelial dysfunction, Blood pressure, Cerebral blood flow, Autoregulation
Introduction
Spinal cord injury (SCI) is often accompanied by cognitive impairment. Nearly 64% of individuals with SCI exhibit cognitive impairment that largely affects their rehabilitation, re-employment, and reintegration into the community [1]. In the present study, Sachdeva et al. utilized a chronic (14-week) SCI animal model with high thoracic (T3) to study whether chronic SCI promotes cerebrovascular dysfunction and remodeling that mimics that seen in SCI patients and to study whether these cerebral vascular complications contribute to the onset and development of cognitive impairment [2]. This is a follow-up study of their previous findings that identified changes in the structure and function of cerebrovasculature in the sub-acute stage (7-week) following high thoracic SCI [3,4]. Consistent with clinical studies, cognitive function was impaired in animals with chronic SCI in a novel object recognition test, indicating short-term memory deficits. Resting cerebral blood flow (CBF) was assessed using arterial spin labeling magnetic resonance imaging. They found that CBF was reduced in the hippocampus, which is responsible for spatial/non-spatial learning and memory. These results suggest that the dysfunction of neurons in the hippocampus in chronic SCI may be due to ischemic brain injury caused by CBF hypoperfusion. To study cerebrovascular contributions to the reduced CBF and cognitive impairment, Sachdeva et al. found that there was reduced cerebrovascular distensibility in the middle cerebral artery (MCA) of adult male Wistar rats with chronic (14-week) high thoracic SCI, which was characterized by increased deposition of collagen in the vascular wall [5] in association with impaired endothelium-dependent vasodilation. Endothelial dysfunction was associated with decreased expression of the transient receptor potential cation channel 4 (TRPV4), which mediates the production of nitric oxide (NO) and epoxyeicosatrienoic acids (EETs) following shear stress and response to carbachol and other endothelium-dependent vasodilators [6]. They also reported that the expression of CD31 was reduced in the MCA, suggesting the possibility of a loss of the number and integrity of endothelial cells lining these cerebral vessels after SCI. Sachdeva et al. are among those who first provide mechanistic evidence of cerebrovascular dysfunction related to cerebral hypoperfusion and cognitive deficits in chronic SCI.
The brain accounts for nearly 2% of body mass but consumes 20% of total energy [7]. Precise regulation of CBF is critical in maintaining constant cerebral perfusion to meet the high energy demands of neurons as they lack the capability of energy storage. Stability of blood pressure is critical in maintaining proper CBF delivered to the brain, especially when blood pressure exceeds the CBF autoregulatory range or when CBF autoregulation is compromised under pathological conditions and genetic abnormalities [5,8–15]. However, SCI patients often develop autonomic dysfunction and exhibit large bidirectional fluctuations in blood pressure [16]. Orthostatic hypotension is frequently found in individuals with high thoracic and cervical SCI, leading to the widely accepted theory that intermittent cerebral hypoperfusion may contribute to cognitive deficits in these patients [16,17]. However, restoring blood pressure to normal via administration of midodrine hydrochloride failed to reverse cognitive deficits in human clinical trials, suggesting that other factors may also contribute to the development of cognitive impairment after SCI [18]. In contrast, blood pressure is often transiently rising to up to 300 mmHg after distension of bladder or the bowels in individuals with SCI above T5 or T6 levels. Such extreme episodic increases in blood pressure can occur >10 times per day, termed autonomic dysreflexia [19]. The drastic fluctuation of blood pressure that exceeds the ability of the vasculature to autoregulate CBF is a life-threatening phenomenon, which can lead to cerebral arteries remodeling and increase transmission pressure to the capillaries. These vascular changes result in blood-brain barrier (BBB) damage, microhemorrhages, localized neuroinflammation, and neurodegeneration.
Chronic hypertension induces inward vascular remodeling and vascular stiffness, promotes endothelial dysfunction, and impairs autoregulation in the cerebral and renal circulation [20–22]. However, whether repetitive transient hypertension can lead to cerebrovascular abnormalities contributing to cognitive impairment in SCI remains not clear. By using a novel clinical-relevant animal model of high thoracic SCI, previous studies from Dr. Krassioukov’s group demonstrated that 7-week SCI, considered as a sub-acute stage, caused MCA inward remodeling with a reduced wall-to-lumen ratio, increased wall stress and decreased vascular distensibility. However, unlike in chronic hypertension, wall thickness was not changed in these sub-acute SCI models. The increase in vascular stiffness was secondary to elevated collagen I, III, and reduced elastin expression [3]. Interestingly, endothelial function was not altered in the sub-acute stage of SCI; however, in a later study with the induction of repetitive transient hypertension by manually triggering autonomic dysreflexia (6 times per day for 20 days) in the sub-acute SCI animals [4], endothelial dysfunction and exacerbated vascular stiffness were found, although the underlying mechanisms were not understood. The new information revealed in the present study is that endothelial dysfunction is occurred along with the structural remodeling of the MCA in rats at the chronic phase of SCI [2]. These studies suggest that the changes in endothelial function and vascular remodeling develop slowly following the onset of SCI and are likely caused by the repetitive transient elevations in blood pressure. The current study is important as it highlights that there may be a critical therapeutic time window to minimize cerebrovascular dysfunction and loss of cognitive function by controlling blood pressure in SCI.
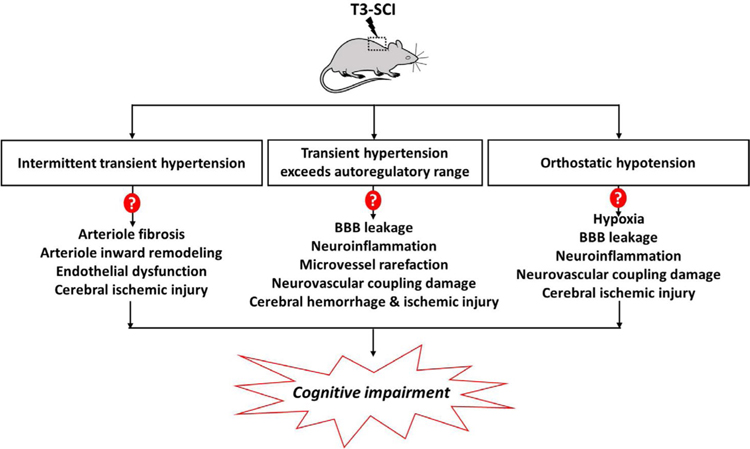
Sachdeva et al. discovered that TRPV4 channels might also be involved in the mechanisms underlying endothelial dysfunction in chronic SCI [2]. Previous studies have shown that change of arterial hemodynamics is associated with endothelial dysfunction [23]; however, it was not clear if the loss of endothelial TRPV4 channels of cerebrovasculature is due to abnormal shear stress after SCI. The present study provided evidence that the large bidirectional fluctuations in blood pressure likely contribute to cerebrovascular remodeling and endothelial dysfunction leading to cognitive impairment. However, further studies are needed to sort out the potential mechanisms involved in SCI induced cerebrovasculature injury, as summarized in Figure 1. For example, it remains to be determined that if repetitive transient hypertension in SCI can result in fibrosis and endothelial dysfunction in arterioles due to abnormal shear stress-induced vascular injury. It is not clear whether high pressure that exceeds CBF autoregulatory range during autonomic dysreflexia in SCI, which could be transmitted to downstream capillaries, leading BBB leakage, microhemorrhage, impaired neurovascular coupling, and cognitive impairment, as found in other pathological or abnormal genetic conditions [5,8–15]. Finally, episodes of orthostatic hypotension can cause cerebral ischemic injury, which could also result in endothelial dysfunction, BBB leakage, neurovascular uncoupling, and neuroinflammation [8]. Hence, the effects of SCI on the structure and function of microvessels need further studies.
Figure 1: Summary of potential mechanisms involved in SCI induced cerebrovasculature injury and cognitive impairment.
Chronic spinal cord injury (SCI) rats following high thoracic (T3) injury is characterized by large bidirectional fluctuations in blood pressure. Intermittent transient hypertension secondary to autonomic dysreflexia may cause fibrosis of cerebral arterioles and inward remodeling, as seen in chronic hypertensive animal models resulting in cerebral ischemic injury. Transient hypertension (blood pressure approximately 300 mmHg) due to autonomic dysreflxia in SCI often exceeds the autoregulatory range leading to increased pressure transmitted to downstream capillaries, which causes blood-brain barrier (BBB) leakage, neuroinflammation, microvessel rarefaction, and impaired neurovascular coupling. Periods of orthostatic hypotension associated with cerebral ischemic injury may also contribute to BBB leakage neuroinflammation, and damaged neurovascular coupling. The dysfunction of cerebral arterioles, and capillaries due to the duration and magnitude of fluctuations in blood pressure could ultimately result in cognitive impairment in SCI.
Conclusion
The current study is an important advance in defining vascular-cognitive impairment following high-thoracic SCI, which provides a novel therapeutic target for the treatment of cognitive impairment in chronic SCI individuals. This group discovered impaired endothelial-dependent vasodilation due to loss of TRPV4 in the endothelium of cerebrovasculature in chronic SCI, together with increased vascular stiffness, contributes to cerebral hypoperfusion and cognitive deficits in chronic SCI. Cerebral parenchymal arterioles are a bottleneck of the cerebral circulation and play a major role in regulating CBF [9]. Remodeling and endothelial dysfunction of cerebral parenchymal arterioles could cause cerebral ischemic injury leading to cognitive impairment. Whether chronic SCI also affects the function and structure of cerebral parenchymal arterioles could be an interesting future direction. In addition, TRPV4 agonist and other pharmacological agents such as jNc-440 that promote TRPV4-KCa2.3 interaction [2] could be used to study their therapeutic effects on cognitive functions in chronic SCI. In conclusion, the studies from Dr. Krassioukov’s group demonstrated that both the duration (Figure 2) and the magnitude of fluctuations in blood pressure (Figure 3) link cerebrovascular dysfunction and cognitive impairment following SCI.
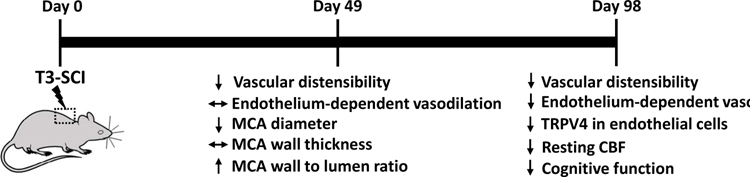
Figure 2: Duration: Structural and functional changes in the cerebrovasculature secondary to sub-acute to chronic high thoracic spinal cord injury.
Changes of cerebrovascular structure and function have been reported in both the sub-acute stage (49 days) and chronic (98 days) phase after high thoracic spinal cord injury (SCI). MCA, middle cerebral artery; CBF, cerebral blood flow; TRPV4, Transient Receptor Potential Cation Channel Subfamily V Member 4.
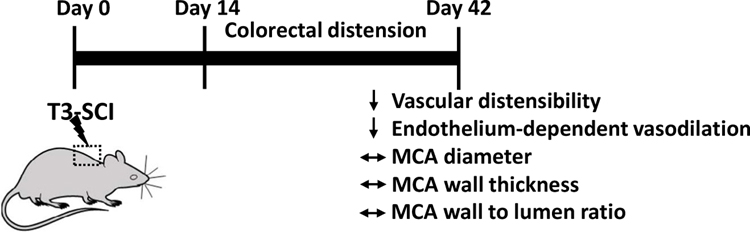
Figure 3: Pressure: Structural and functional changes in the cerebrovasculature caused by repetitive transient hypertension secondary to autonomic dysreflexia following high thoracic spinal cord injury.
Changes in the structure and function of the cerebrovasculature in animals with high thoracic spinal cord injury (SCI) were reported after repetitive transient hypertension induced by colorectal distension (6 times/day) to mimic autonomic dysreflexia in patients with SCI. MCA, middle cerebral artery.
Acknowledgments
Funding
This study was supported by grants AG050049, AG057842, P20GM104357, and HL138685 from the National Institutes of Health; 16GRNT31200036 and 20PRE35210043 from the American Heart Association.
Abbreviations:
- SCI
Spinal Cord Injury
- CBF
Cerebral Blood Flow
- MCA
Middle Cerebral Artery
- TRPV4
Transient Receptor Potential Cation Channel 4
- BBB
Blood-Brain Barrier
Footnotes
Conflicts of Interests
None of the authors declare any conflict of interest.
References
- 1.Sachdeva R, Gao F, Chan CC, Krassioukov AV. Cognitive function after spinal cord injury: A systematic review. Neurology. 2018. September 25;91(13):611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sachdeva R, Jia M, Wang S, Yung A, Zheng MM, Lee AH, et al. Vascular-Cognitive Impairment Following High-Thoracic Spinal Cord Injury is Associated with Structural and Functional Maladaptations in Cerebrovasculature. Journal of Neurotrauma. 2020. May 12(ja). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips AA, Matin N, Frias B, Zheng MM, Jia M, West C, et al. Rigid and remodelled: cerebrovascular structure and function after experimental high-thoracic spinal cord transection. The Journal of Physiology. 2016. March 15;594(6):1677–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips AA, Matin N, Jia M, Squair JW, Monga A, Zheng MM, et al. Transient hypertension after spinal cord injury leads to cerebrovascular endothelial dysfunction and fibrosis. Journal of Neurotrauma. 2018. February 1;35(3):573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S, Zhang H, Liu Y, Li L, Guo Y, Jiao F, et al. Sex differences in the structure and function of rat middle cerebral arteries. American Journal of Physiology-Heart and Circulatory Physiology. 2020. May 1;318(5):H1219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filosa JA, Yao X, Rath G. TRPV4 and the regulation of vascular tone. Journal of Cardiovascular Pharmacology. 2013. February;61(2):113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang S, Lv W, Zhang H, Liu Y, Li L, Jefferson JR, et al. Aging exacerbates impairments of cerebral blood flow autoregulation and cognition in diabetic rats. GeroScience. 2020. July 21:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shekhar S, Varghese K, Li M, Fan L, Booz GW, Roman RJ, et al. Conflicting roles of 20-HETE in hypertension and stroke. International Journal of Molecular Sciences. 2019. January;20(18):4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Zhang C, Liu Y, Gao W, Wang S, Fang X, et al. Influence of dual-specificity protein phosphatase 5 on mechanical properties of rat cerebral and renal arterioles. Physiological Reports. 2020. January;8(2):e14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roman RJ, Fan F. 20-HETE: hypertension and beyond. Hypertension. 2018. July;72(1):12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shekhar S, Wang S, Mims PN, Gonzalez-Fernandez E, Zhang C, He X, et al. Impaired cerebral autoregulation-a common neurovascular pathway in diabetes may play a critical role in diabetes-related Alzheimer’s disease. Current Research in Diabetes & Obesity Journal. 2017. June;2(3):555587. [PMC free article] [PubMed] [Google Scholar]
- 12.Fan F, Ge Y, Lv W, Elliott MR, Muroya Y, Hirata T, et al. Molecular mechanisms and cell signaling of 20-hydroxyeicosatetraenoic acid in vascular pathophysiology. Frontiers in Bioscience (Landmark edition). 2016;21:1427–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan F, Geurts AM, Murphy SR, Pabbidi MR, Jacob HJ, Roman RJ. Impaired myogenic response and autoregulation of cerebral blood flow is rescued in CYP4A1 transgenic Dahl salt-sensitive rat. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2015. March 1;308(5):R379–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warrington JP, Fan F, Murphy SR, Roman RJ, Drummond HA, Granger JP, et al. Placental ischemia in pregnant rats impairs cerebral blood flow autoregulation and increases blood–brain barrier permeability. Physiological Reports. 2014. August;2(8):e12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan F, Geurts AM, Pabbidi MR, Smith SV, Harder DR, Jacob H, et al. Zinc-Finger Nuclease Knockout of Dual-Specificity Protein Phosphatase-5 Enhances the Myogenic Response and Autoregulation of Cerebral Blood Flow in FHH. 1 BN Rats. PloS one. 2014. November 14;9(11):e112878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sachdeva R, Nightingale TE, Krassioukov AV. The blood pressure pendulum following spinal cord injury: implications for vascular cognitive impairment. International Journal of Molecular Sciences. 2019. January;20(10):2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jegede AB, Rosado-Rivera D, Bauman WA, Cardozo CP, Sano M, Moyer JM, et al. Cognitive performance in hypotensive persons with spinal cord injury. Clinical Autonomic Research. 2010. February 1;20(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips AA, Warburton DE, Ainslie PN, Krassioukov AV. Regional neurovascular coupling and cognitive performance in those with low blood pressure secondary to high-level spinal cord injury: improved by alpha-1 agonist midodrine hydrochloride. Journal of Cerebral Blood Flow & Metabolism. 2014. May;34(5):794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Archives of Physical Medicine and Rehabilitation. 2000. April 1;81(4):506–16. [DOI] [PubMed] [Google Scholar]
- 20.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiological Reviews. 2002. January 1;82(1):131–85. [DOI] [PubMed] [Google Scholar]
- 21.Fan F, Roman RJ. Effect of cytochrome P450 metabolites of arachidonic acid in nephrology. Journal of the American Society of Nephrology. 2017. October 1;28(10):2845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan F, Muroya Y, Roman RJ. Cytochrome P450 eicosanoids in hypertension and renal disease. Current opinion in nephrology and hypertension. 2015. January;24(1):37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fry DL. Acute vascular endothelial changes associated with increased blood velocity gradients. Circulation research. 1968. February;22(2):165–97. [DOI] [PubMed] [Google Scholar]