Abstract
In multicellular organisms with specialized cells, the most significant distinction among cell types is between reproductive (germ) cells and non-reproductive/somatic cells (soma). Although soma contributed to the marked increase in complexity of many multicellular lineages, little is known about its evolutionary origins. We have previously suggested that the evolution of genes responsible for the differentiation of somatic cells involved the co-option of life history trade-off genes that in unicellular organisms enhanced survival at a cost to immediate reproduction. In the multicellular green alga, Volvox carteri, cell fate is established early in development by the differential expression of a master regulatory gene known as regA. A closely related RegA-Like Sequence (RLS1) is present in its single-celled relative, Chlamydomonas reinhardtii. RLS1 is expressed in response to stress, and we proposed that an environmentally induced RLS1-like gene was co-opted into a developmental pathway in the lineage leading to V. carteri. However, the exact evolutionary scenario responsible for the postulated co-option event remains to be determined. Here, we show that in addition to being developmentally regulated, regA can also be induced by environmental cues, indicating that regA has maintained its ancestral regulation. We also found that the absence of a functional RegA protein confers increased sensitivity to stress, consistent with RegA having a direct or indirect role in stress responses. Overall, this study (i) provides mechanistic evidence for the co-option of an environmentally induced gene into a major developmental regulator, (ii) supports the view that major morphological innovations can evolve via regulatory changes and (iii) argues for the role of stress in the evolution of multicellular complexity.
Keywords: Volvox carteri, regA, evolution of soma, co-option, stress, development
1. Introduction
The evolution of multicellularity had a profound impact on the diversification of life on Earth. In multicellular organisms with specialized cell types, the most significant distinction among cell types is between reproductive (germ) cells and non-reproductive/altruistic somatic cells (soma). The evolution of soma has contributed to the marked increase in complexity that some lineages (especially animals and plants) achieved, and somatic cell differentiation is a basic process in the development of most multicellular organisms. Yet little is known about the mechanistic and genetic basis for the early evolution of soma.
We have previously suggested that the evolution of germ and soma required a change in the expression of reproductive and survival functions, from a temporal/environmental context (such as during the life cycle of single-celled individuals) into a spatial/developmental context (between somatic and germ cells) [1]. Furthermore, we proposed that the evolution of genes responsible for the differentiation of somatic cells in multicellular lineages involved the co-option of life history trade-off genes that in their single-celled ancestors enhanced survival during challenging environmental conditions, at a cost to immediate reproduction [2]. Nevertheless, the specific scenarios and mechanisms involved in the co-option of such stress-induced adaptive responses into developmental pathways are not understood.
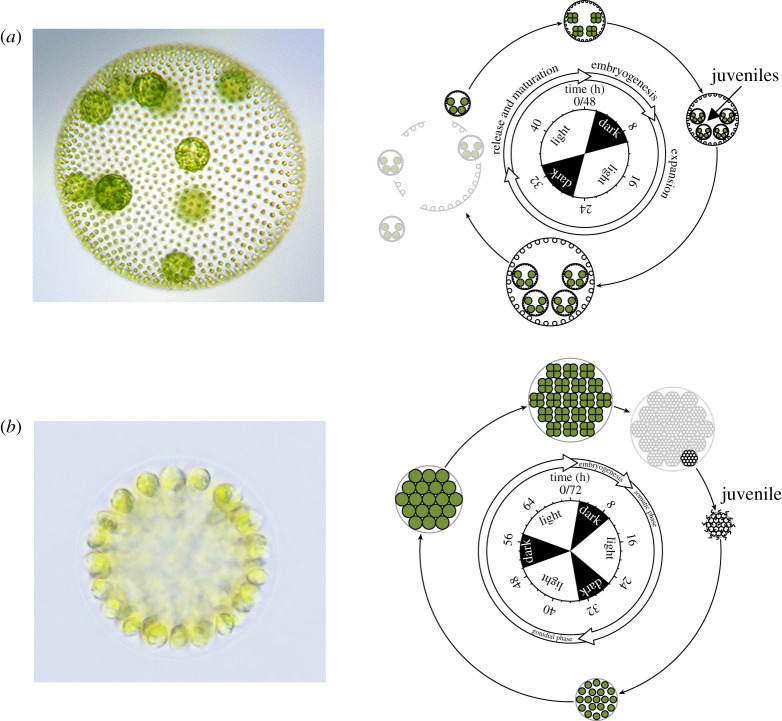
To investigate the genetic and mechanistic basis for the early evolution of soma we have been using the volvocine algae—a group of green algae that comprises both unicellular and multicellular species with or without specialized germ and somatic cells. The most well-studied volvocine species are the single-celled Chlamydomonas reinhardtii and the multicellular Volvox carteri [3–5]. Volvox carteri is a spherical alga consisting of two different cell types (figure 1a): about 2000 biflagellated somatic cells (specialized in motility) with no cell division or re-differentiation potential, and up to 16 non-flagellated cells (gonidia) specialized in asexual reproduction [3]. During the asexual life cycle (figure 1a), each gonidium grows extensively and undergoes a rapid succession of symmetric and asymmetric cell divisions (cleavage) resulting in small and large cells that will later differentiate into somatic and reproductive cells, respectively [7]. The two cell types show extensive differences in their transcriptomic profiles, with gonidia expressing more growth-related genes, while somatic cells express a survival-related programme [8]. The two cell types also differ in the representation of genes diurnally controlled in C. reinhardtii, with the expression of dark-phase and light-phase genes being overrepresented in somatic cells and gonidia, respectively [8].
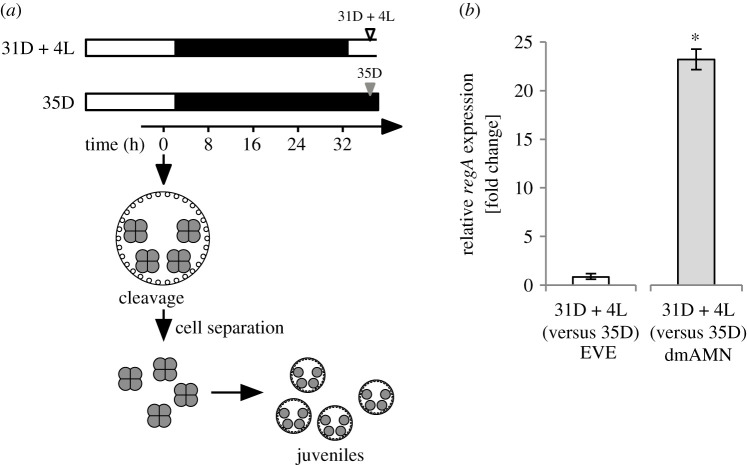
Figure 1.
Micrographs and life cycles for the two V. carteri strains used in this study. (a) Wild-type strain EVE. Left: juvenile with up to 2000 somatic cells (small circles) and up to 16 gonidia (large circles). Right: life cycle (48 h; 16 L : 8 D regime). The first cleavage division of gonidia is defined as time point 0 h and occurs ca 2 h before the first dark period. Embryogenesis takes about 8 h, and after a full day of growth, juveniles hatch from their parents, and the parental somatic cells undergo senescence and die. The gonidia of the released juveniles enter the next round of embryogenesis. (b) regA−/gls− dmAMN mutant strain. Left: juvenile with up to 256 unspecialized cells. Right: life cycle (72 h; 16 L : 8 D regime). The first cleavage division defines the 0 h time point; each cell undergoes 6–8 symmetric divisions to form new juveniles that hatch and swim away. About 24 h later, juveniles enter the reproductive phase; cells lose their flagella, grow and become increasingly vacuolated resembling wild-type gonidia. After 72 h, all cells enter a new round of embryogenesis. Life cycles adapted from [6]. (Online version in colour.)
In V. carteri, cell fate is established early during development through the differential expression of a master regulatory gene known as regA. Specifically, regA is only expressed in cells that at the end of cleavage fall below an 8 µm threshold size, which will differentiate into somatic cells [9]. A series of asymmetric divisions ensures that a number (up to 16) of cells remain large (to avoid the induction of regA) and differentiate into gonidia. Interestingly, the expression of regA is dependent strictly on the size (not cytoplasmic composition) of cells at the end of cleavage [9]. However, the signal that induces regA expression in cells that fall under the 8 µm threshold is yet to be identified.
Mutations in regA alone result in somatic cells regaining their ability to grow and reproduce (i.e. regenerate [10]); in addition, all somatic regenerator mutations have been mapped to regA [11]. Thus, regA is necessary and sufficient to establish somatic cell fate in V. carteri. regA codes for a transcriptional repressor [12] thought to suppress the expression of nuclear-encoded chloroplast proteins [13,14]. Consequently, the growth and division of cells expressing regA are suppressed, resulting in small unproliferative, terminally differentiated somatic cells [12]. RegA's transcriptional activity appears to be related to the presence of a DNA binding motif called the SAND domain that is also present in a series of transcription factors with important roles in plant and animal development (e.g. [15] and references therein).
regA belongs to the VARL (Volvocine Algal RegA-Like) gene family characterized by the presence of the VARL domain—an approximately 100 amino acid (AA) conserved region that includes the SAND domain [16]. The VARL family has 14 members in V. carteri and 12 members in C. reinhardtii [5]. The closest regA homologue (though not an orthologue) in C. reinhardtii is RLS1 (RegA-Like Sequence 1) [2,16]. RLS1 is expressed in response to several types of stress, including extended dark, deprivation of sulfur or phosphorus, stationary phase or the inhibition of the photosynthetic electron transport [2,17]. The expression of RLS1 coincides with the down-regulation of a light-harvesting chloroplast protein-coding gene as well as with a decrease in reproduction, suggesting that RLS1 is part of a general photo-acclimation response to environmental challenges [17].
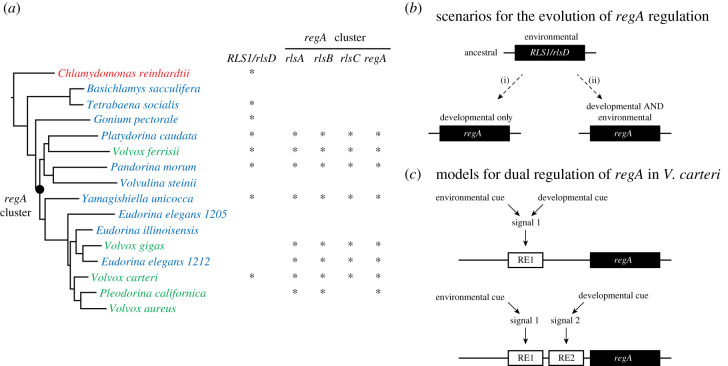
Interestingly, RLS1 orthologues, known as rlsD, are present in multicellular volvocine lineages, both with or without somatic cells [18,19] (figure 2a). Furthermore, in V. carteri, rlsD has been shown to be expressed in response to environmental stress (i.e. phosphorous deprivation; [21]). Phylogenetic analyses indicate that regA evolved from an rlsD-like sequence through a series of tandem duplications that gave rise to the so-called regA cluster [16,19,22]. This gene cluster includes three more rls sequences (rlsA, rlsB and rlsC) that are thought to also be involved in somatic cell differentiation [21]. Nevertheless, regA orthologues with unknown function have been identified in several multicellular volvocine algae that lack somatic cells [18,19], implying that the duplication event that gave rise to regA preceded the evolution of the somatic cell phenotype [18,19] (figure 2a).
Figure 2.
Phylogenetic distribution, evolutionary scenarios and models of regulation of regA. (a) Simplified volvocine phylogeny (adapted from [18]) showing the distribution of RLS1/rlsD and the regA cluster (red, blue and green indicate species that are unicellular, multicellular with no soma and multicellular with soma, respectively). (b) Scenarios for the co-option of an environmentally regulated ancestral RLS1/rlsD into a developmentally regulated regA by (i) replacing the environmental regulation or (ii) adding the developmental regulation. (c) Models for the dual regulation of regA involving either shared components of the ancestral signalling pathway or the evolution of a parallel pathway (RE-regulatory elements); adapted from [20].
Overall, available data suggest that an RLS1/rlsD-like sequence expressed as part of a stress-induced acclimation pathway was co-opted (following a series of duplications) into a developmental pathway in the lineage leading to V. carteri. However, the exact evolutionary scenario and mechanisms responsible for the postulated co-option event with respect to how the developmental regulation of regA was achieved are not known. Theoretically, two scenarios can be envisioned (figure 2b): (i) a new developmental regulation replaced the ancestral environmental regulation of RLS1/rlsD in one paralogous sequence or (ii) a new developmental regulation was acquired while the ancestral regulation was maintained. The two scenarios make very different predictions. While the first scenario postulates that regA is strictly a developmental gene, the second scenario allows for the possibility that regA can still be induced environmentally. To distinguish between these two scenarios, the present study investigated whether regA in V. carteri can be induced in response to environmental cues.
2. Material and methods
This section provides a brief summary of strains and methods employed in this work; for additional details, see electronic supplementary material.
(a). Strains and culturing conditions
We used two V. carteri strains: a female wild-type strain (EVE [23]) and a spontaneous regA−/gls− mutant (dmAMN) isolated in our lab from the regA− male strain UTEX1877. Synchronous cultures of both strains were grown in aerated standard Volvox medium [24] using a photoperiod of 16 h light and 8 h dark (16 L : 8 D).
(b). RNA extraction and quantification
Pelleted algae were flash-frozen in liquid nitrogen. RNA was extracted with the RNeasy Plant Mini Kit (Qiagen). Reverse transcription reactions were performed with SuperScript III Reverse Transcriptase (Invitrogen) and either oligo(dT)20 for reference gene rps18, or gene-specific primers for regA (electronic supplementary material, table S1).
(c). Quantitative real-time PCR
qRT-PCR was performed in a Rotor-Gene 6000 (Corbett Research) using the KAPA SYBR FAST Master Mix Universal (Kapa Biosystems) and gene-specific primers (electronic supplementary material, table S1). Data were collected with the Rotor-Gene 6000 software (v. 1.7.87) and exported to LinRegPCR [25,26] to calculate average PCR efficiency and threshold cycle (CT); CT values for technical replicates were averaged. The relative expression of regA was determined with REST2009 software (v. 2.0.13, Qiagen); the software also determined if differences between control and treatment groups were significant using a randomization test.
(d). Cell viability
Cell viability was assessed using SYTOX Green (Invitrogen). Live and dead cells on one hemisphere of at least 20 individuals per technical replicate were counted using the Fiji image processing package and the cell counter plug-in. Statistical analyses were performed with JMP (v. 10, SAS).
3. Results
(a). Characterization of a new gonidialess mutant and its developmental regA expression
To test for a potential environmental induction of regA independently of its developmental function and effects on differentiation status, we used a V. carteri spontaneous mutant that lacks cell differentiation (dmAMN; see Material and methods). The life cycle of this mutant is reminiscent of multicellular volvocine species without somatic and germ cells: that is, all cells start small and flagellated, but then grow, lose flagella and reproduce (figure 1b). This mutant phenotype is the result of changes affecting two loci: regA and gls. The loss of gls affects the ability to perform asymmetric divisions, which results in the absence of gonidia (i.e. gonidialess). Gonidialess phenotypes can only be propagated on a regA− background as in the presence of a functional RegA all cells will differentiate as somatic. Although the specific gls mutation is not known (several gls loci have been predicted, but only on locus—glsA, has been characterized), the dmAMN mutant shares the same phenotype and life cycle progression as a previously described regA−/gls− mutant (figure 1b) [27]. Such mutants have been previously used to study expression of cell-type-specific genes in V. carteri [14,27,28]. The use of this mutant allowed us to (i) circumvent cell separation protocols that can induce stress responses, (ii) work with a homogeneous population of undifferentiated cells resembling the ancestral undifferentiated state, (iii) avoid the effect that somatic cell differentiated state might have on the environmental expression of regA and (iv) prevent any feedback loops that a functional RegA might have on the regulation of regA.
First, we addressed whether the somatic regenerator phenotype is due to mutations in regA's coding regions or its regulatory elements, by sequencing the entire regA locus. We identified a 365 bp insertion after position +5222 in exon 6. The mutation is predicted to result in a truncated and chimeric protein containing the first 355 AA of the RegA protein (1049 AA) and 33 additional AA encoded by the insertion (electronic supplementary material, figure S3). However, we did not find any mutations in regA's promoter or the introns known to contain regulatory elements (electronic supplementary material, figure S4), suggesting that regA's expression and regulation should be unaffected. A truncated protein (291 AA) was also predicted to be encoded by another regA mutant (HB11A) that has been used in previous studies of regA expression [12,29].
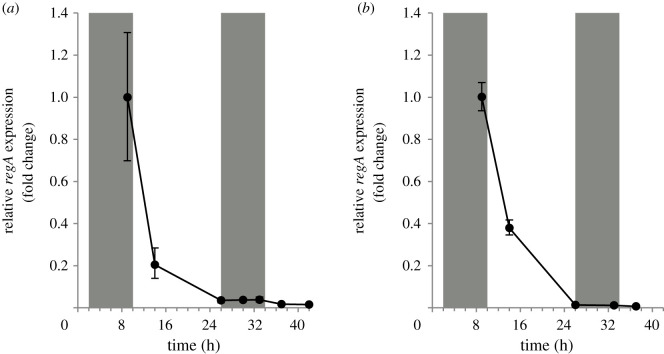
To validate that regA in the dmAMN mutant was still expressed and has maintained a developmental expression pattern similar to that of the wild-type EVE strain, we extracted RNA from both the mutant and EVE strains at several time points during the first two days of their life cycles. Quantitative RT-PCR analyses showed that regA had a similar pattern of expression in both strains (figure 3): regA transcript levels peaked at the end of embryogenesis (when cells fall below 8 µm), dropped abruptly by the end of the first day and stayed low during the second day. These findings confirmed the suitability of the dmAMN mutant as a model system to test the environmental induction of regA in an undifferentiated/ancestral-like cell context.
Figure 3.
Comparison of regA expression in EVE and dmAMN in a standard 16 L : 8 D regime. Isolated EVE (a) or dmAMN (b) embryos were grown in a standard 16 L : 8D regime, and RNA was extracted at various time points (points on x-axis are based on time from start of cleavage). regA transcript levels were quantified (RT-qPCR) and expressed relative (fold change) to the first time point (9 h after start of cleavage) (n = 3, error bars indicate standard errors-s.e.). Along x-axis: shaded and white areas denote dark and light periods, respectively (figure 1).
(b). RegA in the dmAMN mutant can be induced by environmental cues
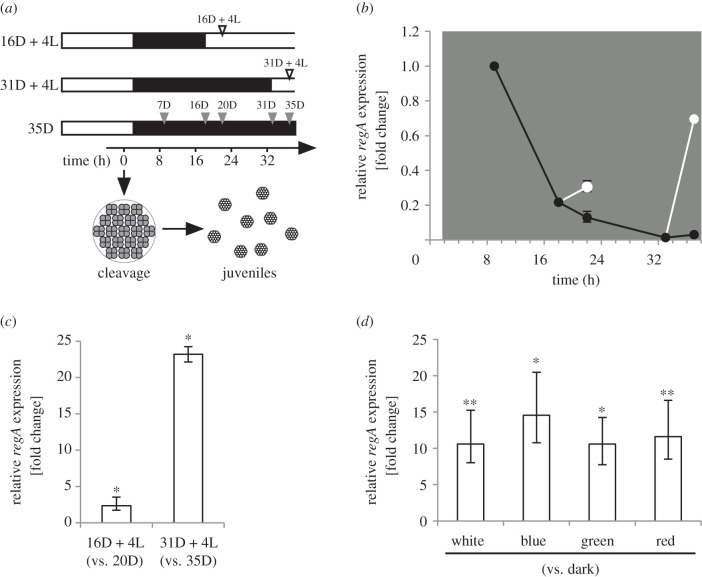
As RLS1 in C. reinhardtii was induced when cells were exposed to prolonged dark periods [2], dmAMN cultures of newly formed juveniles were subjected to up to 2 days of dark, and regA transcript levels were quantified at several points during this time (figure 4a). In contrast with RLS1, regA was not induced by extended dark periods. After the initial developmental induction, regA transcript levels dropped in a similar fashion to that observed during the regular light-dark cycle (figure 4b; electronic supplementary material, figure S5).
Figure 4.
The effect of light following extended dark periods on regA expression in juvenile dmAMN mutants. (a) dmAMN cultures were grown in a standard 16 L : 8 D light regime. At the end of the first dark period, cultures were maintained in dark for a total of 7, 16, 20, 31 or 35 h. Cultures at 16 or 31 h were then exposed to 4 h of light (16D + 4L and 31D + 4L, respectively). White and black bars denote light and dark periods, respectively; arrowheads indicate time points for RNA extraction, during the dark period (grey) and after light exposure (white). (b) regA expression at various times during the dark period (7D, 16D, 31D, 20D and 35D; black lines and circles) and following 4 h of light (16D + 4L and 31D + 4L; white lines and circles) relative to the 7D time point (points on x-axis are based on time from start of cleavage; 7D, 16D and 31D are n = 1, all others n = 3). (c) Comparison between regA expression in cultures exposed to 4 h light after 16 or 31 h of dark (relative to cultures maintained in dark for the entire period; 20 h and 35 h, respectively) (n = 3, bars indicate s.e.; randomization test, *p < 0.05). (d) Comparison among regA expression in cultures exposed to 4 h of light of different qualities (red, green or blue light at 5 µmol photons m−2 s−1 or standard white light at 260 µmol photons m−2 s−1), relative to cultures maintained in dark (n = 3, bars indicate s.e.; randomization test, *p < 0.05; **p < 0.01).
However, we found that returning these dark-maintained cultures to light triggered the expression of regA, and its induction levels were dependent on the length of both the dark period and the light exposure (figure 4b; electronic supplementary material, figure S6). Overall, the highest increase in regA transcript levels was observed when cultures were exposed to regular light (260 µmol photons m−2 s−1) for 4 h after a 31 h-long period of dark. In these conditions, we observed a 23.2-fold increase in regA transcript levels, relative to cultures maintained in the dark for the same amount of time (figure 4c).
To determine if the observed regA induction by light is mediated by a photoreceptor, cultures were exposed to wavelengths (i.e. blue, green and red light) that are in the range of known photoreceptors in V. carteri [30]. The increase in regA transcript levels in cultures exposed to different wavelength (relative to cultures maintained in the dark) ranged from 10.6 (white light) to 14.5-fold (blue light; figure 4d), but overall regA induction did not appear to be dependent on the wavelength of the tested light qualities.
(c). The conditions that induce regA in the dmAMN mutant also induce cell death
To address whether the conditions that induce regA expression are stressful, we tested the viability of cells using SYTOX Green—a DNA-binding fluorescent dye that penetrates only dead cells. Levels of cell death in the dmAMN mutant cultures exposed to the same conditions that triggered high levels of regA induction (4 h of light after 31 h of dark) are significantly increased relative to cultures maintained in the dark for the entire 35 h period (31.5% versus 7.9%) (figure 5a,b). Interestingly, levels of cell death correlated strongly with light intensity (figure 5d). When compared with cultures kept in the dark, cell death levels did not increase significantly at 20 µmol photons m−2 s−1, but they did increase at 70 and 260 µmol photons m−2 s−1. To explore the possibility that cells died via a programmed cell death (PCD) pathway, the DNA laddering assay (which is a hallmark of PCD and was previously reported in V. carteri [31]) was performed. While no DNA laddering was observed in the dark culture, the culture exposed to light showed the DNA laddering pattern specific to PCD (figure 5c).
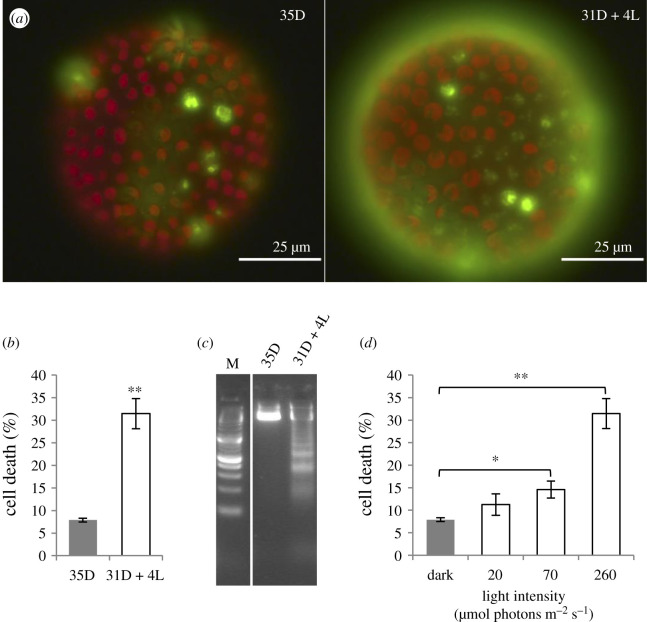
Figure 5.
The effect of light following an extended dark period on the viability of juvenile dmAMN cells. dmAMN cultures were subjected to 31D + 4L and 35D as in figure 4a. (a) Representative fluorescent micrographs of dmAMN juveniles subjected to 35 h of dark (35D) or 31 h of dark followed by 4 h of light (31D + 4L); dead cells appear green (SYTOX Green) and live cells are red (due to chlorophyll autofluorescence). (b) Comparison between percentage of dead cells (per individual) from 35D and 31D + 4L cultures (n = 3; 3 technical replicates with greater than or equal to 20 individuals each; bars indicate s.e.; two-sample t-test, **p < 0.001); (c) total DNA extracted from 35D and 31D + 4L cultures showing the DNA laddering effect characteristic of PCD in the latter. M, DNA marker. (d) Comparison among percentage of dead cells (per individual) from 35D and 31D + 4L cultures exposed to light of different intensities (20, 70 or 260 µmol photons m−2 s−1); 35D and 31D + 4L at 260 µmol photons m−2 s−1 are as in (b) (Tukey HSD, *p < 0.01; **p < 0.001).
(d). The conditions inducing regA and PCD in the dmAMN mutant are ineffective in EVE
To test if regA is also expressed in EVE under the environmental conditions found to induce regA in dmAMN cells, juvenile EVE cultures were also subjected to 31 h of dark followed by 4 h of exposure to light (figure 6a). In contrast to the 23.2-fold regA induction observed in juvenile dmAMN mutants, these conditions had very little effect on regA expression in juvenile EVE cultures (figure 6b). We also addressed whether these experimental conditions can nevertheless induce cell death in EVE, by assessing the number of dead somatic cells in juvenile cultures subjected to either 35 h of dark or 31 h of dark followed by 4 h of light (figure 7a). We found a slight but not significant increase in somatic cell death in light-exposed cultures compared with the dark control (0.8% versus 0.2%; figure 7b). Furthermore, compared with the dmAMN cultures grown in the same experimental conditions (figure 5b) cell death levels in EVE were almost 40-fold lower, for both light and control treatments (figure 7b). In addition, no DNA laddering indicative of PCD was observed in these cultures (figure 7c). Thus, exposure to light following a prolonged dark period did not have a negative effect on the viability of somatic cells in EVE cultures.
Figure 6.
The effect of light following an extended dark period on regA expression in juvenile EVE strain. (a) EVE cultures were grown in a standard 16 L : 8 D light regime. After the onset of embryogenesis, early embryos were isolated and kept in dark for 31 h followed by 4 h light (31D + 4L) or maintained in the dark for the entire 35 h (35D). White and dark bars denote light and dark periods, respectively; arrowheads indicate time points for RNA extraction, during the dark period (grey) and after light exposure (white). (b) regA expression in EVE cultures exposed to 4 h light after 31 h of dark (31D + 4L; left column), relative to cultures maintained in dark for the entire period (35D) (n = 3, error bars indicate s.e.); regA expression in dmAMN under the same conditions is also shown for comparison (right column; in grey).
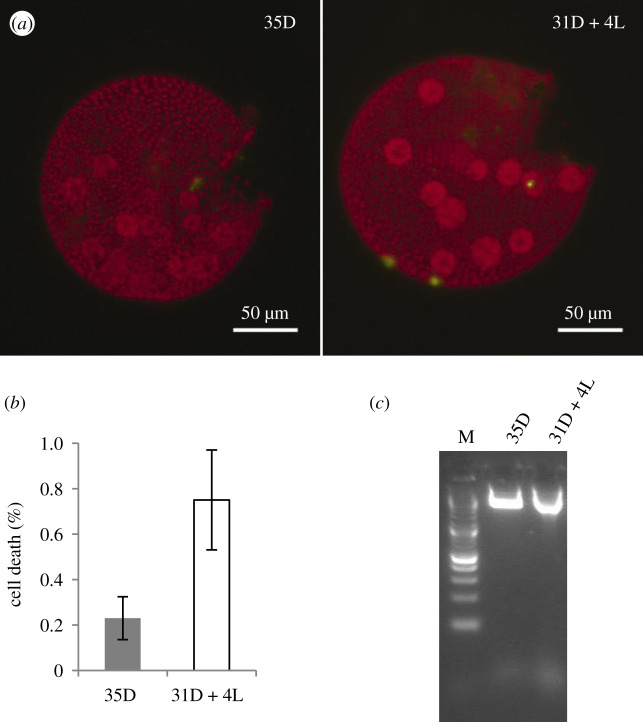
Figure 7.
The effect of light following an extended dark period on the viability of juvenile EVE somatic cells. EVE cultures were subjected to 31D + 4L and 35D as in figure 6a. (a) Representative fluorescent micrographs of EVE juveniles subjected to 35 h of dark (35D) or 31 h of dark followed by 4 h of light (31D + 4L); dead cells appear green (SYTOX Green) and live cells are red (due to chlorophyll autofluorescence). (b) Percentage of dead somatic cells (per individual) from 35D and 31D + 4L cultures (n = 3; 3 technical replicates with greater than or equal to 20 individuals each; bars indicate s.e.); (c) total DNA extracted from 35D and 31D + 4L cultures not showing the DNA laddering effect characteristic of PCD. M, DNA marker.
4. Discussion
(a). RegA is a developmental gene that maintained its ancestral environmental regulation
The data reported here show that in addition to being developmentally regulated, regA can also be induced by environmental cues. Specifically, we showed that exposure to light after a prolonged dark period can trigger the expression of regA. The difference in response to extended periods of dark between RLS1 [2] and regA could be due to (i) the different lengths of dark periods used (up to 49 h for dmAMN versus 3 days for C. reinhardtii), (ii) differences in metabolic states as, in contrast to C. reinhardtii, V. carteri lacks the ability to use organic substrates and to grow in the dark, and/or (iii) the developmental regulation of regA may affect how environmental stimuli affect regA expression. However, the fact that the duration of both dark and light exposure affected its expression levels as well as that these specific conditions can also result in cell death indicate that regA induction is probably triggered by a metabolic imbalance and not an environmentally related developmental perturbation. That is, regA maintained its ancestral environmental regulation. Interestingly, genes with dual regulation are often seen as intermediate steps during the sub-functionalization process that results in two specialized genes (e.g. [32]). It will be of interest to know if regA's paralogues (rlsA, rlsB and rlsC) have retained the ancestral regulation or whether they specialized into specific developmental roles.
(b). Mechanistic models for the evolution of regA's developmental regulation in V. carteri
Previous studies proposed that regA evolved through the co-option of an ancestral RLS1-like gene that was environmentally regulated [2,17]. However, the exact evolutionary scenario and mechanisms responsible for the postulated co-option event remained to be determined. Overall, our findings indicate that the acquisition of regA's developmental regulation did not replace its ancestral environmental regulation (scenario (i) in figure 2b). Rather, a new layer of regulation was added (scenario (ii) in figure 2b). But how did the new developmental regulation evolve? Two potential models can be envisioned: the ancestral signalling pathway was co-opted (that is, the developmental cue simulated the environmental signal) or a completely new signalling pathway evolved. The two models make different predictions as to the dual regulation of regA in V. carteri (figure 2c).
The first model predicts that the same environmentally induced intracellular signal that triggers the expression of RLS1 in C. reinhardtii is also triggered in V. carteri's small cells at the end of embryogenesis. In C. reinhardtii, RLS1 is likely part of the general photo-acclimation response [17] and thus might be induced by an energetic imbalance mediated by a redox signal (e.g. NADPH/NADP+, reactive oxygen species—ROS [33]). A similar signal could also be induced in the dmAMN cells exposed to light after long periods of dark. We suggested that the developmental signal responsible for the induction of regA in small cells can be prompted by an imbalance between membrane-bound proteins (e.g. electron transport carriers) and soluble chloroplast factors (e.g. NADP+) as the surface to volume ratio in these small cells is in favour of membrane proteins (see fig. 2 in [17]). Interestingly, the environmental induction of regA in the dmAMN mutant is also affected by cell size. The same conditions that induce regA expression in the small juvenile dmAMN cells do not elicit a similar response in the larger mature cells associated with the second day of the life cycle (figure 1b) (electronic supplementary material, figure S7). Thus, deciphering the exact signal involved in the environmental induction of regA could provide insights into elucidating the long-standing question as to how small cell size determines somatic cell fate in V. carteri.
The second model predicts that new cis-regulatory and/or trans-acting elements were added to the ancestral RLS1/rlsD gene regulation. In V. carteri, the developmental regulation of regA is known to involve cis-regulatory elements present in its introns (two enhancers in introns 3 and 5, and one silencer in intron 7 [29]). However, the sequence of these postulated regulatory elements as well as the trans-acting factors binding to them are still unknown. Also, nothing is yet known about the regulation of RLS1, whose exon-intron structure and intron sequences differ from those of regA. In animals, new patterns of gene expression affecting developmental genes and resulting in morphological innovation are also thought to evolve through changes in the deployment of trans-acting factors, cis-regulatory elements (de novo, or via modification of pre-existing elements), or a combination of both [34]. Similarly, in the social amoebae, the co-option of ancestral cAMP signalling genes for new developmental roles in Dictyostelium discoideum involved the acquisition of new, distal promoters [35]. The presence of a similar mechanism in volvocine algae would argue for a general role that changes in gene regulation can have in the evolution of major morphological innovations.
(c). The role of regA in protection against environmental stress
This study showed for the first time that regA can be induced outside its developmental context, suggesting that it might play additional roles unrelated to development. Specifically, regA was induced in a mutant lacking cell differentiation in response to specific environmental conditions. However, regA was only slightly induced in EVE. In addition to different patterns of regA expression in response to environmental cues, the two strains also responded differently in terms of cell viability. That is, in contrast to the dmAMN, the viability of EVE somatic cells was only weakly affected. Since at the end of embryogenesis EVE somatic cells and dmAMN cells are developmentally equivalent, the difference in response between the two strains is likely due (directly or indirectly—by establishing a terminally differentiated state) to the presence of a functional RegA protein in EVE but not in the dmAMN mutant.
Specifically, due to regA's role in suppressing the expression of chloroplast proteins, the two strains likely differ in their chloroplast protein composition and these differences can affect the overall metabolic/redox state of their cells [20]. For instance, light might induce a metabolic imbalance in the chloroplast of the RegA-deficient small dmAMN cells exposed to long periods of dark, and this imbalance could trigger a redox signal that induces regA expression in an attempt to adjust the chloroplast composition in response to light (i.e. an acclimation-like response). However, in the absence of a functional RegA protein, these cells will likely be unable to adjust their chloroplast composition and deal with the excess light energy, which would maintain or worsen the imbalance and result in the accumulation of ROS and ultimately trigger PCD [36]. On the other hand, chloroplasts in somatic cells have already adjusted their chloroplast composition in response to the developmental signal (or are able to do so as in response to light as they have a functional RegA protein), which can prevent the imbalance that would trigger regA expression and death. Notably, although gonidia do not express regA, they also appear unaffected by the light stress. This is probably due to their larger cell size and decreased surface to volume ratio (in favour of soluble factors, including NADP+), which can mitigate the imbalance experienced by the small cells. In fact, large dmAMN cells (in the second day of their life cycle; figure 1b) are also unaffected by the light stress (electronic supplementary material, figure S8), suggesting that the light-induced stress is dependent on cell size. Interestingly, RegA might also confer somatic cells protection against heat. Previous work in V. carteri EVE has shown that in response to heat shock, gonidia (which do not express regA during development) undergo PCD, while somatic cells appear unaffected and continue to provide the colony with motility [31].
Overall, these findings indicate that the presence of a functional regA in somatic cells confers them resistance to environmental stress. This is extremely important at the multicellular organism level as somatic cells are responsible for the motility, and thus survival, of the individual. But if somatic cells are already protected (directly or indirectly) by the presence of developmentally expressed RegA protein, why is regA's environmental regulation still maintained? It is likely that regA also plays a direct role in the response to stress in young/small gonidia. For instance, under nutrient deprivation gonidia stop growing and undergo a temporary cessation of reproduction. At the cell level, the inhibition of gonidia growth and reproduction is an acclimation response that prevents the accumulation of ROS-inducing damage, and thus ensures survival. At the multicellular level, this is an adaptive response that is costly in the terms of immediate reproduction but beneficial in terms of offspring quality since it avoids ROS-induced DNA damage and mutations in the gonidia. Nevertheless, when damage is extensive (such as during heat shock) PCD is the best adaptive response [31].
(d). Stress-induced responses and the evolution of complexity
Our finding of a master developmental regulator that both manifests its ancestral environmental regulation and confers stress protection offers a direct link between stress responses and the early evolution of development. Recently, the evolution of several developmental processes—from aggregative multicellularity in D. discoideum to the differentiation of decidual stromal cells in placental mammals—has been linked to pre-existing ancestral stress responses [37–39]. Altogether, our study argues for the role of stress in the evolution of multicellular complexity and provides insights into potential mechanisms involved in the co-option of stress responses into new morphological innovations.
Supplementary Material
Data accessibility
Supporting data are provided as electronic supplementary material.
Authors' contributions
S.G.K. designed the project, performed the experiments, analysed the data, produced the figures and participated in the writing of the manuscript. A.M.N. designed the project, participated in data analyses and production of figures, and wrote the manuscript. Both authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
The work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada to A.M.N. Funding from the Harrison McCain Foundation is also acknowledged.
References
- 1.Nedelcu AM, Michod RE. 2004. Evolvability, modularity, and individuality during the transition to multicellularity in volvocalean green algae. In Modularity in development and evolution (eds Schlosser G, Wagner GP), pp. 466–489. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Nedelcu AM, Michod RE. 2006. The evolutionary origin of an altruistic gene. Mol. Biol. Evol. 23, 1460–1464. ( 10.1093/molbev/msl016) [DOI] [PubMed] [Google Scholar]
- 3.Kirk DL. 1998. Volvox: molecular-genetic origins of multicellularity. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 4.Merchant SS, et al. 2007. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318, 245–250. ( 10.1126/science.1143609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prochnik SE, et al. 2010. Genomic analysis of organismal complexity in the multicellular green alga Volvox carteri. Science 329, 223–226. ( 10.1126/science.1188800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hallmann A, Godl K, Wenzl S, Sumper M. 1998. The highly efficient sex-inducing pheromone system of Volvox. Trends Microbiol. 6, 185–189. ( 10.1016/S0966-842X(98)01234-7) [DOI] [PubMed] [Google Scholar]
- 7.Kirk DL, Kaufman MR, Keeling RM, Stamer KA. 1991. Genetic and cytological control of the asymmetric divisions that pattern the Volvox embryo. Development 113, 67–82. [PubMed] [Google Scholar]
- 8.Matt GY, Umen JG. 2017. Cell-type transcriptomes of the multicellular green alga Volvox carteri yield insights into the evolutionary origins of germ and somatic differentiation programs. G3 8, 531–550. ( 10.1534/g3.117.300253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirk MM, Ransick A, McRae SE, Kirk DL. 1993. The relationship between cell size and cell fate in Volvox carteri. J. Cell Biol. 123, 191–208. ( 10.1083/jcb.123.1.191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huskey RJ, Griffin BE. 1979. Genetic control of somatic cell differentiation in Volvox: analysis of somatic regenerator mutants. Dev. Biol. 72, 226–235. ( 10.1016/0012-1606(79)90113-1) [DOI] [PubMed] [Google Scholar]
- 11.Sessoms AH, Huskey RJ. 1973. Genetic control of development in Volvox: isolation and characterization of morphogenetic mutants. Proc. Natl Acad. Sci. USA 70, 1335–1338. ( 10.1073/pnas.70.5.1335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirk MM, Stark K, Miller SM, Müller W, Taillon BE, Gruber H, Schmitt R, Kirk DL. 1999. regA, a Volvox gene that plays a central role in germ-soma differentiation, encodes a novel regulatory protein. Development 126, 639–647. [DOI] [PubMed] [Google Scholar]
- 13.Meissner M, Stark K, Cresnar B, Kirk DL, Schmitt R. 1999. Volvox germline-specific genes that are putative targets of RegA repression encode chloroplast proteins. Curr. Genet. 36, 363–370. ( 10.1007/s002940050511) [DOI] [PubMed] [Google Scholar]
- 14.Tam LW, Kirk DL. 1991. Identification of cell-type-specific genes of Volvox carteri and characterization of their expression during the asexual life cycle. Dev. Biol. 145, 51–66. ( 10.1016/0012-1606(91)90212-L) [DOI] [PubMed] [Google Scholar]
- 15.Nedelcu AM. 2019. Independent evolution of complex development in animals and plants: deep homology and lateral gene transfer. Dev. Genes Evol. 229, 25–34. ( 10.1007/s00427-019-00626-8) [DOI] [PubMed] [Google Scholar]
- 16.Duncan L, Nishii I, Harryman A, Buckley S, Howard A, Friedman NR, Miller SM. 2007. The VARL gene family and the evolutionary origins of the master cell-type regulatory gene, regA, in Volvox carteri. J. Mol. Evol. 65, 1–11. ( 10.1007/s00239-006-0225-5) [DOI] [PubMed] [Google Scholar]
- 17.Nedelcu AM. 2009. Environmentally induced responses co-opted for reproductive altruism. Biol. Lett. 5, 805–808. ( 10.1098/rsbl.2009.0334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grochau-Wright ZI, Hanschen ER, Ferris PJ, Hamaji T, Nozaki H, Olson BJSC, Michod RE. 2017. Genetic basis for soma is present in undifferentiated volvocine green algae. J. Evol. Biol. 30, 1205–1218. ( 10.1111/jeb.13100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanschen ER, Ferris PJ, Michod RE. 2014. Early evolution of the genetic basis for soma in the Volvocaceae. Evolution 68, 2014–2025. ( 10.1111/evo.12416) [DOI] [PubMed] [Google Scholar]
- 20.König SG, Nedelcu AM. 2016. The mechanistic basis for the evolution of soma during the transition to multicellularity in the volvocine algae. In Multicellularity: origins and evolution (eds Newman S, Niklas K), pp. 43–70. Vienna, Austria: MIT Press. [Google Scholar]
- 21.Harryman A. 2012. Investigating the roles of regA and related genes in the evolution of multicellularity in the volvocine green algae. Baltimore County, MD: University of Maryland. [Google Scholar]
- 22.Duncan L, Nishii I, Howard A, Kirk D, Miller SM. 2006. Orthologs and paralogs of regA, a master cell-type regulatory gene in Volvox carteri. Curr. Genet. 50, 61–72. ( 10.1007/s00294-006-0071-4) [DOI] [PubMed] [Google Scholar]
- 23.Adams CR, Stamer KA, Miller JK, McNally JG, Kirk MM, Kirk DL. 1990. Patterns of organellar and nuclear inheritance among progeny of two geographically isolated strains of Volvox carteri. Curr. Genet. 18, 141–153. ( 10.1007/BF00312602) [DOI] [PubMed] [Google Scholar]
- 24.Kirk DL, Kirk MM. 1983. Protein synthetic patterns during the asexual life cycle of Volvox carteri. Dev. Biol. 96, 493–506. ( 10.1016/0012-1606(83)90186-0) [DOI] [PubMed] [Google Scholar]
- 25.Ramakers C, Ruijter JM, Lekanne Deprez RH, Moorman AFM. 2003. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 339, 62–66. ( 10.1016/S0304-3940(02)01423-4) [DOI] [PubMed] [Google Scholar]
- 26.Ruijter JM, Ramakers C, Hoogaars WMH, Karlen Y, Bakker O, van den Hoff MJBMA. 2009. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 37, e45 ( 10.1093/nar/gkp045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tam L-W KD. 1991. The program for cellular differentiation in Volvox carteri as revealed by molecular analysis of development in a gonidialess/somatic regenerator mutant. Development 112, 571–580. [DOI] [PubMed] [Google Scholar]
- 28.Tam LW, Stamer KA, Kirk DL. 1991. Early and late gene expression programs in developing somatic cells of Volvox carteri. Dev. Biol. 145, 67–76. ( 10.1016/0012-1606(91)90213-M) [DOI] [PubMed] [Google Scholar]
- 29.Stark K, Kirk DL, Schmitt R. 2001. Two enhancers and one silencer located in the introns of regA control somatic cell differentiation in Volvox carteri. Genes Dev. 15, 1449–1460. ( 10.1101/gad.195101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kianianmomeni A, Hallmann A. 2014. Algal photoreceptors: in vivo functions and potential applications. Planta. 239, 1–26. ( 10.1007/s00425-013-1962-5) [DOI] [PubMed] [Google Scholar]
- 31.Nedelcu AM. 2006. Evidence for p53-like-mediated stress responses in green algae. FEBS Lett. 580, 44 ( 10.1016/j.febslet.2006.04.044) [DOI] [PubMed] [Google Scholar]
- 32.Zhang J. 2003. Evolution by gene duplication: an update. Trends Ecol. Evol. 18, 292–298. ( 10.1016/S0169-5347(03)00033-8) [DOI] [Google Scholar]
- 33.Strand DD, et al. 2017. Defects in the expression of chloroplast proteins leads to H2O2 accumulation and activation of cyclic electron flow around photosystem I. Front. Plant Sci. 7, 2073 ( 10.3389/fpls.2016.02073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koshikawa S, Giorgianni MW, Vaccaro K, Kassner VA, Yoder JH, Werner T, Carroll SB. 2015. Gain of cis-regulatory activities underlies novel domains of wingless gene expression in Drosophila. Proc. Natl Acad. Sci. USA 112, 7524–7529. ( 10.1073/pnas.1509022112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvarez-Curto E, Rozen DE, Ritchie AV, Fouquet C, Baldauf SL, Schaap P. 2005. Evolutionary origin of cAMP-based chemoattraction in the social amoebae. Proc. Natl Acad. Sci. USA 102, 6385–6390. ( 10.1073/pnas.0502238102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullineaux PM, Baker NR. 2010. Oxidative stress: antagonistic signaling for acclimation or cell death? Plant Physiol. 154, 521–525. ( 10.1104/pp.110.161406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaap P. 2016. Evolution of developmental signalling in Dictyostelid social amoebas. Curr. Opin. Genet. Dev. 39, 29–34. ( 10.1016/j.gde.2016.05.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner GP, Erkenbrack EM, Love AC. 2019. Stress-induced evolutionary innovation: a mechanism for the origin of cell types. Bioessays 41, 1800188 ( 10.1002/bies.201800188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nedelcu AM, Michod RE. 2020. Stress responses co-opted for specialized cell types during the early evolution of multicellularity. Bioessays 42, 2000029 ( 10.1002/bies.202000029) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supporting data are provided as electronic supplementary material.