Abstract
Climate change is not only causing steady increases in average global temperatures but also increasing the frequency with which extreme heating events occur. These extreme events may be pivotal in determining the ability of organisms to persist in their current habitats. Thus, it is important to understand how quickly an organism's heat tolerance can be gained and lost relative to the frequency with which extreme heating events occur in the field. We show that the California mussel, Mytilus californianus—a sessile intertidal species that experiences extreme temperature fluctuations and cannot behaviourally thermoregulate—can quickly (in 24–48 h) acquire improved heat tolerance after exposure to a single sublethal heat-stress bout (2 h at 30 or 35°C) and then maintain this improved tolerance for up to three weeks without further exposure to elevated temperatures. This adaptive response improved survival rates by approximately 75% under extreme heat-stress bouts (2 h at 40°C). To interpret these laboratory findings in an ecological context, we evaluated 4 years of mussel body temperatures recorded in the field. The majority (approx. 64%) of consecutive heat-stress bouts were separated by 24–48 h, but several consecutive heat bouts were separated by as much as 22 days. Thus, the ability of M. californianus to maintain improved heat tolerance for up to three weeks after a single sublethal heat-stress bout significantly improves their probability of survival, as approximately 33% of consecutive heat events are separated by 3–22 days. As a sessile animal, mussels likely evolved the capability to rapidly gain and slowly lose heat tolerance to survive the intermittent, and often unpredictable, heat events in the intertidal zone. This adaptive strategy will likely prove beneficial under the extreme heat events predicted with climate change.
Keywords: heat acclimatization, heat hardening, heat wave, intertidal zone, physiological plasticity, thermal tolerance
1. Introduction
Anthropogenic climate change is causing mean air and water temperatures to rise globally, with a further 1–4°C increase projected by 2100 [1]. Temperature variability is also increasing, resulting in more frequent extreme temperature events [1]. Phenotypic changes, like acclimatization, that increase thermal tolerance may be critical in enabling organisms to survive under these changing temperature conditions [2–5]. These adaptive changes in phenotype would ideally occur rapidly during an initial period of heat stress and persist long enough to allow the organism to withstand subsequent episodes of extreme heating [6–11]. Improvements in heat tolerance have generally been classified as either (i) rapid heat hardening from a single heat-stress bout, which confers transient heat tolerance that typically lasts less than 32 h [12,13] or (ii) slower heat acclimatization accomplished through repeated heat-stress bouts, which confers heat tolerance that can last for weeks to months [14–16]. Yet despite a large literature based on temperature–phenotype interactions, the time-courses of adaptive physiological changes—both their rate of gain and persistence in the absence of continued thermal stress—remain largely unknown [5,17,18]. Laboratory thermal acclimation studies have typically maintained animals under different constant temperatures for periods of days to weeks [14,15,19], and little attention has been given to the minimal time required for the animal to actually acclimate (i.e. achieve increased heat tolerance [20]) and when this improved heat tolerance subsequently decays. Moreover, by maintaining animals at constant laboratory temperatures, there is a large gap in our understanding of how animals can gain and lose heat tolerance within a more ecologically valid context—one that mimics the stochastic and often unpredictable nature with which animals experience consecutive stressful heat events in the field [21,22].
In this study, we addressed these issues about the time-course of the gain and loss of heat tolerance in the California mussel, Mytilus californianus, a sessile species that experiences wide variations in body temperature [23,24]. Intertidal mussels, as sessile ectotherms, lack the ability to behaviourally thermoregulate and are consequently exposed to intermittent, and often extreme, temperature fluctuations associated with the tidal cycle and terrestrial weather [7,24–27]. Mussels have received substantial investigation in terms of their physiological responses to environmental change, notably temperature. Most studies of mussels' responses to heat stress have involved either a short heat-hardening bout in which mussels’ thermal tolerance was transiently improved for 12 h [9], or longer duration heat acclimation studies which constantly submerge mussels for periods of several days to a few weeks which leads to various physiological changes (e.g. cardiac performance; [14,19]) and biochemical status (e.g. synthesis of heat-shock proteins; [28]). However, to date, none of these laboratory studies have evaluated the rates of gain and loss of heat tolerance within an ecologically valid context, where consecutive heat events might be separated by days to weeks [29,30]. We are only aware of one heat-hardening study in mussels [9], and it did not evaluate any time periods past 12 h. Thus, it is unclear whether this brief improvement in thermal tolerance may be extended to more ecologically relevant periods of time between heat-stress bouts characteristic of the intertidal zone. Here, heat-stress bouts are typically separated by at least 24 h due to a semi-diurnal tidal cycle. Thus, in the context of a semi-diurnal tidal cycle, the findings of Dunphy et al. [9] may not be ecologically relevant. Twelve hours after an extreme heat-stress bout (which likely would occur midday), the temperatures would probably be much cooler as it would be nighttime. Given the unpredictability of heat stress in the intertidal zone, it is important to determine whether a single heat-stress bout may confer prolonged heat tolerance (greater than 24 h) that would provide defense against another heat-stress bout days to weeks later.
The experiments we describe below were designed to determine, firstly, how exposure to short bouts of heating at different sublethal temperatures affected subsequent tolerance to an extreme, potentially lethal heat event. We then determined the time-course over which this improved heat tolerance would last relative to the initial sublethal heat-stress temperature. Our laboratory findings were then placed in the context of an analysis of 4 years of mussel field body temperatures. Our findings offer new insights into heat-stress relationships under field conditions. We show that, in mussels, heat acclimation occurs after a single, brief exposure to heat stress, and that this improved heat tolerance is maintained for up to three weeks. However, the rate with which heat tolerance is gained and lost is dependent on the initial heat-stress temperature. Importantly, when placed in the context of the field data, we found that mussels' adaptive strategy to cope with heat stress—to quickly gain and slowly lose heat tolerance—protects them from the vast majority of intermittent heat-stress bouts in the intertidal zone.
2. Methods
Specimens of M. californianus (Conrad 1837, n = 711) were collected January through May 2020 from the mussel bed of a moderately wave-exposed shore at Hopkins Marine Station in Pacific Grove, California, USA (36.6216°N, 121.9042°W). Intertidal height of sampled mussels ranged from 0.95 to 1.22 m above mean lower low water. To minimize other factors that might affect thermal tolerance (e.g. variation in thermal inertia due to differences in body mass), only adult mussels with shell lengths within an approximately 30 mm range (range: 51–82 mm) were collected. Body mass (digital scale) and shell length (digital callipers) were measured for each individual. The body mass and shell length of the mussels were 29.17 ± 7.87 g and 63.84 ± 5.95 mm (mean ± s.d.), respectively (electronic supplementary material, table S1).
After collection, mussels were kept in a flow-through aquarium system supplied with sand-filtered seawater from Monterey Bay; water temperature in the aquaria matched that of the Bay. During the course of the six-month study, mean ± s.d. water temperature was 13.8 ± 1.0°C (range: 11.9–16.3°C; see electronic supplementary material, table S2 for water temperature data by month). Except for the heating bouts (see below), mussels were not subjected to any other type of abiotic stress (e.g. temperature, pH, dissolved oxygen, or salinity) during the experiments. Under all treatments, mussels were fed a commercial shellfish diet (Shellfish Diet 1800, Reed Mariculture, Campbell, CA, USA) three to four times per week according to the manufacturer's instructions [31].
(a). Survival tests
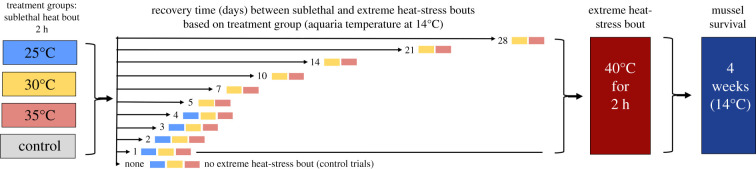
A schematic of the experimental design is given in figure 1. To determine whether a single sublethal heat-stress bout would confer improved heat tolerance during a subsequent more extreme (potentially lethal) heat-stress exposure, mussels were given a sublethal heat-stress bout of either 25, 30 or 35°C for 2 h, and then were placed in aquaria (at approximately 14°C) for 1 to 28 days before undergoing an extreme heat-stress bout at 40°C for 2 h. In order to minimize the effects of seasonality on results, mussels from each of the experimental (i.e. 25, 30 and 35°C) and control groups were collected at the same time, and then tested within the same week for each experimental day 1–28. For example, all mussels that were tested for recovery day 2 were collected on the same day and then tested within the same week of each other. Moreover, for each trial, a group of control mussels (not subjected to sublethal heat stress) were tested alongside the experimental groups—and these control mussels were also collected on the same day as the experimental mussels for that testing period. After being collected, mussels were haphazardly assigned to each of the experimental and control groups. After the extreme heat-stress bout, all mussels were placed back into the flow-through aquaria, and mussel survival was monitored every 2–3 days for four weeks [32]. Mussels that exhibited continuous gaping and were unresponsive to several strong squeezes of the valves were considered dead [9].
Figure 1.
Schematic of the experimental design for the survival tests. Mussels first underwent a sublethal heat-stress bout for 2 h (temperature according to the treatment group: 25, 30 or 35°C, or no sublethal heat-stress control), and then recovered in an aquarium for a certain number of days (i.e. recovery time, 1–28 days), before being subjected to an extreme heat-stress bout (40°C for 2 h). After the extreme heat-stress bout, mussels were placed back into the flow-through aquaria for four weeks, and survival was assessed every 2–3 days throughout that period. The colours after the number of recovery days indicate which groups were tested for each condition. Note that control mussel groups were tested for all conditions (but there are no grey boxes noted on the diagram). The 25°C group was only tested for recovery days 1–4 since we found no differences between the control and 25°C groups; see ‘Methods’ for details.
For the sublethal and extreme heat-stress bouts, mussels were heated in an insulated chamber while exposed to air, as would occur during low tide. Air temperature inside the chamber was increased at a specific rate using temperature control circuitry (Newport Electronics, iSeries Temperature Controller, Omega Engineering, Santa Ana, CA, USA) that regulated a heating element inside the chamber, which in turn received feedback from a resistance temperature detector in the chamber. A small fan circulated air inside the chamber to provide uniform heating. After all mussels were placed inside the chamber and the lid was secured, there was a 10 min equilibration period during which air temperature inside the chamber was held at 22°C. Air temperature was then increased at a rate of 9.0°C h−1 (a heating rate typical of their habitat [24,33]) until reaching the experimental temperature (25, 30, 35 or 40°C) and then was held at that temperature for 2 h. At the conclusion of the trials, mussels were immediately transferred back to the flow-through aquaria (approx. 14°C) where they remained either until their next heat-stress bout (for specimens given sublethal heat-stress treatment) or, if they had been given the extreme (40°C) heat-stress bout, until they died or reached the end of the four-week survival-monitoring period.
The sublethal heat-stress temperatures (25, 30 and 35°C) were selected to span the range over which different types of responses to heat stress have been observed in M. californianus. The lowest temperature, 25°C, is the minimum temperature at which heat-shock proteins have been reported to be upregulated in this species [34]. The highest sublethal heat-stress temperature, 35°C, is approximately 1°C below this species' average critical temperature of cardiac function [33] and approximates the temperature at which massive upregulation occurs for genes that encode several classes of stress-related proteins [35]. However, after the first few rounds of testing, we found that the 25°C group survived similarly to the control group, suggesting that a sublethal heat-stress bout at 25°C does not improve heat tolerance over extended time periods, even though the heat-shock response may be initially induced (see ‘Results’). Consequently, the 25°C group was tested only for trials with 1–4 recovery days between heat-stress bouts. The extreme heat-stress temperature (40°C) was selected because it is the average temperature at which cardiac activity ceases, coinciding with mussels’ lethal temperature (i.e. flatline temperature, mean ± s.d. = 40.4 ± 1.3°C; [36]). Moreover, through a pilot study using control mussels that were not subjected to any sublethal heat-stress bout, 2 h at 40°C led to approximately 75–90% mortality within three to four weeks (data not shown). As each experimental group comprised 20 mussels, if one mussel died, it accounted for a 5% change in overall survival. By using an extreme temperature (40°C) that on average only approximately 13% control mussels survived, it allowed for more sensitive quantification of the survival effects of the various combinations of sublethal heat-stress bouts and recovery durations.
A total of 38 groups were tested (25°C = 5 groups; control, 30 and 35°C = 11 groups each). Each sublethal heat group comprised 16–20 mussels, while each control group comprised 9–20 mussels (see electronic supplementary material, table S1 for details). Note that the ‘none’ recovery groups (figure 1) indicate that mussels were given only the sublethal heat-stress bout without a subsequent extreme heat-stress bout, after which mortality was monitored for four weeks to ensure that these sublethal heat-stress bouts were indeed not lethal. Only one mussel (out of 20) died from the 35°C sublethal heat-stress bout, and no mussels died from the other sublethal heat-stress bouts.
(b). Field body temperature analyses
To interpret the laboratory results in the context of heat-stress events in the field, we reviewed a 4-year record of mussel body temperatures at our study site [37]. These data were obtained in 2005, 2007, 2009 and 2014 using ‘Robomussels’, fabricated from valves of mussels filled with silicon sealant in which iButton temperature loggers were embedded. Each year had at least six months of temperature data, which were acquired every 10 min [37]. To match our laboratory experimental protocol, we used a peak detector function (python scipy.signal.find_peaks) to screen the field body temperature data and identify periods when temperatures fell into the range of 28–41°C for at least 2 h. We defined these occurrences as heat-stress events. We chose 28°C as the lowest temperature (to represent the 30°C sublethal heat-stress bout) because we have previously reported that there is an approximately 2°C temporal lag in mussel body temperatures at a 9°C h−1 heating rate; at the same heating rate, 40°C can be overestimated by approximately 1°C (hence, the 41°C upper limit [33]). For each heat-stress bout detected, we then searched for the next heat-stress bout occurring at least 24 h later, to simulate the minimal time between daytime lower low tides. For each year, we summarized the frequency with which there were a given number of days between any two consecutive heat-stress bouts, relative to the total number of heat-stress bouts in that given year.
(c). Statistical analysis
R 3.5.2 (https://cran.r-project.org/) and R studio (https://www. rstudio.com/) were used for all statistical analyses. To test whether the sublethal heat-stress bouts improved survival under the extreme heat-stress bout, we used two statistical approaches. First, we compared final survival counts at the end of the four-week monitoring period. For each recovery day (i.e. days 1–28 between the sublethal and lethal heat-stress bouts), we used Pearson chi-square tests to compare the sublethal heat-stress groups' final survival versus the control group's final survival. We also compared final survival between each heat-stress treatment (i.e. 35°C versus 30°C group, 35°C versus 25°C group (if applicable) and 30°C versus 25°C group (if applicable)) for each recovery day. To account for conducting repeated chi-square tests, all p-values were corrected with the false discovery rate (FDR; [38]), after which a p < 0.05 was deemed significant.
Secondly, in addition to comparing final survival, we compared the overall survival curves, which describe not just survival at the end of the four weeks, but the responses across time (i.e. survival across the entire four-week monitoring period). We used Kaplan–Meier survival curves to describe the survival function (figure 2a). Comparing across the same groups as above, we tested whether Kaplan–Meier survival curves were statistically different from each other using logrank tests. A significant chi-square statistic (p < 0.05) for the logrank test indicated that there was a difference between two survival curves.
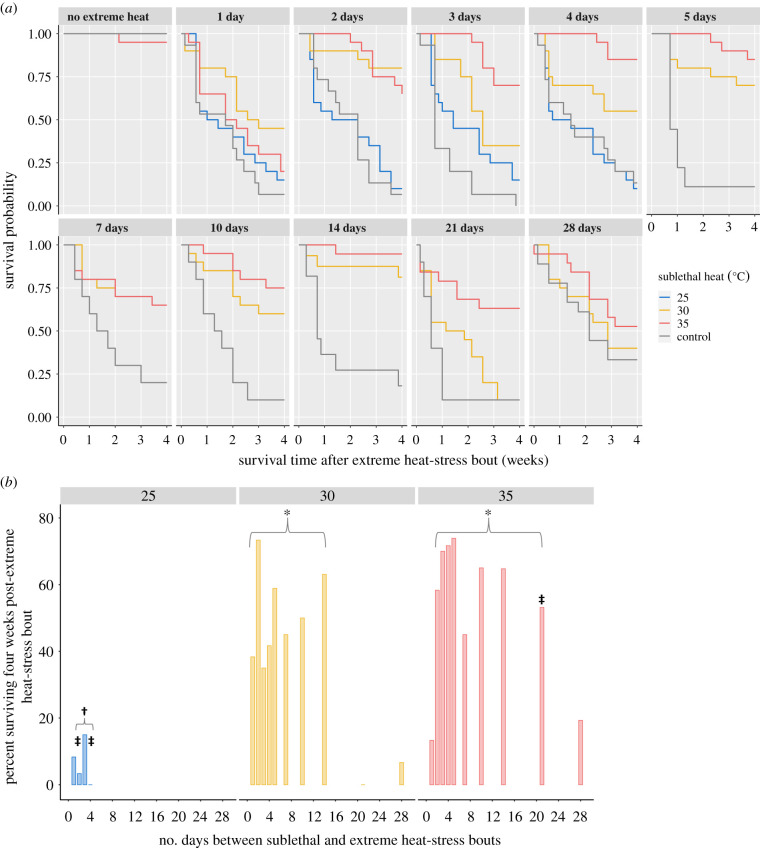
Figure 2.
Mussel survival based on sublethal heat-stress temperature and number of recovery days between consecutive heat-stress bouts. (a) Kaplan–Meier survival curves for each of the experimental (sublethal heat exposure) and control groups over the four-week monitoring period after mussels underwent the extreme heat-stress bout. The number at the top of each panel indicates the recovery time (in days) between the sublethal and extreme heat-stress bouts. Survival probability is indicated on the y-axis, where 1.0 is 100% survival. A separate control group was used for each recovery day. The ‘no extreme heat’ subplot shows mussel survival for four weeks after the sublethal heat-stress bouts alone; only one mussel died after the 35°C sublethal heat-stress bout. (b) Percent survival across the four-week monitoring period (y-axis) based on the number of recovery days between the sublethal and extreme heat-stress bouts (x-axis), separated by experimental groups (25°C in blue, 30°C in yellow and 35°C in red). Each bar on the plot was corrected for by subtracting the control group's final percent survival from each of the experimental group's final percent survival. Superscript symbols indicate significant differences: from the control group*, the 30°C group‡ or the 35°C group† (all p < 0.05) based on the Pearson chi-square tests (see ‘Results’ and electronic supplementary material, table S3 for further details).
3. Results
(a). Survival tests
The three temperatures used to provide the initial sublethal heat-stress bout yielded different effects on survival (figure 2 and electronic supplementary material, table S3). Considering first the final survival counts, the 25°C sublethal heat-stress bout did not confer improved heat tolerance (all p > 0.05 versus control group; electronic supplementary material, table S3). The 30°C sublethal heat-stress bout led to improved survival, starting 24 h later (i.e. 1 day of recovery between heat-stress bouts); this improved heat tolerance was maintained for at least 14 days (all p < 0.05 versus control group) but was completely lost after 21 days (p > 0.05 versus control group). The 30°C sublethal heat-stress bout also led to significantly higher survival compared to the 25°C group on days 2 and 4 (both p < 0.05). The 35°C sublethal heat-stress bout improved heat tolerance beginning 48 h later, and this enhanced tolerance was maintained for at least 21 days (all p < 0.05 versus control group) but was lost by day 28 (p > 0.05 versus control group). The 35°C group's survival was significantly higher than the 25°C group's survival from days 2–4 of recovery (all p < 0.05), but only significantly different from the 30°C group on day 21, when the 30°C group's heat tolerance was lost, while the 35°C group's heat tolerance was maintained (p = 0.006).
When looking not just at final survival but also at the survival curves across the entire four-week monitoring period, all of the above significant differences were still significant (for chi-square values, see electronic supplementary material, table S3). Furthermore, there were three instances where the survival curves were significantly different, but the final survival counts had not differed significantly after the FDR correction. Specifically, the 30°C group's survival curve was significantly different from the 25°C group at day 1 (as well as days 2 and 4), and the 35°C group's survival curve was significantly different from the 30°C group's survival curve on days 3 and 4 in addition to day 21 (all p < 0.05; figure 2 and electronic supplementary material, table S3).
(b). Field body temperature analyses
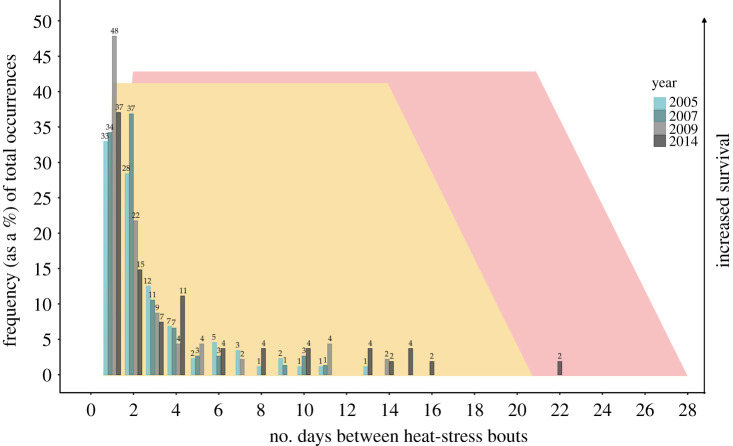
The spacing of heat-stress events varied widely across the 4 years of field body temperature data. We found that approximately 64% of consecutive heat-stress events were separated by 24–48 h, approximately 33% were separated by 3–22 days and approximately 3% were separated by greater than 28 days (figure 3).
Figure 3.
Frequency of two consecutive heat-stress bouts based on field Robomussel data. Field data from Robomussels at Hopkins Marine Station [37], where Robomussel temperatures were collected every 10 min for 6–13 consecutive months in each of the years shown in the figure (2005, 2007, 2009 and 2014). The frequency of heat-stress bouts (on the y-axis) is relative to the total number of heat-stress bouts for that given year. The x-axis indicates the total number of days between any two consecutive heat-stress bouts, where a heat-stress bout was defined as any temperature from 28 to 41°C for at least two consecutive hours (see ‘Methods’ for more details). The numbers above the bars indicate the frequency (as a percentage) with which any two heat-stress bouts occurred for that specific number of days apart, relative to the total number of heat bouts for that given year (e.g. in 2007, 37% of heat events occurred 2 days apart). The yellow and red shading behind the bar plots shows the results from our laboratory-based findings for the 30 and 35°C groups, respectively. Note that the primary y-axis values are not applicable to the yellow- and red-shaded trapezoids, which are only meant as a pictorial representation to synthesize the data from the laboratory with the field body temperature data; the secondary y-axis label of ‘Increasing survival’ is simply to show that the trapezoidal shading indicates when survival increased and decreased over time for each of the two experimental groups. Heat tolerance was improved 1 and 2 days after the initial 30 and 35°C sublethal heat-stress bouts, respectively, and starts to decay at 14 and 21 days later, respectively. Note that the 25°C experimental group data are not pictured here as this treatment did not improve survival.
4. Discussion
In this study, we determined the rates at which heat tolerance is gained and lost in M. californianus in response to different intensities of environmentally realistic sublethal heat-stress bouts and interpreted these data in the context of an extensive set of mussel field body temperature data gathered at our study site. This is the first study that we are aware of to show that mussels can heat acclimate within 24–48 h after a single (short) heat-stress bout, and then maintain this elevated tolerance for two to three weeks in the absence of heat stress. Furthermore, we show that the gain and persistence of heat tolerance is dependent on the initial sublethal heat-stress temperature. Initial heat stress at 35°C led to a slower gain and loss of heat tolerance relative to heat stress at 30°C, while the lowest sublethal heat-stress temperature examined, 25°C, did not enhance heat tolerance.
Although these findings might be characterized as a long-lasting heat-hardening response, we believe that it is more appropriate to view these adaptive responses as rapid heat acclimation. Heat hardening has typically been defined as a transient response that confers improved heat tolerance immediately after the initial heat-stress bout for up to 32 h [9,12,13,39,40], while longer-lasting improvements in heat tolerance are termed as heat acclimation. The fact that the 35°C group did not experience improved survival until 48 h after the initial sublethal heat-stress bout would indicate that this is not a heat-hardening response, but instead is ‘rapid heat acclimation’. The rapid heat acclimation response that we found in our study is contrary to previous work in mussels that assumed heat acclimation (in the sense of improved heat tolerance) takes upwards of two weeks to be completed [14,15,19,41,42]. However, this disparity among studies is likely a reflection of the fact that, to our knowledge, no studies have tested shorter acclimation periods, nor have studies explored the decay in mussels' acquired heat tolerance in the absence of continued heat stress. More research is required to better understand the differences on a cellular and molecular level between heat hardening and heat acclimation, and whether the definition pertains to how quickly the heat tolerance is gained, or also accounts for how long the improved tolerance is maintained.
The mechanistic basis of the different responses to 25, 30 and 35°C was not examined, but the different responses among these three groups may reflect the extent of cellular damage caused by the three sublethal heat-stress temperatures and, thus, the extent to which the cellular stress response (CSR) was activated [43,44]. Studies of the effects of field body temperature on gene expression in M. californianus have shown a strong temperature-dependence of the expression of stress-related genes, e.g. those encoding heat-shock proteins and proteins involved in control of the cell cycle and programmed cell death [35]. These studies suggest that heat-shock protein 70 (HSP70) expression would have increased markedly over the range of sublethal heat-stress temperatures used in the present study. Our finding of a temperature-dependent time-course for the gain and loss of heat tolerance suggests that the intensity of initial temperature stress dictates the recovery time (through activation of the CSR), along with the duration of the changes (i.e. a highly activated CSR may have led to more permanent changes within the cells that led to a longer-lasting heat tolerance [43,44]). This is supported by the fact that the animals in the 35°C sublethal heat-stress bout took double the time (48 versus 24 h) to heat acclimate compared to the 30°C group, but then maintained this improved heat tolerance for 7 days longer.
It may be that mussels have developed superior adaptive capacities (i.e. physiological plasticity) compared to more mobile animals as a result of their inability to behaviourally thermoregulate [5]. Previous research in mussels has found that physiological changes in the initial stages of heat acclimation predominantly occur within the nervous system [9,45], whereas longer-term changes that occur with heat acclimation typically involve cellular, cardiovascular, respiratory, and metabolic changes (or other organ systems that have been largely unexplored to this point) [14,15,19,28,36,41,46,47]. Regardless of the sequence with which these physiological changes occur, it seems that the physiological changes that conferred long-lasting heat tolerance (from this sublethal heat bout) were maximized at 2 and 5 days after the 30 and 35°C heat-stress bouts, respectively, as this is when survival peaked in each group (figure 2). Further research is required to uncover the biochemical and organ-level processes that lead to this rapid heat acclimation, and also whether cessation of these same processes underlies the loss of heat tolerance that occurred over time (in the absence of heat stress).
Lastly, through comparing field temperature data with the laboratory-based findings (figure 3), it is clear that an ability to quickly gain heat tolerance (i.e. within 24–48 h), is crucial for mussel survival in the intertidal zone, as approximately 64% of consecutive heat events occur within this short time frame. Additionally, mussels' ability to maintain this improved heat tolerance for at least 21 days after the initial heat-stress bout is also important in terms of survival, as over one-third (approx. 33%) of consecutive heat events are separated by longer periods of time (3–22 days). In summary, based on these data, it appears that mussels’ rapid gain, and slow loss, of heat tolerance is an advantageous strategy for coping with the intermittent, and often extreme temperatures they experience in the intertidal zone. It is likely that mussels have evolved this adaptive capacity to the heat due to their sessile nature, which precludes escape from heat stress through locomotory behaviour. Further research is needed to determine whether this highly adaptive response is only present in sessile species like mussels or whether mobile marine ectotherms also exhibit this adaptive capability.
5. Conclusion
Understanding organisms' responses to temperature in situ requires complementary analysis of laboratory-based thermal tolerance tests in conjunction with field temperature data. In the present study, we combined these two modes of analysis and found that the time-course with which heat tolerance is gained and lost is dependent on the sublethal heat-stress temperature, whereby a higher heat-stress temperature leads to a slower gain and loss of heat tolerance. We show that mussels’ adaptive strategy to the heat—whereby they rapidly gain, and slowly lose, heat tolerance—is aligned with the frequency in which consecutive heat-stress bouts occur in the intertidal zone. This adaptive strategy may allow mussels to survive the majority of the increased numbers of intermittent and extreme heat events predicted with climate change. Thus, the data we present can be used to forecast mussel survival under the increasingly hotter and more variable temperatures predicted with climate change. Importantly, these findings highlight the importance of accounting not only for rate with which heat tolerance is gained but also for the rate with which it is lost, as both of these components of heat acclimation are important when forecasting animal survival. To our knowledge, this is the first study to show, in any animal, that heat acclimation can be rapidly acquired from a single heat-stress bout, and then maintained for weeks in the absence of heat stress. It may be that this phenotype is present in other animals, and researchers are encouraged to explore the decay of heat acclimation in future work to better understand how animals gain and lose heat tolerance in an ecologically relevant context.
Supplementary Material
Acknowledgements
The authors thank Brian Helmuth and his many collaborators for access to their massive dataset on mussel field body temperatures [37], and their efforts to collect several years of continuous temperature data from the intertidal zone at several locations worldwide. The authors also thank Ben Burford for his feedback on graphics and visuals in preparing this manuscript.
Data accessibility
Field temperature data are available at: https://helmuthlab.cos.northeastern.edu/databases/robomussel/ [37]. Laboratory dataset and code are available from the Dryad Digital Repository at: https://dx.doi.org/10.5061/dryad.0k6djh9z6 [48].
Authors' contributions
Conceptualization: N.E.M., G.N.S. and M.W.D.; methodology: N.E.M., G.N.S. and M.W.D.; statistical analysis: N.E.M. and R.L.C.; data collection and experiments: N.E.M. and R.L.C.; writing—original draft: N.E.M.; writing—review and editing: N.E.M., R.L.C., G.N.S. and M.W.D.; visualization: N.E.M.; project administration: N.E.M. and R.L.C.; funding acquisition: M.W.D. and N.E.M.
Competing interests
The authors declare no competing or financial interests.
Funding
This work is supported by NSF IOS 1655529 to M.W.D. and Myers Oceanographic & Marine Biology Trust to N.E.M.
References
- 1.Masson-Delmotte V, et al. 2018. Special report on global warming of 1.5°C. See https://www.ipcc.ch/site/assets/uploads/sites/2/2019/06/SR15_Full_Report_High_Res.pdf.
- 2.Stillman J. 2019. Heat waves, the new normal: summertime temperature extremes will impact animals, ecosystems, and human communities. Physiology 34, 86–100. ( 10.1152/physiol.00040.2018) [DOI] [PubMed] [Google Scholar]
- 3.Morley SA, Nguyen KD, Peck LS, Lai CH, Tan KS. 2017. Can acclimation of thermal tolerance, in adults and across generations, act as a buffer against climate change in tropical marine ectotherms? J. Therm. Biol. 68, 195–199. ( 10.1016/j.jtherbio.2016.09.007) [DOI] [PubMed] [Google Scholar]
- 4.Burggren W. 2018. Developmental phenotypic plasticity helps bridge stochastic weather events associated with climate change. J. Exp. Biol. 221, jeb161984 ( 10.1242/jeb.161984) [DOI] [PubMed] [Google Scholar]
- 5.Gunderson A, Stillman J. 2015. Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc. R. Soc. B 282, 20150401 ( 10.1098/rspb.2015.0401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breitenbach AT, Carter AW, Paitz RT, Bowden RM. 2020. Using naturalistic incubation temperatures to demonstrate how variation in the timing and continuity of heat wave exposure influences phenotype. Proc. R. Soc. B 287, 20200992 ( 10.1098/rspb.2020.0992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denny MW, Miller LP, Harley CDG. 2006. Thermal stress on intertidal limpets: long-term hindcasts and lethal limits. J. Exp. Biol. 209, 2420–2431. ( 10.1242/jeb.02258) [DOI] [PubMed] [Google Scholar]
- 8.Siegle MR, Taylor EB, O'Connor MI. 2018. Prior heat accumulation reduces survival during subsequent experimental heat waves. J. Exp. Mar. Bio. Ecol. 501, 109–117. ( 10.1016/J.JEMBE.2018.01.012) [DOI] [Google Scholar]
- 9.Dunphy BJ, Ruggiero K, Zamora LN, Ragg NLC. 2018. Metabolomic analysis of heat-hardening in adult green-lipped mussel (Perna canaliculus): a key role for succinic acid and the GABAergic synapse pathway. J. Therm. Biol. 74, 37–46. ( 10.1016/j.jtherbio.2018.03.006) [DOI] [PubMed] [Google Scholar]
- 10.Ma CS, Wang L, Zhang W, Rudolf VHW. 2018. Resolving biological impacts of multiple heat waves: interaction of hot and recovery days. Oikos 127, 622–633. ( 10.1111/oik.04699) [DOI] [Google Scholar]
- 11.Drake MJ, Miller NA, Todgham AE. 2017. The role of stochastic thermal environments in modulating the thermal physiology of an intertidal limpet, Lottia digitalis. J. Exp. Biol. 220, 3072–3083. ( 10.1242/jeb.159020) [DOI] [PubMed] [Google Scholar]
- 12.Sørensen MH, Hamann Sørensen M, Kristensen TN, Mørk J, Lauritzen S, Krog Noer N et al. 2019. Rapid induction of the heat hardening response in an Arctic insect. Biol. Lett. 10, 20190613 ( 10.1098/rsbl.2019.0613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowler K. 2005. Acclimation, heat shock and hardening. J. Therm. Biol. 30, 125–130. ( 10.1016/j.jtherbio.2004.09.001) [DOI] [Google Scholar]
- 14.Pickens P. 1965. Heart rate of mussels as a function of latitude, intertidal height, and acclimation temperature. Physiol. Zool. 38, 390–405. ( 10.1086/physzool.38.4.30152416) [DOI] [Google Scholar]
- 15.Widdows J. 1976. Physiological adaptation of Mytilus edulis to cyclic temperatures. J. Comp. Physiol. B 105, 115–128. ( 10.1007/BF00691115) [DOI] [Google Scholar]
- 16.Huey R, Bennett A. 1990. Physiological adjustments to fluctuating thermal environments: an ecological and evolutionary perspective. In Stress proteins in biology and medicine, pp. 37–59. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 17.Kingsolver JG, Buckley LB. 2017. Quantifying thermal extremes and biological variation to predict evolutionary responses to changing climate. Phil. Trans. R. Soc. B 372, 20160147 ( 10.1098/rstb.2016.0147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Heerwaarden B, Kellermann V.. 2020. Does plasticity trade off with basal heat tolerance? Trends Ecol. Evol. 35, 874–885. ( 10.1016/j.tree.2020.05.006) [DOI] [PubMed] [Google Scholar]
- 19.Braby CE, Somero GN. 2006. Following the heart: temperature and salinity effects on heart rate in native and invasive species of blue mussels (genus Mytilus). J. Exp. Biol. 209, 2554–2566. ( 10.1242/jeb.02259) [DOI] [PubMed] [Google Scholar]
- 20.Lagerspetz KYH. 2006. What is thermal acclimation?. J. Therm. Biol. 31, 332–336. ( 10.1016/j.jtherbio.2006.01.003) [DOI] [Google Scholar]
- 21.Burggren WW. 2019. Inadequacy of typical physiological experimental protocols for investigating consequences of stochastic weather events emerging from global warming. Am. J. Physiol. Regul. Integr. Comp. Physiol. 316, R318–R322. ( 10.1152/ajpregu.00307.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann AA, Sgrò CM. 2018. Comparative studies of critical physiological limits and vulnerability to environmental extremes in small ectotherms: how much environmental control is needed? Integr. Zool. 13, 355–371. ( 10.1111/1749-4877.12297). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denny MW, Dowd WW, Bilir L, Mach KJ. 2011. Spreading the risk: small-scale body temperature variation among intertidal organisms and its implications for species persistence. J. Exp. Mar. Biol. Ecol. 400, 175–190. ( 10.1016/j.jembe.2011.02.006) [DOI] [Google Scholar]
- 24.Miller LP, Dowd WW. 2017. Multimodal in situ data logging quantifies inter-individual variation in thermal experience and persistent origin effects on gaping behavior among intertidal mussels Mytilus californianus. J. Exp. Biol. 220, 4305–4319. ( 10.1242/jeb.164020) [DOI] [PubMed] [Google Scholar]
- 25.Jimenez A, Jayawardene S, Alves S, Dallmer J, Dowd W. 2015. Micro-scale environmental variation amplifies physiological variation among individual mussels. Proc. R. Soc. B 282, 20152273 ( 10.1098/rspb.2015.2273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denny MW, Hunt LJH, Miller LP, Harley CDG. 2009. On the prediction of extreme ecological events. Ecol. Monogr. 79, 397–421. ( 10.1890/08-0579.1) [DOI] [Google Scholar]
- 27.Compton TJ, Rijkenberg MJ, Drent J, Piersma T. 2018. Thermal tolerance ranges and climate variability: a comparison between bivalves from differing climates. J. Exp. Mar. Bio. Ecol. 352, 200–211. ( 10.1016/j.jembe.2007.07.010) [DOI] [Google Scholar]
- 28.Williams E, Somero G. 1996. Seasonal-, tidal-cycle- and microhabitat-related variation in membrane order of phospholipid vesicles from gills of the intertidal mussel Mytilus californianus. J. Exp. Biol. 199, 1587–1596. [DOI] [PubMed] [Google Scholar]
- 29.Helmuth B, et al. 2006. Mosaic patterns of thermal stress in the rocky intertidal zone: implications for climate change. Ecol. Monogr. 76, 461–479. ( 10.1890/0012-9615(2006)076[0461:MPOTSI[2.0.CO;2) [DOI] [Google Scholar]
- 30.Helmuth B. 1999. Thermal biology of rocky intertidal mussels: quantifying body temperatures using climatological data. Ecology 80, 15–34. ( 10.1890/0012-9658(1999)080[0015:TBORIM]2.0.CO;2) [DOI] [Google Scholar]
- 31.Gleason L, Strand E, Hizon B, Dowd W.. 2018. Plasticity of thermal tolerance and its relationship with growth rate in juvenile mussels (Mytilus californianus). Proc. R. Soc. B 285, 20172617 ( 10.1098/rspb.2017.2617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dowd W, Somero G. 2013. Behavior and survival of Mytilus congeners following episodes of elevated body temperature in air and seawater. J. Exp. Biol. 216, 502–514. ( 10.1242/jeb.076620) [DOI] [PubMed] [Google Scholar]
- 33.Moyen NE, Somero GN, Denny MW. 2019. Impact of heating rate on cardiac thermal tolerance in the California mussel, Mytilus californianus. J. Exp. Biol. 222, jeb203166 ( 10.1242/jeb.203166) [DOI] [PubMed] [Google Scholar]
- 34.Roberts D, Hofmann G, Somero G. 1997. Heat-shock protein expression in Mytilus californianus: acclimatization (seasonal and tidal-height comparisons) and acclimation effects. Biol. Bull. 192, 309–320. ( 10.2307/1542724) [DOI] [PubMed] [Google Scholar]
- 35.Gracey AY, Chaney ML, Boomhower JP, Tyburczy WR, Connor K, Somero GN. 2008. Rhythms of gene expression in a fluctuating intertidal environment. Curr. Biol. 18, 1501–1507. ( 10.1016/j.cub.2008.08.049) [DOI] [PubMed] [Google Scholar]
- 36.Moyen N, Somero G, Denny M. 2020. Mussels' acclimatization to high, variable temperatures is lost slowly upon transfer to benign conditions. J. Exp. Biol. 223, jeb.222893 ( 10.1242/jeb.222893) [DOI] [PubMed] [Google Scholar]
- 37.Helmuth B, et al. 2016. Long-term, high frequency in situ measurements of intertidal mussel bed temperatures using biomimetic sensors Sci. Data 3, 87 ( 10.1038/sdata.2016.87) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jafari M, Ansari-Pour N. 2019. Why, when and how to adjust your P values? Cell J. 20, 604–607. ( 10.22074/cellj.2019.5992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moghadam NN, Ketola T, Pertoldi C, Bahrndorff S, Kristensen TN. 2019. Heat hardening capacity in Drosophila melanogaster is life stage-specific and juveniles show the highest plasticity Biol. Lett. 15, 20180628 ( 10.1098/rsbl.2018.0628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorby K, Green M, Dempster T, Jessop T. 2018. Can physiological engineering/programming increase multi-generational thermal tolerance to extreme temperature events? J. Exp. Biol. 221, 1–10. ( 10.1242/jeb.174672) [DOI] [PubMed] [Google Scholar]
- 41.Logan CA, Kost LE, Somero GN. 2012. Latitudinal differences in Mytilus californianus thermal physiology. Mar. Ecol. Prog. Ser. 450, 93–105. ( 10.3354/meps09491) [DOI] [Google Scholar]
- 42.Widdows J. 1973. Effect of temperature and food on the heart beat, ventilation rate and oxygen uptake of Mytilus edulis. Mar. Biol. 20, 269–276. ( 10.1007/BF00354270) [DOI] [Google Scholar]
- 43.Kültz D, Somero GN. 2020. Introduction to the special issue: comparative biology of cellular stress responses in animals. J. Exp. Zool. A Ecol. Integr. Physiol. 333, 345–349. ( 10.1002/jez.2395) [DOI] [PubMed] [Google Scholar]
- 44.Somero GN. 2020. The cellular stress response and temperature: function, regulation, and evolution. J. Exp. Zool. A: Ecol Integr. Physiol. 333, 379–397. ( 10.1002/jez.2344) [DOI] [PubMed] [Google Scholar]
- 45.Senius K. 1975. The thermal resistance and thermal resistance acclimation of ciliary activity in the Mytilus gills. Comp. Biochem. Physiol. 51A, 957–961. [DOI] [PubMed] [Google Scholar]
- 46.Jimenez A, Alves S, Dallmer J, Njoo E, Roa S, Dowd W. 2016. Acclimation to elevated emersion temperature has no effect on susceptibility to acute, heat-induced lipid peroxidation in an intertidal mussel (Mytilus californianus). Mar. Biol. 163, 55 ( 10.1007/s00227-016-2828-8) [DOI] [Google Scholar]
- 47.Newell RC, Bayne BL. 1973. A review on temperature and metabolic acclimation in intertidal marine invertebrates. Neth. J. Sea Res. 7, 421–433. ( 10.1016/0077-7579(73)90063-X) [DOI] [Google Scholar]
- 48.Moyen NE, Crane RL, Somero GN, Denny MW. 2020. Data from: A single heat-stress bout induces rapid and prolonged heat acclimation in the California mussel, Mytilus californianus Dryad Digital Repository. ( 10.5061/dryad.0k6djh9z6) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Moyen NE, Crane RL, Somero GN, Denny MW. 2020. Data from: A single heat-stress bout induces rapid and prolonged heat acclimation in the California mussel, Mytilus californianus Dryad Digital Repository. ( 10.5061/dryad.0k6djh9z6) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Field temperature data are available at: https://helmuthlab.cos.northeastern.edu/databases/robomussel/ [37]. Laboratory dataset and code are available from the Dryad Digital Repository at: https://dx.doi.org/10.5061/dryad.0k6djh9z6 [48].