Abstract
The ecology of coral reefs is rapidly shifting from historical baselines. One key-question is whether under these new, less favourable ecological conditions, coral reefs will be able to sustain key geo-ecological processes such as the capacity to accumulate carbonate structure. Here, we use data from 34 Caribbean reef sites to examine how the carbonate production, net erosion and net carbonate budgets, as well as the organisms underlying these processes, have changed over the past 15 years in the absence of further severe acute disturbances. We find that despite fundamental benthic ecological changes, these ecologically shifted coral assemblages have exhibited a modest but significant increase in their net carbonate budgets over the past 15 years. However, contrary to expectations this trend was driven by a decrease in erosion pressure, largely resulting from changes in the abundance and size-frequency distribution of parrotfishes, and not by an increase in rates of coral carbonate production. Although in the short term, the carbonate budgets seem to have benefitted marginally from reduced parrotfish erosion, the absence of these key substrate grazers, particularly of larger individuals, is unlikely to be conducive to reef recovery and will thus probably lock these reefs into low budget states.
Keywords: geo-ecological functions, net carbonate balance, ecological trend analysis, bioerosion, parrotfish, carbonate budgets state
1. Introduction
The physical structure of natural systems strongly influences ecological dynamics and their capacity to provide ecosystem services. Examples of these ecosystems occur across tropical and subtropical seas with different relative levels of complexity, such as mussel beds, algal rims and stromatolite reefs [1]. In coral reefs, sessile calcifying organisms create a reef carbonate structure over hundreds to thousands of years. The resultant three-dimensionality is a defining feature of most contemporary reefs and promotes high species diversity: tropical reefs are home to one-third of marine biodiversity and support the economy, safety and livelihoods of at least one-tenth of the world's human population who inhabit tropical coastal areas [2]. Sustaining these ecosystem services over time depends partly on reef growth potential, which is largely determined by the interaction between carbonate production and erosion rates [3], in addition to the processes of cementation, lithification and physical material export [4]. The minimum requirement for a coral reef to persist in the relatively short ecological term is for the resultant budgetary state of the reef (the balance between production and erosion/loss) to be at least neutral (i.e. in a state of approximate equilibrium between gross production and erosion); whereas a relatively high net production rate is required to support sustained reef growth at rates that can track sea-level rise [5,6].
Rates of gross production and erosion depend inherently on the abundance of carbonate producing and eroding organisms. While accretion is classically driven by reef-building coral species, in addition to the other scleractinian corals and crustose coralline algae, bioerosion is driven by a diverse range of species of parrotfish, sea urchins, encrusting sponges and macro- and microborers [7]. The patterns of relative abundance of species in many of these groups have however changed considerably across most reef-building regions, leading in many cases to what are now termed ‘shifted' coral reef assemblages. The magnitude of change has, however, been most pervasive in the Caribbean, this resulting from the low functional redundancy of its reef assemblages, and the chronic and acute disturbances experienced in the last five decades, possibly accentuated at the end of last century [6,8]. For instance, high gross carbonate production rates that have historically relied on the monospecific dominance of species such as Acropora spp. or Orbicella spp. in the region, have significantly decreased owing to a series of disease outbreaks, multiple bleaching events and the loss of ecological resilience, which has led to substantial declines in their populations of up to 80% [9]. Likewise, overall erosion rates appear to have declined in the Caribbean, possibly by around 75% compared with historical rates, although past data are sparse on this topic [10]. One especially rapid decline occurred in tropical reefs immediately after the Diadema antillarum die-off, which became functionally extinct throughout the Caribbean in the early 1980s [10,11]. Parrotfish, the most important bioeroders on today's reefs, have shown biomass reductions between 30 to 70% owing to overfishing and habitat loss [12,13]. Encrusting sponge erosion rates also probably declined considerably between the 1970s and the 2000s owing to reductions in substrate availability [10], but have remained relatively stable during the last two decades [14,15]. The described ecological changes occurred during a short time period, at large scale and possibly with no parallel in the fossil record of modern reefs [16,17]. As a result, major reductions in production of at least 50% compared to mid-to late-Holocene rates, have been suggested [17]. Although this reduction is significant, it is likely that the overall decrease in bioerosion rates has, to some extent buffered the impact of net declines in carbonate production which otherwise would perhaps be more marked [10].
An issue with undertaking such modern to historical comparisons in order to understand the magnitudes of contemporary change is the paucity, and disaggregated nature, of temporal bioerosion and carbonate production datasets. Where such temporal records now exist they have mainly been collected as part of efforts to understand the short-term impacts of coral bleaching, which results in near-instantaneous declines in budget states owing to both reduced live coral cover and/or declines in calcification rates [18–20]. Longer-term declines in net carbonate budgets in the Caribbean have also been associated with shifts in coral assemblages [21,22]; whereas, at some sites in the Indo-Pacific, a lack of recovery in the carbonate budgets spanning a 16 year period, were linked to an increase in bioerosion rates [23]. While these findings provide useful insights into net budget changes, mostly in the context of punctuated perturbations, more time-series data analyses are needed. This is particularly the case for assessing the impacts of longer-term and seemingly persistent shifts in coral reef assemblages in order to: (i) better understand whether contemporary low budget state reefs can recover; but also (ii) to grasp how producer and bioeroder rates might be interacting over time. Two recent studies have suggested that bioerosion may play a key role in directing net carbonate budgets under low production scenarios [4,10]; however, detailed analysis of how this might be occurring remains to be explored. Here, we use time-series data from 34 temporally replicated sites spanning 15 years (2004–2018) to test these ideas through an investigation of the temporal dynamics of carbonate budgets at sites along the Mexican Caribbean reef system. Specifically, we use these data: (i) to determine how gross carbonate production, net erosion and net carbonate budgets have transitioned as coral reef assemblages have shifted over recent decades; and (ii) to identify how the abundance and/or size classes of the organisms driving key elements of the budgets have changed over time.
2. Methods
(a). Study area
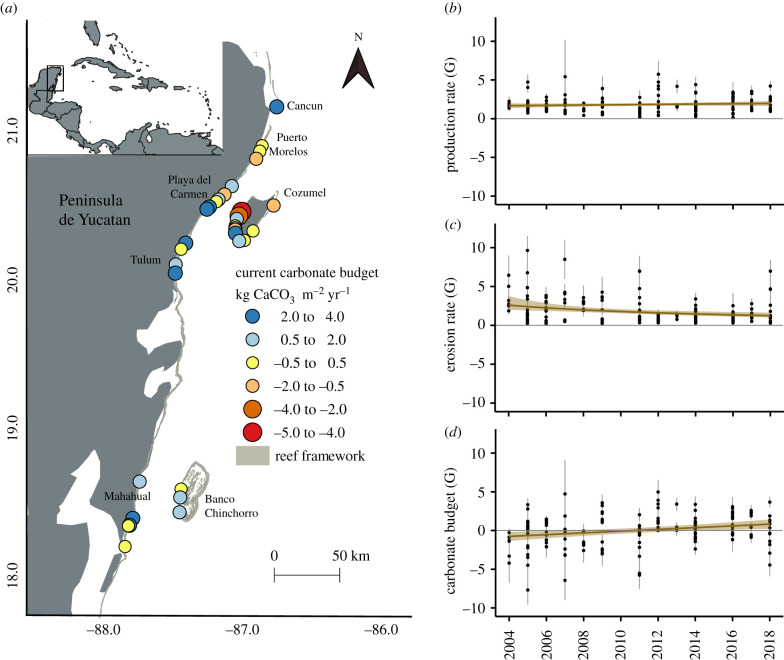
The 34 reef sites are distributed across 400 km surrounding the mainland and two major islands (Cozumel and Banco Chinchorro) in the Mexican Caribbean (figure 1a). Sites span the three main geomorphological zones that define the reefs in the region, the back-reef and crest zone and the fore-reef, at a depth range between 1 and 15 m. This fringing reef system shows some variations in geomorphological development with latitude [24]. However, the broad impact of natural and anthropogenic disturbances, such as the aforementioned outbreak of diseases and thermal stress events, as well as the impact of water quality associated with an unprecedented fast coastal development in this region over the last five decades, has led to low coral cover and more homogeneous ‘shifted coral reef assemblages' [25,26]. These new community types are now relatively stable ecologically, are dominated by what are often referred to as stress-tolerant or weedy species, and which often have reduced calcification rates compared to fast-growing competitive taxa [21,27].
Figure 1.
Temporal carbonate budget dynamics in the Mexican Caribbean. (a) Current (2017–2018) net carbonate budgets of the 34 surveyed reefs; (b) model prediction of gross production from 2004 to 2018; (c) model prediction of erosion rate from 2004 to 2018; and (d) model prediction of net carbonate production from 2004 to 2018. In (b–d), mean site values are represented as black points; standard error for each mean estimated values are presented as vertical grey lines. Model effects are shown as lines (with 95% confidence intervals). Units in kg CaCO3 m−2 yr−1(G). (Online version in colour.)
(b). Data collection
Historical data based on reef surveys or monitoring programs were obtained from several sources, including scientific and monitoring reports from the Marine Park authorities and researchers, as well as from non-governmental organizations such as the Atlantic and Gulf Rapid Reef Assessment (AGRRA) Program and the Healthy Reefs for Healthy People Initiative. To complement these historical time-series data, we conducted, between 2017 and 2018, fieldwork following the ReefBudget methodology V. 2 [28]. Data were curated and integrated into the Caribbean Reef Information System, from the Biodiversity and Reef Conservation Laboratory, UNAM, with records of more than 400 sites in the Mesoamerican Reef System and Gulf of Mexico. Owing to the considerable number of variables necessary to estimate both gross production and erosion, only 34 sites were selected (electronic supplementary material, table S1), based on the following criteria: (i) sites needed to have been systematically monitored for at least a decade between 2004 and 2018; (ii) every temporal survey needed to report both the abundance of carbonate producing taxa (i.e. crustose coralline algae (CCA) cover and live coral cover) and the main eroding taxa (parrotfish, urchin, substrate available for microbioerosion); and (iii) for both historical and contemporary surveys, our target organisms needed to have been identified to species level.
Regarding data from historical sources, benthic data were obtained by two main methodologies: the point intercept method (PIT) and line intercept method (LIT). Both methods have been shown to be comparable [29]. An average of six transects of 10 m length were laid on the reef to obtain live coral cover, CCA cover, sponge cover and other benthic component data. Urchin and fish data were obtained in six and nine belt transects, respectively, of 10 m2 for urchins and between 60 m2 and 120 m2 for fishes.
For our recent surveys, we deployed an average of eight 10 m LIT transects to estimate the live cover of scleractinian coral species, CCA and other benthic organisms. Along the same transects (1 m wide belts), the cover of all species of encrusting sponges and the number and size (in 20 mm size classes) of all species of sea urchins were recorded [28]; however, the number of transects was on average lower (i.e. six transects for urchins and sponges). For parrotfish visual census, an average of eight belt transects of 30 × 2 m were deployed, along which the number of individuals by species, life phase and size class were recorded based on total length (i.e. less than or equal to 5 cm, 6–10 cm and then in 10 cm bins).
Some adjustments were needed to calculate erosion rates with historical data. First, because the size classes of sea urchins were not recorded in any of the historical surveys, we assigned the average test-diameter obtained in contemporary surveys to historical counts (i.e. a mean test-diameter of 60 mm was assigned to Diadema antillarum) to be able to estimate the erosion potential of this group on historical surveys. The rationale for this is that this size class represents the average as reported for different reefs in the Caribbean over the last two decades [30,31]. Second, because the specific record of eroding sponge species was not regularly annotated until recently (i.e. 2017 and 2018), we use the contemporary site-specific reported cover of encrusting sponges as a site-specific constant during the study period. We considered this as a valid approximation because: (i) recent evidence shows that the cover of this groups has been relatively stable since the 2000s [14,15]; and (ii) in general encrusting sponges contribute relatively little to the total erosion rate, although in some sites this number can be more significant [32].
(c). Carbonate budget estimations
We estimated the net carbonate budgets for all sites during all surveys using the ReefBudget methodology V. 2 [28], a census-based approach in which gross carbonate production rate is estimated as follows: first, for each colony (corals and also CCA patches), gross production is derived from species (or nearest equivalent species) specific density (g cm−3) and linear growth rate (cm yr−1). This rate (kg CaCO3 yr−1) is then standardized per unit area according to the transect dimension and reported as kg CaCO3 m−2 yr−1. For coral species, gross carbonate production rates are estimated considering the species colony morphology (see [28] for details on equations and density and growth rates), differentiated as massive, sub-massive, encrusting, foliose-and-plating, branching and columnar. Second, all estimated production rates are summarized at the transect level, and finally, a site average rate is estimated from all surveyed transects (electronic supplementary material, table S2). Bioerosion rates are estimated depending on the eroding organism recorded within each transect. The parrotfish erosion rate is derived from the bite rate (bite h−1, which depends on the total length and life-phase), bite volume (cm3), the proportion of bites leaving scars (%) and the species abundance. Then, the bioerosion rate (kg CaCO3 ind−1 yr−1) is multiplied by 365 (days in a year), standardized per unit area according to the transect dimension, and reported as kg CaCO3 m−2 yr−1. Urchin erosion rate is derived from the species, number and size of urchins observed in each transect, and the already published species-specific regression equation-constants (multiple and exponent), that were obtained from a regression where x-axis values are test-size, and y-axis values are bioerosion rates (g urchin−1 d−1) reported for different sizes in a number of publications [28]. To yield the rate per year, the total daily rate is multiplied by 365 and then divided by the transect area to yield rate per unit area and reported as kg CaCO3 m−2 yr−1. Sponge erosion rate is derived from the species-specific substrate covered area (m2) and the published erosion rates by species (kg CaCO3 m−2 yr−1). Microbioerosion rate is estimated from the % cover of the available hard-substrate that microborers can exploit and an average erosion rate (kg CaCO3 m−2 yr−1) obtained from the published estimated rates of erosion by microendolithic organisms per square meter. All erosion rates obtained per organism were summarized per transect, and then an average of the total erosion rate reported per site (electronic supplementary material, table S2). An estimate of the net carbonate budget can then be obtained from the mathematical difference between the site's gross production and its erosion rate, reported in kg CaCO3 m−2 yr−1, units recognized with a capital G.
To account for the difference between benthic cover estimations obtained from the modified LIT-Reefbudget method and the earlier census using methods different from Reefbudget, we multiplied the recorded species-specific cover (cm) for each transect by the species-specific rugosity index [27], thus allowing us to estimate the true three-dimensional length (cm) of each species and therefore, to make a more accurate estimate of gross production [22]. Additionally, in the case of parrotfish data, the life phase was not recorded in surveys before 2017. To account for this, we used an average between juvenile and adult's published bite volume (cm3) and the proportion of bites leaving scars (%). All of the species-specific rates used for producing and eroding organisms (e.g. bite rates, bite volumes and proportion of bites leaving scars) were obtained from the ReefBudget database, which summarizes all data available to date published in the Caribbean [28].
(d). Data analysis
Temporal carbonate budget dynamics were explored by means of generalized linear models (GLMs), which unlike general linear models, allow for non-normal distribution of the response variable (e.g. gamma, Poisson). We used the function glm in R, with the site average values of net budget, gross production and erosion rate as separate response variables and time as predictor. Site averages were used because differences in the field methods impeded the estimation of carbonate production and erosion rates at transect level. Uncertainty around site level estimates was generally low and consistent through time (figure 1b–d). Data were not transformed for analysis, but we used two different distributions to account for non-normal distribution in one of the response variables, this is gamma error distribution and log link function for erosion rate and Gaussian distribution with identity link function for net carbonate budget and gross production rate. Residual plots were used to validate the model assumptions. To identify whether resultant net budget trajectory was mostly pulled by the interaction of both producing and erosive processes, or rather by one them, we compare GLM resultant rates of change direction and significance. Then, to identify to what extent specific producing and eroding organisms had been responsible for observed changes in the net budget over time, we adjust another set of GLMs with gamma error distribution and log link function and non-transformed data for producing and eroding organism specific rates. Three models for the main carbonate producers were used, these were major reef framework builders such as: (i) Branching Acropora species; and (ii) Massive species (including Orbicella species and other hemispheric growth like species [27]); and (iii) non-framework builders (including foliose-digitiform species, milleporids, other encrusting coral species as well as crustose coralline algae) [21,27]. Another four models were used for bioeroders, these were parrotfish, sea urchins, encrusting sponges and microbioerosion. Residual plots were used to validate the model assumptions.
At this point in the analysis, only the parrotfish erosion rate showed a significant decline. Therefore, to identify the specific parrotfish population metrics driving changes in their erosion capacity (and consequently in the net budget trajectory), which is a function not only of overall abundance but also of species and size, we performed two different analyses. First, to explore total parrotfish frequency size distribution over time, we performed a kernel density estimate, which represents the distribution of a numeric variable as a smoothed version of a histogram. The kernel density estimate gives the probability distribution from the dataset so that the total area under the curve is equal to one (i.e. normalization); a bin with a large number of observations will yield a higher density value. This technique allows for easier comparison of the distributions among years, particularly when comparing several groups [33]. Secondly, we adjusted a GLM, setting parrotfish abundance as a response variable and time as the predictor, using a gamma error distribution and log link function to account for non-normal distribution. Because this last analysis result was indecisive, we decided to explore changes in abundance of parrotfish as a function of differences in their foraging behaviour and bite type, which is related to their capacity to erode the substrate, as ‘excavating' parrotfish (Scarus guacamaia, Scarus coelestinus, Sparisoma viride), ‘scraping’ parrotfish (Scarus iseri, Scarus taeniopterus, Scarus vetula, Scarus coeruleus) and ‘browsing' parrotfish (Sparisoma aurofrenatum, Sparisoma chrysopterum, Sparisoma rubripinne) [34]. Similarly, each group abundance was set as the response variable and time as the predictor, using a gamma error distribution and log link function to account for non-normal distribution in all the response variables.
3. Results
Our data show that the net carbonate budgets of sites along the Mesoamerican reef presently average 0.6 G, ranging from −4.4 G to 3.6 G (figure 1a). Consistent regional north-to south trends are hard to discern but on average sites towards the southern end of our survey area tended to have slightly higher contemporary net positive budgets. Our data do show however, that net carbonate budgets on surveyed shifted coral reef assemblages have changed in the past two decades (figure 1b). Estimates of gross carbonate production suggest that rates have remained relatively stable across sites (p > 0.05), with a mean rate remaining close to 1.8 G over the study period (figure 1b). By contrast, erosion rates have decreased significantly from an average rate of 2 G to 1.2 G (p < 0.05) over the past 15 years (figure 1c). Consequently, the net carbonate budget showed a slight yet significant increase over the surveyed period, increasing on average at an annual rate of approximately 0.1 kg CaCO3 m−2 (p < 0.05). Most significantly, we note that at the onset of the study period, regional mean carbonate budget estimates were net negative (−0.8 G), that is, the erosion rate exceeded the gross production rate. However, over time mean net budgets showed a slight yet significant increase (+1.6 G in 15 years, p < 0.05), such that by the end of the study period, the mean net carbonate budget was net positive (0.8 G; figure 1c). Additionally, we also found that site-specific net budget estimates throughout the surveyed period were high and more strongly correlated with site-specific erosion estimates (R = 0.61, p < 0.01) than with estimated values of gross production (R = 0.39, p < 0.01; electronic supplementary material, figure S1). These results suggest that erosion pressures on these phase-shifted reefs are now acting as the main process driving net budget outcomes and recovery trajectories.
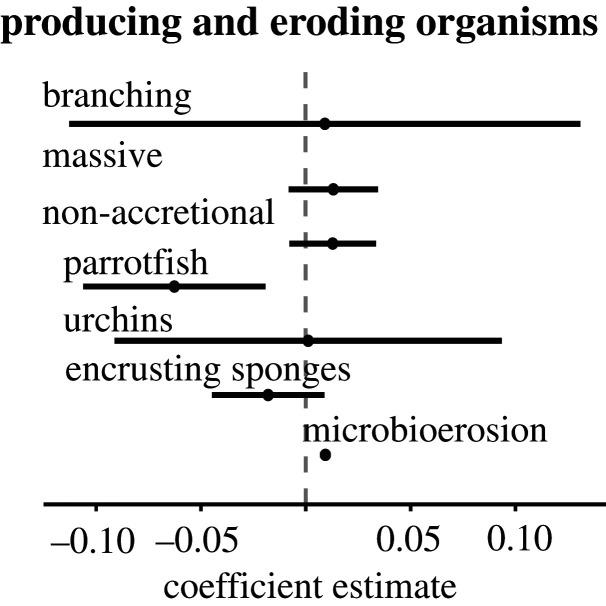
Consistent with the regional trend, gross production rates by different major reef framework builders (i.e. acroporids and massive corals) and non-framework builders showed no significant differences over time (p > 0.05 in all cases; figure 2). In the case of bioeroding organisms, as outlined above, the rate of erosion for encrusting sponges was already fixed as a constant value to account for its contribution to total erosion rate, given the lack of data in past years. Regarding sea urchins, the average rate of erosion did not change over time (p > 0.05; figure 2); microbioerosion, on the other hand, showed a very slight but significant increase over time from 0.024 G to 0.027 G (p < 0.05). Only parrotfish erosion rate showed a significant decreasing trend (from 1.7 G to 0.7 G, p < 0.05) that is consistent with the regional trend of a decrease in the total erosion rate, and with the associated net carbonate budget recovery trend. This is strongly linked with the fact that parrotfish contribute the most (77%) to total estimated bioerosion rate.
Figure 2.
Producing and eroding organisms' coefficient estimates and confidence intervals of the fitted generalized linear models for producers and eroders. Dots and lines represent each model coefficient and 95% confidence interval. (Online version in colour.)
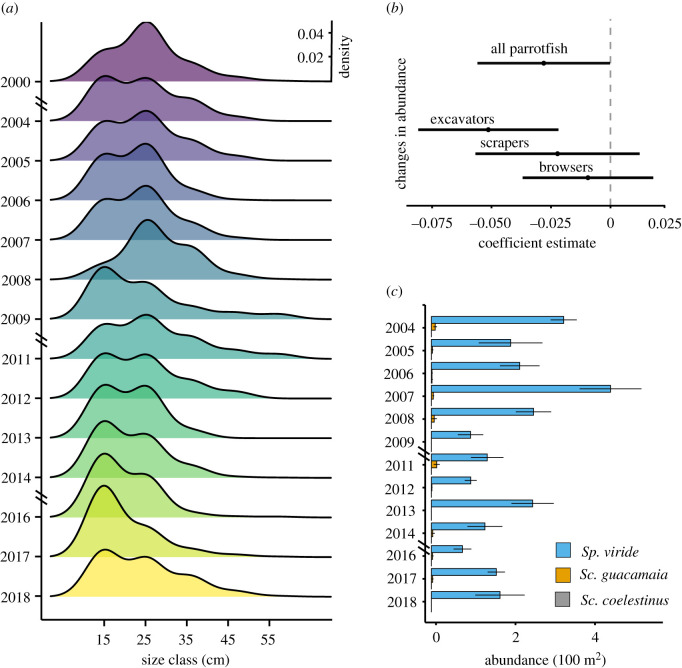
Detailed analysis of the parrotfish data over the survey period also shows that the decrease in the parrotfish erosion rate was driven by changes in both the size structure and abundance of parrotfishes (figure 3a,b). Specifically, the size-frequency distribution of parrotfishes gradually moved towards a shorter body length. At the onset of the study period, the modal length of parrotfishes was 25 cm (21–30 cm size class), whereas in recent years the modal length has reduced to the 11–20 cm size category (figure 3a). This implies a major reduction in the potential of individual fish to bite and erode the substrate while feeding (g CaCO3 ind−1 dy−1) because size is a major determinant of erosion rate [35]. Regarding changes in parrotfish abundance, this did not significantly decrease for the whole group (p = 0.0503). However, when analysing by specific feeding behaviour subgroups (i.e. excavators, scrapers, browsers), there has been a significant reduction in the abundance of excavating parrotfishes (p < 0.05; figure 3b). This functional group is entirely dominated by Sp. viride (figure 3c).
Figure 3.
Temporal changes in parrotfish size and abundance. (a) Kernel density estimates for parrotfish size class (cm) distribution by year. Second y-axis in (a) shows the estimated density value, which is uniform for all curves as it gives the probability distribution from the dataset so that the total area under each curve is equal to one. (b) Coefficient estimates and confidence intervals of the fitted generalized linear models for parrotfish abundance: lines represent each model coefficient and the 95% confidence interval for the overall parrotfishes, for excavating species, for scraping species and for browsing species. (c) Species-specific abundance (ind. 100 m−2) of the excavating parrotfishes over time. (Online version in colour.)
4. Discussion
We found that shifted coral assemblages across our study sites have undergone a modest but significant increase in their net carbonate budget states over the past 15 years, increasing from a mean net rate of −0.8 G in 2004 to 0.8 G in 2018. However, while it might be expected that such a trend would be driven primarily by the recovery of the coral assemblages, given that their decline induced in the first instance the transition to the contemporary low budget states widely observed across the Caribbean [6,17], our data show that coral carbonate production actually remained remarkably stable through the survey period (figure 1b). Instead, the main factor triggering the net positive budget trajectory has been a decrease in biological erosion pressure, largely resulting from ecological changes in the communities of associated parrotfishes. Specifically, we observed both a change in the size-frequency distribution of parrotfishes towards small-bodied individuals and a decline in the abundance of excavating parrotfishes. Understanding how carbonate budgets on sites now dominated by non-framework building corals are transitioning is crucial to predicting and assessing the geo-ecological responses (sensu Perry & Alvarez-Filip [6]) of these ecosystems under currently rapid changing environmental pressures.
Our findings suggest that reefs in the Mexican Caribbean now constitute shifted coral reef assemblages as defined by low coral cover 17% (s.d. ± 9%), a paucity of historically important reef building species, and a low-and-stable carbonate production rate. A series of acute and chronic disturbances led to these major declines in coral cover and associated increases in the coverage of macroalgae and the loss of structural complexity between the late 1970s and early 2000s [36]. Although coral cover has shown some signs of recovery since [36], there are no clear signs that the capacity of the coral communities to increase calcium carbonate accumulation rates have changed, at least at equivalent rates [26,36]. This has been largely owing to a lack of recovery by the most important functional groups in the coral community. This is to say that while historical losses of coral cover resulted mainly from losses of key reef-building species [22,37], what recovery has occurred in terms of coral cover has been mainly associated with small weedy corals such as Agaricia agaricites and Porites astreoides. These species may protect to some extent the substrate from bioerosion [38,39], however, presently they are not fulfilling the role of major reef framework builders [21,27]. The resultant assumption therefore is that coral production rates are likely to remain relatively low, and thus that biological eroding agents are likely to increase in importance as the main drivers of contemporary carbonate budget states.
In this context, we found a decline of 0.8 kg CaCO3 m−2 in the erosion rate over the study period, a decline sufficient to drive the positive shift in the net carbonate budget states of these reefs (figure 1d). This means that while in the mid-2000s the rate at which the carbonate structures were being eroded was higher than the rate at which they were produced, by the mid to late 2010s this balance had changed, favouring the reef's framework persistence. However, because the increase in net carbonate budget was not related to an increase in the gross production, coral reef condition in the region has not truly improved, but rather has been ‘stopped' from transitioning into states of more active erosion and denudation. This has largely arisen as a function of the decline in biomass of the main regional bioeroder group, the parrotfishes [10].
Supporting evidence for important reductions in parrotfishes' abundance and biomass occurring in some Caribbean reefs over the past century have been reported by other authors [13,40]. However, such trends have not been empirically related to an increase in the amount of calcium carbonate accumulating on the region's reefs. According to our findings, the reduced abundance of larger-sized parrotfishes and a decrease in the number of the excavating parrotfishes specifically (Sp. viride, figure 3c), is now acting as an ecological buffer that is for now compensating for the reduced ability of these reefs to produce carbonate [10]. Although the change in the size-frequency distribution of parrotfishes towards small-bodied individuals might not be so evident in terms of the total size spectrum, the impact on the erosional potential of this group is significant because the capacity of parrotfishes to erode substrate (i.e. as a function of bite rate and the amount of mass removed per bite) is highly positively correlated with body size and life-phase [34,41]. Furthermore, it is precisely at the smaller sizes (i.e. under 20 cm) that the capacity of parrotfishes to erode substrate is severely reduced (i.e. the probability of leaving a grazing scar decreases significantly) [34]. This has been exacerbated by the fact that the decrease in abundance was specifically related to Sp. viride, the species, which along with Sc. vetula, have historically been the major substrate eroding parrotfish species in the Caribbean [35].
These observed changes in the parrotfish communities are likely to be a consequence of several potentially interacting factors [40,42,43]. First, selective fishing on larger individuals, not only indirectly increases the relative abundance of small-bodied individuals but also directly reduces the abundance of the organisms that, owing to their larger size, exert a greater effect on the ecosystem [35,41,44,45]. Although, in this region, parrotfishes are not formally extracted for marketing, they can be caught as bycatch, and it has been reported that self-consumption and sporadic commercialization can also occur (e.g. by mislabelling), particularly as large piscivores decline [46–48]. Second, long-term habitat degradation, particularly following important losses in reef structural habitat complexity, coral cover and refuge availability, can negatively affect parrotfish and other tropical fish populations (i.e. abundance and biomass), as these features mediate productivity (g m−3 yr−1), recruitment, competition, predator–prey interactions, as well as the fish size spectrum on reefs [12,43]. Additionally, because parrotfishes occur most commonly in shallow reefal habitats, they are highly vulnerable to the impact of terrestrial anthropogenic activities, including pollution, eutrophication and sedimentation [43]. These can negatively impact not only the coral reefs but also adjacent habitats such as seagrass meadows and mangroves [49] where some parrotfish species occur, particularly during their juvenile phases, as they search for food or nursery habitats [50,51].
Interestingly, the effect that the impaired parrotfish communities exerted on the carbonate budget in this region, is the opposite to the effect described in some reefs in the Indo-Pacific. In these cases, increases in parrotfish abundance and in their erosion rate occurred after coral mass mortality events, stalling the recovery of the net carbonate budgets even after an increase in the gross carbonate production rate [23]. Nevertheless, several factors differ between these regions, for instance neither persistent habitat degradation (i.e. critical losses in coral cover and generalized changes in coral assemblages), widespread persistent transitions to macroalgal dominance, nor regional depletion of herbivores in the Indo-Pacific, have been in general as pervasive as those that have occurred across the wider Caribbean. This is very likely owing to the Caribbean reefs' higher vulnerability and lower functional redundancy [44,52]. They may help explain different dynamics for producing and eroding communities between regions.
Other eroding organisms considered in this study (other than parrotfish) were not related to the reduction in the overall erosion rate we observe. For instance, in the case of sea urchins, although we only use density data to calculate rates of erosion, for this group the observed trend was largely controlled by a consistent absence of these organisms across sites and surveyed periods, as their populations have been depleted for decades [53]. Furthermore, evidence suggests that despite slight recoveries in urchin populations on some reefs, their population growth might be limited by low densities (i.e. by the Allee effect [54]). In the case of encrusting sponges, because we use a constant coverage value through time (see Methods) the observed trend might have to be taken with some caution. However, there is no evidence of important regional increases or declines in the coverage of endolithic sponges over the last 20 years [14,15], and additionally encrusting sponges contribute relatively little to total erosion rates, although in some sites this number can be more significant [32].
Although in the short term the carbonate budget states of these sites, which are dominated by non-framework building corals, seem to be favourable as a function of recent declines in parrotfish populations, the absence of these organisms, particularly of larger individuals, is likely to lock these reefs into low budget states. This is because parrotfishes are also key herbivores that promote reef resilience and coral cover recovery by regulating space competition between benthic organisms such as hermatypic corals and algae [41,55]. A paradox in this context, is that any actions to promote increasing parrotfish biomass on presently degraded reefs (especially excavating species; [56]) would at least in the short-term exacerbate the erosion of the reef carbonate framework. This is particularly relevant because parrotfish protection seems to be effective in promoting coral population resilience under some very narrow sets of environmental conditions such as high coral cover, low algal productivity or sufficient coral settlement [57], that are not met in most of our study sites and elsewhere in the Caribbean [26,57,58]. For Caribbean reefs to truly recover their capacity, there is clearly a need for a recovery towards higher rates of coral carbonate production. This will need to be underpinned by a return of both healthy grazing and coral communities; a scenario that might only be achievable by adopting and complying with management measures that directly address uncontrolled coastal development and construction, poor regulated wastewater, and lack of the enforcement of marine protection in addition to concerted efforts to reduce the rates of global environmental change [25].
Our findings highlight the importance of the ecological-historical context in the interpretation of current carbonate budgetary states and trajectories on now increasingly common ecologically shifted-coral-reef assemblages [6,21], and the importance of identifying those feedback mechanisms that may prevent low gross production rate reefs transitioning to negative carbonate budget states. In order to predict potential paths of coral reef recovery in the context of changing populations of major eroders (i.e. parrotfishes), further research could focus on describing the trade-off between their species-specific roles as herbivores and eroders. We also strongly recommend work on model parametrization with local species-specific rates and further research into understanding what factors might be driving different trends in eroders and producers' abundance in highly perturbed reefs.
Supplementary Material
Acknowledgements
We thank Esmeralda Perez-Cervantes, Nuria Estrada-Saldivar and Fernando Pardo for their great contribution in collecting, curating and systemizing the data used in this study. We also thank the Atlantic Gulf Rapid Reef Assessment (AGRRA) data managers and the individual field researchers who collaborated over the years in collecting part of the data used in this study.
Data accessibility
Provided as the electronic supplementary material.
Authors' contributions
A.M.-H. conceived and designed the study, analysed the data, prepared figures and/or tables, helped write and review drafts of the paper, and approved the final draft. F.J.G.-B. analysed the data, prepared figures and/or tables, helped write and review drafts of the paper, and approved the final draft. C.T.P. conceived and designed the study, helped write and review drafts of the paper, and approved the final draft. L.Á.-F. conceived and designed the study, coordinated the data integration, helped write and review drafts of the paper, and approved the final draft.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by the Universidad Nacional Autónoma de México (UNAM; UNAM-DGAPA-PAPIIT program, project code IN-205019), a Royal Society Newton Advanced Fellowship (grant no. NA150360), and the Mexican Council of Science and Technology (CONACYT; grant no. PDC-247104). A.M.-H. was supported with a PhD scholarship (number 595756) from CONACYT.
References
- 1.Kovalenko KE, Thomaz SM, Warfe DM. 2012. Habitat complexity: approaches and future directions. Hydrobiologia 685, 1–17. ( 10.1007/s10750-011-0974-z) [DOI] [Google Scholar]
- 2.Sale PF, Hixon MA. 2015. Adressing the global decline in coral reefs and forthcoming impacts on fishery yields. In Interrelationships between corals and fisheries (ed. Bortone SA.), pp. 7–18. Boca Raton, FL: CRC Press. [Google Scholar]
- 3.Perry CT, Edinger EN, Kench PS, Murphy GN, Smithers SG, Steneck RS, Mumby PJ. 2012. Estimating rates of biologically driven coral reef framework production and erosion: a new census-based carbonate budget methodology and applications to the reefs of Bonaire. Coral Reefs 31, 853–868. ( 10.1007/s00338-012-0901-4) [DOI] [Google Scholar]
- 4.Lange ID, Perry CT, Alvarez-Filip L. 2020. Carbonate budgets as indicators of functional reef ‘health’: a critical review of data underpinning census-based methods and current knowledge gaps. Ecol. Indic. 110, 105857 ( 10.1016/j.ecolind.2019.105857) [DOI] [Google Scholar]
- 5.Perry CT, et al. 2018. Loss of coral reef growth capacity to track future increases in sea level. Nature 558, 396–400. ( 10.1038/s41586-018-0194-z) [DOI] [PubMed] [Google Scholar]
- 6.Perry CT, Alvarez-Filip L. 2018. Changing geo-ecological functions of coral reefs in the Anthropocene. Funct. Ecol. 33, 976–988. ( 10.1111/1365-2435.13247) [DOI] [Google Scholar]
- 7.Perry CT, Spencer T, Kench PS. 2008. Carbonate budgets and reef production states: a geomorphic perspective on the ecological phase-shift concept. Coral Reefs 27, 853–866. ( 10.1007/s00338-008-0418-z) [DOI] [Google Scholar]
- 8.Alvarez-Filip L, Carricart-Ganivet JP, Horta-Puga G, Iglesias-Prieto R. 2013. Shifts in coral-assemblage composition do not ensure persistence of reef functionality. Sci. Rep. 3, 3486 ( 10.1038/srep03486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aronson RB, Precht WF. 2001. White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia 460, 25–38. ( 10.1023/A:1013103928980) [DOI] [Google Scholar]
- 10.Perry CT, Murphy GN, Kench PS, Edinger EN, Smithers SG, Steneck RS, Mumby PJ. 2014. Changing dynamics of Caribbean reef carbonate budgets: emergence of reef bioeroders as critical controls on present and future reef growth potential. Proc. R. Soc. B 281, 20142018 ( 10.1098/rspb.2014.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lessios HA, Robertson DR, Cubit JD. 1984. Spread of Diadema mass mortality through the Caribbean. Science 226, 335–337. ( 10.1126/science.226.4672.335) [DOI] [PubMed] [Google Scholar]
- 12.Rogers A, Blanchard JL, Mumby PJ. 2014. Vulnerability of coral reef fisheries to a loss of structural complexity. Curr. Biol. 24, 1000–1005. ( 10.1016/j.cub.2014.03.026) [DOI] [PubMed] [Google Scholar]
- 13.Paddack MJ, et al. 2009. Recent region-wide declines in Caribbean reef fish abundance. Curr. Biol. 19, 590–595. ( 10.1016/j.cub.2009.02.041) [DOI] [PubMed] [Google Scholar]
- 14.Gilliam DS.2009. Southeast Florida Coral Reef Evaluation and Monitoring Project 2008 Year 6 Final Report, pp. 1–39. Nova Southeastern University, Miami, FL, USA.
- 15.Ramsby BD, Hoogenboom MO, Whalan S, Webster NS, Thompson A. 2017. A decadal analysis of bioeroding sponge cover on the inshore Great Barrier Reef. Sci. Rep. 7, 2706 ( 10.1038/s41598-017-02196-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandolfi JM, Jackson JBC. 2006. Ecological persistence interrupted in Caribbean coral reefs. Ecol. Lett. 9, 818–826. ( 10.1111/j.1461-0248.2006.00933.x) [DOI] [PubMed] [Google Scholar]
- 17.Perry CT, Murphy GN, Kench PS, Smithers SG, Edinger EN, Steneck RS, Mumby PJ. 2013. Caribbean-wide decline in carbonate production threatens coral reef growth. Nat. Commun. 4, 1402 ( 10.1038/ncomms2409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eakin CM. 2001. A tale of two ENSO events: carbonate budgets and the influence of two warming disturbances and intervening variability, Uva Island, Panama. Bull. Mar. Sci. 69, 171–186. [Google Scholar]
- 19.Perry CT, Morgan KM. 2017. Bleaching drives collapse in reef carbonate budgets and reef growth potential on southern Maldives reefs. Sci. Rep. 7, 40581 ( 10.1038/srep40581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manzello DP, Enochs IC, Kolodziej G, Carlton R, Valentino L. 2018. Resilience in carbonate production despite three coral bleaching events in 5 years on an inshore patch reef in the Florida Keys. Mar. Biol. 165, 99 ( 10.1007/s00227-018-3354-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perry CT, Steneck RS, Murphy GN, Kench PS, Edinger EN, Smithers SG, Mumby PJ. 2015. Regional-scale dominance of non-framework building corals on Caribbean reefs affects carbonate production and future reef growth. Glob. Change Biol. 21, 1153–1164. ( 10.1111/gcb.12792) [DOI] [PubMed] [Google Scholar]
- 22.Estrada-Saldívar N, Jordán-Dalhgren E, Rodríguez-Martínez RE, Perry C, Alvarez-Filip L. 2019. Functional consequences of the long-term decline of reef-building corals in the Caribbean: evidence of across-reef functional convergence. R. Soc. Open Sci. 6, 190298 ( 10.1098/rsos.190298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Januchowski-Hartley FA, Graham NAJ, Wilson SK, Jennings S, Perry CT. 2017. Drivers and predictions of coral reef carbonate budget trajectories. Proc. R. Soc. B 284, 20162533 ( 10.1098/rspb.2016.2533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rioja-Nieto R, Álvarez-Filip L. 2019. Coral reef systems of the Mexican Caribbean: status, recent trends and conservation. Mar. Pollut. Bull. 140, 616–625. ( 10.1016/j.marpolbul.2018.07.005) [DOI] [PubMed] [Google Scholar]
- 25.Suchley A, Alvarez-Filip L. 2018. Local human activities limit marine protection efficacy on Caribbean coral reefs. Conserv. Lett. 11, e12571 ( 10.1111/conl.12571) [DOI] [Google Scholar]
- 26.González-Barrios FJ, Cabral-Tena RA, Alvarez-Filip L. In press. Recovery disparity between coral cover and the physical functionality of reefs with impaired coral assemblages. Glob. Change Biol. gcb.15431 ( 10.1111/gcb.15431) [DOI] [PubMed] [Google Scholar]
- 27.González-Barrios FJ, Álvarez-Filip L. 2018. A framework for measuring coral species-specific contribution to reef functioning in the Caribbean. Ecol. Indic. 95, 877–886. ( 10.1016/j.ecolind.2018.08.038) [DOI] [Google Scholar]
- 28.Perry CT, Lange ID. 2019. ReefBudget Caribbean v2: online resource and methodology. Retrieved from http//geography.exeter.ac.uk/reefbudget/. See https://geography.exeter.ac.uk/media/universityofexeter/schoolofgeography/reefbudget/documents/Reefbudget_CaribbeanV2_methods_June2019.pdf.
- 29.Facon M, Pinault M, Obura D, Pioch S, Pothin K, Bigot L, Garnier R, Quod J. 2016. A comparative study of the accuracy and effectiveness of line and point intercept transect methods for coral reef monitoring in the southwestern Indian Ocean islands. Ecol. Indic. 60, 1045–1055. ( 10.1016/j.ecolind.2015.09.005) [DOI] [Google Scholar]
- 30.Weil E, Torres JL, Ashton M. 2005. Population characteristics of the sea urchin Diadema antillarum in La Parguera, Puerto Rico, 17 years after the mass mortality event. Rev. Biol. Trop. 53(Suppl 3), 219–231. [PubMed] [Google Scholar]
- 31.Tuohy E, Wade C, Weil E. 2020. Lack of recovery of the long-spined sea urchin Diadema antillarum Philippi in Puerto Rico 33 years after the Caribbean-wide mass mortality. PeerJ 8, e8428 ( 10.7717/peerj.8428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glynn PW, Manzello DP.. 2015. Bioerosion and coral reef growth: a dynamic balance. In Coral Reefs in the Anthropocene (ed. Birkeland C.), pp. 67–97. Dordrecht, The Netherlands: Springer Netherlands. [Google Scholar]
- 33.Chen Y-C. 2017. A tutorial on kernel density estimation and recent advances. Biostat. Epidemiol. 1, 161–187. ( 10.1080/24709360.2017.1396742) [DOI] [Google Scholar]
- 34.Adam TC, Duran A, Fuchs CE, Roycroft MV, Rojas MC, Ruttenberg BI, Burkepile DE. 2018. Comparative analysis of foraging behavior and bite mechanics reveals complex functional diversity among Caribbean parrotfishes. Mar. Ecol. Prog. Ser. 597, 207–220. ( 10.3354/meps12600) [DOI] [Google Scholar]
- 35.Bruggemann JH, Van Kessel AM, Van Rooij JM, Breeman AM.. 1996. Bioerosion and sediment ingestion by the Caribbean parrotfish Scarus vetula and Sparisoma viride: implications of fish size, feeding mode and habitat use. Mar. Ecol. Prog. Ser. 134, 59–71. ( 10.3354/meps134059) [DOI] [Google Scholar]
- 36.Contreras-Silva AI, et al. 2020. A meta-analysis to assess long-term spatiotemporal changes of benthic coral and macroalgae cover in the Mexican Caribbean. Sci. Rep. 10, 8897 ( 10.1038/s41598-020-65801-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodríguez-Martínez RE, Banaszak AT, McField MD, Beltrán-Torres AU, Álvarez-Filip L. 2014. Assessment of Acropora palmata in the Mesoamerican Reef System. PLoS ONE 9, e96140 ( 10.1371/journal.pone.0096140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuffner IB, Toth LT. 2016. A geological perspective on the degradation and conservation of western Atlantic coral reefs. Conserv. Biol. 30, 706–715. ( 10.1111/cobi.12725) [DOI] [PubMed] [Google Scholar]
- 39.Toth LT, Kuffner IB, Stathakopoulos A, Shinn EA. 2018. A 3,000-year lag between the geological and ecological shutdown of Florida's coral reefs. Glob. Change Biol. 24, 5471–5483. ( 10.1111/gcb.14389) [DOI] [PubMed] [Google Scholar]
- 40.Jackson JBC, Donovan M, Cramer K, Lam V. 2014. Status and trends of Caribbean coral reefs: 1970–2012. Gland, Switzerland: Global Coral Reef Monitoring Network, IUCN; See https://www.iucn.org/content/status-and-trends-caribbean-coral-reefs-1970-2012. [Google Scholar]
- 41.Shantz AA, Ladd MC, Burkepile DE. 2020. Overfishing and the ecological impacts of extirpating large parrotfish from Caribbean coral reefs. Ecol. Monogr. 90, 1–17. ( 10.1002/ecm.1403) [DOI] [Google Scholar]
- 42.Comeros-Raynal MT, et al. 2012. The likelihood of extinction of iconic and dominant herbivores and detritivores of coral reefs: the parrotfishes and surgeonfishes. PLoS ONE 7, e39825 ( 10.1371/journal.pone.0039825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emslie M, Pratchett MS. 2018. Differential vulnerabilities of parrotfishes to habitat degradation. In Biology of parrotfishes (eds Hoey AS, Bonaldo RM), pp. 355–382. Boca Raton, FL: CRC Press. [Google Scholar]
- 44.Bellwood DR, Hoey AS, Hughes TP. 2012. Human activity selectively impacts the ecosystem roles of parrotfishes on coral reefs. Proc. R. Soc. B 279, 1621–1629. ( 10.1098/rspb.2011.1906) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vallès H, Gill D, Oxenford HA. 2015. Parrotfish size as a useful indicator of fishing effects in a small Caribbean island. Coral Reefs 34, 789–801. ( 10.1007/s00338-015-1295-x) [DOI] [Google Scholar]
- 46.Johnson A. 2010. Reducing bycatch in coral reef trap fisheries: escape gaps as a step towards sustainability. Mar. Ecol. Prog. Ser. 415, 201–209. ( 10.3354/meps08762) [DOI] [Google Scholar]
- 47.Cox CE, Jones CD, Wares JP, Castillo KD, McField MD, Bruno JF. 2013. Genetic testing reveals some mislabeling but general compliance with a ban on herbivorous fish harvesting in Belize. Conserv. Lett. 6, 132–140. ( 10.1111/j.1755-263X.2012.00286.x) [DOI] [Google Scholar]
- 48.Schmitter-Soto JJ, Aguilar-Perera A, Cruz-Martínez A, Herrera-Pavón RL, Morales-Aranda AA, Cobián-Rojas D. 2018. Interdecadal trends in composition, density, size, and mean trophic level of fish species and guilds before and after coastal development in the Mexican Caribbean. Biodivers. Conserv. 27, 459–474. ( 10.1007/s10531-017-1446-1) [DOI] [Google Scholar]
- 49.Ellison AM, Farnsworth EJ. 1996. Anthropogenic disturbance of Caribbean Mangrove ecosystems: past impacts, present trends, and future predictions. Biotropica 28, 549–565. ( 10.2307/2389096) [DOI] [Google Scholar]
- 50.Dorenbosch M, Grol MGG, Nagelkerken I, van der Velde G.. 2006. Seagrass beds and mangroves as potential nurseries for the threatened Indo-Pacific humphead wrasse, Cheilinus undulatus and Caribbean rainbow parrotfish, Scarus guacamaia. Biol. Conserv. 129, 277–282. ( 10.1016/j.biocon.2005.10.032) [DOI] [Google Scholar]
- 51.Dromard CR, Vaslet A, Gautier F, Bouchon-Navaro Y, Harmelin-Vivien M, Bouchon C.. 2017. Resource use by three juvenile scarids (Cryptotomus roseus, Scarus iseri, Sparisoma radians) in Caribbean seagrass beds. Aquat. Bot. 136, 1–8. ( 10.1016/j.aquabot.2016.08.003) [DOI] [Google Scholar]
- 52.Roff G, Mumby PJ. 2012. Global disparity in the resilience of coral reefs. Trends Ecol. Evol. 27, 404–413. ( 10.1016/j.tree.2012.04.007) [DOI] [PubMed] [Google Scholar]
- 53.Lessios HA. 2016. The great Diadema antillarum die-off: 30 years later. Annu. Rev. Mar. Sci. 8, 267–283. ( 10.1146/annurev-marine-122414-033857) [DOI] [PubMed] [Google Scholar]
- 54.Feehan CJ, Brown MS, Sharp WC, Lauzon-Guay J-S, Adams DK. 2016. Fertilization limitation of Diadema antillarum on coral reefs in the Florida Keys. Ecology 97, 1897–1904. ( 10.1002/ecy.1461) [DOI] [PubMed] [Google Scholar]
- 55.Suchley A, Alvarez-Filip L. 2017. Herbivory facilitates growth of a key reef-building Caribbean coral. Ecol. Evol. 7, 11 246–11 256. ( 10.1002/ece3.3620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bellwood DR, Choat JH. 1990. A functional analysis of grazing in parrotfishes (family Scaridae): the ecological implications. In Alternative life-history styles of fishes (ed. Bruton MN.), pp. 189–214. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 57.Bruno JF, Côté IM, Toth LT. 2019. Climate change, coral loss, and the curious case of the parrotfish paradigm: why don't marine protected areas improve reef resilience? Annu. Rev. Mar. Sci. 11, 307–334. ( 10.1146/annurev-marine-010318-095300) [DOI] [PubMed] [Google Scholar]
- 58.Suchley A, McField MD, Alvarez-Filip L. 2016. Rapidly increasing macroalgal cover not related to herbivorous fishes on Mesoamerican reefs. PeerJ 4, e2084 ( 10.7717/peerj.2084) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Provided as the electronic supplementary material.