Abstract
Infectious diseases, including transmissible cancers, can have a broad range of impacts on host behaviour, particularly in the latter stages of disease progression. However, the difficulty of early diagnoses makes the study of behavioural influences of disease in wild animals a challenging task. Tasmanian devils (Sarcophilus harrisii) are affected by a transmissible cancer, devil facial tumour disease (DFTD), in which tumours are externally visible as they progress. Using telemetry and mark–recapture datasets, we quantify the impacts of cancer progression on the behaviour of wild devils by assessing how interaction patterns within the social network of a population change with increasing tumour load. The progression of DFTD negatively influences devils' likelihood of interaction within their network. Infected devils were more active within their network late in the mating season, a pattern with repercussions for DFTD transmission. Our study provides a rare opportunity to quantify and understand the behavioural feedbacks of disease in wildlife and how they may affect transmission and population dynamics in general.
Keywords: transmissible cancer, sickness behaviour, disease transmission, devil facial tumour disease, Tasmanian devil
1. Introduction
Disease can be a strong driver of behavioural interactions among individuals in both human and wildlife populations [1–4]. The extent of influence of disease depends on multiple variables, including the social system of the host, environmental stressors, pathogen load/virulence and the long-term consequences of infection [5–8]. Alterations to behaviour are expected to be contingent on infection stage and driven by gradual physiological changes in the host [9]. As a result, there is often a threshold at which behavioural changes begin to occur or increase in intensity [10]. Individual changes in behaviour influence social interaction dynamics; thus infection-induced alterations in behaviour impact not only individual hosts, but also population-level transmission dynamics [11].
Behavioural responses to disease can be observed in healthy as well as infected individuals. Healthy individuals may alter their behaviour by avoiding sources of infection, while infected individuals may undergo disease-induced behavioural changes. The former is caused by active avoidance of diseased individuals [12], driven by selective pressure on healthy individuals to avoid infection. The propensity for avoidance to occur depends on the transmission mode and relative cost of infection, as well as the social system of the host and the context of interactions [13]. Secondly, infected animals must trade-off energy allocation to different fitness components: fighting infection to survive versus energy devoted to reproduction [14]. The suite of behavioural responses to infection are collectively termed ‘sickness behaviours’ and are generally associated with energy budgeting [5,15]. In some cases, increasing severity of infection may alter behaviour progressively, as body condition of the individual worsens and energetic demands on the immune system increase. Over time, infection can drive animals into social isolation, to avoid potentially costly competition with conspecifics, to conserve energy or to avoid infection of kin [7,16,17]. Alternatively, there may be fitness benefits to aggregating, which can help reduce the cost of an infection [18]. While questions of sickness-induced behavioural changes have been addressed in a theoretical context [19] and under laboratory conditions [12,20], the effects of avoidance and sickness behaviours can be difficult to disentangle in populations of wild animals. For example, Weber et al. [21] found that European badgers infected with bovine tuberculosis (Mycobacterium bovis) were socially isolated from their own groups, but it was unclear whether this was the result of avoidance by healthy individuals or self-isolation of infected individuals. Overall, the effects and progression of sickness behaviour remain relatively poorly studied in free-living wildlife populations.
Studying the effects of disease-induced behavioural changes of individuals requires detailed and time-step knowledge of disease status and interaction patterns. This has been achieved in some group-living species, notably primates, mongooses and mice [7,22,23]. However, obtaining robust data is particularly challenging in non-gregarious species where interactions are less common, and in which those infrequent interactions are essential for pathogen transmission. The difficulty of timely assessment of clinical diagnosis of infection in wild animals [24] presents a further complication. Many diseases can induce behavioural or physiological changes without the host displaying clinical symptoms (e.g. Ross River virus; [25]). Other diseases with high mortality, such as cancer, are particularly difficult to diagnose in wild populations [14,26,27] with the incidence of cancers in wildlife poorly understood [28]. Animals affected by most forms of cancer are generally not diagnosable until tumours are visible externally, and death often occurs before clinical symptoms are apparent [29]. Given the ubiquity of oncogenic processes in most multicellular organisms [30,31], studying their behavioural effects in wild populations is both ecologically and epidemiologically relevant across a broad range of taxa. However, studying sickness behaviour in wildlife requires a system in which (i) individuals display clearly diagnosable clinical signs at an early stage, and (ii) interactions of individuals in a population can be monitored during disease progression.
Here, we follow the progression of cancer-induced behavioural changes in a solitary nocturnal animal, the Tasmanian devil (Sarcophilus harrisii), caused by a transmissible cancer, devil facial tumour disease (DFTD; [32]). DFTD is a clonal cancer cell line [33] transmitted between hosts when they bite one another, predominantly in and around the oral cavity [34]. Transmission is driven by the social and aggressive behaviours of the species, resulting in bite wounds [34,35]. The majority of transmission is expected to occur during the devils mating season, when both interaction and injury rates peak [35]. Once infected, tumours develop around the head and mouth of the host, resulting in death after 6–12 months in most cases ([36]; though see [37,38]). The disease severely impacts the health of infected individuals, particularly as tumour volume increases, resulting in compromised immune function, poor body condition and lack of competitiveness in resource acquisition [39]. As tumour load increases, the metabolic cost of DFTD on the host grows, increasing the need to conserve energy [39]. In some cases, animals can have difficulty feeding, owing to tumours displacing teeth or obstructing the palate and throat. Energy intake is impacted, and progressively reduces ability to compete with conspecifics for resources. Increasing tumour load is thus expected to influence interactions between the host and other individuals, with consequences for transmission dynamics.
In this study, we used proximity-sensing radiotelemetry to generate contact networks and investigated interactions within a population of Tasmanian devils recently infected with DFTD. Over a six-month period, we closely monitored both interactions and disease status of the adult population to test the effects of DFTD and tumour load on contact patterns. We used temporal exponential random graph models (TERGMs), which use social network theory to model an individual's probability of interacting with others in the population over time, while also accounting for changes in disease status. This allows an evaluation of how cancer progression might alter social behaviour over a temporal scale. We predicted that an individual's probability of interacting would decrease on exhibiting clinical symptoms of DFTD and with increasing tumour load.
2. Methods
(a). Proximity loggers
Proximity loggers fitted to adjustable collars (Sirtrack E2, Havelock North, New Zealand) were used to collect data on interactions between devils. Individual collars emit a unique UHF pulse, such that when two, or more, units (i.e. individual devils fitted with collars) are proximal, details of the interaction are recorded on the device's internal memory. Collars were calibrated to detect and log one another at a distance of 30 cm or less—a biologically meaningful distance at which devils could conceivably bite one another. Proximity loggers were set up and calibrated to be consistent with previous research on Tasmanian devil interactions (see the electronic supplementary material, S1, as well as [35,40] for further details of proximity logger calibration and data handling).
(b). Field site and data collection
The study was conducted in a population of Tasmanian devils near Smithton in northwestern Tasmania, Australia (−40.980 E, 145.263 S). Individual devils were caught for collaring by setting 40 traps over a 25 km2 area (a standard monitoring area for long-term devil population studies; [41]) for a period of one month. Traps were custom built from 300 mm polypipe and baited with various meats (predominantly sheep and native macropod). The population had been surveyed for six months prior to commencement of collaring, allowing identification of resident individuals in the core study area (see the electronic supplementary material, S2, for details of individuals collared and background population). All known sexually mature individuals (2 years and older) were caught and collared in January 2017 (12 females, 10 males). Proximity loggers collected data on individual's interactions from January until the end of June. This period covers both mating (February–April) and non-mating periods (May–June), when devil interaction rates differ significantly [35,40]. Timing of the mating season was estimated from extended intersex interactions recorded by the proximity loggers and confirmed by back-dating birth dates of pouch young based on their developmental stage [40,42].
Collared devils were re-trapped on a monthly basis throughout the study period to monitor their disease status, record bite wounds and assess collar fit. Upon capture, devils were thoroughly examined for the presence of facial tumours. For all tumours, length, width and depth were recorded to 0.1 mm using callipers (Mitutoyo Vernier; Kawasaki, Japan). These measurements were used to calculate the volume of each tumour according to the following formula:
Multiple tumours on each individual devil were pooled to obtain a value of tumour load per individual. Tumour load on each individual at each time-step was categorized into four levels (as per [37]); (1) 0.0001–50 cm3, (2) 50–100 cm3, (3) 100–200 cm3 and (4) greater than 200 cm3. Devils were also examined thoroughly for new wounds (see the electronic supplementary material, S3, and [35] for detailed methods).
(c). Network construction
Contact networks were formulated using twelve 14-day periods running from the point at which all adult animals in the population had been collared—19 January. The 14-day period represents enough time to identify new infections (clinical symptoms of DFTD), while being sufficiently temporally fine-scale to identify shifts within seasons. The time series examined encompasses both mating (16 February–26 April) and non-mating (19 January–15 February and 27 April–5 July) periods. In network visualizations, individuals are represented as nodes linked by observed contacts—lines (edges) between nodes are weighted by the frequency of contacts (consistent with previous research into devil networks [35,40]), i.e. edges represent the relative frequency of interactions between each pair of nodes. All networks were produced using the igraph package [43] in R v. 3.4.2 [44].
Within each 14-day period, we calculated four node-level network metrics using igraph. All provide an indication of an individual's position and interactive potential within the network: (i) interaction frequency, (ii) degree (the number of other individuals associated with), (iii) betweenness centrality (the number of shortest paths flowing through an individual; a measure of their importance in connecting disparate parts of a network), and (iv) closeness centrality (sum of all shortest paths flowing through an individual; highlights nodes best placed to influence the entire network most quickly). For each metric within each time-step, we used a node-permuted general linear model to test for differences between healthy and DFTD-infected individuals. To account for the non-independence inherent in network analysis [45,46], these were compared to 10 000 randomized networks that had the disease status of each node allocated at random.
(d). Temporal exponential random graph models
TERGMs were used to investigate whether individual interaction patterns within a contact network differ as a result of infection status or tumour load. TERGMs can be used to examine network structure through time, allowing evaluation of the effect of DFTD on interaction patterns. TERGMs were run using the package btergm [47] in R v. 3.4.2 [44].
Separate TERGMs were independently fitted to examine the effects of DFTD status and tumour load on edge formation within binary fortnightly contact networks. These models were further subdivided into mating and non-mating season to account for known seasonal variability in Tasmanian devil interactions [35,40]. Each model included the following terms; edges—similar to the intercept term in a general linear model (GLM), this gives the probability of edges forming in a network relative to a random network [48]; memory—models if interactions remain consistent over time; nodefactor (Sex)—models sex-based variations in interactions; nodemix (Sex)—accounts for any tendency for sexes to interact preferentially; nodefactor/cov (Wounds)—the number of bite wounds (discrete numerical covariate) accrued over the time periods modelled, effectively a proxy of infection risk in the devil/DFTD system [34]. The final nodefactor/cov() in each model was aimed at examining the tendency of interactions to vary based on the key variables of DFTD status (binomial factorial) or tumour load (continuous numerical covariate). Each model was fitted using bootstrapped pseudo-likelihood estimation, with models bootstrapped 10 000 times to obtain confidence intervals.
3. Results
(a). Contact networks
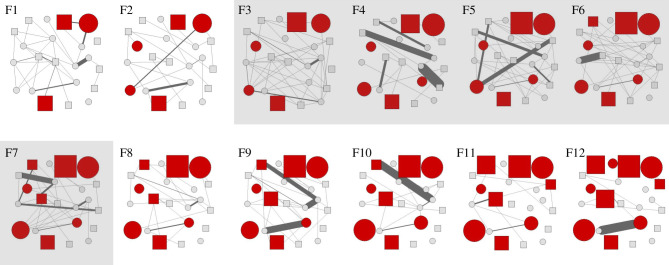
The total number of interactions recorded over the six-month period was 8504 (7273 in the mating season, 1231 in the non-mating season). At the beginning of the study, three individuals had clinical symptoms of DFTD infection, while a further seven individuals began displaying clinical symptoms during the following six months; the remaining 12 devils remained disease-free throughout the study period (figure 1). Network density was significantly higher in mating season contact networks (0.14 ± 0.02 95% confidence interval (CI)) than non-mating season contact networks (0.09 ± 0.02).
Figure 1.
Fortnightly (F) contact networks based on the interactions between individual Tasmanian devils over the course of six months during the early stages of a DFTD outbreak; F1–12 represent 14-day time steps with the mating season highlighted in grey. Squares represent males, while circles represent females. Nodes coloured solid red represent those with clinical symptoms of DFTD, where size is scaled by tumour load category (1, 0.0001–50 cm3; 2, 50–100 cm3; 3, 100–200 cm3; 4, greater than 200 cm3; [37]). Edges between nodes represent interaction frequency within the dyad, the thicker the line, the more interactions between those individuals. Edges have been scaled (dyad interaction frequency/30) to allow depiction of high edge weights without occluding entire networks. (Online version in colour.)
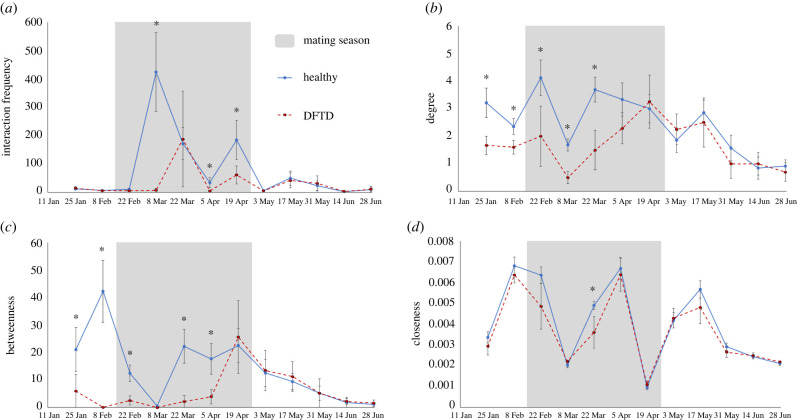
Interaction frequency differed significantly between healthy and DFTD-infected individuals for three periods during the mating season (figure 2a; node-permuted GLMs; F4, p < 0.001; F6, p < 0.001; F7, p < 0.001). Degree was significantly lower in DFTD-infected individuals during the first three periods of the mating season (figure 2b; F3, p = 0.033; F4, p = 0.045; F3, p = 0.012). Betweenness was significantly lower in DFTD-infected animals for the first two non-mating periods in the study (figure 2c; F1, p < 0.001; F2, p < 0.001) and a further three within the mating season (figure 2c; F3, p < 0.001; F5, p < 0.001; F6, p < 0.001). Closeness was only found to be significantly divergent (higher in healthy devils) for one period during the mating season (figure 2d; F1, p = 0.029).
Figure 2.
Mean network metrics of (a) interaction frequency, (b) degree, (c) betweenness, and (d) closeness through fortnightly contact networks for healthy devils (solid, blue) and those with clinical symptoms of DFTD (dashed, red). The Tasmanian devil mating season is shaded in grey. Error bars indicate 95% confidence intervals—periods with non-overlapping error bars represent a significant effect of infection status in node-permuted significance tests (indicated by an asterisk). (Online version in colour.)
(b). Temporal exponential random graph models
The probability of edge formation in fortnightly contact networks was observed to decrease with DFTD infection; this effect was seen across both the mating and non-mating seasons (table 1a). Individuals with DFTD were predicted to be 26% (CI = 0–78%) less likely to form an edge with another individual in the network during the mating season (table 1a) and 22% (CI = 0–48%) less likely during the non-mating season (table 1a), though this effect was not significant in either case. Edges were more likely (mating season estimate = 1.73, CI = 1.55, 2.43; non-mating season estimate = 1.99, CI = 1.75, 2.30; table 1a) to form between individuals that shared edges during the previous time period, indicating the persistence of regular interaction partners through time, regardless of infection status. There was no effect of sex or number of wounds accrued on the probability of edge formation, while sex-mixing was unbiased towards either homophily or heterophily through time in both seasons (table 1a).
Table 1.
Model output for temporal exponential random graph models investigating the influence of (a) DFTD status and (b) tumour load on edge formation within 14-day contact networks during the mating and non-mating seasons. (Confidence intervals (CI) provide the lower and upper bounds of the 95% CI around the model estimate.)
| model term | mating season |
non-mating season |
||
|---|---|---|---|---|
| estimate | CI | estimate | CI | |
| (a) DFTD status | ||||
| edgesa | −2.47 | −3.50, −1.57 | −2.57 | −3.16, −2.10 |
| memorya | 1.73 | 1.57, 2.42 | 2.04 | 1.83, 2.36 |
| sex (M versus F) | 0.27 | −0.63, 0.85 | 0.06 | −0.32, 0.38 |
| same sex versus different sex | −0.05 | −0.42, 0.19 | −0.19 | −0.66, 0.22 |
| wounds | 0.08 | −0.006, 0.18 | 0.10 | −0.11, 0.22 |
| DFTD status (+ ve versus −ve) | −0.26 | −0.78, 0.05 | −0.22 | −0.48, 0.09 |
| (b) tumour load | ||||
| edgesa | −2.78 | −3.87, −1.88 | −2.81 | −3.30, −2.53 |
| memorya | 1.70 | 1.53, 2.40 | 2.02 | 1.78, 2.35 |
| sex (M versus F) | 0.29 | −0.61, 0.87 | 0.07 | −0.32, 0.40 |
| same sex versus different sex | −0.05 | −0.41, 0.18 | −0.19 | −0.67, 0.22 |
| wounds | 0.08 | −0.02, 0.17 | 0.09 | −0.10, 0.20 |
| tumour load (0–4)a | −0.17 | −0.30, −0.05 | −0.15 | −0.25, −0.05 |
aSignificant terms are those for which the confidence intervals do not cross zero.
A significant effect of tumour load was observed in TERGMs investigating the effect of tumours, with devils becoming progressively less likely to form edges with other individuals in the network, with increasing tumour load (table 1b). For each increasing level of category of tumour load (1–4) that an individual progressed through, the likelihood of forming an edge decreased by 17% (CI = 5–30%) in the mating season, and by 15% in the non-mating season (CI = 5–25%; table 1b).
4. Discussion
We provide, to our knowledge, the first empirical study of the progression of a transmissible cancer and its effects on social behaviour through a population of wild animals by closely monitoring their disease status and interactions over a six-month period. An individual Tasmanian devil's probability of interaction declines progressively as DFTD load increases. Consequently, disease progression reduces the connectivity of DFTD-infected animals within their social network, particularly during the mating season when most potential disease transmission opportunities occur [35]. These findings have implications for our understanding of the progression and transmission of DFTD in Tasmanian devils, while also contributing to our knowledge of disease-induced sickness behaviours.
The negative correlation between tumour load and interaction frequency is consistent with expectations that infected devils would reduce their interactions as the cost of infection became higher. Devils with a higher tumour load appear to become increasingly socially isolated, which can be a consequence of both the metabolic and physiological costs of the cancer. Given that most interactions in devils are based around competition (either for food or mating partners; [34,35]), a decrease in interactions is also likely to signal reduced competitive ability. Ruiz-Aravena et al. [39] found that body condition in DFTD-infected devils, particularly males, declines sharply as tumour volume exceeds 3% of body weight. The fact that our data also show a decrease in interaction rate, particularly at high tumour volumes, indicates the presence of a threshold beyond which the effect of disease burden on behaviour becomes pronounced. Reduced interaction rates and network connectivity of devils in the latter stages of DFTD infection should affect transmission dynamics. Individuals are expected to be most likely to transmit DFTD to new hosts when tumours are at their largest because of the greater area of infected tissue [49]. However, we show that devils with high tumour loads interact with other animals relatively infrequently, which reduces potential opportunities for DFTD transmission. Instead, interaction patterns suggest it may be devils in earlier stages of infection, with smaller tumour loads and suffering less from the effects of the disease in terms of overall health, condition and sickness behaviour, that are likely to be driving disease transmission.
Interaction patterns alter in individuals infected with DFTD, a tendency that has measurable effects on their overall connectedness within their social network. The observed effects are driven by reproductive season, with most network metrics aligning in the non-mating season but diverging during the mating season. Notably, the network metrics of DFTD-infected devils were more similar to those of healthy individuals towards the end of the mating season. Thus, there is an increase in connectivity of diseased individuals as the mating season comes to a close—both in terms of the number of individuals they interact with (degree) and whether they occupy key positions capable of reaching disparate parts of the network (betweenness). This period may be important for DFTD transmission dynamics. Not only are infected devils involved in more interactions, those interactions are with individuals already likely to be in poor condition and immunocompromised, making these other individuals particularly vulnerable to infection (a recognized pattern in dasyurids; [50,51]). While aggressive mating season interactions have been identified as key to DFTD spread previously [34,35], our results indicate that late-season interactions may be particularly important sources of transmission events.
It remains difficult to disentangle the potential effects of sickness behaviours from the possibility that healthy devils are actively avoiding those with DFTD. Avoidance behaviour could become particularly pronounced as tumours increase in size and present an increasingly clear visual signal of disease to other individuals. Additionally, DFTD infection is probably associated with olfactory cues, as ulcerated tumours are regularly the source of secondary infections and necrosis. Devils have a keen sense of smell [52], so it is conceivable that they would react to DFTD olfactory cues, potentially influencing contact behaviour. Nonetheless, our results suggest that healthy individuals are not avoiding diseased individuals entirely. First, networks are not segregated into healthy and DFTD-infected subgroups. Second, long-term associations within contact networks continue to persist when one half of a dyad becomes symptomatic. For example, a female–female relationship persisted through the entire six-month study period, including after one female began to develop clinical signs of DFTD. Most of the dyads' interactions took place during the day, indicating the females were regularly denning together (devils are nocturnal; [52]); a behaviour unlikely to result in competitive interactions or injury [35]. Further, this demonstrates a healthy individual that was not actively avoiding a symptomatic individual. While we cannot rule out behavioural avoidance, it is unlikely to be the sole driver of alterations in interaction patterns observed in individuals with DFTD. Future studies combining interaction data with geographical location will be useful to investigate avoidance behaviour further.
Evaluating the effects of disease on behaviour is rare in wildlife studies, owing to the difficulty of diagnosis and of following disease progression in a wild setting. Here, we provide evidence that progression of a transmissible cancer alters interaction rates and position within a social network in Tasmanian devils. This has implications for our understanding of how infectious cancers may evolve and spread. Up to 20% of cancers in humans [53], possibly even more in wildlife [54], have been associated with direct infectious origins. Improved knowledge of the behavioural side effects of infectious diseases can help to further understand their overall ecological and evolutionary effects in wildlife across a broad range of taxa.
Supplementary Material
Acknowledgements
We acknowledge the tarkiner people, traditional owners of the land on which this research was conducted. We thank Sustainable Timber Tasmania for permission to conduct this research on land under their management. We also thank Bonorong Wildlife Sanctuary for assistance with trialling proximity logging collars, and a large number of volunteers who assisted with data collection. Thanks to Catherine Young for comments on an early draft of the manuscript, reviewers for constructive suggestions which improved the manuscript considerably and Manuel Ruiz and Sébastien Comte for useful discussions on the project.
Ethics
Data were collected under permits from the Tasmanian Department of Primary Industries, Parks, Water and the Environment (TFA 16180) and Sustainable Timber Tasmania (no. 1505). Ethical approval for the research was granted by the University of Tasmania Ethics Committee (A15835).
Data accessibility
Data to replicate statistical tests used in this study are available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.xksn02vdp [55].
Authors' contributions
D.G.H., M.E.J., E.Z.C., D.H.K. and R.K.H. conceived the study in consultation with H.M., P.A.H. and A.S. D.G.H. collected and analysed the data in discussion with M.E.J., E.Z.C., D.H.K. and R.K.H. D.G.H. wrote the manuscript with input from all authors.
Competing interests
We declare we have no competing interests.
Funding
Support for this research was provided by a grant from the National Science Foundation (DEB no. 1316549) as part of the joint NSF-NIH-USDA Ecology and Evolution of Infectious Diseases program and by the Australian Research Council (DE170101116). Data collection was supported by funding from the Holsworth Research Endowment awarded to D.G.H.
References
- 1.Telfer S, Lambin X, Birtles R, Beldomenico P, Burthe S, Paterson S, Begon M. 2010. Species interactions in a parasite community drive infection risk in a wildlife population. Science 330, 243–246. ( 10.1126/science.1190333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallana M, Ryser-Degiorgis M-P, Wahli T, Segner H. 2013. Climate change and infectious diseases of wildlife: altered interactions between pathogens, vectors and hosts. Curr. Zool. 59, 427–437. ( 10.1093/czoolo/59.3.427) [DOI] [Google Scholar]
- 3.Lowry H, Lill A, Wong BB. 2013. Behavioural responses of wildlife to urban environments. Biol. Rev. 88, 537–549. ( 10.1111/brv.12012) [DOI] [PubMed] [Google Scholar]
- 4.Kappeler PM, Cremer S, Nunn CL. 2015. Sociality and health: impacts of sociality on disease susceptibility and transmission in animal and human societies. Phil. Trans. R. Soc. B 370, 20140116 ( 10.1098/rstb.2014.0116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart BL. 1988. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 12, 123–137. ( 10.1016/S0149-7634(88)80004-6) [DOI] [PubMed] [Google Scholar]
- 6.Bohn S, Turner J, Warnecke L, Mayo C, McGuire L, Misra V, Bollinger TK, Willis CKR. 2016. Evidence of ‘sickness behaviour’ in bats with white-nose syndrome. Behaviour 153, 981–1003. ( 10.1163/1568539X-00003384) [DOI] [Google Scholar]
- 7.Ghai RR, Fugère V, Chapman CA, Goldberg TL, Davies TJ. 2015. Sickness behaviour associated with non-lethal infections in wild primates. Proc. R. Soc. B 282, 20151436 ( 10.1098/rspb.2015.1436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adelman JS, Córdoba-Córdoba S, Spoelstra K, Wikelski M, Hau M. 2010. Radiotelemetry reveals variation in fever and sickness behaviours with latitude in a free-living passerine. Funct. Ecol. 24, 813–823. ( 10.1111/j.1365-2435.2010.01702.x) [DOI] [Google Scholar]
- 9.Aubert A. 1999. Sickness and behaviour in animals: a motivational perspective. Neurosci. Biobehav. Rev. 23, 1029–1036. ( 10.1016/S0149-7634(99)00034-2) [DOI] [PubMed] [Google Scholar]
- 10.Szyszka O, Kyriazakis I. 2013. What is the relationship between level of infection and ‘sickness behaviour’ in cattle? Appl. Anim. Behav. Sci. 147, 1–10. ( 10.1016/j.applanim.2013.05.007) [DOI] [Google Scholar]
- 11.Ezenwa VO, Archie EA, Craft ME, Hawley DM, Martin LB, Moore J, White L. 2016. Host behaviour-parasite feedback: an essential link between animal behaviour and disease ecology. Proc. R. Soc. B 283, 20153078 ( 10.1098/rspb.2015.3078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behringer DC, Butler MJ, Shields JD. 2006. Avoidance of disease by social lobsters. Nature 441, 421 ( 10.1038/441421a) [DOI] [PubMed] [Google Scholar]
- 13.Fairbanks B, Hawley D, Alexander K. 2014. No evidence for avoidance of visibly diseased conspecifics in the highly social banded mongoose (Mungos mungo). Behav. Ecol. Sociobiol. 69, 371–381. ( 10.1007/s00265-014-1849-x) [DOI] [Google Scholar]
- 14.Vittecoq M, et al. 2015. Animal behaviour and cancer. Anim. Behav. 101, 19–26. ( 10.1016/j.anbehav.2014.12.001) [DOI] [Google Scholar]
- 15.Hart BL, Hart LA. 2019. Sickness behavior in animals: implications for health and wellness. In Encyclopedia of animal behavior (ed. Choe JC.), 2nd edn, pp. 171–175. Oxford, UK: Academic Press. [Google Scholar]
- 16.Johnson RW. 2002. The concept of sickness behavior: a brief chronological account of four key discoveries. Vet. Immunol. Immunopathol. 87, 443–450. ( 10.1016/S0165-2427(02)00069-7) [DOI] [PubMed] [Google Scholar]
- 17.Shorter JR, Rueppell O. 2012. A review on self-destructive defense behaviors in social insects. Insectes Soc. 59, 1–10. ( 10.1007/s00040-011-0210-x) [DOI] [Google Scholar]
- 18.Dawson EH, et al. 2018. Social environment mediates cancer progression in Drosophila. Nat. Commun. 9, 1–7. ( 10.1038/s41467-017-02088-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White LA, Forester JD, Craft ME. 2018. Covariation between the physiological and behavioral components of pathogen transmission: host heterogeneity determines epidemic outcomes. Oikos 127, 538–552. ( 10.1111/oik.04527) [DOI] [Google Scholar]
- 20.Croft DP, Edenbrow M, Darden SK, Ramnarine IW, van Oosterhout C, Cale J. 2011. Effect of gyrodactylid ectoparasites on host behaviour and social network structure in guppies Poecilia reticulata. Behav. Ecol. Sociobiol. 65, 2219–2227. ( 10.1007/s00265-011-1230-2) [DOI] [Google Scholar]
- 21.Weber N, Carter SP, Dall SR, Delahay RJ, McDonald JL, Bearhop S, McDonald RA. 2013. Badger social networks correlate with tuberculosis infection. Curr. Biol. 23, R915–R916. ( 10.1016/j.cub.2013.09.011) [DOI] [PubMed] [Google Scholar]
- 22.Flint BF, Hawley DM, Alexander KA. 2016. Do not feed the wildlife: associations between garbage use, aggression, and disease in banded mongooses (Mungos mungo). Ecol. Evol. 6, 5932–5939. ( 10.1002/ece3.2343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopes PC, Block P, König B. 2016. Infection-induced behavioural changes reduce connectivity and the potential for disease spread in wild mice contact networks. Sci. Rep. 6, 31790 ( 10.1038/srep31790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Artois M, et al. 2009. Wildlife disease surveillance and monitoring: management of disease in wild mammals, pp. 187–213. New York, NY: Springer. [Google Scholar]
- 25.Claflin SB, Webb CE. 2015. Ross River virus: many vectors and unusual hosts make for an unpredictable pathogen. PLoS Pathog. 11, e1005070 ( 10.1371/journal.ppat.1005070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ujvari B, et al. 2016. Cancer and life-history traits: lessons from host–parasite interactions. Parasitology 143, 1–9. ( 10.1017/S0031182016000147) [DOI] [PubMed] [Google Scholar]
- 27.Ujvari B, Fitzpatrick J, Raven N, Osterkamp J, Thomas F. 2019. The ecology of cancer. In Cancer and society: a multidisciplinary assessment and strategies for action (ed. Bernicker EH.), pp. 153–174. New York, NY: Springer. [Google Scholar]
- 28.Vittecoq M, Roche B, Daoust H, Missé D, Abadie J, Labrut S, Renaud F, Gauthier-Clerc M, Thomas F. 2013. Cancer: a missing link in ecosystem functioning? Trends Ecol. Evol. 28, 628–635. ( 10.1016/j.tree.2013.07.005) [DOI] [PubMed] [Google Scholar]
- 29.Thomas F, et al. 2017. The importance of cancer cells for animal evolutionary ecology. Nat. Ecol. Evol. 1, 1592–1595. ( 10.1038/s41559-017-0343-z) [DOI] [PubMed] [Google Scholar]
- 30.Leroi AM, Koufopanou V, Burt A. 2003. Cancer selection. Nat. Rev. Cancer 3, 226–231. ( 10.1038/nrc1016) [DOI] [PubMed] [Google Scholar]
- 31.Pesavento PA, Agnew D, Keel MK, Woolard D. 2018. Cancer in wildlife: patterns of emergence. Nat. Rev. Cancer 18, 646–661. ( 10.1038/s41568-018-0045-0) [DOI] [PubMed] [Google Scholar]
- 32.Woods GM, Fox S, Flies AS, Tovar CD, Jones ME, Hamede RK, Pemberton D, Lyons B, Bettiol SS. 2018. Two decades of the impact of Tasmanian devil facial tumor disease. Integr. Comp. Biol. 58, 1043–1054. ( 10.1093/icb/icy118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearse AM, Swift K. 2006. Allograft theory: transmission of devil facial-tumour disease. Nature 439, 549 ( 10.1038/439549a) [DOI] [PubMed] [Google Scholar]
- 34.Hamede RK, McCallum H, Jones M. 2013. Biting injuries and transmission of Tasmanian devil facial tumour disease. J. Anim. Ecol. 82, 182–190. ( 10.1111/j.1365-2656.2012.02025.x) [DOI] [PubMed] [Google Scholar]
- 35.Hamilton DG, Jones ME, Cameron EZ, McCallum H, Storfer A, Hohenlohe PA, Hamede RK. 2019. Rate of intersexual interactions affects injury likelihood in Tasmanian devil contact networks. Behav. Ecol. 30, 1087–1095. ( 10.1093/beheco/arz054) [DOI] [Google Scholar]
- 36.Hamede RK, Lachish S, Belov K, Woods G, Kreiss A, Pearse AM, Lazenby B, Jones M, McCallum H. 2012. Reduced effect of Tasmanian devil facial tumor disease at the disease front. Conserv. Biol. 26, 124–134. ( 10.1111/j.1523-1739.2011.01747.x) [DOI] [PubMed] [Google Scholar]
- 37.Wells K, Hamede RK, Kerlin DH, Storfer A, Hohenlohe PA, Jones ME, McCallum H. 2017. Infection of the fittest: devil facial tumour disease has greatest effect on individuals with highest reproductive output. Ecol. Lett. 20, 770–778. ( 10.1111/ele.12776) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Margres MJ, et al. 2018. Large-effect loci affect survival in Tasmanian devils (Sarcophilus harrisii) infected with a transmissible cancer. Mol. Ecol. 27, 4189–4199. ( 10.1111/mec.14853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruiz-Aravena M, Jones Menna E, Carver S, Estay S, Espejo C, Storfer A, Hamede RK. 2018. Sex bias in ability to cope with cancer: Tasmanian devils and facial tumour disease. Proc. R. Soc. B 285, 20182239 ( 10.1098/rspb.2018.2239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamede RK, Bashford J, McCallum H, Jones M. 2009. Contact networks in a wild Tasmanian devil (Sarcophilus harrisii) population: using social network analysis to reveal seasonal variability in social behaviour and its implications for transmission of devil facial tumour disease. Ecol. Lett. 12, 1147–1157. ( 10.1111/j.1461-0248.2009.01370.x) [DOI] [PubMed] [Google Scholar]
- 41.Lazenby BT, et al. 2018. Density trends and demographic signals uncover the long-term impact of transmissible cancer in Tasmanian devils. J. Appl. Ecol. 55, 1368–1379. ( 10.1111/1365-2664.13088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hesterman H, Jones SM, Schwarzenberger F. 2008. Pouch appearance is a reliable indicator of the reproductive status in the Tasmanian devil and the spotted-tailed quoll. J. Zool. 275, 130–138. ( 10.1111/j.1469-7998.2008.00419.x) [DOI] [Google Scholar]
- 43.Csardi G, Nepusz T. 2006. The igraph software package for complex network research. InterJournal 1695, 1–9. [Google Scholar]
- 44.R Core Team. 2018. R: a language and environment for statistical computing. Version 3.4.2 Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 45.Croft DP, Madden JR, Franks DW, James R. 2011. Hypothesis testing in animal social networks. Trends Ecol. Evol. 26, 502–507. ( 10.1016/j.tree.2011.05.012) [DOI] [PubMed] [Google Scholar]
- 46.Farine DR, Whitehead H. 2015. Constructing, conducting and interpreting animal social network analysis. J. Anim. Ecol. 84, 1144–1163. ( 10.1111/1365-2656.12418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leifeld P, Cranmer SJ. 2015. A theoretical and empirical comparison of the temporal exponential random graph model and the stochastic actor-oriented model. Netw. Sci. 7, 20–51. ( 10.1017/nws.2018.26) [DOI] [Google Scholar]
- 48.Silk MJ, Croft DP, Delahay RJ, Hodgson DJ, Weber N, Boots M, McDonald RA. 2017. The application of statistical network models in disease research. Methods Ecol. Evol. 8, 1026–1041. ( 10.1111/2041-210X.12770) [DOI] [Google Scholar]
- 49.Obendorf DL, McGlashan ND. 2008. Research priorities in the Tasmanian devil facial tumour debate. Eur. J. Oncol. 13, 229–238. [Google Scholar]
- 50.McDonald I, Lee A, Than K, Martin R. 1986. Failure of glucocorticoid feedback in males of a population of small marsupials (Antechinus swainsonii) during the period of mating. J. Endocrinol. 108, 63–68. ( 10.1677/joe.0.1080063) [DOI] [PubMed] [Google Scholar]
- 51.Dickman C, Braithwaite RW. 1992. Postmating mortality of males in the dasyurid marsupials, Dasyurus and Parantechinus. J. Mammal. 73, 143–147. ( 10.2307/1381875) [DOI] [Google Scholar]
- 52.Rose RK, Pemberton DA, Mooney NJ, Jones ME. 2017. Sarcophilus harrisii (Dasyuromorphia: Dasyuridae). Mamm. Species 49, 1–17. ( 10.1093/mspecies/sex001) [DOI] [Google Scholar]
- 53.Ewald PW, Swain Ewald HA. 2015. Infection and cancer in multicellular organisms. Phil. Trans. R. Soc. B 370, 20140224 ( 10.1098/rstb.2014.0224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamede R, et al. 2020. The ecology and evolution of wildlife cancers: applications for management and conservation. Evol. Appl. 13, 1719–1732. ( 10.1111/eva.12948) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamilton DG, Jones ME, Cameron EZ, Kerlin DH, McCallum H, Storfer A, Hohenlohe PA, Hamede RK. 2020. Data from: Infectious disease and sickness behaviour: tumour progression affects interaction patterns and social network structure in wild Tasmanian devils Dryad Digital Repository. ( 10.5061/dryad.xksn02vdp) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Hamilton DG, Jones ME, Cameron EZ, Kerlin DH, McCallum H, Storfer A, Hohenlohe PA, Hamede RK. 2020. Data from: Infectious disease and sickness behaviour: tumour progression affects interaction patterns and social network structure in wild Tasmanian devils Dryad Digital Repository. ( 10.5061/dryad.xksn02vdp) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data to replicate statistical tests used in this study are available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.xksn02vdp [55].