Abstract
Terrestrial species on islands often show reduced dispersal abilities. For insects, the generality of explanations for island flight loss remains contentious. Although habitat stability is considered the most plausible explanation, others are frequently highlighted. Adopting a strong inference approach, we examined the hypotheses proposed to account for the prevalence of flightlessness in island insect assemblages, for a region long suspected to be globally unusual in this regard—the Southern Ocean Islands (SOIs). Combining comprehensive faunal inventories, species' morphological information, and environmental variables from 28 SOIs, we provide the first quantitative evidence that flightlessness is exceptionally prevalent among indigenous SOI insect species (47%). Prevalence among species which have evolved elsewhere is much lower: Arctic island species (8%), species introduced to the SOIs (17%), and globally (estimated as approx. 5%). Variation in numbers of flightless species and genera across islands is best explained by variation in wind speed, although habitat stability (thermal seasonality proxy) may play a role. Variables associated with insularity, such as island size, are generally poor predictors of flightlessness. The outcomes redirect attention to Darwin's wind hypothesis. They suggest, however, that wind selects for flightlessness through an energy trade-off between flight and reproduction, instead of by displacement from suitable habitats.
Keywords: flightlessness, sub-Antarctic, natural selection, speciation, island biogeography, functional diversity
1. Introduction
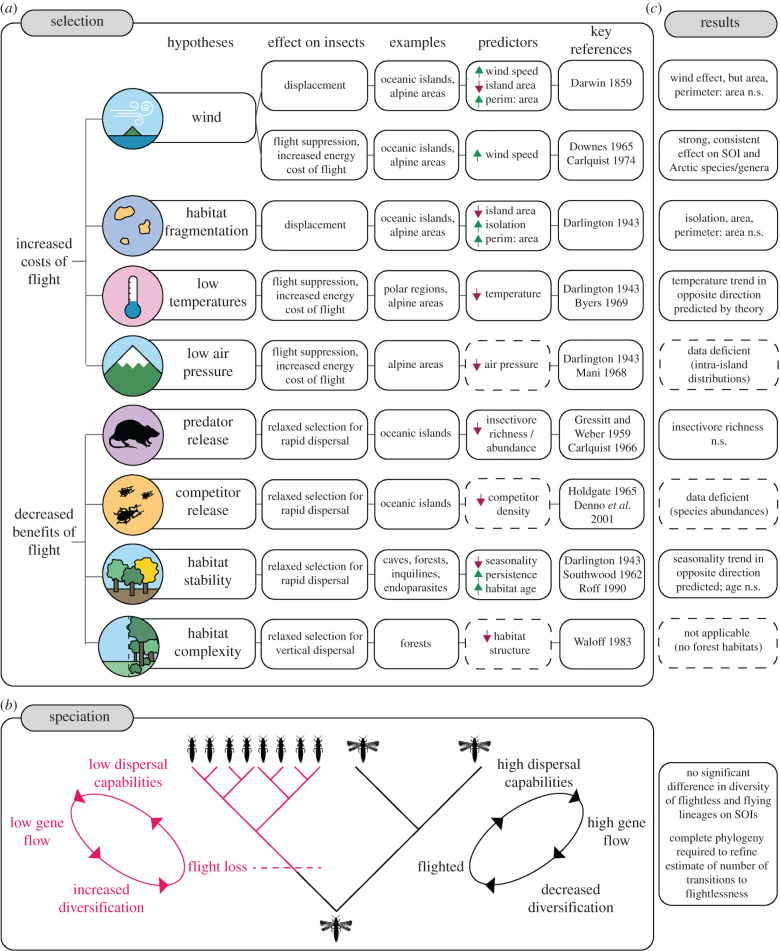
Most insects can fly. Why some have lost that ability secondarily has been widely debated since Darwin and Hooker's disagreement about the causes of flightlessness in island insects. Darwin [1,2] proposed that strong oceanic winds select for flight loss on islands via displacement and/or disuse. He focused, however, on the increased likelihood that flying individuals might be blown out to sea and perish, leaving behind individuals that typically do not fly, to found the next generation. Hooker [3] countered by emphasizing the occurrence of flightless insects in non-island settings where the wind-driven displacement hypothesis is inappropriate. Owing to the energetic costs of building and maintaining the structures underlying flight, and the costs of flight itself [4–8], energy redirection to reproduction rather than flight might be the most important mechanism [9,10]. The central theme of this debate has persisted for more than 160 years, encompassing evidence demonstrating the independent evolution of secondary flight loss in many insect Orders across various environmental settings [11–15]. While the total number of species to have evolved secondary flight loss remains relatively low (approx. 5% of global insect richness) [16], the diversity of groups and environments in which flight loss is found has prompted many hypotheses to account for the phenomenon (figure 1).
Figure 1.
Hypothetical framework for studying insect flightlessness. Diversity of flightless taxa may be driven by selection processes (a), speciation processes (b), or a combination thereof (see [2,12–14,17–25]). Expected positive selection for flightlessness (or relaxed selection for flight) is indicated with upward arrows for predictors, negative selection indicated by downward arrows. Predictors in dashed boxes excluded from our analyses (see electronic supplementary material, Methods, for rationale). Annotations (c) indicate which hypotheses were best supported by the Southern Ocean Islands (SOIs) and Arctic Islands insect data; n.s. indicates non-significant relationships between predictors and the incidence of flightlessness. (Online version in colour.)
The most widely supported selection hypothesis for flight loss focuses on the loss of dispersal ability in stable and persistent habitats, such as in caves and forests and those encountered by inquilines and endoparasites [12–14,17,26–28], or in those cases where predators are largely absent [18]. In these habitats, individuals are under relaxed selection to rapidly evade threats or changing conditions, thus diminishing the advantage of flight. In some species, however, flight also facilitates foraging for resources and mate location, and wings can have a role in other processes, such as courtship, crypsis, and thermoregulation, which may have an influence on the evolution of flightlessness [9]. Alternatively, strong winds, habitat fragmentation, and/or isolation are thought to select for flight loss if flying individuals disperse more frequently into surrounding inhospitable areas than non-flying individuals, and incoming recruitment rates are low, leaving an increasingly flight-reticent population [2,12,27]. Because fragmentation and isolation characterize non-island settings, such as alpine areas and caves, where flight loss is common, this hypothesis is thought to have considerable generality [9]. Based on a theoretical model and consideration of insect flight propensity under different conditions, Roff [10,13], however, considered the displacement mechanism unconvincing because the proportion of flightless insects on islands does not necessarily scale with island size or the predicted rate of displacement. Finally, it has also been proposed that the high energetic costs of developing flight structures and flying in low temperatures [4,29] may favour flight loss in alpine, polar, and oceanic environments [12,19–21,30]. The inefficiency of maintaining flight capability in low-temperature environments, along with fewer opportunities for flying above an individual's flight-temperature threshold [21], may, therefore, select for a diversion of resources from flight to growth and reproduction.
In addition to independent selection on populations, species radiation may also contribute to the diversity of flightless taxa on islands (figure 1). The reduced dispersal abilities associated with flight loss are expected to reduce gene flow between populations and increase speciation [31–35]. Thus, flightless species are expected to speciate more rapidly than flying species. Analytical evidence for such a role of radiation has, thus far, however, not been compelling [15,16,36,37], though large radiations of flightless taxa suggest that the process may be important [38–40].
The most comprehensive reviews of the hypotheses for insect secondary flight loss [13,16] have suggested that habitat fragmentation, stability, or quality are the primary ecological factors selecting for secondary flight loss, that insularity per se is unimportant (therefore rendering Darwin's displacement mechanism irrelevant), and that the radiation of flightless taxa is uncommon [16,41]. Yet, alternative explanations for secondary flight loss continue to be raised, including the importance of insularity and of Darwin's wind hypothesis [36,37,42,43]. Moreover, recent quantitative assessments have emphasized insularity and the associated absence of predation as a major explanation for secondary flight loss in birds [44], and Darwin's wind hypothesis as an important explanation for the decline in dispersal capability in island plants [45,46]. Given these developments and the absence of formal statistical tests of the alternative hypotheses in discussions of secondary flight loss in insects [13], the current view on the mechanisms underlying insect flight loss could be considered unresolved. Such uncertainty is perhaps compounded by the fact that the sub-Antarctic, the region considered most iconic for insect secondary flight loss and potentially where both insularity and Darwin's wind hypothesis may be most applicable [13,43], has yet to be assessed in this context. The most accurate assessment of the proportion of flightless species on the sub-Antarctic islands remains the semi-quantitative one made ca 50 years ago by Gressitt [47], despite significant gains in knowledge about the insect fauna of the region [48]. Furthermore, no studies have sought to make comparisons of the faunas of these islands with those in the Arctic, thus seeking to test an explicit insularity effect relative to some other feature of the sub-Antarctic that promotes flightlessness [13].
Here, building on recent comprehensive faunistic surveys of the Southern Ocean Islands (SOIs), including those of the sub-Antarctic [48,49], we adopt a strong inference approach [50] to examine all of the hypotheses proposed to account for the evolution of flightlessness in insect assemblages. Thus, instead of discounting any hypothesis a priori, we reconsider all of them. First, we quantify the prevalence of flightlessness on these islands by determining the flight capability of described insect species in the region and compare it with secondary flight loss on Arctic islands. We do so specifically to differentiate Roff's [13] ‘sub-Antarctic effect' (i.e. that sub-Antarctic insects have a greater than expected incidence of flightlessness due to some characteristic of these islands, other than their insularity) from the effect of insularity. To determine whether flight capability is an establishment barrier to the sub-Antarctic islands, we also compare the incidence of flight loss between indigenous species, established alien species, and alien species detected in Antarctic pathways of introduction. Alien species are typically good dispersers [51,52] and, due to the relatively short history of human activity in the sub-Antarctic [53], are not expected to have yet had time to evolve flightlessness in response to sub-Antarctic conditions [54]. A prevalence of flightlessness among established aliens would, therefore, indicate that flight capability is an important filter to the establishment on sub-Antarctic islands. Second, we examine relationships between the incidence of secondary flight loss and a suite of environmental factors across the islands in our dataset, with each of the environmental factors closely related to one or more of the hypotheses proposed to account for secondary flight loss in animals (figure 1). Third, we examine taxonomic and geographic patterns of flightlessness to determine the extent to which large radiations of flightless taxa explain the prevalence of flightless species on the SOIs. A complete phylogeny would provide the most accurate estimate of the number of times flightlessness has independently evolved. A fully resolved phylogenetic tree is, however, unavailable for this diverse assemblage—indeed for several taxa their closest ancestors remain the subject of speculation [55]. We, therefore, use several proxies to do so in the absence of a phylogeny for the faunas of these islands.
2. Methods
(a). Region and taxa
The SOIs vary in climate severity, from the temperate northern SOIs, to the colder and windier sub-Antarctic and maritime Antarctic islands (electronic supplementary material figure S1) [53]. Arctic islands are generally less windy and isolated than the SOIs, yet have more extreme and seasonally variable temperatures, photoperiods, and precipitation [56,57]. The terrestrial species composition of the SOIs, and the five Arctic islands considered here, are well-known due to repeated systematic surveys that have been conducted over the last 60 years (e.g. [47,58–63]). To quantify the number of flightless and flying insect species per island, we used a recently updated presence–absence database for the SOIs [48] and compiled species lists for five well-surveyed Arctic islands (Svalbard, Jan Mayen, Ellef Ringnes, Bathurst, and St. Matthew islands) from species inventories in the literature (see Data availability). The ectoparasitic Orders Phthiraptera and Siphonaptera were excluded because these groups have not been well-surveyed in the Southern Ocean region [48]. All species from these Orders are flightless. To quantify the incidence of flight loss among alien insects detected in introduction pathways across the broader Antarctic region, we used species lists of intercepted insects from recent studies of Antarctic and sub-Antarctic stations, vessels, and cargo facilities [64–67].
To classify insect species as either flying or flightless, we used descriptions of the length and functionality of their flight wings, or observations of their flight performance, in species descriptions and the literature (see Data availability). Species with reduced or absent wings (brachypterous/apterous) were classified as flightless. Unless otherwise specified in their descriptions, macropterous species were considered flighted, acknowledging, however, that flight muscle reduction may precede wing reduction in insects and thus some fully winged species may be functionally flightless [29,32,68]. Wing polymorphic species (i.e. species with alate and apterous individuals) were classified as flighted because some individuals are capable of flight. Uncertain occurrence records and undescribed species were excluded from the dataset. The flight capability of 4.2% (n = 40) of the described SOI species, 3.75% (n = 3) of the intercepted alien species, and 2.1% (n = 10) of the Arctic species could not be determined from the literature and were excluded (see Data availability).
(b). Prevalence of flightlessness
To test Roff's [13] ‘sub-Antarctic effect' we used a Kruskal–Wallis rank sum test to compare the proportion of flightless species per island in the sub-Antarctic islands to other island assemblages. We compared the incidence of flightlessness across the endemic, indigenous, and introduced assemblages of the sub-Antarctic islands (as defined by [47]; 12 islands; electronic supplementary material, figure S1), to those assemblages of the temperate northern SOIs (14 islands), the intercepted aliens from Antarctic introduction pathways (four collections of intercepted insects from Antarctic and sub-Antarctic stations, ships, and cargo facilities), and the indigenous Arctic islands assemblages (five islands). If the species in our study support the sub-Antarctic hypothesis, we would expect the indigenous and endemic sub-Antarctic assemblages to have a higher proportion of flightless insects than the temperate SOIs, alien, and Arctic insects. Because most of the introduced species to the SOIs are relatively recent introductions [48], they are not expected to have evolved their dispersal traits in their introduced range. Like Arctic species, established aliens on the sub-Antarctic and temperate SOIs are, therefore, expected to have a lower proportion of flightless species than indigenous SOI insects, if a sub-Antarctic effect occurs. If sub-Antarctic conditions are an establishment barrier to flying insects, established aliens should have a higher proportion of flightless species than alien species that have been detected in Antarctic pathways of introduction (e.g. vessels, cargo facilities), but have not established on the SOIs. Single-island endemic species are expected to have a higher incidence of flight loss than non-endemic indigenous species because their limited distributions suggest they have reduced dispersal tendencies and their evolution in situ may confer more specialization to local conditions, which could include flightlessness if some feature of the sub-Antarctic islands selects for secondary flight loss in insects.
Species that are both indigenous and introduced to various SOIs (11 species) [48] were included in these analyses as indigenous species. To reduce the impact of small sample sizes, 18 island assemblages with fewer than three species were excluded (e.g. no insects have been introduced to the McDonald islands). A non-parametric Kruskal–Wallis test was used to compare the proportion of flightless species per island across assemblages because proportions are bounded variables that do not meet the normality assumptions of a one-way analysis of variance. A post-hoc Dunn's test of multiple comparisons using rank sums, corrected for multiple comparisons with a False Discovery Rate adjustment (Benjamini–Hochberg procedure [69]; α = 0.05), was applied to determine which assemblages differed significantly from each other, using the ‘dunn.test' package (ver. 1.3.5; [70]) in R [71].
(c). Environmental predictors of flightlessness
Hypotheses to explain secondary flight loss are associated with expectations of relationships between particular environmental variables and the extent of secondary flight loss (figure 1a), such as wind speed for Darwin's wind hypothesis. To determine the best environmental predictors of patterns of flightlessness, generalized linear models (GLMs) were used to model the relationships between the number of indigenous flightless and flying insect species per island, as a dependent variable, and a set of environmental variables. Data on topographically corrected island area, isolation from the nearest continent (excluding Antarctica), mean summer land surface temperature (LST), thermal seasonality, mean wind speed, island age (or time since last island-wide glaciation), island perimeter-to-area ratio, and insectivore richness (a proxy for predation pressure, [44]) were derived from high-resolution climate datasets, geographic information systems, and the literature (see electronic supplementary material, Methods). We included mean wind speed, derived from reanalysed surface observations for the decade from 2007 to 2017 for each island, and three measures of displacement risk (island area, isolation, and perimeter-to-area ratio) to explicitly differentiate between the disuse and displacement mechanisms associated with wind-driven selection for flight loss [10]. Thermal seasonality was used as a proxy for habitat stability because these islands are not water limited and therefore thermal variation (including the potential of water unavailability through freezing) plays a large role in determining variation in primary productivity [72]. Including the number of flightless and flying species per island as a response variable weighted the proportion of flightless species by the total number of species to account for differences in insect richness across islands. A quasi-binomial GLM, with logit link function, was fitted to account for over-dispersion, identified during initial data exploration using a binomial distributed model.
Model simplification was undertaken using a bootstrap model selection procedure [73] (see electronic supplementary material, Methods). This model was run on the full set of high-latitude islands with complete environmental data (n = 24) and on the SOIs only (n = 19) to test the consistency of the overall predictor-response relationships to the SOIs (electronic supplementary material, table S1). High-resolution land surface temperature data were not available for nine of the smaller SOIs [74]; therefore, the model was run with and without these islands to ensure their exclusion did not affect the overall trends. Moran's I tests were used to assess spatial autocorrelation in the residuals of the models (‘spdep' R package, ver. 1.1–3; [75]). No significant spatial autocorrelation was detected (results not shown), except for the models run on the full set of islands excluding temperature predictors (electronic supplementary material, table S2). Pseudo-r2 values were calculated as a measure of explained deviance in each model.
To test the consistency of the predictor-response relationships across the major taxonomic groups, the number of indigenous flightless and flying species from each of the five most species-rich insect Orders (Diptera, Coleoptera, Lepidoptera, Hymenoptera, Hemiptera; which account for 96.6% of study species) per island was modelled as a response variable of the most important explanatory variables identified in the bootstrap model simplification. Mean summer LST was excluded from these models, however, due to multiple-collinearity with the included variables in some Order-level models, measured using Variance Inflation Factors (VIFs > 8, ‘car' R package, ver. 3.0–6; [76]). Partial effect plots for these models were drawn using the ‘effects’ R package (ver. 4.0–3; [77]).
To control for possible radiation events within flightless genera, the flight–environment relationships were also examined at the genus level. Genera were classified as either flightless or flying based on the flight capability of their species. Genera containing both flightless and flying species were classified here as flightless because they represent, at a minimum, a single evolutionary transition to flightlessness (following [16]). The number of flightless and flying genera per island were modelled as the response variable in the final bootstrap-selected model from the species-level analysis (electronic supplementary material, table S1).
(d). Transitions to flightlessness
If extensive species radiation occurred after an evolutionary transition to flightlessness, inferences about flight–environment relationships based on the number of flightless species may be confounded by phylogenetic effects [16]. To determine whether large radiations of flightless taxa have occurred within indigenous SOI insect assemblages which may account for the prevalence of flightlessness, we examined two species–trait relationships. These relationships were used to estimate the number of times flightlessness has evolved in lieu of the extensive field collections and genetic analyses that would be required to build a complete phylogenetic tree for the 1077 study species. Phylogenetic relationships for many of these species are currently poorly resolved in the literature (e.g. [55,78]).
First, to test the hypothesis that flightless genera have diversified more than flying genera due to a faster rate of speciation among dispersal-limited taxa [31,34,35], we compared the number of species per genus across genera containing only flying species (197 genera), flightless species (153 genera), or genera with both flying and flightless species (26 genera). A comparison of lineage size across taxa with different flight capabilities has been used elsewhere to estimate the number of independent transitions to flightlessness in insects, in the absence of phylogenetic data [13,16]. If the prevalence of flightlessness across the SOIs was the result of a few large radiations of flightless taxa, we would expect there to be few monomorphically flightless genera, but that these genera would be more species rich than genera containing only flying species. A negative binomial generalized linear mixed effect model (GLMM) was used to determine whether generic richness was related to flight capability, with Order and Family nested as random effects to account for a possible phylogenetic signal in species richness (generic richness∼flight capability + (1|Order/Family)). The GLMM was run using the ‘lme4' package (ver. 1.1-18-1; [79]) in R.
Second, to estimate the number of times flightlessness has evolved among SOI insects and to control for possible radiation events, Families represented by at least one indigenous flightless species in the region were counted as, at a minimum, a single evolutionary transition to flightlessness [16]. This approach assumes that SOI insect Families are monophyletic and do not have a common flightless ancestor at a higher level of classification. Because some insect Families are monomorphically flightless (e.g. Rhaphidophoridae; [16]), considering all flightless species within a given Family as the result of only one independent transition to flightlessness is a more conservative estimate of the number of evolutions of flight loss than the number of flightless genera or species. These estimates may, however, underestimate the number of times flightlessness has evolved in the region because flightlessness can evolve multiple times within insect species [37].
3. Results
(a). Prevalence of flightlessness
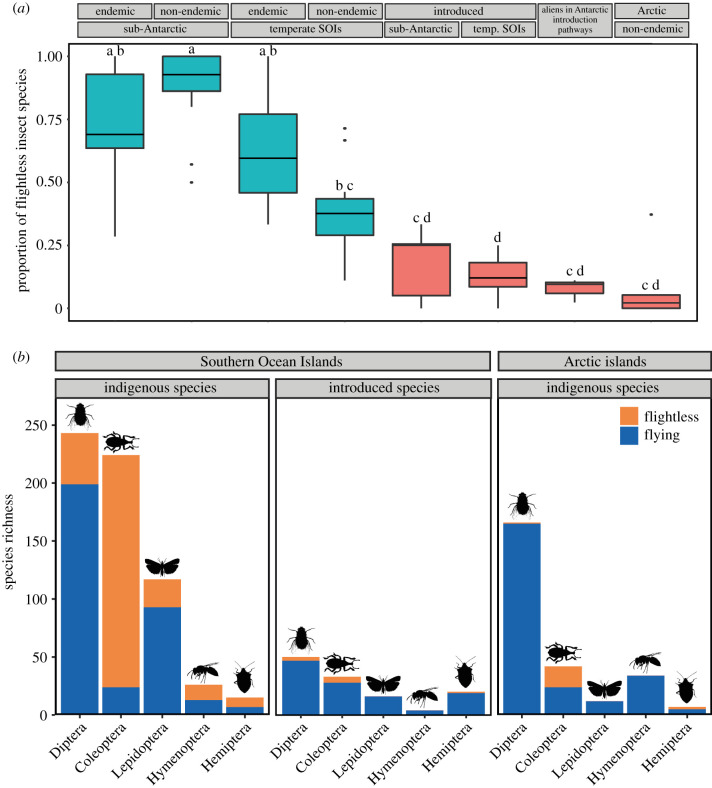
Indigenous SOI insect assemblages have a significantly higher proportion of flightless species than Arctic island and introduced assemblages (Dunn test: figure 2; Kruskal–Wallis test: p < 0.001). Of the 664 indigenous SOI insect species included in this study, 47.4% (n = 315) are monomorphically flightless. By contrast, fewer than 7.9% (n = 21) of Arctic insects, 16.5% (n = 24) of the established SOI aliens, and 6.9% (n = 5) of the alien species detected in Antarctic pathways of introduction, are flightless. As suggested by Roff's [13] ‘sub-Antarctic effect', flightlessness was more prevalent among the indigenous insects of the sub-Antarctic islands than the temperate northern SOIs (figure 2a; electronic supplementary material, figure S1A). No statistically significant difference was found in the incidence of flight loss among the established SOI aliens, alien insects detected in Antarctic introduction pathways, and indigenous Arctic assemblages (figure 2a).
Figure 2.
Prevalence of flightlessness in high-latitude island insects. Proportion of flightless insect species across the indigenous (endemic and non-endemic species) and introduced insect assemblages of the sub-Antarctic (n = 12) and temperate Southern Ocean Islands (SOIs; n = 14), the alien insects detected in Antarctic introduction pathways, and the indigenous insect assemblages of five Arctic islands (a). Boxes indicate median values and interquartile ranges (IQR), whiskers indicate the smallest and largest proportion within 1.5 × IQR, points indicate outliers. Common letters above boxes indicate no significant difference between groups, red boxes indicate assemblages that evolved outside the Southern Ocean region. Number of flightless (orange) and flying (blue) insect species in the five most species-rich insect Orders across the indigenous and introduced assemblages of the SOIs, and the indigenous Arctic island assemblages (b). (Online version in colour.)
(b). Environmental predictors of flightlessness
Model simplification of the generalized linear model identified mean wind speed, thermal seasonality, and mean summer LST as important predictors of the incidence of flightlessness among indigenous insect species across the Southern Ocean and Arctic islands (electronic supplementary material, table S1). These variables had significant positive relationships with the incidence of flightless species and explained 77% of its deviance across islands (table 1 and figure 1). Distance to the nearest continent, island area, and perimeter-to-area ratio, measures of displacement risk, along with insectivore richness and island age, were excluded during model simplification (electronic supplementary material, table S1). Mean wind speed had the strongest effect on the incidence of insect flight loss. The relative effect sizes and fit of the environmental predictors were similar across the species and genus-level models and the models which excluded the Arctic islands from analysis (table 1). In the models including the maximum number of islands in the dataset (where high-resolution temperature data were unavailable for some islands), mean wind speed explained as much as 56% of the deviance in the incidence of flightless species across the Southern Ocean and Arctic islands (electronic supplementary material, table S2). With the exception of Hemiptera, mean wind speed was significantly positively related to the incidence of flightless insects among each of the major insect Orders, and thermal seasonality had no significant effect for any Order (electronic supplementary material, figure S2; and table S3).
Table 1.
Quasi-binomial generalized linear model outcomes for the relationships between the incidence of insect flightlessness across Southern Ocean and Arctic islands and environmental predictors. Response variables were either the number of flightless and flying species per island, or the number of genera containing flightless or flying species per island. Models included all islands with complete environmental data, or the Southern Ocean Islands excluding Arctic sites (SOIs only).
| response | islands | d.f. | predictor | model coeff. | SE | t | p |
|---|---|---|---|---|---|---|---|
| (Pseudo-r2) | |||||||
| species | SOIs & Arctic | 23 (0.77) |
mean wind speed | 1.53 | 0.31 | 4.97 | <0.001 |
| thermal seasonality | 0.21 | 0.06 | 3.29 | 0.004 | |||
| mean summer temp. | 0.22 | 0.07 | 3.40 | 0.003 | |||
| SOIs only | 18 (0.65) |
mean wind speed | 1.61 | 0.39 | 4.12 | 0.001 | |
| thermal seasonality | 0.38 | 0.10 | 3.73 | 0.002 | |||
| mean summer temp. | 0.17 | 0.08 | 2.04 | 0.059 | |||
| genera | SOIs & Arctic | 23 (0.75) |
mean wind speed | 1.13 | 0.24 | 4.67 | <0.001 |
| thermal seasonality | 0.12 | 0.05 | 2.20 | 0.040 | |||
| mean summer temp. | 0.14 | 0.05 | 2.70 | 0.014 | |||
| SOIs only | 18 (0.56) |
mean wind speed | 1.21 | 0.35 | 3.50 | 0.003 | |
| thermal seasonality | 0.27 | 0.10 | 2.82 | 0.013 | |||
| mean summer temp. | 0.11 | 0.07 | 1.47 | 0.161 |
(c). Transitions to flightlessness
No significant difference in the number of species per genus across SOI genera containing only flying or flightless species was found (electronic supplementary material figure S3; and table S4). Genera with both flying and flightless species were more species rich than genera with only flying species (electronic supplementary material figure S3; and table S4). Most indigenous insect genera, irrespective of their flight capability, are represented by only one or two species in the region (electronic supplementary material, figure S3; mean generic richness: flying genera = 1.52, flightless genera = 1.77, flying & flightless genera = 3.62).
If Families represented by at least one indigenous flightless species across the SOIs were considered, at a minimum, a single independent transition to flightlessness to control for possible radiations of flightless species within groups, flightlessness has evolved an estimated 62 times among SOI insects, predominantly among the Coleoptera, Diptera, and Lepidoptera taxa (electronic supplementary material, table S5).
4. Discussion
Sub-Antarctic insects have long been considered the epitome of flightlessness [3,22,58,80,81] and an exception from general explanations regarding its evolution in insects [13]. By using a strong inference, quantitative approach, we show that the situation is more complicated. Flightlessness is indeed remarkably prevalent among SOI insects, especially those from the sub-Antarctic. Nearly half (47.4%) of the indigenous SOI insects are monomorphically flightless (73.6% in the sub-Antarctic), approximately tenfold the estimated global incidence (approx. 5% insect species [16]). Although flightlessness is most common among SOI beetles, its incidence is perhaps more striking among Lepidoptera, Diptera, and Hymenoptera (figure 2b) because, outside the Southern Ocean region, flight loss in both sexes of these insects is exceptionally rare [13,81,82]. Given that flightlessness is uncommon on other islands elsewhere [13,14], including the Arctic islands studied here (figure 2), these findings suggest that some feature of the SOIs, especially those of the sub-Antarctic, other than their insularity, selects for secondary flight loss in insects. These conditions are not, however, an absolute barrier to the establishment of flying species because flying insects persist among both the indigenous and alien SOI assemblages (figure 2).
We found, among the full suite of hypotheses for flight loss (figure 1), that those previously well-supported elsewhere, including habitat stability and predator release [13,14], failed consistently to predict the incidence of flight loss across the Southern Ocean and Arctic islands (table 1; electronic supplementary material, table S3). Instead, secondary flight loss has evolved repeatedly among insects on the windiest SOIs (table 1; electronic supplementary material, figure S1A). Therefore, Darwin's [1] wind hypothesis cannot be rejected.
Darwin [2] proposed two mechanisms by which strong winds could select for insect flightlessness—displacement and disuse. Variables associated with the risk of displacement from island habitats, including island size and isolation, were, however, generally poor predictors of the incidence of flightlessness on the SOIs (figure 1c). Thus, little support is available for Darwin's displacement to sea mechanism. Some evidence exists that winds blow flying insects from SOIs [58,83], but the degree to which this occurs relative to islands elsewhere is unclear. Strong winds can also inhibit normal insect flight activity [5,19,84,85], thereby increasing the energetic costs of flying or maintaining flight structures. This energy trade-off is more complex than Darwin's single-step displacement mechanism because it requires genetic linkage between traits associated with flight ability, flight propensity, and fecundity or survival [10,86]. Yet, such a coevolution of dispersal and reproductive traits has been convincingly demonstrated in insects [4,8,86]. Moreover, reduced flight propensity, or an ‘indolent habit' [2], has been observed in several flight-capable SOI insect species [47,87]. Thus, strong winds may favour secondary flight loss in SOI insects, but not necessarily through the mechanisms Darwin proposed.
Environmental stability and habitat persistence have been previously identified as the most important factors favouring insect flight loss [12–14,17,26–28]. Here, thermal seasonality and mean summer LST, proxies for habitat stability and energy availability, respectively, had significant positive relationships with the incidence of flightlessness, when wind speed was included in the model (table 1). These trends are in the opposite directions predicted by theory (figure 1) because wind speed, surface temperature, and thermal seasonality are themselves correlated across the SOIs. In the case of habitat stability, univariate models were in the negative direction predicted by the habitat stability hypothesis, yet they consistently underperformed relative to multivariate explanations including wind speed (table 1, electronic supplementary material table S1, and table S6), contrary to many past assumptions based on qualitative interpretations [48,54,88,89]. Nonetheless, the climates of the SOIs are highly stable due to the thermal inertia of the Southern Ocean, which moderates insular climates [90,91]. Conversely, variation in Arctic temperatures may maintain selection for flight because flight capability allows Arctic insects to swarm opportunistically during brief periods of warm or calm conditions [19]. Thus, although wind speed had the strongest and most consistent effect on the incidence of flightlessness across Southern Ocean and Arctic insects (table 1; electronic supplementary material, table S3), habitat stability may also play a role in explaining broad-scale patterns of insect flight loss and perhaps especially the widespread retention of flight capability on Arctic islands.
Hypotheses to explain secondary flight loss in insects are not necessarily mutually exclusive [22,48]. Indeed, many of the environmental conditions associated with these hypotheses, such as low predator pressure, low temperatures, and high insularity (figure 1), broadly characterize the SOIs and several factors, or their cumulative effect, may be operating to different degrees on different taxa. These environmental conditions, however, with the exception of the strong and persistent circumpolar winds that exemplify the sub-Antarctic region (electronic supplementary material, figure S1B; [92]), also describe islands elsewhere. Thus, the exceptional degree of flightlessness found on the SOIs and the absence of a consistent island effect on flight loss elsewhere [13,14,36], are most likely caused by the strong and frequent Southern Ocean winds. Because the sub-Antarctic is one of the windiest regions on Earth (electronic supplementary material, figure S1), the degree to which the evolution of insect flight loss is driven by wind pressure may not, however, be generalizable to less windy environments, although other work has suggested that the hypothesis cannot be rejected [20,36,37,58,93].
We show that flightlessness has evolved among SOI insects from several Orders, genera, and islands (figure 2b; electronic supplementary material figure S1A; and table S3). We found no difference in the species richness of flightless and flying genera (electronic supplementary material figure S3; and table S4). These results indicate that flightlessness has not evolved disproportionately in one taxonomic group as would be expected if a single large radiation or a phylogenetic predisposition to flight loss were driving the observed diversity patterns [16]. Instead, repeated secondary flight loss across unrelated taxa and isolated islands indicates extensive convergent evolution of flightlessness in SOI insect communities. These findings are in keeping with previous studies that have not found sufficient radiation among flightless taxa to account for their diversity on islands [16,44]. Using a conservative taxonomic method in lieu of a complete phylogeny [16], we estimated that indigenous SOI insects have transitioned to flightlessness a minimum of 62 times (electronic supplementary material, table S5). This approach likely underestimates the number of independent evolutions of secondary flight loss in the region, because flightlessness has evolved elsewhere multiple times within insect Families and species [16,37]. Nonetheless, this estimate is greater than previous quantifications of the number of times insects have evolved flightlessness, based on North American assemblages (25 times; [16]), indicating that its evolution is more common in the Southern Ocean region than elsewhere. A fully resolved phylogeny of SOI insects is required to further refine these estimates.
Reduced dispersal abilities are often associated with increased extinction risk, especially among island birds, and flightless species may be less able to evade changing conditions or novel predators [13,44]. On the sub-Antarctic islands, several flightless insect populations have declined substantially following the introduction of new predators, suggesting that some flightless species in the region are at risk of local extinction [94,95]. Few studies have, however, quantified the abundance or distribution of whole SOI insect assemblages (but see [83,95,96] for work approaching such comprehensive assessment), or compared the rarity of flightless and flying taxa. Thus, it remains unclear whether insect flightlessness is related to extinction probability owing to recently changed conditions on these islands.
In conclusion, our strong inference, quantitative approach to understanding the evolution of high levels of flightlessness in some insect assemblages, has both provided a plausible explanation for the sub-Antarctic effect and good reason to reconsider Darwin's wind hypothesis as plausible for both remote islands and other settings.
Supplementary Material
Acknowledgements
M.A. McGeoch, G.A. Duffy, J. Lau, R. Dudley, M. Laparie, P.A.V. Borges, and three anonymous reviewers provided helpful comments on previous versions of this manuscript.
Data accessibility
Data are provided at https://doi.org/10.26180/5d47d491c8e8a.
Authors' contributions
R.I.L. and S.L.C. conceived the ideas; R.I.L. collected the data and performed the analysis. Both authors wrote and edited the paper.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Antarctic Circumnavigation Expedition and a Sir James McNeill Foundation Postgraduate Research Scholarship to R.I.L.
References
- 1.Darwin CR. 1855. Letter to J.D Hooker (ed. JD Hooker). See https://www.darwinproject.ac.uk/entry-1643, Darwin Correspondence Project.
- 2.Darwin CR. 1859. On the origin of species by means of natural selection or, The preservation of favoured races in the struggle for life. London, UK: Collector's Library. [PMC free article] [PubMed] [Google Scholar]
- 3.Hooker JD. 1855. Letter to C.R. Darwin. (ed. CR Darwin). See https://www.darwinproject.ac.uk/entry-1644, Darwin Correspondence Project.
- 4.Zera AJ, Denno RF. 1997. Physiology and ecology of dispersal polymorphism in insects. Annu. Rev. Entomol. 42, 207–230. ( 10.1146/annurev.ento.42.1.207) [DOI] [PubMed] [Google Scholar]
- 5.Dudley R. 2000. The biomechanics of insect flight. Princeton, NJ: Princeton University Press. [Google Scholar]
- 6.Nespolo RF, Roff DA, Fairbairn DJ. 2008. Energetic trade-off between maintenance costs and flight capacity in the sand cricket (Gryllus firmus). Funct. Ecol. 22, 624–631. ( 10.2307/20142849) [DOI] [Google Scholar]
- 7.Tigreros N, Davidowitz G. 2019. Flight-fecundity tradeoffs in wing-monomorphic insects. Adv. Insect Physiol. 56, 1–41. ( 10.1016/bs.aiip.2019.02.001) [DOI] [Google Scholar]
- 8.Saglam IK, Roff DA, Fairbairn DJ. 2008. Male sand crickets trade-off flight capability for reproductive potential. J. Evol. Biol. 21, 997–1004. ( 10.1111/j.1420-9101.2008.01548.x) [DOI] [PubMed] [Google Scholar]
- 9.Wagner DL, Liebherr JK. 1992. Flightlessness in insects. Trends Ecol. Evol. 7, 216–220. ( 10.1016/0169-5347(92)90047-F) [DOI] [PubMed] [Google Scholar]
- 10.Roff DA. 2002. Life history evolution. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 11.Jackson DJ. 1928. XXVII.- The inheritance of long and short wings in the Weevil, Sitona hispidula, with a discussion of wing reduction among beetles. Trans. R. Soc. Edinburgh 55, 665–735. ( 10.1017/S0080456800013351) [DOI] [Google Scholar]
- 12.Darlington PJ. 1943. Carabidae of mountains and islands: data on the evolution of isolated faunas, and on atrophy of wings. Ecol. Monogr. 13, 37–61. ( 10.2307/1943589) [DOI] [Google Scholar]
- 13.Roff DA. 1990. The evolution of flightlessness in insects. Ecol. Monogr. 60, 389–421. ( 10.2307/1943013) [DOI] [Google Scholar]
- 14.Denno RF, Hawthorne DJ, Thorne BL, Gratton C. 2001. Reduced flight capability in British Virgin Island populations of a wing-dimorphic insect: the role of habitat isolation, persistence, and structure. Ecol. Entomol. 26, 25–36. ( 10.1046/j.1365-2311.2001.00293.x) [DOI] [Google Scholar]
- 15.Snäll N, Tammaru T, Whahlberg N, Viidalepp J, Ruohomaki K, Sacontaus M.-L, Huoponed K. 2007. Phylogenetic relationships of the tribe Operophterini (Lepidoptera, Geometridae): a case study of the evolution of female flightlessness. Biol. J. Linn. Soc. 92, 241–252. ( 10.1111/j.1095-8312.2007.00834.x) [DOI] [Google Scholar]
- 16.Roff DA. 1994. The evolution of flightlessness: is history important? Evol. Ecol. 8, 639–657. ( 10.1007/BF01237847) [DOI] [Google Scholar]
- 17.Southwood TRE. 1962. Migration of terrestrial arthropods in relation to habitat. Biol. Rev. 37, 171–214. ( 10.1111/j.1469-185X.1962.tb01609.x) [DOI] [Google Scholar]
- 18.Carlquist S. 1966. The biota of long-distance dispersal. I. Principles of dipseral and evolution. Q. Rev. Biol. 41, 247–270. ( 10.1086/405054) [DOI] [PubMed] [Google Scholar]
- 19.Downes JA. 1965. Adaptations of insects in the Arctic. Annu. Rev. Entomol. 10, 257–274. ( 10.1146/annurev.en.10.010165.001353) [DOI] [Google Scholar]
- 20.Mani MS. 1968. Ecological specializations of high altitude insects. In Ecology and biogeography of high altitude insects (ed. Mani MS.), pp. 51–74. The Hague, The Netherlands: Dr W. Junk N. V. Publishers. [Google Scholar]
- 21.Byers GW. 1969. Evolution of wing reduction in crane flies (Diptera: Tipulidae). Evolution 23, 346–354. ( 10.1111/j.1558-5646.1969.tb03517.x) [DOI] [PubMed] [Google Scholar]
- 22.Holdgate MW. 1965. The fauna of the Tristan Da Cunha Islands. Philos. Trans. R. Soc. B 249, 361–402. [Google Scholar]
- 23.Carlquist SJ. 1974. Island biology. New York: NY: Columbia University Press. [Google Scholar]
- 24.Gressitt JL, Weber NA. 1959. Bibliographic introduction to Antarctic-Subantarctic entomology. Pac. Insects 1, 441–480. [Google Scholar]
- 25.Waloff N. 1983. Absence of wing polymorphism in the arboreal, phytophagous species of some taxa of temperate Hemiptera: an hypothesis. Ecol. Entomol. 8, 229–232. ( 10.1111/j.1365-2311.1983.tb00502.x) [DOI] [Google Scholar]
- 26.den Boer PJ. 1970. On the significance of dispersal power for populations of carabid-beetles (Coleoptera, Carabidae). Oecologia 4, 1–28. ( 10.1007/BF00390612) [DOI] [PubMed] [Google Scholar]
- 27.Jarvinen O. 1976. Migration, extinction and alary polymorphism in water-striders (Gerris Fabr). Ann. Acad. Sci. Fenn. A 206, 1–7. [PubMed] [Google Scholar]
- 28.Slater JA. 1977. The incidence and evolutionary significance of wing polymorphism in Lygaeid bugs with particular reference to those of South Africa. Biotropica 9, 217–229. ( 10.2307/2388139) [DOI] [Google Scholar]
- 29.Roff DA. 1986. The evolution of wing dimorphism in insects. Evolution 40, 1009–1020. ( 10.2307/2408759) [DOI] [PubMed] [Google Scholar]
- 30.Hudson GV. 1912. Notes on semi-apterous females in certain species of Lepidoptera, with an attempted explanation. Entomol. Mon. Mag. 23, 269–275. [Google Scholar]
- 31.MacArthur RH, Wilson EO. 1967. The theory of island biogeography. Princeton, NJ: Princeton University Press. [Google Scholar]
- 32.McCulloch GA, Wallis GP, Waters JM. 2009. Do insects lose flight before they lose their wings? Population genetic structure in subalpine stoneflies. Mol. Ecol. 18, 4073–4087. ( 10.1111/j.1365-294X.2009.04337.x) [DOI] [PubMed] [Google Scholar]
- 33.Gillespie RG, Baldwin BG, Waters JM, Fraser CI, Nikula R, Roderick GK. 2012. Long-distance dispersal: a framework for hypothesis testing. Trends Ecol. Evol. 27, 47–56. ( 10.1016/j.tree.2011.08.009) [DOI] [PubMed] [Google Scholar]
- 34.Ikeda H, Nishikawa M, Sota T. 2012. Loss of flight promotes beetle diversification. Nat. Commun. 3, 648 ( 10.1038/ncomms1659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitterboeck TF, Adamowicz SJ. 2013. Flight loss linked to faster molecular evolution in insects. Proc. R. Soc. B 280, 20131128 ( 10.1098/rspb.2013.1128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medeiros MJ, Gillespie RG. 2011. Biogeography and the evolution of flightlessness in a radiation of Hawaiian moths (Xyloryctidae: Thyrocopa). J. Biogeogr. 38, 101–111. ( 10.1111/j.1365-2699.2010.02402.x) [DOI] [Google Scholar]
- 37.McCulloch GA, Foster BJ, Dutoit L, Ingram T, Hay E, Veale AJ, Dearden PK, Waters JM. 2019. Ecological gradients drive insect wing loss and speciation: the role of the alpine treeline. Mol. Ecol. 28, 3141–3150. ( 10.1111/mec.15114) [DOI] [PubMed] [Google Scholar]
- 38.Paulay G. 1985. Adaptive radiation on an isolated oceanic island: the Cryptorhynchinae (Curculionidae) of Rapa revisited. Biol. J. Linn. Soc. 26, 95–187. ( 10.1111/j.1095-8312.1985.tb01554.x) [DOI] [Google Scholar]
- 39.Jago ND. 1994. Odontomelus I. Bolivar 1890 (Orthoptera Acridoidea Acrididae Acridinae): savanna-woodland grasshoppers with a major radiation of flightless species in Eastern Africa. Trop. Zool. 7, 367–450. ( 10.1080/03946975.1994.10539266) [DOI] [Google Scholar]
- 40.Grzywacz B, Lehmann AW, Chobanov DP, Lehmann GU.C. 2018. Multiple origin of flightlessness in Phaneropterinae bushcrickets and redefinition of the tribus Odonturini (Orthoptera: Tettigonioidea: Phaneropteridae). Org. Divers. Evol. 18, 327–339. ( 10.1007/s13127-018-0371-9) [DOI] [Google Scholar]
- 41.Bouchard P, Brooks DR. 2004. Effect of vagility potential on dispersal and speciation in rainforest insects. J. Evol. Biol. 17, 994–1006. ( 10.1111/j.1420-9101.2004.00766.x) [DOI] [PubMed] [Google Scholar]
- 42.Strathdee AT, Bale JS. 1998. Life of the edge: insect ecology in Arctic environments. Annu. Rev. Entomol. 43, 85–106. ( 10.1146/annurev.ento.43.1.85) [DOI] [PubMed] [Google Scholar]
- 43.Gillespie RG, Roderick GK. 2002. Arthropods on islands: colonization, speciation and conservation. Annu. Rev. Entomol. 47, 595–632. ( 10.1146/annurev.ento.47.091201.145244) [DOI] [PubMed] [Google Scholar]
- 44.Wright NA, Steadman DW, Witt CC. 2016. Predictable evolution toward flightlessness in volant island birds. PNAS 113, 4765–4770. ( 10.1073/pnas.1522931113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cody ML, Overton J. 1996. Short-term evolution of reduced dispersal in island plant populations. J. Ecol. 84, 53–61. ( 10.2307/2261699) [DOI] [Google Scholar]
- 46.Riba M, et al. 2009. Darwin's wind hypothesis: does it work for plant dispersal in fragmented habitats? New Phytol. 183, 667–677. ( 10.1111/j.1469-8137.2009.0294) [DOI] [PubMed] [Google Scholar]
- 47.Gressitt JL. 1970. Subantarctic entomology and biogeography. Pac. Insects Monogr. 23, 295–374. [Google Scholar]
- 48.Chown SL, Convey P. 2016. Antarctic entomology. Annu. Rev. Entomol. 61, 119–137. ( 10.1146/annurev-ento-010715-023537) [DOI] [PubMed] [Google Scholar]
- 49.Leihy RI, Duffy GA, Chown SL. 2018. Species richness and turnover among indigenous and introduced plants and insects of the Southern Ocean Islands. Ecosphere 9, e02358 ( 10.1002/ecs2.2358) [DOI] [Google Scholar]
- 50.Platt JR. 1964. Strong inference. Science 146, 347–352. ( 10.1126/science.146.3642.347) [DOI] [PubMed] [Google Scholar]
- 51.Travis JM, Dytham C. 2002. Dispersal evolution during invasions. Evol. Ecol. Res. 4, 1119–1129. [Google Scholar]
- 52.Nahrung HF, Swain AJ. 2015. Strangers in a strange land: do life history traits differ for alien and native colonisers of novel environments? Biol. Invasions 17, 699–709. ( 10.1007/s10530-014-0761-7) [DOI] [Google Scholar]
- 53.Bergstrom DM, Chown SL. 1999. Life at the front: history, ecology and change on southern ocean islands. Trends Ecol. Evol. 14, 472–477. ( 10.1016/S0169-5347(99)01688-2) [DOI] [PubMed] [Google Scholar]
- 54.Laparie M, Vernon P, Cozic Y, Frenot Y, Renault D, Debat V. 2016. Wing morphology of the active flyer Calliphora vicina (Diptera: Calliphoridae) during its invasion of a sub-Antarctic archipelago where insect flightlessness is the rule. Biol. J. Linn. Soc. 119, 179–193. ( 10.1111/bij.12815) [DOI] [Google Scholar]
- 55.Kuschel G, Chown S. 1995. Phylogeny and systematics of the Ectemnorhinus-group of genera (Insecta: Coleoptera). Invertebr. Syst. 9, 841–863. ( 10.1071/IT9950841) [DOI] [Google Scholar]
- 56.French DD, Smith VR. 1985. A comparison between Northern and Southern Hemisphere tundras and related ecosystems. Polar Biol. 5, 5–21. ( 10.1007/BF00446040) [DOI] [Google Scholar]
- 57.Barry RG, Hall-McKim EA. 2018. Polar environments and global change. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 58.Gressitt JL. 1964. Insects of Campbell Island. Honolulu, HI: Bishop Museum. [Google Scholar]
- 59.McAlpine JF. 1965. Insects and related terrestrial invertebrates of Ellef Ringnes Island. Arctic 18, 73–103. ( 10.14430/arctic3455) [DOI] [Google Scholar]
- 60.Danks HV. 1980. Arthropods of Polar Bear Pass, Bathurst Island, Arctic Canada. Syllogeus 25, 1–68. [Google Scholar]
- 61.Jones AG, Chown SL, Webb TJ, Gaston KJ. 2003. The free-living pterygote insects of Gough Island, South Atlantic Ocean. Syst. Biodivers. 1, 213–273. ( 10.1017/S1477200003001142) [DOI] [Google Scholar]
- 62.Greenslade P. 2006. The invertebrates of Macquarie Island. Kingston: Australian Antarctic Division. [Google Scholar]
- 63.Coulson SJ. 2007. The terrestrial and freshwater invertebrate fauna of the High Arctic archipelago of Svalbard. Zootaxa 1448, 41–68. ( 10.11646/zootaxa.1448.1.2) [DOI] [Google Scholar]
- 64.Osyczka P, Mleczko P, Karasiński D, Chlebicki A. 2012. Timber transported to Antarctica: a potential and undesirable carrier for alien fungi and insects. Biol. Invasions 14, 15–20. ( 10.1007/s10530-011-9991-0) [DOI] [Google Scholar]
- 65.Chwedorzewska KJ, Korczak-Abshire M, Olech M, Lityńska-Zając M, Augustyniuk-Kram A. 2013. Alien invertebrates transported accidentally to the Polish Antarctic Station in cargo and on fresh foods. Pol. Polar Res. 34, 55–66. ( 10.2478/popore-2013-0005) [DOI] [Google Scholar]
- 66.Houghton M, McQuillan PB, Bergstrom DM, Frost L, van den Hoff J, Shaw J. 2016. Pathways of alien invertebrate transfer to the Antarctic region. Polar Biol. 39, 23–33. ( 10.1007/s00300-014-1599-2) [DOI] [Google Scholar]
- 67.Newman J, Poirot C, Roper-Gee R, Leihy RI, Chown SL. 2018. A decade of invertebrate colonization pressure on Scott Base in the Ross Sea region. Biol. Invasions 20, 2623–2633. ( 10.1007/s10530-018-1722-3) [DOI] [Google Scholar]
- 68.Harrison RG. 1980. Dispersal polymorphisms in insects. Annu. Rev. Ecol. Syst. 11, 95–118. ( 10.1146/annurev.es.11.110180.000523) [DOI] [Google Scholar]
- 69.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300. [Google Scholar]
- 70.Dinno A. 2017. dunn.test: Dunn's test of multiple comparisons using rank sums. R package, v. 1.3.5.
- 71.R Core Team. 2019. R: A language and environment for statistical computing (v. 3.4.1). Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 72.Clarke A, Gaston KJ. 2006. Climate, energy and diversity. Proc. R. Soc. B 273, 2257–2266. ( 10.1098/rspb.2006.3545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng R. 2017. Bootstrapping linear models. In Non-Standard parametric statistical inference, pp. 317–334. Oxford, UK: Oxford University Press. [Google Scholar]
- 74.Leihy RI, Duffy GA, Nortje E, Chown SL. 2018. High resolution temperature data for ecological research and management on the Southern Ocean Islands. Sci. Data 5, 180177 ( 10.1038/sdata.2018.177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bivand RS, Wong DWS. 2018. Comparing implementations of global and local indicators of spatial association. TEST 27, 716–748. ( 10.1007/s11749-018-0599-x) [DOI] [Google Scholar]
- 76.Fox J, Weisberg S. 2011. An R companion to applied regression, 2nd edn Thousand Oaks, CA: Sage. [Google Scholar]
- 77.Fox J. 2003. Effect displays in R for generalized linear models. J. Stat. Softw. 8, 1–27. [Google Scholar]
- 78.Michaux B, Leschen RAB. 2005. East meets west: biogeology of the Campbell Plateau. Biol. J. Linn. Soc. 86, 95–115. ( 10.1111/j.1095-8312.2005.00511.x) [DOI] [Google Scholar]
- 79.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 80.Brinck P. 1948. Coleoptera of Tristan da Cunha. Results of the Norwegian Scientific Expedition to Tristan da Cunha 1937–1938 17, 1–125. ( 10.1086/220083) [DOI] [Google Scholar]
- 81.Sattler K. 1991. A review of wing reduction in Lepidoptera. Bull. Br. Mus. Nat. Hist. Entomol. 60, 243–288. [Google Scholar]
- 82.Hackman W. 1964. On reduction and loss of wings in Diptera. Not. Entomol. 44, 73–93. [Google Scholar]
- 83.Hawes TC, Greenslade P. 2013. The aerial invertebrate fauna of subantarctic Macquarie Island. J. Biogeogr. 40, 1501–1511. ( 10.1111/jbi.12090) [DOI] [Google Scholar]
- 84.Digby PS. 1958. Flight activity in the blowfly, Calliphora erythrocephala, in relation to wind speed, with special reference to adaptation. J. Exp. Biol. 35, 776–795. [Google Scholar]
- 85.Walters KFA, Dixon AFG. 1984. The effect of temperature and wind on the flight activity of cereal aphids. Ann. Appl. Biol. 104, 17–26. ( 10.1111/j.1744-7348.1984.tb05582.x) [DOI] [Google Scholar]
- 86.Fairbairn D, Desranleau L. 1987. Flight threshold, wing muscle histolysis, and alary polymorphism: correlated traits for dispersal tendency in the Gerridae. Ecol. Entomol. 12, 13–24. ( 10.1111/j.1365-2311.1987.tb00980.x) [DOI] [Google Scholar]
- 87.Hudson GV. 1909. General notes on the entomology of the southern islands of New Zealand. In The subantarctic islands of New Zealand (ed. Chilton C.), pp. 58–66. Wellington, New Zealand: Philosophical Institute of Canterbury. [Google Scholar]
- 88.Vernon P, Vannier G, Trehen P. 1998. A comparative approach to the entomological diversity of polar regions. Acta Oecologica 19, 303–308. ( 10.1016/S1146-609X(98)80034-9) [DOI] [Google Scholar]
- 89.Crafford JE, Scholtz CH, Chown SL. 1986. The insects of sub-Antarctic Marion and Prince Edward Islands; with a bibliography of entomology of the Kerguelen Biogeographical Province. S. Afr. J. Antarct. Res. 16, 42–84. [Google Scholar]
- 90.Bonan G. 2008. Ecological climatology: concepts and applications. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 91.le Roux P. 2008. Climate and climate change. In The Prince Edward Islands: land-sea interactions in a changing ecosystem (eds Chown SL, Froneman PW). Stellenbosch, South Africa: Sun Press. [Google Scholar]
- 92.Stopa JE, Cheung KF, Tolman HL, Chawla A. 2013. Patterns and cycles in the climate forecast system reanalysis wind and wave data. Ocean Model. 70, 207–220. ( 10.1016/j.ocemod.2012.10.005) [DOI] [Google Scholar]
- 93.L'Héritier P, Neefs Y, Teissier G. 1937. Aptérisme de insectes et sélection naturelle. Comptes rendus de l'Académie des Sciences 204, 907–909. [Google Scholar]
- 94.Lebouvier M, et al. 2011. The significance of the sub-Antarctic Kerguelen Islands for the assessment of the vulnerability of native communities to climate change, alien insect invasions and plant viruses. Biol. Invasions 13, 1195–1208. ( 10.1007/s10530-011-9946-5) [DOI] [Google Scholar]
- 95.McClelland GTW, Altwegg R, Van Aarde RJ, Ferreira S, Burger AE, Chown SL.. 2018. Climate change leads to increasing population density and impacts of a key island invader. Ecol. App. 28, 212–224. ( 10.1002/eap.1642) [DOI] [PubMed] [Google Scholar]
- 96.Davies K, Melbourne B, McClenahan J, Tuff T. 2011. Statistical models for monitoring and predicting effects of climate change and invasion on the free-living insects and a spider from sub-Antarctic Heard Island. Polar Biol. 34, 119–125. ( 10.1007/s00300-010-0865-1) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are provided at https://doi.org/10.26180/5d47d491c8e8a.