Abstract
Objective
The widespread use of opioid analgesics to treat chronic nonmalignant pain has contributed to the ongoing epidemic of opioid-related morbidity and mortality. Previous studies have also demonstrated a relationship between opioid analgesic use and unemployment due to disability. These studies have been limited to mainly white European and North American populations. The objective of this study is to explore the relationship between opioid analgesic use for chronic nonmalignant pain in an urban, mainly black and Hispanic, low-income population.
Design
This is a cross-sectional observational study.
Setting
Subjects were recruited from six urban primary care health centers.
Subjects
Adults with chronic neck, back, or osteoarthritis pain participating in an acupuncture trial were included.
Methods
Survey data were collected as a part of the Acupuncture Approaches to Decrease Disparities in Pain Treatment two-arm (AADDOPT-2) comparative effectiveness trial. Participants completed a baseline survey including employment status, opioid analgesic use, the Brief Pain Inventory, the global Patient Reported Outcomes Measurement Information Systems quality of life measure, the Patient Health Questionnaire-9 (PHQ-9), and demographic information. A multivariable logistic regression model was built to examine the association between opioid analgesic use and unemployment.
Results
Opioid analgesic use was associated with three times the odds of unemployment due to disability while controlling for potential confounders, including depression, pain severity, pain interference, global physical and mental functioning, and demographic characteristics.
Conclusions
This study adds to the growing body of evidence that opioid analgesics should be used with caution in chronic nonmalignant pain.
Keywords: Opioid, Narcotic, Chronic Pain, Osteoarthritis, Low Back Pain, Disability, Unemployment
Introduction
Chronic pain affects an estimated 15–30% of the population [1,2] and accounts for a large volume of outpatient visits [2–4]. The widespread use of prescription opioid analgesics to treat chronic nonmalignant pain has contributed to the ongoing epidemic of opioid-related morbidity and mortality [5–7]. Randomized controlled trials of opioids demonstrate good short-term effect on pain and function in patients with nonmalignant pain [8]. There has been longstanding debate about whether opioids are beneficial for chronic nonmalignant pain, particularly in terms of improved physical functioning [9,10]. Some studies have found that patients with chronic nonmalignant pain who respond well to opioid therapy also have improved functional status, at least in the short term [11,12]. Other studies have failed to find a positive effect on function even when analgesia is achieved [13–15]. Many of these studies had a short duration of follow-up.
Patients who use chronic opioid analgesics for nonmalignant pain have high levels of unemployment and depression and poor quality of life [16–19]. Observational studies have found that the use of opioid analgesics for low back pain is associated with chronic work loss, independently of pain severity, injury severity, and initial disability [20–22]. It is unclear whether opioid analgesic use is a marker of higher levels of pain, which are related to disability and unemployment, whether unemployment itself increases opioid analgesic use, and whether opioid analgesic use independently increases disability and unemployment rates. However, the high level of disability and unemployment in patients using chronic opioid analgesics raises concerns about the efficacy of opioids to improve functional status or return to work [18,19,23–25].
Racial and ethnic minority patients experience a higher prevalence of chronic pain [2], more disability related to pain [26], and less adequate treatment [27,28]. On the other hand, recent increases in opioid prescriptions have affected predominantly white communities [29], and the literature on the relationship between opioid analgesic use and unemployment has been explored mainly in Caucasian populations in the United States and Europe [16,17,19–21,30]. Physicians are less likely to prescribe opioids for racial and ethnic minority patients [31–33], and opioid prescribing is more common in rural areas [34–36]. It is unknown whether the association between opioid analgesic prescription and unemployment due to disability is also present in urban ethnic minority populations. The Acupuncture Approaches to Decrease Disparities in Pain Treatment (AADDOPT-2) two-arm comparative effectiveness trial presents an opportunity to explore this association in a population of urban, mainly ethnic and racial minority patients undergoing acupuncture treatment for chronic nonmalignant pain. We aimed to explore the relationship between opioid analgesic use and unemployment due to disability while controlling for the effects of pain severity, sociodemographic characteristics, and depression on this relationship.
Methods
Participants
This is a cross-sectional observational study of baseline data collected as a part of the AADDOPT-2 comparative effectiveness trial. AADDOPT-2 randomized 765 patients between March 2015 and August 2017 to assess whether acupuncture for chronic pain delivered in a group setting is as effective as individual acupuncture in a low-income, underserved, mainly black and Hispanic patient population at risk for health disparities. Patients were recruited from six urban primary care health centers. Eligible patients were at least 21 years old, had chronic pain lasting three or more months due to osteoarthritis, or chronic neck or back pain, were able to provide consent in English or Spanish, and were available for up to 24 weeks. Patients with pain due to cancer were excluded. The Albert Einstein College of Medicine Institutional Review Board (IRB) approved this study protocol (IRB No. 2014-4192).
Measures
The initial research interview was performed after consent to participate in the trial had been obtained but before randomization, three weeks or less before the first session. Interviews were conducted by phone by trained bilingual research assistants. The main outcome of interest for this analysis was employment status, which was collected by asking participants “What is your current working status?” Response options included working full-time, working part-time, unemployed, retired, unable to work due to disability, homemaker, other, and “don’t know.” This outcome was dichotomized as “unable to work due to disability” vs all other responses. Patients who reported that they were retired were excluded from the analysis.
The predictor variable, opioid medication use, was assessed using two questions. The first question was “Do you have a prescription from a health care provider for one or more of the following opioid (narcotic) pain medications?” The researcher then read the following options including both generic and trade names: codeine (Tylenol 3 or Tylenol 4), fentanyl (Duragesic), hydrocodone (Vicodin), oxycodone (Percocet, OxyContin), oxymorphone (Opana), propoxyphene (Darvon), hydromorphone (Dilaudid), meperidine (Demerol), methadone, morphine (Kadian, MS Contin), or “I do not have a prescription for any of these medications.” The second question was “During the past week, on how many days did you take one or more doses of your opioid pain medications?” Patients were categorized as opioid analgesic users if they reported use of any opioid medication for the first question AND they reported a nonzero number for the second question.
Sociodemographic information included age, gender, race, ethnicity, preferred language, household income, nationality, marital status, and number of dependent children. Participants were also asked whether they received household income support. Options listed by the interviewer included Welfare of General Public Assistance; WIC: Supplemental Food Program; Food Stamps; Unemployment Insurance; Housing Support; Child Support; SSI or SS retirement, Disability or Survivor’s Benefit; Payments for Providing Foster Care; No Income Support; or I Don’t Know. This was asked separately from the primary outcome measure of current working status. Level of education and health insurance status were also collected.
The initial interview consisted of several established measures with good reliability, all of which have been validated for use in Spanish-speaking populations. These included the Brief Pain Inventory: Short Form (BPI), which is the primary outcome measure for the parent trial [37]. It includes four pain scales, measuring “pain on average” during the past week, “pain at its worst” during the past week, “pain at its least” during the past week, and “pain right now.” It also includes a validated seven-item scale measuring the extent to which pain interferes with function, including activity, mood, sleep, work, and life enjoyment.
Quality of life was assessed with the 10-item global Patient Reported Outcomes Measurement Information Systems (PROMIS) [38]. Depressive symptoms were assessed with the Patient Health Questionnaire-9 (PHQ-9) measure [39].
Analysis
Associations between sociodemographic variables, pain, quality of life and depression measures, and unemployment due to disability were assessed using chi-square or Fisher exact tests for categorical variables and Student t tests for continuous variables. The α = 0.05 level of significance was used. To test the association between opioid analgesic use and unemployment due to disability, a multivariable logistic regression model was built with unemployment due to disability as the outcome. Variables associated with opioid analgesic use with a P value of α = 0.10 or less in the bivariate association analysis were also included in the model as covariates.
Results
Of the 765 patients who enrolled in the trial and completed baseline interviews, 150 patients were excluded because they reported being retired. An additional 11 patients were excluded due to missing data on opioid analgesic use. Participants who used opioid analgesics (N = 136) were more likely to be born in the United States (64.7% vs 46.8%, P = 0.002), to be male (25.0% vs 17.1%, P = 0.04), and to speak English as their primary language (84.3% vs 76.6%, P = 0.02). Opioid analgesic use was not associated with race or ethnicity (Table 1).
Table 1.
Associations between opioid pain medication use and sociodemographic characteristics (N = 604)
| Characteristic | Opioid Use (N = 136), No. (%) | No Opioid Use (N = 468), No. (%) | P Value |
|---|---|---|---|
| Age, mean (SD), y | 50.9 (9.7) | 51.1 (12.2) | 0.88 |
| Gender | |||
| Female | 102 (75.0) | 388 (82.9) | 0.04 |
| Male | 34 (25.0) | 80 (17.1) | |
| Education | |||
| ≤ High school | 75 (55.1) | 253 (54.1) | 0.82 |
| ≥ Some college | 61 (44.9) | 215 (45.9) | |
| Race | 0.83 | ||
| American Indian/Native | 8 (6.1) | 24 (5.3) | |
| Pacific Islander/Native Hawaiian | 0 (0.0) | 1 (0.2) | |
| Black/African American | 49 (37.4) | 150 (33.2) | |
| White | 19 (14.5) | 59 (13.1) | |
| Multiracial | 18 (13.7) | 60 (13.3) | |
| Asian | 2 (1.5) | 7 (1.5) | |
| Other | 35 (26.7) | 151 (33.4) | |
| Ethnicity | 0.26* | ||
| Hispanic or Latino | 76 (55.9) | 288 (61.7) | |
| Non-Hispanic | 60 (44.1) | 175 (37.5) | |
| Don't know | 0 (0.0) | 4 (0.9) | |
| Marital status | 0.25 | ||
| Married or living with partners | 33 (24.3) | 137 (29.3) | |
| Others | 103 (75.7) | 330 (70.7) | |
| Preferred language | 0.02* | ||
| English | 102 (84.3) | 351 (76.6) | |
| Spanish | 18 (14.9) | 107 (23.4) | |
| Other | 1 (0.8) | 0 (0.0) | |
| Born in the United States | <0.01 | ||
| No | 48 (35.3) | 249 (53.2) | |
| Yes | 88 (64.7) | 219 (46.8) |
Fisher exact test.
Participants who used opioid analgesics reported poorer function on the PROMIS global mental health measure (9.91 vs 11.66, P < 0.001). They also reported poorer global physical health (PROMIS score 8.98 vs 10.13, P < 0.001). Opioid analgesic use was associated with more symptoms of depression as measured by the PHQ-9 (11.31 vs 8.65, P < 0.001). Participants who used opioid analgesics reported greater pain interference (7.30 vs 6.07, P < 0.001) but not greater baseline pain severity (7.22 vs 6.89, P > 0.05) (Table 2).
Table 2.
Associations between opioid pain medication use and measures of pain severity, quality of life, and depression (N = 604)
| Characteristic | Opioid Use (N = 136), Mean (SD) | No Opioid Use (N = 468), Mean (SD) | P Value |
|---|---|---|---|
| Pain severity* | 7.22 (1.70) | 6.89 (1.89) | 0.0712 |
| Pain interference† | 7.30 (2.16) | 6.07 (2.73) | <0.0001 |
| PHQ-9 score‡ | 11.31 (5.99) | 8.65 (6.05) | <0.0001 |
| PHQ-9 categories, No. (%) | 0.0007 | ||
| 0–4 | 16 (11.9) | 132 (28.6) | |
| 5–9 | 43 (32.1) | 144 (31.2) | |
| 10–14 | 32 (23.9) | 93 (20.1) | |
| 15–19 | 30 (22.4) | 69 (14.9) | |
| ≥20 | 13 (9.7) | 24 (5.2) | |
| PROMIS global physical health§ | 8.98 (2.50) | 10.13 (2.81) | <0.0001 |
| PROMIS global mental health¶ | 9.91 (3.54) | 11.66 (3.86) | <0.0001 |
PHQ-9 = Patient Health Questionnaire-9; PROMIS = Patient Reported Outcomes Measurement Information Systems.
Scale of 0 = no pain to 10 = worst pain imaginable.
Scale of 0 = no interference to 10 = completely interferes.
Scale of 0–27, with higher scores indicating greater depression.
Answer to the question “In general, how would you rate your physical health?” with the following answer categories: 5 = excellent, 4 = very good, 3 = good, 2 = fair, and 1 = poor.
Answer to the question “In general, how would you rate your mental health, including your mood and your ability to think?” with the following answer categories: 5 = excellent, 4 = very good, 3 = good, 2 = fair, and 1 = poor.
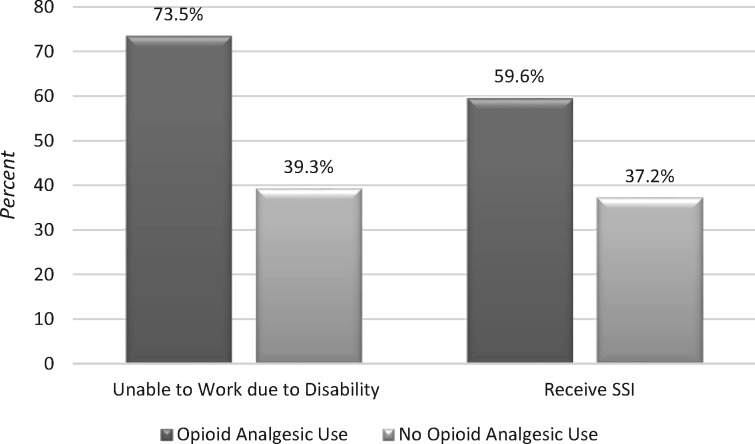
Participants who used opioid analgesics were more likely to be unable to work due to disability (73.5% vs 39.3%, P < 0.001) and to receive social security income (SSI/SSD; 59.6% v 37.2%, P < 0.001) (Figure 1). When controlling for potential confounders in multivariable analysis, patients who used opioid analgesics had three times the odds of being unable to work due to disability compared with nonusers (odds ratio = 3.080, P < 0.001) (Table 3).
Figure 1.
Association between opioid pain medication use and unemployment due to disability and receipt of social security insurance (SSI; P < 0.01 for both comparisons).
Table 3.
Logistic regression analysis of association between opioid pain medication use and unemployment due to disability (N = 604)
| Parameter | Estimate | SE | OR | 95% CI for the OR | P Value | |
|---|---|---|---|---|---|---|
| Intercept | −0.9518 | 1.2265 | 0.44 | |||
| Opioid medication use | 1.1249 | 0.2589 | 3.080 | 1.854 | 5.116 | <0.01 |
| Age | 0.0519 | 0.0102 | 1.053 | 1.032 | 1.075 | <0.01 |
| Gender | −0.1452 | 0.1356 | 0.748 | 0.440 | 1.273 | 0.28 |
| Pain severity | 0.0624 | 0.0739 | 1.064 | 0.921 | 1.230 | 0.40 |
| Pain interference | 0.0629 | 0.0570 | 1.065 | 0.952 | 1.191 | 0.27 |
| PHQ-9 score | 0.0184 | 0.0240 | 1.019 | 0.972 | 1.068 | 0.44 |
| Global physical health | −0.1828 | 0.0595 | 0.833 | 0.741 | 0.936 | <0.01 |
| Global mental health | −0.1199 | 0.0409 | 0.887 | 0.819 | 0.961 | <0.01 |
| Language* | 0.0776 | 0.2874 | 1.081 | 0.615 | 1.898 | 0.79 |
| Born in the United States | 0.0696 | 0.2442 | 1.072 | 0.664 | 1.730 | 0.79 |
CI = confidence interval; OR = odds ratio; PHQ-9 = Patient Health Questionnaire-9.
English vs Spanish and other.
Discussion
In this population of low-income, primarily racial and ethnic minority chronic nonmalignant pain patients in an urban setting, opioid analgesic use was associated with three times the odds of unemployment due to disability. Eriksen et al. found a comparable magnitude association in a cross-sectional study comparing 228 chronic pain patients on opioid medication with 1,678 similar pain patients not on medication in Denmark; the odds ratio for employment was 0.37 in that study [19]. This finding suggests that this association is robust, even in populations that may have less access to opioid analgesics overall.
Previous studies have suggested that depression is an important mediator of the relationship between pain, opioid analgesic use, and poor functional status [40], and pain intensity and depression are independently associated with unemployment in chronic pain patients [30]. However, in this population, even when controlling for pain intensity, pain interference, depression, and global mental and physical health, the association between opioid analgesic use and unemployment remained large and significant.
The main limitation of this study is that it is a cross-sectional observational study. It was not possible to determine the direction of hypothesized causal relationships. For example, unemployment itself may lead to increased opioid analgesic use if patients are less concerned about interference of medication with work activities or if unemployment leads to greater rumination on pain symptoms [35]. This is likely to explain the larger association found in this study compared with prospective studies that link opioid prescribing and future disability and eliminate the possibility of reverse causality [21]. In our study, the temporal relationship between opioid analgesic prescription and unemployment could not be established. Given the cross-sectional nature of the current study and the lower odds ratio of ∼2 reported in the prospective study by Franklin et al. [21], our reported odds ratio of 3 is likely an overestimation of the true relationship. However, although increasing unemployment is associated with increases in opioid prescription rates, these associations are small and unlikely to fully explain the large associations found in both cross-sectional and prospective studies [41]. Another limitation is that there may be misclassification of the outcome of disability. Specifically, about 19% of patients excluded from this study because they identified as “retired” were under 65 years of age. Some of these patients may have, in fact, chosen to retire due to disability. However, this is unlikely to change the overall association between opioid analgesic use and disability.
As in other observational studies, the possibility exists that opioid analgesic use is a consequence of increased pain severity, and that pain severity is actually the proximate cause of disability and unemployment. However, in this population, there was a small and nonsignificant difference in pain severity between opioid analgesic users and nonusers, and pain intensity was not associated with unemployment due to disability in the multivariable analysis. The possibility remains that pain severity would be greater in opioid analgesic users in the absence of these medications; however, mitigating the pain intensity does not appear to increase the likelihood of return to work.
These findings are troublesome given the high prevalence of opioid prescribing among disabled Medicare and Medicaid beneficiaries [42,43]. Future large-scale prospective studies that follow patients from the initiation of opioid analgesic use should be undertaken to evaluate long-term functional and employment outcomes over time. Nonetheless, this study adds to the growing body of evidence that opioid analgesics should be used with caution in chronic nonmalignant pain.
Funding sources: This work was supported by the Patient-Centered Outcomes Research Institute (AD-1402-10857) and the New York State Empire Clinical Research Investigator Program.
Disclosure and conflicts of interest: None of the authors has competing financial interests.
References
- 1. Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: Results of an Internet-based survey. J Pain Society 2010;11(11):1230–9. [DOI] [PubMed] [Google Scholar]
- 2. Riskowski JL. Associations of socioeconomic position and pain prevalence in the United States: Findings from the National Health and Nutrition Examination Survey. Pain Med 2014;15(9):1508–21. [DOI] [PubMed] [Google Scholar]
- 3. Park PW, Dryer RD, Hegeman-Dingle R, et al. Cost burden of chronic pain patients in a large integrated delivery system in the United States. Pain Pract 2016;16(8):1001–11. [DOI] [PubMed] [Google Scholar]
- 4. Upshur CC, Luckmann RS, Savageau JA. Primary care provider concerns about management of chronic pain in community clinic populations. J Gen Intern Med 2006;21(6):652–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep 2016;64(50–51):1378–82. [DOI] [PubMed] [Google Scholar]
- 6. Compton WM, Boyle M, Wargo E. Prescription opioid abuse: Problems and responses. Prev Med 2015;80:5–9. [DOI] [PubMed] [Google Scholar]
- 7. Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: Promises and perils. Pain 2013;154(suppl 1):S94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kalso E. Opioids for persistent non-cancer pain. BMJ 2005;330(7484):156–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med 2003;349(20):1943–53. [DOI] [PubMed] [Google Scholar]
- 10. Abdel Shaheed C, Maher CG, Williams KA, Day R, McLachlan AJ. Efficacy, tolerability, and dose-dependent effects of opioid analgesics for low back pain: A systematic review and meta-analysis. JAMA Intern Med 2016;176(7):958–68. [DOI] [PubMed] [Google Scholar]
- 11. Zenz M, Strumpf M, Tryba M. Long-term oral opioid therapy in patients with chronic nonmalignant pain. J Pain Symptom Manage 1992;7(2):69–77. [DOI] [PubMed] [Google Scholar]
- 12. Simpson RK, Edmondson EA, Constant CF, Collier C. Transdermal fentanyl as treatment for chronic low back pain. J Pain Symptom Manage 1997;14(4):218–24. [DOI] [PubMed] [Google Scholar]
- 13. Moulin DE, Amireh R, Sharpe WKJ, et al. Randomised trial of oral morphine for chronic non-cancer pain. Lancet 1996;347(8995):143–7. [DOI] [PubMed] [Google Scholar]
- 14. Caldwell JR, Rapoport RJ, Davis JC, et al. Efficacy and safety of a once-daily morphine formulation in chronic, moderate-to-severe osteoarthritis pain: Results from a randomized, placebo-controlled, double-blind trial and an open-label extension trial. J Pain Symptom Manage 2002;23(4):278–91. [DOI] [PubMed] [Google Scholar]
- 15. Ashworth J, Green DJ, Dunn KM, Jordan KP. Opioid use among low back pain patients in primary care: Is opioid prescription associated with disability at 6-month follow-up? Pain 2013;154(7):1038–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Campbell G, Nielsen S, Bruno R, et al. The Pain and Opioids IN Treatment study: Characteristics of a cohort using opioids to manage chronic non-cancer pain. Pain 2015;156(2):231–42. [DOI] [PubMed] [Google Scholar]
- 17. Svendsen K, Fredheim OM, Romundstad P, Borchgrevink PC, Skurtveit S. Persistent opioid use and socio-economic factors: A population-based study in Norway. Acta Anaesthesiol Scand 2014;58(4):437–45. [DOI] [PubMed] [Google Scholar]
- 18. Busse JW, Mahmood H, Maqbool B, et al. Characteristics of patients receiving long-term opioid therapy for chronic noncancer pain: A cross-sectional survey of patients attending the Pain Management Centre at Hamilton General Hospital, Hamilton, Ontario. CMAJ Open 2015;3(3):E324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eriksen J, Sjogren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non-cancer pain: An epidemiological study. Pain 2006;125(1–2):172–9. [DOI] [PubMed] [Google Scholar]
- 20. Volinn E, Fargo JD, Fine PG. Opioid therapy for nonspecific low back pain and the outcome of chronic work loss. Pain 2009;142(3):194–201. [DOI] [PubMed] [Google Scholar]
- 21. Franklin GM, Stover BD, Turner JA, Fulton-Kehoe D, Wickizer TM. Early opioid prescription and subsequent disability among workers with back injuries: The Disability Risk Identification Study Cohort. Spine 2008;33(2):199–204. [DOI] [PubMed] [Google Scholar]
- 22. Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine 2007;32(19):2127–32. [DOI] [PubMed] [Google Scholar]
- 23. Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. BMJ 2015;350:g6380.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sites BD, Beach ML, Davis MA. Increases in the use of prescription opioid analgesics and the lack of improvement in disability metrics among users. Reg Anesth Pain Med 2014;39(1):6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morden NE, Munson JC, Colla CH, et al. Prescription opioid use among disabled Medicare beneficiaries: Intensity, trends, and regional variation. Med Care 2014;52(9):852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Green CR, Hart-Johnson T. The association between race and neighborhood socioeconomic status in younger black and white adults with chronic pain. J Pain 2012;13(2):176–86. [DOI] [PubMed] [Google Scholar]
- 27. Green CR, Hart-Johnson T. The adequacy of chronic pain management prior to presenting at a tertiary care pain center: The role of patient socio-demographic characteristics. J Pain 2010;11(8):746–54. [DOI] [PubMed] [Google Scholar]
- 28. Chen I, Kurz J, Pasanen M, et al. Racial differences in opioid use for chronic nonmalignant pain. J Gen Intern Med 2005;20(7):593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuo YF, Raji MA, Chen NW, Hasan H, Goodwin JS. Trends in opioid prescriptions among Part D Medicare recipients from 2007 to 2012. Am J Med 2016;129(2):221.e21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Giladi H, Scott W, Shir Y, Sullivan MJ. Rates and correlates of unemployment across four common chronic pain diagnostic categories. J Occup Rehabil 2015;25(3):648–57. [DOI] [PubMed] [Google Scholar]
- 31. Joynt M, Train MK, Robbins BW, et al. The impact of neighborhood socioeconomic status and race on the prescribing of opioids in emergency departments throughout the United States. J Gen Intern Med 2013;28(12):1604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dominick KL, Bosworth HB, Dudley TK, et al. Patterns of opioid analgesic prescription among patients with osteoarthritis. J Pain Palliat Care Pharmacother 2004;18(1):31–46. [PubMed] [Google Scholar]
- 33. Ringwalt C, Roberts AW, Gugelmann H, Skinner AC. Racial disparities across provider specialties in opioid prescriptions dispensed to Medicaid beneficiaries with chronic noncancer pain. Pain Med 2015;16(4):633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prunuske JP, St Hill CA, Hager KD, et al. Opioid prescribing patterns for non-malignant chronic pain for rural versus non-rural US adults: A population-based study using 2010 NAMCS data. BMC Health Serv Res 2014;14(1):563.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou C, Yu NN, Losby JL. The association between local economic conditions and opioid prescriptions among disabled Medicare beneficiaries. Med Care 2018;56(1):62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heins SE, Sorbero MJ, Jones CM, Dick AW, Stein BD. High-risk prescribing to Medicaid enrollees receiving opioid analgesics: Individual- and county-level factors. Subst Use Misuse 2018;53(10):1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Badia X, Muriel C, Gracia A, et al. Validation of the Spanish version of the Brief Pain Inventory in patients with oncological pain [in Spanish]. Med Clin 2003;120(2):52–9. [DOI] [PubMed] [Google Scholar]
- 38. Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the Patient-Reported Outcomes Measurement Information System (PROMIS) global items. Qual Life Res 2009;18(7):873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Valkanoff TA, Kline-Simon AH, Sterling S, Campbell C, Von Korff M. Functional disability among chronic pain patients receiving long-term opioid treatment. J Soc Work Disabil Rehabil 2012;11(2):128–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kozman D, Graziul C, Gibbons R, Alexander GC. Association between unemployment rates and prescription drug utilization in the United States, 2007-2010. BMC Health Serv Res 2012;12(1):435.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meara E, Horwitz JR, Powell W, et al. State legal restrictions and prescription-opioid use among disabled adults. N Engl J Med 2016;375(1):44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guy GP Jr, Zhang K, Bohm MK, et al. Vital signs: Changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep 2017;66(26):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]