Abstract
Background
Acute alcohol triggers release of cytokines, which are immune signaling molecules. Dysregulated cytokine levels are associated with impaired immune function, and peripheral cytokine levels may communicate with the brain to propagate drinking-related behaviors. This exploratory study aims to characterize the peripheral cytokine response to an alcohol challenge in a well-controlled laboratory setting.
Methods
Moderate alcohol drinkers (n=17), abstinent for >5 days, consumed alcohol calibrated to achieve blood concentrations of 120 mg/dL. Serum cytokine levels (IL-6, IL-8, IL-12, IFN-γ, TNF-α) were measured prior to drinking, 6 hours after drinking, and 24 hours after drinking. Linear mixed models evaluated within-subject differences in cytokine levels over time.
Results
The pro-inflammatory chemokine IL-8 significantly increased 6 hours after alcohol (F(1,34)=4.13, p=0.0002, d’=0.5). In contrast, the pro-inflammatory cytokine TNF-α significantly decreased 6 hours after alcohol (F(1,34)=−3.07, p=0.004, d’=0.3). No cytokines were significantly different from baseline 24 hours after alcohol.
Conclusions
In our exploratory data, acute alcohol challenge (120 mg/dL) elicits dynamic changes in the pro-inflammatory molecules IL-8 and TNF-α. The findings help inform the temporal profile of cytokine response to alcohol, and identify IL-8 as a cytokine of interest for future studies of periphery-brain immune communication.
Keywords: Acute alcohol, cytokines, inflammation, craving
1. Introduction
Alcohol exposure triggers a constellation of immune responses. A hallmark of the innate immune response to alcohol is release of cytokines(Pascual et al., 2018). Cytokine release recruits immune cells to clear or contain an immune stimulus, helping to restore homeostasis. Dysregulated cytokine signaling can lead to immune related pathologies, such as liver cirrhosis or pancreatitis in cases of chronic alcohol use(Szabo and Saha, 2015). Numerous peripheral cytokines are altered in alcohol use disorder(Achur et al., 2010). Mounting evidence also suggests that peripheral cytokines communicate with CNS immune signaling(de Timary et al., 2017). Increased brain immune activity can contribute to ‘sickness behaviors’ such as fatigue, irritability, anhedonia, social withdrawal(Dantzer et al., 2008), symptoms that map on to dimensions of alcohol use disorder. Moreover, increased cytokine levels in rodent brain escalate alcohol drinking behaviors(Blednov et al., 2012, Marshall et al., 2017) and positively correlate with alcohol craving in alcohol dependent people(Leclercq et al., 2012). Therefore, the peripheral cytokine response to alcohol likely influences alcohol’s addiction cycle and negative health consequences of alcohol use.
The temporal profile of cytokine response to binge alcohol drinking in people is only partially characterized. In healthy people, 120–130 mg/dL alcohol decreased pro-inflammatory IL-1β 2–5 hours after alcohol drinking, while increasing anti-inflammatory IL-1RA 20 min-2 hours after alcohol(Neupane et al., 2016, Afshar et al., 2015). In healthy men (n=20), the pro-inflammatory cytokine MCP-1 initially decreases from baseline 2 h after 120 mg/dL alcohol but increases from baseline 12 h after alcohol(Neupane et al., 2016). In contrast, in healthy volunteers (n=15), pro-inflammatory TNF-α and anti-inflammatory IL-10 were not significantly different from baseline up to 5 hours post-drink (130 mg/dL) (Afshar et al., 2015). A small study (n=5) also indicated increased chemokine IL-8 36 hours after 60 g alcohol(Gonzalez-Quintela et al., 2000). Interestingly, in healthy volunteers (n=25) binge alcohol also increased serum endotoxin (or lipopolysaccharide, LPS), a robust immune activator, 0.5–3.0 hours after drinking to 80 mg/dL(Bala et al., 2014), possibly due to increased gut permeability(Leclercq et al., 2014). A less-controlled study examining a full cytokine panel in serum of adolescents hospitalized with alcohol intoxication revealed an interesting profile of numerous cytokines increased in females but not males(Pascual et al., 2016), however blood alcohol levels (BAL) were not reported. Yet, these studies do not capture a full cytokine profile. The goal of this exploratory study was to build on these initial studies by analyzing levels of 5 important cytokines measured during a previously published study featuring well-controlled alcohol challenge (O’Malley at al., 2015). Secondary goals explored relationships of cytokine levels with alcohol craving, motivated by previous findings in abstinent people with alcohol use disorder(Leclercq et al., 2012), and relationships of cytokine levels with bilirubin, based on its role as an antioxidant. The findings extend our understanding of innate immune responses to binge alcohol drinking.
2. Materials and Methods
2.1. Subjects
Data were analyzed from a previously described study(O’Malley et al., 2015). To summarize previously reported subject characteristics, participants were 18 healthy individuals, including 8 smokers (6 men, 2 women) and 10 nonsmokers (5 men, 5 women). Subjects were 33.1±10.5 years old with BMI of 27.6±4.4 kg/m. Cytokine data were not collected for one male nonsmoker. Participants reported a day of drinking sufficient to reach an estimated BAL of ≥100 mg/dL in the past 6 months, but did not meet DSM-IV criteria for alcohol dependence assessed with the SCID. Exclusion criteria included all primary DSM-IV diagnoses, significant medical conditions during the study period, evidence of hepatocellular injury or history of liver disease, and women who were pregnant or nursing. Subjects were instructed to not consume alcohol or use ethanol containing products or foods for at least 5 days prior to and following each session. Written informed consent was obtained, and all procedures were approved by the Human Investigation Committee at Yale University.
2.2. Study Procedures: Alcohol Challenge
The alcohol challenge session was conducted at the Hospital Research Unit of the Yale Center for Clinical Investigation. Participants were instructed to fast after midnight prior to study day. On study day following a light standardized breakfast, subjects received an alcohol dose calculated to achieve BAL of 120 mg/dL using an established fomula(Curtin and Fairchild, 2003). Alcohol was administered as 80-proof vodka with a nonalcoholic mixer (diet cranberry drink with Sucralose) at a 1:3 alcohol:mixer ratio. The total volume was divided into 6 equal drinks, each consumed over a 15-min period. Although other alcohol doses were included in the primary study(O’Malley et al., 2015), cytokine measurements were only made for most subjects at the 120 mg/dL dose.
2.3. Study Procedures: Blood Samples
Blood samples and breath alcohol concentrations (BrAC) were collected on a 30 min basis, beginning 1 hour after initiation of alcohol challenge and ending with negative BrAC, to measure blood alcohol concentration with gas chromatography techniques. Serum samples acquired to measure bilirubin and cytokine concentrations were obtained at the following three timepoints: baseline (15 min before initiation of alcohol challenge); 6 and 24 hours after initiation of alcohol challenge. Serum samples were stored at −80° C for 5–35 months. Total and direct serum bilirubin levels were measured with an FDA approved diazo procedure(Jendrassik, 1938) on the Roche DPP Modular automated chemistry analyzer. Cytokines were analyzed by a multiplexed immunoassay. Meso Scale Discovery® (Meso Scale Diagnostics LLC, 1601 Reseach Boulevard, Rockville, MD 20850) was the source of instrumentation and reagent kits including antibodies and standards. High and low cytokine controls were obtained from Randox Laboratories Ltd.55 Diamond Road,Crumlin,County Antrim,BT29 4QY,United Kingdom.
The following cytokines were measured: IFN-γ, IL-6, IL-8, IL-10, IL-12 p70, and TNF-α. Measurements of IL-10 were not analyzed statistically because ~25% of measurements were below detectable levels.
2.4. Study Procedures: Craving
The Drug Effects Questionnaire (Morean et al., 2012) was administered at 90, 120, and 150 min after initiation of alcohol challenge (0, 30 and 60 min after the end of alcohol consumption). This measurement was only available for 13 of the 18 alcohol challenge sessions. The final visual analog scale measure, ‘Would you like MORE of what you consumed, right now?’, was used as a measure of alcohol craving for exploratory analyses investigating relationships between cytokine levels and craving.
2.5. Statistical Analysis
Cytokine data were log-transformed to better approximate a normal distribution. For each cytokine, a mixed effects model was constructed with time (0=baseline, 1=6h, 2=24h) as a within-subject factor and smoking status and sex as between subject factors. The best-fitting correlation structure was selected using the Bayes Information Criterion. Main effects and interaction effects were considered significant with α<0.01 (Bonferroni correction with m=5).
A first exploratory analysis investigated relationships between reported craving for alcohol and serum IL-8 and TNF-α levels. These cytokines were chosen because they were significantly altered by alcohol challenge. Non-parametric Spearman correlations tested for evidence of relationships between IL-8 or TNF-α levels measured 6 hours after alcohol challenge and craving measured after alcohol consumption. To control for multiple comparisons, a Bonferroni-correction was introduced for testing 2 cytokines at 3 timepoints, therefore findings with α<0.008 were considered statistically significant. A second exploratory analysis inspected for relationships between cytokine levels and the antioxidant bilirubin. Finally, a third exploratory analysis examined for relationships between cytokine levels and peak BAL. Non-parametric Spearman correlations using all data pooled across timepoints tested for evidence of relationships between cytokine and total bilirubin levels.
3. Results
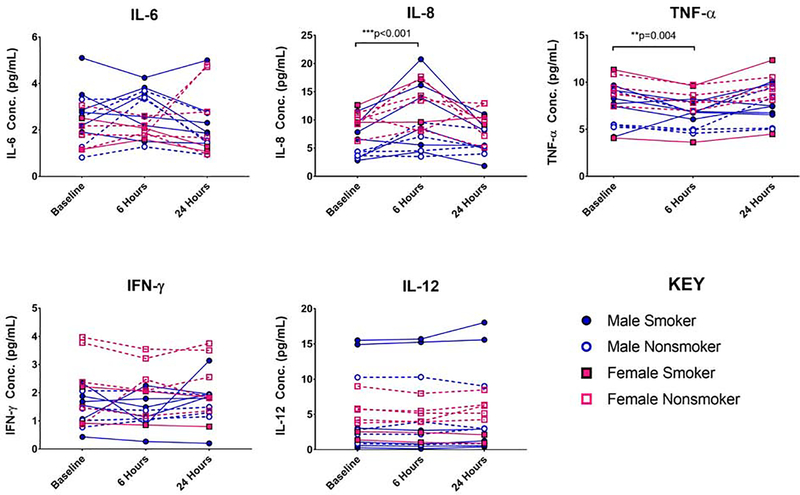
Significant main effects of time were detected for IL-8 (F(2,34)=10.33, p=0.0003) and TNF-α (F(2,34)=10.42, p=0.0003). Pairwise comparisons revealed that IL-8 concentrations increased in 14 of 17 subjects (F(1,34)=4.13, p=0.0002, d’=0.5) by 3.3±3.6 pg/mL 6 hours after initiating alcohol challenge. IL-8 concentrations were not significantly different between baseline and 24 hours after challenge (F(1,34)=0.419, p=0.678). In contrast, TNF-α concentrations decreased in 15 of 17 subjects (F(1,34)=−3.07, p=0.004, d’=0.3) by 1.1±0.8 pg/mL 6 hours after initiating alcohol challenge. TNF-α concentrations were not significantly different between baseline and 24 hours after challenge (F(1,34)=1.39 p=0.173). The effects of an acute alcohol challenge on all cytokine levels are summarized by Figure 1.
Figure 1:
Time course of cytokine concentrations at baseline, 6 hours after 120 mg/dL alcohol, and 24 hours after 120 mg/dL. Cytokines depicted include interleukin-6 (IL-6), interleukin-8 (IL-8), Tissue Necrosis Factor-α (TNF-α), Interferon gamma (IFN-γ), and interleukin-12 (IL-12). Male smokers indicated by closed blue symbols; male nonsmokers indicated by blue open symbols; female smokers indicated by closed red symbols; female nonsmokers indicated by red open symbols. ** indicates statistically significant post-hoc pairwise comparisons with p<0.01. *** indicates statistically significant post-hoc pairwise comparisons with p<0.001.
There was no evidence for statistically significant effects of smoking status or sex. There was a non-significant trend for a main effect of smoking status in TNF-α concentrations (F(1,17)= 1.93, p=0.07). To illustrate this trend, at baseline TNF-α concentrations in nonsmokers of 7.0±1.9 pg/mL were lower (not statistically significant) than smoker levels of 8.7±2.3 pg/mL (see Figure 1 Effect of Smoking on TNF-α panel).
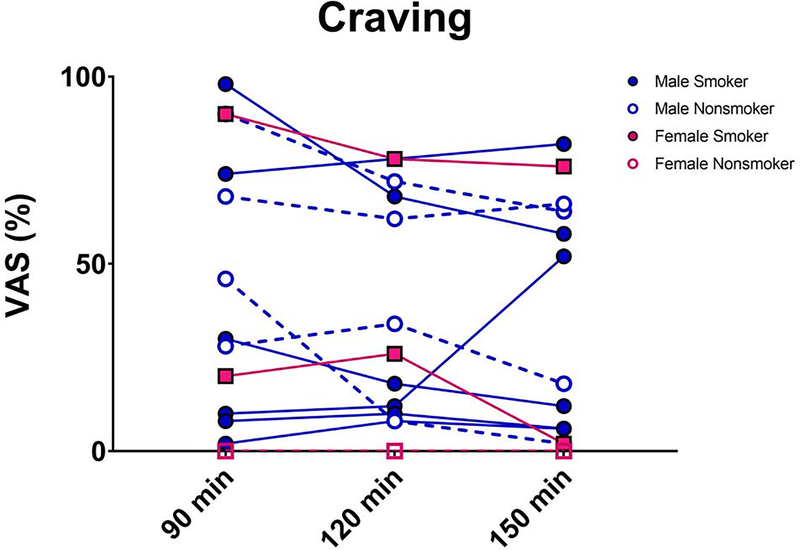
The time course of craving for alcohol is shown in Figure 2. No significant findings were observed between cytokine levels and craving. There was a trend (α<0.008) for a positive relationship between craving measured immediately after end of alcohol and IL-8 levels measured 6 hours after end of alcohol (ρ=0.60; p=0.03). No significant findings were observed between total bilirubin levels and cytokine levels. There was a trend (α<0.008) for a negative association between total bilirubin and IL-8 concentrations (ρ=−0.29; p=0.04). There was no evidence for relationships between peak BAL and cytokine concentrations.
Figure 2:
Time course of craving for more alcohol (Visual Analog Scale). Male smokers indicated by closed blue symbols; male nonsmokers indicated by blue open symbols; female smokers indicated by closed red symbols; female nonsmokers indicated by red open symbols.
4. Discussion
These exploratory data show that acute alcohol (120 mg/dL) elicits dynamic changes in the pro-inflammatory molecules IL-8 and TNF-α. Notably, all cytokine levels were not significantly different from baseline 24 hours after challenge, indicating that the peripheral cytokine response abates by this time. The IL-8 chemokine plays a prominent role in neutrophil recruitment. Our finding of increased IL-8 levels 6 h after alcohol challenge somewhat corroborate a previous report of IL-8 sensitivity to 60 g alcohol(Gonzalez-Quintela et al., 2000), however, that preliminary study (n=5) measured serum 36 h after ingestion, whereas we show that IL-8 that return baseline by 24 h. Interestingly, IL-8 mRNA levels were positively correlated with alcohol craving in alcohol dependent subjects 2 days into detoxification(Leclercq et al., 2014). Motivated by this finding, exploratory analyses on our data similarly suggest evidence for a positive relationship between craving immediately after the alcohol challenge and IL-8 levels 6 hours later. Given the neurocircuitry underpinnings of craving, this elevates interest for future research into IL-8 as a mediator of peripheral-brain immune communication in the context of alcohol’s addiction cycle.
The pro-inflammatory TNF-α cytokine activates key inflammatory transcription pathways of NF-κB and MAPK. TNF-α levels were significantly lower than baseline 6 hours after alcohol challenge, returning to baseline levels after 24 hours. This finding extends previous report of no change in TNF-α up to 5 h after alcohol(Afshar et al., 2015), suggesting that TNF-α may require extended time for the dynamic response. While the present study examined cytokine responses to acute alcohol in healthy volunteers, both IL-8 and TNF-α have well-known associations with alcohol-related liver disease(Hill et al., 1993, Huang et al., 1996, McClain et al., 2002). Therefore, the reported findings are consistent with existing research broadly implicating IL-8 and TNF-α in various contexts of human alcohol use.
Secondary analyses investigated possible roles of sex and smoking status in the cytokine response to alcohol. Although previous reports demonstrated a robust sex effect(Pascual et al., 2016), we did not observe evidence of this effect in our sample, possibly due to the small sample size of women (n=7). Given prominent sex differences in immune function(Eagon, 2010), this remains a critical consideration for future work. Similarly, we did not find significant effects of tobacco smoking, although there was some preliminary evidence for a trend of higher TNF-α levels in tobacco smokers across all samples. This is consistent with previous reports of greater TNF-α levels in tobacco smokers(Petrescu et al., 2010). Thus, both sex and smoking status are important considerations when evaluating immune responses to alcohol.
These cytokine data were acquired as part of a larger study measuring dose-dependent responses of bilirubin to alcohol challenge, where bilirubin increased from baseline 24 h after alcohol challenge in smokers but not nonsmokers across all alcohol doses(O’Malley et al., 2015). Bilirubin is an antioxidant that may explain some favorable health outcomes reported to occur with light alcohol consumption. Interestingly, we found a trend for negative association between IL-8 levels and total bilirubin. This echoes a similar qualitative relationship previously reported(Gonzalez-Quintela et al., 2007), and is consistent with antioxidant effects of bilirubin, since inflammatory responses are associated with increased oxidative species production.
Limitations of this work include the modest sample size, making the results exploratory rather than conclusive due to the limited statistical power. This is particularly important for investigating the effects of sex and smoking status on the cytokine response to alcohol, as our power to fully explore these effects is limited to large effects (f=0.8 given 80% power) with the current sample size. Additionally, multiplex cytokine assays favor within-subject comparisons over across-subject comparisons due to both subject variation in immune state at time of measurement (such as recent cold/sickness or anti-inflammatory drug use). This lends greater confidence to the reported within subject changes in cytokine level following alcohol challenge, but also gives more cautionary weight to the trends of effects of tobacco smoking on cytokine levels and relationship between I L-8 and bilirubin.
6. Conclusion
This work presents preliminary evidence that an acute alcohol challenge (120 mg/dL) elicits elevated IL-8 levels 6 hours after alcohol, while TNF-α levels are decreased at this time. Both cytokines return to baseline levels within 24 hours. The findings help characterize the temporal profile of cytokine response to alcohol, and identify IL-8 as a cytokine of interest for future studies of periphery-brain immune communication.
Highlights.
In a controlled human study, cytokine levels were measured 6 hours and 24 hour after subjects consumed 120 mg/dL alcohol
The pro-inflammatory chemokine IL-8 significantly increased 6 hours after drinking.
The pro-inflammatory cytokine TNF-α significantly decreased 6 hours after drinking.
A trend for positive relationship between IL-8 levels and craving for alcohol was observed.
Acknowledgments
Funding Sources:
This study was supported by a Pilot Grant from the Yale Comprehensive Cancer Center, grants from the National Institutes of Health (R01AA018665; P50AA12870; K01AA024788; K05AA014715; CTSA Grant number UL1 RR024139), and the State of Connecticut, Department of Mental Health and Addiction Services (DMHAS).
Role of the Funding Source
This study was supported by a Pilot Grant from the Yale Comprehensive Cancer Center, grants from the National Institutes of Health (R01AA018665; P50AA12870; K01AA024788; K05AA014715; CTSA Grant number UL1 RR024139), and the State of Connecticut, Department of Mental Health and Addiction Services (DMHAS). The funding sources had no further role in study design; collection, analysis or interpretation of the data; in the writing of the paper or the decision to publish. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism or the National Institutes of Health or DMHAS.
Footnotes
Conflict of Interest
Dr. O’Malley has served as a consultant to or advisory board member for Pfizer, Alkermes, Arkeo Pharmaceuticals, and the Hazelden Foundation; she is a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which is supported by Abbott Laboratories, Eli Lilly & Company, Lundbeck, Pfizer and Ethypharm; and she has received study supplies from Pfizer and a contract from Eli Lilly as a study site for a multi-site trial. None of these activities are related to the current report. No other authors report potential conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achur RN, Freeman WM, Vrana KE (2010) Circulating Cytokines as Biomarkers of Alcohol Abuse and Alcoholism. J. Neuroimmune Pharmacol 5:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshar M, Richards S, Mann D, Cross A, Smith GB, Netzer G, Kovacs E, Hasday J (2015) Acute immunomodulatory effects of binge alcohol ingestion. Alcohol 49:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala S, Marcos M, Gattu A, Catalano D, Szabo G (2014) Acute Binge Drinking Increases Serum Endotoxin and Bacterial DNA Levels in Healthy Individuals. PLoS ONE 9:e96864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA (2012) Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict. Biol 17:108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JJ, Fairchild BA (2003) Alcohol and cognitive control: implications for regulation of behavior during response conflict. J. Abnorm. Psychol 112:424–436. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci 9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Timary P, Starkel P, Delzenne NM, Leclercq S (2017) A role for the peripheral immune system in the development of alcohol use disorders? Neuropharmacology 122:148–160. [DOI] [PubMed] [Google Scholar]
- Eagon PK (2010) Alcoholic liver injury: Influence of gender and hormones. World J. Gastroenterol 16:1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Quintela A, Campos J, Gude F, Perez L-F, Tomé S (2007) Serum concentrations of interleukin-8 in relation to different levels of alcohol consumption. Cytokine 38:54–60. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Quintela A, Dominguez-Santalla M, Perez L, Vidal C, Lojo S, Barrio E (2000) Influence of acute alcohol intake and alcohol withdrawal on circulating levels of IL-6, IL-8, IL-10 and IL-12. Cytokine 12:1437–1440. [DOI] [PubMed] [Google Scholar]
- Hill DL, Marsano LS, McClain CJ (1993) Increased plasma interleukin-8 concentrations in alcoholic hepatitis. Hepatology 18:576–580. [PubMed] [Google Scholar]
- Huang Y-S, Chan C-Y, Wu J-C, Pai C-H, Chao Y, Lee S-D (1996) Serum levels of interleukin-8 in alcoholic liver disease: relationship with disease stage, biochemical parameters and survival. J. Hepatol 24:377–384. [DOI] [PubMed] [Google Scholar]
- Jendrassik L (1938) Vereinfachte photometrische methoden zur bestimmung des blutbilirubins. Biochem Z 297:81–89. [Google Scholar]
- Leclercq S, Cani PD, Neyrinck AM, Starkel P, Jamar F, Mikolajczak M, Delzenne NM, de Timary P (2012) Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav. Immunity 26:911–918. [DOI] [PubMed] [Google Scholar]
- Leclercq S, De Saeger C, Delzenne N, de Timary P, Starkel P (2014) Role of inflammatory pathways, blood mononuclear cells, and gut-derived bacterial products in alcohol dependence. Biol. Psychiatry 76:725–733. [DOI] [PubMed] [Google Scholar]
- Sa Marshall , McKnight KH, Blose AK, Lysle DT, Thiele TE (2017) Modulation of Binge-like Ethanol Consumption by IL-10 Signaling in the Basolateral Amygdala. J. Neuroimmune Pharmacol 12:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain CJ, Hill DB, Song Z, Deaciuc I, Barve S (2002) Monocyte activation in alcoholic liver disease. Alcohol 27:53–61. [DOI] [PubMed] [Google Scholar]
- Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, O’Malley SS (2012) The drug effects questionnaire: psychometric support across three druge types. Psychopharmacol. 227:177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupane SP, Skulberg A, Skulberg KR, Aass HCD, Bramness JG (2016) Cytokine Changes following Acute Ethanol Intoxication in Healthy Men: A Crossover Study. Mediators Inflamm. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley SS, Gueorguieva R, Wu R, Jatlow PI (2015) Acute alcohol consumption elevates serum bilirubin: an endogenous antioxidant. Drug Alcohol Depend. 149:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Montesinos J, Guerri C (2018) Role of the innate immune system in the neuropathological consequences induced by adolescent binge drinking. J. Neurosci. Res 96:765–780. [DOI] [PubMed] [Google Scholar]
- Pascual M, Montesinos J, Marcos M, Torres JL, Costa-Alba P, García-García F, Laso FJ, Guerri C (2016) Gender differences in the inflammatory cytokine and chemokine profiles induced by binge ethanol drinking in adolescence. Addict. Biol 22:1829–1841. [DOI] [PubMed] [Google Scholar]
- Petrescu F, Voican SC, Silosi I (2010) Tumor necrosis factor-α serum levels in healthy smokers and nonsmokers. Int. J. Chron. Obstruct. Pulmon. Dis 5:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Saha B (2015) Alcohol’s Effect on Host Defense. Alcohol Res. Curr. Rev 37:159–170. [PMC free article] [PubMed] [Google Scholar]