Abstract
Background/Aims:
We aimed to evaluate the distribution of abnormal liver-related biomarkers in patients with coronavirus disease (COVID-19) and explore the prognostic value of elevated liver enzymes and abnormal liver synthetic capacity with regards to patient mortality.
Patients and Methods:
This retrospective observational study included 80 laboratory-confirmed COVID-19 cases. Data were collected from the electronic medical record system by a trained team of physicians. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TB), albumin, and prealbumin levels at admission and on day 7 after admission were collected. The primary outcome of the current study was patient mortality.
Results:
Abnormal ALT, AST, TB, albumin, and prealbumin levels were observed in 11 (13.8%), 15 (18.8%), 5 (6.3%), 22 (27.5%), and 31 (38.8%) patients, respectively. Male gender correlated with elevated ALT and AST levels (p = 0.027 and 0.036, respectively). Higher levels of AST and lower levels of albumin and prealbumin were associated with patient mortality (p = 0.009, 0.002, and 0.003, respectively). Multivariate Cox regression analysis identified patient age (p = 0.013, HR 1.108) and prealbumin levels (p = 0.015, HR 0.986) as independent predictors for patient mortality. However, changes in liver-related biomarkers were not associated with poor outcome in multivariate analysis (p > 0.05).
Conclusions:
Abnormalities in albumin and prealbumin levels are common among COVID-19 patients and hypoprealbuminemia independently predicts adverse outcome and should be carefully considered in clinical practice. Moreover, changes in liver-related biomarkers is not a salient feature of COVID-19.
Keywords: COVID-19, hypoprealbuminemia, liver impairment, prognosis
INTRODUCTION
The outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) has spread to 216 countries, areas or territories, with over 7.69 million confirmed cases as of June14, 2020.[1] The lung is the main organ attacked by SARS-Cov-2. However, extrapulmonary organ injuries were also observed in patients with coronavirus disease (COVID-19).[2,3,4,5] Liver impairment or injury has been reported in previous studies on COVID-19; the frequency of abnormal alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in COVID-19 patients ranged from 14% to 53%.[2,3,4,5,6,7] Although liver dysfunction was shown to be more common in COVID-19 patients with severe symptoms,[6,7,8] several studies concluded that liver impairment may not be an independent predictor for adverse outcome of the disease.[5,6,9,10]
However, previous studies on liver impairment focused primarily on changes in ALT, AST, and total bilirubin (TB) levels. As an important biomarker for evaluating the protein synthesis function of the liver, the role of prealbumin was underappreciated and thus, not analyzed in multivariate analyses with other liver-related biomarkers.[5,8,9] Furthermore, the effects exerted by changes in liver-related biomarkers also require further clarification.
Thus, the current study systematically evaluated the effects of abnormal liver-related biomarker levels in COVID-19 patients and explored the prognostic value of elevated levels of liver enzymes and abnormal liver synthetic capacity for patient mortality.
PATIENTS AND METHODS
This was a retrospective observational study. Ward E1-7, Optics Valley District, Tongji Hospital Affiliated to the Tongji Medical College of HUST was a specially designated ward that was taken over by the medical team backing Hubei province from the Second Hospital of Shandong University for COVID-19 management. All patients hospitalized in the ward from February 10th to March 4th, 2020 were consecutively included. Patients were followed-up until Mar 31, 2020, on which day our medical team left Wuhan and 3 of the 80 patients were transferred to Sino-French New City Branch for further treatment.
All included patients were laboratory-confirmed cases of COVID-19, with positive results from nasal or pharyngeal swabs. The detailed procedures for nucleic acid testing are described in previously published studies.[2] All patients admitted to the ward also presented with pneumonia, as confirmed by radiological findings. Therefore, all consecutive patients with complete data were included in the current study.
The study was approved by the Ethical Committee of the Tongji Hospital Affiliated to the Tongji Medical College of HUST and the Second Hospital of Shandong University: on April 2, 2020. The protocol also follows the Declaration of Helsinki.
The data were collected from the electronic medical record system by a trained team of physicians. The following data were collected on admission: patient age, gender, ALT, AST, TB, albumin, and prealbumin levels, white blood cell counts (WBC) and lymphocyte counts (Lym), as well as blood urea nitrogen (BUN), creatinine (Cr), D-dimer, N-terminal pro-B-type natriuretic peptide (NT-proBNP), troponin I (TnI), C-reactive protein (CRP), procalcitonin (PCT), and interleukin-6 (IL-6) levels. Data regarding ALT, AST, TB, albumin, and prealbumin levels at day 7 after admission were also collected.
Patient mortality was designated as the primary outcome of the current study. Abnormal liver-related biomarkers were defined as ALT values of more than 40 U/L, or AST values of more than 40 U/L, or TB values of more than 17.1 μmol/L, or albumin values of less than 35 g/L, or prealbumin values of less than 200 mg/L. Rbiomarkerwas defined as the ratio between the biomarker levels at day 7 after admission and biomarker levels on admission.
Statistical analyses
Variables were presented as mean ± standard deviations, median [interquartile range (IQR)], or numbers (percentages). Moreover, the Student's t-test, Mann-Whitney U-test, chi-square test, and Wilcoxon rank sum test were used according to the characteristics of distribution. Bivariate correlations between liver-related biomarkers and age/gender/patient mortality were analyzed using the Spearman's Rank correlation. The Kaplan-Meier method was used to calculate differences in mortality rates between the hypoprealbuminemia group and the normal prealbumin group. Univariate and multivariate Cox regression analyses (Forward logistic regression method) were performed to investigate predictors for patient outcome. Multivariate logistic regression analysis (Forward logistic regression method) was used to indicate factors associated with hypoprealbuminemia and elevated TB levels. All statistical analyses were performed using the SPSS version 22.0 software (IBM Corp., Armonk, NY, USA).
RESULTS
The clinical data of the 80 hospitalized patients with COVID-19 admitted to the ward were retrospectively collected. No patients with mild disease were included in our cohort; seven patients were critical at admission and two patients progressed to be critical during follow-up. Among these patients, 9 succumbed to the disease between 7 to 40 days after admission. All other patients recovered and were discharged from the hospital (the 3 transferred patients were followed-up by colleagues of Sino-French New City Branch and recovered after treatment). The median age of the included patients was 62 years (IQR 50 to 68 years). Thirty-eight (47.5%) patients were male. The median follow-up time was 47 days (IQR 32 to 49 days). Comorbidities were observed in 37 patients (46.3%), including hypertension in 29 patients (36.3%), diabetes in 13 patients (16.3%), coronary heart disease in seven patients (8.8%), and respiratory diseases in three patients (3.8%). Positive HBsAg was tested in two patients, however, they were judged as HBeAg-positive chronic HBV infection or HBeAg-negative chronic HBV infection, and no therapy was needed. No chronic liver disease or cirrhosis was present. No patient developed liver failure in the present cohort. Baseline characteristics of the 80 included patients are shown in Table 1.
Table 1.
Baseline characteristics of COVID-19 patients at admission
| Characteristics | Patients (n=80) |
|---|---|
| Age, years* | 62 (50, 68) |
| Gender, male (n, %) | 38 (47.5%) |
| Comorbidities (n, %) | 37 (46.3%) |
| WBC (109/L) | 6.31±2.04 |
| Lym (109/L) | 1.42±0.61 |
| PLT (109/L)* | 240.0 (192.5, 320.3) |
| ALT (U/L)* | 22 (14, 33) |
| AST (U/L)* | 25 (15, 35) |
| TB (umol/L)* | 7.9 (6.6, 11.5) |
| Albumin (g/L)* | 38.0 (34.9, 41.7) |
| Prealbumin (mg/L)* | 211.5 (157.0, 244.8) |
| BUN (mmol/L)* | 4.40 (3.43, 5.70) |
| Creatinine (umol/L)* | 71.5 (53.0, 82.0) |
| INR* | 1.07 (1.02, 1.12) |
| D-dimer (ug/ml FEU)* | 0.45 (0.22, 1.43) |
| NT-proBNP (pg/ml)* | 79.5 (27.8, 199.8) |
| TnI (pg/ml)* | 2.40 (1.90, 7.58) |
| CRP (mg/L)* | 3.95 (0.73, 35.43) |
| Procalcitonin (ng/ml)* | 0.06 (0.05, 0.09) |
WBC=white blood cell counts; Lym=lymphocyte counts; PLT=platelet counts; ALT=alanine aminotransferase; AST=aspartate aminotransferase; TB=total bilirubin; BUN=blood urea nitrogen; Cr=creatinine; INR=international normalized ratio; NT-proBNP=N-terminal pro-B-type natriuretic peptide; TnI=troponin I; CRP=C-reactive protein. *median (interquartile range)
Distribution of abnormal liver-related biomarkers
Abnormal ALT, AST, TB, albumin, and prealbumin levels were observed in 11 (13.8%), 15 (18.8%), 5 (6.3%), 22 (27.5%), and 31 (38.8%) patients, respectively.
The results of the bivariate correlations revealed that older age correlated with higher levels of AST [Correlation coefficient (r) =0.383, P < 0.001] and lower levels of albumin and prealbumin (r = -0.517, P < 0.001 and r = -0.360, P = 0.001, respectively). However, there was no significant correlation between ALT or TB levels and age (P = 0.286 and 0.651, respectively).
Bivariate correlations also revealed that elevated ALT and AST levels correlated with the male gender (r = 0.247, P = 0.027 and r = 0.234, P = 0.036, respectively). Moreover, there was no significant correlation between TB, albumin, and prealbumin levels and gender (P = 0.204, 0.579, and 0.981, respectively).
Bivariate correlations showed that platelet level was negatively correlated with albumin level (r = -0.259, P = 0.002). There was no significant correlation between ALT, AST, TB, and prealbumin levels and platelet level (p = 0.744, 0.271, 0.384, and 0.738, respectively). When patients were divided as low platelet (<200 × 109/L) and high platelet (>200 × 109/L) groups, no differences for mortality, age, gender, ALT, AST, TB, PA, NT-proBNP, CRP, WBC, Lym, INR and D-dimer between the two groups were found (P > 0.05), also for the albumin levels (P = 0.060).
Abnormal liver-related biomarkers and patient mortality
The results of the bivariate correlations showed that elevated ALT and TB levels did not correlated with patient mortality. However, higher levels of AST and lower levels of albumin and prealbumin were significantly associated with patient mortality (r = 0.289, P = 0.009, r = -0.349, P = 0.002, and r = -0.324, P = 0.003, respectively).
Univariate Cox regression analysis also indicated that higher levels of AST and lower levels of albumin and prealbumin were predictors for patient mortality (P = 0.008, 0.003, and 0.004, respectively). Multivariate Cox regression analysis, which included age and ALT, AST, TB, albumin, and prealbumin levels, revealed that patient age (P = 0.013, HR 1.108, 95%CI 1.022-1.202) and prealbumin (P= 0.015, HR 0.986, 95%CI 0.975-0.997) were independent predictors of patient mortality [Table 2].
Table 2.
Univariate and multivariate Cox analyses revealing predictors for patient death
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Age | 1.104 (1.035, 1.177) | 0.003 | 1.108 (0.022, 1.202) | 0.013 |
| ALT | 0.997 (0.972, 1.022) | 0.787 | ||
| AST | 1.038 (1.010, 1.068) | 0.008 | ||
| TB | 1.014 (0.960, 1.071) | 0.624 | ||
| Albumin | 0.818 (0.716, 0.935) | 0.003 | ||
| Prealbumin | 0.985 (0.974, 0.995) | 0.004 | 0.986 (0.975-0.997) | 0.015 |
ALT=alanine aminotransferase; AST=aspartate aminotransferase; TB=total bilirubin
Patient characteristics in relation to prealbumin levels
The 30-day mortality rates for patients with hypoprealbuminemia and normal prealbumin levels were 19.4% and 2.0%, respectively [Figure 1]. Moreover, compared to patients with normal prealbumin levels, patients with hypoprealbuminemia were characterized by older age (P = 0.009), lower levels of Lym (P < 0.001), and higher levels of AST (p = 0.006), D-dimer (P < 0.001), NT-proBNP (P < 0.001), TnI (p < 0.001), CRP (P < 0.001), PCT (P < 0.001), and IL-6 (P < 0.001) [Table 3].
Figure 1.
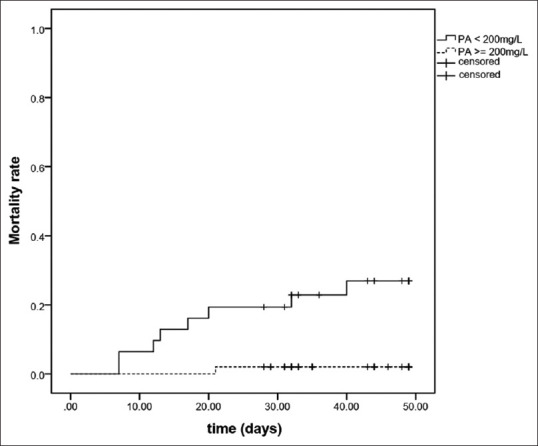
Survival curves in patients with hypoprealbuminemia and normal prealbumin levels. PA, prealbumin
Table 3.
Baseline characteristics of COVID-19 patients with hypoprealbuminemia and normal levels of prealbumin
| Patients with hypoprealbuminemia (n=31) | Patients with normal prealbumin (n=49) | P* | |
|---|---|---|---|
| Age, years† | 66.0 (53.0, 70.0) | 56.0 (46.0, 66.0) | 0.009 |
| Gender, male (n, %) | 14 (35.2%) | 24 (49.0%) | 0.820 |
| ALT (U/L)† | 24.0 (15.0, 36.0) | 21.0 (13.5, 32.0) | 0.621 |
| AST (U/L)† | 29.0 (20.0, 45.0) | 21.0 (14.0, 30.5) | 0.006 |
| TB (umol/L)† | 8.1 (6.7, 11.4) | 7.7 (6.3, 11.6) | 0.913 |
| WBC (109/L) | 6.26±2.23 | 6.34±1.93 | 0.876 |
| Lym (109/L) | 1.04±0.49 | 1.66±0.56 | <0.001 |
| BUN (mmol/L)† | 4.30 (3.60, 5.10) | 4.50 (3.15, 5.85) | 0.984 |
| Creatinine (umol/L)† | 72.0 (53.0, 87.0) | 71.0 (51.5, 79.5) | 0.421 |
| INR† | 1.09 (1.04, 1.17) | 1.07 (1.01, 1.10) | 0.061 |
| D-dimer (ug/ml FEU)† | 1.43 (0.41, 3.41) | 0.29 (0.22, 0.93) | <0.001 |
| NT-proBNP (pg/ml)† | 162.0 (63.0, 429.0) | 54.0 (23.0, 114.0) | <0.001 |
| TnI (pg/ml)† | 6.30 (2.90, 19.20) | 1.90 (1.90, 2.75) | <0.001 |
| CRP (mg/L)† | 37.60 (5.80, 63.60) | 1.50 (0.55, 5.60) | <0.001 |
| Procalcitonin (ng/ml)† | 0.09 (0.06, 0.16) | 0.06 (0.04, 0.06) | <0.001 |
| Interleukin-6 (pg/ml)† | 11.26 (3.62, 43.62)‡ | 2.16 (1.50, 5.16)§ | <0.001 |
ALT=alanine aminotransferase; AST=aspartate aminotransferase; TB=total bilirubin; WBC=white blood cell counts; Lym=lymphocyte counts; BUN=blood urea nitrogen; Cr=creatinine; INR=international normalized ratio; NT-proBNP=N-terminal pro-B-type natriuretic peptide; TnI=troponin I; CRP=C-reactive protein. *Student’s t-test, Mann-Whitney U-test or Chi-square test according to the characteristics of distribution. †median (interquartile range); ‡29 patients; §46 patients
Multivariate logistic regression analysis, which included age and Lym, as well as AST, D-dimer, NT-proBNP, TnI, CRP, PCT, and IL-6 levels, revealed that lower Lym (OR 3.844, 95%CI 1.166 – 12.674, P = 0.027) and higher CRP levels (OR 0.971, 95%CI 0.948 – 0.994, P = 0.013) were factors independently associated with hypoprealbuminemia.
Changes in liver-related biomarkers
The current study also collected data regarding liver-related biomarkers on day 7 after admission (75 patients). In the 9 non-survivors, there were no significant changes in ALT, AST, TB, albumin, and prealbumin levels at day 7 when compared with the biomarker values on admission. In the 66 surviving patients (5 survivors were excluded because of incomplete data at day 7), AST levels were decreased, while TB and prealbumin levels were increased at day 7 (p = 0.004, 0.002, and 0.001, respectively) [Table 4]. However, all Rbiomarkerswere not significant predictors for patient mortality in the multivariate Cox regression analysis [Table 5].
Table 4.
Changes of liver-related biomarkers in survivors and non-survivors
| Survivors (n=66) |
Non-survivors (n=9) |
|||||
|---|---|---|---|---|---|---|
| Day 0 | Day 7 | P* | Day 0 | Day 7 | P* | |
| ALT (U/L)† | 21.0 (13.0, 33.0) | 20.0 (14.0, 33.5) | 0.303 | 20.0 (18.0, 36.5) | 29.0 (17.0, 53.5) | 0.374 |
| AST (U/L)† | 23.5 (15.0, 33.3) | 19.0 (15.0 24.3) | 0.004 | 36.0 (25.0, 60.5) | 33.0 (27.0, 54.0) | 0.441 |
| TB (umol/L)† | 7.7 (6.6, 11.4) | 10.5 (7.6, 14.9) | 0.002 | 9.8 (7.3, 13.4) | 14.2 (10.1, 23.5) | 0.123 |
| Albumin (g/L)† | 38.5 (35.2, 41.7) | 38.3 (34.5, 41.6) | 0.128 | 32.5 (30.6, 36.7) | 30.8 (24.7, 37.0) | 0.173 |
| Prealbumin (mg/L)† | 219.5 (174.8, 245.3) | 235.5 (204.8, 270.8) | 0.001 | 96.0 (82.5, 163.0) | 105.0 (80.0, 153.0) | 0.672 |
ALT=alanine aminotransferase; AST=aspartate aminotransferase; TB=total bilirubin. *Wilcoxon rank sum test. †median (interquartile range)
Table 5.
Univariate and multivariate Cox analyses revealing predictive value of R biomarker for patient death
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Age | 1.101 (1.032, 1.174) | 0.003 | 1.101 (1.032, 1.174) | 0.003 |
| RALT* | 1.936 (0.519, 7.221) | 0.325 | ||
| RAST* | 1.061 (0.265, 4.247) | 0.934 | ||
| RTB* | 1.720 (0.357, 8.287) | 0.499 | ||
| Ralbumin* | 1.627 (0.437, 6.063) | 0.468 | ||
| Rprealbumin* | 0.437 (0.117, 1.633) | 0.219 | ||
ALT=alanine aminotransferase; AST=aspartate aminotransferase; TB=total bilirubin. Rbiomarker=biomarker at day 7/biomarker at admission. *>1 vs. ≤1
Lopinavir/ritonavir and liver-related biomarkers
The bivariate correlations showed that the use of lopinavir/ritonavir did not correlate with changes in ALT, AST, albumin, and prealbumin levels at day 7 after admission (p = 0.291, 0.892, 0.068, and 0.319, respectively). However, increased TB levels at day 7 was associated with the use of lopinavir/ritonavir (r = 0.126, P = 0.041). In the multivariate logistic regression analysis, which included age and the use of lopinavir/ritonavir, arbidol, and glucocorticoid, the drugs showed no differential odds in predicting elevated TB levels.
DISCUSSION
The current study demonstrated that in COVID-19 patients abnormalities in albumin and prealbumin levels may be more common than liver enzymes and TB abnormalities. Hypoprealbuminemia was shown to be an independent predictor for patient mortality, indicating the importance of the biomarker in clinical liver evaluations. On the other hand, changes in liver-related biomarkers were not predictors of adverse outcomes. This study emphasized the importance of prealbumin for outcome prediction and discussed the value of changes in liver-related biomarkers. To our knowledge, there are few studies that reported on the effects of prealbumin variations in COVID-19 patients.
Liver dysfunction is common in patients with COVID-19[3,6,7,9,11] and several mechanisms underlying these abnormalities have been reported. First, SARS-CoV-2 might bind to the upregulated angiotensin-converting enzyme 2 (ACE2) receptor in hepatocytes derived from bile duct epithelial cells and attack the liver tissues directly.[6,7,12] Second, the cytokine storm ensuing the infection with SARS-CoV-2 leads to increased release of inflammatory factors and decreased CD4+ and CD8+ T lymphocyte counts.[13,14] Moreover, over-activation of the inflammatory response, which is common in septic patients, may cause dysfunction of multiple organs, including the liver.[6,12,15] Third, respiratory failure and circulatory disorders caused by COVID-19 may lead to hepatic ischemia and hypoxia. Therefore, hypoxic hepatitis is frequent in these patients, especially those with severe symptoms.[6] Fourth, the contribution of antiviral agents, nonsteroidal anti-inflammatory drugs, glucocorticoids, etc., to drug hepatotoxicity in patients with COVID-19 should not be ignored.[6,7] Cai et al.found that the use of lopinavir/ritonavir was associated with liver injury[11] and our results corroborated this. However, multivariate logistic regression analysis showed no difference in odds. The benefits of lopinavir/ritonavir treatment for COVID-19 patients were observed in a modified intention-to-treat analysis.[16] Thus, the potential adverse events and possible benefits should be carefully balanced when using this antiviral drug. The influence of lopinavir/ritonavir on the liver should also be re-evaluated.
Our study also clarified the correlation between liver-related biomarkers and patient mortality, indicating that higher levels of AST and lower levels of albumin and prealbumin are predictors for adverse outcomes in univariate analysis. Increased AST levels in COVID-19 patients may be due to the impairment of the liver, muscles, and heart.[10] On the other hand, albumin levels may be affected by intravenous albumin infusion during treatment. Therefore, these two indicators may be influenced by other factors that are independent of liver dysfunction.
The functions of albumin include the maintenance of osmotic pressure, transportation of various substances and drugs in the body, and scavenging of free oxygen radicals, as well as antioxidant and antiplatelet effects.[17] The mechanisms involved in the predictive value of albumin for patient outcomes may be summarized as follows:[17] first, albumin levels reflect the basic nutritional status of the patients and cases with poor nutritional status are likely to progress to adverse outcomes; second, albumin deficiency may adversely affect physiological functions by inhibiting the transportation of various substances; third, as a negative acute-phase protein, hypoalbuminemia might be correlated with obvious inflammation. Prealbumin is the precursor to albumin. The half-life of prealbumin is significantly shorter than that of albumin and is not affected by albumin infusion during treatment. Therefore, prealbumin could better reflect the recent nutritional status and albumin reserve of patients.[18]
Zhang et al. showed that prealbumin levels were lower in severe COVID-19 cases when compared to mild cases.[9] However, the predictive value of prealbumin for disease outcome was not explored. In the current study, multivariate Cox regression analysis showed that prealbumin is a promising prognostic biomarker for patient mortality, indicating that the role of prealbumin in clinical practice should not be ignored. Furthermore, the current study revealed that lower Lym and higher CRP levels were factors independently associated with hypoprealbuminemia, which also indicated the relationship between prealbumin and inflammation.
Previous studies also explored the gender-associated distribution of liver impairment in COVID-19 patients and found that it was more common in males.[8,19] Similarly, our results showed that elevated ALT and AST levels were correlated with the male gender. Whether the susceptibility of males to liver dysfunction is due to higher basal levels of liver enzymes or gender differences in COVID-19 evolution requires further investigation. We also found that older age was correlated with higher AST levels and lower albumin and prealbumin levels. Feng et al. hypothesized that older age correlates with a higher risk of liver impairment, considering the absence of liver damage in children with COVID-19.[19] However, Xie et al. found no significant differences in age between patients with and without liver injury.[8] Consistent with previous studies, the results of the current study showed that age was an independent predictor of poor patient outcomes.[5,20,21] Elderly patients tend to be associated with a decline in immune response[22] and relatively poor nutritional status, which then leads to a poor prognosis.
Reduced platelet levels were observed in patients with cirrhosis; however, inflammatory cytokines may induce the release of platelets from megakaryocytes.[23] The negative correlation between platelet and albumin may be due to the influence of inflammation. However, in view of the lack of difference found in albumin levels between low platelet (<200 × 109/L) and high platelet (>200 × 109/L) groups, the clinical value of this negative correlation should be further evaluated.
This study has several limitations. First, this was a retrospective study and the presence of bias cannot be excluded. Second, only a few patients in our cohort had a pre-existing liver disease and, therefore, it was difficult to evaluate the influence of liver-related comorbidities. Third, although the present study might be the first to explore the predictive value of changes in liver-related biomarkers using multivariate analysis, we only discussed changes exhibited in the first week after admission due to the limited survival time in several cases. Thus, further evaluation of the changes in liver-related biomarkers in COVID-19 patients should provide helpful insight for clinicians.
In conclusion, elevated levels of liver enzymes and TB were not rare in patients with COVID-19. However, abnormalities in albumin and prealbumin levels were found to be more common in this patient population. Hypoprealbuminemia was also shown to independently predict adverse outcome and should, therefore, be evaluated and monitored in clinical practice. Moreover, changes in liver-related biomarkers might not be salient characteristics of COVID-19.
Financial support and sponsorship
This study was funded by Shandong Province Natural Science Foundation (No. ZR2019PH 052).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank all members of medical team backing Hubei province from the Second Hospital of Shandong University. The authors also thank colleagues of the Second Hospital of Shandong University and Tongji Hospital who continue to work in their station and give us great support.
We would also like to thank Editage (www.editage.cn) for English language editing.
REFERENCES
- 1.WHO. [cited 2020 Jun 15];Coronavirus disease (COVID-19) outbreak situation. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 . [Google Scholar]
- 2.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;95:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int. 2020;40:1278–81. doi: 10.1111/liv.14470. [DOI] [PubMed] [Google Scholar]
- 7.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–30. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie H, Zhao J, Lian N, Su L, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int. 2020;40:1321–6. doi: 10.1111/liv.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single center in Wuhan city, China. Liver Int. 2020 doi: 10.1111/liv.14455. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 10.Bangash MN, Patel J, Parekh D. COVID-19 and the liver: Little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529–30. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai Q, Huang D, Yu H, Chen J, Liu L, Xu L. Characteristics of liver tests in COVID-19 patients. J Hepatol. 2020;73:566–74. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan GW, Gao L, Wang JW, Wen XJ, Mao TH, Peng SW, et al. Exploring the mechanism of liver enzyme abnormalities in patients with novel coronavirus-infected pneumonia. Zhonghua Gan Zang Bing Za Zhi. 2020;28:100–6. doi: 10.3760/cma.j.issn.1007-3418.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–2. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedersen SF, Ho YC. SARS-CoV-2: A storm is raging. J Clin Invest. 2020;130:2202–5. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392:75–87. doi: 10.1016/S0140-6736(18)30696-2. [DOI] [PubMed] [Google Scholar]
- 16.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–99. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S, McClave SA, Martindale RG, Miller KR, Hurt RT. Hypoalbuminemia and clinical outcomes: What is the mechanism behind the relationship? Am Surg. 2017;83:1220–7. doi: 10.1177/000313481708301123. [DOI] [PubMed] [Google Scholar]
- 18.Smith SH. Using albumin and prealbumin to assess nutritional status. Nursing. 2017;47:65–6. doi: 10.1097/01.NURSE.0000511805.83334.df. [DOI] [PubMed] [Google Scholar]
- 19.Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, et al. COVID-19 and liver dysfunction: current insights and emergent therapeutic strategies. J Clin Transl Hepatol. 2020;8:18–24. doi: 10.14218/JCTH.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu W, Tao ZW, Lei W, Ming-Li Y, Kui L, Ling Z, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J. 2020;133:1032–8. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–43. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goronzy JJ, Fang F, Cavanagh MM, Qi Q, Weyand CM. Naive T cell maintenance and function in human aging. J Immunol. 2015;194:4073–80. doi: 10.4049/jimmunol.1500046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couldwell G, Machlus KR. Modulation of megakaryopoiesis and platelet production during inflammation. Thromb Res. 2019;179:114–20. doi: 10.1016/j.thromres.2019.05.008. [DOI] [PubMed] [Google Scholar]


