Abstract
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19) and has created a worldwide pandemic. Many patients with this infection have an asymptomatic or mild illness, but a small percentage of patients require hospitalization and intensive care. Patients with respiratory tract involvement have a spectrum of presentations that range from scattered ground-glass infiltrates to diffuse infiltrates with consolidation. Patients with the latter radiographic presentation have severe hypoxemia and usually require mechanical ventilation. In addition, some patients develop multiorgan failure, deep venous thrombi with pulmonary emboli, and cytokine storm syndrome. The respiratory management of these patients should focus on using low tidal volume ventilation with low intrathoracic pressures. Some patients have significant recruitable lung and may benefit from higher positive end-expiratory pressure (PEEP) levels and/or prone positioning. There is no well-established anti-viral treatment for this infection; the United States Food and Drug Administration (FDA) has provided emergency use authorization for convalescent plasma and remdesivir for the treatment of patients with COVID-19. In addition, randomized trials have demonstrated that dexamethasone improves outcomes in patients on mechanical ventilators or on oxygen. There are ongoing trials of other drugs which have the potential to moderate the acute inflammatory state seen in some of these patients. These patients often need prolonged high-level intensive care. Hospitals are confronted with significant challenges in patient management, supply management, health care worker safety, and health care worker burnout.
Keywords: Coronavirus, Novel coronavirus, 2019-nCoV, Middle East respiratory syndrome coronavirus, SARS coronavirus, COVID 19, Hypoxia, Remdesivir
Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19) and has created a worldwide pandemic. As of October 4, 2020, there were 34804348 confirmed cases throughout the world (216 countries) with 1030738 deaths (https://covid19.who.int/), translating to an overall mortality rate of approximately 3.0%. The number of confirmed cases in the United States was 7256234 with 207366 deaths. Many patients with this infection have an asymptomatic or mild illness. However, some patients present with severe acute respiratory distress syndrome (ARDS) with significant hypoxemia and require hospitalization and intensive care. This review provides up-to-date information on the characteristics of this virus, the clinical presentations including radiology, pathophysiology, lung pathology, and current treatment options.
Virology
Virus Characteristics
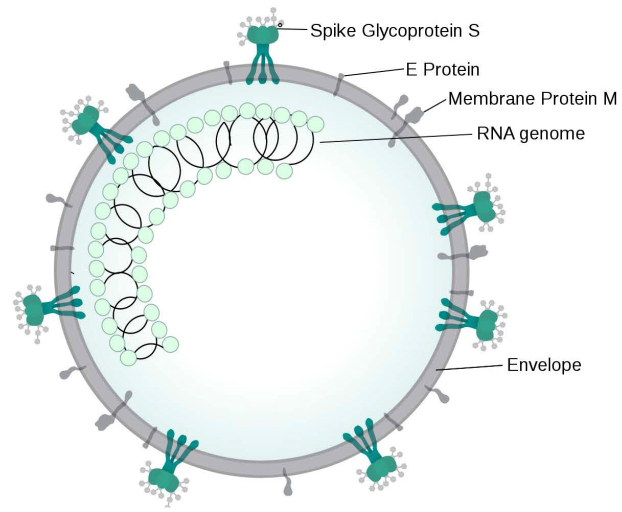
The recent COVID-19 pandemic has led to increased research on the specific characteristics of SARS-CoV-2 to better understand viral structure, macromolecular function, and pathogenicity. SARS-CoV-2 is a β-coronavirus that is single stranded, non-segmented, enveloped, and positive-sense RNA virus measuring about 65–72.5 nm in diameter (Fig 1).1,2 The genome of coronaviruses ranges from 26 to 32 kilobases in length.3 The SARS-CoV-2 genome contains 14 open reading frames that encode for 27 proteins, which include four structural proteins, namely spike protein (S), envelope protein (E), membrane protein (M), and nucleocapsid protein (N); 16 nonstructural proteins (nsp 1–16); and 5–8 accessory proteins. SARS-CoV-2 uses the human angiotensin converting enzyme 2 (ACE2) receptor to enter and infect human cells.4-6 The mechanism of binding and entry of SARS-CoV-2 is mediated mainly by the S protein, which can be further divided into the S1 and S2 subunits.5,6
Figure 1.
SARS-CoV-2 structure. Sourc e: Wikimedia, file: Coronavirus virion structure.svg, accessed September 5, 2020.
Subunit S1 serves as the main protein assisting with viral binding onto a host cell; S2 facilitates fusion of the virus into the host membranes.1 The S protein itself has been shown to exist as a trimer. The S1 trimer is later removed for viral entry.4 To enter a host cell, all coronavirus S proteins are further cleaved at the S2ʹ site by host proteases, changing its conformation and allowing fusion of viral and host membranes. In SARS-CoV-2, a furin cleavage occurs in the Golgi apparatus in a specific site at the boundary between S1 and S2, unique to this viral subtype.4 Experiments with amino acid mutations in the furin cleavage site concluded that the four amino acids present at this site are not necessary for viral entry as both mutated viruses (conservation of only arginine at pos 685) and non-mutated viruses have entry into the host. However, due to the unique nature of the furin cleavage site on SARS-CoV-2 in comparison to other SARS-CoVs, this site has been hypothesized to potentially increase viral infectivity.4
The S1 subunit contains a receptor binding domain that binds to the ACE2 peptidase domain.7 Shang, et al, demonstrated increased binding affinity of SARS-CoV-2 receptor binding domain to ACE2 in comparison to SARS-CoV, due mainly to the presence of a proline-proline-alanine motif in SARS-CoV-2, allowing for a favorable turn to be made when binding.5 Wang, et al, confirmed this increased affinity binding using surface plasmon resonance assays, demonstrating a 4-fold higher affinity of SARS-CoV-2 to human ACE2 in comparison to SARS-CoV.6
Genome sequencing of SARS-CoV-2 compared to other human and animal virus genomes demonstrates a close relation to the several other viruses, including bat-SL-CoV2XC21 and bat-SL-CoV2C45.8 Examination of specific sequences within each viral genome showed a greater than 90% sequence similarity. However, the spike protein on the membrane of the virus demonstrated a lower sequence identity at 80%.8 Lu, et al, further hypothesized that due to a similarity observed in the genome sequences between SARS-CoV and SARS-CoV-2 that perhaps the novel 2019 coronavirus uses the same ACE2 receptor.7 Anderson, et al, mentions the 96% identical nature of RaTG13 from the Rhinolopphus affinus bat with SARS-CoV-2; however, once again the spike exhibits differences in the receptor binding domain, further demonstrating effective adaptive binding of SARS-CoV-2 to human ACE2 and hypothesized evolution of SARS-CoV-2 from RaTg13.5,9 Examination of the genome and sequence identity has aided in understanding the SARS-CoV-2 evolution and may help researchers target specific viral components to reduce pathogenicity.
In Vitro Growth Studies
The isolation of SARS-CoV-2 has allowed the use of cell culture and organoid cultures to study viral characteristics, such as replication, lifecycle, stability, and pathogenesis. Furthermore, the in vitro setting of these studies allows for the evaluation of antiviral drug therapy and antibodies that can block viral infection and could contribute to the development of vaccines.
Cell lines from human epithelial airway cells develop cytopathic effects after infection with SARS-CoV-2. These cells express both ACE2 and transmembrane serine proteinase 2 (TMPRSS2). However, human cell lines are expensive and are not infinitely proliferative.10 Other cell lines, such as Vero cells, derived from the kidney of the African green monkey, can support viral replication resulting in high titers of viral particles and can replicate continuously in cell culture.11 In particular, Vero E6 cells are used to replicate SARS-CoV-2 due to the lack of interferon (IFN), which allows viral entry and replication, and the expression of ACE2 on cell membranes.10 Vieira, et al, reported that the predominant changes in Vero E6 cells after SARS-CoV-2 infection include mitochondrial degeneration, increased ribosomal thickness and quantity, intense smooth vesicle proliferation, and thickening of both the nuclear membrane and rough endoplasmic reticulum.12 These investigators observed nucleocapsids in the cisterns of the rough endoplasmic reticulum, suggesting it as a site for viral assembly. The viral progeny is then released by fusing smooth vesicles with the cell membrane.12
Cell lines can be used to study therapeutic agents. In one experiment, remdesivir demonstrated antiviral activity in an in vitro cell line.13 Studies with Vero E6 cells also demonstrated the immunomodulatory effects of chloroquine as a viral infection and replication inhibitor.13 Still, there are substantial differences between studying cultured cells instead of whole organs, such as the lungs, liver, kidney, and gastrointestinal (GI) tract, infected by the virus.
Using in vitro organoids allows the pathophysiology of SARS-CoV-2 to be studied in a more holistic manner and allows antiviral drug testing without the expense and ethical concerns of animal models.14 Research studies have demonstrated that SARS-CoV-2 can directly infect both human blood vessel and kidney organoids, and that this infection can be inhibited by human recombinant soluble ACE2.15 Furthermore, a study focusing on developing organoids of the GI tract demonstrates that the virus readily replicates in enterocytes, which have a high ACE2 expression.16 This could lead to the possibility that the GI tract serves as a site for viral replication and could help explain why diarrhea is commonly observed in infected patients. Human bronchial organoids also demonstrated permissiveness to the SARS-CoV-2 virus.10 Furthermore, liver organoids had impaired cholangiocytes, which provide bile transportation.10 These organoid studies could have value in therapeutic antiviral trials. One study established human capillary and kidney organoids and implemented a human recombinant soluble ACE2 (hrsACE2) therapy, concluding that hrsACE2 reduces SARS-CoV-2 infections in a dose-dependent manner.15 In another experiment, liver organoids were infected with SARS-CoV-2 and exposed to baricitinib, which reduced the viral load by inhibiting Janus kinases (JAK) and Numb-associated kinases (NAK).17 Although these experimental approaches provide valuable information, it is important to recognize that isolated organoids cannot demonstrate the systemic effects of SARS-CoV-2 as well as an animal model can.18
Diagnosis
The identification of COVID-19 requires upper respiratory tract secretions to identify viral RNA or viral antigens. Viral RNA is identified using reverse transcription polymerase chain reaction (RT-PCR) assays. There are multiple assays available that appear to have good sensitivity.19 Viral infection can also be established by identifying specific viral proteins in upper airway secretions. Most assays try to identify the COVID-19 spike protein. These assays are potentially much faster. However, all assays have the potential to provide false-negative results due to poor specimen collection, laboratory errors, low levels of viral RNA, or low levels of viral protein in secretions.
Clinical Presentation
SARS-CoV-2 infections can be asymptomatic or associated with COVID-19.20 The clinical presentation of these patients includes constitutional symptoms and symptoms specific to various organ systems including the respiratory tract, GI tract, central nervous system (CNS), skin, and oral and nasal tissue.
In a systematic review and meta-analysis of 656 patients reported by Rodriguez, et al, fever occurred in 88.7% of confirmed patients.21 Another meta-analysis of 31 articles and 46959 patients shows that the most common clinical manifestations are fever (87.3%), muscle soreness or fatigue (35.5%), and chest distress (31.2%).22 Fever was the most common symptoms in five retrospective clinical studies reviewed by Lovato, et al, and was reported in 85.6% of 1556 hospitalized patients with COVID-19; 39.4% of these patients also had fatigue.23 Fever was also the most common presenting symptom in a meta-analysis by Tahvildari, et al, who included 80 articles and analyzed 417 COVID-19 patients.24 In this study, it was the most common presenting symptom in patients who tested positive for COVID-19 and was reported in up to 62% of patients in 82% of the analyzed studies. Other symptoms including dizziness and chills, were reported less frequently.24
Katal, et al, found that CNS involvement in coronavirus infection occurs in more than one-third of the hospitalized patients with various degrees of severity ranging from mild to life-threatening conditions. Myalgia, headache, cerebrovascular disease, and encephalopathy are the most common CNS manifestations associated with COVID-19.25
Respiratory manifestations with COVID-19 include a spectrum that ranges from dry cough and dyspnea to pneumonia, pulmonary edema, ARDS, and multiorgan failures, requiring hospitalization in intensive care units and leading to death in severe cases.20 A systematic review with meta-analysis of 660 articles by Rodriguez, et al, reports that cough (57.6%) and dyspnea (45.6%) are the most frequent symptoms.21 A meta-analysis of 46959 patients shows that cough occurs in 58.1%, dyspnea in 38.3%, and chest distress in 31.2% of patients.22 Five retrospective clinical studies of 1556 hospitalized patients with COVID-19 reveal cough as a common symptom (68.7%); nasal congestion is reported in 3.7%; rhinorrhea is rare.23
In addition to the respiratory manifestations, patients with COVID-19 frequently present with GI symptoms, which can occur in up to 26% of patients.2,6 The most common GI presentation in patients with COVID-19 is diarrhea (3.8%–34%) followed by nausea and/or vomiting (3.9%–10.1%) and abdominal pain (1.1%–2.2%). Other common GI symptoms reported in these patients include anorexia, anosmia, and dysgeusia. Moreover, a few COVID-19 case studies and case series have reported cases with GI symptoms preceding respiratory symptoms, with some patients presenting with GI symptoms without respiratory symptoms.26 A systematic review of clinical studies by D'Amico, e t al, reported diarrhea in 2%–50% of cases. It may precede or follow respiratory symptoms. A pooled analysis revealed an overall percentage of diarrhea at onset of 10.4%.27 Pooled data from five retrospective clinical studies of 1556 hospitalized patients with COVID-19 revealed that pharyngodynia is present in 12.4% of patients.23
A systematic review of 1457 patients with different ethnicities by Costa, et al, showed that 885 patients (60.7%) had smell disorders and 822 patients (56.4%) had taste disorders with varying intensity, with women more often affected.28 This olfactory/gustatory dysfunction (OGD) occurred even without nasal obstruction/rhinorrhea and can precede other COVID-19 signs and symptoms. The recovery of smell/taste, when it occurs, usually occurs in the first two weeks after COVID-19 resolution. Olfactory/gustatory dysfunction is a strong predictor of infection by SARS-CoV-2 and should be considered a part of the clinical features of COVID-19, even in mild cases.28 Agyeman, et al, estimated the prevalence of OGDs in COVID-19 patients in a systematic review.29 Twenty-four studies with 8438 patients with confirmed COVID-19 infection from 13 countries were analyzed. The pooled proportions of patients presenting with olfactory or gustatory symptoms were 41.0% and 38.2%, respectively. Increasing age was inversely correlated with the prevalence of OGD. A higher prevalence of olfactory dysfunction was found using objective measurements than with self-reports. No significant differences in the prevalence of OGDs by sex of patient were observed.29
A systematic review of 44 articles that included 507 patients with cutaneous manifestations was summarized by Zhao, et al .30 The skin lesions were polymorphic, erythematous, and chilblain-like. Urticarial lesions were most common, occurring on an average of 9.9 days (range 1–30) after the onset of systemic symptoms.30
Radiology
Early diagnosis of COVID-19 is vital for prompt clinical intervention and reduction of transmission to other individuals. Therefore, imaging has been proposed as a supporting diagnostic method in addition to the gold-standard testing with RT-PCR assays. Chest radiographs have low diagnostic yield in the early stages of the infection. However, infiltrates on computed tomography (CT) scans of the thorax have occasionally preceded symptom onset.31 Given its high sensitivity, multiple studies have established a significant role for CT scans in the diagnosis of COVID-19 patients. However, most radiology organizations and several scientific societies have recommended against the routine use of CT for the screening for COVID-19.32
Ai and colleagues first determined the diagnostic value of chest CT scans in patients with COVID-19. A total of 1014 patients with suspected COVID-19 underwent chest CT scans and RT-PCR. Of those, 601 (59%) had positive RT-PCR results; abnormal chest CT findings were present in 97% of the 601 patients. Using RT-PCR as the gold-standard diagnostic reference for COVID-19, their results show that the sensitivity and specificity of CT scans are 97% and 25%, respectively. The high false-positive rate of CT scans in detecting abnormal lung changes could be attributed to significant overlap with pneumonia caused by other pathogens, such as other respiratory viruses. An additional analysis of 258 patients who underwent multiple RT-PCR tests indicated that 15 of these patients had conversion from an initial negative to a positive test result. The initial chest CT scans were positive in 67% of these patients, and 93% of the patients presented with typical CT images consistent with COVID-19 diagnosis.33
In a systemic review done by Ojha, et al, who included 4410 adult patients with confirmed COVID-19, CT imaging was indicated in patients with specific criteria, including moderate-to-severe pneumonia, worsening respiratory status, possible risk for progression, or hypoxemia after recovery. In those subgroups, CT scans in combination with laboratory testing were used to diagnose COVID-19 and monitor the response to treatment.34 In this review, the most common radiological pattern across all the CT scans performed was ground-glass opacities (GGOs), detected in 50.2%, followed by mixed GGOs plus consolidation pattern in 44.4%, and consolidation in 24.2%. A reticular pattern was found in 9.9%. The hallmark distribution of the opacities was bilateral, mostly peripheral/subpleural, and mainly located posteriorly in the lungs. Less common findings included cavitation (0.1%), pleural effusions (5%), mediastinal lymphadenopathy (5.4%), pericardial effusions (3.6%), and a reverse halo sign (2.4%).34
Wang, et al, described the serial changes in the pulmonary parenchymal abnormalities over time in 90 COVID-19 infected patients. The predominant pattern detected after symptoms onset was GGOs (65% on illness days 0–5), which gradually progressed to a mixed pattern of GGOs plus consolidations (38% in the third week). Most of these patients (94%) had residual lesions at the time of discharge, and the majority of these were GGOs.35
Pan, et al, also described similar findings, with GGOs (75%) being the most common imaging manifestations at 0–4 days from the onset of symptoms progressing to a crazy paving pattern (53%) at 5–8 days.31 Ai, et al, pointed out that consolidations (91%) were the most common manifestation at 9–13 days, with gradual resolution of consolidations (75%) at more than 14 days of symptom onset. The findings peaked at 10 days in this study.35
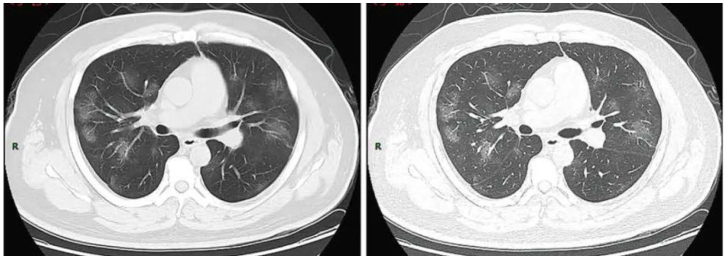
In summary, CT imaging can be used in the early diagnosis, evaluation of disease progression, and monitoring response to therapy. The most common CT manifestations are bilateral, peripheral/subpleural, posterior GGOs with/without consolidations with a lower lobe predominance (Fig 2). Since information is continuously evolving with new studies, it is essential for clinicians and radiologists to have updates on various manifestations of COVID-19 on CT scans, so that they can contribute to the management of these patients.
Figure 2.
Ground-glass opacities on computed tomography in a patient with COVID-19. Source: Wikimedia, file: COVID19CT2.webp, accessed September 5, 2020.
Pathology
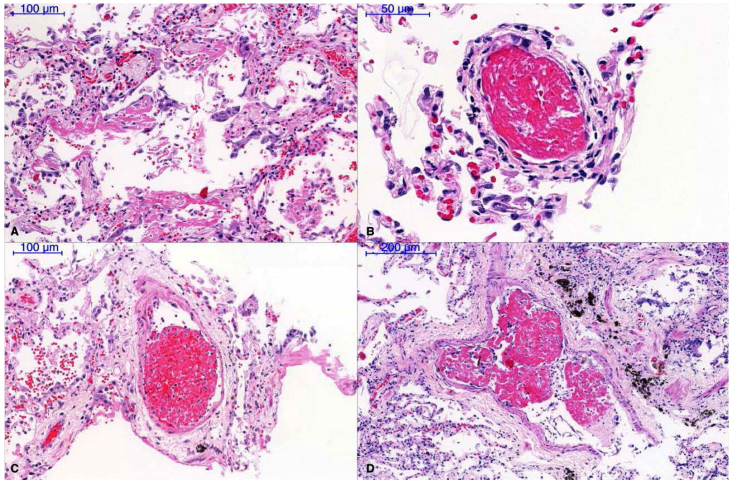
Patients with a COVID-19 and acute respiratory failure usually have abnormal chest x-rays with infiltrates that range from patchy ground-glass infiltrates to diffuse areas of consolidation. Autopsy studies in these patients have shown important abnormalities in the alveolar spaces and pulmonary vasculature. Schaller and colleagues conducted autopsies on 10 patients with COVID-19 who died in a German hospital.36 All patients had disseminated diffuse alveolar damage at different stages, usually more prominent in middle and lower lung fields (Fig 3). Nine patients had acute diffuse alveolar damage with hyaline membranes, intra-alveolar edema, and thickened alveolar septa with perivascular lymphocyte infiltration. Ten patients had organizing diffuse alveolar damage with pronounced fibroblastic proliferation, partial fibrosis, pneumocyte hyperplasia, and patchy lymphocyte infiltration. One patient had fully established fibrosis; four patients had mild lymphocytic myocarditis. At the time of autopsy, the virus was detected in the respiratory tract in all patients. These patients had a mean age of 79 and significant comorbidity.
Figure 3.
Pu lmonary Pathology. A. Diffuse alveolar damage with minimal lymphocyte infiltration; B–D. Fib rinous microthrombi in small-sized pulmonary arterioles. Reused with permission from John Wiley and Sons. Source: Journal of Thrombosis and Haemostasis. doi: 10.111 1/jth.14844 (accessed September 22, 2020).
Carsana, et al, studied 38 patients who died in two hospitals in northern Italy in March 2020.37 All autopsies showed features of exudative and proliferative diffuse alveolar damage with capillary congestion, necrosis of pneumocytes, hyaline membranes, interstitial and intra-alveolar edema, type II pneumocyte hyperplasia, squamous metaplasia, and platelet fibrin thrombi (Fig 3). Inflammatory infiltrates were largely macrophages in the alveolar lumen and lymphocytes in the interstitium. Electron microscopy revealed viral particles in pneumocytes.
Ackermann and co-workers compared seven lungs obtained at autopsy from patients who died of COVID-19 with seven lungs from patients who died from H1N1 influenza.38 The main histologic pattern was diffuse alveolar damage with necrosis of the alveolar lining cells, pneumocyte type II hyperplasia, and intra-alveolar fibrin deposition. Perivascular T-cell infiltration was also present. Lungs from patients with COVID-19 had severe endothelial injury associated with the presence of intracellular virus and disrupted cellular membranes. Pulmonary vessels in these patients had a widespread thrombosis with microangiopathy. Alveolar capillary microthrombi were nine times more prevalent in patients with COVID-19 than in patients who had influenza infection. Patients with COVID-19 infection had significant amounts of new vessel growth based on the mechanism of intussusceptive angiogenesis. These lungs also contained angiogenesis secondary to conventional sprouting; these angiographic features appeared to increase as a function of the length of hospital stay. ACE2 expression was much higher in epithelial cells and endothelial cells from patients in the lungs of patients who died from COVID-19.
Grosse studied 14 lungs from patients who died with COVID-19. All lungs contained bilateral diffuse alveolar damage; 11 of the 14 lungs had acute bronchopneumonia.39 These lungs also contained thrombotic/thromboembolic vascular occlusions. Eleven patients had capillary microthrombi, five patients had thrombi in midsize pulmonary vessels with complete vessel occlusion, one patient had bone marrow emboli, and one patient had septic emboli. All patients had pulmonary hemorrhage that ranged from mild to marked, and two patients had pulmonary infarction. Four patients had deep venous thrombi, but no patient had thrombi in any other organs, including heart, kidney, liver, spleen, and brain.
In summary, lungs from patients with COVID-19 have the typical features of ARDS, which include an exudative phase, an inflammatory phase, and a fibrotic phase. There is extensive injury to the endothelium with new vessel formation based on the mechanism of intussusception, and there is formation of microthrombi in capillaries. There are increased levels of ACE2, and virus is present in endothelial and epithelial cells and in extracellular locations. These pathologic changes should increase diffusion distances for oxygen and carbon dioxide transfer, abnormalities in alveolar spaces, interstitium, and pulmonary vessels create very abnormal ventilation-perfusion relationships, and diffuse infiltrates reduce lung compliance.
Pathophysiology
Acute Respiratory Failure
Hypoxemia
Patients with COVID-19 and acute respiratory failure can have significant hypoxemia but do not always have significant dyspnea associated with the low oxygen saturations. This has led to several commentaries that try to explain these observations.40 This presentation often involves patients who have minimal radiographic infiltration and consequently may not have increased work of breathing or stimulation of the J receptors in the lung parenchyma. Viral effects on the autonomic nervous system, carotid bodies, and the CNS could reduce the transmission of information related to hypoxemia or reduce the CNS’s “interpretation,” ie, dyspnea, of hypoxemia.40 In addition, older patients and patients with diabetes have decreased respiratory responses to hypoxemia. The response to hypoxemia is also significantly increased in patients who also have hypercapnia, which is unusual in patients with COVID-19 during the initial phase of the respiratory syndrome. Finally, this presentation likely occurs in other patients with non-COVID-19 ARDS during the initial phase of the clinical course but has not received much attention from clinicians.
The pathophysiologic explanations for hypoxemia in patients with COVID-19 include pronounced ventilation-perfusion abnormalities (V/Q mismatch), right-to-left shunts, and possibly diffusion limitation. Pathological studies in these patients indicate that they have diffuse lung disease with the typical pathologic features of ARDS. However, they also have significant vascular disease with microthrombi and new vessel formation. The presence of microthrombi should cause redistribution of blood flow from those regions to other regions of the lung, which potentially creates more V/Q mismatch. Reynolds, et al, used contrast enhanced transcranial Doppler of the middle cerebral arteries following the injection of agitated saline to identify shunts in the lung.41 They found that 83% (15/18) of patients had detectable microbubbles. The paO2/fiO2 ratio was inversely correlated with the number of microbubbles. In addition, the number of microbubbles was inversely correlated with lung compliance. This study suggests that patients with COVID-19 have dilatation of the pulmonary vasculature which allows the bubbles of approximately 24 µm in diameter to pass through the lung parenchyma into the systemic circulation. Vascular disease in these patients may limit autoregulation and reduce the possibility of internal adjustment of V/Q relationships to maintain better gas exchange. In addition, changes in vascular permeability increase the potential for fluid accumulation which in turn further aggravates gas exchange. Finally, rapid blood flow through narrowed capillaries could limit the time available for diffusion and contribute to hypoxemia.
Compliance
Some patients requiring mechanical ventilation for significant hypoxemia have had surprisingly normal lung compliance. This has led to the idea that the lung disease in these patients may differ from the usual disease seen in patients with other causes of ARDS. However, information collected on larger numbers of patients who require mechanical ventilation would suggest that compliance tends to be in the expected range for ARDS. Fan, et al, summarized information from seven studies reporting details regarding gas exchange and pressure-volume characteristics of the lung in patients with COVID-19.42 The median paO2/fiO2 ratio ranged from 103 to 182 mm Hg in 1914 patients; the median fiO2 ranged from 70% to 100% in 1581 patients; the median compliance ranged from 26 to 35 mL/cm H2O in 614 patients; the median plateau pressure ranged from 21 to 27 cm H2O in 652 patients; and the median PEEP ranged from 10 to 15 cm H2O in 1928 patients. In general, these patients had very poor gas exchange and reduced lung compliance in the expected range for ARDS patients. Less than 50% of patients in these various studies underwent prone positioning during their management.
Phenotypes
Patients with acute respiratory failure secondary to COVID-19 have a spectrum of clinical presentations that range from significant hypoxemia and relatively clear chest x-rays to significant hypoxemia and very abnormal chest x-rays. These observations have suggested that there are two phenotypes. Some patients have hypoxemia with low V/Q relationships, low lung weights, low lung elastance (high compliance), and low recruitability with PEEP.43 At the other end of the spectrum, some patients have high right-to-left shunts, high lung weights, high lung elastance (low compliance), and high recruitability with PEEP. These two phenotypes suggest that some patients have pulmonary disease with a mild or benign clinical course and do not progress to the point at which they require mechanical ventilation. Other patients have a severe clinical course and eventually require mechanical ventilation and often have high mortality rates.
Mechanical ventilation
The management of patients with acute respiratory distress requiring mechanical ventilation should focus on the standard approach used in other patients with ARDS.44 This approach should include the use of low tidal volumes (6–8 mL/kg ideal body weight) and low plateau pressures (<30 cm H2O). Ideally, the driving pressure should be <15 cm H2O. These patients may benefit from a relatively “high” PEEP level to increase lung recruitment. The fiO2 should be maintained at the lowest possible level resulting in O2 saturations in the range of 88%–92%. Some patients will develop hypercapnia, which does not necessarily require changes in ventilator parameters unless the arterial pH is below 7.2. Clinicians should strongly consider the possibility of prone positioning in patients with severe hypoxemia defined by a paO2/fiO2 ratio <150 mm Hg.45 These patients are at increased risk for self-induced lung injury if they make excessive effort during inspiration and increase transpulmonary pressure.46 This is relatively difficult to detect and is best studied using esophageal balloons to measure intrapleural pressures to calculate transpulmonary pressures.
Hypercoagulability
A prominent complication in patients with severe COVID-19 is hypercoagulability, which leads to thrombosis with more complicated disease courses and worse outcomes. Hypercoagulability can be identified in these patients using routine laboratory tests including increased D-dimer, fibrinogen, factor VIII, and von Willebrand factor (vWF) levels. A retrospective study in Wuhan, China, reported that 25% of hospitalized patients without thromboprophylaxis developed lower extremity venous thromboembolism (VTE).47 A retrospective study in the Netherlands on hospitalized ICU patients with nadroparin thromboprophylaxis demonstrated an incidence of ischemic stroke in 2.5% of patients and an incidence of VTE in 27%, with 81% of those patients developing pulmonary emboli.48 In general, the incidence of arterial thrombosis in various studies was significantly less at about 2.5%–3.7%.49 Several studies done on post-mortem COVID-19 patients have identified microthrombi and widespread thrombosis.38,50 When compared to patients with severe influenza, alveolar microthrombi were nine times more prevalent in COVID-19 patients.38
Several mechanisms could explain the pathophysiology of the hypercoagulable state in COVID-19. These mechanisms include a pro-inflammatory state, vessel wall injury, and stasis of blood flow. In critical illness, such as COVID-19, the coagulation cascade is activated by systemic inflammation to limit the spread of infection.49 The systemic inflammatory response results in adaptive hemostasis and increased cytokine production. The inflammatory cytokines formed include IL-6, IL-7, TNF, CCL2, CCL3, and the IL2 receptor, activating monocytes, neutrophils, and the endothelium.51 In one study, 80% of the bronchoalveolar lavages from COVID-19 patients with severe respiratory failure contained abundant monocyte and macrophages, identified by CD4 and CD16 markers.51 These macrophages have excessive production of IL-6.51 In response to pro-inflammatory cytokines, mainly IL-6, mononuclear cells express tissue factor and promote the generation of thrombin from prothrombin, triggering the coagulation cascade and thus the hypercoagulable state.51
A unique mechanism proposed to induce hypercoagulability with SARS-CoV-2 involves the virus binding to, activating, and directly damaging the endothelium. In the lung autopsies of COVID-19 patients, endothelial injury with an intracellular virus was identified in post-mortem patients.38 The SARS-CoV-2 spike (S) glycoprotein binds to and fuses with the ACE2 receptor on the endothelium to mediate cellular entry, consequently contributing to the shedding of ACE2 that normally degrades angiotensin II (ANGII).52 However, with the loss of ACE2 from viral internalization, there is decreased degradation of ANGII, resulting in excess binding of ANGII to its receptor AT1R, thereby stimulating IL-6 release.52 Excess ANGII induces tissue factor and plasminogen activator 1 expression and increases levels of vWF and factor VIII.53 These factors lead to the generation of thrombin, fibrin clot formation, and the hypercoagulable state of COVID-19, contributing to the poor prognosis in patients.
Multiple observational studies have been performed on COVID-19 patients to identify the effect of anticoagulation on mortality. A study in New York city at Mount Sinai Hospital used Cox proportional hazard models and found that mechanically ventilated COVID-19 patients on treatment-dose anticoagulation had an in-hospital mortality of 29.1% with a median survival of 21 days; patients without treatment-dose anticoagulation had a mortality of 62.7% and median survival of 9 days.54 This study also showed the association between a longer duration of anticoagulation treatment and a reduced risk of mortality. A retrospective observational study in Tongji Hospital in Shanghai, China, on 449 severe patients reported a decrease in 28-day mortality in heparin users compared to non-users for patients with a sepsis-induced coagulopathy score >4 (p=0.029) or D-dimer levels six times the upper limit of normal (p=0.017).55 Many trials, including a double-blind randomized controlled trial (Coagulopathy of COVID-19: A Pragmatic Randomized Controlled Trial of Therapeutic Anticoagulation versus Standard Care as a Rapid Response to the COVID-19 Pandemic, RAPID COVID COAG, www.invent-vte.com/studies/study/~815-rapid-covid-coag), comparing patients randomized to low-dose anticoagulation to those randomized to high-dose anticoagulation, are still underway to determine whether anticoagulation decreases disease progression and death in COVID-19 patients.
Cytokine Storm
Cytokine storm is defined as the hyperactive immune response due to an infection producing an uninhibited, maladaptive release of pro-inflammatory cytokines. In patients infected with COVID-19, studies have suggested that cytokine storm correlates with disease progression in patients.56 In fact, one study demonstrated increased levels of pro-inflammatory cytokines, such as granulocyte colony-stimulating factor, IP-10, MCP-1, macrophage inflammatory protein 1A, and TNF-α, in COVID-19 ICU patients in comparison to patients in the general wards.56 Furthermore, the cytokine storm positively correlates with the development of severe clinical manifestations, such as ARDS and extra-pulmonary organ failure.57
In the early stages of an immune response against viral infections, type 1 interferons (INF) and IFN-a and b have key antiviral roles in the control of infection.58 In vitro cell experiments of COVID-19 demonstrated delayed release of cytokines and chemokines from respiratory epithelial cells, with low levels of antiviral INFs and high levels of IL-IB, IL-6, TNF, CCL2, CCL3, and CCL5 in the later stages of infection.59,60 The abnormal release of IFNs and IFN-a and b cytokines later in the infection cycle hinders the normal antiviral response, and the hyperactive release of cytokines attracts neutrophils and monocytes causing infiltration of inflammatory cells in the lungs and death in a murine model.59 Infiltration of inflammatory cells eventually leads to apoptosis of airway and alveolar epithelial cells.61 Consequently, the impaired pulmonary microvascular and alveolar epithelial cell barriers potentiate vascular leakage, pulmonary edema, and ARDS.
Cytokine storm is strongly associated with disease progression in older patients. Studies have suggested an upregulated cytokine response in elderly patients is a potential reason for the severe deterioration in this age group. In one study on macaques (old world monkeys), despite similar SARS-CoV viral titers in aged macaques and in young macaques, the aged macaques were more likely to develop an increase in differential expression of genes associated with inflammation, potentially causing more severe clinical manifestations in these animals.62 Reducing type 1 IFN diminished the pathologic manifestations in this age group.62 In another study performed on aged SARS-CoV infected mice, viral replication induced delayed release of IFN-a and b with an influx of inflammatory mononuclear macrophages.63 The rapid production of mononuclear macrophages through positive feedback contributed to the dysregulated, elevated pro-inflammatory cytokines, such as TNF, IL-6, IL-1b, and increasing the disease severity.17 When the monocyte-macrophages were depleted and the inflammatory cytokine TNF neutralized, the mice were protected against the lethality of the virus.63 Similarly in humans, patients with a more severe disease course presented with higher levels of IL-2R and IL-6.57 With continuing evidence of immune dysregulation in COVID-19, reducing this response could alleviate the severe manifestations in some patients.
Ye, et al, reviewed the pathogenesis and treatment of cytokine storm in COVID-19.64 Multiple classes of medication were reviewed, including INF-γ, corticosteroids, intravenous immunoglobulins, interleukin antagonists or blockers, and blood purification using plasma exchange, absorption, and filtration. Mehta and coworkers suggested a standardized scoring system should be used to identify patients with cytokine storm to provide more accurate diagnoses.65 Finally, Sinha and colleagues suggested that the inflammatory events associated with COVID-19 are not necessarily more pronounced than the inflammatory events associated with some cohorts of patients with ARDS.66 In particular, patients with hyperinflammatory ARDS have much higher levels of IL-6 than patients with severe COVID-19. Consequently, the best approach to managing cytokine storm is unclear at this time.
Treatment
Various drugs and biological products have so far been proposed and used for the treatment of patients with COVID-19 (Table 1).
| Table 1: Drugs and biological products used for the treatment of patients with COVID-19 | ||||
| Drug | Pharmacology | Dose | Outcome | Side-effects |
|
Convalescent plasma |
Polyclonal antibodies directed against the SARS-CoV-2 virus.67 | 200–600 mL. |
Reduces mortality Increases viral clearance Improve the clinical condition in COVID-19 patients |
Transfusion-associated circulatory overload Transfusion-related lung injury Severe allergic reactions Rash |
| Remdesivir | Nucleoside analog prodrug that inhibits viral RNA-dependent RNA polymerase leading to inhibition of viral replication.72 |
200 mg on the first day, followed by 100 mg daily for the rest of the treatment period.77
Length of treatment differed between trials.77 |
Significantly reduced time-to-recovery compared to placebo (11 vs 15 days, p<0.001).79
There was also a modest increase in survival (mortality at 14 days 8.0% vs 11.6%, p=0.059).79 The US FDA issued an Emergency Use Authorization for use of remdesivir for COVID-19.79 |
One patient in the study by Wang, et al, was noted to have had a cardiac arrest.78
Individual cases of multiple organ dysfunction syndrome and septic shock were reported.78 Multiple reports of liver enzyme abnormalities in the study by Wang, et al .78 Reports of ARDS have also been reported; 16 patients experienced this outcome during treatment in the study by Wang, et al, which led to discontinuation in seven patients.78 |
| Dexamethasone | Inhibition of inflammation to control the hyperactive uninhibited maladaptive immune response believed to be contributing to the cytokine storm, ARDS and multiorgan failure in COVID-19 patients.80 |
20 mg iv daily for 5 days, followed by 10 mg daily for 5 days or until ICU discharge.81
6 mg iv or orally up to 10 days.82 |
Significant reduction in the mean ventilator-free days in the dexamethasone group compared to the standard care group (p=0.04).81
Significant lower 28-day mortality (p<0.001).82 |
Dexamethasone was not associated with increased risk of adverse events in critically ill COVID-19 patients.81,82
This includes need for insulin use for hyperglycemia, infection rate, and bacteremia.81 |
| Tocilizumab | Monoclonal antibody that selectively targets the interleukin-6 (IL-6) receptor.83 |
Dose and number of doses varied per Zhao meta-analysis83
Most studies 400–800 mg per dose 8 mg/kg (max 800 mg per dose) Number of doses: 1–3 |
Studies suggested a benefit, including significant difference in mortality,83,84 and improve patients’ clinical status.85
Others showed no reduction in mortality or clinical status improvement.86 |
No obvious adverse events reported.85,86 |
Convalescent Plasma
Convalescent plasma transfusion (CPT) has been tested in the past during epidemics and pandemics, such as the Spanish Influenza in 1915–1917, severe acute respiratory syndrome (SARS) in 2003, influenza A (H1N1) in 2009, avian influenza A (H5N1), and Ebola. It is obtained from patients who recently recovered from a viral illness, expecting to have maximum levels of polyclonal antibodies directed against that virus. In the absence of definitive therapy for COVID-19 infection thus far, CPT may be a crucial treatment for this disease.67
There is a lack of randomized control trials investigating CPT in COVID-19 patients. Only two trials were published after being stopped prematurely.68,69 The first study was a randomized trial in the Netherlands organized by Gharbharan, et al, comparing CPT with usual care for hospitalized COVID-19 patients. The primary endpoint was 60-day mortality. However, the study was terminated early after enrollment of 86 patients. Of the 66 patients tested, 53 already had anti-SARS-CoV-2 antibodies at baseline, with neutralizeing antibodies in 79% and titers comparable to the donor plasma. Subsequently, the study was discontinued by a data safety monitoring board due to concerns about the lack of benefit.68
The second clinical trial took place in seven medical centers in Wuhan, China, as an open-label, multicenter randomized clinical trial.69 A toal of 103 confirmed COVID-19 patients with severe or life-threatening illness were included. The primary endpoint was clinical improvement at 28 days. There was statistically insignificant clinical improvement in patients in the convalescent plasma group (51.9%) compared to the control group (43.1%). The primary outcome occurred in 91.3% of convalescent plasma severe COVID-19 subgroup compared to the 68.2% of the controls in this subgroup (p=0.03). No significant difference was found in the primary outcome of 28-day mortality between the plasma group and the control group.69
An open-label multicenter cohort study across 2807 acute care centers in the United States with over 35322 severe or life-threatening COVID-19 patients was conducted by Joyner, et al . The endpoints were 7- and 30-day mortality. The 7-day mortality was significantly lower in patients transfused with convalescent plasma within three days of diagnosis compared to those transfused 4 or more days after diagnosis (8.7% vs 11.9%, p<0.001). The results were similar at 30-day mortality endpoint (p<0.001). Moreover, the 7- and 30-day mortality rates were inversely associated with the IgG antibody levels in the transfused plasma, with lower mortality observed with higher concentrations of antibodies.70
Rajendran, et al, analyzed five studies reporting CPT in COVID-19 patients. This review concluded that convalescent plasma reduced mortality in critically ill patients, increased neutralizing antibody titers, accelerated the disappearance of SARS-CoV-2 RNA, and improved clinical symptoms.71 Another systematic review by Sarkar, et al, reached the same conclusion after analyzing seven studies with 5444 patients. The use of CPT seems to reduce mortality, increase viral clearance, and improve the clinical condition in COVID-19 patients.67
Based on these limited data, CPT therapy in COVID-19 patients appears to be safe and reduces mortality. Nonetheless, despite these promising outcomes, more randomized control trials on a large scale need to be conducted for better evaluation.
Remdesivir
Remdesivir (Veklury®, GS-5734, C27H35N6O8P, MW-602.6 g/mole, Gilead Sciences Inc, United States) is a nucleoside analog prodrug that inhibits viral RNA-dependent RNA polymerase.72 It was initially used to treat Ebola virus infections as an adenosine analog that incorporates into viral RNA, leading to inhibition of viral replication.73 In 2019, remdesivir infusion was administered for compassionate use to the first confirmed COVID-19 case in the United States. The patient was a 35-year-old man who received it on the 7th day of hospitalization; it resulted in remarkable clinical improvement and discontinuation of supplemental oxygen by the 8th day.74
Subsequently, a larger study was performed by Grein, et al, with 53 confirmed COVID-19 patients with hypoxia (room air oxygen saturation of ≤94%) who were treated with remdesivir. The loading dose was 200 mg on the first day followed by 9 days of 100 mg infusions. Of those patients, 68% demonstrated clinical improvement based on oxygen support, with 57% of the 30 mechanically ventilated patients extubated and 47% of patients discharged. In total, the mortality rate was 13%; 60% developed adverse reactions, mainly hepatic and renal dysfunction.75 Despite the encouraging results, the study had various limitations, including small sample size, lack of a comparison group, and missing data.76
Clinical trials are currently underway in several countries, including the United States, Norway, Canada, France, and China, in an attempt to adequately assess the efficacy of remdesivir. Although the length of treatment differs slightly, the dose of remdesivir is usually 200 mg on the first day, followed by 100 mg daily for the rest of the treatment period.77 The first randomized controlled clinical trial of remdesivir for treatment of COVID-19 was done in China by Wang, et al . The study analyzed the treatment of 237 patients (158 received remdesivir and 79 received placebo). The primary endpoint was the time needed to achieve clinical improvement. Despite the fact that the remdesivir group had a reduced time to clinical improvement (18 vs 23 days), this outcome was statistically insignificant. No statistically significant mortality or morbidity benefits were associated with remdesivir.78 There are several limitations to these results according to a systemic review published by Musa, et al . First, patients in the remdesivir and placebo groups received additional drugs (INF-α, lopinavir-ritonavir, antibiotics, and corticosteroids) before and after enrollment. These additional drugs made it difficult to differentiate between the effects of remdesivir and other treatments, especially since the placebo group received a higher percentage of these drugs. Second, 36 patients stopped treatment due to adverse reactions, reducing the sample size. Since the trial was terminated early, the statistical power was reduced from 80% to 58% in identifying a difference. Although these findings do not support remdesivir to treat COVID-19, the limitations, missing data, and early termination may have affected the results.76
The Adaptive COVID-19 Treatment Trial (ACTT) is an ongoing double-blind randomized controlled trial of remdesivir. After the data and safety monitoring board performed a preliminary analysis of 1063 patients, the National Institute of Allergy and Infectious Diseases reported in April 2020 that remdesivir significantly reduced time-to-recovery compared to placebo (11 vs 15 days, p<0.001). There was also a modest increase in survival (mortality at 14 days 7.1% vs 11.9%) that approached statistical significance (p=0.059). Although preliminary findings are supportive, it remains possible that the final results may differ at the conclusion of the trial. Nevertheless, based on the ACTT and Gilead open-label trial, the US Food and Drug Administration issued an Emergency Use Authorization (EUA) for use of remdesivir for COVID-19 in May 2020.79
Clinicians should consider remdesivir administration based on the strength of evidence available in the literature so far, which suggests decreased time-to-recovery and possibly increased survival.
Corticosteroids
Hyperimmune responses leading to uncontrolled inflammation could contribute to the ARDS and multiorgan failure seen in COVID-19 patients. For this reason, corticosteroids have been reasonably hypothesized as a possible treatment in COVID-19 infected patients to control this hyperinflammatory response.80
The COVID-19 Dexamethasone (CoDEX) randomized clinical trial by Bruno, et al, is a multicenter, open-label, randomized clinical trial conducted in 41 ICUs in Brazil. A total of 299 patients with COVID-19 and moderate-to-severe ARDS were enrolled. The 151 patients assigned to the treatment group received 20 mg of dexamethasone intravenously daily for 5 days, followed by 10 mg of dexamethasone daily for 5 days or until ICU discharge, plus standard care. The results were compared to the 148 patients who received standard care alone. The results demonstrated a significant (p=0.04) increase in the number of ventilator-free days in the dexamethasone group compared to the standard care group. There was no significant difference in the secondary outcomes, including all-cause mortality, ICU-free days, mechanical ventilation duration, or patients’ clinical status.81
The Randomized Evaluation of COVID-19 Therapy (RECOVERY) study is an open-label randomized controlled trial that compared the mortality in COVID-19 patients who received dexamethasone to COVID-19 patients who received standard care alone in the United Kingdom. A total of 2104 patients received dexamethasone 6 mg iv or orally up to 10 days; 4321 received standard care alone. The study concluded that the use of dexamethasone resulted in significant (p<0.001) lower 28-day mortality. Stratification revealed that the 28-day mortality was lower in those on either invasive mechanical ventilation or oxygen at randomization, but not in those on no respiratory support.82
Tocilizumab
Tocilizumab is a monoclonal antibody that selectively targets the interleukin-6 (IL-6) receptor and is mainly used to treat rheumatoid arthritis. Severe COVID-19 patients present with elevated inflammatory markers, and the elevation of IL-6 has been associated with the severity of infection. Therefore, tocilizumab has been proposed as a potential treatment for severe COVID-19 infections.83
A retrospective cohort study on 630 propensity-matched critically ill COVID-19 patients was done by Biran, et al, where 210 patients received tocilizumab and were compared to the 420 patients in a control group. The mortality rate was 57%, including 49% of the tocilizumab group and 61% of the control group. The median overall survival in patients receiving tocilizumab from date of admission was not reached; in non-tocilizumab users, the median overall survival was 19 days (p=0·003). Cox regression analysis noted an association between use of tocilizumab and decreased hospital related mortality (p=0·004).84
In a single-center cohort study conducted on 154 mechanically ventilated patients by Somers, et al, 79 patients received tocilizumab, and 76 patients served as the control group. This treatment had a 45% reduction in hazard of death with improved status on an ordinal outcome scale. Even though this study showed a statistically significant association between tocilizumab use and an increased risk of superinfections (54% vs 26%, p<0.001), there was still no difference in 28-day case fatality rate in tocilizumab-treated patients with superinfection compared to those without superinfection (22% vs 15%, p=0.42).85 A meta-analysis and systematic review conducted by Zhao included 10 studies involving 1675 severe COVID-19 patients. The result revealed a significant difference in mortality between the tocilizumab group (132/675, 19.5%) and control group (283/1000, 28.3%; p < 0.001), suggesting the efficacy of tocilizumab treatment for severe COVID-19.83
The first global, double-blind, placebo-controlled randomized controlled trial with tocilizumab (COVACTA trial of Actemra/RoActemra [tocilizumab] from Roche) is currently in phase III. However, as of July 29, 2020, a Roche update indicated that this trial did not have promising results. The results of Actemra use showed that patients did not meet the primary endpoint of improved clinical status. Moreover, it did not meet the key secondary endpoint of reducing patient mortality. There was no significant difference in ventilator-free days between groups (median of 22 days in Actemra/RoActemra vs 16.5 days in placebo, p=0.32). Patients treated with tocilizumab did demonstrate shorter inpatient time compared to placebo. Nonetheless, it is not considered significant due to its not meeting the primary endpoint.86
Based on these limited data, there is still inadequate evidence that tocilizumab improves outcomes in the treatment of COVID-19 patients. More randomized controlled trials need to be conducted prior to using tocilizumab in COVID-19.
National Institutes of Health (USA) Expert Panel Recommendations87
i. The COVID-19 Treatment Guidelines Panel recommends using dexamethasone 6 mg/day for up to 10 days for the treatment of COVID-19 in patients who are mechanically ventilated and in patients who require supplemental oxygen but who are not mechanically ventilated.
ii. Because remdesivir supplies are limited, the panel recommends prioritizing remdesivir for use in hospitalized patients with COVID-19 who require supplemental oxygen but who do not require oxygen delivery through a high-flow device, noninvasive ventilation, invasive mechanical ventilation, or extracorporeal membrane oxygenation.
iii. The Panel recommends against the use of chloroquine or hydroxychloroquine for the treatment of COVID-19 in hospitalized patients. The Panel recommends against using hydroxychloroquine plus azithromycin to treat COVID-19, except in a clinical trial.
iv. The Panel recommends against the use of the following immunomodulatory [drugs] for the treatment of COVID-19, except in a clinical trial. Examples include anti-IL-6 receptor monoclonal antibodies (eg, sarilumab and tocilizumab) and anti-IL-6 monoclonal antibody (siltuximab).
v. The panel concluded that there are insufficient data to recommend either for or against the use of convalescent plasma for the treatment of COVID-19.
Hospital Management during the COVID-19 Pandemic
The COVID-19 pandemic has presented significant challenges to hospitals. In particular, the physical workload and emotional stress in health care professionals has required hospitals to create protocols to maintain worker safety and to prevent burnout. Triage protocols for acute respiratory infection admissions start with hospital entry point restrictions, including point of entry mask requirements.88 If patients are COVID-19 suspects, they are immediately taken to an isolation room.88 To prevent transmission among health care providers, teams are usually established to reduce cross-transmission.88 If a member of one team is infected, then the rest of the team must quarantine, and this shifts work to other medical personnel.
The key principle established in the majority of hospitals is central, regular communication between the hospital leadership and medical professionals. Centralized communication is organized and established weekly by one individual to alleviate the mass threads of information sent by multiple individuals. This one individual provides updates on hospital metrics of number of cases, bed capacity, amount of available resources, and available staff.89 Part of the strategy focuses on allowing for two-way communication so that hospital providers are heard and have check-ins on an individual basis to demonstrate a supportive environment.89 To prevent additional burnout, focus leadership groups, targeting PPE, COVID-19 testing, and discharge protocols, have been created to split up additional work load among providers. During the initial phase of the pandemic, to ensure adequate personnel and hospital supplies, elective surgeries were cancelled and these procedural-based providers were directed to assist in screening clinics, phone lines, and emergency departments, to allow for the inpatient hospitalists to focus on their inpatient duties and prevent burnout.89
Conclusions
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19) and can cause severe acute respiratory failure. These patients usually have diffuse pulmonary infiltrates and severe hypoxemia and often require mechanical ventilation and have a relatively high mortality. COVID-19 can also cause an acute inflammatory response, cytokine storm, which contributes to the inflammation in the lung and other organs and is associated with elevated levels of ferritin, D-dimer, CRP, and erythrocyte sedimentation rates. Patients with COVID-19 frequently have coagulopathy and can develop deep venous thrombi and pulmonary emboli. At present, there is no specific antiviral treatment. Corticosteroids improve outcomes in patients on oxygen or on mechanical ventilation. The FDA has provided Emergency Use Authorization for the use of remdesivir and convalescent plasma in hospitalized patients. Multiple other drugs are under study.
This pandemic has had widespread and devastating effects throughout the world. Management of this pandemic will require the development of safe antiviral drugs with specific activity against this virus, the production of effective vaccination, or spontaneous changes in viral virulence. The likelihood of and the timeframe for the development of any of these events are unknown.
Conflicts of Interest:
None declared.
Cite this article as: Maveddat A, Mallah H, Rao S, et al. Severe acute respiratory distress syndrome secondary to coronavirus 2 (SARS-CoV-2). Int J Occup Environ Med 2020;11:157-178. doi: 10.34172/ijoem.2020.2202
References
- 1.Wu C, Liu Y, Yang Y. et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10:766–88. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astuti I, Ysrafil Ysrafil. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab Syndr. 2020;14:407–12. doi: 10.1016/j.dsx.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H, Li X, Li T. et al. The genetic sequence, origin, and diagnosis of SARS-CoV-2. Eur J Clin Microbiol Infect Dis. 2020;39:1629–35. doi: 10.1007/s10096-020-03899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walls AC, Park YJ, Tortorici MA. et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–92 e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shang J, Ye G, Shi K. et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–4. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q, Zhang Y, Wu L. et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894–904 e9. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan R, Zhang Y, Li Y. et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–8. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu R, Zhao X, Li J. et al. Genomic characterisation and epidemiology of 201novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020;395:565–74. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen KG, Rambaut A, Lipkin WI. et al. The proximal origin of SARS-CoV-2. Nat Med. 2020;26:450–2. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takayama K. In vitro and animal models for SARS-CoV-2 research. Trends in Pharmacological Sciences. 2020;41:513–7. doi: 10.1016/j.tips.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ammerman NC, Beier-Sexton M, Azad AF. Growth and maintenance of Vero cell lines. Curr Protoc Microbiol. 2008;Appendix 4:Appendix–4E. doi: 10.1002/9780471729259.mca04es11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vieira DFB, da Silva MAM, Garcia CC. et al. Morphology and morphogenesis of SARS-CoV-2 in Vero-E6 cells. Virology. 2020;??:??. doi: 10.21203/rs.3.rs-40432/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo YR, Cao QD, Hong ZS. et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mallapaty S. Mini organs reveal how the coronavirus ravages the body. Nature. 2020;583:15–6. doi: 10.1038/d41586-020-01864-x. [DOI] [PubMed] [Google Scholar]
- 15.Monteil V, Kwon H, Prado P. et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–13 e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamers MM, Beumer J, van der Vaart J. et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50–4. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schultz MB, Vera D, Sinclair DA. Can artificial intelligence identify effective COVID-19 therapies? EMBO Mol Med. 2020;12:e12817. doi: 10.15252/emmm.202012817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranga A, Gjorevski N, Lutolf MP. Drug discovery through stem cell-based organoid models. Adv Drug Deliv Rev. 2014;69-70:19–28. doi: 10.1016/j.addr.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Mostafa HH, Hardick J, Morehead E. et al. Comparison of the analytical sensitivity of seven commonly used commercial SARS-CoV-2 automated molecular assays. J Clin Virol. 2020;130:104578. doi: 10.1016/j.jcv.2020.104578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D, Hu B, Hu C. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Morales AJ, Cardona-Ospensa JA, Gutierrez-Ocampo E. et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Disease. 2020;??:??. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao Y, Liu X, Xiong L. et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: A systematic review and meta-analysis. J Med Virol. 2020;??:??. doi: 10.1002/jmv.25822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lovato A, de Filippis C. Clinical presentation of COVID-19: a systematic review focusing on upper airway symptoms. Ear, Nose Throat J. 2020;??:??. doi: 10.1177/0145561320920762. [DOI] [PubMed] [Google Scholar]
- 24.Tahvildari A, Arbahi M, Farsi Y. et al. Clinical features, diagnosis, and treatment of COVID-19 in hospitalized patients: a systematic review of case reports and case series. Front Med (Lausanne) 2020;7:231. doi: 10.3389/fmed.2020.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katal S, Balakrishnan S, Gholamrezanezhad A. Neuroimaging findings in COVID-19 and other coronavirus infections: a systematic review in 116 patients. J Neuroradiology. 2020;??:??. doi: 10.1016/j.neurad.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cha MH, Miguel Regueiro M, Sandhu DS. Gastrointestinal and hepatic manifestations of COVID-19: A comprehensive review. World J Gastroenterology. 2020;26:2323–32. doi: 10.3748/wjg.v26.i19.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Amico F, Baumgart DC, Danese S. et al. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention and management. Clin Gastroenterology Hepatology. 2020;??:??. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.da Costa VT, Carnauba ATL, Rocha KW. et al. Olfactory and taste disorders in COVID-19: a systematic review. Brazilian J Otorhinolaryngology. 2020;??:???. doi: 10.1016/j.bjorl.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agyeman AA, Chin KL, Landersdorfer CB. et al. Smell and taste dysfunction in patients with COVID-19: A systematic review and meta-analysis. Mayo Clin Proc. 2020;95:1621–31. doi: 10.1016/j.mayocp.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Q, Fang X, Pang Z. et al. COVID-19 and cutaneous manifestations: A systematic review. J European Academy Dermatology Venereology. 2020;??:??. doi: 10.1111/jdv.16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan Y, Guan H, Zhou S. et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020;30:3306–9. doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. ACR recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID-19 infection | American College of Radiology. Available from www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection (Accessed September 9, 2020).
- 33.Ai T, Yang Z, Hou H. et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology. 2020;296:E32–40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ojha V, Mani A, Pandey NN. et al. CT in coronavirus disease 2019 (COVID-19): a systematic review of chest CT findings in 4410 adult patients. Eur Radiol. 2020;??:1. doi: 10.1007/s00330-020-06975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Dong C, Hu Y. et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: A longitudinal study. Radiology. 2020;296:E55–64. doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaller T, Hirschbühl K, Burkhardt K. et al. Postmortem examination of patients with COVID-19. JAMA. 2020;??:??. doi: 10.1001/jama.2020.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carsana L, Sonzogni A, Nasr A. et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;??:??. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ackermann M, Verleden SE, Kuehnel M. et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–8. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grosse C, Grosse A, Salzer HJF. et al. Analysis of cardiopulmonary findings in COVID-19 fatalities: High incidence of pulmonary artery thrombi and acute suppurative bronchopneumonia. Cardiovasc Pathol. 2020;??:49. doi: 10.1016/j.carpath.2020.107263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tobin MJ, Laghi F, Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020;202:356–60. doi: 10.1164/rccm.202006-2157CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reynolds AS, Lee AG, Renz J. et al. Pulmonary vascular dilatation detected by automated transcranial Doppler in COVID-19 pneumonia. Am J Respir Crit Care Med. 2020;??:??. doi: 10.1164/rccm.202006-2219le. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan E, Beitler JR, Brochard L. et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med. 2020;8:816–21. doi: 10.1016/S2213-2600(20)30304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gattinoni L, Chiumello D, Caironi P. et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323:2329–30. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 45.Carsetti A, Paciarini AD, Marini B. et al. Prolonged prone position ventilation for SARS-CoV-2 patients is feasible and effective. Crit Care. 2020;24:225. doi: 10.1186/s13054-020-02956-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Worsham CM, Banzett RB, Schwartzstein RM. Air hunger and psychological trauma in ventilated patients with COVID-19 An urgent problem. Ann Am Thorac So. 2020;17:926–7. doi: 10.1513/AnnalsATS.202004-322VP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui S, Chen S, Li X. et al. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–4. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klok FA, Kruip MJHA, van der Meer NJM. et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombosis Research. 2020;191:145. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abou-Ismail MY, Diamond A, Kapoor S. et al. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management. Thrombosis Research. 2020;194:101–15. doi: 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Menter T, Haslbauer JD, Nienhold R. et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology August. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nature Reviews Immunology. 2020;20:355–62. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zores F, Rebeaud ME. COVID and the renin-angiotensin system: are hypertension or its treatments deleterious? Frontiers in Cardiovascular Medicine. 2020;7:71. doi: 10.3389/fcvm.2020.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaughan DE, Lazos SA, Tong K. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells: a potential link between the renin-angiotensin system and thrombosis. J Clin Invest. 1995;95:995–1001. doi: 10.1172/JCI117809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paranjpe I, Fuster V, Lala A. et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:122–4. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang N, Bai H, Chen X. et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–9. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang C, Wang Y, Li X. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen L, Liu HG, Liu W. et al. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:203–8. doi: 10.3760/cma.j.issn.1001-0939.2020.03.013. [in Chinese]. [DOI] [PubMed] [Google Scholar]
- 58.Garcia-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: A lesson in détente. Science. 2006;312:879–82. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- 59.Channappanavar R, Fehr AR, Zheng J. et al. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J Clin Invest. 2019;129:3625–39. doi: 10.1172/JCI126363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Law HKW, Cheung CY, Ng HY. et al. Chemokine up-regulation in SARS-coronavirus–infected, monocyte-derived human dendritic cells. Blood. 2005;106:2366–74. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herold S, Steinmueller M, von Wulffen W. et al. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Experimental Med. 2008;205:3065–77. doi: 10.1084/jem.20080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smits SL, de Lang A, van den Brand JMA. et al. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathogens. 2010;6:e1000756. doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rockx B, Baas T, Zornetzer GA. et al. Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. J Virology. 2009;83:7062–74. doi: 10.1128/JVI.00127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘cytokine storm’ in COVID-19. J Infect. 2020;80:607–13. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mehta P, McAuley DF, Brown M. et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–4. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sinha P, Matthay MA, Calfee CS. Is a “cytokine storm” relevant to COVID-19? JAMA Intern Med. 2020;180:1152–4. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 67.Sarkar S, Soni KD, Khanna P. Convalescent plasma is a clutch at straws in COVID-19 management! A systematic review and meta-analysis. J Med Virol. 2020;???:???. doi: 10.1002/jmv.26408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gharbharan A, Jordan CCE, GeurtsvanKessel C. et al. Convalescent plasma for COVID-19 A randomized clinical trial. medRxiv. 2020;??:??. doi: 10.1101/2020.07.01.20139857. [DOI] [Google Scholar]
- 69.Li L, Zhang W, Hu Y. et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: A randomized clinical trial. JAMA. 2020;??:??. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Joyner MJ, Senefeld JW, Klassen SA. et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: Initial three-month experience. medRxiv. 2020;??:??. doi: 10.1101/2020.08.12.20169359. [DOI] [Google Scholar]
- 71.Rajendran K, Krishnasamy N, Rangarajan J. et al. Convalescent plasma transfusion for the treatment of COVID-19: Systematic review. J Med Virol. 2020;??:??. doi: 10.1002/jmv.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eastman RT, Roth JS, Brimacombe KR. et al. Remdesivir: A review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci. 2020;6:672–83. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Warren TK, Jordan R, Lo MK. et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–5. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holshue ML, DeBolt C, Lindquist S. et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–36. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grein J, Ohmagari N, Shin D. et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–36. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Musa A, Warbasse E, Baron DA. et al. Addendum to systematic review of remdesivir for the treatment of COVID-19. West J Emerg Med. 2020;21:742–3. doi: 10.5811/westjem.2020.5.48121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frediansyah A, Nainu F, Dhama K. et al. Remdesivir and its antiviral activity against COVID-19: A systematic review. Clin Epidemiol Glob Health. 2020;??:??. doi: 10.1016/j.cegh.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y, Zhang D, Du G. et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–78. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Clinicaltrials.gov (2020). Adaptive COVID-19 Treatment Trial (ACTT). Available from https://clinicaltrials.gov/ct2/show/NCT04280705?term=remdesivir&draw=2&rank=9 (Accessed September 9, 2020).
- 80.Xiang Z, Liu J, Shi D. et al. Glucocorticoids improve severe or critical COVID-19 by activating ACE2 and reducing IL-6 levels. Int J Biol Sci. 2020;16:2382–91. doi: 10.7150/ijbs.47652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tomazini BM, Maia IS, Cavalcanti AB. et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: The CoDEX randomized clinical trial. JAMA. 2020;??:??. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Group RC, Horby P, Lim WS. et al. Dexamethasone in hospitalized patients with COVID-19 - preliminary report. N Engl J Med. 2020;???:??. doi: 10.1056/NEJMoa2021436. [DOI] [Google Scholar]
- 83.Zhao J, Cui W, Tian BP. Efficacy of tocilizumab treatment in severely ill COVID-19 patients. Crit Care. 2020;24:524. doi: 10.1186/s13054-020-03224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Biran N, Ip A, Ahn J. et al. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;??:??. doi: 10.1016/S2665-9913(20)30277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Somers EC, Eschenauer GA, Troost JP. et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis. 2020;??:??. doi: 10.1101/2020.05.29.20117358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Roche - Roche provides an update on the phase III COVACTA trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 associated pneumonia. July 29. Available from www.roche.com/media/releases/med-cor-2020-07-29.htm (Accessed September 9, 2020).
- 87. NIH-treatment. The Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available from www.covid19treatmentguidelines.nih.gov/ (Accessed September 9, 2020).
- 88.Ağalar C, Öztürk Engin D. Protective measures for COVID-19 for healthcare providers and laboratory personnel. Turk J Med Sci. 2020;50:578–84. doi: 10.3906/sag-2004-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garg M, Wray CM. Hospital medicine management in the time of COVID-19: Preparing for a sprint and a marathon. J Hosp Med. 2020;15:305–7. doi: 10.12788/jhm.3427. [DOI] [PubMed] [Google Scholar]