Abstract
Context
Exercise-related lower leg pain (ERLLP) is common in runners.
Objective
To compare biomechanical (kinematic, kinetic, and spatiotemporal) measures obtained from wearable sensors as well as lower extremity alignment, range of motion, and strength during running between runners with and those without ERLLP.
Design
Case-control study.
Setting
Field and laboratory.
Patients or Other Participants
Of 32 young adults who had been running regularly (>10 mi [16 km] per week) for ≥3 months, 16 had ERLLP for ≥2 weeks and 16 were healthy control participants.
Main Outcome Measure(s)
Both field and laboratory measures were collected at the initial visit. The laboratory measures consisted of alignment (arch height index, foot posture index, navicular drop, tibial torsion, Q-angle, and hip anteversion), range of motion (great toe, ankle, knee, and hip), and strength. Participants then completed a 1.67-mi (2.69-km) run along a predetermined route to calibrate the RunScribe devices. The RunScribe wearable sensors collected kinematic (pronation excursion and maximum pronation velocity), kinetic (impact g and braking g), and spatiotemporal (stride length, step length, contact time, stride pace, and flight ratio) measures. Participants then wore the sensors during at least 3 training runs in the next week.
Results
The ERLLP group had a slower stride pace than the healthy group, which was accounted for as a covariate in subsequent analyses. The ERLLP group had a longer contact time during the stance phase of running (mean difference [MD] = 18.00 ± 8.27 milliseconds) and decreased stride length (MD = −0.11 ± 0.05 m) than the control group. For the clinical measures, the ERLLP group demonstrated increased range of motion for great-toe flexion (MD = 13.9 ± 4.6°) and ankle eversion (MD = 6.3 ± 2.7°) and decreased strength for ankle inversion (MD = −0.49 ± 0.23 N/kg), ankle eversion (MD = −0.57 ± 0.27 N/kg), and hip flexion (MD = −0.99 ± 0.39 N/kg).
Conclusions
The ERLLP group exhibited a longer contact time and decreased stride length during running as well as strength deficits at the ankle and hip. Gait retraining and lower extremity strengthening may be warranted as clinical interventions in runners with ERLLP.
Keywords: medial tibial stress syndrome, shin splints, running, biomechanics
Key Points
Runners with exercise-related lower leg pain (ERLLP) displayed increased contact time and decreased stride length during running.
The ERLLP group exhibited strength deficits in ankle inversion and eversion and hip flexion.
Gait training and strengthening protocols may be warranted to address deficits in runners with ERLLP.
Running is a popular form of physical activity that may lead to overuse injuries, such as exercise-related lower leg pain (ERLLP),1 which refers to a group of injuries that include medial tibial stress syndrome, tibial stress fractures, chronic exertional compartment syndrome (CECS), and tendinopathy of the anterior or posterior tibialis muscles.2 Exercise-related lower leg pain can result in time loss from running, as demonstrated by the 60% of runners with ERLLP who missed at least 1 day of training due to their condition.3 Additionally, 68% of collegiate cross-country runners reported having experienced ERLLP at some point during their running career.3,4 The condition poses a significant problem to runners at all levels of participation. Some discomfort with physical activity is expected, but increased pain may deter people from exercising. Some runners then choose to rest or quit running entirely, which leads to time lost from running, reduced mileage, impaired race performance, decreased fitness, and potential overall health consequences.3,5
To treat this prevalent running injury, we must understand how runners with ERLLP differ from their healthy counterparts. Unfortunately, the causes of ERLLP are not firmly established, although some risk factors have been identified. These risk factors include a navicular drop greater than 10 mm,4,6 increased rearfoot pronation excursion during running,7,8 and increased medial plantar pressure during running.7 Yet these risk factors have been primarily identified via laboratory studies and may not fully represent how runners in normal training environments run. In a motion-capture study, researchers9 found that participant cadence was increased, stride time and stride length were decreased, knee-flexion moment was reduced, and dorsiflexion moment was increased when running on a treadmill compared with running on an overground surface. These differences suggest that running may differ as settings or surface types change. For example, variables such as stride length and cadence are often targeted by gait-retraining studies to reduce ERLLP.10 If these metrics are decreased during treadmill running but not in an outdoor running environment, then they may not be factors that deserve focus during gait-retraining interventions. Therefore, it is necessary to quantify running-gait patterns among runners with ERLLP in outdoor running settings.
Wearable technology makes analyzing running biomechanics in outdoor settings possible. The RunScribe wearable sensors (Scribe Labs Inc, Half Moon Bay, CA) have shown acceptable reliability and concurrent validity compared with laboratory gait analysis11 and face validity when tested on various surface types and at different speeds.12 The RunScribe sensors are small, lightweight pods that are shoe mounted and record kinematic, kinetic, and spatiotemporal measures during a run. Unlike treadmill motion-capture systems that require cumbersome markers to be secured to the participant, RunScribe allows the individual to run naturally, in any location, without hindrance from external materials. Therefore, the purpose of our study was to compare kinematic, kinetic, and spatiotemporal measures during running in field settings, as well as clinical measures of lower extremity alignment, range of motion (ROM), and strength between runners with and runners without ERLLP.
METHODS
Design
We performed a case-control study to compare running-gait biomechanics and lower extremity alignment, ROM, and strength between runners with and those without ERLLP. The primary biomechanical dependent variables were kinematic (pronation excursion and maximum pronation velocity), kinetic (braking g and impact g), and spatiotemporal (stride length, step length, contact time, stride pace, and flight ratio) measures recorded during running. The secondary dependent variables were the clinical laboratory measures for lower extremity alignment, ROM, and strength.
Participants
A total of 32 recreational runners were recruited from a large public university and the surrounding community. Sixteen runners had ERLLP and 16 were healthy control individuals (Table 1). To participate, runners needed to run an average of 10 or more mi (16 km) per week for at least the past 3 months. All runners were between the ages of 18 and 45 years. To be included in the ERLLP group, participants needed to have had pain and tenderness below the knee and above the ankle affecting 5 cm or more of the tibia13 or cramping or burning pain in the lower leg during or after exercise and currently be experiencing pain during physical activity that had persisted for at least 2 weeks. Healthy runners were defined as those with a visual analog scale (VAS) score of <10/100 mm for lower leg pain and no pain with clinical evaluation.
Table 1.
Participant Demographics
| Demographic |
Healthya |
Exercise-Related Lower Leg Paina |
P Value |
Cohen d |
| Age, y | 24 ± 4 | 23 ± 6 | .84 | 0.07 |
| Height, cm | 170.14 ± 8.68 | 171.41 ± 8.35 | .68 | −0.15 |
| Body mass index, kg/m2 | 23.02 ± 3.05 | 23.32 ± 1.89 | .74 | −0.12 |
| Godin Leisure Time Questionnaire, units | 106 ± 58 | 74.6 ± 18 | .05b | 0.73 |
| Exercise-Induced Leg Pain-British version questionnaire, % | 99.53 ± 1.01 | 75.16 ± 9.72 | <.001b | 3.53 |
| Lower Extremity Functional Scale, points | 80 ± 1 | 72 ± 8 | <.001b | 1.39 |
| Running experience, y | 5 ± 4 | 5 ± 3 | .77 | −0.10 |
| Weekly mileage | 23.6 ± 15.0 | 19.8 ± 14.2 | .47 | 0.26 |
| Average pace, min/mile | 7.92 ± 0.98 | 8.54 ± 0.75 | .05b | −0.71 |
| Greatest pain in last week (range = 0–10) | 0 ± 0 | 4.6 ± 2.1 | <.001b | −2.92 |
| Steps analyzed, No. | 300,684 | 270,066 |
Each group had N = 16 (8 males and 8 females). Values are mean ± SD except where indicated.
Significant at P ≤ .05.
Volunteers were excluded if they had a history of lower extremity surgery within the last 12 months, were currently experiencing Achilles tendon pain (as this is an inherently different condition14,15), had been instructed not to run by a physician, or had a recent history of fracture or a prolonged period of inactivity. Additionally, recruits were excluded if they self-reported any condition known to affect gait, such as multiple sclerosis, muscular dystrophy, diabetes mellitus, lumbosacral radiculopathy, neurologic or vestibular disorder, cardiovascular disease, fibromyalgia, pregnancy, Marfan syndrome, previous spine injury or surgery, peripheral artery disease, or deep vein thrombosis. Runners with ERLLP were excluded if they had any lower extremity weight-bearing restrictions,3 a VAS score of >70/100 mm,16 or were in too much pain to complete the runs in the study.14 Healthy participants were excluded if they had any other condition that might have interfered with running, such as low back pain, hip pain, knee pain, or another running-related injury, a recent history of ERLLP (within 12 months), or pain with running in general.
Participants in each group were matched based on sex, age, and years of running experience and were not permitted to wear minimalist running shoes during the runs analyzed for this study. All participants provided informed consent before the study. The study methods were approved by the university's Institutional Review Board for Health Science Research.
Instrumentation
The RunScribe wearable sensors (version 2.3.3) were used to collect kinematic, kinetic, and spatiotemporal measures during participant running. The sensors consisted of a triaxial accelerometer and gyroscope that sampled at 200 Hz. The Foot Posture Index 6-item version and Arch Height Index measurement tool (JAKTOOL Corp, Cranberry, NJ) were used to assess foot morphology. A MicroFET2 digital handheld dynamometer (Hoggan Health Industries, West Jordan, UT) was used to assess strength. A clear, plastic 12-in (30.48-cm) goniometer and a standard tape measure were used to assess ROM. All participants wore their own running shoes during each run and were not permitted to change shoes for running activities for the duration of the study.
Procedures
Participants completed the Godin Leisure Time Questionnaire, Exercise-Induced Leg Pain-British version questionnaire, Lower Extremity Functional Scale, a running history questionnaire, VAS for lower leg pain, and a clinical assessment. The athletic trainer performing clinical assessments was not blinded to group allocation or questionnaire responses. Lower extremity alignment measures of leg length, arch height index (seated and standing), arch rigidity index, arch drop, Foot Posture Index, tibial torsion, Q-angle, and hip anteversion were collected using previously described methods.17,18 Similarly, passive ROM and strength measures for the first metatarsophalangeal (flexion and extension),17 ankle (plantar flexion, dorsiflexion, inversion, and eversion),17 knee (flexion and extension),19 and hip (abduction, flexion, and extension)19 were obtained. The measurement order was not randomized and followed the methods of previous researchers17 who performed clinical assessments of foot alignment, lower extremity ROM, and strength.
Each eligible participant was given a pair of RunScribe sensors (left and right) and instructed on their proper use and care. The RunScribe sensors were placed on the back of the shoes, consistent with earlier studies.11,12,20 The tightest clip size that best fit the shoe was used to ensure reduced levels of motion. No additional materials were used to secure the sensors. Participants completed a calibration run of 1.67 mi (2.69 km) on a predetermined route around the university campus. The runners kept the sensors for the next week, during which time they completed and recorded at least 3 runs on their own, running at their normal pace and in their usual training locations. They were instructed to wear the same shoes for all runs. They were also directed to keep a running log to rate the levels of pain before, during, and after the run, as well as other relevant details from the run (eg, time run, distance, and terrain).
Data Processing
Data were stored in the sensors and then uploaded from the RunScribe mobile phone application via Bluetooth (Bluetooth SIG, Inc, Kirkland, WA) and then to the RunScribe website via wireless transmission. The data were then exported to an Excel (Microsoft Corp, Redmond, WA) document from the website by a study team member. The data were trimmed to eliminate walking steps, which were determined by a decrease in pace to less than 1.79 m/s. We used all remaining steps to calculate the mean values for each dependent measure of each participant's runs except the calibration run.
Statistical Analysis
We performed an a priori sample-size estimate based on previously published data showing a 7.54° difference in pronation excursion between medial tibial stress syndrome and healthy groups. Therefore, assuming variability in pronation excursion of approximately 5.8°,11 we estimated that 12 participants per group would be needed to find statistically significant differences at an α level (type I error) of .05 and power (1−β) of 0.8 and because we anticipated that up to 15% of participants' data might be lost to attrition.
The patient-reported outcome measures for leg pain and physical activity were compared using independent t tests with α set a priori to .05. To determine if any significant underlying factors would need to be controlled for during the running outcome analyses, we used independent t tests to assess group-level differences in running stride pace and demographics. Stride pace was decreased in the ERLLP group (mean difference [MD] = −0.39 ± 0.15 m/s, P = .01) and, thus, was included as a covariate in subsequent running outcome analyses.
Based on the clinical assessment, 13 of the 16 ERLLP runners were identified as having bilateral symptoms. Paired t tests with α set to .05 were performed to assess between-limbs differences within the ERLLP and healthy groups to determine if pooled limb assessments would be a reasonable statistical approach for between-groups comparisons. Of the clinical measures and biomechanical running outcomes, the only difference was increased pronation excursion (MD = 2.15 ± 1.00°, P = .05) for the reported worse limb in the ERLLP group, whereas the healthy group demonstrated no differences between limbs. Given that these were the only discrepancies for all outcome measures, we deemed a pooled limb analysis reasonable for group-level comparisons. Therefore, analyses of covariance (ANCOVAs) with α set to .05 were conducted for the running outcome measures between the ERLLP and healthy groups' mean limb outcomes, treating stride pace as a covariate. Additionally, the clinical laboratory measures were compared between the ERLLP and healthy groups' mean limb scores using independent t tests. To determine the magnitude of differences, we calculated Cohen d effect sizes and interpreted them as ≤0.2 for small, 0.6 for moderate, or ≥0.8 for large effects. All analyses were performed using jamovi (version 0.9.6.8; The jamovi project, Sydney, Australia).
RESULTS
For the patient-reported outcome measures, the ERLLP group presented with decreased physical activity on the Godin questionnaire (MD = −31.63 ± 15.29, P = .05) and decreased scores on the Exercise-Induced Leg Pain-British version questionnaire (MD = −9.63 ± 0.99, P < .001) and Lower Extremity Functional Scale (MD = −7.81 ± 1.99, P < .001), which indicated more pain and worse function than in the healthy group. Additionally, 100-mm VAS pain scale outcomes were higher for the ERLLP group than for the heathy group (MD = 40.5 ± 4.9 mm, P < .001), as expected.
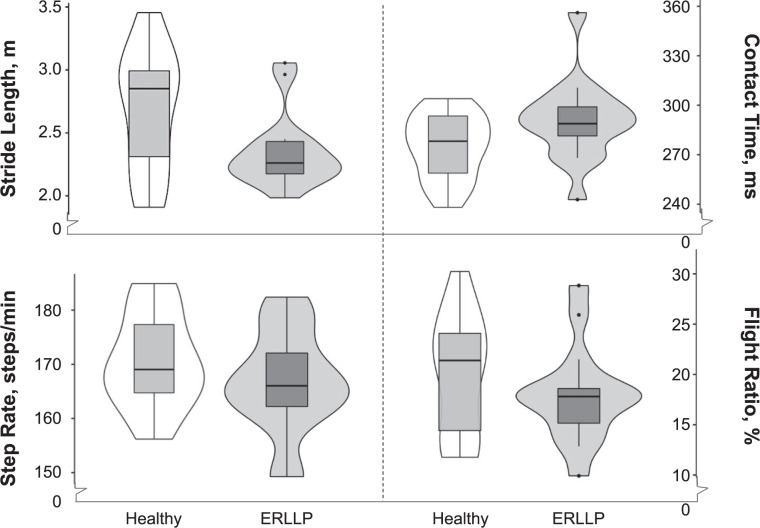
During the assessment of the running biomechanical measures, the ANCOVA reflected that the groups differed in stride length (F = 5.72, P = .02) and contact time (F = 4.36, P = .05). The ERLLP group had decreased stride length (MD = −0.11 ± 0.05 m, P = .02) and increased contact time (MD = 18.0 ± 8.27 milliseconds, P = .05) versus the healthy group (Figure 1). All other running outcomes were comparable across groups (Figures 1–3; Supplementary Table).
Figure 1.
Spatiotemporal outcomes comparing mean limb differences between the healthy and exercise-related lower leg pain (ERLLP) groups. Violin plots including median (central black horizontal lines), interquartile ranges (box), and spread of data (lines extending from boxes, dots for outliers) are used to depict the spatiotemporal biomechanical outcomes, including stride length, contact time, step rate, and flight ratio. Wider areas of the violin plots indicate a greater density of outcomes for the group.
Figure 3.
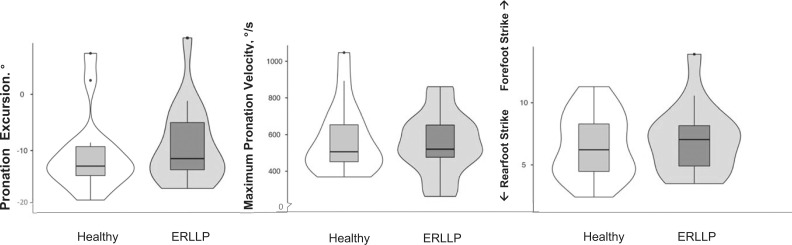
Kinematic outcomes comparing mean limb differences between the healthy and exercise-related lower leg pain (ERLLP) groups. Violin plots including median (central black horizontal lines), interquartile ranges (box), and spread of data (lines extending from boxes, dots for outliers) are used to depict the kinematic biomechanical outcomes, including pronation excursion, maximum pronation velocity, and footstrike type. Wider areas of the violin plots indicate a greater density of outcomes for the group.
Figure 2.
Loading outcomes comparing mean limb differences between the healthy and exercise-related lower leg pain (ERLLP) groups. Violin plots including median (central black horizontal lines), interquartile ranges (box), and spread of data (lines extending from boxes, dots for outliers) are used to depict the loading biomechanical outcomes, including shock, impact, and braking g measures. Wider areas of the violin plots indicate a greater density of outcomes for the group.
When we compared the clinical measures for mean limb scores between groups, no differences were evident for the static alignment measures (Table 2). However, the ERLLP group displayed increased ROM for metatarsophalangeal flexion (MD = 13.9 ± 4.6°, P = .01) and increased ankle eversion (MD = 6.3 ± 2.7°, P = .03) compared with the healthy group (Table 3). Additionally, the ERLLP group had decreased mass-normalized strength for ankle inversion (−0.49 ± 0.23 N/kg, P = .05), ankle eversion (−0.57 ± 0.27 N/kg, P = .04), and hip flexion (−0.99 ± 0.39 N/kg, P = .02) versus the healthy group (Table 4). These differences were associated with moderate to large effect sizes (d = 0.74–1.06). All other clinical laboratory measures were comparable across groups.
Table 2.
Clinical Laboratory Measures: Alignment
| Group, Mean ± SD |
||||
| Measure |
Healthy |
Exercise-Related Lower Leg Pain |
P Value |
Cohen d |
| Leg length, cm | 89.29 ± 6.03 | 89.98 ± 4.99 | .73 | 0.12 |
| Arch height index—seated | 0.05 ± 0.01 | 0.05 ± 0.01 | .66 | 0.16 |
| Arch height index—standing | −1.16 ± 0.70 | −1.02 ± 0.65 | .55 | 0.21 |
| Arch rigidity index | −24.78 ± 17.94 | −20.58 ± 12.77 | .45 | 0.27 |
| Arch drop, mm | 25.56 ± 6.31 | 25.13 ± 6.10 | .85 | 0.07 |
| Foot Posture Index | 4.88 ± 4.41 | 3.22 ± 2.71 | .21 | 0.45 |
| Tibial torsion, ° | 14.88 ± 7.29 | 15.88 ± 6.48 | .69 | 0.14 |
| Q-angle, ° | 10.59 ± 2.64 | 12.25 ± 3.47 | .14 | 0.54 |
| Hip anteversion, ° | 3.84 ± 5.46 | 7.19 ± 3.88 | .06 | 0.71 |
Table 3.
Clinical Laboratory Measures: Range of Motion
| Group, Mean ± SD |
|||||
| Joint |
Measure |
Healthy |
Exercise-Related Lower Leg Pain |
P Value |
Cohen d |
| 1st Metatarsophalangeal | |||||
| Flexion, ° | 27.16 ± 13.51 | 41.03 ± 12.54 | .005a | 1.06 | |
| Extension, ° | 81.00 ± 15.70 | 68.66 ± 20.08 | .06 | 0.68 | |
| Ankle | |||||
| Inversion, ° | 31.59 ± 10.16 | 28.59 ± 11.94 | .45 | 0.27 | |
| Eversion, ° | 8.13 ± 5.49 | 14.47 ± 9.49 | .03a | 0.82 | |
| Weight-bearing dorsiflexion, cm | 11.10 ± 2.84 | 10.23 ± 3.85 | .48 | 0.26 | |
| Dorsiflexion, ° | 4.38 ± 5.79 | 4.16 ± 6.47 | .92 | 0.04 | |
| Plantar flexion, ° | 66.19 ± 10.08 | 68.72 ± 7.34 | .42 | 0.29 | |
| Knee | |||||
| Flexion, ° | 133.72 ± 22.06 | 137.88 ± 10.43 | .50 | 0.24 | |
| Extension, ° | 0.16 ± 2.99 | −0.25 ± 1.35 | .62 | 0.18 | |
| Hip | |||||
| Abduction, ° | 131.28 ± 32.27 | 142.44 ± 10.75 | .20 | 0.46 | |
| Thomas test, cm | 1.84 ± 4.60 | 1.52 ± 2.63 | .81 | 0.08 | |
Significant at P ≤ .05.
Table 4.
Clinical Laboratory Measures: Isometric Strength
| Group, Mean ± SD |
|||||
| Joint |
Measure, N/kg |
Healthy |
Exercise-Related Lower Leg Pain |
P Value |
Cohen d |
| 1st Metatarsophalangeal | Flexion | 1.5 ± 0.46 | 1.46 ± 0.54 | .59 | 0.19 |
| Ankle | Dorsiflexion | 4.52 ± 0.97 | 4.10 ± 0.56 | .15 | 0.53 |
| Plantar flexion | 7.10 ± 2.87 | 6.12 ± 2.31 | .29 | 0.38 | |
| Inversion | 2.73 ± 0.67 | 2.24 ± 0.66 | .05a | 0.75 | |
| Eversion | 2.69 ± 0.82 | 2.13 ± 0.70 | .04a | 0.74 | |
| Knee | |||||
| Flexion | 3.03 ± 0.93 | 2.47 ± 0.62 | .06 | 0.71 | |
| Extension | 6.26 ± 2.73 | 4.98 ± 1.34 | .10 | 0.60 | |
| Hip | |||||
| Flexion | 5.57 ± 1.30 | 4.58 ± 0.84 | .02a | 0.90 | |
| Extension | 4.22 ± 0.90 | 3.75 ± 0.89 | .15 | 0.52 | |
| Abduction | 2.74 ± 0.75 | 2.33 ± 0.77 | .13 | 0.55 | |
Significant at P ≤ .05.
DISCUSSION
To our knowledge, we are the first to thoroughly assess running-gait biomechanics in a field setting over multiple training sessions using wearable sensors in runners experiencing ERLLP. While running in natural running environments, runners with ERLLP had increased contact time and decreased stride length compared with a healthy control group. For the clinical measures, moderate to large strength deficits for ankle inversion and eversion and hip flexion were found in runners with ERLLP. Additionally, the participants with ERLLP had increased toe-flexion and ankle-eversion ROM.
Gait Biomechanics
We identified increased contact time in the ERLLP group (MD = 18 ms) compared with the healthy control group after we controlled for group differences in stride pace. This finding indicates that the feet of the ERLLP group were in contact with the ground longer during the stance phase of running than those of the healthy group. This increased contact time may allow for increased loading of lower extremity musculoskeletal structures, thereby heightening the risk of ERLLP. Although no differences in foot-strike patterns were demonstrated among the runners in our study, contact time was greater in runners who used a rearfoot strike,21 which has been associated with decreased overall running economy.22 Runners who used a rearfoot-strike pattern were also associated with a higher cost of oxygen transport, which may in turn lead to faster onset of fatigue and a 12% decreased ability to attenuate shock.23 Therefore, individualized running assessments are necessary before implementing intervention strategies for runners with ERLLP.
Reducing contact time with gait-retraining cues was effective in reducing pain with running among those with CECS.24 The researchers investigated the outcomes of a gait-training program in which participants with CECS underwent a 6-week intervention to change their foot-strike type from rearfoot to forefoot while running.24 After the intervention, decreases were noted in compartmental pressure, pain, step length, and contact time.24 This strategy may be appropriate for individuals with a natural rearfoot strike; however, not all runners with ERLLP use a rearfoot-strike pattern. In our study, the healthy runners and runners with ERLLP did not differ in foot-strike pattern, yet the runners with ERLLP had greater contact time.
To reduce pain while running, Breen et al10 implemented a variety of gait-retraining strategies in a case series of 10 runners with CECS. Runners were coached individually over a 6-week period to increase hip flexion, increase cadence, maintain an upright torso, and achieve a midfoot strike pattern. Although contact time was not measured, increasing cadence is a strategy that has been used to reduce contact time. After gait retraining, runners' total running distance, subjective function, and pain improved.10 Hence, focusing in-field gait-retraining programs on decreasing contact time and reducing pain in runners with ERLLP appears to be warranted. Reducing ground contact time may decrease the ground reaction forces and, thus, decrease muscle overloading.25–27
No group differences in kinematics and kinetics were found, which was somewhat surprising, as previous authors have shown differences between ERLLP and healthy runners in pronation measures7,8,28 and vertical ground reaction forces.27,29,30 These studies were performed in the laboratory setting, either on an instrumented treadmill or across a short-distance runway. The RunScribe sensors worn by participants in our study do not directly measure the maximum pronation angle but rather the amount of foot excursion from initial contact until maximum pronation. In addition, impact g and braking g are considered surrogate measures for ground reaction forces that are typically measured in Newtons.11 The outcomes from the wearable sensors should not be directly compared with laboratory measures but instead considered an addition to the existing literature.
Lastly, laboratory studies of running biomechanics typically capture 3 to 16 steps for analysis, whereas our RunScribe data were captured for every step taken during each run, resulting in more than 570,000 steps in our analysis. The increased number of steps analyzed may allow for a better representation of the running activity. It is also beneficial that the runners were able to use the wearable sensors during their typical weekly running regimen instead of being observed in a laboratory setting. Differences have occurred between treadmill-running biomechanics and overground-running biomechanics,9 so it is reasonable to speculate the biomechanics of treadmill running versus running outside would be different. More studies need to be performed in the field setting to further validate our results.
Lower Extremity Strength
Strength was lower in the ERLLP group for ankle inversion (MD = 0.49 N/kg), ankle eversion (MD = 0.57 N/kg), and hip flexion (MD = 0.99 N/kg). The main ankle invertors are the tibialis posterior and tibialis anterior, which attach to the tibia along the painful areas described by participants with ERLLP. Whether this decreased ankle strength contributes to or results from ERLLP is unclear. Overuse of these muscles over time could result in damage to the muscles or tendinous attachments or transfer of more force to the tibia, as the muscles are too weak to absorb the forces imposed on them by running. This is important because the ankle invertors functionally control the extent of pronation and medial arch collapse during loaded activity and increased pronation is an intrinsic risk factor for developing ERLLP.31 Although we did not note any differences in pronation velocity or excursion, individuals with ERLLP demonstrated longer foot contact-time outcomes, which may have resulted in increased stress on the arch and supporting soft tissue structures, thereby exacerbating their pain. It is also plausible that the muscles that eccentrically control pronation during the stance phase may be under load for a longer period of time, which may contribute to ERLLP. Future studies in which researchers explore these relationships and muscle activity during running in this injured population are warranted.
Weak hip flexion may indicate weakness of the rectus femoris and iliopsoas muscles. Less rectus femoris activation during ground contact time is associated with a higher cost of oxygen transport, which indicates decreased running economy.22 An individual with weak hip flexors may be unable to achieve sufficient hip flexion during running. Increased hip flexion during running may promote a midfoot or forefoot strike and has been used as an effective gait-retraining cue to reduce pain associated with running.10 Additionally, increasing the step rate above a preferred rate has been shown to increase hip-flexor muscle loading during the swing phase, resulting in less peak force during the loading response.32 Thus, adding hip-flexor strengthening exercises for runners with ERLLP may be appropriate.
Lower Extremity ROM
The ERLLP group had increased ROM for great toe flexion (MD = 13.9°) and ankle eversion (MD = 6.3°) compared with the healthy group. Although we did not identify any differences in navicular drop or foot type, increased passive ankle-eversion ROM may translate to the more pronated foot position during running identified in the laboratory setting.7,8 The RunScribe wearable sensors measure pronation excursion, not the absolute maximum pronation angle. Pronation excursion is the total amount of pronation the foot exhibits from the time it contacts the ground and depends on the initial foot position. Contacting the ground with a more everted foot position may decrease the total amount of pronation excursion an individual can achieve without influencing the absolute maximum pronation angle. Differences in measurement techniques and running environments may have contributed to these various results among studies. Running-related injuries are multifactorial, and these outcomes should be assessed in the future by investigating runners with ERLLP.
Clinical Implications
Treatment for ERLLP may involve gait retraining to reduce ground contact time. Proposed methods for reducing ground contact time via gait retraining may include encouraging the individual to attempt a midfoot or forefoot strike, increase the cadence, or try to lift the foot off the ground higher and earlier than normal.10,24,27 Strength training may also be warranted to address weaknesses in ankle inversion and eversion, as well as hip flexion. Although gait retraining may be warranted after ERLLP has developed, researchers should conduct more prospective studies to understand the underlying alterations that contribute to ERLLP. A better understanding of how ERLLP develops in runners would allow for improved strategies for intervening before the condition develops.
Limitations
Runners in our study ran in natural environments wearing shoe-mounted sensors, and our results are not directly comparable with those of laboratory-based biomechanical studies. This may be considered a limitation at this time due to the small amount of available evidence for quantifying running using wearable sensors. Additionally, our runners' training runs were not the same, which may have affected the biomechanical measures; however, we felt this study design best represented how these participants ran in their natural training environments. To measure knee-extension strength, we needed to place the handheld dynamometer on the distal shin, which could have been painful for runners with ERLLP and altered their results. Lastly, the investigation may have also been limited by the relatively small sample size. As a result, data from men and women were grouped for the statistical analysis. Men and women may display different running biomechanics, but we could not assess this factor due to the small sample size.13
CONCLUSIONS
Runners with ERLLP had longer contact time and decreased stride length during running. These measures may be appropriate for intervention using gait retraining. Furthermore, the ERLLP group had decreased strength in ankle inversion and eversion and hip flexion. Researchers need to better understand how ERLLP develops and affects running mechanics. Additional knowledge of a person's natural running gait in his or her training environment may help to improve the focus of treatment efforts for those with ERLLP.
Supplementary Material
REFERENCES
- 1.Tenforde AS, Sayres LC, McCurdy ML, Collado H, Sainani KL, Fredericson M. Overuse injuries in high school runners: lifetime prevalence and prevention strategies. PM R. 2011;3(2):125–131. doi: 10.1016/j.pmrj.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Reinking MF. Exercise related leg pain (ERLP): a review of the literature. North Am J Sports Phys Ther. 2007;2(3):170–180. [PMC free article] [PubMed] [Google Scholar]
- 3.Reinking MF, Austin TM, Hayes AM. Exercise-related leg pain in collegiate cross-country athletes: extrinsic and intrinsic risk factors. J Orthop Sports Phys Ther. 2007;37(11):670–678. doi: 10.2519/jospt.2007.2534. [DOI] [PubMed] [Google Scholar]
- 4.Reinking MF. Exercise-related leg pain in female collegiate athletes: the influence of intrinsic and extrinsic factors. Am J Sports Med. 2006;34(9):1500–1507. doi: 10.1177/0363546506287298. [DOI] [PubMed] [Google Scholar]
- 5.Miller TL, Jamieson M, Everson S, Siegel C. Expected time to return to athletic participation after stress fracture in Division I Collegiate Athletes. Sports Health. 2018;10(4):340–344. doi: 10.1177/1941738117747868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett JE, Reinking MF, Rauh MJ. The relationship between isotonic plantar flexor endurance, navicular drop, and exercise-related leg pain in a cohort of collegiate cross-country runners. Int J Sports Phys Ther. 2012;7(3):267–278. [PMC free article] [PubMed] [Google Scholar]
- 7.Willems TM, De Clercq D, Delbaere K, Vanderstraeten G, De Cock A, Witvrouw E. A prospective study of gait related risk factors for exercise-related lower leg pain. Gait Posture. 2006;23(1):91–98. doi: 10.1016/j.gaitpost.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Willems TM, Witvrouw E, De Cock A, De Clercq D. Gait-related risk factors for exercise-related lower-leg pain during shod running. Med Sci Sports Exerc. 2007;39(2):330–339. doi: 10.1249/01.mss.0000247001.94470.21. [DOI] [PubMed] [Google Scholar]
- 9.Riley PO, Dicharry J, Franz J, Della Croce U, Wilder RP, Kerrigan DC. A kinematics and kinetic comparison of overground and treadmill running. Med Sci Sports Exerc. 2008;40(6):1093–1100. doi: 10.1249/MSS.0b013e3181677530. [DOI] [PubMed] [Google Scholar]
- 10.Breen DT, Foster J, Falvey E, Franklyn-Miller A. Gait re-training to alleviate the symptoms of anterior exertional lower leg pain: a case series. Int J Sports Phys Ther. 2015;10(1):85–94. [PMC free article] [PubMed] [Google Scholar]
- 11.Koldenhoven RM, Hertel J. Validation of a wearable sensor for measuring running biomechanics. Digit Biomark. 2018;2(2):74–78. doi: 10.1159/000491645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollis CR, Koldenhoven RM, Resch JE, Hertel J. Running biomechanics as measured by wearable sensors: effects of speed and surface. Sports Biomech. 2019:1–11. doi: 10.1080/14763141.2019.1579366. [DOI] [PubMed]
- 13.Yagi S, Muneta T, Sekiya I. Incidence and risk factors for medial tibial stress syndrome and tibial stress fracture in high school runners. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):556–563. doi: 10.1007/s00167-012-2160-x. [DOI] [PubMed] [Google Scholar]
- 14.Nauck T, Lohrer H, Padhiar N, King JB. Development and validation of a questionnaire to measure the severity of functional limitations and reduction of sports ability in German-speaking patients with exercise-induced leg pain. Br J Sports Med. 2015;49(2):113–117. doi: 10.1136/bjsports-2012-091745. [DOI] [PubMed] [Google Scholar]
- 15.Korakakis V, Malliaropoulos N, Baliotis K, et al. Cross-cultural adaptation and validation of the exercise-induced leg pain questionnaire for English- and Greek-speaking individuals. J Orthop Sports Phys Ther. 2015;45(6):485–496. doi: 10.2519/jospt.2015.5428. [DOI] [PubMed] [Google Scholar]
- 16.Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J. 2001;18(3):205–207. doi: 10.1136/emj.18.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser JJ, Koldenhoven RM, Saliba SA, Hertel J. Reliability of ankle-foot morphology, mobility, strength, and motor performance measures. Int J Sports Phys Ther. 2017;12(7):1134–1149. doi: 10.26603/ijspt20171134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shultz SJ, Nguyen AD, Windley TC, Kulas AS, Botic TL, Beynnon BD. Intratester and intertester reliability of clinical measures of lower extremity anatomic characteristics: implications for multicenter studies. Clin J Sport Med. 2006;16(2):155–161. doi: 10.1097/00042752-200603000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Thorborg K, Bandholm T, Hölmich P. Hip- and knee-strength assessments using a hand-held dynamometer with external belt-fixation are inter-tester reliable. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):550–555. doi: 10.1007/s00167-012-2115-2. [DOI] [PubMed] [Google Scholar]
- 20.Gregory C, Koldenhoven RM, Higgins M, Hertel J. External ankle supports alter running biomechanics: a field-based study using wearable sensors. Physiol Meas. 2019;40(4):044003. doi: 10.1088/1361-6579/ab15ad. [DOI] [PubMed] [Google Scholar]
- 21.Di Michele R, Merni F. The concurrent effects of strike pattern and ground-contact time on running economy. J Sci Med Sport. 2014;17(4):414–418. doi: 10.1016/j.jsams.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Tam N, Tucker R, Santos-Concejero J, Prins D, Lamberts RP. Running economy: neuromuscular and joint stiffness contributions in trained runners. Int J Sports Physiol Perform. 2018;14(1):1–22. doi: 10.1123/ijspp.2018-0151. [DOI] [PubMed] [Google Scholar]
- 23.Mercer JA, Bates BT, Dufek JS, Hreljac A. Characteristics of shock attenuation during fatigued running. J Sports Sci. 2003;21(11):911–919. doi: 10.1080/0264041031000140383. [DOI] [PubMed] [Google Scholar]
- 24.Diebal AR, Gregory R, Alitz C, Gerber JP. Forefoot running improves pain and disability associated with chronic exertional compartment syndrome. Am J Sports Med. 2012;40(5):1060–1067. doi: 10.1177/0363546512439182. [DOI] [PubMed] [Google Scholar]
- 25.Tweed JL, Barnes MR. Is eccentric muscle contraction a significant factor in the development of chronic anterior compartment syndrome? A review of the literature. Foot (Edinb) 2008;18(3):165–170. doi: 10.1016/j.foot.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Meardon SA, Derrick TR. Effect of step width manipulation on tibial stress during running. J Biomech. 2014;47(11):2738–2744. doi: 10.1016/j.jbiomech.2014.04.047. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann WO, Bakker EWP. Reducing vertical ground reaction forces: the relative importance of three gait retraining cues. Clin Biomech (Bristol Avon) 2019;69:16–20. doi: 10.1016/j.clinbiomech.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Becker J, James S, Wayner R, Osternig L, Chou LS. Biomechanical factors associated with Achilles tendinopathy and medial tibial stress syndrome in runners. Am J Sports Med. 2017;45(11):2614–2621. doi: 10.1177/0363546517708193. [DOI] [PubMed] [Google Scholar]
- 29.Milner CE, Ferber R, Pollard CD, Hamill J, Davis IS. Biomechanical factors associated with tibial stress fracture in female runners. Med Sci Sports Exerc. 2006;38(2):323–328. doi: 10.1249/01.mss.0000183477.75808.92. [DOI] [PubMed] [Google Scholar]
- 30.Davis IS, Bowser BJ, Mullineaux DR. Greater vertical impact loading in female runners with medically diagnosed injuries: a prospective investigation. Br J Sports Med. 2016;50(14):887–892. doi: 10.1136/bjsports-2015-094579. [DOI] [PubMed] [Google Scholar]
- 31.Moen MH, Tol JL, Weir A, Steunebrink M, De Winter TC. Medial tibial stress syndrome: a critical review. Sports Med. 2009;39(7):523–546. doi: 10.2165/00007256-200939070-00002. [DOI] [PubMed] [Google Scholar]
- 32.Lenhart R, Thelen D, Heiderscheit B. Hip muscle loads during running at various step rates. J Orthop Sports Phys Ther. 2014;44(10):766–774. doi: 10.2519/jospt.2014.5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.