Abstract
Context
Changes in lower limb loading and movement quality after prolonged running and training periods might influence injury risks in runners.
Objectives
To assess (1) the effects of a single prolonged run and a 3-week running training program on peak tibial acceleration (PTA) during running and Functional Movement Screen (FMS) criterion tests, and (2) the relationship between running volume during the 3-week training program and changes in PTA and FMS scores after training.
Design
Case series.
Setting
Research laboratory.
Patients or Other Participants
Ten novice runners (age = 27 ± 7 years) with 15 ± 14 months of running experience, who ran on average 19.6 ± 4.8 km per week at a preferred pace of 7:05 ± 1:30 minutes per km.
Main Outcome Measure(s)
Participants completed a 30-minute submaximal prolonged treadmill run and 3-week training program with 25% increases in weekly running volume. Peak tibial acceleration and the deep-squat and active straight-leg–raise criterion FMS test scores were assessed before and after the prolonged run at enrollment and after the training program (ie, 3 testing sessions).
Results
No differences in PTA or FMS scores were observed among the 3 testing times. Although the changes in PTA (r = 0.57) and FMS aggregate score (r = 0.15) were not significantly correlated with training volume, training volume explained 32% of the variance in the PTA change from before to after training.
Conclusions
Our findings suggest that tibial acceleration and movement quality were not influenced by a single submaximal-effort prolonged run or a 3-week training period. However, novice runners who have a greater increase in running volume might be more susceptible to training-related changes in tibial acceleration than those whose running volume is less.
Keywords: treadmill, biomechanics, lower limb, injury risks
Key Points
Peak tibial acceleration and movement quality were not affected by a prolonged submaximal run.
Peak tibial acceleration and movement quality were not affected by a 3-week training period.
Greater increases in training volume might influence peak tibial acceleration.
Stress fractures are common overuse injuries attributed to running training.1 Distance runners had the highest incidence of stress fractures among sport athletes.2 Tibial stress fractures accounted for 33% to 50% of all stress fractures among both recreational runners and military recruits,3,4 and in the United States, stress fractures accounted for the greatest loss of training days among military recruits.5 Researchers have identified several tibial stress fracture risk factors that can be modified to potentially reduce the risk of these injuries in individuals who run frequently. Considering that many military recruits may not have extensive running experience before enlisting and that novice runners are reported to have high injury incidences (ie, 17.8 injuries per 1000 hours of running),6 it is worthwhile to study injury risk factors in novice runner populations.
Among the modifiable risk factors for tibial stress fracture, peak tibial acceleration (PTA) immediately after foot strike during running tends to be higher in runners with a history of tibial stress fracture7 and in the injured limb of runners with a tibial stress fracture,8 although some findings have conflicted.9 Because PTA can be measured inexpensively, with minimal equipment (eg, wearable accelerometers), and may be related to some running-related injuries (RRIs) such as stress fractures, PTA may be a useful variable for assessing injury risk in runners. Although RRI causes are multifactorial and require an understanding of both musculoskeletal tissue capacity and mechanical loads applied to the tissue,10 PTA is a surrogate for the external load applied to the tibia while running and thus contributes to factors related to risks of tibial stress fractures. Peak tibial acceleration has received much attention in the literature, yet changes in PTA from prolonged running bouts have mostly been studied during single testing sessions and among varied runner populations. Peak tibial acceleration increases after a single prolonged, exhaustive (ie, to volitional exhaustion and test termination) run in novice runners11–13 but does not increase over the course of a 20-minute run at a lactate threshold pace in highly trained runners.14 Thus, PTA increases as a result of a single prolonged run may be more likely in novice runners than in more trained and experienced runners. It is therefore important to study a specific runner population to understand the influence of prolonged running on PTA. Additionally, suboptimal physical training (too much or too little) is a modifiable risk factor for tibial stress fracture,4 potentially because acute training-induced muscle damage may alter joint properties associated with shock attenuation.15 Although better physical fitness test scores appear protective against lower extremity stress fracture,4 increasing training volume, which is required to improve fitness, may increase skeletal distress and contribute to the development of tibial stress fracture. If PTA remains elevated over time, cumulative exposure may result in harmful loading of the tissues of the lower leg,16 which may increase the risk of tibial stress fracture.
Further, assessments of movement quality could also offer insight into injury risks in runners and are frequently used in strength and conditioning and sports medicine settings. The Functional Movement Screen (FMS) consists of 7 movements that help screen for pain and movement quality and may identify risk for certain types of injuries within certain populations.17,18 Two FMS criterion tests, the deep squat (DS) and active straight-leg raise (ASLR), have been used to detect a higher risk for musculoskeletal injury in young male runners,19 whereas the full FMS battery may be less well suited to identifying injury risks in other athletes.20 Thus, these 2 criterion tests may be more effective than the composite score of all 7 FMS tests in predicting the RRI risk.19 To best assess an individual's natural movement tendencies, it is often assumed that clinicians should administer the FMS at consistent timepoints, without the influence of a warmup.21 Whether activities such as running could influence FMS scores is currently unknown. However, because a prolonged run can alter motor control12 and exercise training can alter FMS scores,22 FMS test scores may be altered by a single bout of prolonged running or after a period of running training.
The primary purpose of our study was to assess the effects of a single, submaximal prolonged run and a 3-week running program on PTA and FMS DS and ASLR scores in novice runners. We hypothesized that a single, submaximal prolonged run would increase PTA and reduce FMS scores in novice runners. We also hypothesized that 3 weeks of progressively increasing running volume would increase PTA and reduce FMS scores compared with those before and after the submaximal prolonged run at baseline testing. The secondary purpose of the study was to evaluate the association between total running volume during the 3-week program and the changes in PTA and FMS scores before and after the training program. We expected that a greater total running volume would be associated with larger increases in PTA and larger reductions in FMS scores after training in novice runners.
METHODS
Participants
Seventeen novice runners (9 women) volunteered for the study. Participants were considered novice and included if they ran an average of at least 16 km per week in the 3 weeks before data collection and had been running at least 16 km per week for no longer than 2 years. Volunteers were excluded if they had any lower limb injury, back injury, lower limb surgery (eg, reconstructive, arthroscopic), or other medical contraindication for running within the 3 months before testing. The study and consent were approved by the Institutional Review Board for Human Participants Research. All participants provided written consent before data collection.
Experimental Protocol
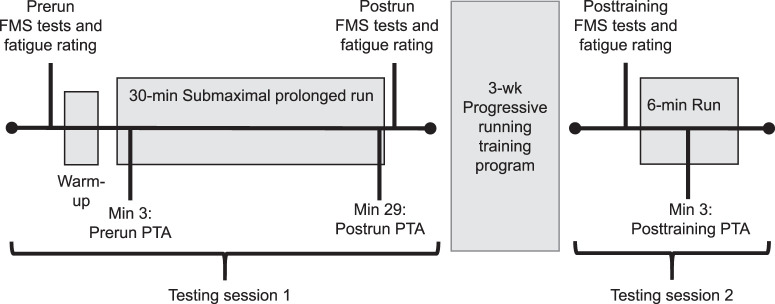
An overview of all testing procedures is presented in Figure 1. Participants were instructed to refrain from exhaustive runs (ie, near-maximal or maximal-effort running sessions) during the 48 hours before testing. They began the first laboratory session by completing a training history questionnaire and providing a rating of their overall (VASOverall) and lower extremity (VASLE) perceived self-reported fatigue using a 10-cm visual analog scale (VAS; 0 indicates no fatigue at all and 10 indicates extreme fatigue).
Figure 1.
Protocol timeline for functional movement screen (FMS) and peak tibial acceleration (PTA) measurements completed at the start (prerun) and end (postrun) of the submaximal prolonged run at baseline. Posttraining measurements were also completed after the 3-week progressive training program.
Participants then performed the DS and ASLR criterion movements of the FMS. These tests were administered by 1 researcher with FMS certification (K.C.) using testing procedures described previously.17,18 Functional Movement Screen procedures were explained to the participant using a script to avoid DS and ASLR performance bias from oral cueing. Using the 100-point FMS scoring system for DS, ASLR, and ASLR asymmetry criterion tests,23 the examiner scores the DS out of a possible 18 points, the ASLR of each leg (ASLRR and ASLRL) out of a possible 6 points each, and ASLR right and left asymmetry (ASLRAssym) out of a possible 4 points. The aggregate score was the sum of the DS, ASLRR, and ASLRL points (ie, maximum = 30 points).19 The 100-point FMS score has demonstrated interrater reliability with intraclass correlation coefficients (ICCs) > 0.9 for all test scores among FMS certified test administrators.23 For improved validity and reliability of results, FMS administrators performing peer-reviewed research should be FMS certified and use the 100-point scoring system.23
After the FMS tests, the same researcher attached a 3-dimensional (3-D) accelerometer (1200 Hz, model 356A26; PCB Piezotronics, Depew, NY) to the distal anteromedial aspect of the tibia and along its longitudinal (Z) axis to collect tibial accelerations.24 The accelerometer was placed 2 to 4 cm anterior and superior to the right medial malleolus. This position was measured for each participant using an anthropometric tape measure to ensure consistent accelerometer placement for the second testing session. Prewrap, self-adhering cohesive wrap, and a hook-and-loop neoprene strap were used to secure the accelerometer to the tibia and minimize unwanted motion. Spherical reflective markers were secured to the leg and foot to measure cadence and foot contact angle during the prolonged treadmill run. A neoprene wrap was also placed around the right shank to secure a 4-marker cluster used to track shank motion during running. A 3-marker semirigid thermoplastic shell was placed over the heel cup of the shoe to track foot motion. Participants wore their own footwear and performed all testing sessions in the same footwear.
After the accelerometer and markers were placed, participants ran on a 0% grade treadmill (model C962i; Precor, Woodinville, WA) for 2 minutes to find their preferred pace, become comfortable with the accelerometer and marker clusters, and ensure that the shank marker cluster settled into a fixed position before the prolonged run. Each person was instructed to find a preferred pace for an easy run, specifically at a pace the participant felt was between 11 and 13 on the 20-point Borg scale for rating of perceived exertion. After a 1- to 2-minute break, participants performed the submaximal prolonged treadmill run for 30 minutes at their preferred pace. A 9-camera 3-D motion-capture system (240 Hz, model Oqus; Qualisys AB, Göteburg, Sweden) was used to track shank and foot markers during the treadmill run. Between the 3- and 4-minute mark (prerun) and during the final minute of the run (postrun), 3D kinematic and accelerometer data were collected in synchrony for 10 seconds (model Track Manager; Qualisys AB). Immediately after the run, VASOverall and VASLE self-reported fatigue were recorded again using the VAS followed by the FMS tests. The time between the end of the run and the start of the FMS tests was recorded using a stopwatch and ranged between 1 and 2 minutes.
At the end of this testing session, calibration of a foot pod (model Pod; MileStone Sports, Columbia, MD) was performed per the manufacturer's instructions separately from any data collection. Participants were instructed on how to download the free mobile app for the foot pod and upload the data to the app after each training run. Data from this device were used to confirm weekly running volume (kilometers) at the end of the 3-week training period.
Within 48 hours of the last run of the 3-week training program, participants returned to the laboratory for their posttraining testing session (posttraining). The VAS self-reported fatigue rating and FMS tests were performed as in the first testing session. Next, for each person, the accelerometer was secured to the same location on the tibia according to the measurements taken during the first session. Reflective marker-placement procedures, warmup procedures, and treadmill procedures and speed were identical to those in the first testing session. Participants then ran on the treadmill for 6 minutes to assess the change in PTA and FMS scores after the 3-week training program. Accelerometer and 3D kinematic data were collected between minutes 3 and 4 of the 6-minute run. Foot-pod data from each individual were downloaded to a spreadsheet (version 1908; Excel, Microsoft Corp, Redmond, WA) to confirm completion of the prescribed running volume during the training program.
Training Program
The research staff prescribed each participant a 3-week running program with weekly volume increases of 25% starting from each person's reported average weekly running volume from the 3 weeks before the study. The authors of a recent systematic review25 concluded that, although evidence was limited, weekly running volume increases >30% compared with 10% were associated with higher injury risks. Thus, we chose the 25% increase in weekly volume to elicit feelings of perceived chronic fatigue after the 3-week running training program while maintaining safe volume increases in these novice runners. To maintain ecological validity in the study design, the training programs were individualized based on the number of weekly runs performed by all participants (eg, 3 to 6 runs per week). Pace during the training runs was not controlled or prescribed, but the run cadence needed to be >140 steps per minute for the foot pod to register a run as opposed to a walk (according to thresholds reported by the manufacturer). Participants were instructed to complete the prescribed weekly volume but were free to vary the weekly frequency of the runs to accommodate their schedules and maximize adherence to their prescribed weekly training volume. They were told to upload the foot-pod data to the mobile app at the end of each run during the training period. Participants were also instructed to report their weekly running volume to the researchers at the end of each training week via e-mail survey. To ensure adherence to the training program, we were in contact with all participants regularly via email during each training week.
Data Analyses
Visual3D (version V6.03.5; C-Motion, Germantown, MD) and MATLAB (The MathWorks, Inc, Natick, MA) software were used to process and analyze the kinematic marker and accelerometer data. Marker data were interpolated using a third-order cubic spline with a maximum gap of 10 frames. Marker data and accelerometer data were filtered using a fourth-order Butterworth low-pass filter with cutoff frequencies of 8 Hz and 60 Hz,26 respectively. Peak tibial acceleration, cadence, and sagittal-plane foot contact angle (FCA) were extracted from 5 consecutive stance phases. The FCA was expressed in the laboratory coordinate system and calculated as the sagittal-plane (2-dimensional) angle between the foot defined by the heel cluster markers and the laboratory anterior-posterior axis. For all kinematic variables collected, the average of the 5 stance phases during the 3 data-collection timepoints (prerun, postrun, and posttraining) was used in the statistical analyses.
Statistical Analyses
A repeated-measures analysis of variance (ANOVA) with time as the within-subjects factor was conducted to assess the effects of the submaximal prolonged run and training program on PTA, 2 FMS criterion tests (DS, ASLR, ASLR asymmetry), self-reported fatigue (VAS), and running kinematics (cadence and FCA; version 22.0; SPSS, IBM Corp, Armonk, NY). When F tests were significant, we calculated paired t tests to compare the means of all dependent variables between all timepoints. The Cohen d effect sizes were also determined to assess effect magnitudes using the interpretation of Hopkins (ie, small: d < 0.6, moderate: 0.6 > d < 1.2; large: d > 1.2).27 Data normality was evaluated using the Kolmogorov-Smirnov test. To assess the effect of the total absolute running volume increase, we used the Pearson r correlation coefficient to measure the association between total running volume during the 3-week program and the changes in PTA and FMS scores before and after the program. The R2 was calculated to assess the degree to which running volume explained the variance in PTA and FMS score changes from before to after the training program. The α level was set at P ≤ .05 for all statistical tests.
RESULTS
Participants and Training Details
Based on the Kolmogorov-Smirnov test, we confirmed that all data were normally distributed (P > .05). Of the 17 participants who completed the first testing session, 7 were excluded from posttraining data analyses because their adherence to training volume fell below 80% due to illness (n = 2), injury (ankle sprain unrelated to running: n = 1), or noncompliance (n = 4). Therefore, data from 10 runners (6 women) were included in the analyses (Table 1). The 10 participants who completed the 3-week program increased their volume, on average, by 34%, 9%, and 15% during weeks 1, 2, and 3, respectively, instead of the prescribed 25% increase in weekly volume. These weekly increases represented an average weekly increase of 19% ± 40% (range = 50% decrease to 193% increase) or 3.2 ± 9.7 km (range = 27-km decrease to 37-km increase). It is important to note that the large ranges in percentage and absolute volume increases were due to data from 1 runner (ie, large increase in week 1, followed by large decrease in week 2). Despite these large ranges in volume performed by this participant, the PTA (prerun = 0.97 ± 0.36g, postrun = 1.13 ± 0.30g, posttraining = 1.80 ± 0.33g), FMS aggregate score (prerun = 14, postrun = 12, posttraining = 12), and VASOverall (prerun = 0, postrun = 4.6, posttraining = 2.3) and VASLE (prerun = 0, postrun = 4.5, posttraining = 2.1) fatigue were all below or similar to the sample mean. Although the planned weekly increases in volume did not match the actual running volume, on average, the actual total training volume matched the planned total training volume (Table 1).
Table 1.
Participant Characteristics and Training Information
| Variable |
Mean ± SD |
Range |
| Age, y | 27 ± 7 | 20–38 |
| Height, m | 1.69 ± 0.13 | 1.52–1.91 |
| Mass, kg | 70.7 ± 17.0 | 49.9–99.8 |
| Training experience, mo | 15 ± 14 | 1–48 |
| Typical training volume, km·wk−1 | 19.6 ± 4.8 | 16.0–31.4 |
| Preferred running pace, min:s·km−1 | 7:05 ± 1:30 | 4:50–9:50 |
| Planned total training volume, km | 83.5 ± 18.8 | 62.2–127.1 |
| Actual total training volume, km | 84.6 ± 15.0 | 64.7–111.7 |
| Overall adherence, % | 102.9 ± 13.6 | 84.0–118.1 |
| Average daily volume, km·day−1 | 4.0 ± 1.1 | 2.7–6.8 |
| Average run volume, km·run−1 | 6.6 ± 1.6 | 4.0–9.0 |
| Training frequency, runs·week−1 | 4.4 ± 0.8 | 3.3–6.0 |
Three of the 10 participants reported noticeable discomfort to the research staff during the final week or run (or both) of the program. Of these, 1 described increased perceived exertion at comparable distances during the last (ie, third) weeks runs versus week 2; the remaining 2 described lower extremity stiffness (1 reported anterior hip discomfort; the other reported anterior thigh and knee discomfort). Despite these concerns, the participants voluntarily completed their training as scheduled.
Time Effects
We observed no time effects for PTA (P = .54) or FMSAgg (P = .47), DS (P = .78), ASLRR (P = 1.00), or ASLRL (P = .13; Table 2). All between-times effect sizes were small (d < 0.4). No differences were found for cadence (P = .87) or FCA (P = .33) between any timepoints. A time effect was present for self-reported VASLE (P = .032). On average, self-reported fatigue was 3 points greater postrun compared with prerun (P = .005), with large effect sizes (Table 3). Self-reported VASOverall also showed a time effect (P = .02): VASOverall, on average, was 2.2 points greater postrun than at prerun (P = .03), with a moderate effect size (Table 3). Neither VASLE nor VASOverall was different posttraining compared with postrun (P > .05), but a moderate effect size was demonstrated for posttraining versus prerun VASLE (Table 3).
Table 2.
Peak Tibial Acceleration and Functional Movement Screen (FMS) Scores Before (Prerun) and After (Postrun) the Prolonged Run and After the Training Program (Posttraining)
| Variable |
Mean ± SD |
Prerun Versus Postrun |
Pretraining Versus Posttraining |
||||
| Prerun |
Postrun |
Posttraining |
Δ, % |
d |
Δ, % |
d |
|
| Peak tibial acceleration,g | 3.4 ± 1.5 | 3.5 ± 1.2 | 3.2 ± 1.1 | 1 | 0.03 | 7 | −0.18 |
| FMSAgg (maximum = 30) | 15.0 ± 3.3 | 14.2 ± 3.2 | 15.2 ± 3.2 | −5 | −0.25 | 1 | 0.06 |
| Deep squat (maximum = 18) | 6.4 ± 1.6 | 6.0 ± 1.6 | 6.2 ± 1.8 | −6 | −0.25 | −3 | −0.12 |
| ASLRR (maximum = 6) | 4.4 ± 1.6 | 4.4 ± 1.3 | 4.4 ± 0.8 | 0 | 0.0 | 0 | 0.0 |
| ASLRL (maximum = 6) | 4.2 ± 1.1 | 3.8 ± 1.5 | 4.6 ± 1.0 | −10 | −0.31 | 10 | 0.38 |
| ASLRAssym (maximu = 4) | 0.6 ± 1.0 | 1.0 ± 1.1 | 0.2 ± 0.6 | 67 | 0.39 | −67 | −0.5 |
Abbreviations: ASLRAssym, absolute difference between ASLRR and ASLRL scores; ASLRL, left active straight-leg raise; ASLRR, right active straight-leg raise; d, Cohen effect size; FMSAgg, sum of DS, ASLRR, ASLRL; g, magnitude of gravitational acceleration (9.81 m/s2).
Table 3.
Secondary Kinematic and Fatigue Variables Before (Prerun) and After the Prolonged Run (Postrun) and After the Training Program (Posttraining)
| Mean ± SD |
Prerun Versus Postrun |
Pretraining Versus Posttraining |
|||||
| Variable |
Prerun |
Postrun |
Posttrain |
Δ, % |
d |
Δ, % |
d |
| Visual analog scale rating (maximum = 10) VASOverall (/10)a | |||||||
| Overall | 2.8 ± 2.2 | 5.0 ± 1.7b | 3.2 ± 1.4 | 76 | 1.12 | 13 | 0.21 |
| Lower extremity | 2.1 ± 1.8 | 5.0 ± 1.7b | 3.5 ± 1.9 | 143 | 1.72 | 69 | 0.78 |
| Cadence, steps/min | 162 ± 13 | 163 ± 14 | 162 ± 13 | <1 | 0.05 | <1 | <0.01 |
| Sagittal-plane foot contact angle, ° | 13.4 ± 6.7 | 14.6 ± 7.2 | 13.5 ± 7.5 | 9 | 0.18 | 3 | 0.06 |
Abbreviation: d, Cohen effect size; VASOverall, overall fatigue on visual analog scale.
Time main effect (P ≤ .05).
Different than prerun.
Association Between Running Volume and Changes in PTA and FMS Score During the Training Program
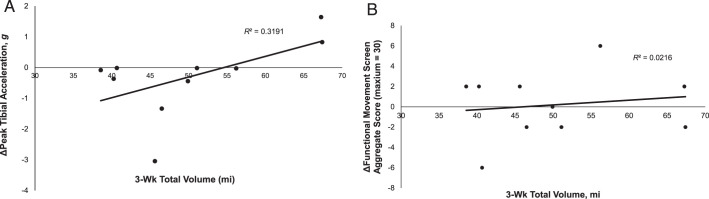
The correlation between total running volume during the 3-week program and the prerun-to-posttraining change in PTA (ΔPTA) was positive, indicating that 31.9% of the variance in ΔPTA was explained by total running volume, but the relationship was not significant (r = 0.57, P = .088; Figure 2A). Only 2.2% of the prerun-to-posttraining change in FMSAgg (ΔFMSAgg) was explained by total running volume during the 3-week program, and this relationship was also not significant (r = 0.15, P = .69; Figure 2B).
Figure 2.
Scatter plot of individual participant mean changes in posttrain and prerun A, peak tibial acceleration and B, Functional Movement Screening aggregate score versus 3-week total running volume.
DISCUSSION
The primary purpose of our study was to assess the effects of a submaximal running bout and a 3-week running program with weekly increases in running volume on PTA and FMS scores in novice runners. Contrary to our hypotheses, a single submaximal prolonged run and 3 weeks of progressively increasing running volume did not affect PTA or FMS scores in novice runners.
The planned 25% increase in weekly volume was not achieved, as participants increased their volume by an average of 19% each week. This average weekly volume increase was slightly less than the reported 20% threshold at which fewer runners sustained injuries compared with those whose weekly increases were between 20% and 60%.28 Peak tibial acceleration and FMS score did not change after the 3-week training period, which suggests that weekly increases in training volume below 20% might be considered a safe progression for preventing increases in tibial shock and reductions in movement quality among novice runners. The manner of weekly volume increases might have affected individual runners and overall PTA changes (Figure 3). Across all participants, the large mean volume increase in week 1 (ie, 34%) might have elicited changes in PTA and movement quality that could have been attenuated after weeks 2 and 3, during which the weekly mean volume increases were smaller (ie, 9% and 15%, respectively). Future authors should address the effects of weekly volume changes on tibial load and movement quality to address this limitation.
Figure 3.
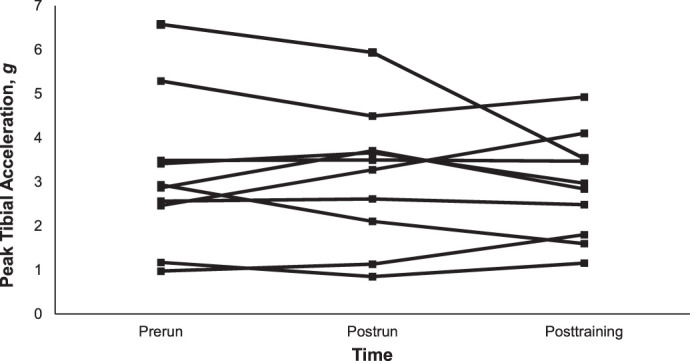
Participant individual mean peak tibial accelerations at each testing timepoint.
The secondary purpose of our study was to assess the association between total running volume during the 3-week program and the changes in PTA and FMS scores from the beginning to the end of the program. Contrary to our hypotheses, total running volume was not significantly associated with the changes in PTA or FMS scores after 3 weeks of running training. However, total volume completed during the 3-week period accounted for approximately 32% of the variance in the PTA change from before to after the training period, while total volume accounted for only about 2% of the variance in the FMS score change (Figure 2). Thus, it appears that total running volume may influence changes in PTA but not movement quality. This finding suggests that it might be important to consider total running volume within a training period and not just weekly volume increases when assessing changes in PTA. Novice runners who run more volume might be more susceptible to training-related changes in PTA than those who run less volume.
The unchanged PTA after the submaximal prolonged run and 3-week training program might also be explained by the lack of large differences in self-reported fatigue ratings after training or the relatively lower fatigue ratings after the submaximal prolonged run. Peak tibial acceleration has been shown to increase with fatigue or continued exercise training exposure.12,13,29 The prolonged submaximal run in our study increased perceived VASOverall and VASLE, but the reported fatigue ratings may not have been large enough to elicit changes in PTA from prerun to postrun (Table 3). After running training, however, 3 participants described noticeable and novel discomfort during the final week or run (or both) of the program. The average changes in weekly running volume and PTA in this subsample were 12% and 18%, respectively. Additionally, these participants' VASLE increased by an average of only 0.73 points after the program. Peak tibial acceleration was unchanged despite a 69% increase in VASLE (d = 0.78), which suggests that perceived fatigue remaining within the 3–5/10 range by the end of training might not have influenced PTA changes associated with accumulated running volume. In addition, longer steps increase PTA while running,30 and step length tends to increase after exhaustive running.31 In our study, given that the treadmill running speed was consistent during in-laboratory tests and was considered an easy pace, the unchanged PTA was a result of the unchanged cadence (and thus step length), and the prolonged run was not exhaustive. Further, the unchanged PTA might be related to the unchanged FCA and, thus, unchanged effective mass at ground contact, after the prolonged run and the training program.32 Therefore, PTA likely remained unchanged because neither FCA nor cadence changed after the run or training.
Our prescribed run was not exhaustive (approximately 12/20 rating of perceived exertion), so our results appear to concur with the previous finding33 that FCA did not change at the end of a 40-minute submaximal treadmill run. A long but submaximal-effort run (ie, 25% of weekly volume) in trained male runners did not change loading rate, foot-strike pattern, or other lower limb joint kinematic variables associated with running injuries.34 The unchanged PTA after the prolonged run and the 3-week training program in our investigation suggest that neither a single submaximal prolonged run nor an approximate 9.7-km increase in volume over a 3-week period (despite being a 54% total increase in volume) increased PTA in novice runners who began the protocol running 16 to 32 km per week. This result may indicate no increased risk of tibial stress fracture in novice runners after a single submaximal prolonged run or a modestly progressive 3-week running training program. However, higher-intensity running (eg, to exhaustion) had various effects on PTA in trained runners14 but increased PTA in novice runners was due to more knee flexion at heel strike (ie, reduced effective mass).29 It is possible that the PTA changes postexhaustive running might reflect muscular or neural fatigue that does not occur in prolonged submaximal running. Therefore, performing biomechanical screening tests after an exhaustive run in novice runners might be more informative to practitioners regarding fatigue-dependent changes in PTA than assessments after a prolonged submaximal run.
With respect to movement quality, no differences were observed between any of the timepoints for the FMS aggregate, DS, or ASLR score. These findings agree with previous research on other populations and follow-up periods, including elite rugby athletes over a season35 or deputy sheriff trainees after a 9-week aerobic and resistance training program.36 After training, our participants perceived relatively more fatigue (+2/10) than at the start of the study but still reported relatively low ratings of fatigue (3–5/10). The low self-reported fatigue ratings and similarity in FMS scores may be related to our cohort's low to moderate absolute initial weekly volumes, the duration of the training period, the lack of uniform adherence to the volume progressions, or differences in self-selected running pace during training. Ultimately, the running training program we used did not appreciably alter the results of the DS or ASLR, possibly because the training program did not greatly increase ratings of self-reported fatigue. It is important to consider that we did not account for influences that may have caused some individuals to respond to the training program or ratings of self-reported fatigue differently than others (eg, other training, life-related stressors). Further, recent evidence37 on the use of the FMS composite score (ie, all criterion tests) might not differentiate injured from uninjured runners in a broader population of runners (eg, girls or women, boys or men, high school or collegiate). Thus, the FMS scores might need to be combined with other factors, including training exposure, to better differentiate injured from uninjured runners. Finally, the data suggest that practitioners and researchers who use these assessments in novice runners should not expect to see different results after either a single submaximal run or a 3-week running training program.
Although the current study may have revealed certain novel findings, some limitations existed. Peak tibial acceleration was not measured bilaterally, and prolonged running or training could have affected the contralateral leg. Because our runners did not have a history of tibial stress fracture, we had no reason to believe that 1 leg was more susceptible to changes in PTA than the other. Also, the between-days intrarater agreements of novice FMS raters were previously reported as 88% for the DS (K = 0.76, 95% confidence interval [CI] = 0.63, 0.85) and 80% for the ASLR (K = 0.60, 95% CI = 0.42, 0.74).38 Although our FMS-certified test administrator had more experience than the novice raters in that earlier study, it is possible that extraneous between-days factors could have affected the FMS criterion test results. In addition, the use of the 2 FMS criterion tests (DS and ASLR) for assessing injury risks has only been evaluated in male high school runners19 and has not been tested in other populations, such as the novice runners in our study. Finally, the small sample size of 10 novice runners limits the generalization of our findings. However, these results still provide valuable information regarding the need to consider other training-related factors when studying biomechanical risk factors in novice runners. Larger studies on this topic will further advance our understanding of training factors and injury risk in runners.
In summary, our data indicated that, for novice runners, neither a submaximal prolonged (ie, 30-minute) run at a self-selected pace nor a 3-week training program of low-intensity running with modest increases in weekly volume caused detectable changes in PTA or movement quality. However, total running volume during the 3-week program explained 32% of the variance in PTA change from before to after training. Novice runners who run more volume at submaximal intensity might be more susceptible to training-related changes in PTA than those who run less volume.
ACKNOWLEDGMENTS
We thank all volunteers for participating in the study.
REFERENCES
- 1.Loringer K, Bedno S, Hauret KG, Jones BH, Kao TC, Mallon TM. Injuries from Participation in Sports Exercise and Recreational Activities Among Active Duty Service Members—Analysis of the April 2008 Status of Forces Survey of Active Duty Members. Aberdeen Proving Ground, MD: US Army Public Health Command; 2011. September 13. [Google Scholar]
- 2.Johnson AW, Weiss CB, Jr, Wheeler DL. Stress fractures of the femoral shaft in athletes–more common than expected. A new clinical test. Am J Sports Med. 1994;22(2):248–256. doi: 10.1177/036354659402200216. [DOI] [PubMed] [Google Scholar]
- 3.Jones BH, Hauschild VD. Physical training, fitness, and injuries: lessons learned from military studies. J Strength Cond Res. 2015;29(Suppl 11):S57–S64. doi: 10.1519/JSC.0000000000001115. [DOI] [PubMed] [Google Scholar]
- 4.Jones BH, Thacker SB, Gilchrist J, Kimsey CD, Jr, Sosin DM. Prevention of lower extremity stress fractures in athletes and soldiers: a systematic review. Epidemiol Rev. 2002;24(2):228–247. doi: 10.1093/epirev/mxf011. [DOI] [PubMed] [Google Scholar]
- 5.Ross RA, Allsopp A. Stress fractures in Royal Marines recruits. Mil Med 2002; 167(7):560–565. [PubMed] [Google Scholar]
- 6.Videbaek S, Bueno AM, Nielsen RO, Rasmussen S. Incidence of running-related injuries per 1000 h of running in different types of runners: a systematic review and meta-analysis. Sports Med 2015; 45(7):1017–1026. doi: 10.1007/s40279-015-0333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milner CE, Ferber R, Pollard CD, Hamill J, Davis IS. Biomechanical factors associated with tibial stress fracture in female runners. Med Sci Sports and Exerc. 2006;38(2):323–328. doi: 10.1249/01.mss.0000183477.75808.92. [DOI] [PubMed] [Google Scholar]
- 8.Zifchock RA, Davis I, Hamill J. Kinetic asymmetry in female runners with and without retrospective tibial stress fractures. J Biomech. 2006;39(15):2792–2797. doi: 10.1016/j.jbiomech.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Bennell K, Crossley K, Jayarajan J, et al. Ground reaction forces and bone parameters in females with tibial stress fracture. Med Sci Sports Exerc. 2004;36(3):397–404. doi: 10.1249/01.mss.0000117116.90297.e1. [DOI] [PubMed] [Google Scholar]
- 10.Bertelsen ML, Hulme A, Petersen J, et al. A framework for the etiology of running-related injuries. Scand J Med Sci Sports. 2017;27(11):1170–1180. doi: 10.1111/sms.12883. [DOI] [PubMed] [Google Scholar]
- 11.Verbitsky O, Mizrahi J, Voloshin A, Treiger J, Isakov E. Shock transmission and fatigue in human running. J Appl Biomech. 1998;14(3):300–311. doi: 10.1123/jab.14.3.300. [DOI] [PubMed] [Google Scholar]
- 12.Mizrahi J, Verbitsky O, Isakov E. Fatigue-related loading imbalance on the shank in running: a possible factor in stress fractures. Ann Biomed Eng. 2000;28(4):463–469. doi: 10.1114/1.284. [DOI] [PubMed] [Google Scholar]
- 13.Voloshin AS, Mizrahi J, Verbitsky O, Isakov E. Dynamic loading on the human musculoskeletal system—effect of fatigue. Clin Biomech (Bristol Avon) 1998;13(7):515–520. doi: 10.1016/s0268-0033(98)00030-8. [DOI] [PubMed] [Google Scholar]
- 14.Clansey AC, Hanlon M, Wallace ES, Lake MJ. Effects of fatigue on running mechanics associated with tibial stress fracture risk. Med Sci Sports Exerc. 2012;44(10):1917–1923. doi: 10.1249/MSS.0b013e318259480d. [DOI] [PubMed] [Google Scholar]
- 15.de Paula Simola RA, Raeder C, Wiewelhove T, et al. Muscle mechanical properties of strength and endurance athletes and changes after one week of intensive training. J Electromyogr Kinesiol. 2016;30:73–80. doi: 10.1016/j.jelekin.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Edwards WB, Ward ED, Meardon SA, Derrick TR. The use of external transducers for estimating bone strain at the distal tibia during impact activity. J Biomech Eng 2009; 131(5):051009. doi: 10.1115/1.3118762. [DOI] [PubMed] [Google Scholar]
- 17.Cook G, Burton L, Hoogenboom BJ, Voight M. Functional movement screening: the use of fundamental movements as an assessment of function—part 2. Int J Sports Phys Ther. 2014;9(4):549–563. [PMC free article] [PubMed] [Google Scholar]
- 18.Cook G, Burton L, Hoogenboom BJ, Voight M. Functional movement screening: the use of fundamental movements as an assessment of function—part 1. Int J Sports Phys Ther. 2014;9(3):396–409. [PMC free article] [PubMed] [Google Scholar]
- 19.Hotta T, Nishiguchi S, Fukutani N, et al. Functional movement screen for predicting running injuries in 18- to 24-year-old competitive male runners. J Strength Cond Res. 2015;29(10):2808–2815. doi: 10.1519/JSC.0000000000000962. [DOI] [PubMed] [Google Scholar]
- 20.Warren M, Lininger MR, Chimera NJ, Smith CA. Utility of FMS to understand injury incidence in sports: current perspectives. Open Access J Sports Med. 2018;9:171–182. doi: 10.2147/OAJSM.S149139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cook G, Burton L, Fields K. The Functional Movement Screen and Exercise Progressions Manual. Functional Movement Screen Web site. 2014 https://www.functionalmovement.com/files/Articles/717a_650a_FMS%20Level%201%20Online%20V1%203-21-2016.pdf Accessed February 21, 2017.
- 22.Bodden JG, Needham RA, Chockalingam N. The effect of an intervention program on functional movement screen test scores in mixed martial arts athletes. J Strength Cond Res. 2015;29(1):219–225. doi: 10.1519/JSC.0b013e3182a480bf. [DOI] [PubMed] [Google Scholar]
- 23.Butler RJ, Plisky PJ, Kiesel K. Interrater reliability of videotaped performance on the functional movement screen using the 100-point scoring scale. Athl Train Sports Health Care. 2012;4(3):103–109. doi: 10.3928/19425864-20110715-01. [DOI] [Google Scholar]
- 24.Clansey AC, Hanlon M, Wallace ES, Nevill A, Lake MJ. Influence of tibial shock feedback training on impact loading and running economy. Med Sci Sports Exerc. 2014;46(5):973–981. doi: 10.1249/MSS.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 25.Damsted C, Glad S, Nielsen RO, Sorensen H, Malisoux L. Is there evidence for an association between changes in training load and running-related injuries? A systematic review. Int J Sports Phys Ther 2018; 13(6):931–942. [PMC free article] [PubMed] [Google Scholar]
- 26.Gruber AH, Boyer KA, Derrick TR, Hamill J. Impact shock frequency components and attenuation in rearfoot and forefoot running. J Sport Health Sci. 2014;3(2):113–121. doi: 10.1016/j.jshs.2014.03.004. [DOI] [Google Scholar]
- 27.Hopkins WG. A New View of Statistics: A Scale of Magnitudes for Effect Statistics. Sportscience Web site. 2002 http://www.sportsci.org/resource/stats/effectmag.html Accessed May 2, 2017.
- 28.Damsted C, Parner ET, Sørensen H, Malisoux L, Hulme A, Nielsen RØ. The association between changes in weekly running distance and running-related injury: preparing for a half marathon. J Orthop Sports Phys Ther. 2019;49(4):230–238. doi: 10.2519/jospt.2019.8541. [DOI] [PubMed] [Google Scholar]
- 29.Derrick TR, Dereu D, McLean SP. Impacts and kinematic adjustments during an exhaustive run. Med Sci Sports Exerc. 2002;34(6):998–1002. doi: 10.1097/00005768-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Mercer JA, Devita P, Derrick TR, Bates BT. Individual effects of stride length and frequency on shock attenuation during running. Med Sci Sports Exerc. 2003;35(2):307–313. doi: 10.1249/01.MSS.0000048837.81430.E7. [DOI] [PubMed] [Google Scholar]
- 31.Hayes PR, Caplan N. Leg stiffness decreases during a run to exhaustion at the speed at VO2max. Eur J Sport Sci. 2014;14(6):556–562. doi: 10.1080/17461391.2013.876102. [DOI] [PubMed] [Google Scholar]
- 32.Edwards WB, Derrick TR. The influence of effective mass on impact force and acceleration. International Society of Biomechanics in Sports Conference; July 14–18. 2008. Seoul, Korea.
- 33.Paquette MR, Milner CE, Melcher DA. Foot contact angle variability during a prolonged run with relation to injury history and habitual foot strike pattern. Scand J Med Sci Sports. 2017;27(2):217–222. doi: 10.1111/sms.12647. [DOI] [PubMed] [Google Scholar]
- 34.Paquette MR, Melcher DA. Impact of a long run on injury-related biomechanics with relation to weekly mileage in trained male runners. J Appl Biomech. 2017;33(3):216–221. doi: 10.1123/jab.2016-0170. [DOI] [PubMed] [Google Scholar]
- 35.Waldron M, Gray A, Worsfold P, Twist C. The reliability of functional movement screening and in-season changes in physical function and performance among elite rugby league players. J Strength Cond Res. 2016;30(4):910–918. doi: 10.1519/JSC.0000000000000270. [DOI] [PubMed] [Google Scholar]
- 36.Rosendale RP. Functional Movement Assessment and Change After a Physical Fitness Training Program in Law Enforcement Personnel [dissertation] University Park: Pennsylvania State University;; 2014. [Google Scholar]
- 37.Bring BV, Chan M, Devine RC, Collins CL, Diehl J, Burkam B. Functional movement screening and injury rates in high school and collegiate runners: a retrospective analysis of 3 prospective observational studies. Clin J Sport Med. 2018;28(4):358–363. doi: 10.1097/JSM.0000000000000459. [DOI] [PubMed] [Google Scholar]
- 38.Teyhen DS, Shaffer SW, Lorenson CL, et al. The Functional Movement Screen: a reliability study. J Orthop Sports Phys Ther. 2012;42(6):530–540. doi: 10.2519/jospt.2012.3838. [DOI] [PubMed] [Google Scholar]