Abstract
Background:
Comorbid mental disorders in patients with tuberculosis (TB) may exacerbate TB treatment outcomes. We systematically reviewed current evidence on the association between mental disorders and TB outcomes.
Methods:
We searched eight databases for studies published from 1990-2018 that compared TB treatment outcomes among patients with and without mental disorders. We excluded studies that did not systematically assess mental disorders and studies limited to substance use. We extracted study and patient characteristics and effect measures and performed a meta-analysis using random-effects models to calculate summary odds ratios (OR) with 95% confidence intervals (CI).
Findings:
Of 7,687 studies identified, ten were included in the systematic review and nine in the meta-analysis. Measurement of mental disorders and TB outcomes were heterogeneous across studies. The pooled association between mental disorders and any poor outcome, loss to follow-up, and non-adherence were OR 2.13 (95% CI: 0.85-5.37), 1.90 (0.33-10.91), and 1.60 (0.81-3.02), respectively. High statistical heterogeneity was present.
Interpretation:
Our review suggests that mental disorders in TB patients increase the risk of poor TB outcomes, but pooled estimates were imprecise due to small number of eligible studies. Integration of psychological and TB services might improve TB outcomes and progress towards TB elimination.
Keywords: Infectious Disease, Lost to Follow-up, Psychiatry, Treatment Adherence and Compliance, Treatment Failure
INTRODUCTION
To eliminate tuberculosis (TB) by 2030, the World Health Organization’s End TB Strategy calls for integrated, patient-centered care and prevention.1 This strategic pillar underscores the management of co-morbidities, including mental disorders, which have been identified in up to 70% of TB patients.2 TB and mental disorders share common risk factors including homelessness, substance use, and HIV infection which may affect health-related behaviours and treatment outcomes.2
A large body of work suggests an association between mental disorders and poor TB treatment outcomes,2-9 yet many studies were not primarily designed to answer this research question.3-6 Not all studies assess mental disorders systematically across all participants,8,9 making results difficult to interpret.10 This gap in evidence greatly impedes progress to formulate an evidence-based strategy to address mental health in TB patients.
The purpose of this study was to systematically review evidence on the impact of mental disorders on TB treatment outcomes. We only included studies that assessed mental disorders systematically for all TB patients. Our primary objective was to determine whether TB patients with concurrent mental disorders have poorer treatment outcomes than patients without mental disorders. We also examined evidence on delayed treatment initiation and medication adherence.
METHODS
This systematic review and meta-analysis was conducted in accordance with PRISMA guidelines and the protocol was registered in the PROSPERO database (CRD42019122382). Ethical approval was not required for this study.
Search strategy
We searched PubMed, Embase Classic, PsycINFO, Global Health, CINAHL, LILACS, Scopus, and Web of Science Core Collection databases for terms related to TB, mental disorder, and TB treatment outcomes. We used both free-text and subject heading (MeSH) terms adapted for use in each database. The MeSH terms search was: “Tuberculosis AND Mental disorders AND (Mortality OR Morbidity OR Death OR Prognosis OR Disease-Free Survival OR Treatment Outcome OR Drug Resistance OR Disease transmission, infectious OR Patient Acceptance of Health Care OR Treatment Failure OR Patient Compliance OR Lost to Follow-Up OR Patient Dropouts OR Treatment Refusal OR Health behavior OR Help-seeking Behavior OR recurrence)”. The full search strategy for PubMed is available in Table 1.
Table 1.
Search strategy for Pubmed
| Search Number |
Query |
|---|---|
| 1 | Tuberculosis [mh] |
| 2 | "tuberculosis" |
| 3 | #1 OR #2 |
| 4 | Mental disorders [mh] mental disorder* OR mental illness* OR mental disease* OR mental health |
| 5 | condition* |
| 6 | psychiatric disorder* OR psychiatric illness* OR psychiatric disease* anxiety disorder* OR mood disorder* OR depression OR "depressive disorder" OR "depressive disorders" OR depressive episode* OR affective disorder* OR |
| 7 | psychotic disorder* OR "psychosis" OR "psychoses" |
| 8 | #5 OR #6 OR #7 |
| 9 | #4 OR #8 Mortality [mh] OR Morbidity [mh] OR Death [mh] OR Prognosis [mh:noexp] OR |
| 10 | Disease-Free Survival [mh] OR Treatment Outcome [mh:noexp] |
| 11 | Drug Resistance [mh] OR Disease transmission, infectious [mh] Patient Acceptance of Health Care [mh:noexp] OR Treatment Failure [mh] OR Patient Compliance [mh] OR Lost to Follow-Up [mh] OR Patient Dropouts [mh] OR Treatment Refusal [mh] OR Health behavior [mh:noexp] OR Help-seeking |
| 12 | Behavior [mh] OR recurrence [mh] |
| 13 | #10 OR #11 OR #12 "Mortality" OR "death" OR "deaths" OR "died" OR "cause of death" OR "morbidity" OR fatal OR fatality OR fatal outcome* OR "prognosis" OR |
| 14 | "prognoses" cure OR cures OR cured OR "disease clearance" OR "pathogen clearance" OR "microbe clearance" OR "microbial clearance" OR "bacteria clearance" OR "bacterial clearance" OR "infection clearance" OR "organism clearance" OR |
| 15 | "disease-free survival" OR "disease-free interval" treatment dropout* OR dropout patient* OR patient dropout* OR no-show |
| 16 | patient* "patient compliance" OR "patient noncompliance" OR "patient adherence" OR "patient nonadherence" OR "treatment compliance" OR "treatment |
| 17 | noncompliance" OR "treatment adherence" OR "treatment nonadherence" "medication adherence" OR "medication nonadherence" OR "medication |
| 18 | compliance" OR "medication noncompliance" "lost to follow-up" OR lost to follow up* OR loss to follow up* OR "loss to follow-up" OR "default" OR "defaults" OR "defaulter" OR "defaulters" OR "defaulting" |
| 19 | OR treatment abandon* treatment outcome* OR treatment success* OR treatment fail* OR treatment complet* OR "completion of treatment" OR "success" OR "successes" OR "succeed" OR "succeeds" OR "succeeded" OR "successful" OR "successfully" |
| 20 | OR favorabl* OR favourabl* OR unfavorabl* OR unfavourabl* |
| 21 | treatment delay* OR treatment refus* |
| 22 | help-seeking behavi* OR treatment seeking behavi* OR health behavi* drug resistan* OR multiple drug resistan* OR multi drug resistan* OR multi-drug resistan* OR multidrug resistant* OR polydrug resistan* OR extensive* drug resistan* OR antibiotic resistan* OR antimicrobial resistan* OR disease transmission* OR resistance amplif* OR "amplification of resistance" OR "relapse" OR "relapses" OR "relapsed" OR "relapser" OR "relapsers" OR |
| 23 | "relapsing" OR "recurrent" OR "recurrence" OR "recurrences" |
| 24 | #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 |
| 25 | #13 OR #24 |
| 26 | #3 AND #9 AND #25 |
Database searches were conducted during October 2018. We included articles published between January 1, 1990 - towards the beginning of the Directly Observed Therapy, Short Course (DOTS) strategy11 - and October 29, 2018. At full-text screening, we reviewed articles published in languages familiar to the co-authors (English, French, Spanish, Portuguese, Korean). We also screened relevant citations in included articles and searched for subsequent publication of study protocols.
Eligibility criteria
Study type and participants
Study types included cross-sectional, case-control, and retrospective and prospective cohort studies, as well as randomized controlled trials. We excluded qualitative studies, case reports and series, theses, literature reviews, meta-analyses, commentaries, editorials, conference abstracts, and latent TB studies. Studies included patients initiating or undergoing treatment for active TB in any setting, with or without multidrug-resistance (MDR).
Exposures and outcomes of interest
We defined mental disorders as mood disorders including depression, anxiety disorders, post-traumatic stress disorder and psychotic disorders. The comparison group was no mental disorders. We only included studies that assessed mental disorders in all TB patients based on the Diagnostic and Statistical Manual of Mental Disorders (DSM), International Classification of Diseases (ICD), or using a validated psychiatric screening instrument. We excluded substance use disorders.
Our primary outcome of interest was TB treatment outcomes as defined by the World Health Organization (WHO): successful (cured, treatment completion) and poor (default or loss to follow-up, failure, death).12 Secondary outcomes were diagnostic delay, defined by time from symptom onset to TB diagnosis or treatment initiation; and medication non-adherence measured by self-report, missed visits, pill count, or physiological tests.
Data analysis
Records found in database searching were uploaded and de-duplicated in EndNote using the Bramer method.13 In duplicate, two reviewers (GL, JS) independently screened abstracts and reviewed the full texts of relevant studies for inclusion. We used Rayyan for screening.14
Data from all studies were extracted independently and duplicated by three authors (GL, JS, EM) using a form based on the Cochrane Data Extraction and Assessment template.15 Data included study characteristics (year, country, study design, sample size) and patient characteristics (type of TB patient, mental health assessment and prevalence, treatment for mental disorder, TB treatment outcomes). Adjusted and unadjusted effect measures (odds, risk, prevalence and hazard ratios) were extracted or calculated for the association between mental disorders and each outcome.
Quality of each study was assessed independently and duplicated among five assessors (GL, JS, EM, JG, AS) using the Risk Of Bias In Non-Randomized Studies of Interventions tool (ROBINS-I).16 Based on the literature, we specified age, sex, and socioeconomic status as key a priori confounders. If studies adjusted for all three confounders, the best bias judgement attributed for the confounding domain was moderate, according to the ROBINS-I tool.16 Disagreements arising in the data extraction and quality assessment process were resolved by consensus.
Statistical analysis
We calculated odds ratios (OR) and 95% confidence intervals (CI) for the association between mental disorders and treatment outcomes. When direct calculation was not possible, we calculated ORs from risk, hazard and prevalence ratios.17,18 Poor outcomes were considered the inverse of successful outcomes. We estimated pooled odds ratios based on the following TB treatment outcomes: any poor outcome (combinations of failure, loss to follow-up, or death), loss to follow-up, and “non-adherence”, which included all definitions of non-adherence across studies. Random-effects models were used, given the clinical heterogeneity of study populations and mental disorders assessed across studies. Heterogeneity was measured using I2 statistics.19 Analysis was done in R (Vienna, Austria).
RESULTS
We considered 5,086 studies for inclusion in the review (Figure 1). Abstract screening eliminated 4,931 articles, leaving 155 for full-text screening. An additional 41 articles were screened from citations within included studies. The most common reasons for exclusion were: lack of relevant exposure or outcome (46.2%), outcome not stratified by exposure (28.5%), or mental illness not systematically assessed (7.0%). In total, ten studies met our inclusion criteria, and nine were included in meta-analysis (Figure 1). We found no studies on TB treatment delay.
Figure 1.
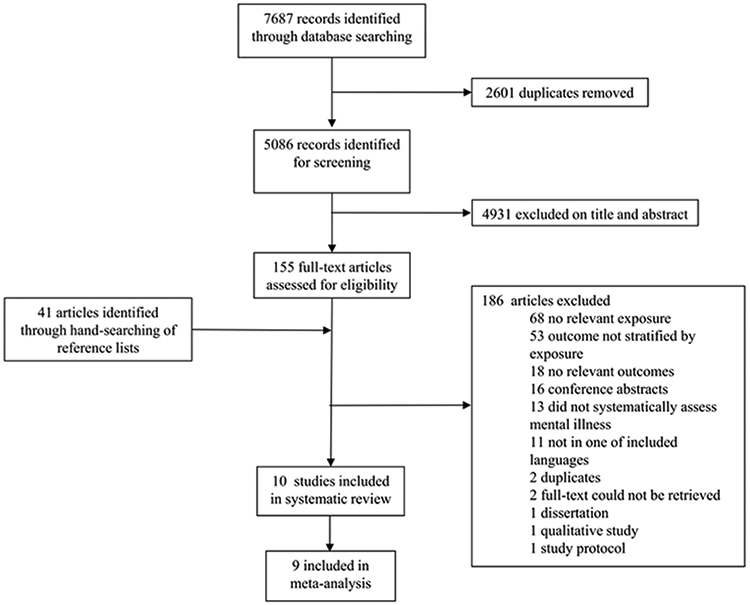
Identification, screening, eligibility, and inclusion of studies, reported using the PRISMA guidelines
Characteristics of included studies
The ten included studies reported data on patients beginning TB treatment between 1999 and 2016 (Table 2). They were conducted in sub-Saharan Africa (South Africa, Ethiopia, Zimbabwe, Zambia, and Tanzania) (n=6),20-25 Peru (n=3);26-28 and China (n=1).29 Six studies were cohort studies,20,21,25-28 three were cross-sectional studies,23,24,29 and one was a case-control study.22 The total sample size was 12,868 (range: 49-4,900). Two studies included MDR-TB patients only.27,28
Table 2.
Characteristics of included studies reporting an association between mental disorders and tuberculosis treatment outcomes.
| Study | Years | Country | Study design | Sample size |
Type of TB patient |
Mental health assessment |
Mental disorder (prevalence) |
Treatment for mental disorder |
TB treatment outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Ambaw | 2014-2016 | Ethiopia | Prospective cohort | 575 | Adults starting sensitive TB treatment | Locally validated Patient Health Questionnaire 9 score of 10 or above at baseline | Depression (53.9%) | Patients responding positively to questionnaire item of suicidal ideation referred to health workers at facilities trained in mental health care as part of WHO’s mhGAPa. | Success Failure Loss to follow-up Death |
| Franke | 1999-2002 | Peru | Retrospective cohort | 613 | Patients starting MDR-TBb treatment | Clinically diagnosed at baseline | Mental disorder (18.9%), predominantly depression | Psychiatric consults and group therapy were offered to patients to address mental health needs, including depression, and increase social support. Patients received psychiatric medications when indicated. | Loss to follow-up Death after loss to follow-up |
| Scuffell | 2010-2012 | Peru | Retrospective cohort | 49 | Adults starting XDR-TBc treatment | Psychiatric evaluations per ICD-10 criteria at baseline | Mental disorder (22.4%), sum of depression, anxiety, mixed anxiety-depressive disorder, psychosis | NId | Poor (failure, loss to follow-up, death) |
| Tola | 2014 | Ethiopia | Prospective cohort | 330 | Adults starting diagnosed with all types of TB on treatment for 1-2 months | Locally validated Kessler K-10 score 16 or above, at baseline | Psychological distress (67.6%) | NId | Success (cure, completion) Poor (loss to follow-up, failure, death) |
| Ugarte-Gil | 2010-2011 | Peru | Prospective cohort | 291 | Adults newly diagnosed with TB starting treatment | Locally validated 5-item Center for Epidemiologic Studies Depression Scale score 6 or above at baseline | Depression (37.5%) | Patients who had scores compatible with depression were referred to the treating physician of the Peruvian National TB Program. | Poor (death, loss to follow-up) |
| Theron | 2011-2012 | South Africa, Zimbabwe, Zambia, Tanzania | Prospective cohort within randomized controlled trial | 1,502 | Adults with TB symptoms | Kessler K-10 scale as a continuous exposure at baseline | Psychological distress (22% with score greater or equal to 30) | NId | Death Non-adherence |
| Govender | NId | South Africa | Case-control | 159 | Newly registered patients starting TB treatment | ICD-10 criteria at baseline, considered as a depression score | Depression (prevalence not recorded) | NId | Non-adherence |
| Naidoo | 2011 | South Africa | Cross-sectional | 3,107 | Adult new TB and new retreatment patients within one month of anti-TB treatment | Kessler K-10 scale score 30 or above | Psychological distress (25.0%) | NId | Non-adherence |
| Peltzer | 2011 | South Africa | Cross-sectional | 4,900 | Adults starting new treatment or retreatment within 1 month of treatment | 4 item Primary Care PTSD Screen score of 2 or above | Post-traumatic stress disorder (29.6%) | NId | Non-adherence |
| Yan | 2013-2014 | China | Cross-sectional | 1,342 | Patients with a diagnosis of TB, without a diagnosis of psychosis | Locally validated 20-item Center for Epidemiologic Studies Depression Scale score 16 or above. Mild depressive symptoms (16-25), severe depressive symptoms (≥26) | Depression (prevalence not reported) | NId | Non-adherence |
mhGAP = Mental Health Gap Action Programme
MDR-TB = Multi drug resistant tuberculosis
XDR-TB = Extensively drug resistant tuberculosis
NI = No information
Mental disorder was measured at baseline either by psychiatric assessment22,27,28 or self-reported screening tools.20,21,23-26,29 (Table 2) The prevalence of mental disorders among TB patients ranged from 18.9%28 to 67.6%.21 Four studies measured depression,20,22,26,29 three psychological distress,21,24,25 and one post-traumatic stress disorder.23 Two studies reported mental disorder as a composite variable.27,28 Only two studies indicated that mental health support was provided to patients outside of usual care.26,28
Primary outcomes
Association between mental disorders and poor TB treatment outcomes
Four studies estimated the association between mental disorders and poor TB treatment outcomes as a composite measure combining some or all of: treatment failure, loss to follow-up, death (Table 3, Figure 2).20,21,26,27 Overall, mental disorders were associated with increased odds of poor TB treatment outcomes (pooled OR 2.13, 95% CI 0.85-5.37, Figure 2). Heterogeneity was high across studies (I2 82%).
Table 3.
Findings of included studies reporting an association between mental disorders and tuberculosis treatment outcomes
| Study | Mental disorder |
TB treatment outcome |
Definition of TB treatment outcome |
Time of outcome assessment |
Adjustment for confoundersa |
Findings |
|---|---|---|---|---|---|---|
| Ambaw | Depression | Success | Cure or treatment course completion | 6 months | Age, Sex, SES | Adjusted RR 0.95 (0.91-1.00) |
| Tola | Psychological distress | Success | Cure or treatment course completion | 6 months | Age, Sex, SES | Adjusted OR 2.87 (1.05-7.81) |
| Scuffell | Mental disorder | Poor | Failure, loss to follow-up, or death | Not applicable | None | Unadjusted OR 1.59 (0.26-17.6) |
| Ugarte-Gil | Depression | Poor | Death or loss to follow-up | 6 months | Age, Sex, SES | Adjusted HR 3.46 (1.48-8.08) |
| Ambaw | Depression | Loss to follow-up | Patient on treatment for at least four weeks and whose treatment was interrupted for eight or more consecutive weeks | 6 months | Age, Sex, SES | Adjusted RR 9.09 (6.72-12.30) |
| Franke | Mental disorder | Loss to follow-up | Patient missed ≥ 30 consecutive days of treatment, prior to physician-approved treatment completion or a physician suspended medications for a patient who was repeatedly nonadherent even after having undergone adherence counseling. | Not applicable | Age, Sex, SES | Unadjusted HR 0.96 (0.50-1.83) |
| Ambaw | Depression | Death | Death of patient (due to any cause) within six months of starting treatment | 6 months | Age, Sex, SES | Adjusted RR 2.99 (1.54-5.78) |
| Theron | Psychological distress | Death | Not specified | 6 months | Not reported | Unadjusted OR for continuous exposure 1.056 (1.035-1.082) |
| Theron | Psychological distress | Non-adherence | Patients who were noted to have missed at a scheduled DOTS visit as recorded on the patient's DOTS clinic card | 2 months | Not specified. Adjusted for site-baseline morbidity, and other clinical and socioeconomic differences. | Adjusted OR 2.29 (1.03-5.10) |
| Govender | Depression | Non-adherence | Patients still taking their anti-TB treatment at the end of the intensive phase. | End of intensive phase | None | Reported mean depression score of 7.14 (6.28-8.00) non-adherence versus 2.55 (1.88-3.22) adherence |
| Naidoo | Psychological distress | Non-adherence | Adherence assessed with the following question: "In your tuberculosis treatment in the past 3-4 weeks how many percent of your anti-tuberculosis medication did you take?" Adherence defined as having taken less than 90% of the anti-TB drugs. | During intensive phase of DOTS programme | Age, Sex, SES | Adjusted OR 0.94 (0.73-1.22) |
| Peltzer | Post-traumatic stress disorder | Non-adherence | Visual Analogue Scale to answer “In past 3–4 weeks how many percent were you taking your anti-tuberculosis medication?”. Non-adherence defined as having taken less than 90% of their anti-TB medication. | Within 1 month of treatment | None | Unadjusted OR 1.00 (0.86-1.17) |
| Yan | Severe depressive symptoms | Non-adherence | 8-item Morisky Medication Adherence Scale score less than 6 being low adherence, 6 to less than 8 being medium adherence, and 8 and higher being high adherence | No information | Age, Sex, SES | Compared to high adherence, adjusted OR 3.67 (2.04-6.61) for low TB treatment adherence |
SES = socioeconomic status
Figure 2.
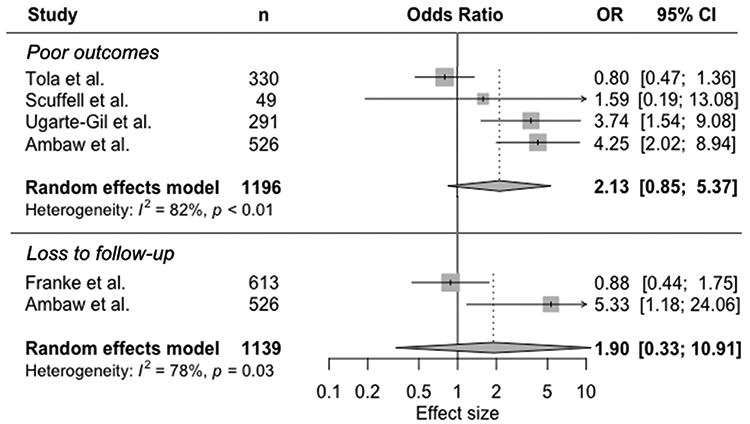
Random-effects meta-analysis of observational studies on mental disorders and tuberculosis treatment outcomes
Association between mental disorders and TB treatment loss to follow-up
Two studies focused on the association between mental disorders and loss to follow-up, but used different definitions (Table 3, Figure 2).20,28 One study defined loss to follow-up as a patient on treatment for at least four weeks and whose treatment was interrupted for eight or more consecutive weeks.20 The other study was defined loss to follow-up as any patient missing 30 or more consecutive days of treatment.26 In the meta-analysis (Figure 2), mental disorder was associated with nearly double the odds of loss to follow-up compared with no mental disorder (pooled OR 1.90, 95% CI 0.33-10.91). There was high heterogeneity (I2 78%).
Association between mental disorders and death
Two studies reported an association between mental disorders and death (Table 3).20,25 In one study, depression was associated with 2.99 times the risk of death (95% CI 1.54-5.78).20 Another study revealed that for each point increase of psychological distress score, odds of death increased by 6% (95% CI 4-8%).25 Meta-analysis was not conducted for this outcome.
Secondary outcomes
Association between mental disorders and non-adherence to TB treatment
TB treatment non-adherence was examined in five studies, but definitions varied (Table 3, Figure 3). Three studies used self-reported non-adherence,23,24,29 one used “discontinuation of treatment beyond the intensive phase of therapy,”22 and the fifth defined it as “missing a scheduled DOTS visit”.25 One study had insufficient information to calculate an effect estimate and was excluded from the meta-analysis.22 Summarizing the effect estimates, there was a trend towards increased odds of non-adherence (OR 1.60, 95% CI 0.84-3.02), with high statistical heterogeneity (I2 86%) (Figure 3).
Figure 3.
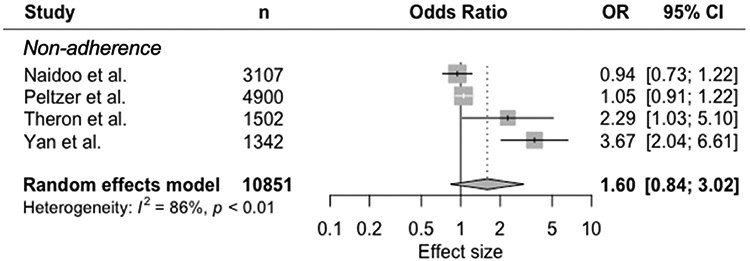
Random-effects meta-analysis of observational studies on mental disorders and tuberculosis treatment non-adherence
Risk of bias assessment
All included studies were observational studies, putting them at risk of bias according to ROBINS-I (Table 4). Half of the studies showed moderate risk of bias related to confounders by adjusting results for age, sex and socioeconomic status,20,24-26,29 while the other half displayed serious risk of bias by not accounting for these confounders.21-23,27,28 Five studies had low risk of bias in selection of participants,20,25-28 but the other five studies were at serious risk of bias with study inclusion being related to mental health status and TB treatment outcome.21-24,29 The most serious methodological concern was risk of classification of exposure. Six studies were at serious risk of bias for this domain, either because mental health was determined at the same time as the outcome or because the definition of mental disorder was not clear.21-25,29 The other four studies had low risk of bias because they used locally validated tools or psychiatric interviews.20,26-28 Two studies reported the provision of psychiatric treatment outside of usual care and were classified as serious risk of bias for deviations from intended exposure.26,28 The other studies were either at low risk of bias or had insufficient information.20-25,27,29 For missing data one study was at serious risk of bias,22 seven were at low risk of bias,20,21,24,25,27-29 and two had no information.23,26 Four studies used self-reported measures and were classified as serious risk of bias due to measurement of outcomes.22-24,29 All except one study24 presented a low risk of bias in selection of reported results.
Table 4.
Risk of bias assessment of observational studies on mental disorder and TB treatment outcomes and non-adherence, according to ROBINS-I criteria
| Risk of bias | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Due to confounding |
Selection of participants into the study |
Classification of exposure |
Deviations from intended exposure |
Missing data |
Measurement of outcomes |
Selection of the reported result |
Overall bias judgement |
| Ambaw | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Franke | Serious | Low | Low | Serious | Low | Low | Low | Serious |
| Govender | Serious | Serious | Serious | ? | Serious | Serious | Low | Serious |
| Naidoo | Moderate | Serious | Serious | ? | Low | Serious | Serious | Serious |
| Peltzer | Serious | Serious | Serious | Low | ? | Serious | Low | Serious |
| Scuffell | Serious | Low | Low | ? | Low | Low | Low | Serious |
| Theron | Moderate | Low | Serious | ? | Low | Low | Low | Serious |
| Tola | Serious | Serious | Serious | ? | Low | Low | Low | Serious |
| Ugarte-Gil | Moderate | Low | Low | Serious | ? | Low | Low | Serious |
| Yan | Moderate | Serious | Serious | Low | Low | Serious | Low | Serious |
? = no information on which to base a judgement about risk of bias for this domain
Low risk of bias (the study is comparable to a well-performed randomized trial with regard to this domain)
Moderate risk of bias (the study is sound for a non-randomized study with regard to this domain but cannot be considered comparable to a well-performed randomized trial)
Serious risk of bias (the study has some important problems)
Critical risk of bias (the study is too problematic to provide any useful evidence on the effects of intervention
DISCUSSION
Our systematic review and meta-analysis found TB patients with mental disorders may have double the risk of loss to follow-up and three times the risk of death compared with patients without mental disorders. Mental disorders were also associated with twice the risk of treatment non-adherence. Still, few studies met criteria for inclusion, resulting in decreased precision of effect estimates, and most included studies had serious risk of bias.
These results are similar to but weaker than associations reported in another recent systematic review focused only on depression.30 This difference may be partially due to variations in outcome definitions and statistical approaches between studies. The strong link between mental disorders and non-adherence to TB treatment is consistent with studies examining medication adherence in patients with mental disorders comorbid with HIV and non-communicable diseases.31-33
We found highly heterogeneous definitions of mental disorders across studies. Of the ten included studies, only three captured mental disorders using ICD or DSM criteria.22,27,28 The remaining seven used screening tools with various cut-off scores.20,21,23-26,29 Given the overlap between some symptoms of TB and mental disorders (e.g. disturbances in appetite, energy, and sleep), screening tools may not be as effective at distinguishing etiology.34 Although half of the studies using screening tools described validation in local populations, none described specific validation among TB patients. This validation is imperative to better evaluate mental health in this population.35
There was also variation in definitions of TB outcomes. The WHO defines loss to follow-up as “a TB patient who did not start treatment or whose treatment was interrupted for 2 consecutive months or more.”12 However, only one study used this definition;19 the other defined loss to follow-up as one month of treatment interruption.28 The five studies evaluating TB treatment adherence22-25,29 had even more disparate definitions: yes or no,22 taking less than 90% of medication,23,24 missing a scheduled DOTS visit,25 and a score qualifying low, medium, or high adherence.29 Variations in outcome definitions may partially explain differences in magnitude and precision of these results.
All included studies except one20 had serious risk of bias using the ROBINS-I tool,18 which limits the interpretation of current evidence. Half of the included studies did not adjust for important confounders of age, sex, and socioeconomic status,34 and we could not account for these in meta-analysis. Residual confounding is likely present since none of the studies measured and adjusted for malnutrition, substance, alcohol or tobacco use.36-39 There are also likely variations in quality and expectations of TB care between studies.
Only one study specifically described mental health intervention provided.28 MDR-TB patients in Peru received group therapy and psychiatric medications to facilitate adherence, which may explain why the association between mental disorders and loss to follow-up was not significant in the study.28 Interventions that aim to directly support TB patients (e.g. psychological therapies and anti-depressant treatment) have been shown to be acceptable40,41 and improve TB outcomes.42 Individual psychotherapy, peer-led “TB clubs”, and psychotropic medications have been shown to improve adherence to TB treatment and TB.10,43 Patient-centered approaches like these either address mental health directly or address TB-related stigma, but are not widely adopted.
Strengths and limitations of study
Our meta-analysis was limited by the small number of studies that met inclusion criteria. For example, 13 studies from retrospective chart review or registry data were excluded because it was unclear if mental disorders were assessed for all patients. To mitigate the small number of studies, we combined all types of mental disorders and both TB and MDR-TB together in the meta-analyses, resulting in high levels of heterogeneity and wide confidence intervals in our pooled estimates. For example, compared to drug-sensitive TB, psychosocial stressors associated with MDR-TB are significantly more complicated;35,44 treatment regimens often include medicines with psychiatric side effects, and treatment duration is much longer.45 As a result, we were unable to form conclusions for specific mental disorders, although different mental disorders may have different impacts on TB outcomes.
CONCLUSIONS
This review emphasizes the scarcity of high quality studies that investigate the impact of mental disorders on TB treatment outcomes. A strong evidence base is essential to close the treatment gap in mental health care and accelerate progress towards achieving the targets of the WHO’s End-TB Strategy. Future studies should systematically assess mental disorders in TB patients using locally validated screening tools or psychiatric evaluations, use standard outcome definitions, and measure and control for confounders. Improving the quality of future studies will showcase the magnitude of associations between mental disorders and TB treatment outcomes.
ACKNOWLEDGEMENTS
We thank Paul Mason, Fred Andayi, Lindokuhle Ndlandla, Lekha Puri, Ajaj Rangaraj, and Rafael Silva Duarte for proposing this systematic review and meta-analyses during a small group session at the McGill Summer Institute of Infectious Diseases.
Funding was provided in part by the US National Institute of Mental Health (NIMH grant K01 MH104514) and National Institute of Allergy and Infectious Diseases (NIAID grant K01 AI118559).
REFERENCES
- 1.World Health Organization. Implementing the end TB strategy: the essentials. Geneva, Switzerland: World Health Organization; 2015. p. 130. Report No.: WHO/HTM/TB/2015.31. [Google Scholar]
- 2.Doherty AM, Kelly J, McDonald C, O’Dywer AM, Keane J, Cooney J. A review of the interplay between tuberculosis and mental health. Gen Hosp Psychiatry. 2013;35(4):398–406. [DOI] [PubMed] [Google Scholar]
- 3.Ribeiro Macedo L, Reis-Santos B, Riley LW, Maciel EL. Treatment outcomes of tuberculosis patients in Brazilian prisons: a polytomous regression analysis. Int J Tuberc Lung Dis. 2013;17(11):1427–34. [DOI] [PubMed] [Google Scholar]
- 4.Duarte EC, Bierrenbach AL, Barbosa da Silva J, Tauil PL, de Fátima Duarte E. Factors associated with deaths among pulmonary tuberculosis patients: a case-control study with secondary data. J Epidemiol Community Health. 2009;63(3):233–8. [DOI] [PubMed] [Google Scholar]
- 5.Wang WB, Zhao Q, Yuan ZA, Jiang WL, Liu ML, Xu B. Deaths of tuberculosis patients in urban China: a retrospective cohort study. Int J Tuberc Lung Dis. 2013;17(4):493–8. [DOI] [PubMed] [Google Scholar]
- 6.Garrido M da S, Penna ML, Perez-Porcuna TM, de Souza AB, Marreiro L da S, Albuquerque BC, et al. Factors associated with tuberculosis treatment default in an endemic area of the Brazilian Amazon: a case control-study. PLoS One. 2012. 12;7(6):e39134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sweetland AC, Jaramillo E, Wainberg ML, Chowdhary N, Oquendo MA, Medina-Marino A, et al. Tuberculosis: an opportunity to integrate mental health services in primary care in low-resource settings. Lancet Psychiatry. 2018;5(12):952–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo S-C, Chen Y-T, Li S-Y, Lee Y-T, Yang AC, Chen T-L, et al. Incidence and outcome of newly-diagnosed tuberculosis in schizophrenics: a 12-year, nationwide, retrospective longitudinal study. BMC Infect Dis. 2013. 29;13:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sikjær MG, Løkke A, Hilberg O. The influence of psychiatric disorders on the course of lung cancer, chronic obstructive pulmonary disease and tuberculosis. Respir Med. 2018;135:35–41. [DOI] [PubMed] [Google Scholar]
- 10.Sweetland A, Oquendo M, Wickramaratne P, Weissman M, Wainberg M. Depression: a silent driver of the global tuberculosis epidemic. World Psychiatry. 2014;13(3):325–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. A guide to understanding the WHO-recommended TB control strategy known as DOTS. Geneva, Switzerland: World Health Organization; 1999. p. 39. Report No.: WHO/CDS/CPC/TB/99.270. [Google Scholar]
- 12.World Health Organization. Definitions and reporting framework for tuberculosis - 2013 revision. Geneva, Switzerland: World Health Organization; 2013. p. 47. Report No.: WHO/HTM/TB/2013.2. [Google Scholar]
- 13.Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104(3):240–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1. [DOI] [PubMed] [Google Scholar]
- 17.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ambaw F, Mayston R, Hanlon C, Medhin G, Alem A. Untreated depression and tuberculosis treatment outcomes, quality of life and disability, Ethiopia. Bull World Health Organ. 2018;96(4):243–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tola HH, Shojaeizadeh D, Garmaroudi G, Tol A, Yekaninejad MS, Ejeta LT, et al. Psychological distress and its effect on tuberculosis treatment outcomes in Ethiopia. Glob Health Action. 2015;8:29019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Govender S, Mash R. What are the reasons for patients not adhering to their anti-TB treatment in a South African district hospital? AJOL. 2009;51(6) [Google Scholar]
- 22.Peltzer K, Naidoo P, Matseke G, Louw J, McHunu G, Tutshana B. Prevalence of post-traumatic stress symptoms and associated factors in tuberculosis (TB), TB retreatment and/or TB-HIV co-infected primary public health-care patients in three districts in South Africa. Psychol Health Med. 2013;18(4):387–97. [DOI] [PubMed] [Google Scholar]
- 23.Naidoo P, Peltzer K, Louw J, Matseke G, McHunu G, Tutshana B. Predictors of tuberculosis (TB) and antiretroviral (ARV) medication non-adherence in public primary care patients in South Africa: a cross sectional study. BMC Public Health. 2013;13:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theron G, Peter J, Zijenah L, Chanda D, Mangu C, Clowes P, et al. Psychological distress and its relationship with non-adherence to TB treatment: a multicentre study. BMC Infect Dis. 2015;15(1):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ugarte-Gil C, Ruiz P, Zamudio C, Canaza L, Otero L, Kruger H, et al. Association of major depressive episode with negative outcomes of tuberculosis treatment. PLoS One. 2013;8(7):e69514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scuffell J, Boccia D, Garcia Velarde F, Leon SR, Raviola G, Lecca L, et al. Mental disorders and drug/alcohol use in patients commencing extensively drug-resistant tuberculosis treatment. Public Health Action. 2017;7(3):237–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franke MF, Appleton SC, Bayona J, Arteaga F, Palacios E, Llaro K, et al. Risk factors and mortality associated with default from multidrug-resistant tuberculosis treatment. Clin Infect Dis. 2008;46(12):1844–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan S, Zhang S, Tong Y, Yin X, Lu Z, Gong Y. Nonadherence to antituberculosis medications: the impact of stigma and depressive symptoms. Am J Trop Med Hyg. 2018;98(1):262–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz-Grosso P, Cachay R, Flor A de la, Schwalb A, Ugarte-Gil C. Association between tuberculosis and depression on negative outcomes of tuberculosis treatment: a systematic review and meta-analysis. PLoS One. 2020;15(1):e0227472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang PS, Bohn RL, Knight E, Glynn RJ, Mogun H, Avorn J. Noncompliance with antihypertensive medications: the impact of depressive symptoms and psychosocial factors. J Gen Intern Med. 2002;17(7):504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eze-Nliam CM, Thombs BD, Lima BB, Smith CG, Ziegelstein RC. The association of depression with adherence to antihypertensive medications: a systematic review. J Hypertens. 2010;28(9):1785–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uthman OA, Magidson JF, Safren SA, Nachega JB. Depression and adherence to antiretroviral therapy in low-, middle- and high-income countries: a systematic review and meta-analysis. Curr HIV/AIDS Rep. 2014;11(3):291–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sweetland AC, Kritski A, Oquendo MA, Sublette ME, Norcini Pala A, Silva LRB, et al. Addressing the tuberculosis-depression syndemic to end the tuberculosis epidemic. Int J Tuberc Lung Dis. 2017;21(8):852–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker IF, Baral SC, Wei X, Huque R, Khan A, Walley J, et al. Multidrug-resistant tuberculosis treatment programmes insufficiently consider comorbid mental disorders. Int J Tuberc Lung Dis. 2017;21(6):603–9. [DOI] [PubMed] [Google Scholar]
- 35.Deiss RG, Rodwell TC, Garfein RS. Tuberculosis and illicit drug use: review and update. Clin Infect Dis. 2009;48(1):72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janse Van Rensburg A, Dube A, Curran R, Ambaw F, Murdoch J, Bachmann M, et al. Comorbidities between tuberculosis and common mental disorders: a scoping review of epidemiological patterns and person-centred care interventions from low-to-middle income and BRICS countries. Infect Dis Poverty. 2020;9(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balinda IG, Sugrue DD, Ivers LC. More than malnutrition: a review of the relationship between food insecurity and tuberculosis. Open Forum Infect Dis. 2019;6(4):ofz102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Najt P, Fusar-Poli P, Brambilla P. Co-occurring mental and substance abuse disorders: a review on the potential predictors and clinical outcomes. Psychiatry Res. 2011;186(2):159–64. [DOI] [PubMed] [Google Scholar]
- 39.Walker IF, Khanal S, Hicks JP, Lamichhane B, Thapa A, Elsey H, et al. Implementation of a psychosocial support package for people receiving treatment for multidrug-resistant tuberculosis in Nepal: a feasibility and acceptability study. PLoS One. 2018;13(7):e0201163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wingfield T, Tovar MA, Huff D, Boccia D, Montoya R, Ramos E, et al. A randomized controlled study of socioeconomic support to enhance tuberculosis prevention and treatment, Peru. Bull World Health Organ. 2017;95(4):270–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rocha C, Montoya R, Zevallos K, Curatola A, Ynga W, Franco J, et al. The innovative socio-economic interventions against tuberculosis (ISIAT) project: an operational assessment. Int J Tuberc Lung Dis. 2011;15(Suppl 2):S50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alipanah N, Jarlsberg L, Miller C, Linh NN, Falzon D, Jaramillo E, et al. Adherence interventions and outcomes of tuberculosis treatment: a systematic review and meta-analysis of trials and observational studies. PLoS Med. 2018;15(7):e1002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanimura T, Jaramillo E, Weil D, Raviglione M, Lönnroth K. Financial burden for tuberculosis patients in low- and middle-income countries: a systematic review. Eur Respir J. 2014;43(6):1763–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khanal S, Elsey H, King R, Baral SC, Bhatta BR, Newell JN. Development of a patient-centred, psychosocial support intervention for multi-drug-resistant tuberculosis (MDR-TB) care in Nepal. PLoS One. 2017;12(1):e0167559. [DOI] [PMC free article] [PubMed] [Google Scholar]


