Abstract
Background:
Most of our knowledge of the biological basis of major depressive disorder (MDD) is derived from studies of chronic stress models in rodents. While these models capture certain aspects of the behavioral and neuroendocrine features of MDD, the extent to which they reproduce the molecular pathology of the human syndrome remains unknown.
Methods:
We systematically compared transcriptional signatures in two brain regions implicated in depression—medial prefrontal cortex (mPFC) and nucleus accumbens (NAc)—of humans with MDD and of three chronic stress models in mice: chronic variable stress (CVS), social isolation in adulthood (SI) and chronic social defeat stress (CSDS). We used differential expression analysis combined with weighted gene co-expression network analysis (WGCNA) to create interspecies gene networks and assess the capacity of each stress paradigm to recapitulate the transcriptional organization of gene networks in human MDD.
Results:
Our results show significant overlap between transcriptional alterations in mPFC and NAc in human MDD and the three mouse chronic stress models, with each of the chronic stress paradigms capturing distinct aspects of MDD abnormalities. CVS and SI better reproduce differentially expressed genes, while CSDS and SI better reproduce gene networks characteristic of human MDD. We also identified several gene networks and their constituent genes that are most significantly associated with human MDD and mouse stress models.
Conclusions:
This study demonstrates the ability of three chronic stress models in mice to recapitulate distinct aspects of the broad molecular pathology of human MDD, with no one mouse model apparently better than another.
Keywords: Major depressive disorder, Mouse models, Chronic stress, Gene expression, Gene networks, Transcriptomics
Introduction
Major depressive disorder (MDD) is one of the most important causes of disability and loss of productivity in the world(1). Past research efforts have highlighted several underlying mechanisms potentially driving the expression of MDD, but little of this research has revealed new therapeutic targets. Restricted access to high quality brain tissue has limited the ability to study the human syndrome directly, with subsequent reliance on animal models driving the field. While several mouse and rat models have been used to study the behavioral impact of stress(2–4), their capacity to reliably reproduce the molecular alterations associated with human MDD remains unknown.
Chronic social defeat stress (CSDS)(5, 6), chronic variable stress (CVS)(7–9) and adult social isolation (SI)(10, 11) represent three chronic stress models in mice that have been shown to reproduce certain phenotypes associated with MDD. All three induce anhedonia and reduce exploratory behavior (interpreted as anxiety-like responses), with CSDS also inducing social avoidance. CSDS has the additional feature of differentiating a subpopulation of mice that are resistant (resilient) to the anhedonia and social deficits seen in susceptible mice.
Global transcriptional analyses in MDD have revealed region-specific alterations in numerous classes of genes in brain(8, 12–17). While similar approaches have demonstrated that CSDS, CVS and SI also induce genome-wide changes in gene expression(7, 8, 18–22), it is not known how these transcriptional profiles in stressed mice overlap with the ones associated with MDD. Indeed, with few exceptions(8, 23, 24), direct comparative studies of human MDD and chronic stress models in rodents are not available.
Recent advances in statistical modelling of large scale genomic, transcriptional and epigenetic data are now providing unbiased data-driven alternative approaches to study the molecular changes underlying the expression of complex diseases in humans, mouse models and cell lines(8, 18, 25–27). These approaches have revealed novel targets and functional pathways underlying stress susceptibility and resilience, and sex-specific features of MDD. In this study, we integrated gene-level and network-level analyses to evaluate how well CSDS, CVS and SI reproduce the transcriptional alterations in brain associated with human MDD.
Materials and Methods
Detailed methods are provided in Supplemental Methods. Briefly, FastQ and metadata files from human MDD (GSE102556), mouse chronic variable stress (GSE102556) and chronic social defeat stress (GSE72343) were obtained from Gene Expression Omnibus. New data for social isolation(10, 11) were generated. Reads were aligned to the GENCODE release 29 (GRCh38.p12) and M21 (GRCm38.p6) for human and mouse, respectively, using STAR and TopHat. Reads for each dataset were counted using HTSeq.
Bioinformatics analyses were performed on human-mouse orthologous genes. Gene expression was transformed and normalized using voom limma(28). Differentially expressed genes (DEGs) were assessed through generalized linear models implemented in limma using phenotype as main factor, with p<0.05. Rank-rank hypergeometric overlap (RRHO)(29, 30) was used to evaluate overlap in gene signatures between human MDD and the mouse models. Enrichment for functional terms in DEGs in human MDD and mouse models was performed using DAVID(31).
We constructed brain region-specific gene networks in each cohort using weighted gene co-expression network analysis (WGCNA)(32, 33). We tested conservation of brain region-specific human modules with each mouse model by analyzing overlap of module membership through Fisher’s exact tests. We examined network phenotypic associations by relating module eigengene expression with phenotype status and calculating module overrepresentation for DEGs in the respective cohort. Finally, we used gene network correlation matrices to identify key regulators for each gene network and compared their interspecies preservation.
Results
To identify converging MDD-relevant transcriptional pathways in mPFC and NAc in three mouse chronic stress models, we used publicly available human MDD(8) and mouse CVS(8) and CSDS(18) datasets, and generated RNAseq profiles for these brain regions in mice after prolonged SI in adulthood(10, 11). We then systematically compared transcriptional profiles associated with each mouse model to human MDD (Figure 1). Details of the human and mouse cohorts are listed in Table 1.
Figure 1. Interspecies assessment of transcriptional signatures in MDD and CSV, SI and CSDS in mice.
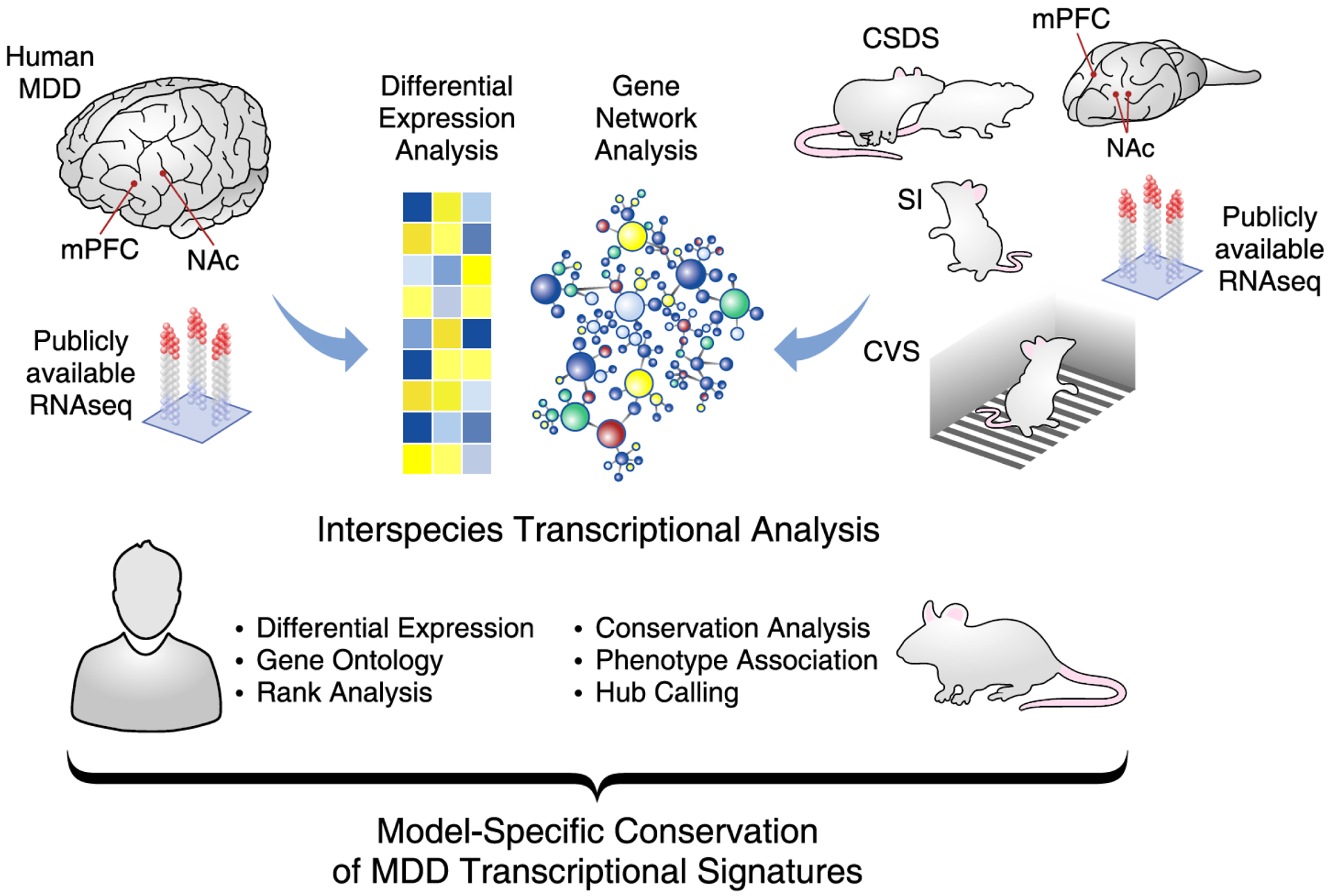
Publically available RNAseq data were obtained from cohorts of human major depressive disorder (MDD) and three mouse models of depressive-like behavior. These mouse models included the chronic variable stress (CVS), prolonged social isolation (SI) and chronic social defeat stress (CSDS) paradigms. Differential expression and weighted gene coexpression network analyses (WGCNA) were performed on each cohort by brain regions, including the medial prefrontal cortex (mPFC) and nucleus accumbens (NAc). Results from these analyses were combined and consistently compared across species. With these dataset, additional analyses including gene ontology overlap, hypergeometric rank, interspecies network conservation, phenotype association and hub calling analyses were performed. The final results allowed to catalogue the capacity of each mouse models to reproduce the transcriptional signatures relevant to MDD across different regions of the human brain.
Table 1.
Human and mouse cohorts’ demographics
| Human | MDD (n=26) | Control (n=22) |
|---|---|---|
| Age | 45.2 ± 17.0 | 48.1 ± 17.0 |
| pH | 6.66 ± 0.25 | 6.56 ± 0.29 |
| PMI | 27.2 ± 16.5 | 27.0 ± 22.7 |
| Sex (M/F) | 13/13 | 13/9 |
| RIN BA25 | 6.42 ± 0.90 | 6.7 ± 0.94 |
| RIN NAc | 7.83 ± 0.75 | 7.35 ± 1.03 |
| Smoking (NA/No/Moderate/Heavy) | 8/6/1/11 | 6/8/0/8 |
| Alcohol | 4 | 1 |
| Drug of abuse | 0 | 1 |
| Antidepressant | 14 | 2* |
| Mouse CVS | CVS (n=20) | Control (n=20) |
| Sex (M/F) | 10/10 | 10/10 |
| RIN mPFC | 8.76 ± 0.40 | 8.91 ± 0.36 |
| RIN NAc | 9.14 ± 0.24 | 9.15 ± 0.32 |
| Mouse SI | SI (n=30) | Control (n=15) |
| Sex (M/F) | 30/0 | 15/0 |
| RIN mPFC | 8.87 ± 0.19 | 9.0 ± 0.18 |
| RIN NAc | 9.29 ± 0.21 | 9.39 ± 0.13 |
| Mouse CSDS | Susceptible (n=11) | Resilient (n=11) | Control (n=11) |
|---|---|---|---|
| Sex (M/F) | 11/0 | 11/0 | 11/0 |
| RIN mPFC | 8.46 ± 0.30 | 8.39 ± 0.34 | 8.56 ± 0.26 |
| RIN NAc | 8.62 ± 0.23 | 8.55 ± 0.17 | 8.90 ± 0.28 |
± SD;
Prescribed for sleep disorders
Shared transcriptional regulation revealed by differential expression analysis
We first identified 9,888 interspecies gene orthologues that are expressed in mPFC or NAc of both humans and mice. All subsequent analyses were performed on this gene set. Our analysis identified transcriptional changes shared between human MDD and mouse CVS, SI or CSDS (p<0.05) in both brain regions (Supplemental Tables 1,2), among which are numerous shared alterations demonstrated previously, such as Dusp4-6, Egr1–2 and Nr4a3(8) (Figures 2A,D). We also identified transcriptional changes in GABAergic markers (Sst, Gabra4, others) and transcription factors (Crem, Creb, cFos). Figures 2A,D exhibit the degree and directionality of differential expression of these genes in mPFC and NAc in CVS, SI and mice susceptible to CSDS.
Figure 2. Differential expression analysis reveals gene signatures associated with MDD in humans and CVS, SI and CSDS in mice.

A, Representative list of genes differentially expressed in the mPFC of human MDD and mouse models. Colors represent fold changes values with bleu showing genes downregulated and yellow showing gene upregulated in MDD or stressed conditions compared to controls. The radius of the circles represents the statistical significance of the differential expression. Blank compartments represent genes being non significantly differentially expressed (p>0.1). B, CVS (yellow), SI (green) and susceptible mice to CSDS (red) each reproduce specific transcriptional changes found in the mPFC of human with MDD (blue). Values in the different portions of the Venn diagram represent the number of genes differentially expressed in common between humans and each of the mouse models of depressive-like behaviors. C, Unbiased rank-rank hypergeometric overlap (RRHO) analysis reveals shared transcriptional changes in the mPFC between human MDD and all three mouse models of depressive-like behaviors. The bar on the right of the plots show levels of significance for the rank overlap between humans and each of the mouse models with maximum Fisher’s Exact Test [FET] p<1.0E-742. D, Representative list of genes differentially expressed in the NAc of human MDD and mouse models. Colors represent fold changes values with bleu showing genes downregulated and yellow showing gene upregulated in MDD or stressed conditions compared to controls. The radius of the circles represents the statistical significance of the differential expression. Blank compartments represent genes being non significantly differentially expressed (p>0.1). E, CVS (yellow), SI (green) and susceptible mice to CSDS (red) each reproduce specific transcriptional changes found in the NAc of human with MDD (blue). Values in the different portions of the Venn diagram represent the number of genes differentially expressed in common between humans and each of the mouse models of depressive-like behaviors. F, Unbiased rank-rank hypergeometric overlap (RRHO) analysis reveals shared transcriptional changes in the NAc between human MDD and all three mouse models of depressive-like behaviors. Similar transcriptional overlap is also observed across mouse models. The bar on the right of the plots show levels of significance for the rank overlap between humans and each of the mouse models with maximum Fisher’s Exact Test [FET] p<1.0E-742.
We compared gene ontology (GO) categories enriched for DEGs across species (Supplemental Table 3,4) and found that alterations of mitochondrial functions are common between human MDD and every mouse model in both mPFC and NAc (Supplemental Figure 1A,B). MDD-relevant mPFC alterations of metabolic processes, DNA methylation and translational elongation were uniquely reproduced in CVS, SI and susceptible CSDS mice, respectively (Supplemental Figure 1A). In NAc, alterations of histone arginine methylation, immune responses and regulation of glutamate secretion were common between human MDD and CVS, SI and susceptible CSDS mice, respectively (Supplemental Figure 1B). Interestingly, GO terms enriched for DEGs in mPFC and NAc of mice resilient to CSDS showed better similarity with human MDD than the susceptible mice, with important differences observed between the susceptible vs resilient phenotypes in both brain regions (Supplemental Figure 1A,B).
Next, we compared DEGs in human MDD with those induced by CVS, SI or CSDS. Figure 2B shows that 16.9% of DEGs in mPFC of human MDD are also differentially expressed in mouse mPFC after CVS (58/344; 24 common, 34 opposite), 18.6% following SI (64/344; 39 common, 25 opposite) and 5.2% in CSDS susceptible mice (18/344; 16 common, 2 opposite). Similarly, in NAc, 20.0% of DEGs in human MDD are also differentially expressed in mouse NAc after CVS (257/1280; 150 common, 107 opposite), 26.7% following SI (342/1280; 210 common, 132 opposite) and only 2.7% in CSDS susceptible mice (34/1280; 14 common, 20 opposite; Figure 1E). Globally, our analysis identified 3 genes differentially expressed in NAc of human MDD and all three mouse models (Zranb1 [H: logFC: −0.054; p=0.045, CVS: logFC:0.09; p=0.015, SI: logFC:−0.08; p=3.23E-5, CSDS susceptible: logFC:0.13; p=0.040)], Copg2 [H: logFC: −0.13; p=0.001, CVS: logFC: 0.08; p=0.002, SI: logFC=−0.071; p=0.0037, CSDS susceptible: logFC:−0.13; p=0.025], 2410131k1Rik [H: logFC: −0.14; p=6.7E-4, CVS: logFC=0.07; p=0.03, SI: logFC=0.07; p=0.037, CSDS susceptible: logFC: −0.13; p=0.038]). No genes were commonly differentially expressed across all three models in mPFC. Our analysis also revealed overlap with human MDD and CSDS resilient mice in mPFC (15.7%; 54/344; 52 common, 2 opposite; Supplemental Figure 2A) and NAc (9.2%; 118/1280; 67 common, 51 opposite; Supplemental Figure 2B).
Because DEG analysis depends upon significance cut-offs and the statistical test used—with widely divergent DEGs identified by different approaches, we extended our interspecies analysis using an unbiased, threshold-free rank-rank hypergeometric overlap (RRHO) procedure(30). In mPFC, dramatic shared regulation of genes was observed between human MDD and CSDS susceptible mice, with much weaker shared regulation observed for SI and CVS (maximum Fisher’s Exact Test [FET] padj<1.0E-742, Figure 2C). A weaker, but significant signal was found in NAc where coordinated up and downregulation of transcriptional signatures was found between human MDD and all three mouse models (maximum FET padj<1.0E-742, Figure 2F). Comparison of human MDD and CSDS resilient mice revealed strong overlap for genes commonly up and downregulated in mPFC (maximum FET padj<1.0E-742, Supplemental Figure 2C), with weaker concordance in NAc (Supplemental Figure 2D).
Overall, results from DEG and RRHO analyses show that each chronic stress model recapitulates a significant subset of gene expression changes seen in mPFC and NAc of human MDD, with DEG analysis showing stronger effects for SI and CVS and RRHO analysis showing stronger effects for CSDS. Explanations for this difference are considered in the Discussion. Another important observation is that the specific genes that comprise the overlap with human MDD are largely different for each mouse stress model.
Shared transcriptional regulation revealed by gene network analyses
Recent work in mouse and human has revealed the value of going beyond differential expression analysis to characterizing gene networks, which reveal distinct but equally important transcriptional insight(8, 18, 32, 33). To determine the conservation of gene networks in human MDD and mouse stress models, we constructed brain region-specific co-expression gene networks for human MDD and mouse CVS, SI and CSDS. We identified 26, 58, 46 and 25 mPFC gene networks, and 39, 29, 31 and 42 NAc gene networks, in MDD, CVS, SI and CSDS, respectively. Each of these gene networks was given an arbitrary color name and was attributed a functional term based on their constituent genes.
Next, we calculated module conservation between the human and mouse cohorts by directly comparing the genes contained within each module. To avoid artifactual overlap due to module size, we first tested different odds ratio thresholds (OR>1.0, >2.0, >3.0 and >5.0) on gene networks showing significant interspecies enrichment (padj<0.05; Supplemental Figure 3). In mPFC, we found that the proportion of overlap between human and each of the mouse models does not vary much with increasing OR thresholds: the proportion of human modules being conserved in one, two or every mouse model varied from 92% at OR>1 to 77% with OR>5.0. In contrast, in NAc, this proportion went from 85% at OR>1.0 down to 36% at OR>5.0. This suggests that, while a significant number of human gene networks in mPFC and NAc are conserved in mice, those from mPFC are better conserved.
Based on this analysis, we used an OR>3.0 for further analyses. At this threshold, 81% of human gene networks in mPFC are conserved in at least one of the mouse models (FET padj< 0.05; OR>3.0), with 27% of the human networks conserved in all three mouse models (Figure 3A). More specifically, 69% of human networks are conserved in CVS and 54% in both SI and CSDS (Figure 3A). In NAc, 69% of human networks are conserved in at least one of the three mouse models (FET padj< 0.05; OR>3.0), and 18% are conserved in all three mouse models, with 31% of human networks conserved in CVS and 49% and 46% preserved in SI and CSDS, respectively (Figure 3B). GO terms associated with these shared modules in mPFC and NAc are shown in Figure 3. Together, these results show that CVS, SI and CSDS each reproduces common but also unique transcriptional features of gene networks in human MDD.
Figure 3. CVS, SI and CSDS reproduce the transcriptional structure of gene network in human brain.
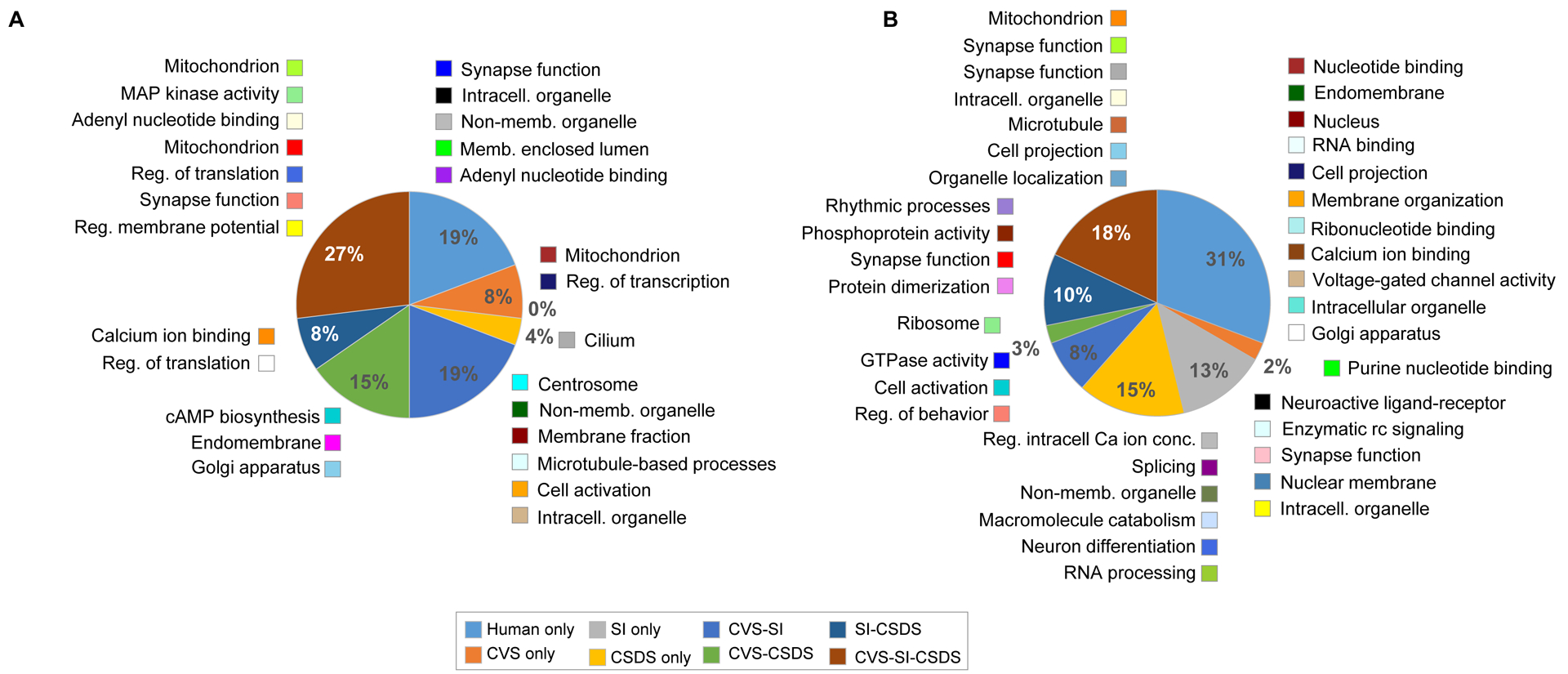
Human-mouse interspecies module conservation in the mPFC (A) and in the NAc (B). Enrichment was performed on module membership by brain region with padj<0.05 at odd ratio (OR)>3. Pie charts are depicting the % of human modules conserved in each of the mouse models. Color legend is shown at the bottom of the figure. Besides each section of the pie appear the human modules (colored square) along with their ontological term. CVS: chronic variable stress, SI: social isolation, CSDS chronic social defeat stress.
Transcriptional organization of gene networks associated with human MDD and mouse chronic stress
In the previous section, we determined which human gene networks were conserved in each mouse model. We then extended these analyses by testing whether the preserved genes networks were associated with “phenotype”: namely, with MDD in humans and with stress exposure in the three mouse models. To determine which gene networks are most strongly associated with these phenotypes, we integrated our network and DEG analyses. We started by correlating the first principal component of each gene network (“module eigengene”) with phenotype status to determine which networks are most strongly associated with DEGs in both species. Our analyses revealed that seven (4 upregulation; 3 downregulation; Figure 4A) gene networks in mPFC and 20 (5 upregulation; 15 downregulation; Figure 4C) in NAc are associated with MDD, while several gene networks in both brain regions of each mouse model associated with chronic stress (Supplemental Figures 4, 5 and 6).
Figure 4. The transcriptional organization of gene networks associates with MDD in human and stress phenotypes in CVS, SI and CSDS.
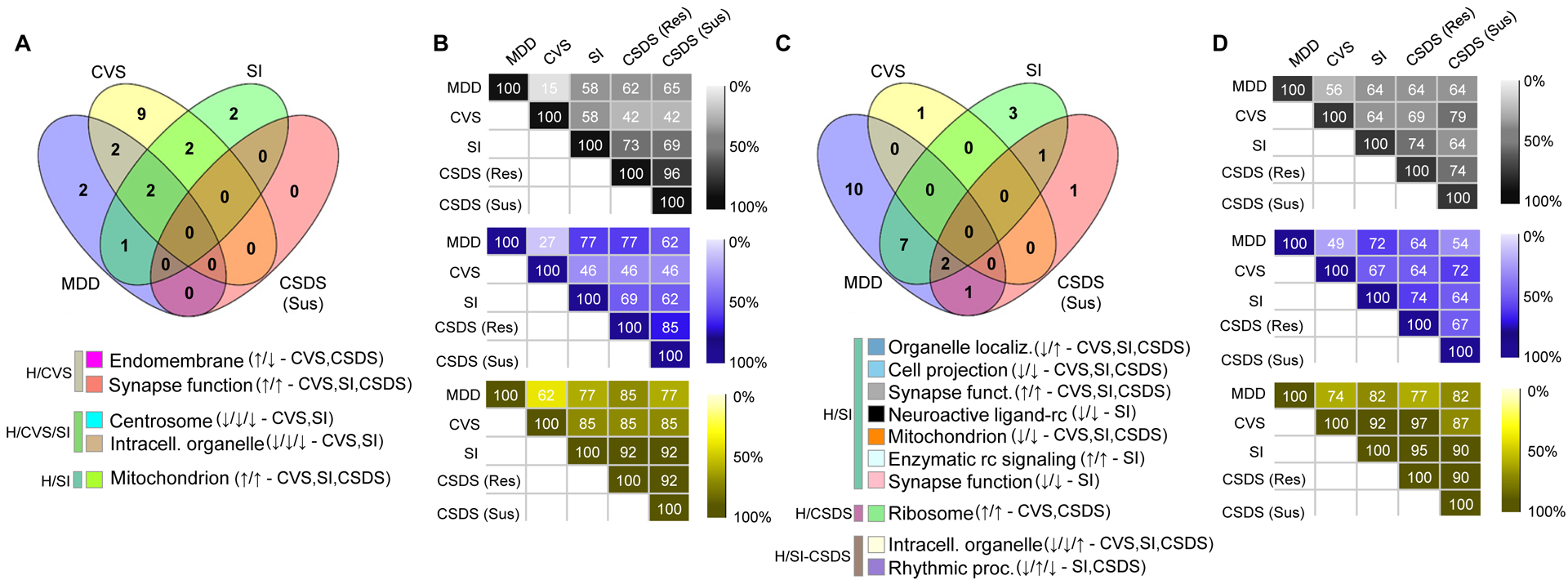
A, C The proportion of interspecies preserved gene networks associated with MDD in human and stress in CVS, SI and CSDS susceptible differs by stress paradigms in the (A) mPFC and (C) NAc. Values in the Venn diagrams represent the number of gene networks preserved in human and mouse (padj<0.05; OR>3.0) and associated with MDD and stress (p<0.2) in human (blue) and CVS (yellow), SI (green) and CSDS susceptible (red). The legends below Venn diagrams in A and C represent the gene network (colored square) associated with their ontological terms commonly associated with the phenotype in human and mice. The arrows in the legend indicate directionality of the association in human MDD (up: increased in MDD compared to control, down: decreased in MDD vs control) followed by directionality of the association in respective mouse models. Human, CVS, SI and CSDS susceptible indications at the end of each network line represent whether each networks are conserved (based on module membership) in CVS, SI or CSDS or not (human only). B,D Heat maps showing the capacity of each mouse model to reproduce patterns of enrichment of genes differentially expressed in the human gene networks in the (B) mPFC and (D) NAc. Numbers in the maps show the percentage of human gene networks showing the same enrichment patterns (black: up and downregulated genes combined, blue: downregulated genes only and yellow upregulated genes only) for genes differentially expressed in each of the mouse models.
We next directly compared the capacity of each mouse model to reproduce the associations found in human MDD. In mPFC, we identified two gene networks associated with human MDD that are also associated with CVS, while one with SI, two with CVS and SI but none with CSDS susceptible mice (Figure 4A). In NAc, we identified seven gene networks commonly associated with MDD and SI, one with CSDS susceptibility, and two with both SI and CSDS susceptibility (Figure 4C). GO terms associated with these shared networks are shown in Figure 4A,C. Similar associations with CSDS resilience are provided in Supplemental Figure 6.
We also assessed the capacity of each mouse model to reproduce the overrepresentation of DEGs in human gene networks. Our analyses in mPFC show that CVS has a limited capacity to reproduce the enrichment patterns for DEGs: when combining genes significantly up- and downregulated, CVS reproduced only 15% of the human enrichment patterns, while 58% were reproduced by SI and 65% by CSDS susceptible mice (Figure 4B). These patterns did not change when considering genes up- or downregulated uniquely in mPFC, but increased to 62% for CVS, 77% for SI and 85% for CSDS susceptible mice when considering only significantly downregulated genes (Figure 4B). In contrast, our results in NAc suggest that all three chronic stress models similarly reproduced human DEG enrichment patterns in gene networks: 56% for CVS and 64% for both SI and CSDS susceptible mice (Figure 4D).
Identification of mouse model-specific hub genes relevant to human MDD
We integrated these analyses to rank each human gene network by their “relevance” based on: a) association with MDD or stress exposure and b) enrichment of DEGs in human MDD and in each of the three mouse models for mPFC (Figure 5A) and NAc (Figure 5C). GO terms associated with these shared networks are shown in these figures.
Figure 5. CVS, SI and CSDS recapitulate the transcriptional organization of gene networks associated with MDD in human mPFC.
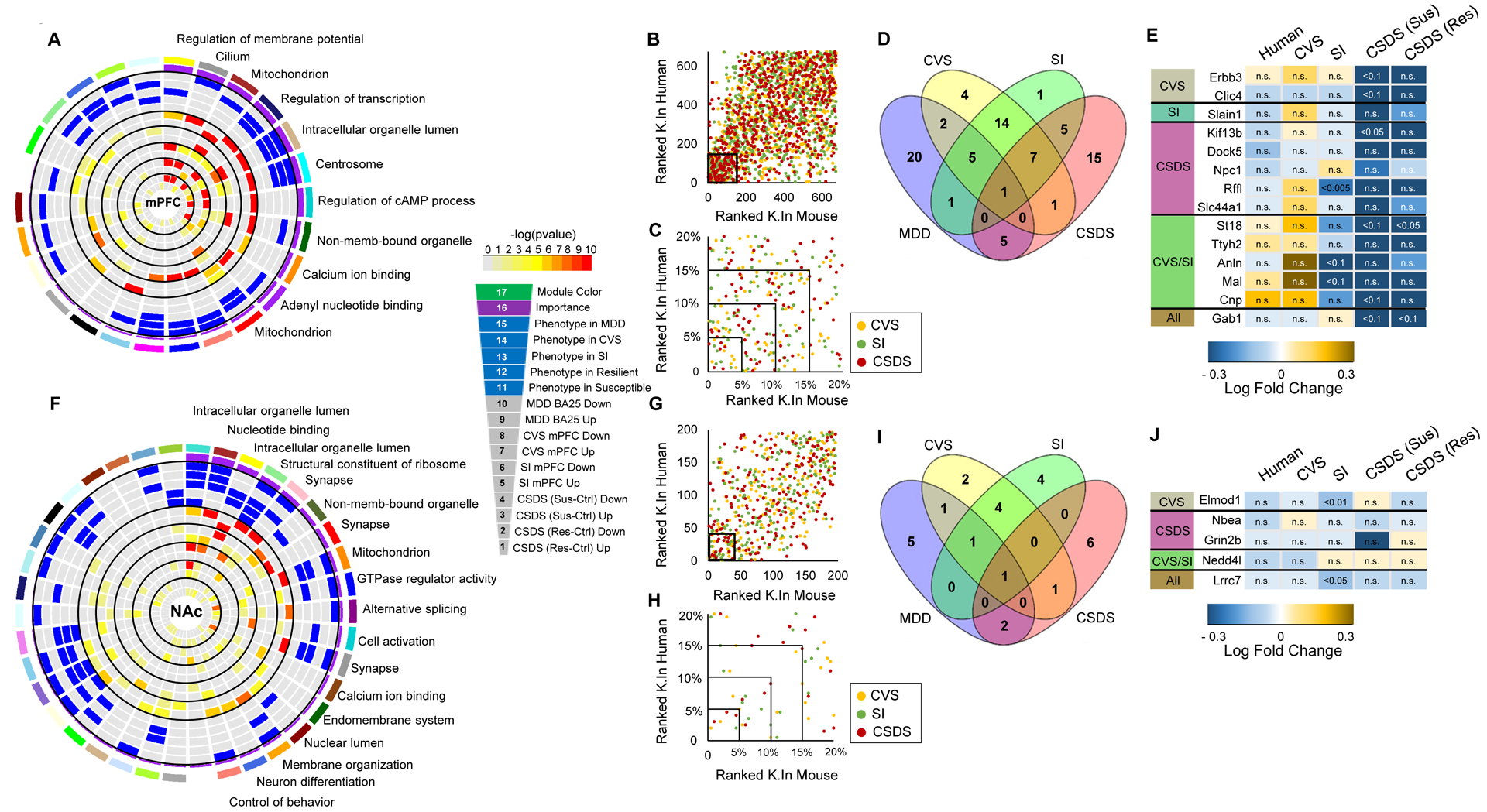
A Circos plots displaying the degree of enrichment for DEGs (p<0.05) in human gene networks in mPFC. Each slice of the chart represents a gene network. The key for the concentric circles is shown on the right of the graph. The outermost rectangle is an arbitrary color as the network name, followed by module “importance” (a composite measure considering the degree of enrichment for DEGs and association with phenotype in human, CVS, SI and CSDS susceptible or resilient). The innermost concentric circles represent the degree to which DEGs in humans and each of the mouse models are contained within a given module from the mPFC (colors reflect corrected FET pval with key provided to the right of the plot). B Scatter plot comparing hYellow network gene ranking (connectivity K.In) in humans and each mouse model (yellow: CVS, green: SI and red: CSDS). The inner square represents the 20% most connected genes. C Scatter plot showing the proportion of most highly connected genes in human and each mouse stress model (enlargement of inner square in B) with the top 5% representing interspecies conserved hub genes in the hYellow gene network. D Venn diagram depicting the number of hub genes within hYellow preserved in each mouse model (blue: human, yellow: CVS, green: SI and red: CSDS). E Table listing hYellow hub genes preserved in human and each of the mouse models with colors in the table indicating fold change in the expression of each gene over respective control conditions (scale presented below the table). n.s.: non-significant transcriptional change. F Circos plots displaying the degree of enrichment for DEGs (p<0.05) in human gene networks in NAc. The innermost concentric circles represent the degree to which DEGs in humans and each mouse model are contained within a given module from the NAc (colors reflect corrected FET pval with key provided to the right of the plot). G Scatter plot comparing hSalmon network gene ranking (connectivity K.In) in humans and each mouse model (yellow: CVS, green: SI and red: CSDS). The inner square represents the 20% most connected genes. H Scatter plot showing the proportion of most highly connected genes in human and each mouse model of chronic stress (enlargement of inner square in G) with the top 5% representing interspecies conserved hub genes in the hSalmon gene network. I Venn diagram depicting the number of hub genes within hSalmon preserved in each mouse model (blue: human, yellow: CVS, green: SI and red: CSDS). J Table listing hSalmon hub genes preserved in human and each of the mouse model with colors in the table indicating fold change in the expression of each gene over respective control conditions (scale presented below the table). n.s.: non-significant transcriptional change.
Next, we calculated and compared hub genes in all human and mouse networks. Hub genes are highly connected with neighboring genes within a given module and have been shown to be significantly associated with disease traits in other experimental systems(8, 18, 24, 32, 33). In mPFC, we concentrated our interest on the hYellow gene network (Figure 5B), which is composed of 675 genes out of which 34 (top 5% most connected genes) are hub genes. This module is involved in the regulation of membrane potential. ~6% of genes in this module are enriched in oligodendrocytes (padj<2.2E-14; OR>6.0; Supplemental Figure 7A). hYellow is conserved in every mouse model and is significantly associated with stress in SI, an observation consistent with previous findings showing significant alterations of oligodendrocyte structure and function induced by SI in mice(10) and with recent postmortem observations in suicide completers with a history of child abuse(34, 35). hYellow is also enriched for genes significantly downregulated in human MDD (padj<4.0E-4, OR>1.3), SI (padj<2.4E-12, OR> 1.8) and both CSDS susceptible (padj<5.4E-30, OR>2.6) and CSDS resilient (padj<4.1E-29, OR> 2.5) mice, with no association seen for CVS (Figure 5A). Our analysis shows that the mouse equivalent of hYellow in CVS shares 172 genes, while SI and CSDS equivalent networks share 148 and 39 genes, respectively, with their human counterpart. Our analysis also shows that human hub genes in hYellow are well conserved in all three mouse models (Figure 5B). For instance, restricting our analysis to the 5% most highly connected (hub) genes, we found that, out of the 34 human hub genes within the hYellow network, 8 conserved their hub status in CVS, 7 in SI and 6 in CSDS (Figure 5C,D). Figure 5E lists conserved hub genes between all human and mouse models. Our analysis shows that the GRB2-associated-binding protein 1 (Gab1) gene serves as a hub in human and every mouse model suggesting that it may have similar control over this network activity in both species.
In NAc, we focused our investigation on the hSalmon network (Figure 5F), which is composed of 196 genes (10 hub genes) involved in control of behavior. hSalmon is significantly associated with human MDD and is enriched for genes significantly downregulated in human MDD (padj<0.005, OR>1.8), CVS (padj<0.05, OR>1.5) and CSDS susceptible mice (padj<0.05, OR>1.4; Figure 5F). The mouse equivalent of hSalmon in CVS shares 12 genes, while SI and CSDS mouse equivalent networks share 8 and 26 genes, respectively, with their human counterpart. Additionally, out of the 10 hub genes within the hSalmon network, 3 conserved their hub status in CVS, 2 in SI and 3 in CSDS susceptible mice (Figure 5G–I). We found Lrrc7, encoding leucine rich repeat containing 7 protein, as a common hub between humans and all three mouse models (Figure 5J).
Discussion
Dissecting the molecular underpinnings of MDD has relied heavily on the use of rodent stress models, the validation of which is based on construct, face and predictive validity(3, 36). However, there has recently been increased scrutiny of mouse chronic stress models given the inherent difficulty of achieving face validity by relating behavioral abnormalities in mice vs. humans(4). A major goal of the present study was to ascertain face validity of mouse chronic stress models at the molecular level through genome-wide transcriptional analysis which is inherently far more objective than behavioral comparisons.
Leveraging DEGs and WGCNA, we evaluated the capacity of CVS, SI and CSDS, three of the most widely used mouse chronic stress models, to reproduce the transcriptional profiles associated with human MDD in two brain regions—mPFC and NAc—widely implicated in depression. Our results show that each model efficiently reproduces common but also unique transcriptional features of the human syndrome. A striking finding is that each of several bioinformatics approaches identified distinct overlaps between human MDD and the three mouse stress models in mPFC and NAc. These differences reflect the knowledge that DEGs, RRHO and WGCNA each captures only partly overlapping modes of transcriptional regulation. Overall, the results of this comprehensive analytical pipeline suggest that no one mouse model is better than another, consistent with the interpretation that each model captures a significant portion of the molecular pathology associated with the broad and heterogeneous human syndrome.
Our analyses culminated with the identification of network hub genes as performed previously for human MDD(8) and mouse CSDS and CVS(8, 18, 24). This multilevel integrative analysis allowed us to identify hub genes for each gene network and evaluate whether these hubs are preserved in each mouse model. We used this approach to rank gene networks integrating simultaneously their association with human MDD or stress exposure in mice with DEG patterns across species. In mPFC, we identified hYellow as being involved in the regulation of membrane potential, including numerous genes enriched in oligodendrocytes. Disruption of white matter integrity has been associated with MDD(37). Recent findings in human postmortem brain showed impaired myelin-related gene expression and reduced myelin thickness in the anterior cingulate of suicide completers with childhood abuse(34). Elevated numbers of mature oligodendrocytes along with decreased expression of myelin basic protein has also been reported in mPFC of abused suicide completers(35). Our results show that hYellow is conserved in all three mouse models and enriched for DEGs in human MDD and SI and CSDS but not CVS in mice. Interestingly, SI(10) and CSDS(38) have been associated with consistent changes in oligodendrocytic genes along with loss of myelin integrity in mPFC. Overall, our results are in accordance with previous findings linking altered oligodendrocytic functions with MDD and provide a transcriptional substrate through which these alterations in MDD could be reproduced by these mouse stress model.
We successfully identified a series of hub genes consistently preserved in human MDD and each of the three mouse models. Of particular interest, GAB1 was the only human hub gene in the hYellow network conserved in all mouse models. Gab1 is known to enhance PI3K/AKT activation and to extend the duration of Ras/MAPK signalling(39); it is also downregulated by glucocorticoids(40). Additionally, PKA activates ERBB2 and its effector GAB1 to elevate Oct6 and Egr2 expression and trigger myelination(41).
Similarly, in NAc we identified hSalmon as a gene network enriched with genes associated with the GO term “control of behavior”. We found the Lrrc7 hub gene, which encodes Densin-180, conserved in all mouse models. Densin-180 is found in postsynaptic densities (PSDs) where it interacts with key synaptic signaling proteins including α-actinin, CamKIIα, shank, maguin-1 and β- and δ-catenin(42). Densin-180 controls positioning of CamKIIα at the PSD(43), and its deletion impairs glutamate receptor-dependent forms of long-term depression and modifies spine morphology(44). Thus, by playing central roles in crucial intracellular pathways, Gab1 and Llrc7 likely modulate the expression of adaptive responses to chronic stress, although more work is required to elucidate their specific contributions in CVS, SI and CSDS.
Another interesting finding emerging from our study refers to the degree of overlap that SI shares with CSDS and, to a lower extent, with CVS, while CVS and CSDS share very few similarities in terms of network association with human MDD. This may refer to the type of chronic stressors constituting these different paradigms. CVS uses physical stressors (e.g., foot shocks, tail suspension and tube restraint)(7, 8), whereas SI and CSDS rely on psychosocial stressors either social exclusion(10, 11) or social defeat(5, 6). The molecular similarity between SI and CSDS may therefore reflect the involvement of common coping strategies triggered by psychosocial stress. Our results raise the possibility that each of three chronic stress models might capture a distinct subtype or aspect of the heterogeneous MDD syndrome. Analysis of much larger human cohorts will be needed to test whether a given mouse model is associated with a given symptom cluster or historical feature (e.g., early life stress) of MDD.
Interestingly, our results highlighted a significant level of overlap between human MDD and CSDS resilience. This overlap could reflect the fact that all organisms mount resilient-related adaptations in an attempt to cope with stress. Indeed, numerous molecular adaptations induced by chronic stress in mouse have been linked causally to behavioral resilience(18, 19). Another possibility is that prior antidepressant medication, although clinically ineffective, may have triggered the activation of resilient-like molecular programs, as shown before in mice following CSDS(19). While we controlled for the effect of medication in our human cohort, we cannot exclude the possibility that such treatment may have induced long-term transcriptional changes. Future work with larger human cohorts is also needed to address this question.
mPFC and NAc differ considerably at the molecular, cellular, and circuit levels between mouse and human, and such divergence represents an obvious limitation intrinsic to interspecies comparative studies. This being said, significant degrees of conservation have also been demonstrated for both brain regions(45–50), with significant preservation in processing of rewarding and motivational cues and in the top-down control of emotions. The high levels of interspecies transcriptional convergence observed in the present study presumably reflects this conservation.
The transcriptional signatures of MDD exhibit strong sexual dimorphism(8, 17, 51). Despite this important fact, we performed our study by controlling statistically for the effects of sex. Only our human and CVS cohorts included females. This may, unfortunately, limit our interpretation of the data and may have potentially masked some effects, as we(8) and others(17) have shown dramatic transcriptional sex differences with several examples of opposite changes in gene expression seen in males vs. females. The statistical approach we used, controlling for the main effect of sex in humans and CVS accounted for this effect. SI and CSDS(52) can be performed in females, and it will be interesting in future studies to assess our predictions in the two sexes.
To conclude, by identifying the transcriptional signatures shared between human MDD and CVS, SI and CSDS in mice, our analyses provide a framework supporting the use of mouse models for the study of MDD and guide their selection for studying specific aspects of the syndrome. Clearly none of the mouse models fully recapitulates MDD nor would they be expected to(3). Rather, they replicate aspects of the syndrome. This is not an impediment, but instead an opportunity to use these three and presumably other mouse models to more fully integrate molecular mechanisms of MDD. The approaches used in this study are highly reliable when associating specific molecular signatures with precise clinical and behavioral assessments in humans(27) and mice(25). This enterprise is certainly ambitious, especially considering the intrinsic limitations associated with postmortem tissue, although studies using peripheral tissue (e.g., blood) would be worth exploring. Nevertheless, by knowing that transcriptionally speaking CVS, SI and CSDS each reproduces certain details of the transcriptional signatures of MDD, future studies can more precisely associate these transcriptional signatures with specific symptomatic and historical profiles of the syndrome.
Supplementary Material
Acknowledgement
BL holds a Sentinelle Nord Research Chair, is supported by a NARSAD young investigator award, a CIHR (SVB397205) and Natural Science and Engineering Research Council (NSERC; RGPIN-2019-06496) grants and receives FRQS Junior-1 salary support. EJN is supported by the National Institute of Mental Health (P50MH096890 and R01MH051399) and by the Hope for Depression Research Foundation (HDRF).
Footnotes
Financial disclosures
Authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.(2017): Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 390:1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menard C, Hodes GE, Russo SJ (2016): Pathogenesis of depression: Insights from human and rodent studies. Neuroscience. 321:138–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nestler EJ, Hyman SE (2010): Animal models of neuropsychiatric disorders. Nat Neurosci. 13:1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bale TL, Abel T, Akil H, Carlezon WA Jr., Moghaddam B, Nestler EJ, et al. (2019): The critical importance of basic animal research for neuropsychiatric disorders. Neuropsychopharmacology. 44:1349–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. (2006): Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 311:864–868. [DOI] [PubMed] [Google Scholar]
- 6.Golden SA, Covington HE 3rd, Berton O, Russo SJ (2011): A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 6:1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, et al. (2015): Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. J Neurosci. 35:16362–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labonte B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, et al. (2017): Sex-specific transcriptional signatures in human depression. Nat Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaPlant Q, Chakravarty S, Vialou V, Mukherjee S, Koo JW, Kalahasti G, et al. (2009): Role of nuclear factor kappaB in ovarian hormone-mediated stress hypersensitivity in female mice. Biol Psychiatry. 65:874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, et al. (2012): Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci. 15:1621–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iniguez SD, et al. (2009): CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci. 12:200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, Vawter MP, et al. (2013): Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc Natl Acad Sci U S A. 110:9950–9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sequeira A, Klempan T, Canetti L, ffrench-Mullen J, Benkelfat C, Rouleau GA, et al. (2007): Patterns of gene expression in the limbic system of suicides with and without major depression. Mol Psychiatry. 12:640–655. [DOI] [PubMed] [Google Scholar]
- 14.Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V, et al. (2009): Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS One. 4:e6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sequeira A, Morgan L, Walsh DM, Cartagena PM, Choudary P, Li J, et al. (2012): Gene expression changes in the prefrontal cortex, anterior cingulate cortex and nucleus accumbens of mood disorders subjects that committed suicide. PLoS One. 7:e35367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang SS, Kamphuis W, Huitinga I, Zhou JN, Swaab DF (2008): Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: the presence of multiple receptor imbalances. Mol Psychiatry. 13:786–799, 741. [DOI] [PubMed] [Google Scholar]
- 17.Seney ML, Huo Z, Cahill K, French L, Puralewski R, Zhang J, et al. (2018): Opposite Molecular Signatures of Depression in Men and Women. Biol Psychiatry. 84:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagot RC, Cates HM, Purushothaman I, Lorsch ZS, Walker DM, Wang J, et al. (2016): Circuit-wide Transcriptional Profiling Reveals Brain Region-Specific Gene Networks Regulating Depression Susceptibility. Neuron. 90:969–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagot RC, Cates HM, Purushothaman I, Vialou V, Heller EA, Yieh L, et al. (2017): Ketamine and Imipramine Reverse Transcriptional Signatures of Susceptibility and Induce Resilience-Specific Gene Expression Profiles. Biol Psychiatry. 81:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. (2007): Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 131:391–404. [DOI] [PubMed] [Google Scholar]
- 21.Lisowski P, Juszczak GR, Goscik J, Wieczorek M, Zwierzchowski L, Swiergiel AH (2011): Effect of chronic mild stress on hippocampal transcriptome in mice selected for high and low stress-induced analgesia and displaying different emotional behaviors. Eur Neuropsychopharmacol. 21:45–62. [DOI] [PubMed] [Google Scholar]
- 22.Malki K, Mineur YS, Tosto MG, Campbell J, Karia P, Jumabhoy I, et al. (2015): Pervasive and opposing effects of Unpredictable Chronic Mild Stress (UCMS) on hippocampal gene expression in BALB/cJ and C57BL/6J mouse strains. BMC Genomics. 16:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin LC, Lewis DA, Sibille E (2011): A human-mouse conserved sex bias in amygdala gene expression related to circadian clock and energy metabolism. Mol Brain. 4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorsch ZS, Hamilton PJ, Ramakrishnan A, Parise EM, Wright WJ, Salery M, et al. (2019): Zfp189 Mediates Stress Resilience Through a CREB-Regulated Transcriptional Network in Prefrontal Cortex. Nature Neuroscience. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang P, Scarpa JR, Fitzpatrick K, Losic B, Gao VD, Hao K, et al. (2015): A systems approach identifies networks and genes linking sleep and stress: implications for neuropsychiatric disorders. Cell reports. 11:835–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parikshak NN, Luo R, Zhang A, Won H, Lowe JK, Chandran V, et al. (2013): Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell. 155:1008–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang B, Gaiteri C, Bodea LG, Wang Z, McElwee J, Podtelezhnikov AA, et al. (2013): Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell. 153:707–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smyth GK (2005): Limma: linear models for microarray data In: Gentleman VC R, Dudoit S, Irizarry R, Huber W editor. Bioinformatics and Computational Biology Solutions using R and Bioconductor. New York: Springer,, pp 397–420. [Google Scholar]
- 29.Plaisier SB, Taschereau R, Wong JA, Graeber TG (2010): Rank-rank hypergeometric overlap: identification of statistically significant overlap between gene-expression signatures. Nucleic Acids Res. 38:e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stein JL, de la Torre-Ubieta L, Tian Y, Parikshak NN, Hernandez IA, Marchetto MC, et al. (2014): A quantitative framework to evaluate modeling of cortical development by neural stem cells. Neuron. 83:69–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang da W, Sherman BT, Lempicki RA (2009): Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 4:44–57. [DOI] [PubMed] [Google Scholar]
- 32.Horvath S (2011): Weighted Network Analysis Applications in Genomics and Systems Biology: Springer. [Google Scholar]
- 33.Zhang B, Horvath S (2005): General framework for weighted gene coexpression analysis. Statistical Applications in Genetics and Molecular Biology 4. [DOI] [PubMed] [Google Scholar]
- 34.Lutz PE, Tanti A, Gasecka A, Barnett-Burns S, Kim JJ, Zhou Y, et al. (2017): Association of a History of Child Abuse With Impaired Myelination in the Anterior Cingulate Cortex: Convergent Epigenetic, Transcriptional, and Morphological Evidence. Am J Psychiatry. appiajp201716111286. [DOI] [PubMed] [Google Scholar]
- 35.Tanti A, Kim JJ, Wakid M, Davoli MA, Turecki G, Mechawar N (2018): Child abuse associates with an imbalance of oligodendrocyte-lineage cells in ventromedial prefrontal white matter. Mol Psychiatry. 23:2018–2028. [DOI] [PubMed] [Google Scholar]
- 36.Willner P (1984): The validity of animal models of depression. Psychopharmacology (Berl). 83:1–16. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Leonards CO, Sterzer P, Ebinger M (2014): White matter lesions and depression: a systematic review and meta-analysis. J Psychiatr Res. 56:56–64. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Yan G, Xu H, Fang Z, Zhang J, Zhang J, et al. (2016): The recovery trajectory of adolescent social defeat stress-induced behavioral, (1)H-MRS metabolites and myelin changes in Balb/c mice. Scientific reports. 6:27906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiyatkin A, Aksamitiene E, Markevich NI, Borisov NM, Hoek JB, Kholodenko BN (2006): Scaffolding protein Grb2-associated binder 1 sustains epidermal growth factor-induced mitogenic and survival signaling by multiple positive feedback loops. J Biol Chem. 281:19925–19938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Juszczak GR, Stankiewicz AM (2018): Glucocorticoids, genes and brain function. Prog Neuropsychopharmacol Biol Psychiatry. 82:136–168. [DOI] [PubMed] [Google Scholar]
- 41.Ghidinelli M, Poitelon Y, Shin YK, Ameroso D, Williamson C, Ferri C, et al. (2017): Laminin 211 inhibits protein kinase A in Schwann cells to modulate neuregulin 1 type III-driven myelination. PLoS Biol. 15:e2001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thalhammer A, Trinidad JC, Burlingame AL, Schoepfer R (2009): Densin-180: revised membrane topology, domain structure and phosphorylation status. J Neurochem. 109:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strack S, Robison AJ, Bass MA, Colbran RJ (2000): Association of calcium/calmodulin-dependent kinase II with developmentally regulated splice variants of the postsynaptic density protein densin-180. J Biol Chem. 275:25061–25064. [DOI] [PubMed] [Google Scholar]
- 44.Carlisle HJ, Luong TN, Medina-Marino A, Schenker L, Khorosheva E, Indersmitten T, et al. (2011): Deletion of densin-180 results in abnormal behaviors associated with mental illness and reduces mGluR5 and DISC1 in the postsynaptic density fraction. J Neurosci. 31:16194–16207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geschwind DH, Rakic P (2013): Cortical evolution: judge the brain by its cover. Neuron. 80:633–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Defelipe J (2011): The evolution of the brain, the human nature of cortical circuits, and intellectual creativity. Frontiers in neuroanatomy. 5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hodge RD, Bakken TE, Miller JA, Smith KA, Barkan ER, Graybuck LT, et al. (2019): Conserved cell types with divergent features in human versus mouse cortex. Nature. 573:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duzel E, Bunzeck N, Guitart-Masip M, Wittmann B, Schott BH, Tobler PN (2009): Functional imaging of the human dopaminergic midbrain. Trends Neurosci. 32:321–328. [DOI] [PubMed] [Google Scholar]
- 49.McCutcheon RA, Abi-Dargham A, Howes OD (2019): Schizophrenia, Dopamine and the Striatum: From Biology to Symptoms. Trends Neurosci. 42:205–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strand AD, Aragaki AK, Baquet ZC, Hodges A, Cunningham P, Holmans P, et al. (2007): Conservation of regional gene expression in mouse and human brain. PLoS Genet. 3:e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fatma M, Labonte B (2019): Molecular programs underlying differences in the expression of mood disorders in males and females. Brain Research. 89–103. [DOI] [PubMed] [Google Scholar]
- 52.Harris AZ, Atsak P, Bretton ZH, Holt ES, Alam R, Morton MP, et al. (2017): A Novel Method for Chronic Social Defeat Stress in Female Mice. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


