Abstract
Fluctuations in blood oxygenation and flow are widely used to infer brain activity during resting-state functional magnetic resonance imaging (fMRI). However, there are strong systemic and vascular contributions to resting-state signals that are unrelated to ongoing neural activity. Importantly, these non-neural contributions to haemodynamic signals (or ‘rude mechanicals’) can be as large as or larger than the neurally evoked components. Here, we review the two broad classes of drivers of these signals. One is systemic and is tied to fluctuations in external drivers such as heart rate and breathing, and the robust autoregulatory mechanisms that try to maintain a constant milieu in the brain. The other class comprises local, active fluctuations that appear to be intrinsic to vascular tissue and are likely similar to active local fluctuations seen in vasculature all over the body. In this review, we describe these non-neural fluctuations and some of the tools developed to correct for them when interpreting fMRI recordings. However, we also emphasize the links between these vascular fluctuations and brain physiology and point to ways in which fMRI measurements can be used to exploit such links to gain valuable information about neurovascular health and about internal brain states.
This article is part of the theme issue ‘Key relationships between non-invasive functional neuroimaging and the underlying neuronal activity’.
Keywords: resting-state fMRI, cerebrovascular physiology, neurovascular coupling, systemic vascular fluctuations, vasomotion
1. Introduction
It is now widely understood that functional magnetic resonance imaging (fMRI) does not directly measure neural but rather haemodynamic activity. The success of blood oxygen level dependent (BOLD) and cerebral blood volume (CBV) based fMRI signals as proxies for local neural activity makes it easy to forget their vascular basis. But there are at least two reasons to keep this basis in mind.
First, the neural mechanisms driving the vascular response are complex and can vary between brain regions. This determines how we interpret haemodynamic measurements in terms of local neural activity [1]. The interpretation can change between different cortical areas [2]. It can vary nonlinearly between cortex, thalamus and brainstem for a sensory pathway [3]. It even reverses sign [4,5]. This complex ‘neurovascular coupling’ linking vascular responses to neural activity is an area of considerable research, however, and is well covered by other recent reviews [6–8].
Next, in addition to neurally evoked responses, brain vasculature exhibits large fluctuations in arterial diameter and hence in blood volume and flow independent of local neural activity. Although such fluctuations have long been noted, and these fluctuations can be as large as or even larger than neurally evoked ones [9,10], they are relatively less well studied. These are the subject of the current review. There appear to be two broad classes of vascular fluctuations. One class is systemic and can be related to physiological drivers such as heart rate or breathing [11]; or ‘systemic low-frequency oscillations' that move through the brain vascular network like a travelling wave but can also be detected in the fingertips and toes [12]. The other class is local and comprises fluctuations in blood vessel diameter that correlates over much shorter distances, e.g. over 100s of microns in the mouse, though they may be bilaterally symmetric [9,13]. These local fluctuations may be a form of ‘vasomotion’, i.e. rhythmic dilations and constrictions that were first reported in veins in the bat wing [14] and also have been described since in many blood vessels, particularly arteries, throughout the body [15]. Vasomotion is intrinsic to blood vessels and is generated locally without the need for external drivers, although the fluctuations can be neurally modulated and synchronized (see §4). Imaging studies often treat both systemic and local vascular fluctuations as confounds to be removed. But they have important physiological relevance. The systemic fluctuations are a valuable probe of neurovascular health. The local fluctuations are likely critical for normal vascular perfusion and in shaping the local neurovascular response, and could carry information about internal brain states of arousal and engagement in a task. These points are elaborated further in this review.
2. Systemic haemodynamic fluctuations
Systemic fluctuations in brain vascular responses are tied to physiological processes such as heart rate, breathing and blood pressure (BP). Importantly, the processes relevant in the brain comprise not just the external drivers (in humans, heart rate approximately 1–2 Hz, breathing approximately 0.5 Hz and their slower fluctuations) but also the brain autoregulatory mechanisms triggered by resultant changes in BP and CO2, as described in the next paragraph. The overall systemic processes are low frequency (approx. 0.1 Hz) and can cause dynamic cerebrovascular changes at similar frequencies. Since fMRI cannot resolve individual micro-vessels, rather giving information averaged over relatively large areas of cortex, it is strongly modulated by these systemic processes. Their overlap with frequencies attributed to slow fluctuations in neural activity (approx. 0.01–0.1 Hz) makes it particularly problematic to interpret low-frequency fluctuations in BOLD fMRI signals such as intrinsic or resting-state measurements [16,17]. BOLD is a vascular signal that depends not only on the cerebral metabolic rate of oxygen (CMRO2—which can be assumed to be a proxy of neural activity) but also on other factors such as cerebral blood flow (CBF) and CBV [18], both of which can change independently of neural activity. More importantly, since these dynamic changes occur at the same frequency as the supposed neural fluctuations, they cause issues with the interpretation of resting BOLD fluctuations as purely neural. For example, by increasing test–retest reliability [19] significant differences in default mode network connectivity between Alzheimer's disease patients and controls can disappear once non-neural fluctuations are accounted for [20]. However, this also provides an opportunity to use fMRI to investigate the vascular fluctuations themselves, opening the possibility of using fMRI as a tool for investigating cerebrovascular health, for example, assessing cerebrovascular reactivity (CVR) after stroke [21], investigating the beneficial effects of exercise [22] or linking vascular rigidity to cognitive ageing [23].
When discussing the relationship between systemic processes and the BOLD signal, it is useful to frame blood flow in large feeding arteries in the brain in terms of two mechanistically differing physiological processes: CVR and cerebral autoregulation (CA) [24,25]. CVR is the dilation response to a vasoactive stimulus, such as CO2, that increases CBF. By imposing large physiological challenges, we can gain good insight into how the purely vascular components of fMRI signals can be measured, thus informing us on how natural fluctuations in vascular dynamics might be disentangled from other neural-related fluctuations. CA is the brain's ability to maintain a constant CBF despite changes in systemic BP. To date, CA has mainly been measured with transcranial Doppler (techniques [26–28], which are restricted to large arteries.
An effective way to assess CVR is by altering the mixture of inhaled gases of subjects. This manipulation changes blood properties, which in turn causes cerebral haemodynamic changes that are measurable with fMRI. CO2 is a potent vasodilator that increases CBF—and thus BOLD signals—which can be used to measure CVR. Breath-holds are a simple way of increasing CO2 concentrations in the blood that can provide a repeatable measure of CVR [29]. However, administering specific levels of CO2 through gas masks in the scanner provides a more controlled approach [30]. Using this approach, the calibrated BOLD method allows for the separation of vascular and neural components of fMRI signals to calibrate task-related signals resulting in a measure of CMRO2 changes [31]. Extending this approach by combining CO2 and O2 gas challenges in the scanner facilitates the separation of vascular and neural components of fMRI signals, allowing the measurement of absolute baseline CMRO2 [32–34]. Baseline levels of CBF can be altered using CO2, emulating different disease states. Neurovascular coupling—that is, the flow-metabolism ratio of responses to neural activity (known as n, where n = ΔCBF/ΔCMRO2)—is sensitive to the baseline CBF state, which demonstrates that differing vascular states can confound the interpretation of fMRI BOLD signals [35–38]. Combining tasks that invoke neural activity with CO2 administration shows that there may be two components of BOLD signals organized into spatially overlapping networks: a component representing neural activity and another representing vascular function [39]. Thus, in addition to responding to localized metabolic changes, the brain's vasculature may be regulated in a coordinated manner that mimics (and potentially supports) specific functional brain networks.
To measure CA, a stimulus that changes BP and systemic tone is required. Clinical assessment using gravitational stressors such as tilt-table tests [40] and orthostasis [41] (standing) is widely carried out, but these stressors are not suitable in the magnetic resonance imaging (MRI) environment for obvious reasons. Lower body negative pressure allows the participant to stay supine while simulating gravitational shifts in central blood volume, which results in reductions in central venous pressure, cardiac output and increases in total peripheral resistance and heart rate [42]. The cerebrovascular response to such a stimulus has been successfully measured using MRI, suggesting a differential change in arterial CBV dependent on baseline arterial CBV [43]. These results may indicate that there are differential changes in vascular tone within different segments of the vascular tree; that is, different sized vessels respond differently to BP changes, with large arteries decreasing volume (approx. −50% change in CBV) and smaller arterioles increasing volume (approx. 150% change in CBV). If this size-dependent property of the vasculature holds true, it further complicates the relationship between resting fluctuations in BP and measured fluctuations in BOLD signals. Some areas will show a positive relationship between BP and BOLD, some a negative relationship, dependent on the relative ratio of large and small vessels within a voxel. This could be problematic when comparing fluctuations across different brain regions within a subject. While comparing groups with different vascular structures (such as those with higher tortuosity of vessels owing to arterial stiffening), spurious differences in fluctuations may result.
Additionally, work in rodents [44] and larger, anaesthetized animals [45,46] has shown that arterial oxygen saturation fluctuates with respiration rate and within the breath-to-breath cycle. The oxygenation of blood will thus vary in time. Because of the transit time delays present in the cerebral vasculature [47–49], different brain regions will experience the fluctuations at different times, though these signals will be bilaterally symmetrical, mimicking patterns of BOLD activation. It is also worth noting that fluctuations in the respiration rate are linked with the behavioural state [50,51], and that there are strong interconnections between respiratory brain regions and neuromodulatory centres [52,53]. How this complicated interaction between respiration, blood oxygenation and activity levels in neuromodulatory regions plays out in the human brain is not at all understood.
It is clear from the previous paragraphs that imposed changes in physiology provide measurable changes in fMRI signals. MRI with advanced physiological challenges can provide a comprehensive assessment of vessel health along the cerebrovascular tree and can help inform when neurovascular coupling breaks down during disease. However, when considering the haemodynamic fluctuations that are the topic of this paper, we are discussing fluctuations during the baseline state, that is, those related to natural fluctuations in systemic physiology. In the field of resting-state fMRI, much work has been done to measure systemic fluctuations (or to infer them from the data), and then to remove them as confounds in the hope that what is left is related solely to oscillations in neural activity [11,54]. Cardiac and respiratory cycles can be measured with a pulse oximeter and respiratory bellows, respectively. Related variance in BOLD signals has been removed using techniques such as RETROICOR [55] (which determines changes related to the phase of cardiac and respiratory cycles). Slower changes (less than 0.1 Hz) in heart rate variability [56] and respiratory volume (RVT) [57] have also been measured and removed. A large proportion of BOLD fluctuation variance can be explained by these methods. For example, at 7T it was shown that in voxels across grey matter (after neglecting low-frequency drifts and thermal noise), RETROICOR, RVT and heart rate variability can explain approximately 10% of the variance each, with the remaining 70% deemed to be spontaneous fluctuations [58]. End-tidal gas tensions can be measured using nasal cannulae and gas analysers. Such measurements show that fluctuations in ventilation rate and volume alter the amount of CO2 in the blood, in the same low‐frequency range. This changes CBF and thus the BOLD signals, with end-tidal CO2 explaining approximately 15% of the regional BOLD signals [59]. Since these early methods were developed, there has been a proliferation of techniques that refine physiological confound removal [11,54]. Recently, synchronized low-frequency oscillations in BP and BOLD signals have demonstrated that a proportion of BOLD fluctuations are due to localized control of blood flow that is independent of activity and related to general systemic autoregulatory processes [60]. On a group level, this effect is quite weak when averaged across grey matter, explaining 2.2% of the variance. However, in some highly vascular areas of the brain, the variance explained at the group level can be as high as 9%. In individual subjects, the effect sizes can be even higher.
3. Ongoing local vascular fluctuations not driven by neural activity
In addition to the systemic vascular fluctuations described above, haemodynamic measurements are also strongly affected by ongoing local vascular fluctuations; these are uncorrelated with the systemic fluctuations, although the two overlap in frequency. Here, we argue that these ongoing local vascular fluctuations are driven not by neural activity but rather mostly reflect processes intrinsic to the blood vessels. Local ongoing fluctuations of about 0.1 Hz in cerebral blood vessel diameter have been reported at least since the early 1980s [61]. Such fluctuations are present in all species studied, including humans [62–64], and drive fluctuations in capillary blood flow that are coherent over at least several hundred micrometers [65]. They occur both during wakefulness and under anaesthesia, although the dynamics change with anaesthesia. Even the earliest reports proposed a purely intrinsic, vascular origin akin to vasomotion [61] although later groups also suggested neuromodulatory mechanisms [66]. Recent imaging studies with concurrent multi-unit spiking and local field potential recordings have made it possible to regress away the neurally predicted haemodynamic components and thereby separate out and characterize the residual ongoing component [9,67,68]. A comparison shows that although uncorrelated, the two components are comparable in amplitude and sum together linearly in the net measured haemodynamics. Thus, predictions of haemodynamics using neural activity account for only about 40% of the net measured haemodynamic variance in awake subjects, even during epochs with strong sensory stimulation and correspondingly strong evoked neural activity [9,10]. The variance explained by neural predictors drops to 20% during periods with no extraneous sensory stimulation where neural activity is correspondingly weak [9]. Separating the ongoing haemodynamic component has also made it possible to test for the effects of systematically blockading neural activity and thereby constrain possible underlying mechanisms. Such blockades of net neural activity (using muscimol), local excitatory postsynaptic activity (with CNQX and APV) and adrenergic influence (by blocking receptors) just partially reduced the ongoing haemodynamic fluctuation even when the concurrent neural activity was strongly suppressed [9]. These negative results reinforce the idea that at least a portion of the ongoing component is intrinsically vascular and not neural.
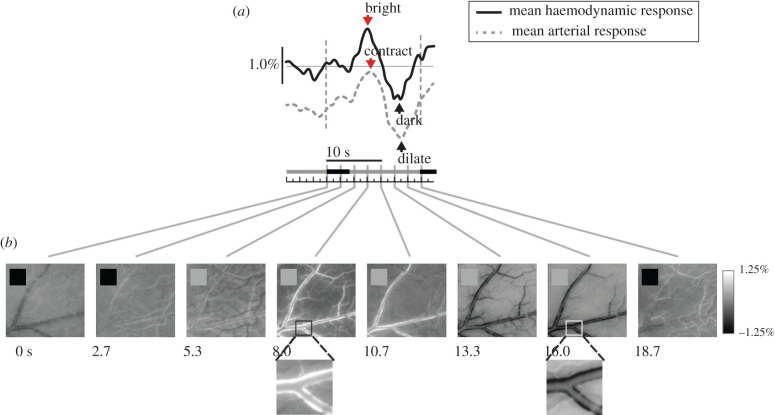
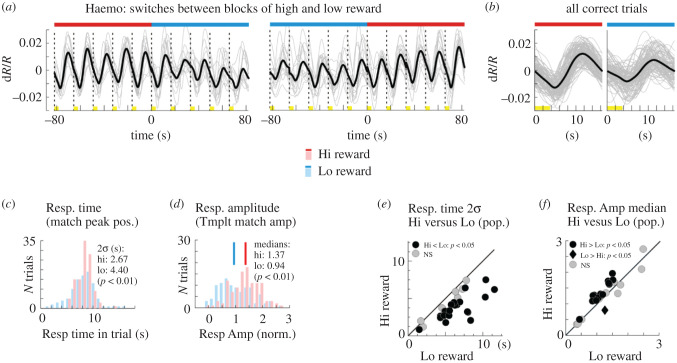
The timing of the local ongoing fluctuations provides further support for a vasomotion-like mechanism. The link is clearest when the animal is awake but not engaged in a task. The ongoing fluctuations show an intrinsic time scale of about 10 s, but with considerable variability and no long-term temporal correlations, consistent with free-running vasomotor fluctuation (see §4 on vasomotion). The situation appears to change when the animal engages in a periodic task. The haemodynamic fluctuation also becomes periodic and entrains robustly to the task timing, over all task periods tested, ranging from about 6–30 s [67,69]. At the vascular level this corresponds to a task-entrained, cyclic fluctuation in blood vessel diameter that is synchronized over the network of pial arteries (figure 1). The precision of this entrainment depends on the animal's level of engagement in the task. When the animal is particularly engaged—for example, when working for a high reward—the task-related response is temporally precise and tightly aligned to the task timing as well as being slightly higher in amplitude [70]. When the animal is less engaged, for example, when working for a low reward, the temporal alignment is less precise, with greater variability in timing, as well as slightly weaker (also see the last paragraph of §4). Notably, there are no other ongoing haemodynamic fluctuations when the task-entrained ones are present. An attractive and parsimonious hypothesis is that both ongoing and task-entrained local haemodynamic fluctuation reflects the same intrinsic vascular mechanism as vasomotion found elsewhere in the body. This could result in the spontaneous vascular fluctuations during wakeful rest but could get synchronized by a task-linked timing signal when the subject is engaged in a task.
Figure 1.
Task-related haemodynamic response: pial arteries show rhythmic contraction and dilation at the task period. (a) Time course of responses measured from primary visual cortex of monkey performing simple periodic fixation task in a dark room. Trial period: 18.7 s, starting 0.0 s. Solid line: mean haemodynamic response over recorded area (recording of CBV, at 536 nm. Vertical scale bar: dR/R, i.e. change in light reflected off cortical surface, as a fraction of the trial mean). Dashed line: mean arterial response (measured using a mask selective for the artery; arbitrary units). (b) Sequence of images (536 nm) showing arterial contraction (turning white: reduced CBV) and dilation (turning black: increased CBV; greyscale: dR/R, defined as in (a)). Black squares in individual panels mark times when monkey fixated periodically. All panels: adapted from Sirotin and Das [67].
4. Vasomotion is an intrinsic property of blood vessels
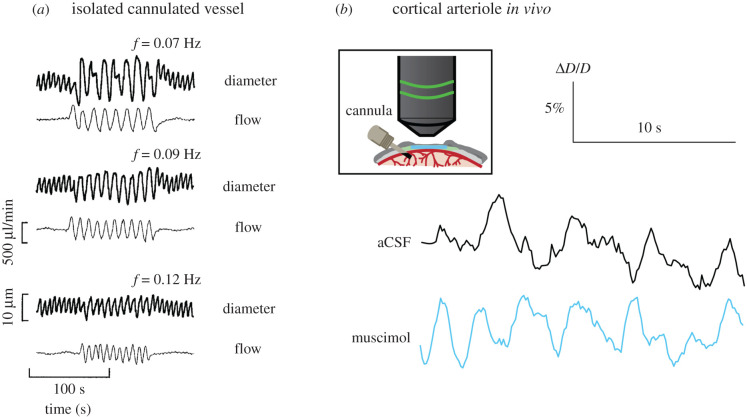
Arteries and arterioles are not simply passive conduits of flow but also contain a large complement of ion channels that are gated by voltage, calcium, pressure and other mechanical forces that can combine together to generate emergent dynamics [71], often referred to as vasomotion [72]. To study these vascular dynamics in a controlled environment, vessel segments can be dissected out and cannulated, allowing the study of their electrical and contractile properties under conditions where the pressure and flow are controlled by the experimenter. Isolated vessels will show spontaneous oscillations in diameter [71,73,74], sometimes with a clearly defined frequency in the 0.1–0.3 Hz range or lower, and other times with no clear peak frequency. The amplitude and frequency of vasomotion in isolated arterioles can be modulated by internal pressure [75] and spontaneous calcium dynamics, which presumably drive the constriction of the vessels. It is suppressed by neural activity in parenchymal arterioles [76]. Vasomotor activity is also suppressed by beta adrenergic input and facilitated by alpha adrenergic input [77]. Thus, despite the intrinsically local nature of vasomotion, it could plausibly be correlated bilaterally through neuromodulatory or callosal connections. The mechanism(s) underlying the diameter oscillations is not understood, though several models have been hypothesized [78,79]. What is known is that the frequency and amplitude of spontaneous arteriole diameter oscillations are greatly reduced by anaesthesia [80], possibly contributing to the differences seen in functional connectivity in awake and anaesthetized animals [81]. While excised vessel experiments show that arteries have the intrinsic capacity to generate spontaneous fluctuations, they do not reveal the extent to which this happens in vivo. When local neural activity is reduced by approximately 90% by infusion of muscimol, there is only a minimal reduction in the amplitude of arterial diameter oscillations, CBV and tissue oxygenation fluctuations [9,44] in mice at rest (figure 2). Amplitude-wise, these spontaneous fluctuations are of order ±3% of diameter. Sensory-evoked arterial dilations to brief stimuli are of order 5–10% [83]. These results suggest that arterioles are able to generate spontaneous oscillations in diameter at the frequencies seen in resting-state studies, independent of neural activity. Arterial dilations will increase blood flow, which will increase tissue oxygenation, which is correlated with the BOLD signal [84]. Recordings of tissue oxygenation in un-anaesthetized mice have shown that the spontaneous fluctuations in oxygenation have a standard deviation of approximately 1.5 mmHg, and these fluctuations in oxygen tension are not affected by blockade of neural activity [44], while sensory-evoked changes in tissue oxygenation are around 2–3 mmHg [44]. Both arterial diameter measurements and oxygen measurements suggest that these ongoing fluctuations that are not of neural origin are approximately half the size of the largest sensory-evoked fluctuations.
Figure 2.
Oscillations in arterial diameter in the absence of neural activity. (a) Diameter and fluid flow through an isolated, cannulated arteriole subjected to different oscillations in flow. The spontaneous oscillations can lock to the imposed input. (b) Spontaneous arteriole diameter measurements in vivo after local infusion of either artificial cerebro-spinal fluid (aCSF) or muscimol. Two photon microscopy was used to measure the spontaneous diameter fluctuations of the same cortical arteriole after aCSF infusion or muscimol infusion (which decreases neural activity by approximately 90% within a radius of 1–2 mm from the infusion cannula). This shows that spontaneous oscillations of cortical arteriole diameter in vivo can occur in the absence of neural input. (a): Adapted from Stergiopulos et al. [82]. (b): Adapted from Winder et al. [9].
In addition to their intrinsic oscillatory capability, arteries throughout the body, including the brain, possess gap junctions between endothelial cells and smooth muscles. These gap junctions electrically couple the cells so that a localized hyperpolarization (causing relaxation of smooth muscles) or a depolarization (causing contractions of smooth muscles) will propagate throughout the vascular tree. The propagation of this electrical signal will follow the cable equation, so the hyperpolarization/depolarization will decay exponentially with distance [85,86] with a length constant in the range of approximately 1 mm for isolated arterioles [87,88]. The conducted response will tend to ‘blur’ the arterial vasodilation, limiting the spatial resolution of any haemodynamic signal [89], and will also serve to couple the oscillations over long distances. The net effect of this coupling is that the arterial network can be thought of as a network of spatially coupled oscillators. Some of these vessels will be receiving similar tonic and oscillatory inputs (e.g. pressure, sympathetic drive), though there may be disparate drives across the network (e.g. local release of vasodilators).
Low-frequency fluctuations in blood flow might have other causes than changes in vessel diameter. Blood is a non-Newtonian fluid [90], so fluctuations in the flow may be enhanced by the nonlinear rheology of blood. Leucocytes are known to plug vessels [91,92] in both the periphery and the brain, leading to large increases in resistance to flow. The presence of leucocytes can drive oscillations of 0.1 Hz or lower frequency in flow in microfluidic networks [93]. The structure of these networks mimics vascular structure but channels lack the ability to dilate and constrict, and the presence of the flow oscillations in these networks under constant pressure shows that the intrinsic properties of the blood, not just vessel diameter fluctuations, may also drive these oscillations in flow. It is likely that the low-frequency fluctuations in flow seen in vivo do not have a single mechanism, but emerge from the interaction of many different drivers that span the cellular to systemic scales.
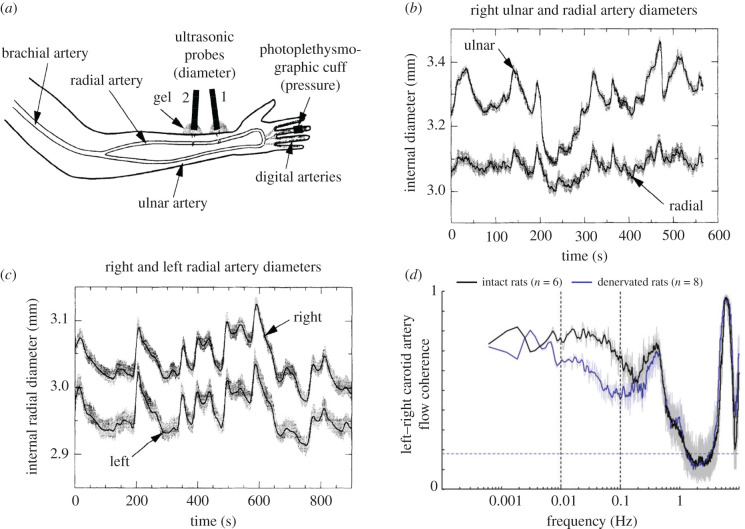
Finally, it is important to keep in mind that fluctuations in blood flow and arterial diameter (and correlations) with power in the 0.01–0.1 Hz range, like those in resting-state signals, are seen throughout the body. For example, the left and right carotid arteries that supply the brain show coherent fluctuations in diameter and/or flow in both humans [48,94] and animals [95] in the absence of any overt stimulation (figure 3). Similar fluctuations in oxygenation and blood flow are also present in the skeletal muscle and tumours [97]. Fluctuations in oxygen in the cat and monkey retina show pronounced oscillation in the 0.06–0.18 Hz range, far from respiration or cardiac frequencies, and are likely owing to arterial dilations at these frequencies. Whether these fluctuations show spatial patterns like those found in the brain is not known, but these fluctuations in arterial diameter are known to be bilaterally symmetric [96], and the fluctuations in blood flow may be more correlated in anatomically related regions (e.g. left and right radial arteries) than between nearby locations (ulnar and radial artery) (figure 3), as is seen in arteriole networks in the brain [13]. These fluctuations in diameter are approximately ±3%, similar in amplitude to those seen in cortical arterioles when neural activity is blocked [9]. These peripheral arterial dilations are coherent with low-frequency oscillations in the cerebral BOLD signal [98], suggesting that they have a common origin or are synchronized somehow. In some of the voxels in the brain, the coherence between the peripheral signal and the BOLD can reach 0.7, indicating that up to about half of the variance in the signal is shared between the BOLD oscillations and systemic fluctuations. Although the origin of these BOLD oscillations in humans is unknown, in animal experiments it has been shown that bilateral sympathetic input drives fluctuations in flow in the same frequency range [95]. These patterns of apparent ‘functional connectivity’ thus appear to be common throughout the body's vasculature, suggesting that care should be taken when interpreting such patterns in the brain as evidence of functional neural connectivity, as they could have substantial contributions from unrelated intrinsic vascular fluctuations.
Figure 3.
‘Functional connectivity’ in vascular networks can occur outside the brain. (a) Schematic showing non-invasive measurement of artery diameter in the arms of human subjects using ultrasound. (b) Simultaneous measurement of ulnar and radial artery diameter on the same side. (c) Simultaneous measurement of left and right radial artery diameters. Note that the diameter oscillations are more similar in contralaterally mirrored arteries than nearby arteries, similar to the functional connectivity found in the brain. (d) Coherence between blood flow in the left and right carotid arteries in conscious rats before and after sympathectomy. Bilaterally synchronous sympathetic drive contributes to some of the coherence below 0.3 Hz, though most of the fluctuations have another source. The peak at approximately 0.3 Hz is vasomotion. (a)–(c) adapted from Porret et al. [96]; (d) adapted from Revel et al. [95].
What do these local fluctuations mean for the interpretation of fMRI measurements? And can estimating them provide more insight into local brain responses? As noted earlier in this review, local vascular fluctuations could be comparable to or larger than neurally evoked responses [9,10]. However they can to some extent be corrected for as they are uncorrelated with the neural response and can be averaged away in the mean. This could be done by randomized stimulus presentation, or by using stimulus contrast in a block design to remove the common task-entrained fluctuations [10,99]. At a more interesting level, however, the local vascular fluctuations could have important physiological roles integral to the normal functioning of the vasculature. They likely enhance normal vascular perfusion [15] and help flush out harmful metabolites or solutes independent of increased blood flow [100]. They could also complement the neurovascular response to increased local neural activity by back-propagating vasodilation along feeding arterioles. This could act as a local impedance match and capacitive buffer in the vascular circuit to facilitate increased blood flow to the activated location [83,101]. Finally, local vascular fluctuations could be a marker of brain state. An attractive hypothesis is that the periodic task-related haemodynamic response seen in area V1 of monkeys engaged in a visual task may be similar to the ‘task structure related’ BOLD fMRI modulations seen in human visual cortex [102]. As in the human measurements, those from monkey V1 are also spatially extensive and homogeneous over the imaged cortical area, and entrain to task timing [69]. As noted earlier, the task-related haemodynamic response in monkey becomes more sharply entrained to task timing as well as getting slightly stronger with higher reward (figure 4) [70]. Since vasomotion is strongly influenced by neuromodulators [77], it would be valuable to test if the effect of task engagement is mediated by sympathetic-like neuromodulatory input. If so, the task-related BOLD fMRI response in human visual cortex could provide useful information about the level of vigilance and engagement [70,103]. It is important to keep these likely functional roles in mind when designing brain imaging experiments.
Figure 4.
Task-related haemodynamic response is more sharply aligned to task timing and grows modestly stronger, with higher reward. (a) Sequences of CBV responses measured from V1 of monkey performing periodic fixation task in a dark room, at transitions between blocks of high and blocks of low reward. Red bars: trials with high reward; blue bars: trials with low reward. Dashed vertical lines: trial onsets. Yellow highlights on time line: fixation periods. (b) Set of all correct trials at high or low reward. (c) Response (Resp.) timing (obtained by matching to a template) is better aligned to trial timing for high versus low reward trials. (d) Response amplitude (obtained by dot product with a template) is modestly higher for high versus low reward trials. ((a) through (d) are from the same experiment). (e) Scatter plot of the width of the response timing, for high versus low reward, across all experiments. (f) Scatter plot of response amplitudes, for high versus low reward, across all experiments. Adapted from Cardoso et al. [70].
5. Conclusions. Relevance of intrinsic vascular dynamics to fMRI, and their functional consequences
fMRI is an easily implemented, high signal-to-noise (SNR) method of measuring in vivo haemodynamic fluctuations in the human brain. BOLD imaging is the most extensively used; however, other lower SNR MRI-based methods exist, such as arterial spin labelling [104] and vascular space occupancy [105,106]. In the field of resting-state fMRI, low-frequency fluctuations in BOLD signal (typically less than 0.1 Hz) are thought to reflect intrinsic fluctuations in neural dynamics. Similarity in these dynamics across different brain regions suggests similar neural functions, thus the regions are taken to represent a functional connectivity network. The existence of multiple networks across the brain has been repeatedly demonstrated [107] and has been shown to relate to functional networks activated by particular tasks [108]. Differences in connectivity strengths between regions are thought to underlie neurodegenerative diseases [109], neurological disorders [110,111] and normal behavioural differences.
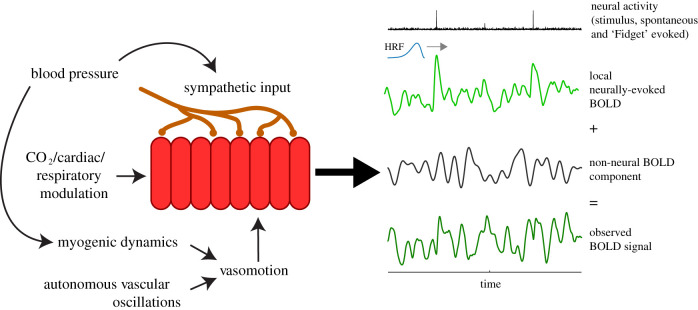
Neurally evoked responses are never seen in isolation, however. They are unavoidably combined with systemic and vascular fluctuations owing to the vascular basis of fMRI (figure 5). Interpreting fMRI results thus requires some care since non-neural effects can be large, as indicated in the numbers reported through this review. When considering systemic fluctuations, cardiac and respiratory effects can account for up to 30% of individual variance, with end-tidal CO2 on its own accounting for up to 15%. However, these numbers are difficult to quantify and vary by a large amount dependent on the location of the signals and how they are aggregated across voxels/regions. When considering the effect of vascular fluctuations, it is noteworthy that arterial diameters oscillate spontaneously with amplitudes approximately half that of sensory-evoked responses. But their contribution depends on their spatial correlation over cortex. When vascular fluctuations are spatially correlated through entrainment to a periodic task, their net contribution could be as large as, or larger than, the visual response [10,67,102]. Without this spatially extensive correlation, the net effect could be weaker, but that is an important question that remains to be fully resolved [9]. The influence of these non-neural effects also depends on additional experimental details. As they are mostly additive, these non-neural effects are a bigger problem in voxels with poorer SNR and weaker neural responses. Non-neural haemodynamic effects can also vary considerably with subject groups, being different, for example, between Alzheimer's disease patients and controls [20]).
Figure 5.
Summary figure. Neural, systemic and vascular components that are proposed in this review to comprise the net measured haemodynamic response. HRF, haemodynamic response function.
Removing variance related to these systemic processes is done with the aim that the low-frequency BOLD fluctuations will more closely follow neural activity. However, an important caveat is that these fluctuations in systemic physiology might change neural activity itself [112]. For example, it has been demonstrated that variations in arterial CO2 caused by natural variations in breathing modulate neural rhythmicity and oscillatory power as measured by magneto-encephalography [113] Changes in this more direct measure of neural activity demonstrate that if we remove CO2-related fluctuations from BOLD signals, we could remove true neural activity-related signals. It is unclear how large this effect might be and whether it would significantly alter the interpretation of the BOLD results as we want to relate them to neural activity. This highlights the difficulty in separating neural and vascular BOLD components from each other. Attempting to remove too many physiological regressors at the same time may remove variance of interest related to neural activity [114]. Pre-processing steps such as bandpass filtering and temporal delay optimization will inflate false positives, again removing variance of interest [115]. Furthermore, if the vasculature is also organized into networks similar to neural activity, we cannot rely on spatial information to help distinguish neural and vascular signals. However, the largest issue may be related to physiological processes that we cannot measure. The vasomotion processes shown above will influence low-frequency BOLD signals. It is unclear to what extent these manifest in the signal and confound results because we have no independent in vivo measure.
Finally, it is important to emphasize that these non-neural processes carry valuable information about vascular function and brain state. Most of the field considers systemic or vascular fluctuations a nuisance confound. In this review we show why such a view is impoverished. Targeted measurements of the systemic components can monitor cerebrovascular health after a stroke [21] or as a consequence of ageing [23], and can probe the neural benefits of exercise [22]; meanwhile, vascular fluctuations are likely integral to normal cerebrovascular function [15,83,100,101] and could also be a measure of brain states like arousal and vigilance [70,103]. These are just a few examples of ways that fMRI studies can be designed to incorporate these prominent and informative non-neural haemodynamic processes.
This review highlights one of the biggest issues when using fMRI to examine low-frequency neural oscillations. We know that BOLD is not only a neural signal but also includes vascular signals, some of which we can independently measure (systemic physiological processes) and some we cannot (local fluctuations, vasomotion). Without a ground truth, though, it is not possible to optimize fMRI analysis techniques to separate these two components. We cannot know how much vasomotion, for example, affects functional connectivity measures because we are measuring a single BOLD signal that is a mixture of neural and vascular. A further complication is that the close symbiotic relationship in the neurovascular unit [112,115] probably means that the two aspects influence each other. Since they cannot exist in isolation, it may be the case that we can never separate neural and vascular processes and that we should consider treating them as a single unit. For resting BOLD fMRI, this would mean that any changes in connectivity should be interpreted as both a neural and vascular change without trying to attribute them to either specific process.
Data accessibility
This article has no additional data.
Authors' contributions
All authors contributed equally to this opinion piece.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Wellcome Trust [Senior Research Fellowship WT200804 (K.M.)]; the National Institutes of Health [grant nos. R01NS078168 and R01NS101353 (P.J.D.) and R01EY025673 and R01EY025330 (A.D.)].
References
- 1.Handwerker DA, Ollinger JM, D'Esposito M. 2004. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. NeuroImage 21, 1639–1651. ( 10.1016/j.neuroimage.2003.11.029) [DOI] [PubMed] [Google Scholar]
- 2.Huo BX, Smith JB, Drew PJ. 2014. Neurovascular coupling and decoupling in the cortex during voluntary locomotion. J. Neurosci. 34, 10 975–10 981. ( 10.1523/JNEUROSCI.1369-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devonshire IM, Papadakis NG, Port M, Berwick J, Kennerley AJ, Mayhew JEW, Overton PG. 2012. Neurovascular coupling is brain region-dependent. NeuroImage 59, 1997–2006. ( 10.1016/j.neuroimage.2011.09.050) [DOI] [PubMed] [Google Scholar]
- 4.Shih Y-YI, Chen CC, Shyu BC, Lin ZJ, Chiang YC, Jaw FS, Chen YY, Chang C. 2009. A new scenario for negative functional magnetic resonance imaging signals: endogenous neurotransmission. J. Neurosci. 29, 3036–3044. ( 10.1523/JNEUROSCI.3447-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shih Y-YI, Chiang YC, Shyu BC, Jaw FS, Duong TQ, Chang C. 2012. Endogenous opioid–dopamine neurotransmission underlie negative CBV fMRI signals. Exp. Neurol. 234, 382–388. ( 10.1016/j.expneurol.2011.12.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA. 2010. Glial and neuronal control of brain blood flow. Nature 468, 232–243. ( 10.1038/nature09613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillman EMC. 2014. Coupling mechanism and significance of the BOLD signal: a status report. Annu. Rev. Neurosci. 37, 161–181. ( 10.1146/annurev-neuro-071013-014111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drew PJ. 2019. Vascular and neural basis of the BOLD signal. Curr. Opin. Neuriobiol. 58, 61–69. ( 10.1016/j.conb.2019.06.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winder AT, Echagarruga C, Zhag Q, Drew PJ. 2017. Weak correlations between hemodynamic signals and ongoing neural activity during the resting state. Nat. Neurosci. 20, 1761–1769. ( 10.1038/s41593-017-0007-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardoso MMB, Sirotin YB, Lima BR, Glushenkova E, Das A. 2012. The neuroimaging signal is a linear sum of neurally distinct stimulus- and task-related components. Nat. Neurosci. 15, 1298–1306. ( 10.1038/nn.3170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy K, Birn RM, Bandettini PA. 2013. Resting-state fMRI confounds and cleanup. NeuroImage 80, 349–359. ( 10.1016/j.neuroimage.2013.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong Y, Hocke LM, Frederick BB. 2019. Low frequency systemic hemodynamic ‘noise’ in resting state BOLD fMRI: characteristics, causes, implications, mitigation strategies, and applications. Front. Neurosci. 13, 787 ( 10.3389/fnins.2019.00787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mateo C, Knutsen PM, Tsai PS, Shih AY, Kleinfeld D. 2017. Entrainment of arteriole vasomotor fluctuations by neural activity is a basis of blood-oxygenation-level-dependent ‘resting-state’ connectivity. Neuron 96, 936–948. ( 10.1016/j.neuron.2017.10.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones TW. 1852. Discovery that the veins of the bat's wing (which are furnished with valves) are endowed with rythmical contractility, and that the onward flow of blood is accelerated by each contraction. Phil. Trans. R. Soc. Lond. B 142, 131–136. ( 10.1098/rstl.1852.0011) [DOI] [Google Scholar]
- 15.Aalkjær C, Boedtkjer D, Matchkov V. 2011. Vasomotion – what is currently thought? Acta Psychol. 202, 253–269. ( 10.1111/j.1748-1716.2011.02320.x) [DOI] [PubMed] [Google Scholar]
- 16.Kelly C, Biswal BB, Craddock RC, Castellanos FX, Milham MP. 2012. Characterizing variation in the functional connectome: promise and pitfalls. Trends Cog. Sci. 16, 181–188. ( 10.1016/j.tics.2012.02.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuo XN, Biswal BB, Poldrack RA. 2019. Editorial: reliability and reproducibility in functional connectomics. Front. Neurosci. 13, 117 ( 10.3389/fnins.2019.00117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buxton RB. 2009. Introduction to functional magnetic resonance imaging: principles and techniques, 2nd edn Cambridge, UK: Cambridge University Press. [Google Scholar]
- 19.Birn RM, Cornejo MD, Molloy EK, Patriat R, Meier TB, Kirk GR, Nair VA, Meyerand ME, Prabhakaran V. 2014. The influence of physiological noise correction on test–retest reliability of resting-state functional connectivity. Brain Connectivity 4, 511–522. ( 10.1089/brain.2014.0284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li YT, Chang CY, Hsu YC, Fuh JL, Kuo WJ, Yeh JN, Lin FH. 2020. Impact of physiological noise in characterizing the functional MRI default-mode network in Alzheimer's disease. In press. J. Cereb. Blood Flow Metab. ( 10.1177/0271678X19897442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geranmayeh F, Wise RS, Leech R, Murphy K. 2015. Measuring vascular reactivity with breath-holds after stroke: a method to aid interpretation of group-level BOLD signal changes in longitudinal fMRI studies. Hum. Brain Mapp. 36, 1755–1771. ( 10.1002/hbm.22735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steventon JJ, Foster C, Furby H, Helme D, Wise RG, Murphy K. 2020. Hippocampal blood flow is increased after 20 min of moderate-intensity exercise. Cereb. Cortex 30, 525–533. ( 10.1093/cercor/bhz104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gauthier CJ, et al. 2015. Hearts and minds: linking vascular rigidity and aerobic fitness with cognitive aging hearts and minds: linking vascular rigidity and aerobic fitness with cognitive aging. Neurobiol. Aging 36, 304–314. ( 10.1016/j.neurobiolaging.2014.08.018) [DOI] [PubMed] [Google Scholar]
- 24.Mitsis GD, Zhang R, Levine BD, Marmarelis VZ. 1985. Cerebral hemodynamics during orthostatic stress assessed by nonlinear modeling. J. Appl. Physiol. 101, 354–366. ( 10.1152/japplphysiol.00548.2005) [DOI] [PubMed] [Google Scholar]
- 25.Ursino M, Ter Minassian A, Lodi CA, Beydon L. 2000. Cerebral hemodynamics during arterial and CO2 pressure changes: in vivo prediction by a mathematical model. Am. J. Physiol. Heart Circ. Physiol. 279, H2439–H2455. ( 10.1152/ajpheart.2000.279.5.H2439) [DOI] [PubMed] [Google Scholar]
- 26.Mader G, Olufsen M, Mahdi A. 2020. Modeling cerebral blood flow velocity during orthostatic stress. Ann. Biomed. Eng. 43, 1748–1758. ( 10.1007/s10439-014-1220-4) [DOI] [PubMed] [Google Scholar]
- 27.Panerai RB. 2009. Transcranial Doppler for evaluation of cerebral autoregulation. Clin. Auton. Res. 19, 197–211. ( 10.1007/s10286-009-0011-8) [DOI] [PubMed] [Google Scholar]
- 28.Panerai RB, Hudson V, Fan L, Mahony P, Yeoman PM, Hope T, Evans DH. 2002. Assessment of dynamic cerebral autoregulation based on spontaneous fluctuations in arterial blood pressure and intracranial pressure. Physiol. Meas. 23, 59–72. ( 10.1088/0967-3334/23/1/306) [DOI] [PubMed] [Google Scholar]
- 29.Bright MG, Murphy K. 2013. Reliable quantification of BOLD fMRI cerebrovascular reactivity despite poor breath-hold performance. NeuroImage 83, 559–568. ( 10.1016/j.neuroimage.2013.07.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wise RG, et al. 2007. Dynamic forcing of end-tidal carbon dioxide and oxygen applied to functional magnetic resonance imaging. J. Cereb. Blood Flow Metab. 27, 1521–1532. ( 10.1038/sj.jcbfm.9600465) [DOI] [PubMed] [Google Scholar]
- 31.Davis TL, Kwong KK, Weisskoff RM, Rosen BR. 1998. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc. Natl Acad. Sci. USA 95, 1834–1839. ( 10.1073/pnas.95.4.1834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wise RG, Harris AD, Stone AJ, Murphy K. 2013. Measurement of OEF and absolute CMRO2: MRI-based methods using interleaved and combined hypercapnia and hyperoxia. NeuroImage 83, 135–147. ( 10.1016/j.neuroimage.2013.06.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bulte DP, Kelly M, Germuska M, Xie J, Chappell MA, Okell TW, Bright MG, Jezzard P. 2012. Quantitative measurement of cerebral physiology using respiratory-calibrated MRI. NeuroImage 60, 582–591. ( 10.1016/j.neuroimage.2011.12.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gauthier CJ, Desjardins-Crépeau L, Madjar C, Bherer L, Hoge RD. 2012. Absolute quantification of resting oxygen metabolism and metabolic reactivity during functional activation using QUO2 MRI. NeuroImage 63, 1353–1363. ( 10.1016/j.neuroimage.2012.07.065) [DOI] [PubMed] [Google Scholar]
- 35.Whittaker JR, Driver ID, Bright MG, Murphy K. 2016. The absolute CBF response to activation is preserved during elevated perfusion: implications for neurovascular coupling measures. NeuroImage 125, 198–207. ( 10.1016/j.neuroimage.2015.10.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corfield DR, Murphy K, Josephs O, Adams L, Turner R. 2001. Does hypercapnia-induced cerebral vasodilation modulate the hemodynamic response to neural activation? NeuroImage 13, 1207–1211. ( 10.1006/nimg.2001.0760) [DOI] [PubMed] [Google Scholar]
- 37.Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. 1999. Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: the deoxyhemoglobin dilution model. Magn. Reson. Med. 42, 849–863. () [DOI] [PubMed] [Google Scholar]
- 38.Cohen ER, Ugurbil K, Kim S-G. 2002. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. J. Cereb. Blood Flow Metab. 22, 1042–1053. ( 10.1097/00004647-200209000-00002) [DOI] [PubMed] [Google Scholar]
- 39.Bright MG, Whittaker JR, Driver ID, Murphy K. 2020. Vascular physiology drives functional brain networks. NeuroImage 217, 116907 ( 10.1016/j.neuroimage.2020.116907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heckmann JG, Hilz MJ, Hagler H, Mück-Weymann M, Neundörfer B. 1999. Transcranial Doppler sonography during acute 80 degrees head-down tilt (HDT) for the assessment of cerebral autoregulation in humans. Neurol. Res. 21, 457–462. ( 10.1080/01616412.1999.11740959) [DOI] [PubMed] [Google Scholar]
- 41.Levine BD, Giller CA, Lane LD, Buckey JC, Blomqvist CG. 1994. Cerebral versus systemic hemodynamics during graded orthostatic stress in humans. Circulation 90, 298–306. ( 10.1161/01.CIR.90.1.298) [DOI] [PubMed] [Google Scholar]
- 42.Wolthuis RA, Bergman SA, Nicogossian AE. 1974. Physiological effects of locally applied reduced pressure in man. Physiol. Rev. 54, 566–595. ( 10.1152/physrev.1974.54.3.566) [DOI] [PubMed] [Google Scholar]
- 43.Whittaker JR, Bright MG, Driver ID, Babic A, Khot S, Murphy K. 2019. Changes in arterial cerebral blood volume during lower body negative pressure measured with MRI. NeuroImage 187, 166–175. ( 10.1016/j.neuroimage.2017.06.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Q, Roche M, Gheres KW, Chaigneau E, Kederasetti RT, Hasalden WD, Charpak S, Drew PJ. 2019. Cerebral oxygenation during locomotion is modulated by respiration. Nat. Commun. 10, 5515 ( 10.1038/s41467-019-13523-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Purves MJ. 1966. The effect of a single breath of oxygen on respiration in the newborn lamb. Respiration Physiol. 1, 297–307. ( 10.1016/0034-5687(66)90048-X) [DOI] [PubMed] [Google Scholar]
- 46.Purves MJ. 1966. Fluctuations of arterial oxygen tension which have the same period as respiration. Respiration Physiol. 1, 281–296. ( 10.1016/0034-5687(66)90047-8) [DOI] [PubMed] [Google Scholar]
- 47.Tong Y, Lindsey KP, Hocke LM, Vitaliano G, Mintzopoulos D, Frederick BdB. 2017. Perfusion information extracted from resting state functional magnetic resonance imaging. J. Cereb. Blood Flow Metab. 37, 564–576. ( 10.1177/0271678X16631755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tong Y, Yao JF, Chen J, Frederick BdB. 2018. The resting-state fMRI arterial signal predicts differential blood transit time through the brain. J. Cereb. Blood Flow Metab. 39, 1148–1160. ( 10.1177/0271678X17753329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aso T, Urayama S, Fukuyama H, Murai T. 2019. Axial variation of deoxyhemoglobin density as a source of the low-frequency time lag structure in blood oxygenation level-dependent signals. PLoS ONE 14, e0222787 ( 10.1371/journal.pone.0225489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perl O, Ravia A, Rubinson M, Eisen A, Soroka T, Mor N, Secundo L, Sobel N. 2019. Human non-olfactory cognition phase-locked with inhalation. Nat. Hum. Behav. 3, 501–512. ( 10.1038/s41562-019-0556-z) [DOI] [PubMed] [Google Scholar]
- 51.Shea SA. 1996. Behavioural and arousal-related influences on breathing in humans. Exp. Physiol. 81, 1–26. ( 10.1113/expphysiol.1996.sp003911) [DOI] [PubMed] [Google Scholar]
- 52.Yackle K, Schwarz LA, Kam K, Sorokin JM, Huguenard JR, Feldman JL, Luo L, Krasnow MA. 2017. Breathing control center neurons that promote arousal in mice. Science 355, 1411–1415. ( 10.1126/science.aai7984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang CF, Feldman JL. 2018. Efferent projections of excitatory and inhibitory preBotzinger complex neurons. J. Comp. Neurol. 526, 1389–1402. ( 10.1002/cne.24415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caballero-Gaudes C, Reynolds RC. 2017. Methods for cleaning the BOLD fMRI signal. NeuroImage 154, 128–149. ( 10.1016/j.neuroimage.2016.12.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glover GH, Li T-Q, Ress D. 2000. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn. Reson. Med. 44, 162–167. () [DOI] [PubMed] [Google Scholar]
- 56.Shmueli K, van Gelderen P, de Zwart JA, Horovitz SG, Fukunaga M, Jansma JM, Duyn JH. 2007. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. NeuroImage 38, 306–320. ( 10.1016/j.neuroimage.2007.07.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Birn RM, Diamond JB, Smith MA, Bandettini PA. 2006. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. NeuroImage 31, 1536–1548. ( 10.1016/j.neuroimage.2006.02.048) [DOI] [PubMed] [Google Scholar]
- 58.Bianciardi M, Fukunaga M, van Gelderen P, Horovitz SG, de Zwart JA, Shmueli K, Duyn JH. 2009. Sources of functional magnetic resonance imaging signal fluctuations in the human brain at rest: a 7T study. Magn. Reson Imaging 27, 1019–1029. ( 10.1016/j.mri.2009.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wise RG, Ide K, Poulin MJ, Tracey I. 2004. Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. NeuroImage 21, 1652–1664. ( 10.1016/j.neuroimage.2003.11.025) [DOI] [PubMed] [Google Scholar]
- 60.Whittaker JR, Driver ID, Venzi M, Bright MG, Murphy K. 2019. Cerebral autoregulation evidenced by synchronized low frequency oscillations in blood pressure and resting-state fMRI. Front. Neurosci. 13, 433 ( 10.3389/fnins.2019.00433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Auer LM, Gallhofer B. 1981. Rhythmic activity of cat pial vessels in vivo. Eur. Neurol. 20, 448–468. ( 10.1159/000115278) [DOI] [PubMed] [Google Scholar]
- 62.Mayhew JEW, Askew S, Zheng Y, Porrill J, Westby GWM, Redgrave P, Rector DM, Harper RM. 1996. Cerebral vasomotion: a 0.1-Hz oscillation in reflected light imaging of neural activity. NeuroImage 4, 183–193. ( 10.1006/nimg.1996.0069) [DOI] [PubMed] [Google Scholar]
- 63.Kleinfeld D, Mitra PP, Helmchen F, Denk W. 1998. Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc. Natl Acad. Sci. USA 95, 15 741–15 746. ( 10.1073/pnas.95.26.15741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rayshubskiy A, et al. 2014. Direct, intraoperative observation of ∼0.1 Hz hemodynamic oscillations in awake human cortex: implications for fMRI. NeuroImage 87, 323–331. ( 10.1016/j.neuroimage.2013.10.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Drew PJ, Shih AY, Driscoll JD, Knutsen PM, Blinder P, Davalos D, Akassoglou K, Tsai PS, Kleinfeld D. 2010. Chronic optical access through a polished and reinforced thinned skull. Nat. Methods 7, 981–984. ( 10.1038/nmeth.1530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pisauro MA, Benucci A, Carandini M. 2016. Local and global contributions to hemodynamic activity in mouse cortex. J. Neurophysiol. 115, 2931–2936. ( 10.1152/jn.00125.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sirotin YB, Das A. 2009. Anticipatory haemodynamic signals in sensory cortex not predicted by local neuronal activity. Nature 457, 475–479. ( 10.1038/nature07664) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herman MC, Cardoso MMB, Lima BR, Sirotin YB, Das A. 2017. Simultaneously estimating the task-related and stimulus-evoked components of hemodynamic imaging measurements. Neurophotonics 4, 031223 ( 10.1117/1.NPh.4.3.031223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sirotin YB, Cardoso MMB, Lima BR, Das A. 2012. Spatial homogeneity and task-synchrony of the trial-related hemodynamic signal. NeuroImage 59, 2783–2797. ( 10.1016/j.neuroimage.2011.10.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cardoso MMB, Lima BR, Sirotin YB, Das A. 2019. Task-related hemodynamic responses are modulated by reward and task engagement. PLoS Biol. 17, e3000080 ( 10.1371/journal.pbio.3000080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haddock RE, Hill CE. 2007. Rhythmicity in arterial smooth muscle. J. Physiol. 566, 645–656. ( 10.1113/jphysiol.2005.086405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nilsson H, Aalkjær C. 2003. Vasomotion: mechanisms and physiological importance. Mol. Interv. 3, 79–89. ( 10.1124/mi.3.2.79) [DOI] [PubMed] [Google Scholar]
- 73.Osol G, Halpern W. 1988. Spontaneous vasomotion in pressurized cerebral arteries from genetically hypertensive rats. Am. J. Physiol. 254, H28–H33. ( 10.1152/ajpheart.1988.254.1.H28) [DOI] [PubMed] [Google Scholar]
- 74.Gustafsson H, Bülow A, Nilsson H. 1994. Rhythmic contractions of isolated, pressurized small arteries from rat. Acta. Physiolo. Scand. 152, 145–152. ( 10.1111/j.1748-1716.1994.tb09794.x) [DOI] [PubMed] [Google Scholar]
- 75.Achakri H, Stergiopulos H, Hoogerwerf N, Hayoz D, Brunner HR, Meister JJ. 1995. Intraluminal pressure modulates the magnitude and the frequency of induced vasomotion in rat arteries. J. Vasc. Res. 32, 237–246. ( 10.1159/000159098) [DOI] [PubMed] [Google Scholar]
- 76.Filosa JA, Bonev AD, Nelson MT. 2004. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ. Res. 95, e73–e81. ( 10.1161/01.RES.0000148636.60732.2e) [DOI] [PubMed] [Google Scholar]
- 77.Colantuoni A, Bertuglia S, Intaglietta M. 1984. The effects of α- or β-adrenergic receptor agonists and antagonists and calcium entry blockers on the spontaneous vasomotion. Microvasc. Res. 28, 143–158. ( 10.1016/0026-2862(84)90014-1) [DOI] [PubMed] [Google Scholar]
- 78.Arciero JC, Secomb TW. 2012. Spontaneous oscillations in a model for active control of microvessel diameters. Mat. Med. Biol. 29, 163–180. ( 10.1093/imammb/dqr005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gonzalez-Fernandez JM, Ermentrout B. 1994. On the origin and dynamics of the vasomotion of small arteries. Math. Biosci. 119, 127–167. ( 10.1016/0025-5564(94)90074-4) [DOI] [PubMed] [Google Scholar]
- 80.Colantuoni A, Bertuglia S, Intaglietta M. 1984. Quantitation of rhythmic diameter changes in arterial microcirculation. Am. J. Physiol. 246, H508–H517. ( 10.1152/ajpheart.1984.246.4.H508) [DOI] [PubMed] [Google Scholar]
- 81.Gao YR, Ma Y, Zhang Q, Winder AT, Liang Z, Antinori L, Drew PJ, Zhang N. 2017. Time to wake up: studying neurovascular coupling and brain-wide circuit function in the un-anesthetized animal. NeuroImage 153, 382–398. ( 10.1016/j.neuroimage.2016.11.069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stergiopulos N, Porret CA, De Brouwer S, Meister JJ. 1998. Arterial vasomotion: effect of flow and evidence of nonlinear dynamics. Am. J. Physiol. 274, H1858–H1864. ( 10.1152/ajpheart.1998.274.6.H1858) [DOI] [PubMed] [Google Scholar]
- 83.Drew PJ, Shih AY, Kleinfeld D. 2011. Fluctuating and sensory-induced vasodynamics in rodent cortex extend arteriole capacity. Proc. Natl Acad. Sci. USA 108, 8473–8478. ( 10.1073/pnas.1100428108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bentley WJ, Li JM, Snyder AZ, Raichle ME, Snyder LH. 2016. Oxygen level and LFP in task-positive and task-negative areas: bridging BOLD fMRI and electrophysiology. Cereb Cortex 26, 346–357. ( 10.1093/cercor/bhu260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hill CE. 2012. Long distance conduction of vasodilation: a passive or regenerative process? Microcirculation 19, 379–390. ( 10.1111/j.1549-8719.2012.00169.x) [DOI] [PubMed] [Google Scholar]
- 86.Hald BO, Jensen LJ, Sørensen PG, Holstein-Rathlou NH, Jacobsen JC. 2012. Applicability of cable theory to vascular conducted responses. Biophys. J. 102, 1352–1362. ( 10.1016/j.bpj.2012.01.055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Segal SS, Damon DN, Duling BR. 1989. Propagation of vasomotor responses coordinates arteriolar resistances. Am. J. Physiol. 256, H832–H837. ( 10.1152/ajpheart.1989.256.3.H832) [DOI] [PubMed] [Google Scholar]
- 88.Dietrich HH, Kajita Y, Dacey RG Jr. 1996. Local and conducted vasomotor responses in isolated rat cerebral arterioles. Am. J. Physiol. 271, H1109–H1116. ( 10.1152/ajpheart.1996.271.3.H1109) [DOI] [PubMed] [Google Scholar]
- 89.Vazquez AL, Fukuda M, Crowley JC, Kim S-G. 2014. Neural and hemodynamic responses elicited by forelimb- and photo-stimulation in channelrhodopsin-2 mice: insights into the hemodynamic point spread function. Cereb. Cortex 24, 2908–2919. ( 10.1093/cercor/bht147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pries AR, Secomb TW, Gaehtgens P. 1996. Biophysical aspects of blood flow in the microvasculature. Cardiovasc. Res. 32, 654–667. ( 10.1016/S0008-6363(96)00065-X) [DOI] [PubMed] [Google Scholar]
- 91.Warnke KC, Skalak TC. 1992. Leukocyte plugging in vivo in skeletal muscle arteriolar trees. Am. J. Physiol. 262, H1149–H1155. ( 10.1152/ajpcell.1992.262.5.C1149) [DOI] [PubMed] [Google Scholar]
- 92.Cruz-Hernández JC, et al. 2019. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer's disease mouse models. Nat. Neurosci. 22, 413–420. ( 10.1038/s41593-018-0329-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Forouzan O, Yang X, Sosa JM, Burns JM, Shevkoplyas SS. 2012. Spontaneous oscillations of capillary blood flow in artificial microvascular networks. Microvasc. Res. 84, 123–132. ( 10.1016/j.mvr.2012.06.006) [DOI] [PubMed] [Google Scholar]
- 94.Panerai RB. 2009. Complexity of the human cerebral circulation. Phil. Trans. R. Soc. A 367, 1319–1336. ( 10.1098/rsta.2008.0264) [DOI] [PubMed] [Google Scholar]
- 95.Revel A, Gallet C, Oréa V, Chapuis B, Barrès C, Julien C. 2012. Effect of chronic cervical ganglionectomy on the spontaneous variability of internal carotid blood flow in the conscious rat. Exp. Physiol. 97, 564–571. ( 10.1113/expphysiol.2011.062455) [DOI] [PubMed] [Google Scholar]
- 96.Porret CA, Stergiopulos N, Hayoz D, Brunner HR, Meister JJ. 1995. Simultaneous ipsilateral and contralateral measurements of vasomotion in conduit arteries of human upper limbs. Am. J. Physiol. 269, H1852–H1858. ( 10.1152/ajpheart.1995.269.6.H1852) [DOI] [PubMed] [Google Scholar]
- 97.Braun RD, Lanzen JL, Dewhirst MW. 1999. Fourier analysis of fluctuations of oxygen tension and blood flow in R3230Ac tumors and muscle in rats. Am. J. Physiol. 277, H551–H568. ( 10.1152/ajpheart.1999.277.2.H551) [DOI] [PubMed] [Google Scholar]
- 98.Tong Y, Hocke LM, Licata SC, Frederick BdB. 2012. Low-frequency oscillations measured in the periphery with near-infrared spectroscopy are strongly correlated with blood oxygen level-dependent functional magnetic resonance imaging signals. J. Biomed. Opt. 17, 106004 ( 10.1117/1.JBO.17.10.106004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lima BR, Cardoso MMB, Sirotin YB, Das A. 2014. Stimulus-related neuroimaging in task-engaged subjects is best predicted by concurrent spiking. J. Neurosci. 34, 13 878–13 891. ( 10.1523/JNEUROSCI.1595-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Veluw SJ, Hou SS, Calvo-Rodriguez M, Arbel-Ornath M, Snyder AC, Frosch MP, Greenberg SM, Bacskai BJ. 2020. Vasomotion as a driving force for paravascular clearance in the awake mouse brain. Neuron 105, 549–561.e5 ( 10.1016/j.neuron.2019.10.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen BR, Bouchard MB, McCaslin AF, Burgess SA, Hillman EMC. 2011. High-speed vascular dynamics of the hemodynamic response. NeuroImage 52, 1021–1030. ( 10.1016/j.neuroimage.2010.09.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jack AI, Shulman GL, Snyder AZ, McAvoy MP, Corbetta M. 2006. Separate modulations of human V1 associated with spatial attention and task structure. Neuron 51, 135–147. ( 10.1016/j.neuron.2006.06.003) [DOI] [PubMed] [Google Scholar]
- 103.Özbay PS, Chang C, Picchioni D, Mandelkow H, Chappel-Farley MG, van Gelderen P, de Zwart JA, Duyn J. 2019. Sympathetic activity contributes to the fMRI signal. Commun. Biol. 2, 421 ( 10.1038/s42003-019-0659-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alsop DC, et al. 2015. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Med. 73, 102–116. ( 10.1002/mrm.25197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lu H, Golay X, Pekar JJ, Van Zijl PC. 2003. Functional magnetic resonance imaging based on changes in vascular space occupancy. Magn. Reson. Med. 50, 263–274. ( 10.1002/mrm.10519) [DOI] [PubMed] [Google Scholar]
- 106.Huber L, Ivanov D, Krieger SN, Streicher MN, Mildner T, Poser BA, Möller HE, Turner R. 2014. Slab-selective, BOLD-corrected VASO at 7 Tesla provides measures of cerebral blood volume reactivity with high signal-to-noise ratio. Magn. Reson. Med. 72, 137–148. ( 10.1002/mrm.24916) [DOI] [PubMed] [Google Scholar]
- 107.Beckmann CF, DeLuca M, Devlin JT, Smith SM. 2005. Investigations into resting-state connectivity using independent component analysis. Phil. Trans. R. Soc. B 360, 1001–1013. ( 10.1098/rstb.2005.1634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smith SM, et al. 2009. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl Acad. Sci. 106, 13 040–13 045. ( 10.1073/pnas.0905267106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pievani M, Filippini N, van den Heuvel MP, Cappa SF, Frisoni GB. 2014. Brain connectivity in neurodegenerative diseases—from phenotype to proteinopathy. Nat. Rev. Neurol. 10, 620–633. ( 10.1038/nrneurol.2014.178) [DOI] [PubMed] [Google Scholar]
- 110.Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. 2009. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci. Biobehav. Rev. 33, 279–296. ( 10.1016/j.neubiorev.2008.09.002) [DOI] [PubMed] [Google Scholar]
- 111.Whittaker JR, Foley SF, Ackling E, Murphy K, Caseras X. 2018. The functional connectivity between the nucleus accumbens and the ventromedial prefrontal cortex as an endophenotype for bipolar disorder. Biol. Psychiatry 84, 803–809. ( 10.1016/j.biopsych.2018.07.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang JH, Badaut J, Tang J, Obenaus A, Hartman R, Pearce WJ. 2012. The vascular neural network—a new paradigm in stroke pathophysiology. Nat. Rev. Neurol. 8, 711–716. ( 10.1038/nrneurol.2012.210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Driver ID, Whittaker JR, Bright MG, Muthukumaraswamy SD, Murphy K. 2016. Arterial CO2 fluctuations modulate neuronal rhythmicity: implications for MEG and fMRI studies of resting-state networks. J. Neurosci. 36, 8541–8550. ( 10.1523/JNEUROSCI.4263-15.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bright MG, Murphy K. 2015. Is fMRI ‘noise’ really noise? Resting state nuisance regressors remove variance with network structure. NeuroImage 114, 158–169. ( 10.1016/j.neuroimage.2015.03.070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Del Zoppo GJ. 2013. Toward the neurovascular unit. A journey in clinical translation: 2012 Thomas Willis Lecture. Stroke 44, 263–269. ( 10.1161/STROKEAHA.112.653618) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.