Abstract
Mind-wandering has become a captivating topic for cognitive neuroscientists. By now, it is reasonably well described in terms of its phenomenology and the large-scale neural networks that support it. However, we know very little about what neurobiological mechanisms trigger a mind-wandering episode and sustain the mind-wandering brain state. Here, we focus on the role of ascending neuromodulatory systems (i.e. acetylcholine, noradrenaline, serotonin and dopamine) in shaping mind-wandering. We advance the hypothesis that the hippocampal sharp wave-ripple (SWR) is a compelling candidate for a brain state that can trigger mind-wandering episodes. This hippocampal rhythm, which occurs spontaneously in quiescent behavioural states, is capable of propagating widespread activity in the default network and is functionally associated with recollective, associative, imagination and simulation processes. The occurrence of the SWR is heavily dependent on hippocampal neuromodulatory tone. We describe how the interplay of neuromodulators may promote the hippocampal SWR and trigger mind-wandering episodes. We then identify the global neuromodulatory signatures that shape the evolution of the mind-wandering brain state. Under our proposed framework, mind-wandering emerges due to the interplay between neuromodulatory systems that influence the transitions between brain states, which either facilitate, or impede, a wandering mind.
This article is part of the theme issue ‘Offline perception: voluntary and spontaneous perceptual experiences without matching external stimulation'.
Keywords: mind-wandering, spontaneous thought, hippocampal sharp wave-ripple, neuromodulation, noradrenergic, cholinergic
1. Introduction
Mind-wandering is a mental state where thoughts arise spontaneously, relatively free from constraints and intentions [1]. Behaviour that is shaped by prior intentions, action plans and external constraints necessarily narrows the scope of possible states available to a system [2,3]. By contrast, mind-wandering suggests a widening of possibilities and a system untethered to constraints imposed by the external world. It reflects a system engaged in abstract, descriptive processes, shifted away from immediate sensorimotor goals or interactive behaviour with external affordances [4,5]. These characteristics are reflected in the phenomenology of mind-wandering: free-wheeling, undirected thoughts, with variable content and unpredictable trajectories. Such system properties and phenomenological characteristics are not unique to mind-wandering, but feature across the related phenomena of creativity, dreaming and hallucinations [6–8]. Collectively, these modes characterized by a lack of constraints on thoughts, and on the transitions between thoughts, are termed ‘spontaneous thought' [1].
As mind-wandering captured the attention of cognitive neuroscientists, the default network quickly became front and centre [9–11]. Although a more complex story has emerged, as an increased activity within the default network, and its relative engagement with attentional, control and sensorimotor networks, are the brain activation patterns most consistently associated with mind-wandering [1,12–14]. Yet, despite the reasonably detailed picture we now have of the attendant brain networks, we know very little about what might trigger mind-wandering and what neurobiological mechanisms control transitions in and out of the mind-wandering state.
Ongoing behaviour and cognitive function are strongly determined by fluctuations in brain state [15]. These states reflect an interaction between sensory input and intrinsically generated activity. In this way, brain state is dually determined by environmental engagement and by endogenously generated rhythms [16]. Despite this equilibrium process, most of our understanding of cognition has derived from studying brain activity and behaviours in response to external stimuli. Such ‘behaviour-yoked' paradigms tend to overlook the influence of underlying intrinsic brain states, which continuously shape and constrain ongoing cognitive, sensory and motor processes [17]. Indeed, many brain states, particularly during quiet periods of stillness or sleep, are dominated by stochastic, intrinsic activity patterns that are not driven by external inputs [18,19]. Endogenously generated activity seems particularly relevant to mind-wandering, which by its nature arises in a spontaneous, undirected manner, often with little or no identifiable influence from external stimuli. Here, we identify a hippocampal brain state, the sharp wave-ripple (SWR), as an intrinsically driven brain state that is particularly conducive to mind-wandering.
A key mechanism for regulating brain states are broadly projecting neuromodulatory systems [15,20,21]. The influence of ascending neuromodulatory systems plays a vital role across all aspects of behaviour, including higher order cognitive function [22,23]. By modulating the relatively fixed structural connectome of the brain to meet environmental challenges, neuromodulators support our capacity to flexibly transition across diverse modes of behaviour [24,25]. Chemical neuromodulation from these systems alters the properties of target cells by increasing, or decreasing, the likelihood of their firing (i.e. altering their gain; [26]). Neuromodulators can exert their influence over varying timescales, acting on target neurons to hyperpolarize or depolarize them, or to alter the plasticity of their synapses [27]. The resulting change in responsivity of target neurons is referred to as a change in neural gain, which can be thought of as a measure of neural signal amplification [28,29]. These properties of neuromodulators can be distinguished from the faster, direct signalling provided by classical neurotransmitters, such as GABA and glutamate [30].
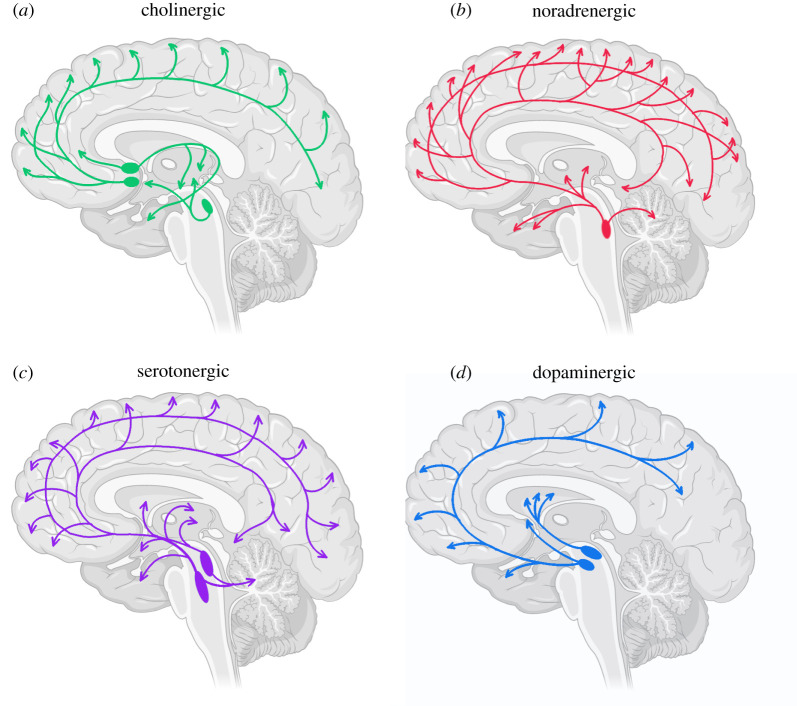
While there are many families of neuromodulatory neurotransmitters, we focus on four of the major ascending neuromodulatory systems: acetylcholine, noradrenaline, serotonin and dopamine (figure 1). Although each neuromodulatory system has unique characteristics, certain general principles apply. Despite their extensive projections, each system is capable of remarkably specialized regional effects; the systems are self-regulating, via actions at auto-receptors and descending projections; and, finally, they follow an inverted-U shaped curve (known as a Yerkes-Dodson-like function [31]), whereby efficacy of performance is governed by an optimal level of neuromodulatory influence, with too much or too little resulting in deleterious effects on behaviour [25].
Figure 1.
Ascending neuromodulatory pathways. The ascending neuromodulatory systems and their projection nuclei, which send broad projections to different parts of the brain. (a) The cholinergic system, projections from the basal forebrain, including the septal nuclei (top) and basal nucleus of Meynert (bottom); and from the brainstem pedunculopontine/laterodorsal tegmental complex; (b) the noradrenergic system, from the locus coeruleus; (c) the serotonergic system, from the dorsal (top) and median (bottom) raphe nuclei; (d) the dopaminergic system, from the substantia nigra (top) and ventral tegmental area (bottom). (Online version in colour.)
To date, the limited attention that neuromodulators have received in mind-wandering has focused on the influence of tonic noradrenaline levels (e.g. see [32,33]). Extending these ideas, we examine the hypothesis that a balance between neuromodulatory neurotransmitters shapes brain states that promote spontaneous thought. We argue that tonic activity within specific combinations of neuromodulatory projection nuclei acts to influence the transitions between brain states that either facilitate (or interfere with) the ability to mind-wander. In addition, we describe how neuromodulatory tone might influence the phenomenology of spontaneous thought. We focus initially on the hippocampal SWR as a brain state that can trigger mind-wandering, highlighting the neuromodulatory influences that promote this hippocampal rhythm. We then describe more global neuromodulatory signatures that shape the subsequent evolution of the mind-wandering brain state.
2. Hippocampal sharp wave-ripples: a brain state for mind-wandering
(a). Hippocampal sharp wave-ripples and their functional relevance for mind-wandering
The link between behaviour and brain states has been well studied in the hippocampus, particularly with respect to theta and SWR hippocampal brain states, which both have their own characteristic oscillatory rhythms and behavioural correlates [16,19]. During awake, task-engaged behaviours, hippocampal activity is dominated by theta oscillations, promoting sensory processing and information sampling. By contrast, during slow-wave sleep and quiescent periods of wakefulness, and also during consummatory periods or following receipt of a reward, distinct neural patterns known as SWR complexes occur in the hippocampus (figure 2). The sharp wave refers to high-amplitude depolarization of a large subset of CA1 neurons, driven by excitatory, recurrent activity in the upstream CA3 region; these sharp waves commonly co-occur with ‘ripples', which are brief, high-frequency oscillations in large populations of CA1 pyramidal neurons [18,34]. Importantly, SWR bursts are not induced but ‘released' in the absence of suppression mechanisms such as particular patterns of neuromodulatory tone [18,35]. The synchronous discharge of these pyramidal neurons in the hippocampal–entorhinal output pathway has powerful diffuse effects, leading to both up- and downregulation of widespread network activity [36,37]. Of particular relevance is that SWRs are consistently associated with hippocampal-prefrontal activation [38], and ripples have been linked to selectively increasing ongoing activity in the default network [39].
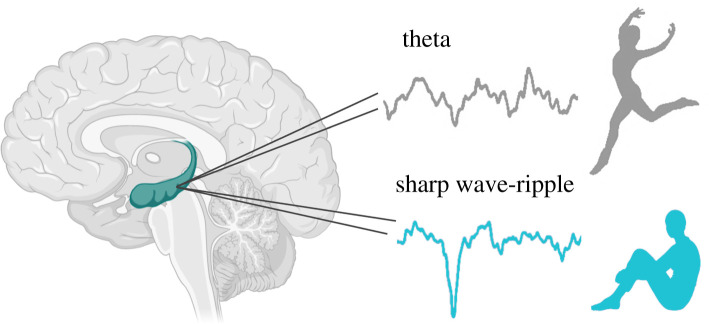
Figure 2.
Hippocampal brain states. An example of the hippocampal theta rhythm and the sharp wave-ripple (SWR). Illustrated beside each recording is the associated behavioural state: locomotion and task engagement (theta), and a quiescent behavioural state, conducive to mind-wandering episodes (SWR). (Online version in colour.)
SWRs are a critical event in memory consolidation and retrieval. SWR neuronal activity can correspond to a previous experience, constituting a time-compressed ‘replay' or reactivation of that event. This activity may reflect events relevant to the immediate environment or can be drawn from more remote memories [40]. Coordinated hippocampal–cortical activity following SWRs is thought to promote ‘systems consolidation', whereby hippocampally mediated associations are progressively embedded within the synaptic weights that comprise neocortical networks, a process driven by the recurrent retrieval of memories over time [41]. This process is predicted by two-stage models of memory formation, where the encoding of new memories in the hippocampal formation occurs during active waking, and consolidation is promoted via random hippocampal reactivation during offline states of rest and sleep [42].
There is a good deal of flexibility in how these neuronal sequences are reactivated, suggesting that mere replay does not capture their diversity of function [43]. Part of the richness and diversity of SWR sequences may derive from the fact that they are often preceded by neocortical activity, consistent with the possibility that their content may be biased by information held in long-term memory [44,45]. During SWRs, neuronal sequences can be recapitulated in the forwards or reverse order of an experienced event [46], and activity can reflect novel combinations of past experiences or predicted future actions not previously experienced [47–49], sometimes comprising completely random, novel sequences [50]. Therefore, apart from a role in consolidating memories and retrieving them to aid immediate decision-making and planning, SWRs may also contribute to our ability to re-combine stored memories and simulate novel scenarios—abilities that underpin imagination [34]. The potential for SWRs to evoke memory and imagination and to activate the default network, raises the intriguing possibility that they may act as a trigger for episodes of mind-wandering.
(b). Hippocampal sharp wave-ripples drive default network activity and may promote conscious thought content
Hippocampal rhythms are capable of driving widespread neuronal activity. By synchronizing and coordinating large populations of hippocampal neurons, hippocampal waves can orchestrate the co-activation of distributed neuronal ensembles throughout the subcortex and neocortex [19,51]. This makes them ideally suited to support memory-related processes, which rely on information encoded across anatomically distinct brain regions [52]. Common neural regions are engaged by various memory-related processes, including remembering the past, simulating the future and imagination [53]. Regions activated during these processes reliably involve the default network and its context-dependent engagement with sensory, attentional and control networks [54]. Mind-wandering uses neural regions similar to those that support memory processes; however, it is initiated spontaneously and sustained in an undirected manner, which differs from deliberative, goal-directed processes that can typify memory retrieval and future planning [1]. The SWR hippocampal brain state is one such mechanism that can recruit neural regions involved in memory-related processes, but in a spontaneous, undirected way.
Dense hippocampal projections to core regions of the default network, including retrosplenial and posterior cingulate cortices, make this network a primary target for propagating hippocampal SWR activity [39]. Spontaneous SWR activation in the hippocampus and hippocampal–entorhinal output pathway has the potential to engage the default network more broadly, supporting the conscious elaboration of memories and simulations. One hypothesis is that the SWR acts as a subconscious search mechanism, which can bias subsequent thought content by priming the relevant circuits and assembly sequences necessary for an item to enter into consciousness [18]. Such biasing may occur via pattern completion processes, whereby a stored memory trace is activated with only a partial or degraded cue [55–58].
Pattern completion is classically attributed to the CA3 region of the hippocampus, on the basis of the recurrent collateral connections of its pyramidal cell population and its attractor dynamics [59–62]. This recurrent circuitry provides the basis of an ‘auto-associative network', capable of instantiating attractor dynamics that promote stable firing patterns. More concretely, the associativity of the network means that an entire neuronal population encoding an episodic memory may be activated based on activation of any subset of that population [63,64]. These dynamics support the highly associative nature of episodic memory and they are well described by two-stage models of memory recollection. Such models predict a priming of relational associations in the hippocampus—a rapid and unconscious process—which can either end, or evolve into a slower, conscious process associated with hippocampal–cortical interaction [65,66]. Pattern completion processes may also contribute to the itinerant and often highly associative nature of mind-wandering [67], where we can drift across a loosely connected train of thought, or certain contexts spontaneously evoke-related memories or imaginings. A memory recalled during pattern completion may trigger further pattern completions, which would support dynamic thought trajectories with variable content, but at the same time allow for thematic relationships and partial associations to persist across consecutive mental states [68]. Converging evidence from human studies shows that a considerable proportion of mind-wandering tends to be unconstrained or ‘freely moving' [69], and that when external demands are reduced, thoughts can tend towards novel, exploratory associations [70,71]. Hippocampal CA3 sharp wave activity has been linked to pattern completion attractor dynamics [72], providing a mechanistic link between the SWR brain state, and these associative mnemonic processes that may underpin mind-wandering.
It is currently unclear to what extent SWRs, which represent only a small fraction of hippocampally mediated activity in the awake state, are associated with conscious recollection versus a subconscious, mnemonic process [43]. Indeed, much of what we know about SWRs is based on rodents and non-human primates, so we lack detailed descriptions of their phenomenological content. Importantly, however, there is a good deal of human functional magnetic resonance imaging (fMRI) evidence demonstrating the spontaneous reactivation of previously learnt information during rest periods [73]. An even closer link with animal studies was recently established, with fMRI evidence for sequential offline replay identified in the human hippocampus following a non-spatial decision-making task [74]. Furthermore, decoded magnetoencephalography signals have been identified that are consistent with the temporally compressed replay of abstract rule sequences learnt during a task [75]. Importantly, these replay events coincided with ripple-band power increases (120–150 Hz) and could be localized to the medial temporal lobe, consistent with the hippocampal–neocortical regions activated during SWRs. Only very recently was the conscious content of hippocampal SWRs directly probed in human subjects, using intracranial electrophysiological recordings in neurosurgical patients. A transient increase in SWRs was observed just prior to the spontaneous free recall of images, which was related to SWR rates measured when participants originally viewed the images [76]. The anticipatory nature of their occurrence suggests a role for SWRs in the initiation of spontaneous recollection, providing evidence that SWR activation has the potential to influence the content of conscious thought.
A critical role for the hippocampus in influencing both the frequency and content of mind-wandering has been highlighted in recent work showing that hippocampal atrophy is associated with reduced mind-wandering in dementia [77] and reduced diversity in terms of content in individuals with selective bilateral hippocampal damage [78]. The SWR may be one mechanism that underpins the contribution of the hippocampus to mind-wandering. However, there is no reason to assume that all SWR events should provoke mind-wandering, as they are likely to be functionally heterogeneous, and both the anatomical locations of SWRs within the hippocampus [79] and timing of the sharp wave to ripple coupling [80] lead to different patterns of downstream brain activation. It could be anticipated that only certain subtypes of SWRs might promote mind-wandering.
Based on the evidence reviewed above, we suggest that SWRs represent a compelling candidate for a brain state that may trigger mind-wandering. The SWR hippocampal brain state meets many criteria relevant to mind-wandering: it is associated with relatively quiescent behavioural states; activity is spontaneously evoked and can be propagated via the default network; and it is functionally associated with recollective, associative, imagination and simulation processes. Through these mechanisms, SWRs may trigger episodes of mind-wandering, which are subsequently sustained and elaborated via the default network, and its engagement with attentional, control and sensorimotor networks [1]. In the next section, we summarize the relationship between SWRs and neuromodulatory tone, and the broader role of neuromodulators in larger scale brain dynamics, identifying how these might promote and sustain the mind-wandering brain state.
3. Neuromodulatory influences over hippocampal sharp wave-ripples and the global dynamics that sustain the mind-wandering brain state
The occurrence of SWRs is strongly determined by hippocampal neuromodulatory tone, suggesting that interactions between the neuromodulatory system and hippocampal SWRs may be a key component in the neuropharmacological signature of mind-wandering. Coupling between the hippocampus and subcortical neuromodulatory structures may provide an important means of orchestrating the brain-wide dynamics that follow SWRs [81,82], which are likely to be instrumental in sustaining mind-wandering. Neuromodulators are also important for constraining the brain-wide dynamics that shape the evolution of a specific mind-wandering episode. That is, although a specific set of circumstances may be required to trigger an SWR, the dynamic recruitment of different neuromodulatory systems may influence how a given mind-wandering episode plays out over time. Here, we describe the major neuromodulatory systems of the brain (figure 1), and how they interact with the presence (or absence) of SWRs and the evolution of the mind-wandering brain state.
(a). Cholinergic system
Acetylcholine is released in widespread brain areas from projection nuclei in two main sites: the basal forebrain cholinergic system and the brainstem cholinergic system (figure 1a) [83–86]. The basal forebrain cholinergic system includes cells in the medial septal nucleus and nucleus basalis of Meynert, which send projections to the neocortex and limbic system. Brainstem cholinergic neurons are clustered in the pedunculopontine tegmental nucleus and laterodorsal pontine tegmental nuclei, which together project to the thalamus, basal ganglia and basal forebrain.
The cholinergic system plays a major role in modulating brain states owing to its ability to promote high-conductance states in the cortex [87], which are associated with high-frequency cortical activation, reduced low-frequency fluctuations and less synchronized activity among neuronal populations [16]. The high-conductance state is linked to enhanced sensory processing, improved task performance and increased task engagement [88–92] and in the past has been described as desynchronized (though see [16,93]).
Cholinergic tone is at its highest during wakeful, task-engaged behaviours and also during rapid eye movement (REM) sleep, suggesting that the cortical activation in high cholinergic states need not necessarily be associated with behavioural activation [15]. Although the cholinergic system innervates many brain regions, there is increasing recognition that its projections are relatively localized [94–96], meaning that it influences target regions much more precisely than was previously thought [97]. In this way, while acetylcholine can have global effects that promote an activated brain state, it also plays highly specific roles in cognitive function. Acetylcholine promotes encoding, attentional selectivity and sensory processing, via various physiological effects that serve to improve the signal-to-noise ratio of neural processing and enhance processing of extrinsic/feed-forward inputs, relative to intrinsic feedback [86,98].
Cholinergic innervation of the hippocampus mainly derives from the septohippocampal pathway, with projections from the septal area (medial forebrain, rostral to the corpus callosum) innervating all regions of the hippocampus [99,100]. In the hippocampus, acetylcholine increases the amplitude of the theta rhythm, a state which is comparable to the high-conductance state in the cortex [16]. By contrast, the presence of acetylcholine is known to suppress SWRs, which has been demonstrated in vitro [101] and in both awake and anesthetized animals [102]. High cholinergic tone has been shown to simultaneously promote theta rhythms and suppress SWRs [72,102], suggesting that hippocampal theta functions in an antagonistic manner to SWRs as a function of cholinergic input. This is consistent with the opposing behavioural and cognitive correlates of the theta and SWR states. High cholinergic levels in the hippocampus, promoting the theta state, favour externally driven sensory processing and engagement with the environment [103], whereas the lower cholinergic tone promoting the SWR state supports internally driven processes [104]. The low cholinergic state enhances intrinsic hippocampal dynamics, allowing internal connections to be reorganized and strengthened based on previously encoded associations [105]. Taken together, a relatively low cholinergic tone in the hippocampus would bias intrinsic hippocampal dynamics over extrinsic input, promoting the spontaneous activation of SWRs.
At the global level, similar to the SWR hippocampal brain state, we hypothesize that mind-wandering should be associated with relatively low cholinergic tone. As described earlier, despite projecting to many areas of the cerebral cortex, cholinergic projections follow a highly topographic and differentiated, rather than diffuse, organization [106]. This organizational feature supports functionally and spatially selective signalling [107]. Cholinergic activity promotes selective neuronal population coding and a cortical network that is less driven by global fluctuations from diffuse inputs [96]. This is achieved by selectively boosting the neuronal gain in target regions, enhancing feed-forward connections and network specificity [108–110]. A direct prediction from these studies is that at the macroscopic network level, heightened cholinergic tone should promote segregated (i.e. tightly connected communities of brain regions with weak inter-connections) patterns of information processing [111]. Such segregated topology is optimized for functionally selective operations and sensory processing, which contrasts with the distributed, integrated information processing that would support mind-wandering.
A possible contention with the notion that relatively low cholinergic tone promotes mind-wandering is that cholinergic tone is also highest during REM sleep, and there are recognized phenomenological and cortical activation similarities between mind-wandering and dreaming [7,112]. However, contrary to the long-held view that dreaming is synonymous with REM sleep, dreaming is also known to occur during non-REM/slow-wave sleep [113–116]. While non-REM dreams often have a shorter and fragmented quality, they can also exhibit phenomenological characteristics indistinguishable from REM dreams, being longer, vivid and with a self-narrative [117]. When drawing parallels between dreams and mind-wandering, it is also important to note that neuromodulatory tone differs significantly across waking versus sleep. Serotonergic and noradrenergic systems considerably reduce their activity in non-REM and become inactive in REM, whereas cholinergic systems are virtually silent during non-REM, becoming highly active in REM [6,117,118]. Together, this highlights that similar phenomenological experiences can occur across waking and non-REM/REM brain states, each associated with very different neuromodulatory levels. In this respect, the low cholinergic state promoting SWRs may be considered one route to mind-wandering in the awake state, while different combinations of neuromodulators drive this phenomenology in other brain states.
(b). Noradrenergic system
The locus coeruleus (LC), situated deep in the pons, is the primary source of noradrenaline in the brain. This small nucleus has widespread projections innervating most brain regions, with the exception of the basal ganglia, and it provides descending input to the spinal cord and autonomic nuclei [119] (figure 1b). Highly collateralized LC projections can release noradrenaline via non-synaptic or paracrine mechanisms, enabling diffuse influence over cortical activation and behavioural arousal [118]. At the larger scale brain network level, LC-noradrenaline activation facilitates reorganization of functional networks in response to changing environmental demands. Through the simultaneous action of noradrenaline at multiple target structures, ongoing functional interactions can be interrupted and reconfigured to promote a change in behaviour [120].
Early reports of LC-noradrenaline function focused on its role in general arousal and vigilance, based on its links with the sleep-wake cycle. However, increasingly more fine-grained functions have been related to the LC-noradrenaline system, including a central role in cognition and behavioural flexibility [121]. One way that noradrenaline regulates behaviour is by negotiating the balance between ongoing focus on a task and the need to shift focus to alternative options. Termed the ‘exploitation-exploration trade-off', this underpins an organism's ability to persist with a behaviour while it is useful and to explore more advantageous opportunities when use decreases. These modes of behaviour are mapped to temporally distinct firing patterns in the LC and changes in tonic noradrenaline levels [122]. When tonic noradrenaline levels are optimal, phasic firing occurs in response to task-relevant stimuli, and this is associated with task focus and good performance. Following a Yerkes-Dodson-like function, optimal task performance is typically associated with the middle range of tonic noradrenaline levels. Lower tonic levels are associated with low arousal and reduced alertness, which negatively impacts task performance. High tonic levels are associated with distractibility and an exploratory mode, facilitating the organism to disengage and pursue another behaviour.
Limited in vitro evidence using hippocampal slices suggests a receptor-dependant modulatory role of noradrenaline in SWRs, with α1 adrenoreceptor activation associated with SWR suppression and SWR expression associated with β1 adrenoreceptor activation [123,124]. Consistent with this, during certain SWR subtypes brain-wide activity shows downregulation of the LC, suggesting a modulatory role from this system in SWR events [80]. These studies raise the possibility that hippocampal noradrenaline levels may have both suppressive and potentiating effects on the occurrence of SWRs.
The LC densely innervates the hippocampus and regulates cellular excitability, cellular reorganization, synaptic plasticity and long-term potentiation, influencing all aspects of memory formation [125–128]. In addition to facilitating encoding, the LC-noradrenaline system is reactivated in the window after initial learning, at the stage of offline memory consolidation [129,130]. This phasic reactivation may relate to hippocampal SWR events. There is indirect evidence for this phenomenon, as delayed LC excitation after learning was found to occur exclusively during slow-wave sleep, a state in which SWRs are known to occur [130]. During slow-wave sleep, noradrenergic activity is at a relatively low tonic level; however, the LC is not completely silent [131]. The LC fires transient bursts during slow-wave sleep that coincide with hippocampal SWRs [132,133]. This suggests that phasic bursts of noradrenergic activity during states of relatively low (but not silent) noradrenergic activity may be conducive to SWR events in slow-wave sleep [134]. We speculate that similar noradrenergic factors—i.e. those that occur when one is awake but not particularly focused or stressed—may be conducive to SWR events in the awake state.
Co-activation of the noradrenergic system during replay could serve to promote plasticity and stability in the distributed cell assemblies reactivated during an SWR, contributing to systems consolidation in the offline state [121,130,135]. The noradrenergic release associated with an SWR-mediated phasic increase in LC activity would act to bring both the cortex and hippocampus into a more excitable, highly conductive state that would promote cross-regional interactions [136,137]. At the systems-level, the activation of relatively low-affinity α1 adrenergic receptors promotes a ‘reset' of large-scale networks [120]. In this way, the noradrenergic activity coinciding with an SWR may be sufficient to reset cortical dynamics and in turn promote a brain state conducive to mind-wandering.
The effect of noradrenaline at the brain network level can be contrasted with the cholinergic system. The broad reach of gain modulation from the noradrenergic system is a key mechanism for enabling dynamic reorganization of cortical networks [138]. Noradrenergic function has been linked to increases in the strength and clustering of functional connectivity [29] and increased network integration [139], suggesting a role for noradrenaline in promoting distributed information processing across widespread brain regions, via its capacity for diffuse alterations in response gain [111]. Relatively higher global tonic noradrenaline, with respect to cholinergic tone, may promote increased integration across the brain to facilitate the coordinated information processing required to sustain episodes of mind-wandering.
(c). Serotonergic system
The serotonergic system innervates most brain regions, with the majority of ascending projections arising from the dorsal and median raphe nuclei in the brainstem (figure 1c) [140,141]. Serotonergic projections arborize across diverse brain regions, with some providing paracrine transmission that enables diffuse influence over target regions. The serotonergic system acts on a large and diverse family of receptors [142], adding to the diverse functional capacity that is already conferred by its diffuse projections [143]. The highly selective distribution of receptor subtypes across cortical layers means that in addition to the ability to modulate brain-wide dynamics, the serotonergic system can exert very precise modulation over specific neuronal populations [144].
There is in vitro evidence that high levels of hippocampal serotonin can suppress SWRs [145]. In vivo, recordings from the serotonergic median raphe region (which projects to the entire hippocampal formation) showed that many of these neurons were inactive at the time of SWRs [146]. Furthermore, optogenetic excitation and inhibition of median raphe neurons respectively suppressed and enhanced SWR activity [146]. During certain SWR subtypes, brain-wide activity shows downregulation of the serotonergic dorsal raphe, in keeping with a modulatory role from this system in SWR events [80]. Therefore, inhibition of serotonergic hippocampally projecting neurons may be a critical factor in promoting the occurrence of SWRs. This action probably occurs via modulation of serotonergic 5HT1A receptors, which are abundant in the hippocampal CA1 subregion [147,148] and are typically associated with inhibitory G-protein-coupled effects [149].
The concentration of serotonin in different brain regions could also influence the evolution of mind-wandering episodes after an SWR has occurred. There is compelling evidence that serotonin is involved in the alteration of global dynamics in a way that is consistent with the phenomenology of the mind-wandering brain state. Serotonergic psychedelics, such as lysergic acid diethylamide (LSD), psilocybin and ayahuasca/dimethyltryptamine (DMT), provide unique insights into the impact of brain serotonin upon the neurophenomenology of perception, as well as the boundaries between spontaneous thought and dreaming. While psychedelics do not act exclusively upon serotonin receptors, extensive work has revealed the dominant role for a specific serotonin receptor subclass (5HT2A) in psychedelic neurophenomenology [150,151]. Recent work has suggested that 5HT2A agonists may shift the brain into a more entropic or anarchic mode of processing, in which the brain shifts between states in an irregular manner that is much less tethered to the external world than normal, waking consciousness [152,153]. By selectively increasing the gain of layer V pyramidal-tract-type cells (the major output population of the cerebral cortex), 5HT2A agonists are suggested to flatten the attractor landscape [153], which means that novel patterns can form in the place of neuronal ensembles that are typically activated according to well-established firing patterns. This is consistent with the novel associations that may form during mind-wandering, raising the possibility that a mind-wandering episode occurring in the context of higher cortical serotonin may follow more novel, associative trajectories.
These lines of reasoning suggest dual effects of serotonin, with intermediate levels in the hippocampus promoting SWRs and higher levels in the cerebral neocortex promoting the evolution of a mind-wandering episode. Speculatively, these effects can be partly explained by actions at different serotonergic receptor subtypes (5HT1A versus 5HT2A). This highlights the complexity of neuromodulatory influences over behaviour, as actions at different subtypes have varied and nuanced effects, which are mostly unexplored in relation to mind-wandering.
(d). Dopaminergic system
Dopamine may serve to promote SWRs in order to bias encoding and retrieval of salient or rewarding events [43]. There is a well-established role for dopamine in the formation and consolidation of memories, most notably via dopamine-driven mechanisms of hippocampal long-term potentiation. Midbrain dopaminergic neurons primarily modulate the hippocampus via a loop involving direct projections from the ventral tegmental area (VTA) to the hippocampus, which itself outputs (via the subiculum) excitatory projections to the nucleus accumbens, inhibiting the ventral pallidum and releasing VTA dopaminergic neurons from tonic inhibition [154,155]. In contrast with the diffusely branching collaterals of the noradrenergic and serotonergic systems that innervate multiple areas, dopaminergic projections (like cholinergic projections) are relatively more segregated and modulate more specific brain regions (figure 1d) [156]. Consistent with this mechanism, the dopaminergic innervation of the hippocampus from the VTA is relatively sparse, potentially allowing it to have more specific, nuanced effects on hippocampal function [157]. Recently, an additional source of hippocampal dopamine was identified, as neurons projecting from the LC to the hippocampus co-release both noradrenaline and dopamine [158,159].
In relation to SWRs, direct application of dopamine to hippocampal slices in vitro results in a long-lasting increase in SWR frequency [160]. Furthermore, activity in the dopaminergic VTA-hippocampal pathway at the time of encoding enhances subsequent offline SWR activation [161]. This is consistent with earlier work showing enhanced SWR activity following rewarded outcomes [162]. Hippocampal dopamine at the time of experiencing novel, salient or rewarded events may therefore bias the content and frequency of subsequent SWR activity [163]. The role of dopamine may also extend to reinforcing reactivated sequences during offline periods, as dopaminergic input coordinates with hippocampal activity during SWRs in the offline state. This is demonstrated by reward responsive VTA neurons coordinating their firing with hippocampal SWR replayed sequences during offline quiet wakefulness [164].
These findings suggest a dual role for dopamine in increasing the likelihood of SWR reactivation of particular experiences, as well as further reinforcing reactivation patterns offline. While this presumably occurs as a means of promoting consolidation of salient events into long-term memory, it suggests that dopaminergic tone in the hippocampus may impact the subsequent spontaneous recall of information and, in this way, influence the content and the reinforcing aspects of mind-wandering. A link between the dopaminergic system and mind-wandering may substantiate recent theoretical assertions that a goal of mind-wandering is to generate potentially rewarding cognitive affordances [165,166].
(e). Interactions between neuromodulatory systems may trigger hippocampal sharp wave-ripples and sustain the mind-wandering brain state
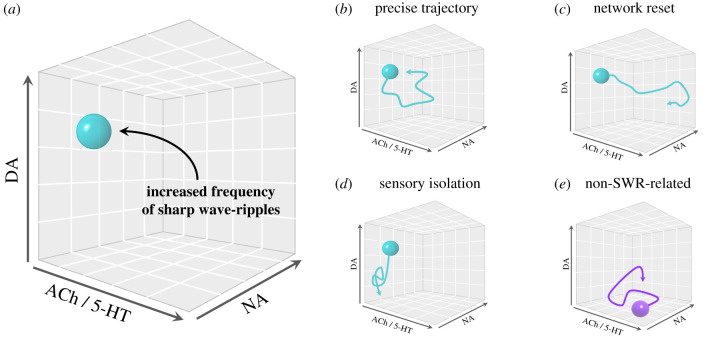
Based on the evidence reviewed above, we propose that the combined influence of neuromodulators defines the likelihood that an SWR will occur in the hippocampus, and by extension, the propensity for an individual to enter into a mind-wandering episode (figure 3). Lower cholinergic tone facilitates an overall dampening of sensory processing, disengagement from the external environment and a relatively quiescent behavioural state. This promotes hippocampal SWRs, which may trigger a mind-wandering episode. Similarly, low levels of hippocampal serotonin also facilitate the occurrence of SWRs. By contrast, high dopaminergic tone in the hippocampus can enhance the activity of SWRs and make it more likely for a neuronal sequence to be reactivated in the future, via reinforcing properties. Finally, phasic bursts during relatively low levels of noradrenaline may promote SWRs. Together, these patterns suggest that mind-wandering should occur during periods of relative quiescence (i.e. low cholinergic and serotonergic tone) but moderate arousal (i.e. low noradrenergic tone) in which a rewarding stimulus is chanced upon (i.e. heightened dopaminergic response), either via an external or internal cue (figure 3a; teal sphere).
Figure 3.
Neuromodulatory interplay that promotes the hippocampal sharp wave-ripple (SWR) brain state. It shows the balance of neuromodulatory tone that promotes hippocampal SWRs, which by extension is the neuromodulatory balance that may trigger a mind-wandering episode. (a) The teal sphere reflects the pro-SWR zone, that is, the balance of hippocampal neuromodulatory levels that is conducive to SWR occurrence; (b) an example of a precise state-space trajectory following an SWR; (c) if the SWR triggers increased noradrenaline, this will probably lead to a ‘network reset' [120]; (d) if the SWR recruits low cholinergic tone, this will facilitate further sensory isolation; (e) heightened serotonergic tone can trigger non-SWR-related mind-wandering. DA = dopamine; ACh = acetylcholine; 5-HT = serotonin; NA = noradrenaline. (Online version in colour.)
Once triggered, the occurrence of an SWR has the potential to drive brain networks into a precise, information-rich (i.e. low entropy) configuration (figure 3b). Specifically, a hippocampal ripple re-activates distributed neuronal ensembles that were either related to the neuronal sequence that was embedded into the network during the original encoding event, or through the Hebbian processes that occur over the course of learning. In this way, a ripple event would act to re-energise a unique constellation of regions that were tangentially related to some aspect of the replay or simulation, opening up particular ways to engage with the current brain state over time (e.g. by re-exploring the same memory, or combining it in new ways).
As we have argued above, neuromodulatory tone plays an important role in shaping the likelihood of the hippocampus undergoing an SWR, and hence, how likely it would be to trigger a mind-wandering episode. However, the relative concentration of different neuromodulators within the pro-SWR zone will also probably play a role in defining the mind-wandering state. For instance, phasic noradrenergic activity at the time of the SWR may enhance the activation of distributed neuronal ensembles throughout the subcortex and neocortex, shaping the amount of integration present between otherwise disparate brain regions (figure 3c). Higher concentrations of noradrenaline would probably facilitate a ‘reset' of the global brain state into one where a specific memory, association, or simulation has been primed into conscious contents, with the potential to trigger a mind-wandering episode. If the concentration of noradrenaline is too high (or low), this could render the phasic burst less (or more) impactful, as the phasic activity can potentially be obscured against the tonic background activity of the LC.
Once a mind-wandering episode has been triggered, how (or if) it evolves will also be further influenced by the neuromodulatory tone in the epoch immediately following a SWR. For instance, the continued low cholinergic tone in the basal forebrain system could downregulate the impact of incoming sensory input and bias the system towards intrinsic processes (figure 3d). Lower cholinergic tone would also theoretically lessen the constraints on segregated network topology, allowing for more flexible integration across tangentially related neuronal ensembles [111]. Noradrenergic tone further determines the level of engagement with the current external or internal environment. If noradrenaline levels are optimal for whatever cognitive or behavioural task is at hand, it is likely that a person will maintain their focus on that task. However, if levels are still within an intermediate range but outside of task-optimal levels, then the triggered mind-wandering episode may take hold and allow the person to engage in spontaneous thought. Together, the cholinergic and noradrenergic systems have considerable influence upon overall brain states [167], working in cooperation to shape the extent to which we are driven by engagement with the external world, versus being driven by intrinsically generated activity.
While inhibition of hippocampal serotonin facilitates the occurrence of SWRs, wider-spread activation of the serotonergic system may shape the evolving content of spontaneous thought, which may not necessarily be triggered by an SWR (figure 3e). Based on work using 5HT2A receptor agonists [152,153], there is evidence that elevated serotonergic gain in layer V pyramidal-tract-type cells may release neuronal populations from their stereotypic firing patterns allowing novel patterns to form, consistent with the novel, unpredictable trajectory of certain spontaneous thought episodes. This mode of mind-wandering is consistent with a high-entropic state, as opposed to the low entropy state that may follow an SWR.
4. Relationship between neuromodulation and the phenomenology of spontaneous thought
Several lines of evidence link our proposed model of neuromodulatory influences with the phenomenology of spontaneous thought. We have suggested that low-to-intermediate levels of noradrenaline, albeit outside of task-optimal levels, are conducive to mind-wandering. In agreement with this, reduced pupil diameter (a proxy for tonic LC function [168]) indicative of low levels of arousal is associated with an increased frequency of hippocampal SWRs [88,169]. Evidence from human studies supports the idea that mind-wandering may occur when tonic noradrenaline levels are outside of task-optimal levels, as studies using pupillometry have identified off-task thought in the context of both elevated and reduced baseline pupils [32,33,170–173]. We suggest that spontaneous thought occurring in the context of relatively low tonic noradrenaline/low arousal most resembles the fleeting and transitory nature of mind-wandering. At the more extreme end of the low arousal/low tonic noradrenaline spectrum, thoughts may become increasingly disjointed and transient, with less awareness of thoughts, which at its extreme may be experienced as a mind-blanking state (i.e. thinking of ‘nothing') [174–176].
By contrast, relatively higher levels of tonic noradrenaline and arousal that facilitate an exploratory mode of behaviour may also engender an exploratory mode of spontaneous thought. This could be realized as exploratory creative thinking, which is subject to greater deliberative constraints than mind-wandering but still considered within the family of spontaneous thought [1]. In keeping with this, during divergent thinking—a creative thought process where many possible associations are explored—larger baseline pupil levels predict the generation of original ideas [177], suggesting a link between higher tonic noradrenaline levels and exploratory, creative thoughts. This contrasts with studies where lowered noradrenaline levels (via β-adrenergic antagonists) improved convergent thinking (i.e. zeroing in on a single creative response) [178], suggesting that noradrenaline may influence the extent to which creative thought is exploitative (convergent) versus explorative (divergent). Relatively high tonic noradrenaline, which would be characterized by high global gain and increased integration across brain regions [117,152,153], may support a brain state that promotes the dynamic default-executive network coupling that has been associated with divergent thinking [179,180].
Based on work using 5HT2A receptor agonists, we have also suggested that serotonergic activity may facilitate the formation of novel patterns of neuronal ensembles as a mind-wandering episode evolves. Psilocybin is shown to enhance the activation of indirect semantic associations [181], which fits well with a recent proposal that certain aspects of mind-wandering may be driven by unconstrained semantic associations [67]. Furthermore, LSD was found to increase individuals' susceptibility to imagining themselves experiencing novel, creative scenarios [182]. Dreamlike states, with vivid visual imagery and cognitive bizarreness, induced by psychedelics [183–185] are also consistent with the possibility of novel pattern formation. Although the psychedelic state may be an intensified form of spontaneous thought, similar effects probably contribute to the formation of novel associations in the mind-wandering brain state.
5. Concluding remarks
Mind-wandering induces a shift from a pragmatic mode where an organism explores its environment, to a mode where mental states are explored. This process may represent a key evolutionary development, emerging as more complex systems interacted with more complex environments, where such offline processing enabled consolidation and refinement of learnt associations without being under the pressure of immediate goal-directed pursuits [186]. Such processing enables organization (and re-organization) of knowledge about the world into a high-dimensional space to support adaptive and flexible behaviour—an idea that has been conceptualized as a ‘cognitive map' [187,188]. The recurrent offline instantiation of memories and novel simulations that occurs with SWRs makes them an ideal candidate for maintaining and modulating these cognitive maps [189,190]. This possibility is supported by recent work showing that replay events in humans can constitute abstract rule sequences, suggesting they may function as a mechanism to generalize knowledge across experiences [75]. Considering a link between SWRs and mind-wandering, the possibility emerges that an adaptive function of mind-wandering may well be to augment these abstract knowledge structures we cultivate over our lifespan.
Here, we have defined spontaneous thought in terms of the possible brain states that might trigger and sustain it, focusing on the role of neuromodulatory systems in shaping the mind-wandering brain state. It is clear that each neuromodulatory system discussed plays a role in orchestrating the mind-wandering state. Indeed, the interplay between ascending arousal systems cannot be underestimated, as all of these systems contribute to waking consciousness and there are substantial interactions between them [191]. Continued understanding of this interplay remains important across all attempts to link behaviour and cognition with neuromodulatory systems. Likewise, although we have not focused on it here, a key principle of neuromodulatory systems that enables their exquisite flexibility is that they receive top-down regulation via the very regions that they are modulating [192,193]. Considering these reciprocal interactions also remains an important focus for reconciling neuromodulatory influences over brain-behaviour states. Finally, it is important to note that we have discussed brain states in somewhat absolute terms. It is now appreciated that brain states can at times be a complex mix of overlapping global and local sub-states [20], which combine to influence behaviour and cognition.
The field of cognitive neuroscience has made great strides in establishing the brain networks recruited during the mind-wandering brain state; however, we are only in the nascent stages of understanding how neuromodulators affect this state. Much of what we have presented here remains speculative; however, we hope that these early ideas may provoke the future work necessary to uncover the nuanced roles that neuromodulators undoubtedly play in mind-wandering.
Data accessibility
This article has no additional data.
Authors' contributions
C.O. was involved in conceptualization, writing original draft, review and editing the manuscript, and making figures. I.C.W. was involved in conceptualization, review and editing the manuscript. J.M.S. was involved in conceptualization; review and editing the manuscript and making figures
Competing interests
We declare we have no competing interests.
Funding
C.O. is supported by a Neil Hamilton Fairley Fellowship from the National Health and Medical Research Council (no. 1091310). J.M.S. is supported by a Robinson Fellowship from the University of Sydney and a project grant from the National Health and Medical Research Council (no. 1156536).
References
- 1.Christoff K, Irving ZC, Fox KCR, Spreng RN, Andrews-Hanna JR. 2016. Mind-wandering as spontaneous thought: a dynamic framework. Nat. Rev. Neurosci. 17, 718–731. ( 10.1038/nrn.2016.113) [DOI] [PubMed] [Google Scholar]
- 2.Bratman M. 1987. Intention, plans, and practical reason. Cambridge, MA: Harvard University Press. [Google Scholar]
- 3.Juarrero A. 2000. Dynamics in action: intentional behavior as a complex system. Emergence 2, 24–57. ( 10.1207/S15327000EM0202_03) [DOI] [Google Scholar]
- 4.Gibson JJ. 1979. The ecological approach to visual perception. Boston, MA: Houghton, Mifflin and Company. [Google Scholar]
- 5.Cisek P, Kalaska JF. 2010. Neural mechanisms for interacting with a world full of action choices. Annu. Rev. Neurosci. 33, 269–298. ( 10.1146/annurev.neuro.051508.135409) [DOI] [PubMed] [Google Scholar]
- 6.Hobson JA. 2009. REM sleep and dreaming: towards a theory of protoconsciousness. Nat. Rev. Neurosci. 10, 803–813. ( 10.1038/nrn2716) [DOI] [PubMed] [Google Scholar]
- 7.Fox KCR, Nijeboer S, Solomonova E, Domhoff GW, Christoff K. 2013. Dreaming as mind wandering: evidence from functional neuroimaging and first-person content reports. Front. Hum. Neurosci. 7, 412 ( 10.3389/fnhum.2013.00412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walpola IC, Muller AJ, Hall JM, Andrews-Hanna JR, Irish M, Lewis SJG, Shine JM, O'Callaghan C. 2020. Mind-wandering in Parkinson's disease hallucinations reflects primary visual and default network coupling. Cortex 125, 233–245. ( 10.1016/j.cortex.2019.12.023) [DOI] [PubMed] [Google Scholar]
- 9.Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. 2007. Wandering minds: the default network and stimulus-independent thought. Science 315, 393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. 2009. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc. Natl Acad. Sci. USA 106, 8719–8724. ( 10.1073/pnas.0900234106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. 2010. Evidence for the default network's role in spontaneous cognition. J. Neurophysiol. 104, 322–335. ( 10.1152/jn.00830.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zabelina DL, Andrews-Hanna JR. 2016. Dynamic network interactions supporting internally-oriented cognition. Curr. Opin. Neurobiol. 40, 86–93. ( 10.1016/j.conb.2016.06.014) [DOI] [PubMed] [Google Scholar]
- 13.Kucyi A. 2017. Just a thought: how mind-wandering is represented in dynamic brain connectivity. Neuroimage 180, 505–514. ( 10.1016/j.neuroimage.2017.07.001) [DOI] [PubMed] [Google Scholar]
- 14.Fox KCR, Spreng RN, Ellamil M, Andrews-Hanna JR, Christoff K. 2015. The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage 111, 611–621. ( 10.1016/j.neuroimage.2015.02.039) [DOI] [PubMed] [Google Scholar]
- 15.Lee S-H, Dan Y. 2012. Neuromodulation of brain states. Neuron 76, 209–222. ( 10.1016/j.neuron.2012.09.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris KD, Thiele A. 2011. Cortical state and attention. Nat. Rev. Neurosci. 12, 509–523. ( 10.1038/nrn3084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kay K, Frank LM. 2019. Three brain states in the hippocampus and cortex. Hippocampus 29, 184–238. ( 10.1002/hipo.22956) [DOI] [PubMed] [Google Scholar]
- 18.Buzsáki G. 2015. Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188. ( 10.1002/hipo.22488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colgin LL. 2016. Rhythms of the hippocampal network. Nat. Rev. Neurosci. 17, 239–249. ( 10.1038/nrn.2016.21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zagha E, McCormick DA. 2014. Neural control of brain state. Curr. Opin. Neurobiol. 29, 178–186. ( 10.1016/j.conb.2014.09.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marder E. 2012. Neuromodulation of neuronal circuits: back to the future. Neuron 76, 1–11. ( 10.1016/j.neuron.2012.09.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldman-Rakic PS. 1995. Cellular basis of working memory. Neuron 14, 477–485. ( 10.1016/0896-6273(95)90304-6) [DOI] [PubMed] [Google Scholar]
- 23.Robbins TW, Arnsten AFT. 2009. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu. Rev. Neurosci. 32, 267–287. ( 10.1146/annurev.neuro.051508.135535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robbins TW. 2005. Chemistry of the mind: neurochemical modulation of prefrontal cortical function. J. Comp. Neurol. 493, 140–146. ( 10.1002/cne.20717) [DOI] [PubMed] [Google Scholar]
- 25.Cools R. 2019. Chemistry of the adaptive mind: lessons from dopamine. Neuron 104, 113–131. ( 10.1016/j.neuron.2019.09.035) [DOI] [PubMed] [Google Scholar]
- 26.Salinas E, Thier P. 2000. Gain modulation: a major computational principle of the central nervous system. Neuron 27, 15–21. ( 10.1016/s0896-6273(00)00004-0) [DOI] [PubMed] [Google Scholar]
- 27.Dayan P. 2012. Twenty-five lessons from computational neuromodulation. Neuron 76, 240–256. ( 10.1016/j.neuron.2012.09.027) [DOI] [PubMed] [Google Scholar]
- 28.Silver RA. 2010. Neuronal arithmetic. Nat. Rev. Neurosci. 11, 474–489. ( 10.1038/nrn2864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eldar E, Cohen JD, Niv Y. 2013. The effects of neural gain on attention and learning. Nat. Neurosci. 16, 1146–1153. ( 10.1038/nn.3428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iversen LL, Iversen SD, Bloom FE, Roth RH. 2009. Introduction to neuropsychopharmacology. Oxford, UK: Oxford University Press. [Google Scholar]
- 31.Robbins TW. 2000. Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp. Brain Res. 133, 130–138. ( 10.1007/s002210000407) [DOI] [PubMed] [Google Scholar]
- 32.Smallwood J, Brown KS, Baird B, Mrazek MD, Franklin MS, Schooler JW. 2012. Insulation for daydreams: a role for tonic norepinephrine in the facilitation of internally guided thought. PLoS ONE 7, e33706 ( 10.1371/journal.pone.0033706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mittner M, Hawkins GE, Boekel W, Forstmann BU. 2016. A neural model of mind wandering. Trends Cogn. Sci. 20, 570–578. ( 10.1016/j.tics.2016.06.004) [DOI] [PubMed] [Google Scholar]
- 34.Joo HR, Frank LM. 2018. The hippocampal sharp wave–ripple in memory retrieval for immediate use and consolidation. Nat. Rev. Neurosci. 19, 744–757. ( 10.1038/s41583-018-0077-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buzsáki G, Leung LW, Vanderwolf CH. 1983. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 287, 139–171. ( 10.1016/0165-0173(83)90037-1) [DOI] [PubMed] [Google Scholar]
- 36.Chrobak JJ, Buzsáki G. 1996. High-frequency oscillations in the output networks of the hippocampal-entorhinal axis of the freely behaving rat. J. Neurosci. 16, 3056–3066. ( 10.1523/JNEUROSCI.16-09-03056.1996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Logothetis NK, Eschenko O, Murayama Y, Augath M, Steudel T, Evrard HC, Besserve M, Oeltermann A. 2012. Hippocampal-cortical interaction during periods of subcortical silence. Nature 491, 547–553. ( 10.1038/nature11618) [DOI] [PubMed] [Google Scholar]
- 38.Tang W, Jadhav SP. 2019. Sharp-wave ripples as a signature of hippocampal-prefrontal reactivation for memory during sleep and waking states. Neurobiol. Learn. Mem. 160, 11–20. ( 10.1016/j.nlm.2018.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaplan R, Adhikari MH, Hindriks R, Mantini D, Murayama Y, Logothetis NK, Deco G. 2016. Hippocampal sharp-wave ripples influence selective activation of the default mode network. Curr. Biol. 26, 686–691. ( 10.1016/j.cub.2016.01.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karlsson MP, Frank LM. 2009. Awake replay of remote experiences in the hippocampus. Nat. Neurosci. 12, 913–918. ( 10.1038/nn.2344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dudai Y, Karni A, Born J. 2015. The consolidation and transformation of memory. Neuron 88, 20–32. ( 10.1016/j.neuron.2015.09.004) [DOI] [PubMed] [Google Scholar]
- 42.Buzsáki G. 1989. Two-stage model of memory trace formation: a role for ‘noisy’ brain states. Neuroscience 31, 551–570. ( 10.1016/0306-4522(89)90423-5) [DOI] [PubMed] [Google Scholar]
- 43.Pfeiffer BE. 2020. The content of hippocampal ‘replay’. Hippocampus 30, 6–18. ( 10.1002/hipo.22824) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abadchi K, Nazari-Ahangarkolaee J, Gattas M, Bermudez-Contreras S, Luczak E, McNaughton BL, Mohajerani MH. 2020. Spatiotemporal patterns of neocortical activity around hippocampal sharp-wave ripples. eLife 9, e51972 ( 10.7554/eLife.51972) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rothschild G, Eban E, Frank LM. 2017. A cortical-hippocampal-cortical loop of information processing during memory consolidation. Nat. Neurosci. 20, 251–259. ( 10.1038/nn.4457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diba K, Buzsáki G. 2007. Forward and reverse hippocampal place-cell sequences during ripples. Nat. Neurosci. 10, 1241–1242. ( 10.1038/nn1961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gupta AS, van der Meer MAA, Touretzky DS, Redish AD. 2010. Hippocampal replay is not a simple function of experience. Neuron 65, 695–705. ( 10.1016/j.neuron.2010.01.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dragoi G, Tonegawa S. 2012. Preplay of future place cell sequences by hippocampal cellular assemblies. Nature 469, 397–401. ( 10.1038/nature09633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfeiffer BE, Foster DJ. 2013. Hippocampal place-cell sequences depict future paths to remembered goals. Nature 497, 74–79. ( 10.1038/nature12112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stella F, Baracskay P, O'Neill J, Csicsvari J. 2019. Hippocampal reactivation of random trajectories resembling Brownian diffusion. Neuron 102, 450–461.e7. ( 10.1016/j.neuron.2019.01.052) [DOI] [PubMed] [Google Scholar]
- 51.Sutherland GR, McNaughton B. 2000. Memory trace reactivation in hippocampal and neocortical neuronal ensembles. Curr. Opin. Neurobiol. 10, 180–186. ( 10.1016/s0959-4388(00)00079-9) [DOI] [PubMed] [Google Scholar]
- 52.Buzsáki G, Chrobak JJ. 1995. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr. Opin. Neurobiol. 5, 504–510. ( 10.1016/0959-4388(95)80012-3) [DOI] [PubMed] [Google Scholar]
- 53.Spreng RN, Mar RA, Kim ASN. 2009. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci. 21, 489–510. ( 10.1162/jocn.2008.21029) [DOI] [PubMed] [Google Scholar]
- 54.Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. 2012. the future of memory: remembering, imagining, and the brain. Neuron 76, 677–694. ( 10.1016/j.neuron.2012.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marr D. 1971. Simple memory: a theory for archicortex. Phil. Trans. R. Soc. Lond. B 262, 23–81. ( 10.1098/rstb.1971.0078) [DOI] [PubMed] [Google Scholar]
- 56.O'Reilly RC, McClelland JL. 1994. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus 4, 661–682. ( 10.1002/hipo.450040605) [DOI] [PubMed] [Google Scholar]
- 57.McNaughton BL, Morris RG. 1987. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 10, 408–415. [Google Scholar]
- 58.Treves A, Rolls ET. 1992. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus 2, 189–199. ( 10.1002/hipo.450020209) [DOI] [PubMed] [Google Scholar]
- 59.Guzman SJ, Schlögl A, Frotscher M, Jonas P. 2016. Synaptic mechanisms of pattern completion in the hippocampal CA3 network. Science 353, 1117–1123. ( 10.1126/science.aaf1836) [DOI] [PubMed] [Google Scholar]
- 60.Rolls ET. 2013. The mechanisms for pattern completion and pattern separation in the hippocampus. Front. Syst. Neurosci. 7, 74 ( 10.3389/fnsys.2013.00074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neunuebel JP, Knierim JJ. 2014. CA3 retrieves coherent representations from degraded input: direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron 81, 416–427. ( 10.1016/j.neuron.2013.11.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cembrowski MS, Spruston N. 2019. Heterogeneity within classical cell types is the rule: lessons from hippocampal pyramidal neurons. Nat. Rev. Neurosci. 20, 193–204. ( 10.1038/s41583-019-0125-5) [DOI] [PubMed] [Google Scholar]
- 63.Rolls ET. 2009. Attractor networks. WIREs Cogn. Sci. 1, 119–134. ( 10.1002/wcs.1) [DOI] [PubMed] [Google Scholar]
- 64.Knierim JJ, Neunuebel JP. 2016. Tracking the flow of hippocampal computation: pattern separation, pattern completion, and attractor dynamics. Neurobiol. Learn. Mem. 129, 38–49. ( 10.1016/j.nlm.2015.10.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moscovitch M. 2008. The hippocampus as a ‘stupid,’ domain-specific module: implications for theories of recent and remote memory, and of imagination. Can. J. Exp. Psychol. 62, 62–79. ( 10.1037/1196-1961.62.1.62) [DOI] [PubMed] [Google Scholar]
- 66.Moscovitch M, Cabeza R, Winocur G, Nadel L. 2016. Episodic memory and beyond: the hippocampus and neocortex in transformation. Annu. Rev. Psychol. 67, 105–134. ( 10.1146/annurev-psych-113011-143733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mildner JN, Tamir DI. 2019. Spontaneous thought as an unconstrained memory process. Trends Neurosci. 42, 763–777. ( 10.1016/j.tins.2019.09.001) [DOI] [PubMed] [Google Scholar]
- 68.Mills C, Herrera-Bennett A, Faber M, Christoff K. 2018. Why the mind wanders: how spontaneous thought's default variability may support episodic efficiency and semantic optimization. In The Oxford handbook of spontaneous thought, mind-wandering, creativity, and dreaming, pp. 11–22. Oxford, UK: Oxford University Press. [Google Scholar]
- 69.Mills C, Raffaelli Q, Irving ZC, Stan D, Christoff K. 2018. Is an off-task mind a freely-moving mind? Examining the relationship between different dimensions of thought. Conscious Cogn. 58, 20–33. ( 10.1016/j.concog.2017.10.003) [DOI] [PubMed] [Google Scholar]
- 70.Baror S, Bar M. 2016. Associative activation and its relation to exploration and exploitation in the brain. Psychol. Sci. 27, 776–789. ( 10.1177/0956797616634487) [DOI] [PubMed] [Google Scholar]
- 71.Maillet D, Beaty RE, Adnan A, Fox KCR, Turner GR, Spreng RN. 2019. Aging and the wandering brain: age-related differences in the neural correlates of stimulus-independent thoughts. PLoS ONE 14, e0223981-14 ( 10.1371/journal.pone.0223981) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hunt DL, Linaro D, Si B, Romani S, Spruston N. 2018. A novel pyramidal cell type promotes sharp-wave synchronization in the hippocampus. Nat. Neurosci. 21, 985–995. ( 10.1038/s41593-018-0172-7) [DOI] [PubMed] [Google Scholar]
- 73.Tambini A, Davachi L. 2019. Awake reactivation of prior experiences consolidates memories and biases cognition. Trends Cogn. Sci. 23, 876–890. ( 10.1016/j.tics.2019.07.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schuck NW, Niv Y. 2019. Sequential replay of nonspatial task states in the human hippocampus. Science 364, eaaw5181-11 ( 10.1126/science.aaw5181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y, Dolan RJ, Kurth-Nelson Z, Behrens TEJ. 2019. Human replay spontaneously reorganizes experience. Cell 178, 640–652 e14. ( 10.1016/j.cell.2019.06.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Norman Y, Yeagle EM, Khuvis S, Harel M, Mehta AD, Malach R. 2019. Hippocampal sharp-wave ripples linked to visual episodic recollection in humans. Science 365, eaax1030-16 ( 10.1126/science.aax1030) [DOI] [PubMed] [Google Scholar]
- 77.O'Callaghan C, Shine JM, Hodges JR, Andrews-Hanna JR, Irish M. 2019. Hippocampal atrophy and intrinsic brain network dysfunction relate to alterations in mind wandering in neurodegeneration. Proc. Natl Acad. Sci. USA 116, 3316–3321. ( 10.1073/pnas.1818523116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCormick C, Rosenthal CR, Miller TD, Maguire EA. 2018. Mind-wandering in people with hippocampal damage. J. Neurosci. 38, 2745–2754. ( 10.1523/JNEUROSCI.1812-17.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sosa M, Joo HR, Frank LM. 2019. Dorsal and ventral hippocampal sharp-wave ripples activate distinct nucleus accumbens networks. Neuron 105, 725–741; e8. ( 10.1016/j.neuron.2019.11.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramirez-Villegas JF, Logothetis NK, Besserve M. 2015. Diversity of sharp-wave-ripple LFP signatures reveals differentiated brain-wide dynamical events. Proc. Natl Acad. Sci. USA 112, E6379–E6387. ( 10.1073/pnas.1518257112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Skelin I, Kilianski S, McNaughton BL. 2019. Hippocampal coupling with cortical and subcortical structures in the context of memory consolidation. Neurobiol. Learn. Mem. 160, 21–31. ( 10.1016/j.nlm.2018.04.004) [DOI] [PubMed] [Google Scholar]
- 82.Todorova R, Zugaro M. 2020. Hippocampal ripples as a mode of communication with cortical and subcortical areas. Hippocampus 30, 39–49. ( 10.1002/hipo.22997) [DOI] [PubMed] [Google Scholar]
- 83.Mesulam MM, Mufson EJ, Wainer BH, Levey AI. 1983. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience 10, 1185–1201. ( 10.1016/0306-4522(83)90108-2) [DOI] [PubMed] [Google Scholar]
- 84.Mesulam MM, Mufson EJ, Levey AI, Wainer BH. 1983. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J. Comp. Neurol. 214, 170–197. ( 10.1002/cne.902140206) [DOI] [PubMed] [Google Scholar]
- 85.Everitt BJ, Robbins TW. 1997. Central cholinergic systems and cognition. Annu. Rev. Psychol. 48, 649–684. ( 10.1146/annurev.psych.48.1.649) [DOI] [PubMed] [Google Scholar]
- 86.Newman EL, Gupta K, Climer JR, Monaghan CK, Hasselmo ME. 2012. Cholinergic modulation of cognitive processing: insights drawn from computational models. Front. Behav. Neurosci. 6, 24 ( 10.3389/fnbeh.2012.00024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Destexhe A, Rudolph M, Paré D. 2003. The high-conductance state of neocortical neurons in vivo. Nat. Rev. Neurosci. 4, 739–751. ( 10.1038/nrn1198) [DOI] [PubMed] [Google Scholar]
- 88.McGinley MJ, David SV, McCormick DA. 2015. Cortical membrane potential signature of optimal states for sensory signal detection. Neuron 87, 179–192. ( 10.1016/j.neuron.2015.05.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vinck M, Batista-Brito R, Knoblich U, Cardin JA. 2015. Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron 86, 740–754. ( 10.1016/j.neuron.2015.03.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beaman CB, Eagleman SL, Dragoi V. 2017. Sensory coding accuracy and perceptual performance are improved during the desynchronized cortical state. Nat. Commun. 8, 1308–1314. ( 10.1038/s41467-017-01030-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jacobs EAK, Steinmetz NA, Carandini M, Harris KD. 2018. Cortical state fluctuations during sensory decision making. bioRxiv 348193 ( 10.1101/348193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marguet SL, Harris KD. 2011. State-dependent representation of amplitude-modulated noise stimuli in rat auditory cortex. J. Neurosci. 31, 6414–6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Steriade M, Amzica F, Contreras D. 1996. Synchronization of fast (30–40 Hz) spontaneous cortical rhythms during brain activation. J. Neurosci. 16, 392–417. ( 10.1523/JNEUROSCI.16-01-00392.1996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zaborszky L, Csordas A, Mosca K, Kim J, Gielow MR, Vadasz C, Nadasdy Z. 2015. Neurons in the basal forebrain project to the cortex in a complex topographic organization that reflects corticocortical connectivity patterns: an experimental study based on retrograde tracing and 3D reconstruction. Cereb. Cortex 25, 118–137. ( 10.1093/cercor/bht210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gielow MR, Zaborszky L. 2017. The input-output relationship of the cholinergic basal forebrain. CellReports 18, 1817–1830. ( 10.1016/j.celrep.2017.01.060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thiele A, Bellgrove MA. 2018. Neuromodulation of Attention. Neuron 97, 769–785. ( 10.1016/j.neuron.2018.01.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hasselmo ME, Sarter M. 2011. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology 36, 52–73. ( 10.1038/npp.2010.104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hasselmo ME. 2006. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 16, 710–715. ( 10.1016/j.conb.2006.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dutar P, Bassant MH, Senut MC, Lamour Y. 1995. The septohippocampal pathway: structure and function of a central cholinergic system. Physiol. Rev. 75, 393–427. ( 10.1152/physrev.1995.75.2.393) [DOI] [PubMed] [Google Scholar]
- 100.Teles-Grilo Ruivo LM, Mellor JR. 2013. Cholinergic modulation of hippocampal network function. Front. Synaptic Neurosci. 5, 2 ( 10.3389/fnsyn.2013.00002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zylla MM, Zhang X, Reichinnek S, Draguhn A, Both M. 2013. Cholinergic plasticity of oscillating neuronal assemblies in mouse hippocampal slices. PLoS ONE 8, e80718-11 ( 10.1371/journal.pone.0080718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vandecasteele M, Varga V, Berényi A, Papp E, Barthó P, Venance L, Freund TF, Buzsáki G. 2014. Optogenetic activation of septal cholinergic neurons suppresses sharp wave ripples and enhances theta oscillations in the hippocampus. Proc. Natl Acad. Sci. USA 111, 13 535–13 540. ( 10.1073/pnas.1411233111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Girardeau G, Zugaro M. 2011. Hippocampal ripples and memory consolidation. Curr. Opin. Neurobiol. 21, 452–459. ( 10.1016/j.conb.2011.02.005) [DOI] [PubMed] [Google Scholar]
- 104.Tarder-Stoll H, Jayakumar M, Dimsdale-Zucker HR, Günseli E, Aly M. 2020. Dynamic internal states shape memory retrieval. Neuropsychologia 138, 107328 ( 10.1016/j.neuropsychologia.2019.107328) [DOI] [PubMed] [Google Scholar]
- 105.Hasselmo ME, McGaughy J. 2004. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Prog. Brain Res. 145, 207–231. ( 10.1016/S0079-6123(03)45015-2) [DOI] [PubMed] [Google Scholar]
- 106.Zaborszky L. 2002. The modular organization of brain systems. Basal forebrain: the last frontier. Prog. Brain Res. 136, 359–372. ( 10.1016/s0079-6123(02)36030-8) [DOI] [PubMed] [Google Scholar]
- 107.Ballinger EC, Ananth M, Talmage DA, Role LW. 2016. Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron 91, 1199–1218. ( 10.1016/j.neuron.2016.09.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Runfeldt MJ, Sadovsky AJ, MacLean JN. 2014. Acetylcholine functionally reorganizes neocortical microcircuits. J. Neurophysiol. 112, 1205–1216. ( 10.1152/jn.00071.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Soma S, Shimegi S, Osaki H, Sato H. 2012. Cholinergic modulation of response gain in the primary visual cortex of the macaque. J. Neurophysiol. 107, 283–291. ( 10.1152/jn.00330.2011) [DOI] [PubMed] [Google Scholar]
- 110.Thiele A, Herrero JL, Distler C, Hoffmann K-P. 2012. Contribution of cholinergic and GABAergic mechanisms to direction tuning, discriminability, response reliability, and neuronal rate correlations in macaque middle temporal area. J. Neurosci. 32, 16 602–16 615. ( 10.1523/JNEUROSCI.0554-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shine JM. 2019. Neuromodulatory influences on integration and segregation in the brain. Trends Cogn. Sci. 23, 572–583. ( 10.1016/j.tics.2019.04.002) [DOI] [PubMed] [Google Scholar]
- 112.Fox KCR, Andrews-Hanna JR, Christoff K. 2016. The neurobiology of self-generated thought from cells to systems: integrating evidence from lesion studies, human intracranial electrophysiology, neurochemistry, and neuroendocrinology. Neuroscience 335, 134–150. ( 10.1016/j.neuroscience.2016.08.020) [DOI] [PubMed] [Google Scholar]
- 113.Nielsen TA. 2000. A review of mentation in REM and NREM sleep: ‘Covert’ REM sleep as a possible reconciliation of two opposing models. Behav. Brain Sci. 23, 851–866, discussion 904–1121 ( 10.1017/s0140525x0000399x) [DOI] [PubMed] [Google Scholar]
- 114.Stickgold R, Malia A, Fosse R, Hobson JA. 2001. Brain-mind states: I. Longitudinal field study of sleep/wake factors influencing mentation report length. Sleep 24, 171–179. ( 10.1093/sleep/24.2.171) [DOI] [PubMed] [Google Scholar]
- 115.Siclari F, Baird B, Perogamvros L, Bernardi G, LaRocque JJ, Riedner B, Boly M, Postle BR, Tononi G. 2017. The neural correlates of dreaming. Nat. Neurosci. 20, 872–878. ( 10.1038/nn.4545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang J, Wamsley EJ. 2019. EEG predictors of dreaming outside of REM sleep. Psychophysiology 56, e13368 ( 10.1111/psyp.13368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nir Y, Tononi G. 2010. Dreaming and the brain: from phenomenology to neurophysiology. Trends Cogn. Sci. 14, 88–100. ( 10.1016/j.tics.2009.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jones BE. 2019. Arousal and sleep circuits. Neuropsychopharmacology 45, 6–20. ( 10.1038/s41386-019-0444-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Samuels ER, Szabadi E. 2008. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organisation. Curr. Neuropharmacol. 6, 235–253. ( 10.2174/157015908785777229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bouret S, Sara SJ. 2005. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 28, 574–582. ( 10.1016/j.tins.2005.09.002) [DOI] [PubMed] [Google Scholar]
- 121.Sara SJ, Bouret S. 2012. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron 76, 130–141. ( 10.1016/j.neuron.2012.09.011) [DOI] [PubMed] [Google Scholar]
- 122.Aston-Jones G, Cohen JD. 2005. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450. ( 10.1146/annurev.neuro.28.061604.135709) [DOI] [PubMed] [Google Scholar]
- 123.Haq U, Liotta R, Kovacs A, Rösler R, Jarosch MJ, Heinemann U, Behrens CJ. 2012. Adrenergic modulation of sharp wave-ripple activity in rat hippocampal slices. Hippocampus 22, 516–533. ( 10.1002/hipo.20918) [DOI] [PubMed] [Google Scholar]
- 124.Ul Haq R, Anderson M, Liotta A, Shafiq M, Sherkheli MA, Heinemann U. 2016. Pretreatment with β-adrenergic receptor agonists facilitates induction of LTP and sharp wave ripple complexes in rodent hippocampus. Hippocampus 26, 1486–1492. ( 10.1002/hipo.22665) [DOI] [PubMed] [Google Scholar]
- 125.Dahl D, Sarvey JM. 1989. Norepinephrine induces pathway-specific long-lasting potentiation and depression in the hippocampal dentate gyrus. Proc. Natl Acad. Sci. USA 86, 4776–4780. ( 10.1073/pnas.86.12.4776) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Harley CW. 2007. Norepinephrine and the dentate gyrus. Prog. Brain Res. 163, 299–318. [DOI] [PubMed] [Google Scholar]
- 127.Sara SJ. 2009. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 10, 211–223. ( 10.1038/nrn2573) [DOI] [PubMed] [Google Scholar]
- 128.Kaufman AM, Geiller T, Losonczy A. 2020. A role for the locus coeruleus in hippocampal ca1 place cell reorganization during spatial reward learning. Neuron 105, 1026.e4 ( 10.1016/j.neuron.2019.12.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tronel S, Feenstra MGP, Sara SJ. 2004. Noradrenergic action in prefrontal cortex in the late stage of memory consolidation. Learn. Mem. 11, 453–458. ( 10.1101/lm.74504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Eschenko O, Sara SJ. 2008. Learning-dependent, transient increase of activity in noradrenergic neurons of locus coeruleus during slow wave sleep in the rat: brain stem–cortex interplay for memory consolidation? Cereb. Cort. 18, 2596–2603. ( 10.1093/cercor/bhn020) [DOI] [PubMed] [Google Scholar]
- 131.Swift KM, et al. 2018. Abnormal locus coeruleus sleep activity alters sleep signatures of memory consolidation and impairs place cell stability and spatial memory. Curr. Biol. 28, 3599–3609; e4. ( 10.1016/j.cub.2018.09.054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mather M, Clewett D, Sakaki M, Harley CW. 2016. Norepinephrine ignites local hotspots of neuronal excitation: how arousal amplifies selectivity in perception and memory. Behav. Brain Sci. 39, e200 ( 10.1017/S0140525X15000667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Diekelmann S, Born J. 2010. The memory function of sleep. Nat. Rev. Neurosci. 11, 114–126. ( 10.1038/nrn2762) [DOI] [PubMed] [Google Scholar]
- 134.Rasch B, Born J. 2013. About sleep's role in memory. Physiol. Rev. 93, 681–766. ( 10.1152/physrev.00032.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sara SJ. 2010. Reactivation, retrieval, replay and reconsolidation in and out of sleep: connecting the dots. Front. Behav. Neurosci. 4, 1–5. ( 10.3389/fnbeh.2010.00185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Eschenko O, Magri C, Panzeri S, Sara SJ. 2012. Noradrenergic neurons of the locus coeruleus are phase locked to cortical up-down states during sleep. Cereb. Cortex 22, 426–435. ( 10.1093/cercor/bhr121) [DOI] [PubMed] [Google Scholar]
- 137.Novitskaya Y, Sara SJ, Logothetis NK, Eschenko O. 2016. Ripple-triggered stimulation of the locus coeruleus during post-learning sleep disrupts ripple/spindle coupling and impairs memory consolidation. Learn. Mem. 23, 238–248. ( 10.1101/lm.040923.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Haider B, McCormick DA. 2009. Rapid neocortical dynamics: cellular and network mechanisms. Neuron 62, 171–189. ( 10.1016/j.neuron.2009.04.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shine JM, van den Brink RL, Hernaus D, Nieuwenhuis S, Poldrack RA. 2018. Catecholaminergic manipulation alters dynamic network topology across cognitive states. Netw. Neurosci. 2, 381–396. ( 10.1162/netn_a_00042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Azmitia EC, Segal M. 1978. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J. Comp. Neurol. 179, 641–667. ( 10.1002/cne.901790311) [DOI] [PubMed] [Google Scholar]
- 141.Hornung J-P. 2003. The human raphe nuclei and the serotonergic system. J. Chem. Neuroanat. 26, 331–343. ( 10.1016/j.jchemneu.2003.10.002) [DOI] [PubMed] [Google Scholar]
- 142.Okaty BW, Commons KG, Dymecki SM. 2019. Embracing diversity in the 5-HT neuronal system. Nat. Rev. Neurosci. 20, 397–424. ( 10.1038/s41583-019-0151-3) [DOI] [PubMed] [Google Scholar]
- 143.Scarr E, Gibbons AS, Neo J, Udawela M, Dean B. 2013. Cholinergic connectivity: its implications for psychiatric disorders. Front. Cell. Neurosci. 7, 55 ( 10.3389/fncel.2013.00055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Puig MV, Gulledge AT. 2011. Serotonin and prefrontal cortex function: neurons, networks, and circuits. Mol. Neurobiol. 44, 449–464. ( 10.1007/s12035-011-8214-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ul Haq R, Anderson ML, Hollnagel J-O, Worschech F, Sherkheli MA, Behrens CJ, Heinemann U. 2016. Serotonin dependent masking of hippocampal sharp wave ripples. Neuropharmacology 101, 188–203. ( 10.1016/j.neuropharm.2015.09.026) [DOI] [PubMed] [Google Scholar]
- 146.Wang DV, Yau H-J, Broker CJ, Tsou J-H, Bonci A, Ikemoto S. 2015. Mesopontine median raphe regulates hippocampal ripple oscillation and memory consolidation. Nat. Neurosci. 18, 728–735. ( 10.1038/nn.3998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lanfumey L, Hamon M. 2000. Central 5-HT1A Receptors: regional distribution and functional characteristics. Nucl. Med. Biol. 27, 429–435. ( 10.1016/S0969-8051(00)00107-4) [DOI] [PubMed] [Google Scholar]
- 148.Palomero-Gallagher N, Kedo O, Mohlberg H, Zilles K, Amunts K. 2020. Multimodal mapping and analysis of the cyto- and receptorarchitecture of the human hippocampus. Brain Struct. Funct. 225, 881–907. ( 10.1007/s00429-019-02022-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Barnes NM, Sharp T. 1999. A review of central 5-HT receptors and their function. Neuropharmacology 38, 1083–1152. [DOI] [PubMed] [Google Scholar]
- 150.Nichols DE. 2016. Psychedelics. Pharmacol. Rev. 68, 264–355. ( 10.1124/pr.115.011478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nichols DE. 2004. Hallucinogens. Pharmacol. Ther. 101, 131–181. ( 10.1016/j.pharmthera.2003.11.002) [DOI] [PubMed] [Google Scholar]
- 152.Carhart-Harris RL, Friston KJ. 2019. REBUS and the anarchic brain: toward a unified model of the brain action of psychedelics. Pharmacol. Rev. 71, 316–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Carhart-Harris RL, Leech R, Hellyer PJ, Shanahan M, Feilding A, Tagliazucchi E, Chialvo DR, Nutt D. 2014. The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front. Hum. Neurosci. 8, 20 ( 10.3389/fnhum.2014.00020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lisman JE, Grace AA. 2005. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron 46, 703–713. ( 10.1016/j.neuron.2005.05.002) [DOI] [PubMed] [Google Scholar]
- 155.Shohamy D, Adcock RA. 2010. Dopamine and adaptive memory. Trends Cogn. Sci. 14, 464–472. ( 10.1016/j.tics.2010.08.002) [DOI] [PubMed] [Google Scholar]
- 156.Grace AA. 2016. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 17, 524–532. ( 10.1038/nrn.2016.57) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Duszkiewicz AJ, McNamara CG, Takeuchi T, Genzel L. 2019. Novelty and dopaminergic modulation of memory persistence: a tale of two systems. Trends Neurosci. 42, 102–114. ( 10.1016/j.tins.2018.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER. 2016. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc. Natl Acad. Sci. USA 113, 14 835–14 840. ( 10.1073/pnas.1616515114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Takeuchi T, et al. 2016. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537, 357–362. ( 10.1038/nature19325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Miyawaki T, Norimoto H, Ishikawa T, Watanabe Y, Matsuki N, Ikegaya Y. 2014. Dopamine receptor activation reorganizes neuronal ensembles during hippocampal sharp waves in vitro. PLoS ONE 9, e104438-13 ( 10.1371/journal.pone.0104438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McNamara CG, Tejero-Cantero Á, Trouche S, Campo-Urriza N, Dupret D. 2014. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat. Neurosci. 17, 1658–1660. ( 10.1038/nn.3843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Singer AC, Frank LM. 2009. Rewarded outcomes enhance reactivation of experience in the hippocampus. Neuron 64, 910–921. ( 10.1016/j.neuron.2009.11.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Atherton LA, Dupret D, Mellor JR. 2015. Memory trace replay: the shaping of memory consolidation by neuromodulation. Trends Neurosci. 38, 560–570. ( 10.1016/j.tins.2015.07.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gomperts SN, Kloosterman F, Wilson MA. 2015. VTA neurons coordinate with the hippocampal reactivation of spatial experience. eLife 4, 321 ( 10.7554/eLife.05360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Metzinger TK. 2017. The problem of mental action. In Philosophy and predicitive processing (eds Metzinger TK, Wiese W). Frankfurt am Main, Germany: MIND Group; ( 10.15502/9783958573208) [DOI] [Google Scholar]
- 166.Shepherd J. 2019. Why does the mind wander? Neurosci. Conscious 2019, 242–249. ( 10.1093/nc/niz014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Edeline J-M. 2012. Beyond traditional approaches to understanding the functional role of neuromodulators in sensory cortices. Front. Behav. Neurosci. 6, 45 ( 10.3389/fnbeh.2012.00045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Joshi S, Li Y, Kalwani RM, Gold JI. 2016. Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron 89, 221–234. ( 10.1016/j.neuron.2015.11.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.McGinley MJ, Vinck M, Reimer J, Batista-Brito R, Zagha E, Cadwell CR, Tolias AS, Cardin JA, McCormick DA. 2015. Waking state: rapid variations modulate neural and behavioral responses. Neuron 87, 1143–1161. ( 10.1016/j.neuron.2015.09.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mittner M, Boekel W, Tucker AM, Turner BM, Heathcote A, Forstmann BU. 2014. When the brain takes a break: a model-based analysis of mind wandering. J. Neurosci. 34, 16 286–16 295. ( 10.1523/JNEUROSCI.2062-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Unsworth N, Robison MK. 2016. Pupillary correlates of lapses of sustained attention. Cogn. Affect. Behav. Neurosci. 16, 601–615. ( 10.3758/s13415-016-0417-4) [DOI] [PubMed] [Google Scholar]
- 172.Unsworth N, Robison MK. 2017. A locus coeruleus-norepinephrine account of individual differences in working memory capacity and attention control. Psychon. Bull. Rev. 24, 1282–1311. ( 10.3758/s13423-016-1220-5) [DOI] [PubMed] [Google Scholar]
- 173.Konishi M, Brown K, Battaglini L, Smallwood J. 2017. When attention wanders: pupillometric signatures of fluctuations in external attention. Cognition 168, 16–26. ( 10.1016/j.cognition.2017.06.006) [DOI] [PubMed] [Google Scholar]
- 174.Unsworth N, Robison MK. 2018. Tracking arousal state and mind wandering with pupillometry. Cogn. Affect. Behav. Neurosci. 18, 638–664. ( 10.3758/s13415-018-0594-4) [DOI] [PubMed] [Google Scholar]
- 175.Andrillon T, Windt J, Silk T, Drummond SPA, Bellgrove MA, Tsuchiya N. 2019. Does the mind wander when the brain takes a break? Local sleep in wakefulness, attentional lapses and mind-wandering. Front. Neurosci. 13, 949 ( 10.3389/fnins.2019.00949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ward AF, Wegner DM. 2013. Mind-blanking: when the mind goes away. Front. Psychol. 4, 650 ( 10.3389/fpsyg.2013.00650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.de Rooij A, Vromans RD, Dekker M. 2018. Noradrenergic modulation of creativity: evidence from pupillometry. Creativity Res. J. 30, 339–351. ( 10.1080/10400419.2018.1530533) [DOI] [Google Scholar]
- 178.Beversdorf DQ. 2019. Neuropsychopharmacological regulation of performance on creativity-related tasks. Curr. Opin. Behav. Sci. 27, 55–63. ( 10.1016/j.cobeha.2018.09.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Beaty RE, Benedek M, Silvia PJ, Schacter DL. 2016. Creative cognition and brain network dynamics. Trends Cogn. Sci. 20, 87–95. ( 10.1016/j.tics.2015.10.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Beaty RE, Benedek M, Kaufman SB, Silvia PJ. 2015. Default and executive network coupling supports creative idea production. Sci. Rep. 5, 10964 ( 10.1038/srep10964) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Spitzer M, Thimm M, Hermle L, Holzmann P, Kovar KA, Heimann H, Gouzoulis-Mayfrank E, Kischka U, Schneider F. 1996. Increased activation of indirect semantic associations under psilocybin. Biol. Psychiatry 39, 1055–1057. ( 10.1016/0006-3223(95)00418-1) [DOI] [PubMed] [Google Scholar]
- 182.Carhart-Harris RL, Kaelen M, Whalley MG, Bolstridge M, Feilding A, Nutt DJ. 2015. LSD enhances suggestibility in healthy volunteers. Psychopharmacology (Berl.) 232, 785–794. ( 10.1007/s00213-014-3714-z) [DOI] [PubMed] [Google Scholar]
- 183.Carhart-Harris RL, et al. 2012. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc. Natl Acad. Sci. USA 109, 2138–2143. ( 10.1073/pnas.1119598109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Muthukumaraswamy SD, et al. 2013. Broadband cortical desynchronization underlies the human psychedelic state. J. Neurosci. 33, 15 171–15 183. ( 10.1523/JNEUROSCI.2063-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kraehenmann R, Pokorny D, Vollenweider L, Preller KH, Pokorny T, Seifritz E, Vollenweider FX. 2017. Dreamlike effects of LSD on waking imagery in humans depend on serotonin 2A receptor activation. Psychopharmacology (Berl.) 234, 2031–2046. ( 10.1007/s00213-017-4610-0) [DOI] [PubMed] [Google Scholar]
- 186.Cisek P. 2019. Resynthesizing behavior through phylogenetic refinement. Atten. Percept. Psychophys. 81, 2265–2287. ( 10.3758/s13414-019-01760-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tolman EC. 1948. Cognitive maps in rats and men. Psychol. Rev. 55, 189. [DOI] [PubMed] [Google Scholar]
- 188.Behrens TEJ, Muller TH, Whittington JCR, Mark S, Baram AB, Stachenfeld KL, Kurth-Nelson Z. 2018. What is a cognitive map? Organizing knowledge for flexible behavior. Neuron 100, 490–509. ( 10.1016/j.neuron.2018.10.002) [DOI] [PubMed] [Google Scholar]
- 189.Wikenheiser AM, Redish AD. 2015. Decoding the cognitive map: ensemble hippocampal sequences and decision making. Curr. Opin. Neurobiol. 32, 8–15. ( 10.1016/j.conb.2014.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kaplan R, Schuck NW, Petro L, Doeller CF. 2017. The role of mental maps in decision-making. Trends Neurosci. 40, 256–259. ( 10.1016/j.tins.2017.03.002) [DOI] [PubMed] [Google Scholar]
- 191.Brezina V. 2010. Beyond the wiring diagram: signalling through complex neuromodulator networks. Phil. Trans. R. Soc. B 365, 2363–2374. ( 10.1098/rstb.2010.0105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Robbins TW, Roberts AC. 2007. Differential regulation of fronto-executive function by the monoamines and acetylcholine. Cereb. Cortex 17(Suppl. 1), i151–i160. ( 10.1093/cercor/bhm066) [DOI] [PubMed] [Google Scholar]
- 193.Dembrow N, Johnston D. 2014. Subcircuit-specific neuromodulation in the prefrontal cortex. Front. Neural Circuits 8, 54 ( 10.3389/fncir.2014.00054) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.