Abstract
Patients with CTCL are at increased risk for bacteremia which is a leading cause of morbidity and mortality. We assessed risk factors for and the impact of bacteremia on survival in a retrospective cohort of 188 CTCL patients at a single US academic institution treated between 1990 and 2018. With a median follow up of 6.2 years, 20% of patients (n = 36) developed 79 bacteremia events. Risk factors for bacteremia included advanced stage, female gender, African American (AA) race, invasive lines, and chemotherapy. Bacteremia was associated with an increased risk of death on univariate and multivariable models. Bacteremia is associated with an increased risk of death in patients with CTCL. The greatest avoidable risk factors included chemotherapy treatment and presence of an invasive line.
Keywords: CTCL, mycosis fungoides, sepsis, staphylococcus, bacteremia
Introduction
Infections are a major cause of morbidity, mortality, and resource utilization among patients with CTCL [1]. CTCL patients are particularly susceptible to cutaneous infection due to widespread disruptions in their skin barrier [1]. In particular, staphylococcal infections and colonization are common in patients with CTCL, with colonization present in up to 63% of patients [2,3]. In addition to contributing to localized and systemic infections, staphylococcus aureus also has an etiologic role in the development and progression of this malignancy [4,5]. S. aureus super-antigens promote tumor progression while aggressive, transient antibiotic treatment results in clinical improvement and regression of CTCL as evidenced by inhibition of malignant T cells in lesional skin [3,5,6]. Areas of cracked skin, ulceration, and tumors predispose to cutaneous infections and may contribute to increased risk for subsequent systemic infection. Furthermore, patients with CTCL may have reduced immunity due to disease-and treatment-related factors. The tumor microenvironment in the skin inhibits local immune response with a shift toward an increasingly immunosuppressive TME to with advancing stage [7]. Similarly, patients with Sézary syndrome have reduction in functional effector responses for viral immunity [8,9]. Chemotherapy and novel agents further diminish immune response through reduction of neutrophils and lymphocytes [10,11].
While the risks and incidence of cutaneous infections have been reported, there is very limited data on risks of bacteremia in CTCL patients [1,12,13]. Bacteremia is an important contributor to death and hospitalizations in this vulnerable population. Prior data has demonstrated that infection was leading cause to death in up to 60% of patients when accompanied by a gram negative superinfection [13]. Yet a comprehensive assessment of infections including bacteremia has not been published in over 20 years [1,13]. There is also limited guidance on best practices regarding reducing the risk for bacteremia or the appropriate use of prophylactic antibiotics. The primary purpose of this project was assess risk factors for bacteremia among CTCL patients and to determine the impact of bacteremia on patient outcomes.
Methods
Data collection
We conducted a single center retrospective analysis at the Winship Cancer Institute of Emory University. The study was performed according to the Declaration of Helsinki protocol, and only healthcare data that are routinely used for clinical reasons in CTCL patients. We selected patients from an existing cutaneous lymphoma database diagnosed with CTCL between 2 January 1990 and June 1, 2019. Patients without at least 2 visits with a provider in dermatology or hematology/oncology were excluded. We assessed for the presence or absence of bacteremia at any point following diagnosis. Bacteremia was defined as identification of at least one microbe in the bloodstream on culture. Baseline demographics included gender, ethnicity/race, age, and diagnosis. Disease and treatment related factors included diagnosis and date of diagnosis, stage by International Society of Cutaneous Lymphoma (ISCL), treatment, and timing of treatment, use of skin prophylactic antibiotics and outpatient dermatology consult at any time. Prophylactic skin antibiotics were defined in the notes as ‘prophylactic’ i.e. not being used to treat active cellulitis or other infections. CTCL treatment included phototherapy, topical nitrogen mustard (NM), topical corticosteroids (CS), chemotherapy, romidepsin, vorinostat, mogamulizumab, methotrexate, brentuximab, interferon (INF), extracorporeal photopheresis (ECP), oral retinoids, total skin electron beam (TSEB) therapy, and radiation therapy (RT).
Infection characteristics were also recorded for both cutaneous cultures and bacteremia. These included culture data, sensitivities, symptoms, antibiotic treatment and response. Symptomatic bacteremias were defined as patients with symptoms which required hospitalization due to the bacteremia which included a fever greater than 38 Celsius, hypotension, chills, or wound infection. To assess risk factors, we assessed presence of invasive devices (central venous catheter, peripherally inserted central catheters (PICC), and hardware) placed prior to bacteremia, duration of hospitalization before bacteremia and laboratory values at admission.
Additionally, we explored patterns of skin cultures and concurrent bacteremia, antibiotic use in the outpatient setting and their association with bacteremia incidence and outcomes.
Statistics
Associations between clinical and biological parameters and the first infectious event (yes, no) were investigated using chi-square tests, Fisher’s exact tests, or ANOVA, where appropriate. Univariate analysis was used to analyze each factor of interest for each different outcome using logistic regression for binary outcomes and Cox proportional hazards models for time-to-event outcomes. Multivariable analysis was then conducted with selected factors. Associations with p < .05 were deemed statistically significant. Overall survival (OS) was defined as time from diagnosis to death or last follow-up, where those alive at last follow-up were censored. Bacteremia-free survival was defined as time from diagnosis to bacteremia or last follow-up, where those without bacteremia at last follow-up were censored. Survival distributions were estimated using the Kaplan Meier method, and survival was compared using log-rank tests. All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Patient characteristics
Among 548 patients in our institutional cutaneous lymphoma database, 180 patients with complete follow up were included in this analysis. Patients were excluded for incomplete data (221), pathologic review only (83), or limited follow up of less than 1 year (64).
In the overall population the median follow up time was 6.2 years (range 0.1–28 years) there was equal male: female predominance, 48.3% were African American (AA) and 51.7% were Caucasian or Hispanic. Mycosis fungoides (MF) was the most common diagnosis (75%); 10% had SS, with 15.0% other CTCL diagnoses. The stage at diagnosis early stage (1–2 A) in 48.8% and advanced (2 b–4) in 51.2%, and missing in 12 patients; 53.4% of patients received oral antibiotics for prophylaxis of skin infection.
Thirty-six of 180 patients (20.0%) developed at least one episode of bacteremia, with 80 episodes recorded (range 0–10 per patient). Fifteen patients had one episode of bacteremia and 21 had > 1 episode. The baseline patient characteristics by group for 144 without bacteremia and 36 with are presented in Table 1. By subgroup, the median age at diagnosis was 57.5 in the non-bacteremia patients and 49.5 in those who developed bacteremia. More patients who developed bacteremia were AA race. There were also different distributions by stage and patterns of treatment in the bacteremia vs. no bacteremia groups.
Table 1.
Patient characteristics.
| Variable | Level | N=180 | % |
|---|---|---|---|
| Age at diagnosis | Median (range) | 56.5 (12–95) | |
| Sex | Female | 86 | 47.8 |
| Male | 94 | 52.2 | |
| Race | White/Hispanic | 93 | 51.7 |
| Black | 87 | 48.3 | |
| Diagnosis | MF | 135 | 75.0 |
| SS | 18 | 10.0 | |
| CD30+ | 6 | 3.3 | |
| NOS | 12 | 6.7 | |
| Other/Unknown | 9 | 5.0 | |
| Stage | 1 | 49 | 29.2 |
| 2A | 33 | 19.6 | |
| 2B | 41 | 24.4 | |
| 3A-B | 15 | 8.9 | |
| 4A-B | 30 | 17.9 | |
| Missing | 12 | - | |
| Bacteremia | No | 144 | 80.0 |
| Yes | 36 | 20.0 | |
| Bacteremia episodes | Range | 0–10 | |
| Skin prophylaxis | No | 82 | 46.6 |
| Yes | 94 | 53.4 | |
| Missing | 4 | - | |
| Treatment | |||
| Chemotherapy | 106 | 73.1 | |
| Romidepsin | 24 | 13.3 | |
| Vorinostat | 40 | 22.2 | |
| Mogamulizumab | 12 | 6.7 | |
| Methotrexate | 32 | 17.8 | |
| Brentuximab | 16 | 8.9 | |
| IFN | 51 | 28.3 | |
| ECP | 24 | 13.3 | |
| Oral retinoids | 89 | 49.4 | |
| Topical CS | 35 | 19.6 | |
| Topical NM | 60 | 33.5 | |
| Phototherapy (UVB or PUVA) | 62 | 34.4 | |
| Outpatient dermatology consult | 113 | 73.9 | |
| TSEB | 65 | 36.3 | |
| Localized RT | 55 | 30.7 | |
| Device at any time* | Yes | 29 | 16.1 |
| Type of line | Port | 6 | 13.6 |
| PICC | 26 | 59.1 | |
| CVC | 7 | 15.9 | |
| Other tunneled line | 5 | 11.4 | |
| Missing | 136 | - | |
| Length of line | ≤30 days | 21 | 53.8 |
| >30 days | 18 | 46.2 | |
| Missing | 141 | - | |
MF: mycosis fungoides; SS: Sézary syndrome; CTCL: NOS cutaneous T-cell lymphoma not otherwise specified; IFN: interferon; ECP: extracorporeal photopheresis; NM: nitrogen mustard; UV: ultraviolet B or A; TSEB: total skin electron beam therapy; RT: radiation therapy; PICC: peripherally inserted central catheter; CVC: central venous catheter (non-tunneled).
Invasive line placed prior to first bacteremia event, or at any time in patients without bacteremia.
Factors associated with first incidence of bacteremia
We identified several factors associated with bacteremia incidence (Table 2). African American race and female gender were correlated with increased odds of bacteremia. Additional factors associated with increased odds of bacteremia included advanced stage, and treatment with chemotherapy, romidepsin, vorinostat, methotrexate, brentuximab vedotin, interferon, and total-skin electron beam therapy (TSEB). By far, the treatment associated with the greatest odds was chemotherapy with an odds ratio (OR) of 15.71, 95% CI 2.06–119.50; p = .008. Mogamulizumab, ECP, topical NM, phototherapy, and localized RT were not correlated with bacteremia. By stage, the greatest odds was identified in those with stage 3 disease vs. stage 1 (OR 17.52, 95% CI 3.73–82.31; p<.001). Stage 2 A and 4 A-B also had statistically significant increased odds compared to stage 1, whereas stage 2B vs. stage 1 had an odds ratio > 1, but did not meet statistical significance.
Table 2.
Odds of bacteremia.
| Covariate | Level | N | Odds ratio (95% CI) | OR p value |
|---|---|---|---|---|
| Sex | Male | 94 | 0.44 (0.21–0.94) | .033 |
| Female | 86 | - | - | |
| Race | Black | 87 | 2.57 (1.19–5.54) | .016 |
| White/Hispanic | 93 | - | - | |
| Age at diagnosis | > =65 | 51 | 0.55 (0.22–1.35) | .190 |
| <65 | 129 | - | - | |
| Stage | 4A-B | 30 | 5.58 (1.35–23.09) | .018 |
| 3A-B | 15 | 17.52 (3.73–82.31) | <.001 | |
| 2B | 41 | 3.72 (0.92–15.08) | .066 | |
| 2A | 33 | 5.75 (1.42–23.24) | .014 | |
| 1 | 49 | - | - | |
| Stage (early/late) | 2B-4 | 86 | 2.26 (1.04–4.89) | .039 |
| 1–2A | 82 | - | - | |
| Outpatient dermatology consult | Yes | 113 | 0.25 (0.11–0.56) | <.001 |
| No | 40 | - | - | |
| Chemotherapy | Yes | 106 | 15.71 (2.06–119.50) | .008 |
| Romidepsin | Yes | 24 | 4.43 (1.79–11.01) | .001 |
| Vorinostat | Yes | 40 | 3.40 (1.54–7.50) | .002 |
| Mogamulizumab | Yes | 12 | 3.16 (0.94–10.61) | .063 |
| Methotrexate | Yes | 32 | 2.58 (1.11–6.01) | .028 |
| Brentuximab | Yes | 16 | 3.62 (1.25–10.51) | .018 |
| Oral retinoids | Yes | 89 | 1.56 (0.75–3.28) | .235 |
| IFN | Yes | 51 | 2.89 (1.36–6.18) | .006 |
| ECP | Yes | 24 | 1.80 (0.68–4.75) | .233 |
| Topical CS | Yes | 35 | 0.20 (0.04–0.86) | .031 |
| Topical NM | Yes | 60 | 0.50 (0.21–1.18) | .113 |
| Phototherapy (UVB or PUVA) | Yes | 62 | 0.80 (0.37–1.76) | .583 |
| Localized RT | Yes | 55 | 1.58 (0.74–3.39) | .237 |
| TSEB | Yes | 65 | 3.15 (1.49–6.68) | .003 |
| Invasive linea | Yes | 29 | 5.47 (2.32–12.90) | <.001 |
| Type of line (at any point) | Other tunneled line | 5 | 0.25 (0.02–3.77) | .317 |
| CVC (non-tunneled) | 7 | 6.00 (0.42–85.23) | .186 | |
| PICC | 26 | 2.71 (0.44–16.75) | .282 | |
| Port | 6 | - | - | |
| Longest length of line | >30 days | 18 | 3.18 (0.78–12.94) | .106 |
| ≤30 days | 21 | - | - | |
| Skin prophylaxis | Yes | 94 | 21.64 (5.00–93.71) | <.001 |
| No | 82 | - | - |
MF: mycosis fungoides; SS: Sézary syndrome; CTCL: NOS cutaneous T-cell lymphoma not otherwise specified; IFN: interferon; ECP: extracorporeal photopheresis; NM: nitrogen mustard; UV: ultraviolet B or A; TSEB: total skin electron beam therapy; RT: radiation therapy; PICC: peripherally inserted central catheter; CVC: central venous catheter (non-tunneled).
Invasive line placed prior to first bacteremia event, or at any time in patients without bacteremia.
Presence of an invasive line was associated with increased odds of bacteremia, but the type of line (port, peripherally inserted central catheter or others) and length of line did not reach statistical significance. It is worth noting, however, that the median duration of an invasive line in patients who developed bacteremia was 40 days compared to only 7.5 in those who did not develop bacteremia.
Regular outpatient dermatology follow up was associated with a reduced odds of bacteremia (OR 0.25 95% CI 0.11–0.56, p<.001), as was treatment with corticosteroids.
Survival analysis
We assessed the impact of bacteremia on survival in our cohort in univariate and multivariable models (Table 3). Two patients were set to missing for OS, and 4 patients were set to missing for time to bacteremia, resulting in 178 and 176 patients respectively for the survival analysis. We found that age > 65, diagnosis, advanced stage, and development of bacteremia were associated with an increased risk of death on univariate analysis. On multivariable analysis controlling for age, diagnosis, stage, and race, only age and bacteremia remained significant. Outpatient dermatology follow up was not associated with survival. In this model, bacteremia was highly significant for risk of death with a hazard ratio of 6.73 95% CI 2.48–18.26; p<.001.
Table 3.
Univariate analysis for overall survival.
| Covariate | Level | N | Hazard Ratio (95% CI) | HR p value | Log-rank p value |
|---|---|---|---|---|---|
| Bacteremia | Yes | 36 | 3.32 (1.53–7.19) | .002 | .001 |
| No | 142 | - | - | ||
| Diagnosis | MF | 133 | 0.92 (0.27–3.15) | .900 | .047 |
| SS | 18 | 3.04 (0.72–12.77) | .129 | ||
| Other | 27 | - | - | ||
| Stage (early/late) | 2B-4 | 86 | 4.01 (1.65–9.75) | .002 | .001 |
| 1–2A | 80 | - | - | ||
| Race | Black | 87 | 0.51 (0.23–1.12) | .093 | .087 |
| White/Hispanic | 91 | - | - | ||
| Sex | Male | 93 | 1.22 (0.56–2.65) | .611 | .610 |
| Female | 85 | - | - | ||
| Age at diagnosis | ≥65 | 51 | 3.66 (1.65–8.13) | .001 | <.001 |
| <65 | 127 | - | - | ||
| Outpatient dermatology consult | Yes | 112 | 0.96 (0.35–2.66) | .944 | .945 |
| No | 40 | - | - |
By Kaplan–Meier assessment, overall survival was significantly shorter in patients who developed bacteremia (p = .0012) (Figure 1). Fourteen patients without (10%) and 12 patients with (33%) bacteremia died during follow up. The absolute difference in survival was 10.7% at five years. Accounting for stage (early vs. advanced) this difference remained (log rank p = .0003) and further stratified patients. Five-year survival was 100%, 91.7%, 81.8%, and 76.4% in those with no bacteremia and early stage, bacteremia and early stage, no bacteremia and advanced stage, and bacteremia and advanced stage, respectively. Additionally, time to bacteremia was shorter in patients with advanced stage vs. early stage (Figure 2). Five-year bacteremia free survival was 98.5% in those with stage 1–2 A disease compared to 68.3% in those with advanced stage.
Figure 1.
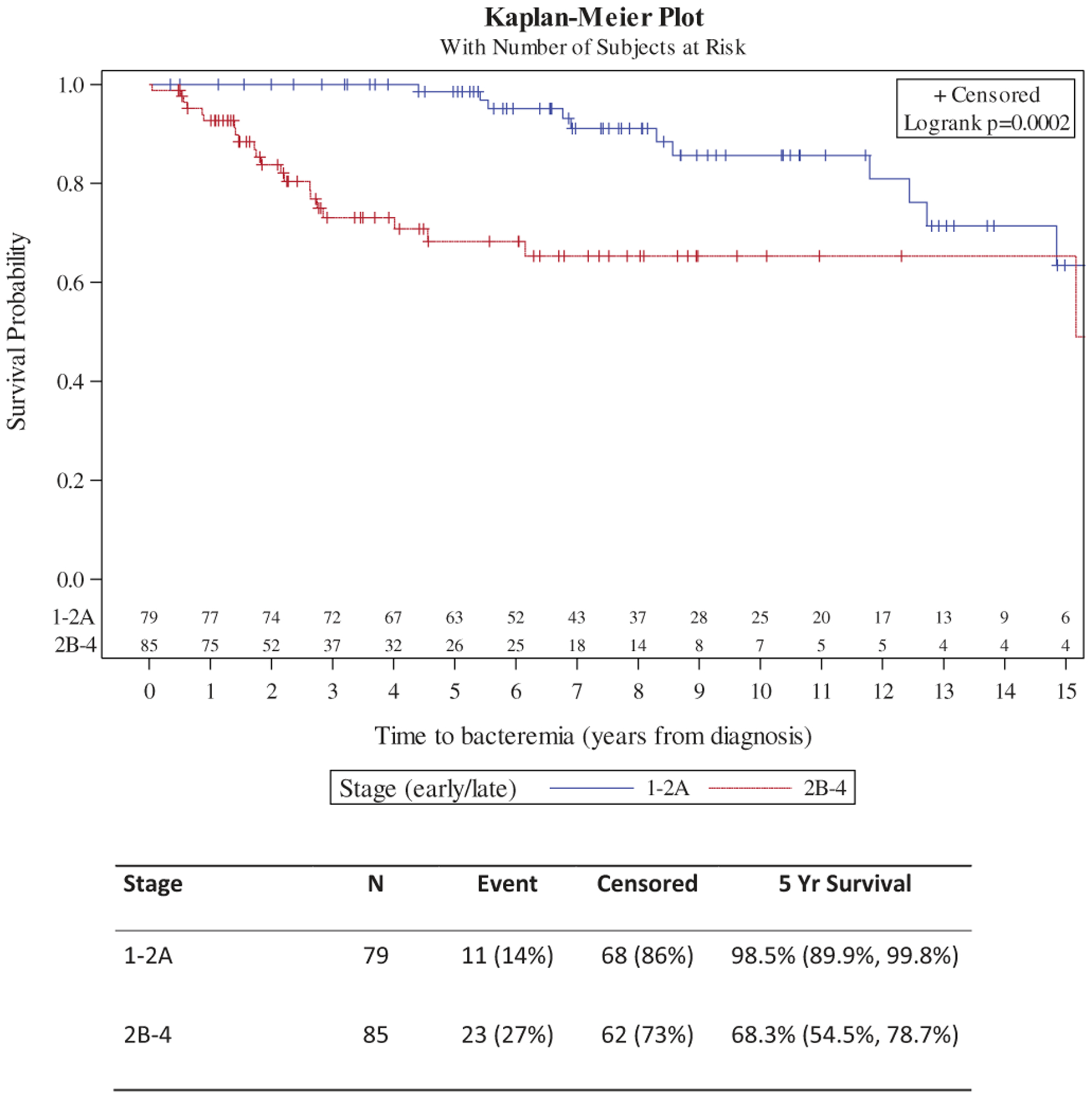
(a) Overall survival by bacteremia; (b) Overall survival by bacteremia and stage.
Figure 2.
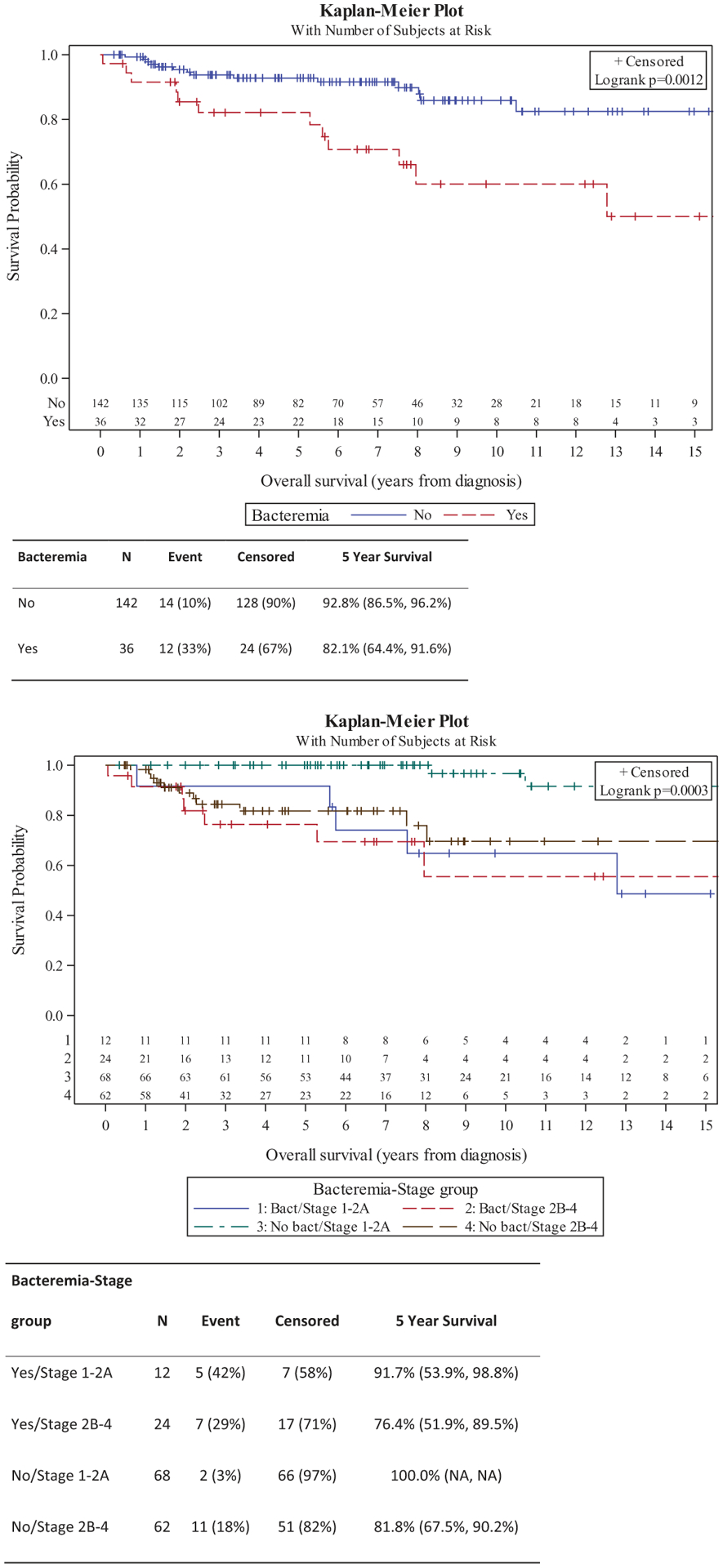
Time to bacteremia in early and advanced stage cutaneous T-cell lymphoma.
Skin prophylaxis and culture data
We additionally assessed use of oral prophylactic antibiotics in this population. We found that there was increased usage of oral antibiotic prophylaxis among those who developed bacteremia with 42% prophylaxis in those without bacteremia and 92% in those who did (p < .001). We also assessed skin culture data, and found that 31 patients had positive skin cultures prior to bacteremia and 38 negative cultures; 19 of the positive skin cultures matched subsequent bacteremia cultures and thus were likely related. For those on antibiotics prior to bacteremia with positive skin cultures, 21 were sensitive to the antibiotic prescribed and 8 were resistant.
Discussion
With a median follow up time of > 6 years, 20.0% of patients (n = 36) in our cohort developed 79 discrete episodes of bacteremia. Risk factors for bacteremia in our cohort included advanced stage, female gender, African American race, presence of an invasive line, and certain treatments, particularly chemotherapy. Bacteremia was associated with >15x increased risk of death accounting for stage and other baseline features.
The largest prior comprehensive review of infections in this population was published 28 years ago and there has been a single update in a small single center institution in recent years [1,12]. Our data are generally consistent these publications, identifying advanced stage and chemotherapy as risk factors for bacteremia [1,12,13]. In the largest series to date, 36 episodes of bacteremia were identified in 356 patients with MF/SS [1]. The authors noted that bacteremia cases were often nosocomial (77% of cases) and were a major infectious cause of death in this population. In our population, nosocomial infection was not the major source, with only 15 of 79 documented nosocomial cases in our population.
Also consistent with our report, multi-agent chemotherapy was associated with increased risk of bacteremia in 2 prior publications [1,13]. In our population, treatment with chemotherapy was associated with greater than 15x increased risk of bacteremia. Interestingly, this appears largely independent of the risk of neutropenia, as neutropenia is rare in this population [1,12]. Only 1 patient was neutropenic at the time of bacteremia in our study. That patient had stage 4B disease and prior treatment with methotrexate, romidepsin, chemotherapy, and was receiving carfilzomib at the time of infection. Similarly, 9.5% of patients receiving chemotherapy in the study by Axelrod et al. were neutropenic during chemotherapy. Many other systemic treatments and TSEB were associated with bacteremia. However, biologics and traditional therapy used for Sézary such as ECP, oral retinoids, and mogamulizumab were not. Given the risk of morbidity and mortality with bacteremia, these data may further support the use of these biologic therapies in appropriate patients prior to cytotoxic or immunosuppressive agents.
We found interesting patterns by disease stage. Patients with stage 3 comprised a minority of our population (n = 15), but may pose the greatest risk for bacteremia in our population compared to stage 1 with an odds ratio >17. Stage 2 A and 4 represented similar risks, while patients with patients with stage 2B disease did not reach statistical significance. We hypothesized the over-representation of stage 2 A, 3, and 4 among bacteremic patients may be related to erythroderma or extent of skin involvement, however, T stage did not predict for infection in our model (data not shown). It is possible that a more detailed analysis using percentage of body surface area or the modified severity weighted index would better assess this correlation. However, it is interesting to note that Axelrod et al. similarly reported increased risk of infection with advanced stage independent of T stage, suggesting that factors associated with advanced stage itself may predispose to infection [1].
We identified novel associations of African American race and female gender with increased risk of bacteremia. The reason for these associations are unclear. Other reports have noted aggressive presentations with more advanced disease in AA females, so it is possible that this correlation is related to a higher prevalence of advanced stage in this group [14]. Compared to other institutions, we have a higher proportion of AA patients. Our population consisted of nearly 50% AA patients, thus increasing our power to detect differences by race. Consistent with prior studies, age was not related to bacteremia but did predict for inferior survival [1,12,13].
As demonstrated in other reports, invasive lines were associated with increased risk of bacteremia. However, we lacked power to detect which types of lines were the highest risk. We noted that the median length of line was longer in those who developed bacteremia at 40 vs. 7.5 days in those who did not, however when adjusting for time before line placement this was 20 vs. 7.5 days. Of 47 lines in all cases of bacteremia, 29 were documented to be removed at the time of hospitalization.
We were interested in use of prophylactic antibiotics for skin infection as a mitigating factor for bacteremia. The increased administration of oral antibiotic prophylaxis among those who developed bacteremia likely suggests that clinicians were able to identify those at risk for infection. While the intricate role of staph aureus and antibiotics in disease progression has been recently elucidated, a much larger study would be necessary to assess its utility in preventing systemic infection [6]. In our cohort, there was a high rate of resistance to oral antibiotics, with matching multidrug-resistant infections in skin and blood cultures. The protective relationship of outpatient dermatology follow up was also of note. While this may be related to a skewing of lower stage patients, it would be interesting to assess whether improved skin care may also reduce risks of bacteremia in future analyses.
Our study had some limitations including those inherent to a single center retrospective study. The long interval of follow up including older data originating from 1990 increased the heterogeneity of treatment and data capture. We therefore had to exclude a large number of older cases due to incomplete data, while those diagnosed prior to 2000 were abstracted from paper records. Our definition of bacteremia included any patient with at least 1 positive blood culture. While we did assess whether patients were symptomatic it is possible some of these cases were due to contaminant rather than infection. It is worth noting that among 79 bacteremic episodes, 49 cases were symptomatic, but there was no difference in outcomes between the symptomatic and asymptomatic cases.
Overall, we provide a detailed update on risk factors for bacteremia in patients with CTCL and demonstrated its detrimental impact on overall survival. Altogether, 47 cases of bacteremia were associated with lines, 15 were nosocomial, and 19 were associated with prior skin infection or colonization. Based on these data we would recommend treatment with non-immune suppressing modalities such as biologics when possible, avoidance of central line placement and prompt removal when necessary. There remain gaps in knowledge regarding the best use of antibiotic prophylaxis and effective methods to prevent bacteremia and associated poor outcomes, however in absence of more robust data we would advise adequate skin care including regular dermatology follow up and appropriate treatment of colonization and infection in select patients.
KEY POINTS.
20% of patients developed bacteremia at any point in time in this analysis.
Bacteremia is associated with an increased risk of death in patients with CTCL
Risk factors for bacteremia include advanced stage, female gender, AA race, invasive line, and chemotherapy.
Funding
Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure statement
MJL reports personal fees from Kyowa Karin, personal fees from Soligenix, outside the submitted work; PA reports Advisory board from Imbrium and Bayer, outside the submitted work.
References
- [1].Axelrod PI, Lorber B, Vonderheid EC. Infections complicating mycosis fungoides and Sezary syndrome. JAMA. 1992;267(10):1354–1358. [PubMed] [Google Scholar]
- [2].Nguyen V, Huggins RH, Lertsburapa T, et al. Cutaneous T-cell lymphoma and Staphylococcus aureus colonization. J Am Acad Dermatol. 2008;59(6): 949–952. [DOI] [PubMed] [Google Scholar]
- [3].Talpur R, Bassett R, Duvic M. Prevalence and treatment of Staphylococcus aureus colonization in patients with mycosis fungoides and Sézary syndrome. Br J Dermatol. 2008;159(1):105–112. [DOI] [PubMed] [Google Scholar]
- [4].Willerslev-Olsen A, Krejsgaard T, Lindahl LM, et al. Staphylococcal enterotoxin A (SEA) stimulates STAT3 activation and IL-17 expression in cutaneous T-cell lymphoma. Blood. 2016;127(10):1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Blumel E, Willerslev-Olsen A, Gluud M, et al. Staphylococcal alpha-toxin tilts the balance between malignant and non-malignant CD4+ T cells in cutaneous T-cell lymphoma. Oncoimmunology. 2019;8(11): e1641387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lindahl LM, Willerslev-Olsen A, Gjerdrum LMR, et al. Antibiotics inhibit tumor and disease activity in cutaneous T-cell lymphoma. Blood. 2019;134(13): 1072–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kim EJ, Hess S, Richardson SK, et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115(4):798–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wysocka M, Zaki MH, French LE, et al. Sézary syndrome patients demonstrate a defect in dendritic cell populations: effects of CD40 ligand and treatment with GM-CSF on dendritic cell numbers and the production of cytokines. Blood. 2002;100(9):3287–3294. [DOI] [PubMed] [Google Scholar]
- [9].Papadavid E, Economidou J, Psarra A, et al. The relevance of peripheral blood T-helper 1 and 2 cytokine pattern in the evaluation of patients with mycosis fungoides and Sezary syndrome. Br J Dermatol. 2003; 148(4):709–718. [DOI] [PubMed] [Google Scholar]
- [10].Whittaker S, Hoppe R, Prince HM. How I treat mycosis fungoides and Sézary syndrome. Blood. 2016;127(25): 3142–3153. [DOI] [PubMed] [Google Scholar]
- [11].Kim YH, Bagot M, Pinter-Brown L, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192–1204. [DOI] [PubMed] [Google Scholar]
- [12].Blaizot R, Ouattara E, Fauconneau A, et al. Infectious events and associated risk factors in mycosis fungoides/Sezary syndrome: a retrospective cohort study. Br J Dermatol. 2018;179(6):1322–1328. [DOI] [PubMed] [Google Scholar]
- [13].Posner LE, Fossieck BE Jr., Eddy JL, et al. Septicemic complications of the cutaneous T-cell lymphomas. Am J Med. 1981;71(2):210–216. [DOI] [PubMed] [Google Scholar]
- [14].Balagula Y, Dusza SW, Zampella J, et al. Early-onset mycosis fungoides among African American women: a single-institution study. J Am Acad Dermatol. 2014; 71(3):597–598. [DOI] [PubMed] [Google Scholar]


