Abstract
Background: Generalized anxiety disorder (GAD) is one of the most common psychiatric disorders associated with substantial dysfunction and socioeconomic burden. Pharmacotherapy is the first choice for GAD. Remission [Hamilton Anxiety Scale (HAM-A) score ≤7] is regarded as a crucial treatment goal for patients with GAD. There is no up-to-date evidence to compare remission rate and tolerability of all available drugs by using network meta-analysis. Therefore, the goal of our study is to update evidence and determine the best advantageous drugs for GAD in remission rate and tolerability profiles.
Method: We performed a systematic review and network meta-analysis of double-blind randomized controlled trials (RCTs). We searched PubMed, EMBASE, Cochrane Central Register of Controlled Trials, Chinese National Knowledge Infrastructure, wanfang data, China Biology Medicine and ClinicalTrials.gov from their inception to March 2020 to identify eligible double-blind, RCTs reporting the outcome of remission in adult patients who received any pharmacological treatment for GAD. Two reviewers independently assessed quality of included studies utilizing the Cochrane Collaboration’s risk of bias tool as described in Cochrane Collaboration Handbook and extracted data from all manuscripts. Our outcomes were remission rate (proportion of participants with a final score of seven or less on HAM-A) and tolerability (treatments discontinuations due to adverse events). We calculated summary odds ratios (ORs) and 95% confidence intervals (CIs) of each outcome via pairwise and network meta-analysis with random effects.
Results: Overall, 30 studies were included, comprising 32 double-blind RCTs, involving 13,338 participants diagnosed as GAD by DSM-IV criteria. Twenty-eight trials were rated as moderate risk of bias, four trials as low. For remission rate, agomelatine (OR 2.70, 95% CI 1.74–4.19), duloxetine (OR 1.88, 95% CI 1.47–2.40), escitalopram (OR 2.03, 95% CI 1.48–2.78), paroxetine (OR 1.74, 95% CI 1.25–2.42), quetiapine (OR 1.88, 95% CI 1.39–2.55), and venlafaxine (OR 2.28, 95% CI 1.69–3.07) were superior to placebo. For tolerability, sertraline, agomelatine, vortioxetine, and pregabalin were found to be comparable to placebo. However, the others were worse than placebo in terms of tolerability, with ORs ranging between 1.86 (95% CI 1.25–2.75) for tiagabine and 5.98 (95% CI 2.41–14.87) for lorazepam. In head-to-head comparisons, agomelatine, duloxetine, escitalopram, quetiapine, and venlafaxine were more efficacious than tiagabine in terms of remission rate, ORs from 1.66 (95% CI 1.04–2.65) for duloxetine to 2.38 (95% CI 1.32–4.31) for agomelatine. We also found that agomelatine (OR 2.08, 95% CI 1.15–3.75) and venlafaxine (OR 1.76, 95% CI 1.08–2.86) were superior to vortioxetine. Lorazepam and quetiapine were poorly tolerated when compared with other drugs.
Conclusions: Of these interventions, only agomelatine manifested better remission with relatively good tolerability but these results were limited by small sample sizes. Duloxetine, escitalopram, venlafaxine, paroxetine, and quetiapine showed better remission but were poorly tolerated.
Keywords: remission rate, tolerability, pharmacotherapy, network meta-analysis, generalized anxiety disorder
Introduction
Generalized anxiety disorder (GAD) is a typically chronic mental disorder characterized by excessive, uncontrollable, and persistent worrying and tension. It is associated with clinical manifestations including palpitations, tremor, restlessness, fatigue, and difficulty concentrating, among others, which cause marked functional impairment across multiple aspects of productivity, family activity and socialization and associated reduced quality of life (Doyle and Pollack, 2003; Tyrer and Baldwin, 2006; Baldwin D. S. et al., 2011). In Europe the 12-months prevalence of GAD is approximately 0.2–4.2% and the life prevalence is approximately 4.3–5.9% (Wittchen and Jacobi, 2005). In urban China, the prevalence of GAD also has been estimated to be approximately 2.4–8.9%. Among those patients, one third self-reported receiving no therapy or even counsels (Yu et al., 2018). Therapies include pharmacological treatment, psychological treatment, or a combination of both.
Psychological interventions for GAD such as cognitive behavior therapy are widely considered preferable to anxiolytic drugs because of their efficacy and harmlessness, but often they cannot be implemented due to limited resources (Gould et al., 1997; Tyrer et al., 2006). Pharmacological therapy is probably still the main clinical treatment for GAD. In clinical practice, clinicians may have difficulty prescribing optimal drugs and facing various obstacles. In a previous survey, Yu and colleagues reported that diazepam, pregabalin, and alprazolam were the most common prescription medications for GAD in urban China, although selective serotonin reuptake inhibitors (SSRIs) and serotonin and norepinephrine reuptake inhibitors (SNRIs) have been indicated as first-line treatments for GAD in guidelines and by meta-analyses (Yu et al., 2018; National Institute For Health and Care Excellence, 2011; Slee et al., 2019).
Achieving response is the traditional goal of GAD therapy and such responses has been defined as either a clinically significant improvement or a meaningful reduction in HAM-A scale or Clinical Global Impressions (CGI) scale score, but many patients exhibit residual symptoms and are at a high risk of recurrence after initially responding to therapy (Mandos et al., 2009; Baldwin D. S. et al., 2011). Thus, the ultimate treatment goal is complete remission with no symptoms of anxiety in addition to complete recovery to premorbid functioning (Doyle and Pollack, 2003; Mandos et al., 2009). In a mixed-treatment meta-analysis comparing nine drugs in 27 trials published between 1980 and 2009 fluoxetine exhibited the best remission rate, and sertraline exhibited the highest tolerability (Baldwin D. et al., 2011). Since that meta-analysis, several new antidepressants such as agomelatine, vilazodone and vortioxetine have demonstrated considerable effects on anxiety symptoms. Therefore, the current network meta-analysis was conducted to compare all available drugs in patients with GAD using data from double-blind, randomized, controlled trials.
Materials and Methods
Our systematic review and network meta-analysis were performed in accordance to the checklist of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) extension statement for reporting of systematic reviews incorporating network meta-analyses of healthcare interventions (Hutton et al., 2015).
Search Strategy and Study Selection
Seven electronic databases including PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, the Chinese National Knowledge Infrastructure, wanfang data, China Biology Medicine, and ClinicalTrials.gov were systematically searched from their inception to March 2020 to identify trial reports. The search terms used were “anxiety”, “anxiety disorder”, “generalized anxiety disorder”, “randomized controlled trials”, and “RCT”. The references lists of relevant meta-analyses, reviews, pooled analyses, and included trials were also reviewed to obtain additional studies. The languages were limited to Chinese and English. Unpublished trials were excluded, because the reliability of data derived from them could not be assured. The search strategy is presented in detail in Table 1.
TABLE 1.
Search strategies.
| Electronic databases | Search strategies |
|---|---|
| Pubmed | #17 #5 and #16 |
| #16 14 NOT #15 | |
| #15 (“Animals” [Mesh]) NOT “Humans” [Mesh] | |
| #14 #6∼13 or | |
| #13 groups [tiab] | |
| #12 trial [tiab] | |
| #11 randomly [tiab] | |
| #10 drug therapy [sh] | |
| #9 placebo [tiab] | |
| #8 randomized [tiab] | |
| #7 controlled clinical trial [PT] | |
| #6 randomized controlled trial [PT] | |
| #5 #1∼4 or | |
| #4 generali* anxiety disorder | |
| #3 GAD | |
| #2 anxiety disorder | |
| #1 “Anxiety Disorders” [Mesh] | |
| CENTRAL | #5 #1∼4 or |
| #4 generali* anxiety disorder | |
| #3 GAD | |
| #2 anxiety disorder | |
| #1 anxiety disorder [MeSH] | |
| Embase | #10 #5 and #9 |
| #9 #6∼#8 or | |
| #8 double-blind:ti,ab | |
| #7 placebo:ab,ti,lnk | |
| #6 random:ti,ab | |
| #5 #1∼4 or | |
| #4 generali* anxiety disorder | |
| #3 GAD | |
| #2 anxiety disorder | |
| #1 “anxiety disorder”/exp | |
| ClinicalTrials.gov | Anxiety disorder |
*represents truncation searching.
Two reviewers (KWQ, WXL) independently conducted study selection in accordance with pre-specified inclusion criteria, and any disagreements was settled via discussion. After removing duplicates, they then screened the titles and abstracts of remaining records, read the remaining reports in full text and identified eligible studies.
Inclusion Criteria
The inclusion criteria were 1) double-blind randomized controlled trials (RCTs) comparing active drugs with placebo or another agent as oral monotherapy in adults with a primary diagnosis of GAD with major comorbidities except those with major depression disorder, substance abuse, schizophrenia, organ diseases or alcohol addiction; 2) standard diagnostic criteria included Diagnostic and Statistical Manual of Mental Disorders, Third Edition (DSM-III), Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V), International Classification of Diseases, 9th Revision (ICD-9), International Classification of Diseases, 10th Revision (ICD-10) or Chinese Classification of Mental Disorders, Third Edition (CCMD-3); 3) remission rates were reported. Articles reporting studies investigating refractory GAD, relapses or changing to another drug were excluded.
Data Extraction
Two investigators (KWQ, WXL) independently extracted data using pre-designed data extraction forms. The data extracted from each report included basic study characteristics (first author, publication year, study duration, total sample size, attrition rate, sponsor), baseline patient characteristics (sex ratio, mean HAM-A, mean age, diagnostic criteria), interventions (drugs and doses), and outcomes (remission rate and tolerability). Remission rate was defined as the proportion of patients who had achieved remission (HAM-A scores ≤7) at the study end-point. Tolerability was determined based on treatment discontinuations due to adverse events. The intention-to-treat (ITT) population consisting of all randomized participants who received at least one dose of study medication was abstracted for two outcomes. In cases of missing or unclear data, first authors or corresponding authors were contacted via email for supplementary information. Any discrepancies were settled by discussion.
Risk of Bias Assessment
The quality of the trials included was assessed in accordance with the Cochrane Collaboration’s risk of bias tool as described in the Cochrane Collaboration Handbook (Higgins et al., 2011). Two investigators (KWQ, WXL) independently determined risks bias to be low, unclear, or high based on the presence or absence of random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessors (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and “other source of bias” (other bias). Subsequently, we divided study quality into three rates from low risk to high risk on the basis of the method described by two articles (Cipriani et al., 2018; He et al., 2019). Discrepancies were resolved via discussion.
Data Analysis
Pairwise meta-analyses using RevMan5.2 software were performed first, to compare the results of mixed treatment meta-analyses. Summary odds ratios (ORs) with 95% confidence intervals (CIs) were calculated via Mantel-Haenszel’s method. A random-effects model was used to derive pooled estimates across studies, because it takes between-study differences into account. Between-study heterogeneity was quantitatively assessed using the I 2 statistic, with I 2 of >50% indicating high heterogeneity and <50% indicating low heterogeneity (Higgins and Thompson, 2002).
Network meta-analyses were then performed using STATA software. A frequentist framework was applied to combine evidence from direct and indirect comparisons with a random effects model (Chaimani et al., 2013). Loop inconsistency was assessed in every closed triangular or quadratic loop via the “loop-specific” approach, wherein a 95% CI excluding zero suggests that the loop is inconsistent (Higgins et al., 2012; Li et al., 2017). The “design-by-treatment” interaction model was used to assess global consistency in networks (Li et al., 2017). The surface under the cumulative ranking curve (SUCRA) and the mean ranks were calculated to rank the treatments for each outcome (Salanti et al., 2011). The comparison-adjusted funnel plots were generated to investigate whether there are study-small effects in the intervention network (Chaimani and Salanti, 2012). The robustness of conclusions was evaluated via Bayesian analysis.
Results
Study Selection and Study Characteristics
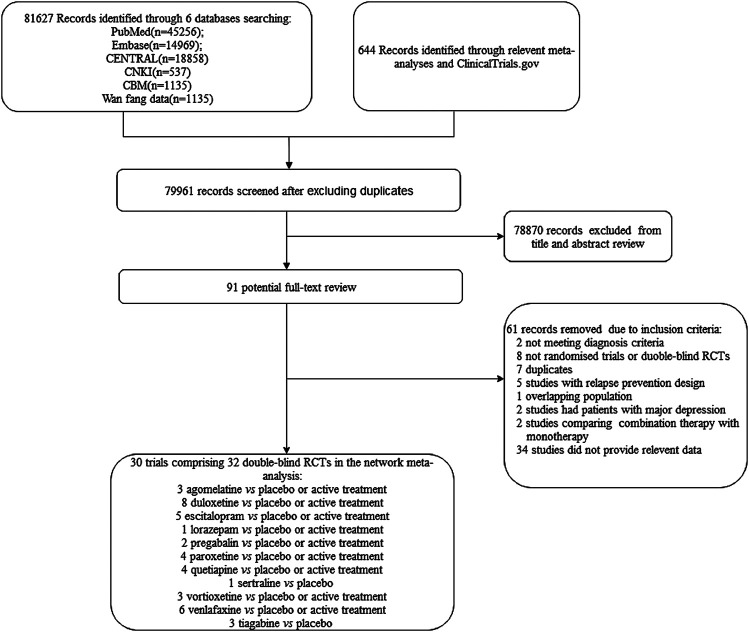
The literature screening process is shown in Figure 1. Electronic searches yielded 82,271 citations, and the full text versions of 91 publications were subsequently reviewed. Of these, 61 were rejected based on the inclusion criteria. Thirty studies (Pollack et al., 2001; Feltner et al., 2003; Lenox-Smith and Reynolds, 2003; Rickels et al., 2003; Allgulander et al., 2004; Boyer et al., 2004; Davidson et al., 2004; Nimatoudis et al., 2004; Hartford et al., 2007; Koponen et al., 2007; Bose et al., 2008; Montgomery et al., 2008; Pollack et al., 2008a; Pollack et al., 2008b; Rynn et al., 2008; Stein et al., 2008; Nicolini et al., 2009; Bandelow et al., 2010; Coric et al., 2010; Khan et al., 2011; Wu et al., 2011; Bidzan et al., 2012; Merideth et al., 2013; Mezhebovsky et al., 2012; Rothschild et al., 2012; Alaka et al., 2014; Kasper et al., 2014; Mahableshwarkar et al., 2014; Stein et al., 2014; Ball et al., 2015; Stein et al., 2017) comprising 32 double-blind RCTs were ultimately included in the network meta-analysis and all of them were published in English.
FIGURE 1.
Flow diagram of study selection.
Basic information derived from each of the 32 RCTs is shown in Table 2. Collectively they involved 13,338 participants diagnosed via the DSM-IV, published between 2003 and 2017. Of these participants 4,848 were randomly assigned to a placebo group and 8,490 were randomly assigned to an active medication group. Thirteen drugs or placebo were included in the analysis. The study’s sample sizes ranged from 46 to 951.60.0% (8,007/13,338) of the participants were female. The vast majority of patients were Caucasian. The medication dosage was flexible in 17 trials. The majority of participants had moderate-to-severe GAD, with a mean HAM-A scale score between 22.6 and 29.0. The adult patient groups had mean ages between 36.3 and 72.4 years. Dropout rates ranged from 6.9 to 50%. Four studies exclusively included people aged >65 years (Montgomery et al., 2008; Mezhebovsky et al., 2013; Alaka et al., 2014; Ball et al., 2015). The study durations ranged from 4 to 24 weeks (median 10 weeks). All studies were placebo-controlled trials. Fourteen of the thirty-two trials (43.8%) randomly allocated patients to three or more groups, and 28/32 trials (87.5%) were funded by pharmaceutical companies.
TABLE 2.
Characteristic of included studies.
| References | Sample size | Interventions | Main race | Female (%) | Mean HAMA | Mean age (Year) | Attrition rate (%) | Sponsor | Follow-up time (weeks) | Diagnosis criteria |
|---|---|---|---|---|---|---|---|---|---|---|
| Lenox-Smith et al., 2003 | 244 | Venlafaxine:75 ∼ 150 mg/day | — | 61.5 | 28.0 | 48.0 | 13.2 | Wyeth | 24 | DSM-IV |
| Placebo | 56.6 | 28.0 | 46.0 | 19.8 | ||||||
| Mahableshwarkar et al., 2014 | 781 | Vortioxetine:2.5 mg/day | White | 69.9 | 25.3 | 39.2 | 23.1 | Takeda | 8 | DSM-IV |
| Vortioxetine:5 mg/day | 64.1 | 25.0 | 37.7 | 25.0 | ||||||
| Vortioxetine:10 mg/day | 67.3 | 25.3 | 39.8 | 28.8 | ||||||
| Duloxetine:60 mg/day | 72.4 | 25.0 | 39.5 | 32.1 | ||||||
| Placebo | 65.0 | 24.4 | 36.8 | 22.9 | ||||||
| Khan et al., 2011 | 951 | Quetiapine:50 mg/day | White | 57.1 | 24.6 | 39.0 | 30.8 | AstraZeneca | 8 | DSM-IV |
| Quetiapine:150 mg/day | 62.8 | 24.5 | 40.7 | 36.1 | ||||||
| Quetiapine:300 mg/day | 60.7 | 24.5 | 41.0 | 42.3 | ||||||
| Placebo | 65.8 | 24.9 | 39.2 | 29.8 | ||||||
| Feltner et al., 2003 | 271 | Pregabalin:600 mg/day | White | 50.0 | 25.4 | 36.3 | 30.3 | Pfizer | 4 | DSM-IV |
| Lorazepam:6 mg/day | 58.8 | 24.7 | 39.2 | 47.1 | ||||||
| Placebo | 50.7 | 24.8 | 37.8 | 28.4 | ||||||
| Stein et al., 2014 | 412 | Agomelatine:25–50 mg/day | — | 74.8 | 28.6 | 43.6 | 16.5 | Servier | 12 | DSM-IV |
| Escitalopram:10–20 mg/day | 68.3 | 28.6 | 41.2 | 26.7 | ||||||
| Placebo | 71.8 | 28.2 | 43.0 | 17.6 | ||||||
| Hartford et al., 2007 | 487 | Duloxetine:60–120 mg/day | Caucasian | 64.2 | 25.6 | 40.4 | 45.7 | Eli Lilly | 10 | DSM-IV |
| Venlafaxine:75–225 mg/day | 62.2 | 24.9 | 40.1 | 37.8 | ||||||
| Placebo | 61.5 | 25.0 | 41.9 | 38.5 | ||||||
| Stein et al., 2017 | 412 | Agomelatine:10 mg/day | — | 67.9 | 28.6 | 43.6 | 13.7 | Servier | 12 | DSM-IV |
| Agomelatine:25 mg/day | 71.9 | 29.0 | 44.1 | 9.4 | ||||||
| Placebo | 63.4 | 28.8 | 44.1 | 21.1 | ||||||
| Alaka et al., 2014 | 291 | Duloxetine:60–120 mg/day | Caucasian | 75.5 | 24.6 | 71.4 | 25.0 | Eli Lilly | 10 | DSM-IV |
| Placebo | 80.0 | 24.4 | 71.7 | 24.0 | ||||||
| Montgomery et al., 2008 | 273 | Pregabalin:50–600 mg/day | White | 79.0 | 27.0 | 72.4 | 24.9 | Pfizer | 8 | DSM-IV |
| Placebo | 75.0 | 26.0 | 72.2 | 28.0 | ||||||
| Merideth et al., 2012 | 854 | Quetiapine:150 mg/day | White | 68.0 | 25.0 | 38.2 | 28.8 | Pfizer | 10 | DSM-IV |
| Quetiapine:300 mg/day | 71.0 | 25.2 | 39.0 | 39.1 | ||||||
| Escitalopram:10 mg/day | 66.0 | 24.6 | 40.4 | 27.7 | ||||||
| Placebo | 64.0 | 25.3 | 36.6 | 21.4 | ||||||
| Stein et al., 2008 | 121 | Agomelatine25–50 mg/day | — | 68.8 | 29.0 | 42.7 | 8.1 | Servier | 12 | DSM-IV |
| Placebo | 68.8 | 28.6 | 41.7 | 6.9 | ||||||
| Allgulander et al., 2004 | 378 | Sertraline:50–150 mg/day | White | 59.0 | 24.6 | 40.3 | 20.0 | NA | 12 | DSM-IV |
| Placebo | 51.0 | 25.0 | 42.2 | 27.0 | ||||||
| Davidson et al., 2004 | 315 | Escitalopram:10–20 mg/day | Caucasian | 52.5 | 23.6 | 39.5 | 25.0 | Forest laboratories | 8 | DSM-IV |
| Placebo | 52.9 | 23.2 | 39.5 | 22.0 | ||||||
| Nicolini et al., 2009 | 581 | Duloxetine:20 mg/day | Caucasian | 57.1 | 27.4 | 42.8 | 25.0 | Eli Lilly | 10 | DSM-IV |
| Duloxetine:60–120 mg/day | 29.1 | |||||||||
| Venlafaxine:75–225 mg/day | 27.2 | |||||||||
| Placebo | 40 | |||||||||
| Rickels et al., 2003 | 566 | Paroxetine:20 mg/day | White | 54.0 | 24.1 | 40.2 | 23.9 | GlaxoSmithKline | 8 | DSM-IV |
| Paroxetine:40 mg/day | 56.0 | 23.8 | 40.5 | 27.4 | ||||||
| Placebo | 56.0 | 24.4 | 40.8 | 22.2 | ||||||
| Bandelow et al., 2010 | 873 | Quetiapine:50 mg/day | White | 68.0 | 26.9 | 40.7 | 25.8 | AstraZeneca | 8 | DSM-IV |
| Quetiapine:150 mg/day | 66.7 | 26.6 | 42.3 | 25.2 | ||||||
| Paroxetine:20 mg/day | 64.5 | 27.1 | 41.6 | 20.3 | ||||||
| Placebo | 62.2 | 27.3 | 41.2 | 18.9 | ||||||
| Bose et al., 2008 | 404 | Escitalopram 10–20 mg/day | White | 64.6 | 24.2 | 38.2 | 19.7 | Forest laboratories | 8 | DSM-IV |
| Venlafaxine:75∼225 mg/day | 59.7 | 23.8 | 37.1 | 25.6 | ||||||
| Placebo | 62.5 | 23.7 | 37.6 | 23.5 | ||||||
| Nimatoudis et al., 2004 | 46 | Venlafaxine:75 mg/day | — | 66.7 | 27.1 | 41.0 | 21 | NA | 8 | DSM-IV |
| Placebo | 68.2 | 28.5 | 44.0 | 50 | ||||||
| Boyer et al., 2004 | 541 | Venlafaxine:37.5 mg/day | — | 42.0 | 26.6 | 45.0 | 27.1 | Wyeth-Ayerst | 24 | DSM-IV |
| Venlafaxine:75 mg/day | 39.0 | 26.3 | 44.0 | 24.6 | ||||||
| Venlafaxine:150 mg/day | 35.0 | 26.3 | 45.0 | 22.6 | ||||||
| Placebo | 42.0 | 26.7 | 46.0 | 34.6 | ||||||
| Ball et al., 2015 | 291 | Duloxetine:30–120 mg/day | Caucasian | 77.7 | 24.5 | 71.6 | NA | Eli Lilly | 10 | DSM-IV |
| Placebo | ||||||||||
| Bidzan et al., 2012 | 301 | Vortioxetine:5 mg/day | white | 68.7 | 26.3 | 45.0 | 14.7 | Takeda | 8 | DSM-IV |
| Placebo | 61.6 | 26.8 | 45.3 | 16.6 | ||||||
| Mezhebovsky et al., 2013 | 450 | Quetiapine 50–300 mg/day | white | 72.1 | 25.2 | 70.3 | 20.2 | AstraZeneca | 9 | DSM-IV |
| Placebo | 69.0 | 25.1 | 70.6 | 26.0 | ||||||
| Koponen et al., 2007 | 513 | Duloxetine:60 mg/day | Caucasian | 64.3 | 25.0 | 43.1 | NA | Eli Lilly | 9 | DSM-IV |
| Duloxetine:120 mg/day | 72.3 | 25.2 | 44.1 | |||||||
| Placebo | 66.9 | 25.8 | 44.1 | |||||||
| Kasper et al., 2014 | 273 | Paroxetine:20 mg/day | Caucasian | 77.3 | 25.8 | 45.8 | 21.2 | Eli Lilly | 10 | DSM-IV |
| Placebo | 73.7 | 25.1 | 44.6 | 13.2 | ||||||
| Rothschild et al., 2012 | 304 | Vortioxetine:5 mg/day | Caucasian | 67.8 | 24.7 | 41.0 | 17.8 | Takeda | 8 | DSM-IV |
| Placebo | 63.8 | 24.6 | 41.4 | 25.0 | ||||||
| Wu et al., 2011 | 210 | Duloxetine:60–120 mg/day | Chinese | 46.3 | 24.5 | 37.3 | 24.1 | Eli Lilly | 15 | DSM-IV |
| Placebo | 54.9 | 24.2 | 38.0 | 27.5 | ||||||
| Coric et al., 2010 | 157 | Escitalopram 20 mg/day | White | 100.0 | 23.5 | 38.8 | 20.8 | Bristol-Myers | 8 | DSM-IV |
| Placebo | 100.0 | 24.7 | 39.3 | 23.1 | ||||||
| Rynn et al., 2008 | 327 | Duloxetine:60–120 mg/day | Caucasian | 61.3 | 22.6 | 42.2 | 44.6 | Eli Lilly | 10 | DSM-IV |
| Placebo | 62.3 | 23.5 | 41.0 | 31.4 | ||||||
| Pollack et al., 2001 | 326 | Paroxetine:20–50 mg/day | White | 60.9 | 24.2 | 39.7 | 21.1 | GlaxoSmithKline | 8 | DSM-IV |
| Placebo | 66.3 | 24.1 | 41.3 | 18.4 | ||||||
| Pollack et al., 2008a † | 910 | Tiagabine:4 mg/day | — | 62.0 | 27.0 | 37.6 | 36.0 | GlaxoSmithKline | 10 | DSM-IV |
| Tiagabine:8 mg/day | 64.0 | 26.8 | 39.4 | 44.0 | ||||||
| Tiagabine:12 mg/day | 67.0 | 27.0 | 38.4 | 47.0 | ||||||
| Placebo | 67.0 | 26.5 | 38.2 | 37.0 | ||||||
| Pollack et al., 2008b † | 468 | Tiagabine:4–16 mg/day | — | 67.0 | 26.8 | 37.8 | 41.0 | GlaxoSmithKline | 10 | DSM-IV |
| Placebo | 61.0 | 26.6 | 39.9 | 30.0 | ||||||
| Pollack et al., 2008b † | 452 | Tiagabine:4–16 mg/day | — | 61.0 | 27.3 | 39.4 | 29.0 | GlaxoSmithKline | 10 | DSM-IV |
| Placebo | 58.0 | 26.7 | 40.8 | 24.0 |
†Pollack., et al 2008 that contained three trials was considered separately.
Risk of Bias
Random sequence generation was appropriate in 13 trials (40.6%), and allocation concealment was adequately conducted in 10 trials (31.3%). Fourteen trials (43.8%) clearly reported how they had performed the blinding of participants and personnel, and only 2 (6.3%) reported a masked outcome assessor, although all studies were double-blind RCTs. Twenty-three trials (71.9%) were considered to entail a high risk of bias based on incomplete outcome data either because they used the last observation carried forward method to handle missing data (Stack et al., 2013; Cipriani et al., 2018), or they had a high dropout rate (>20%). The risk of other bias was unclear in 28 trials (87.5%) because they were funded by pharmaceutical companies. Overall, four trials (12.5%) were rated as low risk (Stein et al., 2008; Bidzan et al., 2012; Alaka et al., 2014; Stein et al., 2014), and the other 28 were rated as moderate risk. The results of quality assessment are shown in Table 3.
TABLE 3.
The quality assessment for included studies.
| ID | Adequate sequence generation | Allocation concealment | Blinding of participant and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias | Overall quality |
|---|---|---|---|---|---|---|---|---|
| Lenox-Smith et al., 2003 | Low | Unclear | Unclear | Unclear | High | Low | Unclear | Medium a |
| Mahableshwarkar et al., 2014 | Low | Low | Low | Unclear | High | Low | Unclear | Medium |
| Khan et al., 2011 | Low | Unclear | Unclear | Unclear | High | Low | Unclear | Medium |
| Feltner et al., 2003 | Unclear | Unclear | Low | Unclear | High | Low | Unclear | Medium |
| Stein et al., 2014 | Low | Low | Low | Low | Low | Low | Unclear | Low b |
| Hartford., et al., 2007 | Unclear | Unclear | Unclear | Unclear | High | Low | Unclear | Medium |
| Stein., et al., 2017 | Low | Unclear | Low | Unclear | High | Low | Unclear | Medium |
| Alaka., et al., 2014 | Low | Low | Low | Low | Unclear | Low | Unclear | Low |
| Montgomery., et al., 2008 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear | Medium |
| Merideth., et al., 2012 | Unclear | Unclear | Low | Unclear | High | Low | Unclear | Medium |
| Stein., et al., 2008 | Low | Low | Low | Unclear | Unclear | Low | Unclear | Low |
| Allgulander., et al., 2004 | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low | Medium |
| Davidson., et al., 2004 | Unclear | Unclear | Unclear | Unclear | High | Unclear | Unclear | Medium |
| Nicolini., et al., 2009 | Low | Low | Unclear | Unclear | High | Unclear | Low | Medium |
| Rickels., et al., 2003 | Unclear | Unclear | Unclear | Unclear | High | Low | Unclear | Medium |
| Bandelow., et al., 2010 | Low | Low | Low | Unclear | High | Low | Unclear | Medium |
| Bose., et al., 2008 | Unclear | Unclear | Unclear | Unclear | High | Low | Unclear | Medium |
| Nimatoudis., et al., 2004 | Unclear | Unclear | Low | Unclear | High | Low | Low | Medium |
| Boyer., et al., 2004 | Low | Unclear | Unclear | Unclear | High | Low | Unclear | Medium |
| Ball., et al., 2015 | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Medium |
| Bidzan., et al., 2012 | Unclear | Low | Low | Unclear | Low | Low | Unclear | Low |
| Mezhebovsky., et al., 2012 | Low | Unclear | Low | Unclear | High | Low | Unclear | Medium |
| Koponen., et al., 2007 | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Medium |
| Kasper., et al., 2014 | Low | Low | Low | Unclear | High | Low | Unclear | Medium |
| Rothschild., et al., 2012 | Unclear | Low | Low | Unclear | High | Low | Unclear | Medium |
| Wu., et al., 2011 | Unclear | Unclear | Unclear | Unclear | High | Low | Unclear | Medium |
| Coric., et al., 2010 | Unclear | Low | Low | Unclear | High | Low | Unclear | Medium |
| Rynn., et al., 2008 | Unclear | Unclear | Unclear | Unclear | High | Low | Unclear | Medium |
| Pollack., et al., 2001 | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Medium |
| Pollack., et al., 2008a; Pollack., et al., 2008b | Low | Unclear | Unclear | Unclear | High | Low | Unclear | Medium |
Medium-risk studies had one high-risk item or more than four items of unclear risk.
Low-risk studies had no high-risk items and fewer than three items of unclear risk.
Network Meta-analysis
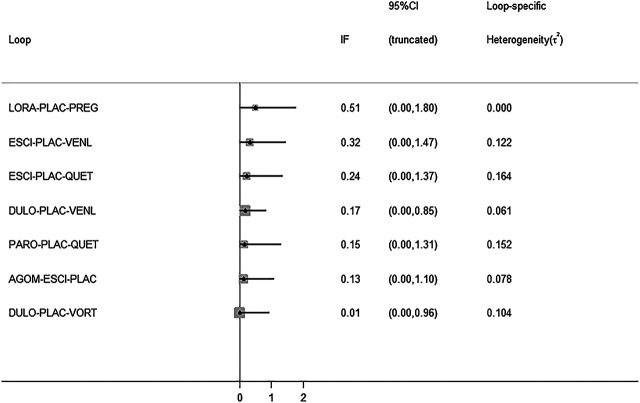
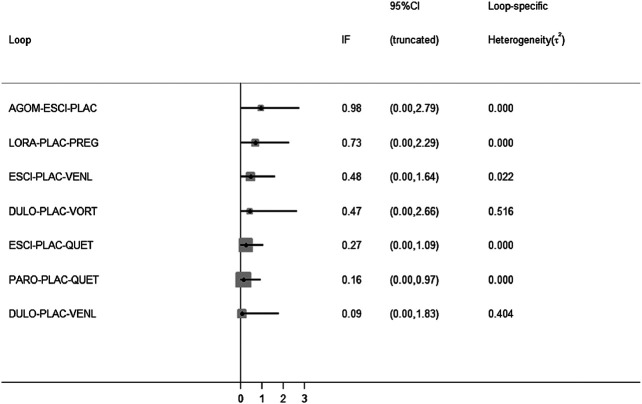
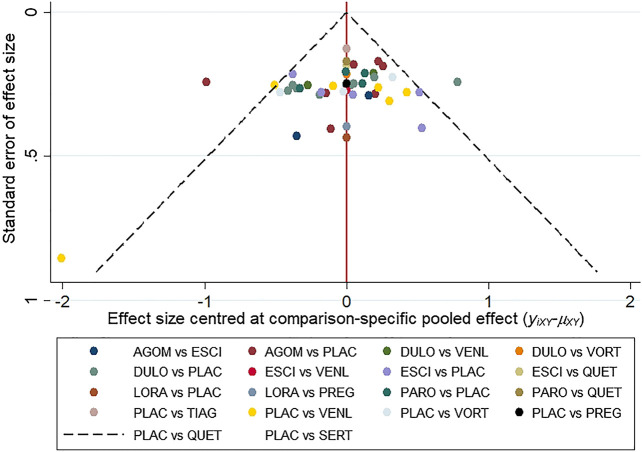
All active drugs were involved in at least one placebo-controlled trial. All active drugs except sertraline and tiagabine were directly compared with at least one other drug in two network plots. Three trials investigating the efficacy and safety of agomelatine were deemed to entail a low-risk quality. Trial network plots are shown in Figures 2, 3. The results of pairwise meta-analyses were generally consistent with those from network meta-analyses with regard to remission rates and tolerability. The comparative results are shown in Table 4. Both networks generated seven closed loops, and there was no inconsistency in any loop. Loop inconsistency plots are shown in Figures 4, 5. No global inconsistency was detected the within any network (p = 0.82 for remission rate, p = 0.77 for tolerability). Comparison-adjusted plots were approximately symmetric, suggesting a lack of small-study effects (Figures 6, 7).
FIGURE 2.
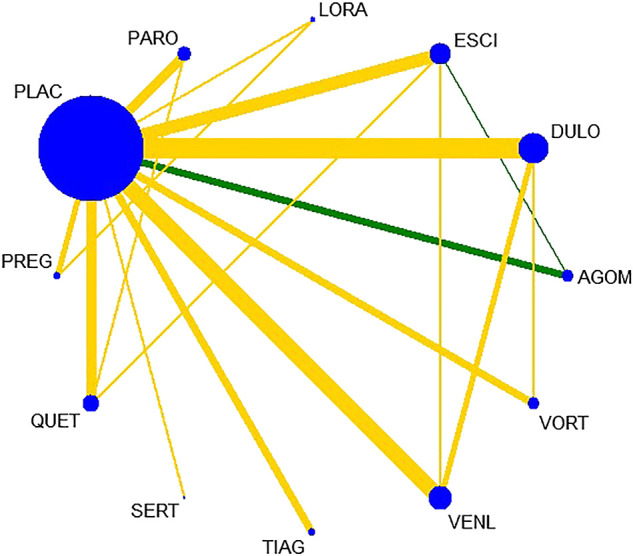
Network plot for remission rate.
FIGURE 3.
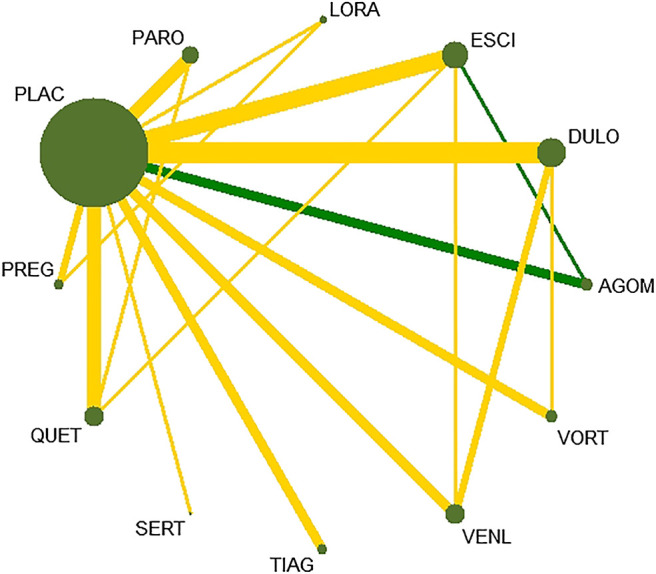
etwork plot for tolerability.
TABLE 4.
Comparative results for remission rate and tolerability.
| Remission rate | ||||
|---|---|---|---|---|
| Network meta-analysis | Pairwise meta-analysis | |||
| OR with 95% CI | Study | OR with 95% CI | Heterogeneity (%) | |
| DULO vs. PLAC | 1.88 (1.47, 2.40) | 8 | 1.84 (1.38, 2.44) | 61 |
| PARO vs. PLAC | 1.74 (1.25, 2.42) | 4 | 1.70 (1.36, 2.13) | 0 |
| LORA vs. PLAC | 1.51 (0.62, 3.70) | 1 | 1.81 (0.77, 4.24) | — |
| PREG vs. PLAC | 1.64 (0.89, 3.02) | 2 | 1.59 (0.99, 2.55) | 0 |
| QUET vs. PLAC | 1.88 (1.39, 2.55) | 4 | 1.85 (1.13, 3.04) | 85 |
| VORT vs. PLAC | 1.30 (0.88,1.92) | 3 | 1.26 (0.80, 1.99) | 59 |
| AGOM vs. PLAC | 2.70 (1.74, 4.19) | 3 | 2.71 (1.91, 3.85) | 0 |
| TIAG vs. PLAC | 1.13 (0.76, 1.68) | 3 | 1.12 (0.86, 1.45) | 0 |
| PREG vs. LORA | 1.09 (0.46, 2.59) | 1 | 1.25 (0.58, 2.72) | — |
| ESCI vs. PLAC | 2.03 (1.48, 2.78) | 5 | 1.89 (1.31, 2.73) | 54 |
| SERT vs. PLAC | 2.01(0.99, 4.10) † | 1 | 2.01(1.24, 3.28) † | — |
| PARO vs. QUET | 0.92 (0.61, 1.39) | 1 | 1.06 (0.76, 1.48) | — |
| ESCI vs. QUET | 1.08 (0.72, 1.61) | 1 | 0.94 (0.65, 1.34) | — |
| VENL vs. PLAC | 2.28 (1.69, 3.07) | 6 | 2.42 (1.60, 3.66) | 64 |
| ESCI vs. AGOM | 0.75 (0.46, 1.23) | 1 | 1.25 (0.76, 2.06) | — |
| ESCI vs. VENL | 0.89 (0.59, 1.34) | 1 | 1.02 (0.60, 1.74) | — |
| DULO vs. VENL | 0.83 (0.58, 1.17) | 1 | 1.11 (0.73, 1.68) | — |
| DULO vs. VORT | 1.45 (0.94, 2.23) | 1 | 1.50 (0.99, 2.29) | — |
| Tolerability | ||||
| DULO vs. PLAC | 2.15 (1.49, 3.11) | 6 | 2.86 (1.34, 6.11) | 76 |
| PARO vs. PLAC | 2.32 (1.56, 3.44) | 4 | 2.17 (1.43, 3.27) | 0 |
| LORA vs. PLAC | 5.98 (2.41, 14.87) | 1 | 8.59 (2.79, 26.50) | — |
| PREG vs. PLAC | 1.52 (0.75, 3.07) | 2 | 1.51 (0.76, 3.00) | 4 |
| QUET vs. PLAC | 4.05 (2.89, 5.65) | 4 | 3.82 (2.70, 5.40) | 0 |
| VORT vs. PLAC | 0.87 (0.49, 1.52) | 3 | 1.19 (0.41, 3.48) | 55 |
| AGOM vs. PLAC | 0.83 (0.28, 2.43) | 3 | 1.14 (0.36, 3.65) | 0 |
| TIAG vs. PLAC | 1.86 (1.25, 2.75) | 1 | 1.85 (1.27, 2.70) | 15 |
| PREG vs. LORA | 0.25 (0.12, 0.543) | 1 | 0.28 (0.14, 0.56) | — |
| ESCI vs. PLAC | 1.68 (1.11, 2.53) | 5 | 1.75 (1.14, 2.70) | 0 |
| SERT vs. PLAC | 0.77 (0.35, 1.66) | 1 | 0.77 (0.38, 1.57) | — |
| PARO vs. QUET | 0.57 (0.34, 0.90) | 1 | 0.53 (0.30, 0.95) | — |
| ESCI vs. QUET | 0.42 (0.27, 0.65) | 1 | 0.38 (0.22, 0.64) | — |
| VENL vs. PLAC | 2.25 (1.43, 3.55) | 3 | 2.61 (1.12, 6.04) | 59 |
| ESCI vs. AGOM | 2.04 (0.69, 5.88) | 1 | 3.84 (1.04, 14.06) | — |
| ESCI vs. VENL | 0.75 (0.43, 1.29) | 1 | 0.50 (0.22, 1.17) | — |
| DULO vs. VENL | 0.95 (0.62, 1.48) | 1 | 1.34 (0.69, 2.59) | — |
| DULO vs. VORT | 2.48 (1.45, 4.24) | 1 | 2.75 (1.53, 4.94) | — |
†Significant differences between pairwise analysis and network meta-analysis were in bold.
FIGURE 4.
Loop inconsistency plot for remission rate.
FIGURE 5.
Loop inconsistency plot for tolerability.
FIGURE 6.
The comparison-adjusted plot for remission rate.
FIGURE 7.
The comparison-adjusted plot for tolerability.
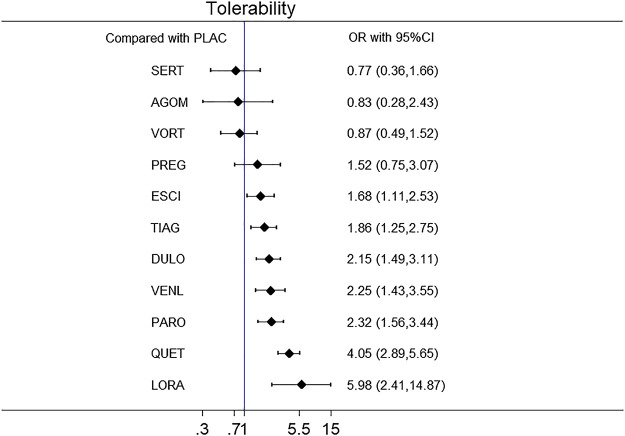
A forest plot of network meta-analysis of all trials for remission rate is shown in Figure 8. With regard to remission rate (comprising 32 RCTs including 13,338 patients), agomelatine (OR 2.70, 95% CI 1.74–4.19), duloxetine (OR 1.88, 95% CI 1.47–2.40), escitalopram (OR 2.03, 95% CI 1.48–2.78), paroxetine (OR 1.74, 95% CI 1.25–2.42), quetiapine (OR 1.88, 95% CI 1.39–2.55) and venlafaxine (OR 2.28, 95% CI 1.69–3.07) were superior to placebo. Tiagabine, vortioxetine, lorazepam, and sertraline were comparable to placebo. A forest plot of network meta-analysis of all trials for tolerability is shown in Figure 9. With regard to tolerability (comprising 25 RCTs involving 12,057 patients), all of the drugs except sertraline, agomelatine, vortioxetine, and pregabalin were worse than placebo, with ORs ranging from 1.68 (95% CI 1.11–2.53) for escitalopram to 5.98 (95% CI 2.41–14.87) for lorazepam.
FIGURE 8.
The forest plot of active drugs vs. placebo for remission rate.
FIGURE 9.
The forest plot of active drugs vs. placebo for tolerability.
In head-to-head comparisons of outcomes to determine the differences between drugs, with respect to remission rate agomelatine (OR 2.08, 95% CI 1.15–3.75) and venlafaxine (OR 1.76, 95% CI 1.08–2.86) were more effective than vortioxetine. Agomelatine, duloxetine, escitalopram, quetiapine, and venlafaxine were associated with higher remission rates than tiagabine (ORs ranging between 1.66 and 2.38). With respect to tolerability quetiapine and lorazepam were worse than the other drugs, with ORs ranging between 1.75 and 7.79. The results of head-to-head comparisons for remission rate and tolerability are summarized in Table 5. The results of Bayesian analysis of remission rates were consistent with those obtained using the frequentist method, with exception of quetiapine vs. tiagabine. The results of Bayesian analysis of tolerability for agomelatine vs. venlafaxine, and tiagabine vs. sertraline or vortioxetine differed significantly from those obtained using the frequentist method. The results of sensitivity analysis are presented in Tables 6, 7. Treatments were ranked in terms of remission rate and tolerability. Agomelatine was ranked the best for remission rate, and tiagabine was ranked the worst. Sertraline was ranked the best for tolerability, and quetiapine and lorazepam were ranked the worst for tolerability. The ranking of drugs based on SUCRAs and mean ranks are shown in Table 8.
TABLE 5.
Network meta-analysis results of head-to-head comparisons for remission rate (lower triangle) and tolerability (upper triangle).
| AGOM | 0.39 (0.13, 1.19) | 0.49 (0.17, 1.44) | 0.14 (0.03, 0.56) | 0.36 (0.12, 1.11) | 0.55 (0.15, 1.97) | 0.20 (0.07, 0.62) | 1.08 (0.29, 4.02) | 0.45 (0.14, 1.39) | 0.37 (0.12, 1.16) | 0.96 (0.29, 3.18) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1.44 (0.87, 2.37) | DULO | 1.28 (0.76, 2.16) | 0.36 ( 0.14, 0.95 ) | 0.93 (0.55, 1.57) | 1.42 (0.64, 3.13) | 0.53 ( 0.33, 0.87 ) | 2.79 ( 1.19, 6.53 ) | 1.16 (0.68, 1.96) | 0.95 (0.62, 1.48) | 2.48 ( 1.45, 4.24 ) |
| 1.33 (0.81, 2.17) | 0.92 (0.62, 1.37) | ESCI | 0.28 ( 0.10, 0.76 ) | 0.72 (0.42, 1.24) | 1.11 (0.49, 2.49) | 0.42 ( 0.27, 0.65 ) | 2.18 (0.91, 5.20) | 0.90 (0.52, 1.58) | 0.75 (0.43, 1.29) | 1.94 (0.99, 3.81) |
| 1.78 (0.66, 4.84) | 1.24 (0.49, 3.15) | 1.34 (0.52, 3.47) | LORA | 2.58 (0.96, 6.93) | 3.94 ( 1.89, 8.23 ) | 0.42 ( 0.27, 0.65 ) | 7.79 ( 2.32, 26.18 ) | 3.22 ( 1.20, 8.64 ) | 2.66 (0.97, 7.30) | 6.91 ( 2.39, 19.98 ) |
| 1.55 (0.90, 2.69) | 1.08 (0.72, 1.63) | 1.17 (0.74, 1.84) | 0.87 (0.33, 2.26) | PARO | 1.53 (0.68, 3.42) | 0.57 ( 0.34, 0.90 ) | 3.01 ( 1.27, 7.12 ) | 1.25 (0.72, 2.16) | 2.66 (0.97, 7.30) | 2.68 ( 1.36, 5.26 ) |
| 1.64 (0.77, 3.47) | 1.14 (0.59, 2.20) | 1.24 (0.62, 2.45) | 0.92 (0.39, 2.19) | 1.06 (0.53, 2.11) | PREG | 0.37 ( 0.17, 0.82 ) | 1.97 (0.69, 5.58) | 0.82 (0.37, 1.83) | 0.67 (0.29, 1.55) | 1.75 (0.72, 4.29) |
| 1.43 (0.85, 2.42) | 1.00 (0.68, 1.47) | 1.08 (0.72, 1.61) | 0.80 (0.31, 2.06) | 0.92 (0.61, 1.39) | 0.87 (0.44, 1.72) | QUET | 5.25 ( 2.27, 12.1 ) | 2.18 ( 1.30, 3.65 ) | 1.80 ( 1.04, 3.11 ) | 4.67 ( 2.44, 8.93 ) |
| 1.34 (0.58, 3.09) | 0.93 (0.44, 1.98) | 1.01 (0.46, 2.20) | 0.75 (0.24, 2.36) | 0.86 (0.39, 1.89) | 0.82 (0.32, 2.08) | 0.94 (0.43, 2.03) | SERT | 0.42 ( 0.18, 0.98 ) | 0.34 ( 0.14, 0.84 ) | 0.89 (0.34, 2.30) |
| 2.38 ( 1.32, 4.31 ) | 1.66 ( 1.04, 2.65 ) | 1.80 ( 1.08, 2.98 ) | 1.34 (0.50, 3.56) | 1.54 (0.91, 2.58) | 1.45 (0.70, 3.01) | 1.66 ( 1.01, 2.74 ) | 1.78 (0.79, 4.02) | TIAG | 0.82 (0.46, 1.48) | 2.14 ( 1.09, 4.20 ) |
| 1.18 (0.70, 2.01) | 0.83 (0.58, 1.17) | 0.89 (0.59, 1.34) | 0.66 (0.26, 1.71) | 0.76 (0.49, 1.19) | 0.72 (0.37, 1.42) | 0.83 (0.54, 1.26) | 0.88 (0.41, 1.91) | 0.50 ( 0.30, 0.82 ) | VENL | 2.60 ( 1.36, 5.26 ) |
| 2.08 ( 1.15, 3.75 ) | 1.45 (0.94, 2.23) | 1.57 (0.95, 2.59) | 1.17 (0.44, 3.10) | 1.34 (0.80, 2.24) | 1.27 (0.62, 2.62) | 1.45 (0.89, 2.38) | 1.55 (0.69, 3.50) | 0.87 (0.50, 1.53) | 1.76 ( 1.08, 2.86 ) | VORT |
Drugs are reported in alphabetical order. Data are ORs (95% CI) in the column-defining treatment compared with the row-defining treatment. For remission rate, ORs higher than 1 favor the column-defining treatment. For tolerability, ORs lower than 1 favor the first drug in alphabetical order. Significant results are in bold and underscored.
TABLE 6.
Sensitivity results for remission rate. Remission rate (Results from Bayesian method were presented in upper triangle, Results from frequentist method were presented in lower triangle).
| AGOM | 1.40 (0.83, 2.50) | 1.30 (0.77, 2.30) | 1.80 (0.63, 5.00) | 1.60 (0.85, 2.90) | 1.70 (0.75, 3.60) | 1.40 (0.83, 2.50) | 1.40 (0.56, 3.50) | 2.40 (1.30, 4.70) | 1.20 (0.67, 2.00) | 2.10 (1.10, 3.90) | 2.70 (1.70, 4.40) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.44 (0.87, 2.37) | DULO | 0.93 (0.58, 1.40) | 1.20 (0.48, 3.10) | 1.10 (0.67, 1.70) | 1.20 (0.57, 2.30) | 1.00 (0.64, 1.50) | 0.95 (0.42, 2.20) | 1.70 (1.00, 2.80) | 0.81 (0.56, 1.20) | 1.50 (0.90, 2.30) | 1.90 (1.40, 2.50) |
| 1.33 (0.81, 2.17) | 0.92 (0.62, 1.37) | ESCI | 1.30 (0.52, 3.50) | 1.20 (0.70, 2.00) | 1.20 (0.59, 2.60) | 1.10 (0.70, 1.60) | 1.00 (0.43, 2.40) | 1.80 (1.00, 3.20) | 0.86 (0.57, 1.40) | 1.60 (0.90, 2.70) | 2.00 (1.50, 2.90) |
| 1.78 (0.66, 4.84) | 1.24 (0.49, 3.15) | 1.34 (0.52, 3.47) | LORA | 0.88 (0.33, 2.40) | 0.90 (0.35, 2.20) | 0.78 (0.28, 2.00) | 0.74 (0.21, 2.40) | 1.30 (0.46, 3.60) | 0.64 (0.23, 1.60) | 1.10 (0.40, 3.10) | 1.51 (0.63, 3.70) |
| 1.55 (0.90, 2.69) | 1.08 (0.72, 1.63) | 1.17 (0.74, 1.84) | 0.87 (0.33, 2.26) | PARO | 1.10 (0.49, 2.20) | 0.93 (0.58, 1.40) | 0.87 (0.36, 2.00) | 1.60 (0.87, 2.70) | 0.75 (0.45, 1.20) | 1.30 (0.76, 2.30) | 1.80 (1.20, 2.50) |
| 1.64 (0.77, 3.47) | 1.14 (0.59, 2.20) | 1.24 (0.62, 2.45) | 0.92 (0.39, 2.19) | 1.06 (0.53, 2.11) | PREG | 0.86 (0.43, 1.80) | 0.81 (0.30, 2.30) | 1.50 (0.68, 3.10) | 0.70 (0.34, 1.50) | 1.30 (0.58, 2.80) | 1.60 (0.87, 3.20) |
| 1.43 (0.85, 2.42) | 1.00 (0.68, 1.47) | 1.08 (0.72, 1.61) | 0.80 (0.31, 2.06) | 0.92 (0.61, 1.39) | 0.87 (0.44, 1.72) | QUET | 0.95 (0.41, 2.20) | 1.70 ( 0.97, 2.90 ) | 0.81 (0.51, 1.30) | 1.50 (0.84, 2.50) | 1.90 (1.40, 2.70) |
| 1.34 (0.58, 3.09) | 0.93 (0.44, 1.98) | 1.01 (0.46, 2.20) | 0.75 (0.24, 2.36) | 0.86 (0.39, 1.89) | 0.82 (0.32, 2.08) | 0.94 (0.43, 2.03) | SERT | 1.80 (0.60, 5.00) | 0.86 (0.37, 2.00) | 1.50 (0.63, 3.80) | 2.00 (0.91, 4.40) |
| 2.38 (1.32, 4.31) | 1.66 (1.04, 2.65) | 1.80 (1.08, 2.98) | 1.34 (0.50, 3.56) | 1.54 (0.91, 2.58) | 1.45 (0.70, 3.01) | 1.66 ( 1.01, 2.74 ) | 1.78 (0.79, 4.02) | TIAG | 0.48 (0.28, 0.82) | 0.86 (0.46, 1.60) | 1.10 (0.73, 1.80) |
| 1.18 (0.70, 2.01) | 0.83 (0.58, 1.17) | 0.89 (0.59, 1.34) | 0.66 (0.26, 1.71) | 0.76 (0.49, 1.19) | 0.72 (0.37, 1.42) | 0.83 (0.54, 1.26) | 0.88 (0.41, 1.91) | 0.50 (0.30, 0.82) | VENL | 1.80 (1.00, 3.10) | 2.40 (1.70, 3.30) |
| 2.08 (1.15, 3.75) | 1.45 (0.94, 2.23) | 1.57 (0.95, 2.59) | 1.17 (0.44, 3.10) | 1.34 (0.80, 2.24) | 1.27 (0.62, 2.62) | 1.45 (0.89, 2.38) | 1.55 (0.69, 3.50) | 0.87 (0.50, 1.53) | 1.76 (1.08, 2.86) | VORT | 1.30 (0.87, 2.00) |
| 2.70 (1.74, 4.19) | 1.88 (1.47, 2.40) | 2.03 (1.48, 2.78) | 1.51 (0.62, 3.70) | 1.74 (1.25, 2.42) | 1.64 (0.89, 3.02) | 1.88 (1.39, 2.55) | 2.01 (0.99, 4.10) | 1.13 (0.76, 1.68) | 2.28 (1.69, 3.07) | 1.30 (0.88.1.92) | PLAC |
Results from Bayesian method were presented in upper triangle, Results using frequentist method were presented in lower triangle.Data are ORs (95% CI) in the column-defining treatment compared with the row-defining treatment. For remission rate, ORs higher than 1 favour the column-defining treatment. For tolerability, ORs lower than 1 favour the first drug in alphabetical order. Significant results are in bold and underscored.
TABLE 7.
Sensitivity results for tolerability. Tolerability (Results from Bayesian method were presented in upper triangle, Results using frequentist method were presented in lower triangle).
| AGOM | 0.32 (0.12, 1.00) | 0.43 (0.17, 1.40) | 0.13 (0.03, 0.59) | 0.34 (0.12, 1.20) | 0.52 (0.16, 1.70) | 0.20 (0.07, 0.60) | 1.10 (0.27, 4.60) | 0.42 (0.14, 1.30) | 0.31 (0.11, 0.96) | 0.78 (0.25, 2.70) | 0.82 (0.31, 2.30) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.39 (0.13, 1.19) | DULO | 1.40 (0.81, 2.30) | 0.38 (0.14, 1.00) | 1.00 (0.57, 1.80) | 1.50 (0.64, 3.40) | 0.59 (0.35, 0.99) | 3.10 (1.30, 8.30) | 1.30 (0.71, 2.30) | 0.94 (0.61, 1.50) | 2.50 (1.20, 4.30) | 2.40 (1.70, 3.50) |
| 0.49 (0.17, 1.44) | 1.28 (0.76, 2.16) | ESCI | 0.28 (0.09, 0.77) | 0.74 (0.43, 1.30) | 1.10 (0.46, 2.50) | 0.42 (0.25, 0.71) | 2.40 (0.87, 6.20) | 0.90 (0.49, 1.70) | 0.70 (0.39, 1.20) | 1.80 (0.86, 3.50) | 1.70 (1.20, 2.70) |
| 0.14 (0.03, 0.56) | 0.36 (0.14, 0.95) | 0.28 (0.10, 0.76) | LORA | 2.90 (0.98, 7.80) | 4.00 (1.60, 9.50) | 1.60 (0.54, 4.50) | 8.80 (2.60, 33.00) | 3.50 (1.20, 9.60) | 2.50 (0.90, 7.20) | 6.30 (2.20, 18.00) | 6.30 (2.40, 17.00) |
| 0.36 (0.12, 1.11) | 0.93 (0.55, 1.57) | 0.72 (0.42, 1.24) | 2.58 (0.96, 6.93) | PARO | 1.40 (0.60, 3.40) | 0.56 (0.35, 0.97) | 3.30 (1.20, 8.20) | 1.20 (0.67, 2.30) | 0.90 (0.50, 1.90) | 2.40 (1.20, 4.60) | 2.40 (1.50, 3.70) |
| 0.55 (0.15, 1.97) | 1.42 (0.64, 3.13) | 1.11 (0.49, 2.49) | 3.94 (1.89, 8.23) | 1.53 (0.68, 3.42) | PREG | 0.38 (0.17, 0.93) | 2.20 (0.67, 6.70) | 0.88 (0.35, 2.00) | 0.62 (0.27, 1.50) | 1.60 (0.64, 4.10) | 1.60 (0.77, 3.60) |
| 0.20 (0.07, 0.62) | 0.53 (0.33, 0.87) | 0.42 (0.27, 0.65) | 0.42 (0.27, 0.65) | 0.57 (0.34, 0.90) | 0.37 (0.17, 0.82) | QUET | 5.60 (2.10, 14.00) | 2.20 (1.20, 3.90) | 1.60 (0.92, 3.00) | 4.20 (2.00, 8.00) | 4.20 (2.80, 6.10) |
| 1.08 (0.29, 4.02) | 2.79 (1.19, 6.53) | 2.18 (0.91, 5.20) | 7.79 (2.32, 26.18) | 3.01 (1.27, 7.12) | 1.97 (0.69, 5.58) | 5.25 (2.27, 12.1) | SERT | 0.39 ( 0.15, 1.10 ) | 0.31 (0.11, 0.78) | 0.78 (0.26, 2.10) | 0.74 (0.32, 1.80) |
| 0.45 (0.14, 1.39) | 1.16 (0.68, 1.96) | 0.90 (0.52, 1.58) | 3.22 (1.20, 8.64) | 1.25 (0.72, 2.16) | 0.82 (0.37, 1.83) | 2.18 (1.30, 3.65) | 0.42 ( 0.18, 0.98 ) | TIAG | 0.77 (0.38, 1.50) | 2.00 ( 0.94, 3.80 ) | 1.90 (1.20, 3.00) |
| 0.37 ( 0.12, 1.16 ) | 0.95 (0.62, 1.48) | 0.75 (0.43, 1.29) | 2.66 (0.97, 7.30) | 2.66 (0.97, 7.30) | 0.67 (0.29, 1.55) | 1.80 (1.04, 3.11) | 0.34 (0.14, 0.84) | 0.82 (0.46, 1.48) | VENL | 2.60 (1.10, 5.20) | 2.50 (1.50, 4.10) |
| 0.96 (0.29, 3.18) | 2.48 (1.45, 4.24) | 1.94 (0.99, 3.81) | 6.91 (2.39, 19.98) | 2.68 (1.36, 5.26) | 1.75 (0.72, 4.29) | 4.67 (2.44, 8.93) | 0.89 (0.34, 2.30) | 2.14 ( 1.09, 4.20 ) | 2.60 (1.36, 5.26) | VORT | 0.96 (0.60, 1.90) |
| 0.83 (0.28, 2.43) | 2.15 (1.49, 3.11) | 1.68 (1.11, 2.53) | 5.98 (2.41, 14.87) | 2.32 (1.56, 3.44) | 1.52 (0.75, 3.07) | 4.05 (2.89, 5.65) | 0.77 (0.35, 1.66) | 1.86 (1.25, 2.75) | 2.25 (1.43, 3.55) | 0.87 (0.49, 1.52) | PLAC |
Results from Bayesian method were presented in upper triangle, Results using frequentist method were presented in lower triangle.Data are ORs (95% CI) in the column-defining treatment compared with the row-defining treatment. For remission rate, ORs higher than 1 favour the column-defining treatment. For tolerability, ORs lower than 1 favour the first drug in alphabetical order. Significant results are in bold and underscored.
TABLE 8.
The ranking of all treatments.
| Outcomes | Treatments | SUCRA | Mean rank |
|---|---|---|---|
| Remission rate | Agomelatine | 89.7 | 2.1 |
| Venlafaxine | 77.2 | 3.5 | |
| Escitalopram | 67.1 | 4.6 | |
| Sertraline | 64 | 5.0 | |
| Duloxetine | 57.6 | 5.7 | |
| Quetiapine | 58.6 | 5.9 | |
| Paroxetine | 49.2 | 6.6 | |
| Pregabalin | 46.3 | 6.9 | |
| Lorazepam | 41.2 | 7.5 | |
| Vortioxetine | 23.9 | 9.4 | |
| Tiagabine | 19 | 9.9 | |
| Placebo | 6.2 | 11.3 | |
| Tolerability | Sertraline | 88.2 | 2.3 |
| Vortioxetine | 85.6 | 2.6 | |
| Agomelatine | 82.9 | 2.9 | |
| Placebo | 79.8 | 3.2 | |
| Pregabalin | 57.2 | 5.7 | |
| Escitalopram | 52.3 | 6.2 | |
| Tiagabine | 46.0 | 6.9 | |
| Duloxetine | 35.2 | 8.1 | |
| Paroxetine | 30.7 | 8.6 | |
| Venlafaxine | 31.6 | 8.6 | |
| Quetiapine | 7.4 | 11.2 | |
| Lorazepam | 3.2 | 11.7 |
Discussion
To our knowledge the current analysis constitutes the most up-to-date evidence with respect to comparisons of remission rate associated with pharmacological treatments obtained by pooling direct and indirect comparisons. A similar network meta-analysis was published by Baldwin D. S. et al., 2011 (Baldwin D. et al., 2011) but in the current study the newest interventions including agomelatine and vortioxetine were analyzed after a more broad-reaching search. Thus, the results of the present analysis may inform clinicians about how to choose appropriate treatments when various therapies are available.
Strict eligibility criteria ensured that high-quality studies were included in the meta-analysis. Trials that included patients with comorbidities were excluded to ensure the similarity assumption of network meta-analysis. Furthermore, the exclusion of studies of GAD with comorbidities allows us to speculate that the anxiolytic effect of drugs in GAD is independent from their effects on comorbidities. Consistency with reference to the similarity of different sources of evidence is an important component when evaluating the reliability and accuracy of network meta-analyses (Higgins et al., 2012; He et al., 2019) and in the present analysis no inconsistency between the overall results and the results of pairwise analysis was evident. These advantages strengthen the reliability and validity of our conclusions.
Paroxetine, duloxetine, quetiapine, escitalopram, venlafaxine, and agomelatine were better than placebo as determined by HAM-A scores ≤7. Patients administered these treatments may ultimately experience minimal symptoms of anxiety and achieve a full recovery after completing follow-up duration. Notably, however, of these six drugs only agomelatine was well tolerated. Other drugs did not exhibit superiority over a placebo. Paroxetine, duloxetine, quetiapine, escitalopram, venlafaxine, and lorazepam exhibited poor tolerability as defined by withdrawal due to adverse events. Tiagabine was the poorest with regard to remission rates, and agomelatine and venlafaxine were more efficacious than vortioxetine.
Currently most guidelines and meta-analyses recommend SSRIs and SNRIs as the first-line pharmacotherapies for GAD. In current analysis venlafaxine (six trials including 2,218 patients), duloxetine (8 trials including 3,392 patients), escitalopram (five trials including 2,093 patients) and paroxetine (four trials including 1,594 patients) were good in terms of remission and were comparable to other drugs in terms of tolerability, which is consistent with previous meta-analyses (Baldwin D. et al., 2011; He et al., 2019). Sertraline, recommended as first choice for GAD by the National Institute for Health and Care Excellence, was not significantly superior to placebo in terms of remission on the basis of the only relevant study in the present analysis (Allgulander et al., 2004). In contrast, in pairwise analysis sertraline was favorable in terms of remission rate. A small sample population may have reduced the accuracy of estimates for sertraline. It is hoped further trials will clarify conclusions pertaining to sertraline in the future. It was ranked the best in terms of tolerability in the current network meta-analysis, which is concordant with Baldwin D. S. et al., 2011 (Baldwin D. et al., 2011).
Comorbidity is common in GAD in clinical practice with approximately 62.4% of patients suffering from comorbid major depression and approximately 39.5% exhibiting dysthymia (Judd et al., 1998). Therefore, GAD subjects with the two major comorbidities can be treated with SSRIs or SNRIS. Although SSRIs and SNRIs are widely considered the first choice for GAD, their slow onset of action and unfavorable side effects including increased risk of bleeding of the gastrointestinal tract preclude their application in some patients (Tyrer et al., 2006; Carvalho et al., 2016; Laporte et al., 2017).
In the current analysis quetiapine (four trials including 3,036 patients) yielded better remission than placebo but exhibited worse tolerability than placebo or other drugs, which is consistent with previous meta-analyses (Stein et al., 2011; Maneeton et al., 2016). Quetiapine may be considered as an alternative treatment in patients with GAD comorbid with sleep disturbance because it can reportedly reduce the symptoms of anxiety and improve sleep (Monti and Monti, 2000; Maneeton et al., 2016). Agomelatine is currently approved for the treatment of GAD and major depressive disorder. It has a unique mechanism of action and functions as a melatonin receptor agonist on MT1 and MT2, and as a selective serotonin receptor antagonist on 5-HT2C receptors, which confers its capacity to treat relevant disorders (Millan et al., 2003; Guardiola-Lemaitre et al., 2014). Among the drugs included in the current analysis, agomelatine (three trials including 938 patients) had the largest effect on remission and exhibited the relatively good tolerability. Given its benefits, agomelatine may be an attractive option for the treatment of GAD with concurrent depression and insomnia. Unfortunately, in a systematic review published by Freiesleben et al. agomelatine associated with a markedly higher rate of liver injury than placebo, paroxetine, sertraline, escitalopram, and fluoxetine (Freiesleben and Furczyk, 2015). Hepatotoxicity may limit its use in practice.
Benzodiazepines are frequently used to treat GAD. Only one relevant RCT (including 191 patients) was included in the present analysis, and in that trial lorazepam was not superior to placebo or other drugs with regard to remission, and it exhibited the poorest tolerability. Disadvantages including a lack of the capacity to alleviate depression, dependence and side effects, together with poor tolerability, impede the use of benzodiazepines to treat anxiety disorders. In practice clinicians tend to combine an antidepressant and a benzodiazepine, then the benzodiazepine is gradually tapered off when the antidepressant shows effectiveness (Tyrer et al., 2006; Baldwin D. S. et al., 2011).
In the current analysis tiagabine (three trials including 1,791 patients) and pregabalin (two trials including 464 patients) yielded remission rate that were slightly but not statistically significantly better than placebo. Tiagabine was the poorest in terms of remission, which is consistent with the work of Baldwin D. S. et al., 2011 (Baldwin D. et al., 2011). Vortioxetine, a multimodal antidepressant, has been licensed for major depressive disorder since 2013. In a systematic review and meta-analysis of four placebo-controlled trials including GAD patients vortioxetine exhibited no superiority over placebo in terms of remission, and it was well tolerated (Qin et al., 2019), which is consistent with the results of the present analysis. Agomelatine and venlafaxine were better than vortioxetine in the current analysis.
There are, however, some important limitations. The substantial heterogeneity between the trials hindered some comparisons. Differences stemming from both baseline demographic characteristics and trial designs contribute to this existence of heterogeneity, particularly with regard to comorbidities and the severity of GAD. The analysis did not exclude patients with low-to-moderate depression or other anxiety disorders comorbid with GAD given that these comorbidities are common in clinical practice. Notably, however, it is extremely hard to determine the extent to which these comorbidities affected the results. The meta-analysis intentionally did not include unpublished studies, and this may have resulted in a degree of associated bias. Most of the studies included were sponsored by pharmaceutical manufactures, and this may have resulted in some reporting bias. Some studies with small samples reduced the strength and validity of some treatment comparisons. For example, only one eligible study was identified for sertraline and lorazepam, two studies for pregabalin, and three studies for agomelatine, vortioxetine and tiagabine. Studies with small samples may lead to conflicting results with regard to some comparisons for tolerability due to comparatively sensitivity. Thus, conclusions pertaining to these drugs should be drawn and interpreted conservatively. The vast majority of patients included in the current network meta-analysis were Caucasian, thus it is uncertain whether the findings are applicable to other ethnic groups. The use of the last observation carried forward approach and high dropout rates in some studies potentially resulted in attrition bias. Only short-term treatment was included (median duration 8 weeks), whereas GAD is known to be a chronic disorder that requires long-term treatment. Noteworthily, in a previous study has demonstrated that early improvement of GAD was associated with endpoint remission (Pollack et al., 2008a; Pollack et al., 2008b). We suspect that achieving remission via acute treatment is more beneficial to patients with GAD in long-term treatment.
In summary, the findings of this network meta-analysis constitute the latest evidence to consider when contemplating viable treatment options for GAD with respect to remission and tolerability profiles. All comparisons between current drugs should be considered within the context of the limitations of this network meta-analysis and patient’s specific situations. We hope that this meta-analysis provides helpful perspectives facilitating informed decisions by patients and clinicians.
Author Contributions
WK and HD designed this study. WK and XW selected the studies, extracted data and analyzed data. WK, JW, YZ, and YLZ drafted this manuscript. BS checked this manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations: PLAC, Placebo; DULO, Duloxetine; PARO, Paroxetine; SERT, Sertraline; ESCI, Escitalopram; VENL, Venlafaxine; QUET, Quetiapine; VORT, Vortioxetine; AGOM, Agomelatine; TIAG, Tiagabine; LORA, Lorazepam; PREG, Pregabalin.
References
- Alaka K. J., Noble W., Montejo A., Dueñas H., Munshi A., Strawn J. R., et al. (2014). Efficacy and safety of duloxetine in the treatment of older adult patients with generalized anxiety disorder: a randomized, double-blind, placebo-controlled trial. Int. J. Geriatr. Psychiatr. 29, 978–986. 10.1002/gps.4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allgulander C., Dahl A. A., Austin C., Morris P. L. P., Sogaard J. A., Fayyad R., et al. (2004). Efficacy of sertraline in a 12-week trial for generalized anxiety disorder. Am. J. Psychiatr. 161, 1642–1649. 10.1176/appi.ajp.161.9.1642 [DOI] [PubMed] [Google Scholar]
- Baldwin D., Woods R., Lawson R., Taylor D. (2011a). Efficacy of drug treatments for generalised anxiety disorder: systematic review and meta-analysis. BMJ 342, d1199 10.1136/bmj.d1199 [DOI] [PubMed] [Google Scholar]
- Baldwin D. S., Waldman S., Allgulander C. (2011). Evidence-based pharmacological treatment of generalized anxiety disorder. Int. J. Neuropsychopharmacol. 14, 697–710. 10.1017/s1461145710001434 [DOI] [PubMed] [Google Scholar]
- Ball S. G., Lipsius S., Escobar R. (2015). Validation of the geriatric anxiety inventory in a duloxetine clinical trial for elderly adults with generalized anxiety disorder. Int. Psychogeriatr. 27, 1533–1539. 10.1017/s1041610215000381 [DOI] [PubMed] [Google Scholar]
- Bandelow B., Chouinard G., Bobes J., Ahokas A., Eggens I., Liu S., et al. (2010). Extended-release quetiapine fumarate (quetiapine xr): a once-daily monotherapy effective in generalized anxiety disorder. Data from a randomized, double-blind, placebo- and active-controlled study. Int. J. Neuropsychopharmacol. 13, 305–320. 10.1017/s1461145709990423 [DOI] [PubMed] [Google Scholar]
- Bidzan L., Mahableshwarkar A. R., Jacobsen P., Yan M., Sheehan D. V. (2012). Vortioxetine (Lu AA21004) in generalized anxiety disorder: results of an 8-week, multinational, randomized, double-blind, placebo-controlled clinical trial. Eur. Neuropsychopharmacol 22, 847–857. 10.1016/j.euroneuro.2012.07.012 [DOI] [PubMed] [Google Scholar]
- Bose A., Korotzer A., Gommoll C., Li D. (2008). Randomized placebo-controlled trial of escitalopram and venlafaxine XR in the treatment of generalized anxiety disorder. Depress. Anxiety. 2, 854–861. 10.1002/da.20355 [DOI] [PubMed] [Google Scholar]
- Boyer P., Mahé V., Hackett D. (2004). Social adjustment in generalised anxiety disorder: a long-term placebo-controlled study of venlafaxine extended release. Eur. Psychiatr. 19, 272–279. 10.1016/j.eurpsy.2004.05.010 [DOI] [PubMed] [Google Scholar]
- Carvalho A. F., Sharma M. S., Brunoni A. R., Vieta E., Fava G. A. (2016). The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature. Psychother. Psychosom. 85, 270–288. 10.1159/000447034 [DOI] [PubMed] [Google Scholar]
- Chaimani A., Higgins J. P. T., Mavridis D., Spyridonos P., Salanti G. (2013). Graphical tools for network meta-analysis in STATA. PLoS One 8, e76654 10.1371/journal.pone.0076654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaimani A., Salanti G. (2012). Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Res. Synth. Methods 3, 161–176. 10.1002/jrsm.57 [DOI] [PubMed] [Google Scholar]
- Cipriani A., Furukawa T. A., Salanti G., Chaimani A., Atkinson L. Z., Ogawa Y., et al. (2018). Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 391, 1357–1366. 10.1016/s0140-6736(17)32802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coric V., Feldman H. H., Oren D. A., Shekhar A., Pultz J., Dockens R. C., et al. (2010). Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder. Depress. Anxiety 27, 417–425. 10.1002/da.20695 [DOI] [PubMed] [Google Scholar]
- Davidson J. R. T., Bose A., Korotzer A., Zheng H. (2004). Escitalopram in the treatment of generalized anxiety disorder: double-blind, placebo controlled, flexible-dose study. Depress. Anxiety 19, 234–240. 10.1002/da.10146 [DOI] [PubMed] [Google Scholar]
- Doyle A. C., Pollack M. H. (2003). Establishment of remission criteria for anxiety disorders. J. Clin. Psychiatr. 64 Suppl 15, 40–45. [PubMed] [Google Scholar]
- Feltner D., Crockatt J., Dubovsky S., Cohn C., Shrivastava R., Targum S., et al. (2003). A randomized, double-blind, placebo-controlled, fixed-dose, multicenter study of pregabalin in patients with generalized anxiety disorder. J. Clin. Psychopharmacol. 23, 240–249. 10.1097/01.jcp.0000084032.22282.ff [DOI] [PubMed] [Google Scholar]
- Freiesleben S. D., Furczyk K. (2015). A systematic review of agomelatine-induced liver injury. J. Mol. Psychiatry 3, 4 10.1186/s40303-015-0011-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould R. A., Otto M. W., Pollack M. H., Yap L. (1997). Cognitive behavioral and pharmacological treatment of generalized anxiety disorder: a preliminary meta-analysis. Behav. Ther. 28, 285–305. 10.1016/s0005-7894(97)80048-2 [DOI] [Google Scholar]
- Guardiola-Lemaitre B., De Bodinat C., Delagrange P., Millan M. J., Munoz C., Mocaër E. (2014). Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br. J. Pharmacol. 171, 3604–3619. 10.1111/bph.12720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartford J., Kornstein S., Liebowitz M., Pigott T., Russell J., Detke M., et al. (2007). Duloxetine as an SNRI treatment for generalized anxiety disorder: results from a placebo and active-controlled trial. Int. Clin. Psychopharmacol. 22, 167–174. 10.1097/yic.0b013e32807fb1b2 [DOI] [PubMed] [Google Scholar]
- He H., Xiang Y., Gao F., Bai L., Gao F., Fan Y., et al. (2019). Comparative efficacy and acceptability of first-line drugs for the acute treatment of generalized anxiety disorder in adults: a network meta-analysis. J. Psychiatr. Res. 118, 21–30. 10.1016/j.jpsychires.2019.08.009 [DOI] [PubMed] [Google Scholar]
- Higgins J. P. T., Altman D. G., Gotzsche P. C., Jüni P., Moher D., Oxman A. D., et al. (2011). The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 343, d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P. T., Jackson D., Barrett J. K., Lu G., Ades A. E., White I. R. (2012). Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res. Synth. Methods 3, 98–110. 10.1002/jrsm.1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P. T., Thompson S. G. (2002). Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- Hutton B., Salanti G., Caldwell D. M., Chaimani A., Schmid C. H., Cameron C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of Health Care interventions: checklist and explanations. Ann. Intern. Med. 162, 777–784. 10.7326/m14-2385 [DOI] [PubMed] [Google Scholar]
- Judd L. L., Kessler R. C., PauIus M. P., Zeller P. V., Wittchen H.-U., Kunovac J. L. (1998). Comorbidity as a fundamental feature of generalized anxiety disorders: results from the national comorbidity study (NCS). Acta Psychiatr. Scand. 98, 6–11. 10.1111/j.1600-0447.1998.tb05960.x [DOI] [PubMed] [Google Scholar]
- Kasper S., Gastpar M., Müller W. E., Volz H.-P., Möller H.-J., Schläfke S., et al. (2014). Lavender oil preparation silexan is effective in generalized anxiety disorder–a randomized, double-blind comparison to placebo and paroxetine. Int. J. Neuropsychopharmacol. 17, 859–869. 10.1017/s1461145714000017 [DOI] [PubMed] [Google Scholar]
- Khan A., Joyce M., Atkinson S., Eggens I., Baldytcheva I., Eriksson H. (2011). A randomized, double-blind study of once-daily extended release quetiapine fumarate (quetiapine XR) monotherapy in patients with generalized anxiety disorder. J. Clin. Psychopharmacol. 31, 418–428. 10.1097/jcp.0b013e318224864d [DOI] [PubMed] [Google Scholar]
- Koponen H., Allgulander C., Erikson J., Dunayevich E., Pritchett Y., Detke M. J., et al. (2007). Efficacy of duloxetine for the treatment of generalized anxiety disorder: implications for primary Care physicians. Prim. Care Companion J. Clin. Psychiatry 09, 100–107. 10.4088/pcc.v09n0203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte S., Chapelle C., Caillet P., Beyens M.-N., Bellet F., Delavenne X., et al. (2017). Bleeding risk under selective serotonin reuptake inhibitor (SSRI) antidepressants: a meta-analysis of observational studies. Pharmacol. Res. 118, 19–32. 10.1016/j.phrs.2016.08.017 [DOI] [PubMed] [Google Scholar]
- Lenox-Smith A. J., Reynolds A. (2003). A double-blind, randomised, placebo controlled study of venlafaxine XL in patients with generalised anxiety disorder in primary Care. Br. J. Gen. Pract. 53, 772–777. [PMC free article] [PubMed] [Google Scholar]
- Li D., Wang T., Shen S., Fang Z., Dong Y., Tang H. (2017). Urinary tract and genital infections in patients with type 2 diabetes treated with sodium-glucose Co-transporter 2 inhibitors: a meta-analysis of randomized controlled trials. Diabetes Obes. Metabol. 19, 348–355. 10.1111/dom.12825 [DOI] [PubMed] [Google Scholar]
- Mahableshwarkar A. R., Jacobsen P. L., Chen Y., Simon J. S. (2014). A randomised, double-blind, placebo-controlled, duloxetine-referenced study of the efficacy and tolerability of vortioxetine in the acute treatment of adults with generalised anxiety disorder. Int. J. Clin. Pract. 68, 49–59. 10.1111/ijcp.12328 [DOI] [PubMed] [Google Scholar]
- Mandos L. A., Reinhold J. A., Rickels K. (2009). Achieving remission in generalized anxiety disorder. What are the treatment options?. Psychiatr. Times 26, 38–41. Available at: www.psychiartrictimes.com/article/10168/1370513 (Accessed February 2, 2009). 20830312 [Google Scholar]
- Maneeton N., Maneeton B., Woottiluk P., Likhitsathian S., Suttajit S., Boonyanaruthee V., et al. (2016). Quetiapine monotherapy in acute treatment of generalized anxiety disorder: a systematic review and meta-analysis of randomized controlled trials. Drug Des. Devel. Ther. 10, 259–276. 10.2147/dddt.s89485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merideth C., Cutler A. J., She F., Eriksson H. (2012). Efficacy and tolerability of extended release quetiapine fumarate monotherapy in the acute treatment of generalized anxiety disorder: a randomized, placebo controlled and active-controlled study. Int. Clin. Psychopharmacol. 27, 40–54. 10.1097/yic.0b013e32834d9f49 [DOI] [PubMed] [Google Scholar]
- Mezhebovsky I., Mägi K., She F., Datto C., Eriksson H. (2013). Double-blind, randomized study of extended release quetiapine fumarate (quetiapine XR) monotherapy in older patients with generalized anxiety disorder. Int. J. Geriatr. Psychiatr. 28, 615–625. 10.1002/gps.3867 [DOI] [PubMed] [Google Scholar]
- Millan M. J., Gobert A., Lejeune F., Dekeyne A., Newman-Tancredi A., Pasteau V., et al. (2003). The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J. Pharmacol. Exp. Ther. 306, 954–964. 10.1124/jpet.103.051797 [DOI] [PubMed] [Google Scholar]
- Montgomery S., Chatamra K., Pauer L., Whalen E., Baldinetti F. (2008). Efficacy and safety of pregabalin in elderly people with generalised anxiety disorder. Br. J. Psychiatry 193, 389–394. 10.1192/bjp.bp.107.037788 [DOI] [PubMed] [Google Scholar]
- Monti J. M., Monti D. (2000). Sleep disturbance in generalized anxiety disorder and its treatment. Sleep Med. Rev. 4, 263–276. 10.1053/smrv.1999.0096 [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence (2011). Generalised anxiety disorder and panic disorder in adults: management. Available at: https://www.nice.org.uk/guidance/CG113 (Accessed January 26, 2011). [PubMed]
- Nicolini H., Bakish D., Duenas H., Spann M., Erickson J., Hallberg C., et al. (2009). Improvement of psychic and somatic symptoms in adult patients with generalized anxiety disorder: examination from a duloxetine, venlafaxine extended-release and placebo-controlled trial. Psychol. Med. 39, 267–276. 10.1017/s0033291708003401 [DOI] [PubMed] [Google Scholar]
- Nimatoudis I., Zissis N. P., Kogeorgos J., Theodoropoulou S., Vidalis A., Kaprinis G. (2004). Remission rates with venlafaxine extended release in Greek outpatients with generalized anxiety disorder. A double-blind, randomized, placebo controlled study. Int. Clin. Psychopharmacol. 19, 331–336. 10.1097/00004850-200411000-00003 [DOI] [PubMed] [Google Scholar]
- Pollack M. H., Kornstein S. G., Spann M. E., Crits-Christoph P., Raskin J., Russell J. M. (2008a). Early improvement during duloxetine treatment of generalized anxiety disorder predicts response and remission at endpoint. J. Psychiatr. Res. 42, 1176–1184. 10.1016/j.jpsychires.2008.02.002 [DOI] [PubMed] [Google Scholar]
- Pollack M. H., Tiller J., Xie F., Trivedi M. H. (2008b). Tiagabine in adult patients with generalized anxiety disorder: results from 3 randomized, double-blind, placebo-controlled, parallel-group studies. J. Clin. Psychopharmacol. 28, 308–316. 10.1097/jcp.0b013e318172b45f [DOI] [PubMed] [Google Scholar]
- Pollack M. H., Zaninelli R., Goddard A., McCafferty J. P., Bellew K. M., Burnham D. B., et al. (2001). Paroxetine in the treatment of generalized anxiety disorder: results of a placebo-controlled, flexible-dosage trial. J. Clin. Psychiatr. 62, 350–357. 10.4088/jcp.v62n0508 [DOI] [PubMed] [Google Scholar]
- Qin B., Huang G., Yang Q., Zhao M., Chen H., Gao W., et al. (2019). Vortioxetine treatment for generalised anxiety disorder: a meta-analysis of anxiety, quality of life and safety outcomes. BMJ Open. 9, e033161 10.1136/bmjopen-2019-033161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickels K., Zaninelli R., McCafferty J., Bellew K., Iyengar M., Sheehan D. (2003). Paroxetine treatment of generalized anxiety disorder: a double-blind, placebo-controlled study. Am. J. Psychiatry 160, 749–756. 10.1176/appi.ajp.160.4.749 [DOI] [PubMed] [Google Scholar]
- Rothschild A. J., Mahableshwarkar A. R., Jacobsen P., Yan M., Sheehan D. V. (2012). Vortioxetine (Lu AA21004) 5 Mg in generalized anxiety disorder: results of an 8-week randomized, double-blind, placebo-controlled clinical trial in the United States. Eur. Neuropsychopharmacol 22, 858–866. 10.1016/j.euroneuro.2012.07.011 [DOI] [PubMed] [Google Scholar]
- Rynn M., Russell J., Erickson J., Detke M. J., Ball S., Dinkel J., et al. (2008). Efficacy and safety of duloxetine in the treatment of generalized anxiety disorder: a flexible-dose, progressive-titration, placebo-controlled trial. Depress. Anxiety 25, 182–189. 10.1002/da.20271 [DOI] [PubMed] [Google Scholar]
- Salanti G., Ades A. E., Ioannidis J. P. A. (2011). Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J. Clin. Epidemiol. 64, 163–171. 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- Slee A., Nazareth I., Bondaronek P., Liu Y., Cheng Z., Freemantle N. (2019). Pharmacological treatments for generalised anxiety disorder: a systematic review and network meta-analysis. Lancet 393, 768–777. 10.1016/s0140-6736(18)31793-8 [DOI] [PubMed] [Google Scholar]
- Stack C. B., Localio A. R., Griswold M. E., Goodman S. N., Mulrow C. D. (2013). Handling of rescue and missing data affects synthesis and interpretation of evidence: the sodium-glucose cotransporter 2 inhibitor example. Ann. Intern. Med. 159, 285–288. 10.7326/0003-4819-159-4-201308200-00009 [DOI] [PubMed] [Google Scholar]
- Stein D. J., Ahokas A. A., de Bodinat C. (2008). Efficacy of agomelatine in generalized anxiety disorder: a randomized, double-blind, placebo-controlled study. J. Clin. Psychopharmacol. 28, 561–566. 10.1097/jcp.0b013e318184ff5b [DOI] [PubMed] [Google Scholar]
- Stein D. J., Bandelow B., Merideth C., Olausson B., Szamosi J., Eriksson H. (2011). Efficacy and tolerability of extended release quetiapine fumarate (quetiapine XR) monotherapy in patients with generalised anxiety disorder: an analysis of pooled data from three 8-week placebo-controlled studies. Hum. Psychopharmacol. Clin. Exp. 26, 614–628. 10.1002/hup.1256 [DOI] [PubMed] [Google Scholar]
- Stein D. J., Ahokas A., Márquez M. S., Höschl C., Oh K. S., Jarema M., et al. (2014). Agomelatine in generalized anxiety disorder: an active comparator and placebo-controlled study. J. Clin. Psychiatr. 75, 362–368. 10.4088/jcp.13m08433 [DOI] [PubMed] [Google Scholar]
- Stein D. J., Ahokas A., Jarema M., Avedisova A. S., Vavrusova L., Chaban O., et al. (2017). Efficacy and safety of agomelatine (10 or 25 Mg/day) in non-depressed out-patients with generalized anxiety disorder: a 12-week, double-blind, placebo-controlled study. Eur. Neuropsychopharmacol 27, 526–537. 10.1016/j.euroneuro.2017.02.007 [DOI] [PubMed] [Google Scholar]
- Tyrer P., Baldwin D. (2006). Generalised anxiety disorder. Lancet 368, 2156–2166. 10.1016/s0140-6736(06)69865-6 [DOI] [PubMed] [Google Scholar]
- Wittchen H.-U., Jacobi F. (2005). Size and burden of mental disorders in europe–a critical review and appraisal of 27 studies. Eur. Neuropsychopharmacol 15, 357–376. 10.1016/j.euroneuro.2005.04.012 [DOI] [PubMed] [Google Scholar]
- Wu W. Y., Wang G., Ball S. G., Desaiah D., Ang Q. Q. (2011). Duloxetine versus placebo in the treatment of patients with generalized anxiety disorder in China. Chin. Med. J. 124, 3260–3268. 10.3760/cma.j.issn.0366-6999.2011.20.010. [DOI] [PubMed] [Google Scholar]
- Yu W., Singh S. S., Calhoun S., Zhang H., Zhao X., Yang F. (2018). Generalized anxiety disorder in urban China: prevalence, awareness, and disease burden. J. Affect. Disord. 234, 89–96. 10.1016/j.jad.2018.02.012 [DOI] [PubMed] [Google Scholar]