Background:
Chronic heart failure with reduced ejection fraction impairs health-related quality of life (HRQL). Omecamtiv mecarbil (OM)—a novel activator of cardiac myosin—improves left ventricular systolic function and remodeling and reduces natriuretic peptides. We sought to evaluate the effect of OM on symptoms and HRQL in patients with chronic heart failure with reduced ejection fraction and elevated natriuretic peptides enrolled in the COSMIC-HF trial (Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure).
Methods:
Patients (n=448) were randomized 1:1:1 to placebo, 25 mg of OM BID, or to pharmacokinetically guided dose titration (OM-PK) for 20 weeks. The Kansas City Cardiomyopathy Questionnaire was administered to assess HRQL at baseline, 16 weeks, and 20 weeks. The primary scores of interest were the Total Symptom Score, Physical Limitation Scale, and Clinical Summary Score.
Results:
Mean change in score from baseline to 20 weeks for the Total Symptom Score was 5.0 (95% CI, 1.8–8.1) for placebo, 6.6 (95% CI, 3.4–9.8) for OM 25 mg (P=0.32 versus placebo), and 9.9 (95% CI, 6.7–13.0) for OM-PK (P=0.03 versus placebo); for the Physical Limitation Scale, it was 3.1 for placebo (95% CI, −0.3 to 6.6), 6.0 (95% CI, 3.1–8.9) for OM 25 mg (P=0.12), and 4.3 (95% CI, 0.7–7.9) for OM-PK (P=0.42); for the Clinical Summary Score, it was 4.1 (95% CI, 1.4–6.9) for placebo, 6.3 (95% CI, 3.6–9.0) for OM 25 mg (P=0.19), and 7.0 (95% CI, 4.1–10.0) for OM-PK (P=0.14). Differences between OM and placebo were greater in patients who were more symptomatic at baseline.
Conclusions:
HRQL as measured by the Total Symptom Score improved in patients with heart failure with reduced ejection fraction assigned to the OM-PK group relative to placebo. Ongoing trials are prospectively testing whether OM improves symptoms and HRQL in heart failure with reduced ejection fraction.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01786512.
Keywords: heart failure, longitudinal studies, quality of life, stroke volume, systole
What is New?
Omecamtiv mecarbil is a novel potential treatment for heart failure with reduced ejection fraction that works by directly targeting cardiac myosin to improve systolic function.
The phase II COSMIC study (Chronic Oral Study of Myosin Activation to Increase Contractility) demonstrated that omecamtiv mecarbil lowers natriuretic peptide concentrations and induces favorable ventricular remodeling.
The current report presents additional data from COSMIC on the effects of omecamtiv mecarbil on health-related quality of life. Twenty weeks of treatment with omecamtiv mecarbil resulted in numerically improved Kansas City Cardiomyopathy Questionnaire scores for the Total Symptom Score, Physical Limitations Score, and Clinical Summary Score compared with placebo, which was statistically significant for the Total Symptom Score.
What are The Clinical Implications?
Impaired quality of life is a major problem for patients with heart failure with reduced ejection fraction.
If the magnitude of improvements in Kansas City Cardiomyopathy Questionnaire scores reported here are confirmed in larger studies such as the ongoing Global Approach to Lowering Adverse Cardiac outcomes Through Improving Contractility and Multicenter Exercise Tolerance Evaluation of Omecamtiv Mecarbil Related to Increased Contractility in Heart Failure trials, omecamtiv mecarbil would represent a significant advance in medical therapy for heart failure with reduced ejection fraction.
Chronic heart failure (HF) is characterized by both a high risk of mortality and morbidity and by impairments in functional capacity and health-related quality of life (HRQL). For patients with HF and reduced ejection fraction (HFrEF), the development of effective treatments (collectively called guideline-directed medical therapy) has resulted in progressive improvements in morbidity and mortality. Improvement in functional capacity and HRQL with these therapies has been less well documented. Many patients with chronic HFrEF continue to have a high symptom burden and poor HRQL despite current treatments. Improving symptom burden and HRQL, especially in highly symptomatic patients, is, therefore, an important goal of treatment in HF,1 as has been recently emphasized in regulatory guidance for drug development in HF.2
Omecamtiv mecarbil (OM) is a selective cardiac myosin activator (myotrope) that improves cardiac contraction by binding to cardiac myosin and increasing the probability of force generating interactions between myosin and actin during the cardiac cycle.3,4 This pharmacology has been shown to result in dose-dependent increases in stroke volume in both healthy volunteers5 and patients with HF.6 Given that impaired systolic performance could be a major contributor to symptom burden in patients with HFrEF, there is substantial interest in the effect of OM on symptoms in patients with HF. The COSMIC-HF study (Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure) was a phase II study of OM in chronic HFrEF evaluating its effects on pharmacodynamics, cardiac remodeling, natriuretic peptides, and patient-reported outcomes. The results of COSMIC-HF demonstrated dose-dependent improvements in cardiac function, plasma concentrations of natriuretic peptides, and left ventricular (LV) remodeling after 20 weeks of treatment with OM compared with placebo.7 Herein we present data describing the effects of OM on the prespecified exploratory end point of HF symptoms and HRQL in the COSMIC-HF study.
Methods
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/clinical-trial-data-sharing-request. Details of the design and primary results of the COSMIC-HF study (https://www.clinicaltrials.gov; unique identifier: NCT01786512) have been published.7 Briefly, this study evaluated the effects 20 weeks of OM in patients with stable HF in 448 outpatients with chronic symptomatic HFrEF (New York Heart Association II or III), LV ejection fraction ≤40%, and elevated natriuretic peptides NT-proBNP (N-terminal pro-B-type natriuretic peptide) ≥200 pg/mL (≥1200 pg/mL if the patient was in atrial fibrillation). Patients were randomized 1:1:1 to placebo, 25 mg of oral OM BID (25 mg), or pharmacokinetically guided dose titration (OM-PK; initial 25 mg BID dose, increased to 50 mg BID depending on plasma concentrations after 2 weeks). The entry criteria for COSMIC-HF mandated clinical stability and optimized background HF therapy at the time of study entry, making it well suited to assess the effects of OM on HRQL in HFrEF. The study was approved by the relevant institutional review boards or ethics committees at participating sites, and all patients provided written informed consent.
Patient-Reported Outcome Measurements
Participants completed self-administered Kansas City Cardiomyopathy Questionnaire (KCCQ) at baseline, 16 weeks, and 20 weeks. The KCCQ is a 23-item self-administered, disease-specific HRQL instrument that has demonstrated good measurement properties in patients with HFrEF.8 The KCCQ is made up of 8 domains evaluating specific aspects of the patient experience. The 8 domains include physical limitation, symptom stability, symptom frequency, symptom burden, Total Symptom Score (TSS), HRQL, self-efficacy, and social limitations. The TSS is a composite of symptom frequency and symptom burden. The Physical Limitations Score (PLS) measures specific physical limitations (eg, dressing, showering). The Clinical Summary Score (CSS) is a composite of the TSS and the PLS. The TSS, PLS, and CSS were recently qualified by the Food and Drug Administration for use in measuring these concepts in drug development.9 Each domain and summary score is scaled from 0 to 100, with higher scores indicating better health status. Based on prior work, ≥5 points is generally considered a minimally clinically meaningful difference in KCCQ scores.10 In addition to the KCCQ, patients and clinicians also assessed the overall burden of symptoms using the Patient Global Rating of Severity and Clinician Global Rating of Severity, which are 6 point Likert scale assessments rating the severity of symptoms from none to very severe. In additional analyses assessing the effect of baseline symptoms severity on the treatment effect of OM, we grouped patients by whether they had rated their baseline symptoms as none, very mild, or mild (n=242) or as moderate, severe, or very severe (n=202).
Statistical Analysis
Descriptive statistics are presented as means (SD), medians (interquartile range), and percentages, as appropriate. Summary statistics were produced using observed data only without applying models or imputation. Participants with missing data were considered nonresponders in categorical responder analyses. P<0.05 was considered to represent statistical significance. There was no adjustment for multiple comparisons.
Treatment group differences for changes in TSS, PLS, and CSS were estimated by using a repeated measures model (ANOVA) fitted separately for each variable and included the stratification factor of the presence or absence of atrial fibrillation/flutter at randomization, baseline value, treatment group, visit, and the treatment group by visit interaction. An unstructured covariance matrix was used to account for the correlation between visits within a subject. Pearson correlation coefficients were calculated to measure the correlation of QOL with natriuretic peptides and LV dimensions.
Results
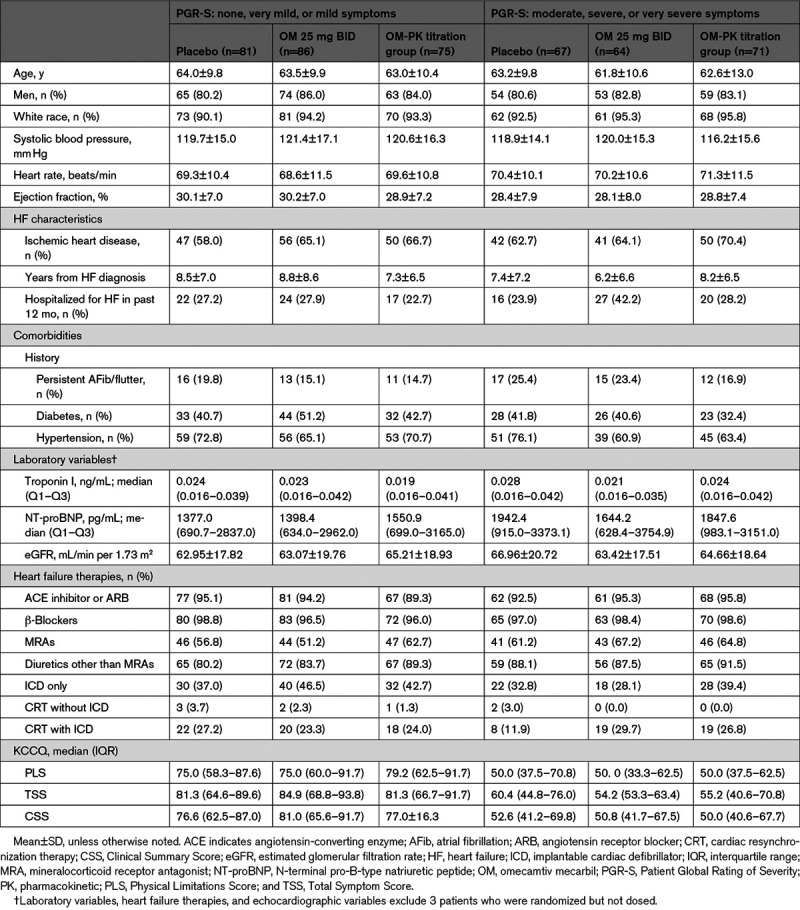
Baseline characteristics dichotomized by patient-reported symptom severity (as assessed by Patient Global Rating of Severity) are shown in Table 1. In general, patients with less severe symptoms and patients with more severe symptoms at baseline were broadly similar, except for KCCQ scores. For placebo, OM 25 mg, and OM-PK groups, the median baseline TSS was 70.8 (56.3–87.5), 74.5 (52.1–90.6), and 68.8 (53.1–85.4), respectively; the median baseline PLS was 62.5 (45.8–83.3), 66.7 (50.0–83.3), and 62.5 (45.8–85.0), respectively, and the median baseline CSS was 69.2 (52.1–82.3), 68.7 (49.9–85.4), and 67.2 (50.0–83.3), respectively. These scores are consistent with moderate limitation in health status and represented more symptomatic patients compared with those reported in other recent trials in chronic HFrEF (eg, median CSS was 72 in Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure and 76 in Prospective Comparison of ARNI [Angiotensin Receptor -Neprilysin Inhibitor] with ACEI [Angiotensin-Converting -Enzyme Inhibitor] to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial).11,12 Rates of missingness for KCCQ data were generally low (≤20 missing values for each treatment group and time point).
Table 1.
Baseline Characteristics Stratified by Symptom Severity
Effect of OM on Symptoms and HRQL
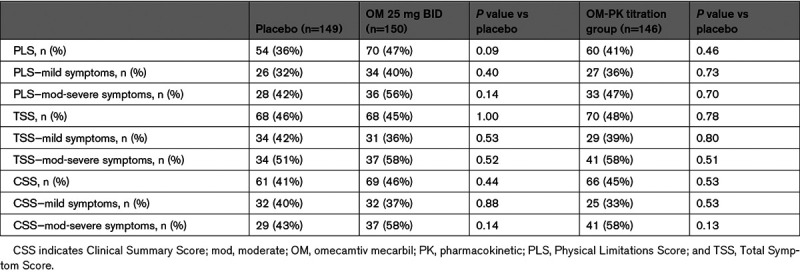
The overall effects of OM on TSS, PLS, and CSS measured at 16 and 20 weeks are shown in Figure 1. For the TSS, the mean change in score from baseline to 20 weeks was 5.0 for placebo, 6.6 for OM 25 mg (P=0.32 versus placebo), and 9.9 for OM-PK (P=0.03 versus placebo). For the PLS, the mean change in score from baseline to 20 weeks was 3.1 for placebo, 6.0 for OM 25 mg (P=0.12 versus placebo), and 4.3 for OM-PK (P=0.42 versus placebo). For the CSS, the mean change in score from baseline to 20 weeks was 4.1 for placebo, 6.3 for OM 25 mg (P=0.19 versus placebo), and 7.0 for OM-PK (0.14 versus placebo). As shown in Figure 1, these trends on KCCQ were evident by 16 weeks of treatment and persisted to week 20. Given that a 5-point change in KCCQ score has been proposed as a minimum clinically important change, we assessed the proportion of patients with an improvement of that amount or greater in each group for each domain (ie, responders). In this analysis, the proportion of responders did not differ significantly between placebo and OM for any of the KCCQ scores (Table 2).
Figure 1.
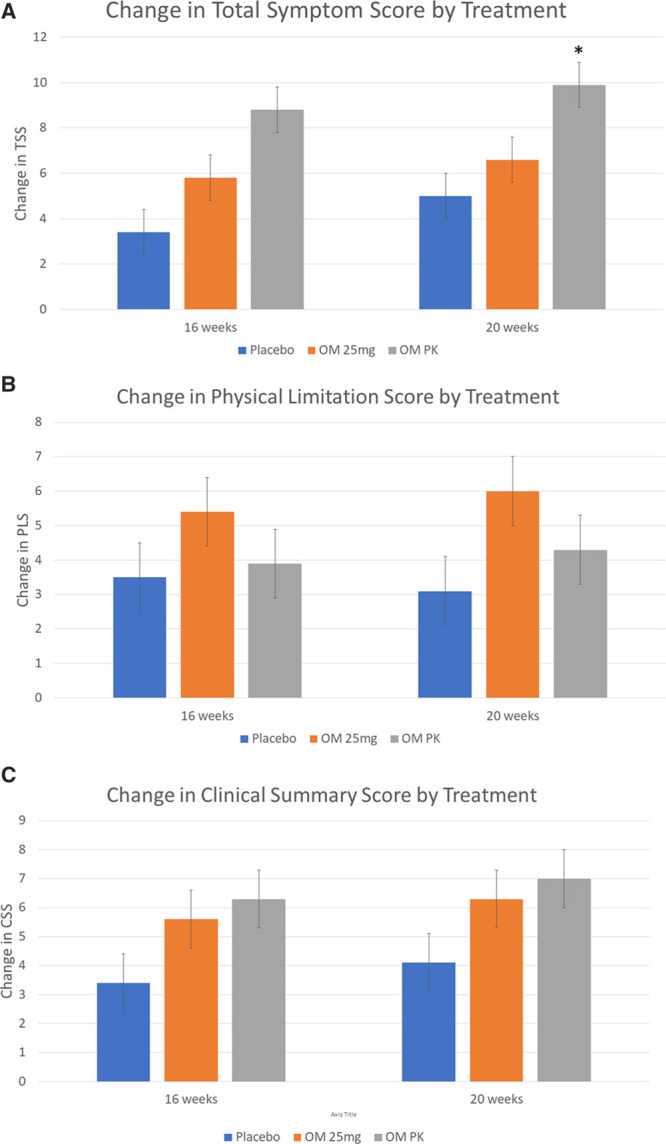
Mean change in Kansas City Cardiomyopathy Questionnaire from baseline through weeks 16 and 20 for Total Symptom Score (TSS), Physical Limitations Score (PLS), and Clinical Summary Score (CSS). Error bars show ±SE. OM indicates omecamtiv mecarbil; and PK, pharmacokinetic. *P≤0.05, all other comparisons, P>0.05. Placebo = blue bars, OM 25mg = Orange bars, OM PK = Grey bars.
Table 2.
Proportion of Patients With Minimally Clinically Important Difference in Kansas City Cardiomyopathy Questionnaire Scores by Treatment, Stratified by Baseline Symptom Severity
Change in Symptoms and HRQL by Baseline Symptoms Severity
Since the COSMIC-HF study enrolled patients with a spectrum of symptoms (from minimally to highly symptomatic), we performed additional analyses stratifying the population by severity of baseline symptoms. The rationale for these analyses was that patients who were more symptomatic at baseline had a greater opportunity for improvement than those with milder symptoms at baseline. For these analyses, we utilized the baseline Patient Global Rating of Severity score, in which patients rated their symptom burden on a 6-point Likert scale from none to very severe, to stratify participants based on their self-reported symptom severity. We grouped patients by whether they had rated their symptoms as none, very mild, or mild (n=242) or as moderate, severe, or very severe (n=202). In this analysis, the improvement in symptoms with OM compared with placebo was greater for those with more severe symptoms (Figure 2). Similarly, the differences in the proportion of responders (≥5-point improvement in the KCCQ score) between OM and placebo were numerically greater in patients with moderate to severe symptoms at baseline, although none of these differences were statistically significant.
Figure 2.
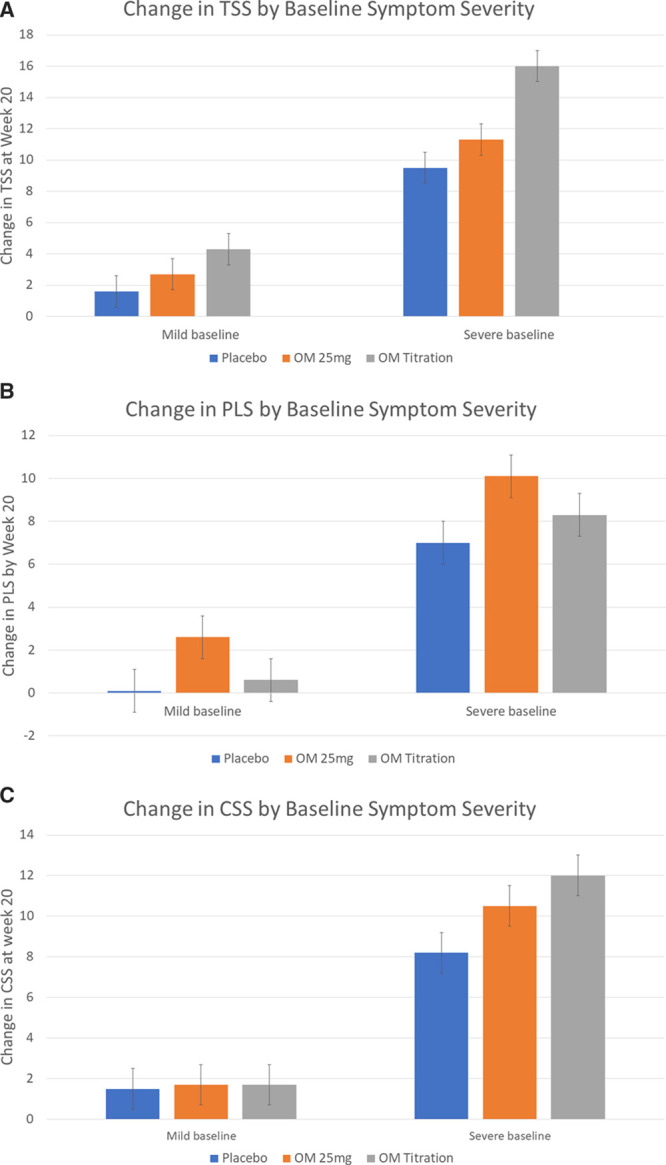
Change in Total Symptom Score (TSS), Physical Limitations Score (PLS), and Clinical Summary Score (CSS) based on symptom severity at baseline. Placebo = blue bars, OM 25mg = Orange bars, OM PK = Grey bars. OM indicates omecamtiv mecarbil; and PK, pharmacokinetic.
Correlation of QOL With Natriuretic Peptides and LV Dimensions
Given that an important objective of the COSMIC-HF study was to assess the impact of OM on ventricular remodeling and natriuretic peptides, we assessed the relationship between improvements in KCCQ and changes in both ventricular remodeling and NT-proBNP observed in COSMIC-HF. As reported previously, treatment with OM in COSMIC-HF improved LV remodeling (ventricular volumes and LV ejection fraction) and reduced plasma NT-proBNP compared with placebo.7 In the current analysis, there was a modest relationship between improvements in KCCQ and decrease in NT-proBNP for PLS (r=−0.08, P=0.098), CSS (r=−0.14, P=0.007), and TSS (r=−0.15, P=0.002). These relationships were the strongest for patients assigned to the pharmacokinetic titration arm; PLS (r=−0.15, P=0.093), CSS (r=−0.23, P=0.009), and TSS (r=−0.26, P=0.003). There was no significant relationship between changes in LV remodeling and changes in KCCQ scores (data not shown).
Discussion
Improvement in patient-reported outcomes is an important goal in the management of HF. In the current analysis, administration of OM improved HRQL as measured by the TSS compared with placebo. Other domains of the KCCQ were not significantly different. This analysis focused on those domains of the KCCQ, specifically the TSS, PLS, and CSS, that have recently been qualified by the Food and Drug Administration for assessing the benefits of interventions for HF. As might be anticipated, observed changes were the greatest in patients who were most symptomatic at baseline, whereas the changes in KCCQ in patients with no or minimal symptoms at baseline were negligible.
These data should be compared with reports of the effects of other interventions for HFrEF on KCCQ scores. In general, effective HF therapies have a variable effect on HRQL. For older therapies such as β-blockers and ACE (angiotensin-converting enzyme) inhibitors, there are few data directly assessing HRQL compared with placebo and none using the KCCQ. β-Blockers have notable effects on ventricular remodeling, morbidity, and mortality but appear to exert little or no effect on improving HFQoL.13 More recently developed therapies for HFrEF had undergone more rigorous assessment of their effect on HRQL. The largest reported effects are in the 2- to 3-point improvement in KCCQ range, specifically for dapagliflozin,11 or exercise training.14 The CARE-HF study (Cardiac Resynchronisation-Heart Failure) of cardiac resynchronization therapy showed a more marked improvement on HRQL but used the Minnesota Living With Heart Failure Score,15 as did the African American Heart Failure Trial study of nitrates and hydralazine in self-identified Black patients.16 Notably, the angiotensin receptor blocker/neprilysin inhibitor sacubitril-valsartan improved the CSS by a more modest mean change of 0.9 points after 8 months of treatment compared with enalapril,12 despite the large observed differences in morbidity and mortality between the two treatment arms.17 In this context, the improvements seen in HRQL for the OM-PK titration group over placebo at 20 weeks of treatment by TSS (4.9 points) equal or exceed those of common effective HF therapies. These improvements are numerically greater when limited to the subgroup of patients who were more symptomatic at baseline (6.5 for TSS).
The specific mechanisms underlying the improvements in QOL with effective HF therapies are poorly defined and probably diverse. Given the mechanism of action of OM is to improve LV performance, we analyzed whether improvement in symptoms and HRQL might mirror changes in LV remodeling or natriuretic peptides, both of which were improved by OM treatment compared with placebo in COSMIC. However, we found only modest relationships between improvements in KCCQ scores and declines in plasma NT-proBNP and none with changes in ventricular function or remodeling. These results underscore the complex nature of symptom and HRQL improvement in HF.
Limitations
COSMIC-HF was a phase 2 trial and was not powered to provide definitive evidence of beneficial effects on symptoms or HRQL for OM. Given the smaller sample size and shorter duration of treatment (20 weeks), and the violation of normality (changes from the baseline of PLS), the CIs around the effect estimates on HRQL are broad in comparison to those from larger phase 3 studies of other therapies, and most of the observed differences in KCCQ did not reach statistical significance. It is possible that our results are related to the play of chance rather than a true treatment effect. Given the sample size of COSMIC-HF and the modest number of clinical events, we are not able to assess the relationship between the observed KCCQ improvements and clinical outcomes.
Conclusions
In COSMIC-HF, randomization to OM led to —statistically significant improvement in TSS in the OM-PK titration group compared with placebo. Point estimates of the effect of KCCQ were of a similar or greater magnitude to those seen with other effective pharmacological interventions for HF. The effect of OM on HF symptoms, specifically the TSS of the KCCQ, is being further tested prospectively as a predefined secondary end point, in ongoing trials focused on morbidity and mortality (Global Approach to Lowering Adverse Cardiac outcomes Through Improving Contractility in Heart Failure; https://www.clinicaltrials.gov; unique identifier: NCT02929329)18 and exercise capacity (Multicenter Exercise Tolerance Evaluation of Omecamtiv Mecarbil Related to Increased Contractility in Heart Failure; https://www.clinicaltrials.gov; unique identifier: NCT03759392), which will further inform the clinical benefits of this potential therapy in patients with HFrEF.
Sources of Funding
The COSMIC-HF trial (Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure) was funded by Amgen and Cytokinetics.
Disclosures
Dr Felker has received research grants from the National Heart, Lung, and Blood Institute (NHLBI), American Heart Association, Amgen, Bayer Merck, Cytokinetics, Myokardia, Bayer, and Roche Diagnostics; he has acted as a consultant to Novartis, Amgen, Bristol Myers Squibb, Cytokinetics, Medtronic, Cardionomic, V-Wave, Myokardia, Innolife, EBR Systems, Arena, Abbott, Roche Diagnostics, Alnylam, LivaNova, Eidos Therapeutics, Rocket Pharma, Reprieve, and SC Pharma. Dr Solomon has received research grants from Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, Bristol Myers Squibb, Celladon, Cytokinetics, Eidos, Gilead, Glaxo Smith Kline, Ionis, Lone Star Heart, Mesoblast, MyoKardia, National Institutes of Health/NHLBI, Neurotronik, Novartis, Respicardia, Sanofi Pasteur, and Theracos and has consulted for Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Bristol Myers Squibb, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, Gilead, Glaxo Smith Kline, Ironwood, Merck, Myokardia, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions, Tenaya, Sanofi Pasteur, Dinaqor, Tremeau, CellProThera, and Moderna. Dr McMurray reports consultancies paid to Glasgow University with Alnylam, Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Cardurion, Cytokinetics, DalCor, Glaxo Smith Kline, Merck, Novartis, Pfizer, Servier Theracos, and Vifor-Fresenius. Dr Cleland reports grants and personal fees from Bristol Myers Squibb, Amgen, Bayer, Novartis, Myokardia, Pharmacosmos, Vifor, Bristol Myers Squibb, and Servier; personal fees from Abbott; and personal fees and nonfinancial support from Medtronic. Drs Abbasi, Zhang, and Globe are employees of Amgen. Dr Malik is an employee of Cytokinetics. Dr Teerlink reports research grants and consulting fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb, Cytokinetics, EBR Systems, Medtronic, Merck, and Novartis.
Nonstandard Abbreviations and Acronyms
- ACE
- angiotensin-converting enzyme
- CARE-HF
- Cardiac Resynchronisation-Heart Failure
- COSMIC-HF
- Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure
- CSS
- Clinical Summary Score
- HF
- heart failure
- HFrEF
- heart failure with reduced ejection fraction
- HRQL
- health-related quality of life
- KCCQ
- Kansas City Cardiomyopathy Questionnaire
- LV
- left ventricle
- NT-proBNP
- N-terminal pro-B-type natriuretic peptide
- OM
- omecamtiv mecarbil
- OM-PK
- Omecamtiv Mecarbil Pharmacokinetically guided dose titration
- PLS
- Physical Limitation Score
- TSS
- Total Symptom Score
For Sources of Funding and Disclosures, see page 721.
Contributor Information
Scott D. Solomon, Email: ssolomon@rics.bwh.harvard.edu.
John J.V. McMurray, Email: john.mcmurray@glasgow.ac.uk.
John G.F. Cleland, Email: j.cleland@imperial.ac.uk.
Siddique A. Abbasi, Email: saa960@mail.harvard.edu.
Fady I. Malik, Email: fmalik@cytokinetics.com.
Hanze Zhang, Email: hzhang05@amgen.com.
Gary Globe, Email: gglobe@amgen.com.
John R. Teerlink, Email: john.teerlink@ucsf.edu.
References
- 1.Psotka MA, von Maltzahn R, Anatchkova M, Agodoa I, Chau D, Malik FI, Patrick DL, Spertus JA, Wiklund I, Teerlink JR. Patient-reported outcomes in chronic heart failure: applicability for regulatory approval. JACC Heart Fail. 2016;4:791–804. doi: 10.1016/j.jchf.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). U.S. Department of Health and Human Services Food and Drug Administration CfDEaRC, Center for Biologics Evaluation and Research (CBER), editor. In: Treatment for heart failure: endpoints for drug development guidance for industry. 2019. Silver Spring, MD: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/treatment-heart-failure-endpoints-drug-development-guidance-industry [Google Scholar]
- 3.Psotka MA, Gottlieb SS, Francis GS, Allen LA, Teerlink JR, Adams KF, Jr, Rosano GMC, Lancellotti P. Cardiac calcitropes, myotropes, and mitotropes: JACC review topic of the week. J Am Coll Cardiol. 2019;73:2345–2353. doi: 10.1016/j.jacc.2019.02.051 [DOI] [PubMed] [Google Scholar]
- 4.Malik FI, Hartman JJ, Elias KA, Morgan BP, Rodriguez H, Brejc K, Anderson RL, Sueoka SH, Lee KH, Finer JT, et al. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science. 2011;331:1439–1443. doi: 10.1126/science.1200113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teerlink JR, Clarke CP, Saikali KG, Lee JH, Chen MM, Escandon RD, Elliott L, Bee R, Habibzadeh MR, Goldman JH, et al. Dose-dependent augmentation of cardiac systolic function with the selective cardiac myosin activator, omecamtiv mecarbil: a first-in-man study. Lancet. 2011;378:667–675. doi: 10.1016/S0140-6736(11)61219-1 [DOI] [PubMed] [Google Scholar]
- 6.Cleland JG, Teerlink JR, Senior R, Nifontov EM, Mc Murray JJ, Lang CC, Tsyrlin VA, Greenberg BH, Mayet J, Francis DP, et al. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet. 2011;378:676–683. doi: 10.1016/S0140-6736(11)61126-4 [DOI] [PubMed] [Google Scholar]
- 7.Teerlink JR, Felker GM, McMurray JJ, Solomon SD, Adams KF, Jr, Cleland JG, Ezekowitz JA, Goudev A, Macdonald P, Metra M, et al. ; COSMIC-HF Investigators. Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC-HF): a phase 2, pharmacokinetic, randomised, placebo-controlled trial. Lancet. 2016;388:2895–2903. doi: 10.1016/S0140-6736(16)32049-9 [DOI] [PubMed] [Google Scholar]
- 8.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3 [DOI] [PubMed] [Google Scholar]
- 9.FDA Gov. Qualification of the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score and its Component Scores A Patient-Reported Outcome Instrument for Use in Clinical Investigations in Heart Failure. 2020FDA Guidance Document. Accessed October 18, 2020. https://www.fda.gov/media/136862/download
- 10.Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, et al. ; Cardiovascular Outcomes Research Consortium. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150:707–715. doi: 10.1016/j.ahj.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 11.Kosiborod MN, Jhund PS, Docherty KF, Diez M, Petrie MC, Verma S, Nicolau JC, Merkely B, Kitakaze M, DeMets DL, et al. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA-HF trial. Circulation. 2020;141:90–99. doi: 10.1161/CIRCULATIONAHA.119.044138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis EF, Claggett BL, McMurray JJV, Packer M, Lefkowitz MP, Rouleau JL, Liu J, Shi VC, Zile MR, Desai AS, et al. Health-related quality of life outcomes in PARADIGM-HF. Circ Heart Fail. 2017;10:e003430. [DOI] [PubMed] [Google Scholar]
- 13.Dobre D, van Jaarsveld CH, deJongste MJ, Haaijer Ruskamp FM, Ranchor AV. The effect of beta-blocker therapy on quality of life in heart failure patients: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. 2007;16:152–159. doi: 10.1002/pds.1234 [DOI] [PubMed] [Google Scholar]
- 14.Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, et al. ; HF-ACTION Investigators. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1451–1459. doi: 10.1001/jama.2009.457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleland JG, Calvert MJ, Verboven Y, Freemantle N. Effects of cardiac resynchronization therapy on long-term quality of life: an analysis from the Cardiac Resynchronisation-Heart Failure (CARE-HF) study. Am Heart J. 2009;157:457–466. doi: 10.1016/j.ahj.2008.11.006 [DOI] [PubMed] [Google Scholar]
- 16.Taylor AL, Ziesche S, Yancy C, Carson P, D’Agostino R, Jr, Ferdinand K, Taylor M, Adams K, Sabolinski M, Worcel M, et al. ; African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–2057. doi: 10.1056/NEJMoa042934 [DOI] [PubMed] [Google Scholar]
- 17.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, et al. ; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 18.Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, Legg JC, Büchele G, Varin C, Kurtz CE, et al. Omecamtiv mecarbil in chronic heart failure with reduced ejection fraction: rationale and design of GALACTIC-HF. JACC Heart Fail. 2020;8:329–340. doi: 10.1016/j.jchf.2019.12.001 [DOI] [PubMed] [Google Scholar]


