Background:
Bronchoscopic diagnosis of small peripheral lung lesions suspected of lung cancer remains a challenge. A successful endobronchial diagnosis comprises navigation, confirmation, and tissue acquisition. In all steps, 3-dimensional information is essential. Cone-beam computed tomography (CBCT) imaging can provide computed tomography information and 3-dimensional augmented fluoroscopy imaging. We assessed whether CBCT imaging can improve navigation and diagnosis of peripheral lesions by 2 clinical workflows with a cross-over design: (1) a primary CBCT and radial endobronchial ultrasound mini probe imaging–based approach and (2) a primary electromagnetic navigation (EMN) and radial endobronchial ultrasound mini probe imaging–based approach.
Methods:
All patients with a peripheral lung lesion biopsy indication were eligible for study inclusion and randomly assigned to study arms. Commercially available equipment was used. The main study goals were to assess CBCT-confirmed navigation success and diagnostic accuracy. Surgery or unambiguous clinical follow-up served as the gold standard.
Results:
Eighty-seven patients with 107 lesions were included. Lesion mean longest axis size in the CBCT arm was 16.6 mm (n=47) and 14.2 mm in the EMN arm (n=40). The primary CBCT approach and primary EMN approach had 76.3% and 52.2% navigation success, respectively. Addition of EMN to the CBCT approach increased navigation success to 89.9%. Addition of CBCT imaging to the EMN approach significantly increased navigation success to 87.5% per lesion. The overall diagnostic accuracy per patient was significantly lower than the navigation success, being 72.4%.
Conclusion:
CBCT imaging is a valuable addition to navigation bronchoscopy. Although overall navigation success was high, the diagnostic accuracy remains to be improved. Future research should focus on improving the tissue acquisition methodology.
Key Words: augmented fluoroscopy, bronchoscopy, cone beam computed tomography, CBCT, electromagnetic navigation, navigation bronchoscopy, peripheral lung lesion, transbronchial lung biopsy
Small peripheral pulmonary lesions suspected of lung cancer remain a diagnostic challenge. Despite computed tomography (CT) screening programs being implemented and a preference for minimally invasive diagnostics where possible,1–6 there are no unambiguous guidelines on the use of technology for an endobronchial approach. Over the last decade, several endobronchial techniques have become available that are able to increase the diagnostic yield of the conventional fluoroscopy-guided transbronchial biopsy approach (pooled yield 31.3%).7 Techniques such as radial ultrasound mini-probe imaging (rEBUS), ultrathin bronchoscopy, virtual navigation bronchoscopy and electromagnetic navigation bronchoscopy (EMN) have been successfully implemented in clinical routine.8 A meta-analysis by Wang et al9 showed that the diagnostic yield of these techniques ranged from 67% to 73%, and that their overall pooled diagnostic yield was ∼70%. The prospective multicenter NAVIGATE trial that evaluated EMN further substantiated this diagnostic yield in a total of 1105 patients. By EMN, in combination with fluoroscopy (91% of cases) and rEBUS imaging (57% of cases), they achieved a combined overall diagnostic yield of 73% and 67.3% in lesions <20 mm.10 Although these are promising results when compared with the conventional transbronchial biopsy, a diagnostic yield of ∼70% still demands further improvement.
Navigation bronchoscopy can technically be divided into 3 steps: navigation, confirmation, and acquisition.11 A successful diagnosis can only be obtained if all 3 steps are accurately performed. The integration of robotics in endoscopy is a new approach that could enhance accuracy in all 3 steps.12,13 Yet, as lesions become smaller, have no bronchus sign, or are positioned at tight angulations relative to the airway, precise 3-dimensional (3D) imaging information likely becomes an essential need for accurate navigation and (transbronchial) biopsy. Furthermore, 3D information will also enable minimally invasive transbronchial treatment.11,14
Cone-beam computed tomography (CBCT) imaging is a relatively new modality in interventional pulmonology, but, readily available as ceiling-mounted or floor-mounted C-arm system in the interventional radiology unit or hybrid operating room. CBCT imaging allows for CT imaging as well as fluoroscopy. An initial CBCT scan does not only provide 3D information, it can be further used to delineate and overlay the lesion and pathway toward the lesion on regular fluoroscopy images (Figs. 2, 3). This overlay, also termed augmented fluoroscopy (AF), is accurately maintained in 3D during every movement of the C-arm. Repeated CBCT scans can provide exact 3D confirmation of lesion and biopsy-tool positioning. CBCT imaging can thus provide navigation guidance, confirmation of lesion access, and biopsy guidance. Several recent studies investigated CBCT imaging, for its CT and fluoroscopic capabilities,15 in combination with ultrathin bronchoscopy and rEBUS,16 EMN,17,18 or transthoracic needle aspiration,19 reaching diagnostic yields of 70% to 84%.15–19 However, to the best of our knowledge, no studies have systematically studied whether CBCT and AF specifically improves navigation and diagnosis in an initial electromagnetic- and rEBUS-based navigation. Similarly, while multislice CT-aided navigation bronchoscopy has been reported,15,20–24 no studies have reported how CBCT and an AF-based navigation can provide for a do-it-all guidance modality.
FIGURE 2.
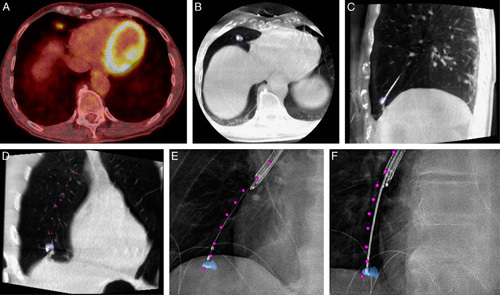
Case example of CBCT and augmented fluoroscopy–based navigation. A, Preprocedural PET-CT showing FDG uptake in 12×11×7 mm solid lesion in the lower right lobe, near the diaphragm. B–D, Conformational CBCT after CBCT and AF-based navigation. E and F, Augmented fluoroscopy under 2 different angles for verification of biopsy positioning. Lesion delineated in blue. Envisioned endobronchial pathway, as segmented on the workstation intraprocedurally, augmented as purple dots. Histopathology analysis of biopsy specimens found granulomatous disease, further proven to be granulomatosis with polyangiitis through clinical follow-up. AF indicates augmented fluoroscopy; CBCT, cone-beam computed tomography; CT, computed tomography; FDG, Fluorodeoxyglucose (18F); PET, positron emission tomography.
FIGURE 3.
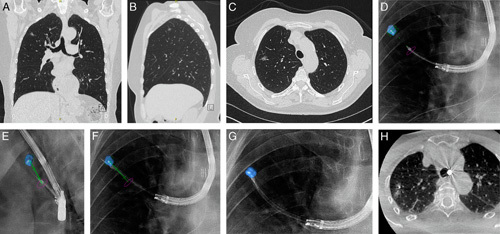
Case example of CBCT and augmented fluoroscopy-based transparenchymal navigation. A–C, Preprocedural CT scan where a ground-glass opacity of 10×9×6 mm in the right upper lobe is visible. D and E, Multiangle augmented fluoroscopy for verifying whether the transparenchymal access tool is positioned correctly. F, Utilizing the transparenchymal access tool for transparenchymal navigation toward the lesion. G and H, After completing transparenchymal navigation, the distal end of the catheter resides within the lesion. Histopathologic analysis of biopsy specimens led to a diagnosis of adenocarcinoma in situ. CBCT indicates cone-beam computed tomography; CT, computed tomography.
In this study, we prospectively assessed CBCT and AF image guidance for navigational bronchoscopy of peripheral pulmonary lesions. Using a combination of modalities in 2 separate workflows and utilizing a cross-over design, we investigated (1) whether CBCT with AF and rEBUS-based navigation bronchoscopy can enable successful navigation and biopsy guidance, and (2) assessed the added value of CBCT and AF imaging on a primary electromagnetic- and rEBUS-based navigation. In this study, navigation success was the primary outcome measure. If accessing the lesion by primary navigation methodology was unsuccessful on CBCT imaging, a cross-over between workflows was applied. The secondary outcome measure was diagnostic accuracy, relating true positives and true negatives to the subjects included.
MATERIALS AND METHODS
Patient Inclusion
This prospective single-center study was approved by the local institutional review body. All patients with a peripheral pulmonary lesion suspected of lung cancer with an indication for minimally invasive biopsy were eligible for study inclusion. Clinical indication to perform a minimally invasive biopsy followed local clinical practice and was defined in our multidisciplinary tumor board in accordance with international guidelines.25 Patients were eligible after informed consent was obtained and when contraindications for endobronchial procedures were absent. Patients with lesions that could be diagnosed without the need for any navigation guidance (ie, <5th-generation endobronchial lesions or mediastinal lymph node involvement on node involvement-CT) were excluded from this study. There was no further preselection for study inclusion based on patient or lesion characteristics. Randomization into one of both study arms was determined by CBCT scanner room availability, which alternated weekly. The primary CBCT-based workflow utilized a ceiling-mounted Philips Allura Clarity FD20 scanner (Philips, Best, The Netherlands). The primary EMN-based workflow utilized Medtronic’s SuperDimension EMN system (version 7.0; Medtronic, Minneapolis, MN) in combination with the floor-mounted Siemens Artis Zeego CBCT system (Siemens Healthineers, Forchheim, Germany). See Figure 1 for a CONSORT flow diagram of subject inclusion.
FIGURE 1.
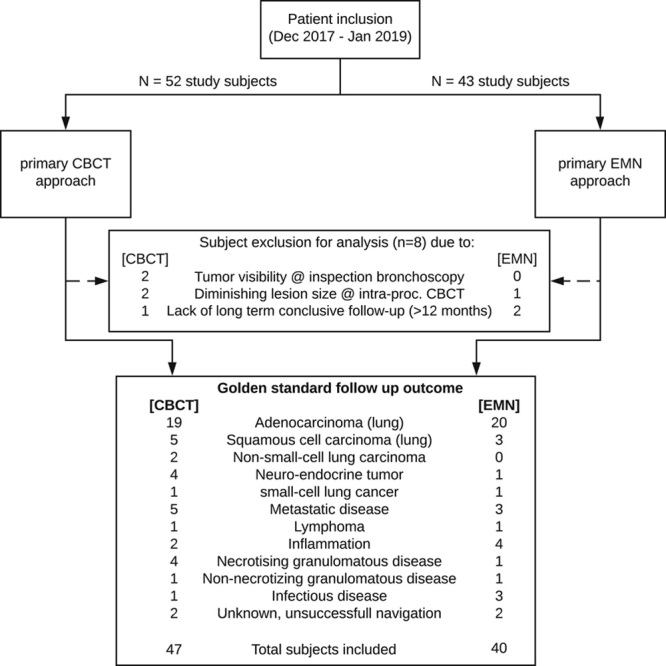
CONSORT flow diagram of study inclusion and exclusion. CBCT indicates cone-beam computed tomography; EMN, electromagnetic navigation.
Materials and Procedural Workflow
Preprocedural CT (≤1.0 mm resolution) and/or positron emission tomography-CT imaging was available in all patients. The preprocedural CT was used to plan a navigation trajectory on the EMN planning platform irrespective of the primary study approach. Procedures were performed under general anesthesia, preferably via laryngeal mask (and if deemed impossible, by endotracheal tube). Standard flexible video bronchoscopes with a 2.8 mm working channel were used for inspection bronchoscopy and consecutive catheter guidance (EB19-J10; Pentax Medical, Tokyo, Japan).
The primarily CBCT-based navigation started with an inspection bronchoscopy and a first CBCT scan (8s roll, Lungsuite; Philips) to identify and segment the lesion and pathway. The 3D segmentations were automatically overlaid during fluoroscopy at any given angle. This AF under multiple angles in combination with an rEBUS-based (UM-S20-17S Radial EBUS miniprobe; Olympus, Tokyo, Japan) confirmation was used for 3D guidance (Figs. 2, 3). Straight catheters with steerable curette (Guide sheath kit; Olympus) or catheters with preformed curvature (Medtronic Extended Working Channel, Minneapolis, MN) were used to navigate. Additional CBCT scans were carried out if AF confirmed positioning while rEBUS imaging remained uncertain, or vice versa. After confirming accurate positioning, tissue sampling was performed under AF-guidance. If the CBCT and AF-based approach remained unsuccessful, additional EMN guidance was added (cross-over).
The primary EMN workflow utilized the preprocedurally segmented CT-based pathway from the EMN planning platform for navigation guidance. After inspection bronchoscopy, the EMN system was calibrated. Navigation was consecutively performed as per the manufacturer’s instruction using catheters with preformed curvature (Medtronic Extended Working Channel). If positioning was estimated to be accurate by the EMN system, the sensor was exchanged for the rEBUS probe. If rEBUS imaging confirmed lesion access, further confirmatory CBCT imaging was performed (6s roll, Syngo DynaCT; Siemens Healthineers). If no confirmation was obtained after repeated EMN and rEBUS-based attempts, CBCT scanning was performed to assess needed repositioning. Once a CBCT was performed, CBCT and AF imaging were used for subsequent biopsy guidance, and, if necessary, navigation guidance (cross-over).
When endobronchial access was difficult in either approach, transparenchymal access was pursued. In all cases, this was carried out with the help of CBCT and AF. Tools to facilitate transparenchymal access varied, from TBNA needles and consecutive guide sheaths, to using a dedicated device (Cross-Country needle, SuperDimension).
After successful navigation, tissue sampling was performed using commercially available tools in an identical order: brush when central in lesion, followed by needle and forceps biopsy for all others, and then, if possible, and deemed safe, cryobiopsy. Rapid on-site evaluation (ROSE) of cytopathology was always available for confirming lesion access. Histology samples were obtained regardless of ROSE outcome.
Analysis
All procedural actions, conclusions, considerations, and their timing were noted. Procedural follow-up outcomes were found through electronic health records. Navigation outcome was termed “centered” if CBCT imaging unambiguously showed center lesion access of tools. Navigations were termed “in contact with” if CBCT imaging showed tool contact with the outer boundary of the lesion, but not a central access of the lesion. Navigations were termed unsuccessful if intraprocedural CBCT verification showed that the lesion was not reached. Navigation success was thus defined as CBCT imaging-confirmed tool positioning residing centrally within the lesion or in contact with the lesion (range: central—in contact with—unsuccessful). The diagnostic accuracy was defined on a patient level, where the count of true-positive and true-negative findings (ie, analysis of at least one nodule corresponding to follow-up outcome) are related to the total amount of patients included. In case tissue biopsy showed malignancy, it was considered a true-positive diagnosis. In case tissue biopsy was found to be benign or nonrepresentative (eg, blood, normal lung tissue), follow-up with CT-guided transthoracic needle aspiration, surgical biopsy, or unambiguous long-term monitoring outcome (ie, ≥12 mo) served as gold standard to provide false-negative or true-negative diagnostic procedural outcome.
Statistical analysis was performed using R.26 Our sample size calculation showed that, to prove a 70% diagnostic accuracy with 15% margin of error and 95% confidence and to be further able to directly compare approaches by cross-over design, a minimum sample size of 40 in every study arm was needed. A binomial proportions test using Pearson χ2 test statistics was performed to assess whether a study approach had a significantly better chance of success (P<0.05). A Student t test was used to compare means.
RESULTS
Between December 2017 and January 2019, 87 study subjects were included. Subject inclusion rate for both study arms unexpectedly varied due to altering room availability, resulting in a slightly higher inclusion in the CBCT arm (Table 1, Fig. 1). A total of 8 study subjects were excluded from analysis due to the following reasons: visible tumor during inspection bronchoscopy (n=2); considerably altered lesion dimensions at procedural imaging (indicative of infectious disease rather than malignancy, n=3); and lack of sufficient follow-up (n=3). In the primary CBCT and AF-based approach, 47 patients and 59 lesions were included. Combined, these lesions had a mean long-axis diameter of 16.6 mm (1.12 cm3 median volume). In the primary EMN approach, 40 patients and 48 lesions were included. Their mean lesion long-axis diameter was 14.2 mm (0.80 cm3 median volume). Detailed patient and lesion characteristics are summarized in Table 1. A comparison of essential parameters showed that malignancy prevalence, lesion volume, and bronchus sign presence did not significantly differ between study arms.
TABLE 1.
Patient and Nodule Characteristics Across Both Study Arms
| Study Characteristics | Primary CBCT and AF Approach | Primary EMN Approach | ||
|---|---|---|---|---|
| Patients (male/female) | 47 (16/31) | 40 (20/20) | ||
| Age (minimum-maximum) (y) | 65 (41-85) | 65 (44-81) | ||
| Lesions | 59 | 48 | ||
| Malignancy prevalence* (lesions/patients) | 83%/83% | 72.9%/72.5% | ||
| Median lesion volume* (cm3) | 1.1 (0.1-19.9) | 0.8 (0.2-18.8) | ||
| Mean long-axis diameter* (mm) | 16.6 (5-43) | 14.2 (7-48) | ||
| Bronchus sign*† (≤1 mm CT) (%) | 62.7 | 70.8 | ||
| Within lesion navigations [n/N (%)] | 32/45 (71.1) | 28/36 (77.8) | ||
| In contact with lesion navigations [n/N (%)] | 2/8 (25) | 3/6 (50) | ||
| Unsuccessful navigations [n/N (%)] | 3/6 (50) | 3/6 (50) | ||
| Lesion Locations | Overall | Malignant | Overall | Malignant |
| LUL/RUL | 9/29 | 7/25 | 15/15 | 13/11 |
| Lingula/RML | 1/3 | 1/2 | 1/2 | 0/2 |
| LLL/RLL | 8/9 | 6/8 | 5/10 | 2/7 |
*Not significantly different between study arms. Significant of italic values: Malignancy prevalence: P=0.3583; Median lesion volume: P=0.5204; Mean Long-axis diameter: P=0.2155; Bronchus sign: P=0.497.
†Bronchus sign, as assessed on preprocedural CT (≤1 mm slice thickness) related to navigation outcome (range: within-edge-unsuccessful), as found by CBCT imaging.
AF indicates augmented fluoroscopy; CBCT, cone-beam computed tomography; CT, computed tomography; EMN, electromagnetic navigation; LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
Navigation Success
Navigation success rate using CBCT with AF and rEBUS as primary guidance modality was 76.3%, which is significantly better than that of the primary EMN-based and rEBUS-based approaches, being 52.2% (P=0.016, Table 2). The addition of EMN sensor guidance to the primary CBCT-approach improved navigation success from 76.3% to 89.9% per lesion (+13.6%, P=0.043). Oppositely, addition of CBCT and AF guidance led to a 35.3% increase in navigation success in the primary EMN-approach, from 52.2% to 87.5% per lesion (P=0.0002, Table 2). Performing a primary CBCT and AF-based navigation resulted in a significantly higher fluoroscopy time and number of CBCTs when compared with a primary EMN approach (Table 3). A bronchus sign (based on ≤1 mm resolution CT) was found less often in unsuccessful navigations and in navigations only in contact with the lesion (42% of cases vs. 66.4% overall, P=0.006, Table 1).
TABLE 2.
Navigation Outcome and Diagnostic Outcome for Both Study Arms
| Primary CBCT and AF Approach (%) | Primary EMN Approach (%) | |||||
|---|---|---|---|---|---|---|
| Navigation Outcome (Lesion) | Center | In Contact | Total | Center | In Contact | Total |
| Primary navigation | 66.1 | 10.2 | 76.3* | 50.1 | 2.1 | 52.2* |
| Combined with EMN/CBCT | +10.2 | +3.4 | +13.6† | +25.0 | +10.4 | +35.4† |
| Total | 76.3 | 13.6 | 89.9‡ | 75.0 | 12.5 | 87.5‡ |
| Total (per patient) | 87.2 | 8.5 | 95.7‡ | 75.0 | 15.0 | 90.0‡ |
| Diagnostic Accuracy (Patient) | Overall [n (%)] | Overall [n (%)] | ||||
| Primary navigation | 29 (61.7) | 20 (50) | ||||
| Combined with EMN/CBCT | 4 (+8.5) | 10 (+25) | ||||
| Overall | 33 (70.2§) | 30 (75§) | ||||
The navigation outcome corresponds to the navigation success of the primary workflow on a per lesion basis. Navigation success was determined by tools proving center lesion access (center) or tools being in contact with but not centered within the lesion (in contact). The “combined with EMN/CBCT” row showcases outcome when all guidance modalities (CBCT+AF+EMN+rEBUS) were used in combination. The diagnostic accuracy is on a per patient basis.
*Significant navigation success difference between arms (P=0.016).
†Significant increases in navigation success by addition of EMN-guidance to primary CBCT-based guidance and by addition of CBCT to primary EMN-based guidance (P=0.043 and 0.0002, respectively).
‡Nonsignificant differences in final navigation success between study arms. Significant italics values P=0.4974 and P=0.5291 for a per nodule and per patient comparison, respectively.
§Diagnostic accuracy significantly lower than navigation success (P=0.0007, but nonsignificant differences between study arms).
AF indicates augmented fluoroscopy; CBCT, cone-beam computed tomography; CT, computed tomography; EMN, electromagnetic navigation; rEBUS, radial endobronchial ultrasound mini probe imaging.
TABLE 3.
Procedural Tool Use and Timing
| Procedural Characteristics | Primary CBCT and AF Approach | Primary EMN Approach | P |
|---|---|---|---|
| Navigation time (min) | 26.9 (2-69) | 35 (4-74) | 0.039 |
| EMN system calibration time (min) | — | 5.7 (3-11) | — |
| Biopsy time (min) | 22.4 (9-55) | 25.5 (2-50) | NS |
| No. biopsy tools | 3.1 (1-7) | 3.7 (1-7) | 0.042 |
| No. tissue samples | 10.0 (1-23) | 9.1 (1-18) | NS |
| CBCTs made | 2.2 (1-5) | 1.5 (0-3) | 0.0014 |
| CBCT preparation time (min) | 4.3 (2-10) | 7.9 (3-11) | <0.0001 |
| CBCT segmentation time (min) | 5.3 (2-10) | 6.4 (2-14) | NS |
| Fluoroscopy time (min) | 9.9 (1-22) | 7.3 (2-23) | 0.021 |
Average times and counts, as derived from procedural report forms. Minimum to maximum range in between brackets. Navigation time definition: from start of navigation modality until decision to start biopsy. Primary EMN navigation also includes the calibration process in timing. Biopsy time definition: From stop of navigation until the last biopsy was taken. Biopsy tools and samples are calculated from lesions wherein at least 1 biopsy was taken. CBCTs and fluoroscopy time derived from total amount of procedures.
AF indicates augmented fluoroscopy; CBCT, cone-beam computed tomography; EMN, electromagnetic navigation; NS, nonsignificant.
Diagnostic Accuracy
Whereas combining all navigation modalities (rEBUS, EMN, CBCT) resulted in an overall navigation success in 93% of patients, the overall diagnostic accuracy in the combination of both study arms was significantly lower (72.4%, P=0.0007). The diagnostic accuracy with the combined use of technologies was 70.2% for the primary CBCT-arm and 75% for the primary EMN-arm (P=0.797, nonsignificant difference). For this, a mean of ∼10 biopsies were taken using 3.4 different biopsy tools across both study arms. Using this combination of biopsy tools with intermittent image verification took ∼ 24 minutes in both study arms (Table 3).
Navigation Time
Average navigation time across study arms was 27.4 minutes (Table 3). Navigation time in the EMN arm was considerably higher than in the CBCT arm, largely due to the calibration time of the EMN system (5.7 min). In both arms, a CBCT was carried out for verification in all but 3 primary EMN cases, wherein rEBUS combined with fluoroscopy gave unambiguous confirmation, and the operator decided to perform first sampling preceding to CBCT verification, which then showed malignancy in ROSE. The time to obtain a CBCT was different in both study arms, using the 2 different systems. It took an average of 7.9 minutes on the floor-mounted system (EMN-arm) and 4.3 minutes for the ceiling-mounted system (CBCT-arm, P<0.0001, Table 3). Segmenting the lesion and pathway for augmentation on the work station after having obtained a CBCT was often carried out in parallel with other procedural tasks. This took an average of 6.4 minutes on the floor-mounted system and 5.3 minutes on the ceiling-mounted system (time from having obtained CBCT to first use of AF by the physician, nonsignificant differences).
Added Navigation Guidance
Reasons to add EMN-guidance in the CBCT-arm were a need for guidance combined with catheter steerability (n=7 patients) and 1 case wherein the pathway on preprocedural planning could not be found during intraoperative CBCT imaging. Causes for adding CBCT and AF-imaging to the EMN arm were cases wherein the planned pathway was shown to be incorrect intraprocedurally (n=2), wherein EMN had difficulty distinguishing multiple parallel bronchi (n=1), EMN and rEBUS suggested having reached the location while CBCT imaging showed inaccurate positioning (n=5), EMN guidance was shown to be inaccurate due to tissue displacement (because of lower segment breathing motion or due to scope-induced tissue manipulation, n=4), or there was need for a transparenchymal approach (n=6).
Transparenchymal Navigation
In both study arms combined, a transparenchymal approach was performed in 18 lesions. CBCT Image guidance was used in all these cases (Fig. 3). In the EMN arm, transparenchymal navigation was successful in 5 of 8 lesions, with 4 leading to an accurate diagnosis. In the CBCT arm, it was successful in 8 of 10 cases, resulting in an accurate diagnosis in 7 cases.
Complications
Observed complications across both study arms were as follows; pneumothorax (n=3), COPD exacerbation following the procedure (n=1), moderate bleeding intraprocedurally following cryobiopsy (n=1), and minor fever (<4 h, n=1). All study subjects—except 1 having pneumothorax requiring chest tube and 1 case of COPD exacerbation—were able to return home the same day.
DISCUSSION
We show that CBCT and AF-based 3D image guidance can be used successfully as a primary tool for navigation bronchoscopy. The addition of CBCT and AF imaging to a workflow wherein EMN and rEBUS are already being used furthermore significantly improved navigation success. By combining all available guidance modalities, almost 90% of small lesions in these patients were reached. As a single guidance modality, CBCT AF guidance had a significantly higher navigation success than EMN-guidance, but, required higher radiation dosage. However, the overall diagnostic accuracy remains considerably lower than the navigation success in both study arms. Improving the tissue acquisition methodology may be crucial to enhancing navigation bronchoscopy success.
The discrepancy between navigation success and diagnostic accuracy, as found in this study, is significant. We believe that the rigidity of the tools used for sampling, breathing motion, and movement due to manipulation of the endoscope and catheter are likely to cause small displacements and thus reduce diagnostic accuracy. In our centimeter-sized lesions, the used EMN system was often found inaccurate. Chen et al27 reported an average of 17.6 mm lesion movement from inspiration to expiration, which is larger than our average lesion long-axis diameter. To our opinion, the lack of intuitiveness in combining multiple modalities and tools may be additional important factors in the diagnostic accuracy. In that regard, EMN guidance is a useful tool, building on pre-existing endoscopic imaging experience, although CBCT-based navigation is likely more susceptible to a learning curve. We furthermore cannot assess whether other commercially available EMN systems would have resulted in different findings. Similarly, we do not know whether other technology such as robotic bronchoscopes or far smaller bronchoscopes with an outer diameter of <3 mm would have improved both navigation and diagnostic outcome. That being observed, we hypothesize that the combined use of technology needs to become further integrated, adaptive, and intuitive.
Although the discrepancy in navigation success to diagnostic accuracy might be caused by tool use,28,29 we did not design the study to compare differences in tool outcome. In this study, we however did aim at performing systematic specimen acquisition by using tools in an identical order (brush when central in lesion, followed by needle and forceps biopsy for all others, and then, if possible and deemed safe, cryobiopsy). In all cases, we had both CBCT-imaging and ROSE available for confirmation of biopsy accuracy. Although CBCT had proven lesion access, we found that ROSE often did not provide unambiguous outcome. Despite this observation, our final analysis would prove diagnostic in the majority of cases. A similar experience has been reported by others.28 Interestingly, Pritchett and colleagues17 describe fully relying on ROSE while performing repeated attempts and report an impressive diagnostic yield of 83.7% in 93 lesions with 16.0 mm median size.
We corroborate previous findings that both lesion size and bronchus sign presence are stringent indicators of navigation success.9,30 Yet, the presence of a bronchus sign remains subject to debate, as it correlates to CT imaging quality and observer interpretation. A bronchus sign that can only be identified by repeatedly following branching of the bronchial tree on high-resolution CT with 0.5 mm slice thickness is likely invisible on a coarser 3 mm slice thickness reconstruction.
Utilizing CBCT and AF provides for the option to meticulously reposition and verify access. This is not only essential for gaining accurate transparenchymal access but also when performing and monitoring endoscopic treatments.18,31,32 A transparenchymal approach in 18 cases had 72% navigation success leading to an accurate diagnosis in 61% of cases in our study. During the conduct of the study, we were limited to only 2 differently curved catheters. We feel that the lack of availability of catheters with higher curvature or active steerability prevented lesion access in several cases, as was also postulated by others.13,17
This study assigned patients to a clinical workflow based on procedure room availability. Because of unanticipated variations in room availability, the group size ended up not being equal. As the essential parameters affecting navigation outcome (ie, lesion volume and bronchus sign presence) were found insignificantly different, we feel this did not influence our conclusion. Furthermore, in every primary EMN case, rEBUS was extensively attempted. Only when proven inaccurate with CBCT, additional CBCT image guidance was used. Having CBCT imaging and AF available could have affected our decision making. In addition, once a confirmation CBCT was made in either study arm, AF was routinely used. It is likely that the addition of AF enhanced EMN diagnostic accuracy and procedure time, as it provided additional biopsy guidance.
CONCLUSIONS
CBCT and AF imaging are highly valuable as a stand-alone means of guiding endobronchial navigation toward peripheral pulmonary lesions. Furthermore, CBCT imaging can significantly enhance navigation success in a workflow where EMN and rEBUS are already being used. Although navigation success using all guidance modalities is high, the diagnostic accuracy was significantly lower. Improving the tissue acquisition methodology may be crucial to further enhance navigation bronchoscopy diagnostic accuracy in the future.
ACKNOWLEDGMENTS
The authors wish to acknowledge and thank for the support and help given by the endoscopy, anesthesia, radiology and pathology team as well as the statistics department and hospital administration. They are appreciative of the technical advice and support from William van der Sterren, Stephanie Schampaert, Lucia Fonseca, and Alessandro Radaelli from the Philips Imaged Guided Therapy team. They further would like to thank the Siemens Healthineers team for their support.
Footnotes
The research was conducted ethically in accordance with the latest World Medical Association Declaration of Helsinki. The study protocol was approved by the independent local medical ethical committee (Arnhem-Nijmegen) and institutional review body before start of subject inclusion. Informed consent was obtained. The study is registered and can be found on ClinicalTrials.gov (identifiers NCT03355586 and NCT03274609).
R.L.J.V. and E.H.F.M.v.d.H. had full access to all the data in the study and take responsibility for the integrity of the data, collection of data, and the accuracy of the data analysis, including and especially any adverse effects. W.H. and J.J.F. contributed substantially to data collection and the revision of the manuscript.
Supported by unrestricted research grants from Philips, the Radboudumc, the Ankie Hak fund, Astra Zeneca Oncology Netherlands and in-kind support of Pentax Medical Europe.
The results of this study have been presented as oral presentations at both the 2019 IASLC world conference in Barcelona and the 2019 ERS congress in Madrid. Interim (preliminary) results have furthermore been presented at the 2019 European Congress for Bronchology and Interventional Pulmonology in Dubrovnik.
Disclosure: R.L.J.V. reports grants and nonfinancial support from Philips, grants from the Radboudumc, personal fees and nonfinancial support from Medtronic, and in kind support from Pentax Medical Europe and Siemens Healthineers, during the conduct of the study; grants from AstraZeneca Oncology, grants from the Ankie Hak Fund, outside the submitted work. E.H.F.M.v.d.H. reports grants from Philips, grants from the Radboudumc, personal fees and nonfinancial support from Medtronic and Pentax Medical Europe, during the conduct of the study; grants from AstraZeneca Oncology, grants from the Ankie Hak Fund, personal fees from Cook Medical, outside the submitted work. W.H. reports an unrestricted study grant from Insmed Inc., outside the submitted work. J.J.F. reports grants and nonfinancial support from Siemens Healthineers, during the conduct of the study.
REFERENCES
- 1.Yousaf-Khan U, van der Aalst C, de Jong PA, et al. Final screening round of the NELSON lung cancer screening trial: the effect of a 2.5-year screening interval. Thorax. 2016;1:1–9. [DOI] [PubMed] [Google Scholar]
- 2.The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rzyman W, Szurowska E, Adamek M. Implementation of lung cancer screening at the national level: polish example. Transl Lung Cancer Res. 2019;8(suppl 1):S95–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedersen JH, Sørensen JB, Saghir Z, et al. Implementation of lung cancer CT screening in the Nordic countries. Acta Oncol (Madr). 2017;56:1249–1257. [DOI] [PubMed] [Google Scholar]
- 5.Pastorino U, Sverzellati N, Sestini S, et al. Ten-year results of the Multicentric Italian Lung Detection trial demonstrate the safety and efficacy of biennial lung cancer screening. Eur J Cancer. 2019;118:142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oudkerk M, Devaraj A, Vliegenthart R, et al. European position statement on lung cancer screening. Lancet Oncol. 2017;18:e754–e766. [DOI] [PubMed] [Google Scholar]
- 7.Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl):93–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl):e142S–e165S. [DOI] [PubMed] [Google Scholar]
- 9.Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest. 2012;142:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folch EE, Pritchett MA, Nead MA, et al. Electromagnetic navigation bronchoscopy for peripheral pulmonary lesions: one-year results of the prospective, multicenter NAVIGATE study. J Thorac Oncol. 2019;14:445–458. [DOI] [PubMed] [Google Scholar]
- 11.Casal RF. Cone beam CT-guided bronchoscopy: here to stay? J Bronchology Interv Pulmonol. 2018;25:255–256. [DOI] [PubMed] [Google Scholar]
- 12.Fielding DIK, Bashirzadeh F, Son JH, et al. First human use of a new robotic-assisted fiber optic sensing navigation system for small peripheral pulmonary nodules. Respiration. 2019;98:142–150. [DOI] [PubMed] [Google Scholar]
- 13.Yarmus L, Akulian J, Wahidi M, et al. A prospective randomized comparative study of three guided bronchoscopic approaches for investigating pulmonary nodules (The PRECISION-1 Study). Chest. 2020;157:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinfort DP, Vrjlic I, Irving LB. Augmented fluoroscopy for guidance of bronchoscopic biopsy of pulmonary nodules. J Bronchology Interv Pulmonol. 2019;26:E27–E29. [DOI] [PubMed] [Google Scholar]
- 15.Park SC, Kim CJ, Han CH, et al. Factors associated with the diagnostic yield of computed tomography-guided transbronchial lung biopsy. Thorac Cancer. 2017;8:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casal RF, Sarkiss M, Jones AK, et al. Cone beam computed tomography-guided thin/ultrathin bronchoscopy for diagnosis of peripheral lung nodules: a prospective pilot study. J Thorac Dis. 2018;10:6950–6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pritchett MA, Schampaert S, De Groot JAH, et al. Cone-beam CT with augmented fluoroscopy combined with electromagnetic navigation bronchoscopy for biopsy of pulmonary nodules. J Bronchology Interv Pulmonol. 2018;25:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobieszczyk MJ, Yuan Z, Li W, et al. Biopsy of peripheral lung nodules utilizing cone beam computer tomography with and without trans bronchial access tool: a retrospective analysis. J Thorac Dis. 2018;10:5953–5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hohenforst-Schmidt W, Zarogoulidis P, Vogl T, et al. Cone beam computer tomography (CBCT) in interventional chest medicine—high feasibility for endobronchial realtime navigation. J Cancer. 2014;5:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ost D, Shah R, Anasco E, et al. A randomized trial of CT fluoroscopic-guided bronchoscopy vs conventional bronchoscopy in patients with suspected lung cancer. Chest. 2008;134:507–513. [DOI] [PubMed] [Google Scholar]
- 21.Heyer CM, Kagel T, Lemburg SP, et al. Transbronchial biopsy guided by low-dose MDCT: a new approach for assessment of solitary pulmonary nodules. Am J Roentgenol. 2006;187:933–939. [DOI] [PubMed] [Google Scholar]
- 22.Hautmann H, Henke MO, Bitterling H. High diagnostic yield from transbronchial biopsy of solitary pulmonary nodules using low-dose CT-guidance. Respirology. 2010;15:677–682. [DOI] [PubMed] [Google Scholar]
- 23.Shinagawa N, Yamazaki K, Onodera Y, et al. CT-guided transbronchial biopsy using an ultrathin bronchoscope with virtual bronchoscopic navigation. Chest. 2004;125:1138–1143. [DOI] [PubMed] [Google Scholar]
- 24.Tsushima K, Sone S, Hanaoka T, et al. Comparison of bronchoscopic diagnosis for peripheral pulmonary nodule under fluoroscopic guidance with CT guidance. Respir Med. 2006;100:737–745. [DOI] [PubMed] [Google Scholar]
- 25.British Thoracic Society Pulmonary Nodule Guideline Development Group. BTS Guidelines for the Investigation and Management of Pulmonary Nodules. Thorax. 2015;70:ii2–ii54. [DOI] [PubMed] [Google Scholar]
- 26.R Core Team. R: a language and environment for statistical computing, (version 4.0.1); 2020.
- 27.Chen A, Pastis N, Furukawa B, et al. The effect of respiratory motion on pulmonary nodule location during electromagnetic navigation bronchoscopy. Chest. 2015;147:1275–1281. [DOI] [PubMed] [Google Scholar]
- 28.Gildea T, Folch E, Khandhar S, et al. The Impact of Biopsy Tool Choice and Rapid on-Site Evaluation on the Diagnostic Accuracy for Malignant Lesions in the Prospective, Multicenter Navigate Study. In: Chest. American College of Chest Physicians; 2019. p. A827-A829. [DOI] [PMC free article] [PubMed]
- 29.Fielding D, Oki M. Technologies for targeting the peripheral pulmonary nodule including robotics. Respirology. 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 30.Ali MS, Sethi J, Taneja A, et al. Computed tomography bronchus sign and the diagnostic yield of guided bronchoscopy for peripheral pulmonary lesions. A systematic review and meta-analysis. Ann Am Thorac Soc. 2018;15:978–987. [DOI] [PubMed] [Google Scholar]
- 31.Bowling MR, Brown C, Anciano CJ. Feasibility and safety of the transbronchial access tool for peripheral pulmonary nodule and mass. Ann Thorac Surg. 2017;104:443–449. [DOI] [PubMed] [Google Scholar]
- 32.Lau K, Spiers A, Pritchett M, et al. Bronchoscopic image-guided microwave ablation of peripheral lung tumours—early results. J Thorac Oncol. 2018;13:S542. [Google Scholar]


