Abstract
Background:
COVID-19 has caused severe outbreaks in Canadian long-term care facilities (LTCFs).
Objective:
To evaluate the effect of mitigation measures in LTCFs including routine testing of staff and vaccination of staff and residents.
Design:
Agent-based transmission model parameterized with disease-specific estimates, temporal sensitivity of nasopharyngeal (NP) and saliva testing, preliminary results of vaccine efficacy trials, and data from initial COVID-19 outbreaks in LTCFs in Ontario, Canada.
Setting:
Characteristics of staff and residents were included in the model with age-dependent risk of hospitalization and deaths, calibrated to the cumulative incidence of COVID-19 reported in these settings.
Participants:
Synthetic staff and resident populations.
Interventions:
Routine NP and saliva testing of staff; vaccination of residents and staff.
Measurements:
Daily incidence and attack rates in the LTCF using large-scale model simulations; estimates of hospitalizations and deaths and their 95% credible intervals.
Results:
Weekly routine testing of staff with 2-day turnaround time reduced infections among residents by at least 20.3% (95% CrI: 18.7–21.8%), compared to baseline measures of mask-wearing, symptom screening, and staff cohorting alone. A similar reduction of hospitalizations and deaths was achieved in residents. Vaccination averted 2–4 times more infections in both staff and residents as compared to routine testing, and markedly reduced hospitalizations and deaths among residents by 81.4% (95% CrI: 80.6–82.2%), and 82.1% (95% CrI: 81.5–82.7%), respectively.
Limitations:
Timelines of vaccine distribution and compliance rates with routine testing are key parameters affecting strategy outcomes.
Conclusion:
Routine testing of staff reduces silent transmission in LTCFs. Vaccination could have a substantial impact on mitigating disease burden among residents, but may not eliminate the need for other measures before population-level control of COVID-19 is achieved.
Introduction
The novel coronavirus disease 2019 (COVID-19) has led to severe outbreaks in Canadian long-term care facilities (LTCFs). LTCF residents are particularly vulnerable to COVID-19 due to a high prevalence of comorbid conditions (1) and their advanced age. Since the start of COVID-19 pandemic, a number of strategies have been implemented to prevent infection and disease transmission in LTCFs, including non-pharmacological measures such as isolation, visitor restrictions, hand hygiene, and mask-wearing (2,3). While COVID-19 mitigation measures have had a significant impact on reducing transmission on a population level (4), control of outbreaks in LTCFs continues to be challenging, in part due to silent transmission from infected asymptomatic or pre-symptomatic visitors and staff (5,6).
In Canada, over 80% of reported COVID-19 deaths have been attributed to LTCFs (7–9). While advanced age and comorbid medical conditions are risk factors for a more severe course of disease among residents (10–12), recent studies have highlighted the inadequacy of the systemic response to COVID-19 in Canadian LTCFs (13–15). Shortages of staff and personal protective equipment (PPE), limited testing capacity with reliance on symptom-based screening, and inadequate space to implement efficient cohorting measures appear to have contributed to the extraordinary disease toll in these settings (14). Recent promising vaccine efficacy results from phase III clinical trials (16–18) suggest that prioritizing residents and staff for vaccination could prevent LTCF outbreaks. However, whether vaccination alone is sufficient to protect these vulnerable populations from COVID-19 remains undetermined. Furthermore, the effectiveness of infection control measures in LTCFs depend on a number of factors such as the contact network between residents and staff, in addition to disease characteristics, such as the presence of symptoms and severity of illness, at the individual level.
We sought to investigate the impact of COVID-19 mitigation measures on controlling COVID-19 in LTCFs by developing an agent-based model of disease transmission dynamics. We parameterized the model with disease-specific estimates and data from initial outbreaks in LTCFs in the province of Ontario, Canada (19). We also used movement and contact network data collected from the largest veterans’ care facility in Canada, located at Sunnybrook Health Sciences Centre (20,21). We evaluated the effect of case isolation, mask-wearing, cohorting and routine testing of staff in the absence of vaccination. We further expanded the model to include vaccination and evaluate the need for other interventions.
Methods
Ethics Statement
The study was approved by the York University Ethics Review Board (Project: 2020–269). The study was categorized as minimal risk, with no requirement for individual consent or participation.
Model structure and population
We developed an agent-based simulation model of COVID-19 transmission dynamics in a LTCF with resident and staff populations (Appendix, Figure A1). The model structure was informed by population demographics of LTCFs in Ontario, Canada (e.g., age (1,22), staff-to-resident ratio, distribution of rooms and occupancy (13)). The interaction within and between staff and resident populations were parameterized using the distributions derived from close-range movement and contact network data collected through wearable sociometric tags in a Canadian LTCF (21). The model included three working shifts of morning, afternoon, and night, each covering 8 hours of daily interactions.
Based on demographic data from the Ontario Long Term Care Association (1), the resident population included individuals of age 50–64 (6.6%), 65–74 (11.4%), 75–84 (27.3%), 85–94 (43.9%), and 95+ (10.8%). A total of 120 residents (i.e., the average size of a LTCF) were included in the model and assigned to 84 rooms, with a distribution corresponding to 48 single and 36 double occupancy rooms.
The model considered a staff population of 68 individuals, aged 20–64 years of age (22), which included direct care providers (i.e., personal support workers, nurses), dieticians and housekeeping personnel. The distribution of staff by classification and staff-to-resident ratio for each daily shift were informed by correspondence with the management teams of 10 LTFCs affected by COVID-19 outbreaks in Ontario.
Daily contacts among residents were sampled from a previously inferred distribution with a mean of 6.8 contacts per resident per day (20,21). Daily numbers of contacts varied from 6 to 9 between residents and direct healthcare providers (nurses and personal support workers). We assumed that there was no contact between residents and other service staff based on correspondence with the management teams of LTFCs. Given visiting restrictions during outbreaks, we did not include visitors in the model. Contacts among staff were distributed in each shift (Appendix, Table A1) depending on the responsibility of staff in the LTCF.
Disease dynamics
We encapsulated the natural history of COVID-19 with epidemiological statuses as susceptible; latently infected (not yet infectious); asymptomatic (and infectious); pre-symptomatic (and infectious); symptomatic with either mild or severe/critical illness; recovered; and dead (Figure 1). We assumed that infection was introduced into the LTCF through infected staff during the silent asymptomatic or pre-symptomatic stages of disease. A population point-prevalence in the range of 0.05%–0.1% was considered for infection of staff outside the LTCF prior to the start of each shift (23). Staff with symptomatic COVID-19, whether infected outside or during the daily shifts in the LTCF, were screened and removed from the model simulations for a minimum of 14 days (and until complete recovery).
Figure 1.
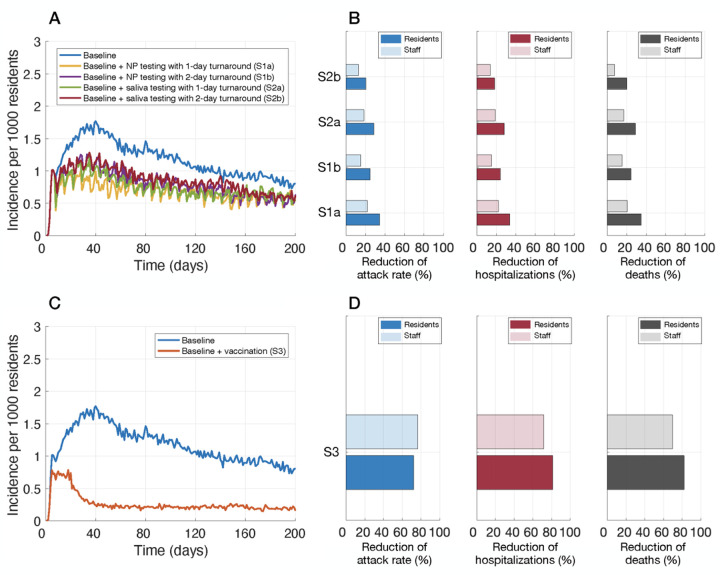
Incidence of infection per 1000 residents (A,C), and relative reduction of cumulative infections (attack rate), hospitalizations, and deaths with routine testing of staff (B) and vaccination of residents and staff (D). Intervention scenarios (in addition to baseline control measures) included: weekly routine testing of staff with NP sampling and 1-day (S1a) and 2-day (S1b) turnaround; saliva sampling and 1-day (S2a) and 2-day (S2b) turnaround; and vaccination (S3).
Disease transmission inside the LTCF was implemented probabilistically for contacts between susceptible and infectious individuals in asymptomatic and pre-symptomatic, or symptomatic stages of the disease. Infected individuals started in the latent (non-infectious) stage, and then proceeded to a silent infectious stage (i.e., either asymptomatic or pre-symptomatic). A proportion of infected individuals remained asymptomatic until recovery (24–27), with an infectious period that was sampled from a Gamma distribution with a mean of 5 days (28,29). Others developed symptoms following a pre-symptomatic stage as part of the incubation period. The incubation and pre-symptomatic periods were sampled from Log-Normal and Gamma distributions with mean values of 5.2 and 2.3 days, respectively (30,31). The infectious period post-symptom onset was also sampled from a Gamma distribution with a mean of 3.2 days (28). Symptomatic cases had an age-dependent probability of developing mild or severe/critical illness. We assumed that recovery from a primary infection provided adequate immunity for the remainder of the simulation, preventing re-infection. Compared to the probability of transmission during the pre-symptomatic stage, the relative risks of transmission were 0.11, 0.44, and 0.89 for the asymptomatic, mild symptomatic, and severe symptomatic stages, respectively (32,33).
Infection outcomes
Residents with symptomatic disease and their roommates, based on outbreak guidelines, were immediately isolated upon symptom onset within the LTCF (34). Contacts of isolated cases were limited to only direct healthcare providers. A proportion of symptomatic residents who developed severe illness were transferred and hospitalized, and therefore excluded from the dynamics within the LTCF until their return upon recovery. For those who were hospitalized, the time from symptom onset to admission was sampled in the range of 2–5 days (35,36). The length of hospital stay was sampled from a Gamma distribution with mean of 12.4 days (37). We parameterized the model with age-specific hospitalization rates of LTCF residents from January to June 2020 (Table 1). The case fatality rate among residents was age-dependent and based on data reported by Public Health Ontario (Table 1).(19)
Table 1.
Model parameters and their estimated value.
| Model parameter | Staff | Resident | Source | |||||
|---|---|---|---|---|---|---|---|---|
| Age group | 20–49 | 50–64 | <60 | 60–69 | 70–79 | 80–89 | 90+ | |
| Transmission probability per contact during pre-symptomatic stage | 0.0476 Calibrated to incidence data for LTCFs in Ontario |
(19) | ||||||
| Incubation period (days) | Log-Normal(shape: 1.434, scale: 0.661) | (30) | ||||||
| Asymptomatic period (days) | Gamma(shape: 5, scale: 1) | Derived from (29,31) | ||||||
| Pre-symptomatic period (days) | Gamma(shape: 1.058, scale: 2.174) | (31) | ||||||
| Infectious period from onset of symptoms (days) | Gamma(shape: 2.768, scale: 1.1563) | Derived from (28) | ||||||
| Proportion of individuals with comorbidities | 0.18 | 0.38 | 1 | (1,46) | ||||
| Proportion of infected individuals developing asymptomatic infection | 0.29 | 0.29 | 0.18 | 0.18 | 0.18 | 0.13 | 0.13 | (47) |
| Proportion of symptomatic cases developing severe illness | 0.15 | 0.40 | 0.8 | (36) | ||||
| Hospitalization rate of symptomatic cases | 23.5% | 23.5% | 18.2% | 15.1% | 13.4% | 9.3% | 7.1% | (19) |
| Case fatality rate | 0.21% | 0.21% | 8.4% | 18.8% | 24.1% | 27.9% | 35.4% | (19) |
| Length of hospital stay | Gamma(shape: 4.5, scale: 2.75) | (37) | ||||||
Interventions
The baseline scenario of control measures included: (i) isolation of symptomatic residents with hospitalization of a proportion who developed severe illness; (ii) screening of staff for symptomatic illness followed by isolation; (iii) cohorting of healthcare providers; and (iv) mask-wearing by all staff. We assumed that all staff wore surgical masks during their shift, but switched to an N95 respirator when caring for isolated residents. A recent meta-analysis indicates a 67% (95% CI: 39% – 83%) risk reduction in respiratory infections when surgical masks are used (38). Thus, the transmission probability per contact was reduced by a factor (1- effsur) for staff-resident, and (1- effsur)2 for staff-staff interactions. When an N95 was used by staff caring for isolated residents, the probability of transmission was reduced by (1-effN95) per contact, with an efficacy effN95 = 0.95 (38). For staff cohorting, we assigned each healthcare provider to a specific group of residents. Personal support workers only interacted with a predetermined group of 9, 9, and 20 residents during the three daily shifts of morning, afternoon, and night. Similarly, nurses interacted with predetermined groups of 30, 30, and 60 residents during the corresponding shifts. Given baseline interventions, we compared two additional measures: routine testing of all staff and vaccination of staff and residents.
Routine testing.
To prevent disease importation and transmission during the silent asymptomatic and pre-symptomatic stages of infection, we implemented routine nasopharyngeal (NP) and saliva PCR testing of staff with a frequency of 7 days (39). The probability of case detection at the time of testing post-infection was determined by the temporal diagnostic sensitivity (40) inferred from fitting a sensitivity function to the percent positivity data of NP testing (41). Both tests were assumed to have a specificity of 100%. We considered time delays of 24–48 hours in turnaround time from sampling to results, during which staff continue their shifts. Staff isolated for a period of 14 days following a positive result, during which they were excluded from interactions in the LTCF.
Vaccination.
Considering LTCFs as one of the priority groups for COVID-19 vaccination, we implemented a two-dose vaccine strategy, with coverages of 90% for staff and 75% for residents. These coverages reflect the reported uptake of seasonal influenza vaccines in Canada in these populations (42,43). We considered a vaccine efficacy of 90% (Ve) against infection following 2 doses, administered 28 days apart, in vaccinated staff (17,18). We implemented a 14-day interval after the first vaccine dose to reach half of Ve. The protection of Ve was reached one week after the second dose. The vaccine efficacy was reduced to Vp = (1-q)Ve in vaccinated residents, where q was sampled uniformly from the 10%–50% range based on observed reductions in influenza vaccine effectiveness among frail and comorbid individuals (44,45). Vaccine efficacy was implemented in the model as a reduction of disease transmission. We also assumed that the risk of developing severe illness was reduced by the vaccine efficacy if infection occurred post-vaccination, thereby affecting hospitalization rates.
Model implementation and calibration
The model was implemented in Julia language using parameter estimates in Table 1. To determine the transmission probability, we calibrated the model to the cumulative incidence data reported for LTCFs in Ontario, Canada, from January to June 2020 (19). For this calibration, we considered case isolation and hospitalization of infected residents, screening of staff for symptomatic illness, and mask-wearing by all staff as measures implemented during initial outbreaks of COVID-19. In the scenario with vaccination, we assumed that the first dose of vaccine was given two weeks before the introduction of the first infection into the LTCF, which resulted in partial effectiveness of vaccination when the outbreak simulations began. The second dose was offered two weeks after the start of simulations. Simulations were seeded with one infected individual among staff with a time-step of 1 hour. The computational model is available at https://github.com/thomasvilches/LTCF-covid.
Results
In the baseline scenario of model calibration, the attack rate was 37% among residents, and 25.6% among staff. Hospitalization and deaths among residents were projected to be 32.9 and 83.8 per 1000 population, respectively. The corresponding rates for staff were 9.5 and 0.5 per 1000 population.
Routine testing of staff
Compared to baseline measures only, augmenting with weekly routine testing of staff reduced the attack rate among residents by 20.3% (95% CrI: 18.7% – 21.8%) using saliva sampling with a 2-day turnaround. The highest reduction was 34.4% (95% CrI: 32.9% – 36.0%) using a weekly NP sampling and a 1-day turnaround time (Figure 1, Table 2). For each scenario, the observed reductions of hospitalizations and deaths were similar to the reduction of attack rate among residents (Table 2).
Table 2.
Mean and 95% credible intervals for the reduction of cumulative infections, hospitalizations, and deaths among residents achieved by additional measures of 7-day routine testing of staff, and vaccination of staff and residents as compared with baseline measures alone.
| Measure | Mean relative reduction (%) and 95% CrI | |||
|---|---|---|---|---|
| 7-day routine testing | Infection | Hospitalization | Death | |
| NP sampling | 1-day turnaround | 34.4 (32.9, 36.0) | 33.7 (31.6, 35.8) | 34.8 (33.0, 36.5) |
| 2-day turnaround | 24.8 (23.1, 26.4) | 24.1 (21.6, 26.5) | 24.4 (22.4, 26.3) | |
| Saliva sampling | 1-day turnaround | 28.7 (27.1, 30.3) | 28.3 (26.0, 30.6) | 29.0 (27.1, 30.8) |
| 2-day turnaround | 20.3 (18.7, 21.8) | 18.5 (15.8, 20.8) | 20.1 (18.3, 22.0) | |
| Vaccination | 72.3 (71.6, 72.9) | 81.4 (80.6, 82.2) | 82.1 (81.5, 82.7) | |
Weekly routine testing led to lower reduction of attack rates among staff, as compared to residents, ranging from 12.8% (95% CrI: 11.7% – 13.9%) to 21.9% (95% CrI: 20.9% – 23.0%) with a 2-day turnaround with saliva testing and a 1-day turnaround with NP testing, respectively (Figure 1, Table 3). The reduction in hospitalizations among staff was similar to their reduction of attack rates in the corresponding scenarios. However, routine testing, irrespective of the type and turnaround time, had no significant effect on reducing deaths in staff compared to baseline measures (Table 3).
Table 3.
Mean and 95% credible intervals for the reduction of cumulative infections, hospitalizations, and deaths among staff achieved by additional measures of 7-day routine testing of staff, and vaccination of staff and residents as compared with baseline measures alone.
| Measure | Mean relative reduction (%) and 95% CrI | |||
|---|---|---|---|---|
| 7-day routine testing | Infection | Hospitalization | Death | |
| NP sampling | 1-day turnaround | 21.9 (20.9, 23.0) | 22.1 (17.6, 26.3) | 21.0 (−1.6, 39.5) |
| 2-day turnaround | 15.2 (14.1, 16.3) | 15.2 (10.5, 19.7) | 15.2 (−7.4, 34.4) | |
| Saliva sampling | 1-day turnaround | 18.3 (17.2, 19.3) | 19.0 (12.2, 23.4) | 17.1 (−5.2, 35.7) |
| 2-day turnaround | 12.8 (11.7, 13.9) | 13.7 (9.2, 18.2) | 7.7 (−16.1, 28.2) | |
| Vaccination | 76.7 (76.4, 77.1) | 71.8 (69.3, 74.0) | 69.8 (58.0, 79.7) | |
Vaccination of residents and staff
When vaccination was implemented, the incidence among residents sharply declined after 3 weeks (Figure 1), as compared with baseline measures alone. The attack rate was reduced by 72.3% (95% CrI: 71.6% – 72.9%) among residents and 76.7% (95% CrI: 76.4% – 77.1%) among staff (Figure 1, Table 2,3), significantly higher than reductions achieved in any scenario of routine testing in both populations (Wilcoxon rank-sum test, p-values<0.001). The reduction of hospitalizations and deaths attributed to vaccination was higher among residents as compared to staff, despite higher vaccine coverage and vaccine efficacy in staff (Tables 2,3). Similar to hospitalizations, vaccination also reduced deaths significantly by 82.1% (95% CrI: 81.5% – 82.7%) among residents and 69.8% (95% CrI: 58.0% – 79.7%) in staff.
Discussion
The COVID-19 pandemic has disproportionately affected geriatric populations and especially residents of LTCFs with devastating outcomes (7,9,48–50). The incidence rate ratio for COVID-19 related deaths among LTCF residents in Ontario, Canada has been estimated to be 13 times higher than that among community-living adults older than 69 years (51), with case fatality rates exceeding 27% (15). In the absence of vaccination, the control of COVID-19 outbreaks in these vulnerable settings has been challenging largely due to staff shortages and frequent staff turnover, low staff-to-resident ratios, crowded settings without room to implement physical distancing, insufficient training and inadequate precaution measures, as well as silent transmission of disease.
Our evaluation of multi-pronged strategies in LTCFs indicate that augmenting non-pharmaceutical measures with routine testing of staff can reduce the rates of silent transmission and would decrease infections and their adverse clinical outcomes considerably among residents. We found that with a 2-day or longer time-delay from sample collection to results, the impact of this measure on hospitalizations and deaths among residents decreases by at least 9% compared to a 1-day turnaround time in both NP and saliva testing. Vaccination with a similar uptake to seasonal influenza vaccine would significantly (2–4 times more than routine testing of staff) reduce infection and outcomes among residents and staff. While vaccines will reduce the incidence of infection and may decrease transmission, the practice of other measures (i.e., mask-wearing, social distancing, hand hygiene) will still be needed for some time until community transmission is controlled since vaccine efficacy will not be absolute.
Limitations
Our results should be interpreted within the study assumptions and limitations. For the model structure, staff-to-resident ratio, and population interactions, we relied on existing data and correspondence with LTCFs affected by COVID-19 in Ontario, Canada. We did not include visitation by community members in the model during the outbreak, which may be allowed with specific guidelines for visitors. We also did not include other modes of disease transmission such as aerosolization of the virus without adequate ventilation. For evaluation of routine testing, we assumed a 100% compliance rate among staff. While this may be a reasonable assumption for non-invasive, self-administered saliva testing, compliance will likely be affected by practical challenges of a relatively invasive NP testing. We assumed that staff cohorting can be effectively and sustainably implemented as described; however, staff shortages and the use of overtime to replace staff may affect the effectiveness of this strategy. We assumed that the protective efficacy of a vaccine would be reduced by 10% to 50% in comorbid and elderly residents, similar to observations for influenza vaccines (44,45). If the efficacy of a COVID-19 vaccine was not affected by age or frailty, vaccine impact would be even greater than projected here among residents.
Conclusions
Our study highlights the importance of multifaceted strategies for protecting vulnerable residents. Furthermore, without population-level control of disease, the risk of infection and silent transmission by staff or visitors cannot be discounted even with a highly efficacious vaccine.
Supplementary Material
Funding/Support:
Seyed Moghadas: CIHR (OV4 — 170643), COVID-19 Rapid Research; Natural Sciences and Engineering Research Council of Canada; and Canadian Foundation for Innovation. Alison Galvani: NSF (RAPID - 2027755), NIH (1RO1AI151176-01). Thomas N. Vilches: São Paulo Research Foundation (FAPESP), grant 2018/24811-1. Lauren Cipriano: Society for Medical Decision Making COVID-19 Decision Modeling Initiative funded by the Gordon and Betty Moore Foundation through Grant GBMF9634 to Johns Hopkins University and a Western University Catalyst Research Grant. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures. Dr. Joanne M. Langley reports that her institution has received funding for research studies from Sanofi Pasteur, GlaxoSmithKline, Merck, Janssen and Pfizer. Dr. Joanne M. Langley also holds the CIHR-GSK Chair in Pediatric Vaccinology at Dalhousie University. Other authors declare no competing interests.
References
- 1.This is long term care 2019, report provided by Ontario Long term Care Association https://www.oltca.com/OLTCA/Documents/Reports/TILTC2019web.pdf (accessed 2020 September 23).
- 2.Preparing for COVID-19 in Nursing Homes, Centre for Disease Control and Prevention, https://www.cdc.gov/coronavirus/2019-ncov/hcp/long-term-care.html (accessed November 2020).
- 3.Wang J, Yang W, Pan L, Ji JS, Shen J, Zhao K, et al. Prevention and control of COVID-19 in nursing homes, orphanages, and prisons. Environ Pollut [Internet]. 2020. Nov [cited 2020 Nov 27];266:115161. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0269749120327627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang K, Vilches TN, Tariq M, Galvani AP, Moghadas SM. The impact of mask-wearing and shelter-in-place on COVID-19 outbreaks in the United States. Int J Infect Dis [Internet]. 2020. Dec [cited 2020 Nov 27];101:334–41. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1201971220322049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ladhani SN, Chow JY, Janarthanan R, Fok J, Crawley-Boevey E, Vusirikala A, et al. Investigation of SARS-CoV-2 outbreaks in six care homes in London, April 2020. EClinicalMedicine [Internet]. 2020. Sep [cited 2020 Nov 27];26:100533. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2589537020302777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouslander JG, Grabowski DC. COVID-19 in Nursing Homes: Calming the Perfect Storm. J Am Geriatr Soc [Internet]. 2020. Oct [cited 2020 Nov 27];68(10):2153–62. Available from: https://onlinelibrary.wiley.com/doi/10.1111/jgs.16784 [DOI] [PubMed] [Google Scholar]
- 7.Barnett ML, Grabowski DC. Nursing Homes Are Ground Zero for COVID-19 Pandemic. JAMA Health Forum [Internet]. 2020. Mar 24 [cited 2020 Oct 20];1(3):e200369. Available from: https://jamanetwork.com/channels/health-forum/fullarticle/2763666 [DOI] [PubMed] [Google Scholar]
- 8.Hsu A, Lane N, Sinha S, Dunning J, Dhuper M, Kahiel Z, et al. Report: Understanding the impact of COVID-19 on residents of Canada’s long-term care homes — ongoing challenges and policy responses. Article in LTCcovidorg Int Long-Term Care Policy Network CPEC-LSE [Internet]. 2020. Jun 4; Available from: https://ltccovid.org/wp-content/uploads/2020/06/LTCcovid-country-reports_Canada_June-4-2020.pdf [Google Scholar]
- 9.Pandemic Experience in the Long-Term Care Sector: How Does Canada Compare With Other Countries? 2020; Available from: https://www.cihi.ca/sites/default/files/document/covid-19-rapid-response-long-term-care-snapshot-en.pdf
- 10.Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging [Internet]. 2020. Apr 8 [cited 2020 Nov 28];12(7):6049–57. Available from: https://www.agingus.com/lookup/doi/10.18632/aging.103000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis [Internet]. 2020. May [cited 2020 Nov 28];94:91–5. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1201971220301363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garnier-Crussard A, Forestier E, Gilbert T, Krolak-Salmon P. Novel Coronavirus (COVID-19) Epidemic: What Are the Risks for Older Patients? J Am Geriatr Soc [Internet]. 2020. May [cited 2020 Nov 28];68(5):939–40. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/jgs.16407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stall NM, Jones A, Brown KA, Rochon PA, Costa AP. For-profit long-term care homes and the risk of COVID-19 outbreaks and resident deaths. Can Med Assoc J [Internet]. 2020. Aug 17 [cited 2020 Oct 20];192(33):E946–55. Available from: http://www.cmaj.ca/lookup/doi/10.1503/cmaj.201197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu M, Maxwell CJ, Armstrong P, Schwandt M, Moser A, McGregor MJ, et al. COVID-19 in long-term care homes in Ontario and British Columbia. Can Med Assoc J [Internet]. 2020. Nov 23 [cited 2020 Nov 27];192(47):E1540–6. Available from: http://www.cmaj.ca/lookup/doi/10.1503/cmaj.201860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown KA, Jones A, Daneman N, Chan AK, Schwartz KL, Garber GE, et al. Association Between Nursing Home Crowding and COVID-19 Infection and Mortality in Ontario, Canada. JAMA Intern Med [Internet]. 2020. Nov 9 [cited 2020 Nov 27]; Available from: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2772335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen Jon. Incredible milestone for science.’ Pfizer and BioNTech update their promising COVID-19 vaccine result. 2020. Nov; Available from: https://www.sciencemag.org/news/2020/11/covid-19-vaccine-trial-complete-pfizer-and-biontech-update-their-promising-result [Google Scholar]
- 17.Pfizer and BioNTech Conclude Phase 3 Study of COVID-19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints. 2020. Nov; Available from: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine
- 18.Mahase E. Covid-19: Moderna vaccine is nearly 95% effective, trial involving high risk and elderly people shows. BMJ [Internet]. 2020. Nov 17 [cited 2020 Nov 27];m4471. Available from: https://www.bmj.com/lookup/doi/10.1136/bmj.m4471 [Google Scholar]
- 19.Enhanced epidemiological summary: COVID-19 in long-term care home residents in Ontario – January 15, 2020 to June 1, 2020. 2020;
- 20.Champredon D, Najafi M, Laskowski M, Chit A, Moghadas SM. Individual movements and contact patterns in a Canadian long-term care facility. AIMS Public Health. 2018;5(2):111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Najafi M, Laskowski M, de Boer PT, Williams E, Chit A, Moghadas SM. The Effect of Individual Movements and Interventions on the Spread of Influenza in Long-Term Care Facilities. Med Decis Mak. 2017;37(8):871–81. [DOI] [PubMed] [Google Scholar]
- 22.Canada Statistics. Labour force characteristics by sex and detailed age group, annual, inactive [Internet]. Government of Canada; [cited 2020 Nov 27]. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1410001801 [Google Scholar]
- 23.COVID-19 Point Prevalence Map, Defence Research and Development Canada. Available from: https://decision-support-tools.com/map
- 24.Poline J, Gaschignard J, Leblanc C, Madhi F, Foucaud E, Nattes E, et al. Systematic Severe Acute Respiratory Syndrome Coronavirus 2 Screening at Hospital Admission in Children: A French Prospective Multicenter Study. Clin Infect Dis [Internet]. 2020. Jul 25 [cited 2020 Nov 16];ciaa1044. Available from: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa1044/5876373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buitrago-Garcia D, Egli-Gany D, Counotte MJ, Hossmann S, Imeri H, Ipekci AM, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. Ford N, editor. PLOS Med [Internet]. 2020. Sep 22 [cited 2020 Nov 16];17(9):e1003346. Available from: https://dx.plos.org/10.1371/journal.pmed.1003346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Xu W, Dozier M, He Y, Kirolos A, Theodoratou E. The role of children in transmission of SARS-CoV-2: A rapid review. J Glob Health [Internet]. 2020. Jun [cited 2020 Nov 16];10(1):011101. Available from: http://jogh.org/documents/issue202001/jogh-10-011101.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeBiasi RL, Delaney M. Symptomatic and Asymptomatic Viral Shedding in Pediatric Patients Infected With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Under the Surface. JAMA Pediatr [Internet]. 2020. Aug 28 [cited 2020 Nov 16]; Available from: https://jamanetwork.com/journals/jamapediatrics/fullarticle/2770149 [DOI] [PubMed] [Google Scholar]
- 28.Li R, Pei S, Chen B, Song Y, Zhang T, Yang W, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science. 2020. 01;368(6490):489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gatto M, Bertuzzo E, Mari L, Miccoli S, Carraro L, Casagrandi R, et al. Spread and dynamics of the COVID-19 epidemic in Italy: Effects of emergency containment measures. Proc Natl Acad Sci [Internet]. 2020. May 12 [cited 2020 Nov 27];117(19):10484–91. Available from: http://www.pnas.org/lookup/doi/10.1073/pnas.2004978117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med [Internet]. 2020. May 5 [cited 2020 Nov 27];172(9):577–82. Available from: https://www.acpjournals.org/doi/10.7326/M20-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–5. [DOI] [PubMed] [Google Scholar]
- 32.Moghadas SM, Fitzpatrick MC, Sah P, Pandey A, Shoukat A, Singer BH, et al. The implications of silent transmission for the control of COVID-19 outbreaks. Proc Natl Acad Sci U S A. 2020. 28;117(30):17513–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferretti L, Wymant C, Kendall M, Zhao L, Nurtay A, Abeler-Dörner L, et al. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science [Internet]. 2020. May 8 [cited 2020 Nov 27];368(6491):eabb6936. Available from: https://www.sciencemag.org/lookup/doi/10.1126/science.abb6936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ministry of Health. COVID-19 Outbreak Guidance for LongTerm Care Homes (LTCH). 2020. Apr; Available from: http://www.health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/LTCH_outbreak_guidance.pdf
- 35.Shoukat A, Wells CR, Langley JM, Singer BH, Galvani AP, Moghadas SM. Projecting demand for critical care beds during COVID-19 outbreaks in Canada. Can Med Assoc J [Internet]. 2020. May 11 [cited 2020 Jun 10];192(19):E489–96. Available from: http://www.cmaj.ca/lookup/doi/10.1503/cmaj.200457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moghadas SM, Shoukat A, Fitzpatrick MC, Wells CR, Sah P, Pandey A, et al. Projecting hospital utilization during the COVID-19 outbreaks in the United States. Proc Natl Acad Sci [Internet]. 2020. Apr 21 [cited 2020 Jun 10];117(16):9122–6. Available from: https://www.pnas.org/content/117/16/9122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanche S, Lin YT, Xu C, Romero-Severson E, Hengartner N, Ke R. High Contagiousness and Rapid Spread of Severe Acute Respiratory Syndrome Coronavirus 2. Emerg Infect Dis [Internet]. 2020. Jul [cited 2020 Nov 27];26(7):1470–7. Available from: http://wwwnc.cdc.gov/eid/article/26/7/20-0282_article.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schünemann HJ, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet Lond Engl. 2020 27;395(10242):1973–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iacobucci G. Covid-19: NHS staff must be tested weekly to keep services running, say MPs. BMJ [Internet]. 2020. Oct 1 [cited 2020 Nov 28];m3804. Available from: https://www.bmj.com/lookup/doi/10.1136/bmj.m3804 [DOI] [PubMed] [Google Scholar]
- 40.Zhang K, Shoukat A, Crystal W, Langley JM, Galvani AP, Moghadas SM. Routine saliva testing for the identification of silent COVID-19 infections in healthcare workers [Internet]. medRxiv; 2020. Nov [cited 2020 Nov 30]. Available from: http://medrxiv.org/lookup/doi/10.1101/2020.11.27.20240044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller TE, Garcia Beltran WF, Bard AZ, Gogakos T, Anahtar MN, Astudillo MG, et al. Clinical sensitivity and interpretation of PCR and serological COVID-19 diagnostics for patients presenting to the hospital. FASEB J [Internet]. 2020. Oct [cited 2020 Nov 28];34(10):13877–84. Available from: https://onlinelibrary.wiley.com/doi/10.1096/fj.202001700RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaccine uptake in Canadian Adults 2019, Government of Canada. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/2018-2019-influenza-flu-vaccine-coverage-survey-results.html
- 43.Moran K, Maaten S, Guttmann A, Northrup D, Kwong JC. Influenza vaccination rates in Ontario children: Implications for universal childhood vaccination policy. Vaccine [Internet]. 2009. Apr [cited 2020 Nov 27];27(17):2350–5. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0264410X09002333 [DOI] [PubMed] [Google Scholar]
- 44.Andrew MK, Shinde V, Ye L, Hatchette T, Haguinet F, Dos Santos G, et al. The Importance of Frailty in the Assessment of Influenza Vaccine Effectiveness Against Influenza-Related Hospitalization in Elderly People. J Infect Dis [Internet]. 2017. Aug 15 [cited 2020 Nov 27];216(4):405–14. Available from: https://academic.oup.com/jid/article/216/4/405/4036225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhakal S, Klein SL. Host Factors Impact Vaccine Efficacy: Implications for Seasonal and Universal Influenza Vaccine Programs. Coyne CB, editor. J Virol [Internet]. 2019. Aug 7 [cited 2020 Nov 27];93(21):e00797–19, /jvi/93/21/JVI.00797–19.atom. Available from: https://jvi.asm.org/content/93/21/e00797-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adams ML, Katz DL, Grandpre J. Population-Based Estimates of Chronic Conditions Affecting Risk for Complications from Coronavirus Disease, United States. Emerg Infect Dis. 2020;26(8):1831–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2020;25(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.ECDC Public Health Emergency Team, Danis K, Fonteneau L, Georges S, Daniau C, Bernard-Stoecklin S, et al. High impact of COVID-19 in long-term care facilities, suggestion for monitoring in the EU/EEA, May 2020. Eurosurveillance [Internet]. 2020. Jun 4 [cited 2020 Nov 27];25(22). Available from: https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.22.2000956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burton JK, Bayne G, Evans C, Garbe F, Gorman D, Honhold N, et al. Evolution and effects of COVID-19 outbreaks in care homes: a population analysis in 189 care homes in one geographical region of the UK. Lancet Healthy Longev [Internet]. 2020. Oct [cited 2020 Nov 27];1(1):e21–31. Available from: https://linkinghub.elsevier.com/retrieve/pii/S266675682030012X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bell D, Comas-Herrera A, Henderson D, Jones S, Lemmon E, Moro M, et al. COVID-19 mortality and long term care: a UK comparison. Article in LTCcovid.org, International Long-Term Care Policy Network, CPEC-LSE, August 2020. 2020; Available from: https://ltccovid.org/2020/08/28/covid-19-mortality-and-long-term-care-a-uk-comparison/
- 51.Fisman D, Lapointe-Shaw L, Bogoch I, McCready J, Tuite A. Failing our Most Vulnerable: COVID-19 and Long-Term Care Facilities in Ontario [Internet]. Infectious Diseases (except HIV/AIDS); 2020. Apr [cited 2020 Nov 27]. Available from: http://medrxiv.org/lookup/doi/10.1101/2020.04.14.20065557 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.