Abstract
Background
Coronavirus disease 2019 (COVID-19) is an emerging infectious disease, related to severe acute respiratory syndrome coronavirus 2 infection. Few data are available in patients with end-stage renal disease (ESRD).
Methods
We conducted an observational cohort study of COVID-19 patients at 11 dialysis centres in two distinct districts of France to examine the epidemiological and clinical characteristics of COVID-19 in this population, and to determine risk factors of disease severity (defined as a composite outcome including intensive care unit admission or death) and mortality.
Results
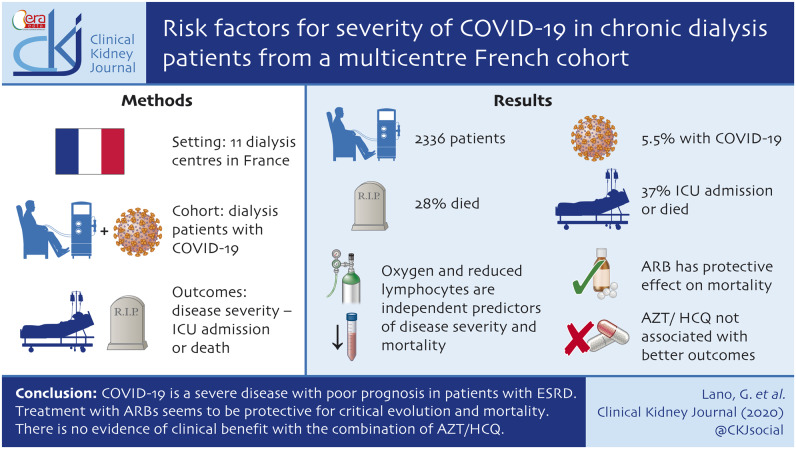
Among the 2336 patients enrolled, 5.5% had confirmed COVID-19 diagnosis. Of the 122 patients with a follow-up superior to 28 days, 37% reached the composite outcome and 28% died. Multivariate analysis showed that oxygen therapy on diagnosis and a decrease in lymphocyte count were independent risk factors associated with disease severity and with mortality. Chronic use of angiotensin II receptor blockers (ARBs) (18% of patients) was associated with a protective effect on mortality. Treatment with azithromycin and hydroxychloroquine (AZT/HCQ) (46% of patients) were not associated with the composite outcome and with death in univariate and multivariate analyses.
Conclusions
COVID-19 is a severe disease with poor prognosis in patients with ESRD. Usual treatment with ARBs seems to be protective of critical evolution and mortality. There is no evidence of clinical benefit with the combination of AZT/HCQ.
Keywords: angiotensin II receptor blockers, COVID-19, dialysis, hydroxychloroquine, lymphocytes
Graphical Abstract
Graphical Abstract.
INTRODUCTION
The coronavirus disease 2019 (COVID-19) is an emerging infectious disease that was first reported in December 2019 in Wuhan, China [1]. COVID-19 has spread worldwide in just >3 months, and the World Health Organization designated COVID-19 as a global pandemic. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can lead to lethal pneumonia associated with high rates of hospitalization in intensive care units (ICUs) [2, 3].
The largest series of patients with COVID-19 in China [1], Italy [4] and in a recent meta-analysis [5], reported comorbidities such as hypertension, cardiovascular diseases, diabetes, obesity and immunodeficiency associated with increased mortality in COVID-19. Furthermore, chronic kidney disease (CKD) is an independent factor in mortality during COVID-19 associated with poor hospital outcomes [6, 7]. Unfortunately, few data are available on the incidence and severity of COVID-19 in patients on chronic dialysis. In fact, only three small series have studied such patients at this point in time (May 2020) [8–10].
Chronic dialysis patients are at increased risk of viral transmission. They interact three times a week with medical transporters, nurses, paramedics, medical workers and other patients from their dialysis facility. In addition to CKD, they display frequent associated comorbidities such as hypertension, cardiovascular diseases and diabetes. They also have impaired immune responses. Haemodialysis units have stringent hygiene protocols, and specific recommendations have been recently published by a European working group of nephrologists [11]. Thus, the risk of hand-transmitted disease is reduced by the establishment of these systematic protective measures. However, the measures limiting the risk of transmission by air are not similarly controlled. Data on incidence and mortality of COVID-19 and associated risk factors are limited in dialysis centres.
This multicentre observational cohort study describes the clinical setting, treatment and clinical outcomes of COVID-19 in patients with CKD Stage 5D from 11 dialysis centres in two French regions.
MATERIALS AND METHODS
Study design
We conducted an observational cohort multicentre study to describe COVID-19 in a large French cohort of patients on chronic haemodialysis. The data included in this study were anonymized, approved and registered at the Health Data Portal of Assistance Publique-Hôpitaux de Marseille under the references PADS-20-154 and 2020-58. The patients received written information about this study and could withdraw consent for the use of their health data.
Participants
From 5 March to 8 May 2020, we included dialysis patients (haemodialysis or peritoneal dialysis) with COVID-19 from 11 dialysis centres.
The inclusion criteria were: diagnosis of COVID-19 by nasopharyngeal real-time reverse transcriptase–polymerase chain reaction (RT-PCR) positive for SARS-CoV-2 and/or a positive chest computed tomography (CT) scan (presence of bilateral lesions like ground-glass opacity, crazy paving consolidation or pleural effusion). The exclusion criteria were age <18 years and had renal replacement therapy initiated <1 month before. At the end of the study, patients still hospitalized with a follow-up inferior to 28 days and not transferred to the ICU were excluded from the final analysis. All data were collected in the period prior to ICU admission. Some patients from this study have already been included in another study published previously [12].
Data source/measurement
Baseline and clinical data
The following patients’ baseline characteristics were collected from electronic medical records: age, gender, body mass index (BMI), obesity (BMI >30 kg/m2), location at diagnosis and classical comorbidities. Their significant usual treatments [angiotensin-converting enzyme inhibitor (ACEI), angiotensin II receptor blockers (ARB), antiplatelet agent, anticoagulation regimen (vitamin K antagonist or others), immunosuppressive therapy] were also collected and verified with the patients during the initial examination. Initial clinical symptoms and vital constants at the first day of hospitalization were collected. After diagnosis of COVID-19 and admission to the hospital, resuscitation status was established in a multidisciplinary consultation, involving nephrologists and ICU medical personnel. Non-admission to the ICU criteria were: age >80 years, institutionalized or advanced neurological disease or dementia, advanced metastatic neoplasia, chronic respiratory disease requiring oxygen, liver cirrhosis with Child–Pugh Score C.
Laboratory and radiological procedures
Blood examinations at inclusion were: complete blood count, serum albumin, C-reactive protein (CRP), coagulation tests, liver function, lactate dehydrogenase (LDH) and myocardial enzymes (Troponine T).
Chest CT scan was performed to evaluate the signs of COVID-19 pneumonia—ground-glass opacity, crazy paving, consolidation and pleural effusion—and to assess the severity of radiological lung involvement.
Treatment of COVID-19 and oxygen therapy
We recorded medications used for treatment of COVID-19: azithromycin and hydroxychloroquine (AZT/HCQ) combination, interleukin (IL)-1 and/or IL-6 inhibitors, antiretroviral therapy, corticosteroids, heparin and antibiotics. We also collected data concerning oxygen therapy, namely, oxygen therapy requirement, duration and maximal flow rate.
Outcomes and objective
The primary aim of this study was to determine risk factors for critical evolution (first event of a composite outcome including ICU admission or death) and mortality in patients on chronic dialysis with COVID-19. Specifically, we wanted to assess if current use of ARBs and the treatment of COVID-19 with AZT/HCQ were associated with critical evolution. We also aimed to describe: the weekly incidence of COVID-19 diagnosis during the outbreak between 5 March and 8 May 2020; the cause of death ( acute respiratory distress syndrome and respiratory failure secondary to pneumonia), cardiovascular (sudden death, heart failure, arterial or venous thrombosis, myocarditis), sepsis or other.
Statistical analysis
Continuous and categorical variables were presented as median [interquartile range (IQR)] and n (%), respectively. We used the Mann–Whitney U-test, χ2 test or Fisher’s exact test to compare differences between groups when appropriate. All tests were two-tailed. Multiple logistic regression analysis was used to determine whether each variable was an independent factor for the composite outcome defined. For this multivariate analysis, the degree of significance is ⩽0.05. A patient was excluded from the analysis if data or value were lacking for the variable of interest. Covariates of interest for the multivariate logistic regression analysis were selected based on a P < 0.2 in a univariate analysis, and <10% missing data. The Kaplan–Meier method was used to estimate the cumulative mortality and/or transfer to ICU depending on group of patients. The log-rank test was used to compare the Kaplan–Meier curves. All statistical analyses were performed using JMP® and Graphpad PRISM® software.
RESULTS
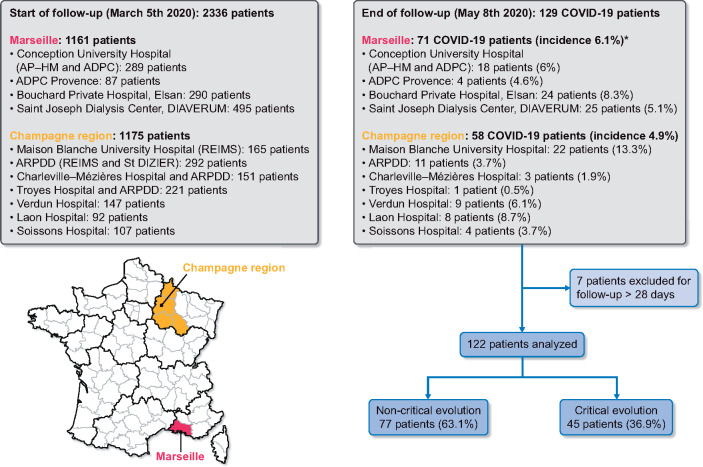
From 5 March to 8 May 2020, 129 chronic dialysis patients were diagnosed with COVID-19, in a cohort of 2336 patients in 11 French dialysis centres. The global incidence was 5.5% (6.1% in Marseille and 4.9% in the Champagne region, P = 0.14), including 97.5% of patients on haemodialysis and 2.5% on peritoneal dialysis. Incidences from the different centres in both regions are shown in the flow chart on Figure 1. Because of unknown outcomes (currently hospitalized and follow-up <28 days), 7 patients were excluded from the final analysis, so that 122 patients were analysed. Any patients with follow-up <28 days were still in hospital. COVID-19 diagnosis was established by RT–PCR for 111 (91%) and by thoracic CT scanning for 11 (9%) patients. Research for COVID-19 was performed because clinical symptoms were present in most of the cases, but systematic screening was also being carried out in some dialysis centres. At the end of the follow-up, 77 (63%) patients were alive and 45 (37%) patients had been transferred to the ICU or had died (critical evolution), and at the final timepoint, 38 (31%) had died.
FIGURE 1:
Flow chart *P = 0.14, no statistical difference between Marseille and Champagne region for COVID-19 incidence in dialysis patients.
ADPC, Association des dialysés Provence Corse; ARDPP, association régionale de promotion dialyse à domicile.
Incidence per week of new COVID-19 cases
The kinetics of the epidemic during the 9 weeks of follow-up is the same in both regions but with a time lag of 1 week for the peak (Figure 2).
FIGURE 2:
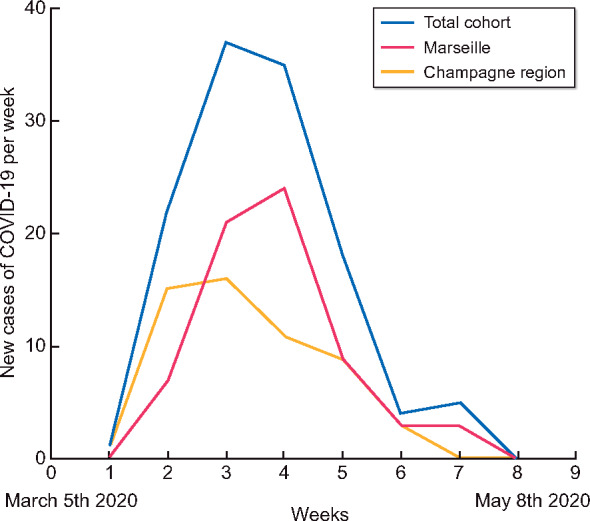
Number of new cases of COVID-19 per week in dialysis patients in Marseille and Champagne region.
Baseline characteristics
Baseline characteristics of the 122 COVID-19 patients are presented in Table 1. Mean age was 73.5 years (IQR = 64.2–81.2), 43 (35%) patients were female. The median dialysis vintage was 3.0 years (IQR = 1.0–5.5). About 97.5% of patients were on haemodialysis and 2.5% on peritoneal dialysis. At the time of the COVID-19 diagnosis, 67% of the patients were at home, 20% in an institution and 13% were already hospitalized. The most prevalent comorbidities and the most frequent drugs taken are listed in Table 1.
Table 1.
Baseline characteristics of patients according to their outcomes: critical evolution (ICU admission or death before 28 days after diagnosis) or non-critical evolution
| Characteristics | All patients (n = 122) | Non-critical evolution (n = 77) | Critical evolution (n = 45) | P-value |
|---|---|---|---|---|
| Age, median (IQR), years | 73.5 (64.2–81.2) | 73.0 (61.0–81.5) | 74.0 (66.6–84.0) | 0.07 |
| Female, n (%) | 43 (35) | 26 (34) | 17 (38) | 0.69 |
| BMI, median (IQR), kg/m2 | 25.3 (22.4–28.8) | 25.1 (22.4–28.6) | 26.5 (22.2–30.0) | 0.56 |
| ESRD vintage, median (IQR), years | 3.0 (1.0–5.5) | 2.7 (0.9–5.1) | 3.3 (1.4–7.2) | 0.23 |
| Haemodialysis, n (%)/peritoneal dialysis, n (%) | 119 (97.5)/3 (2.5) | |||
| Previous transplant, n (%) | 7 (6) | 7 (9) | 0 (0) | 0.05 |
| Location at COVID-19 diagnosis, n (%) | ||||
| At home | 82 (67) | 51 (66) | 31 (69) | 0.88 |
| In institution | 24 (20) | 15 (19) | 9 (20) | |
| Hospitalized | 16 (13) | 11 (14) | 5 (11) | |
| Champagne region | 67 (55) | 44 (66) | 23 (34) | 0.54 |
| Marseille | 55 (45) | 33 (60) | 22 (40) | |
| Cause of ESRD, n (%) | ||||
| Diabetes | 40 (33) | 22 (29) | 18 (40) | 0.03 |
| Hypertension | 31 (26) | 18 (23) | 13 (29) | |
| Glomerulonephritis | 19 (15) | 16 (21) | 3 (7) | |
| Genetic | 5 (4) | 1 (1) | 4 (9) | |
| Undetermined/other | 27(22) | 20 (26) | 7 (16) | |
| Comorbidities, n (%) | ||||
| Congestive heart failure (LVEF <45%) | 13 (11) | 5 (6) | 8 (18) | 0.07 |
| Ischaemic heart disease | 34 (28) | 18 (24) | 16 (36) | 0.21 |
| Atrial fibrillation | 41 (34) | 19 (25) | 22 (49) | 0.01 |
| Hypertension | 95 (78) | 62 (81) | 33 (73) | 0.37 |
| Diabetes | 64 (52) | 37 (48) | 27 (60) | 0.26 |
| Peripheral vascular disease | 34 (28) | 17 (22) | 17 (38) | 0.09 |
| Current smoker | 12 (10) | 6 (8) | 6 (13) | 0.36 |
| Chronic respiratory disease | 14 (11) | 9 (13) | 5 (11) | 0.99 |
| Cancer | 32 (26) | 21 (27) | 11 (24) | 0.83 |
| Obesity (BMI ≥30 kg/m²), n (%) | 25 (20) | 34 (35) | 11 (24) | 0.48 |
| Medication, n (%) | ||||
| ACEIs | 17 (14) | 10 (13) | 7 (16) | 0.78 |
| ARBs | 22 (18) | 18 (23) | 4 (9) | 0.05 |
| Antiplatelet agent | 65 (53) | 39 (51) | 26 (58) | 0.45 |
| Vitamin K antagonist | 26 (21) | 14 (18) | 12 (27) | 0.36 |
| Immunosuppressive therapy | 8 (7) | 6 (7) | 2 (4) | 0.71 |
Quantitative data are expressed in median (IQR 25–75% quartile).
LVEF, left ventricular ejection fraction.
Previous transplantation was less frequent in the critical-evolution group (0% versus 9%; P = 0.05). The medical reasons for end-stage renal disease (ESRD) were different in the two groups, particularly regarding diabetes nephropathy, which was more frequent in the critical-evolution group (40% versus 29%; P = 0.03). Concerning comorbidities, atrial fibrillation was more frequent in the critical-evolution group (49% versus 25%; P = 0.01). Regarding drugs, use of ARB was less frequent in the critical-evolution group (9% versus 23%; P = 0.05).
Clinical symptoms
The initial symptoms are presented in Table 2. About 8% of patients were asymptomatic (but were tested because of viral exposure or systematic screening). Dyspnoea on admission was more frequent in critical evolution patients than in the non-critical-evolution group (59% versus 31%; P = 0.003).
Table 2.
Clinical and paraclinical characteristics of patients according to their outcomes: critical evolution (ICU admission or death before 28 days after diagnosis) or non-critical evolution
| Characteristics | All patients (n = 122) | Non-critical evolution (n = 77) | Critical evolution (n = 45) | P-value |
|---|---|---|---|---|
| Initial symptoms | ||||
| No, n (%) | 10 (8) | 9 (12) | 1 (2) | 0.09 |
| Yes, n (%) | 112 (92) | 68 (88) | 44 (98) | |
| Flu-like symptoms, n (% of symptomatic) | 34 (30) | 23 (34) | 11 (25) | 0.33 |
| Fever, n (% of symptomatic) | 81 (72) | 53 (78) | 28 (64) | 0.10 |
| Cough, n (% of symptomatic) | 77 (69) | 49 (72) | 28 (64) | 0.35 |
| Dyspnoea, n (% of symptomatic) | 47 (42) | 21(31) | 26 (59) | 0.003 |
| Digestive, n (% of symptomatic) | 21 (19) | 14 (21) | 7 (16) | 0.54 |
| Anosmia and/or ageusia, n (% of symptomatic) | 5 (4) | 4 (6) | 1 (2) | 0.37 |
| SBP, median of symptomatic (IQR), mmHg | 129 (111–190) | 130 (113–144) | 134 (106–141) | 0.42 |
| SBP, median of symptomatic (IQR), mmHg | 68 (60–79) | 70 (60–78) | 63 (57–79) | 0.13 |
| Temperature, median of symptomatic (IQR), °C | 37.6 (36.8–38.3) | 37.7 (36.6–38.3) | 37.7 (36.8–38.5) | 0.90 |
| Heart rate, median of symptomatic (IQR), b.p.m. | 78 (70–90) | 76 (65–88) | 83 (72–94) | 0.05 |
| Respiratory rate, median of symptomatic (IQR), c.p.m. | 21 (16–25) | 20 (16–25) | 20 (16–26) | 0.67 |
| Oxygen therapy on diagnosis, n (% of all patients) | 55 (45) | 25 (32) | 30 (67) | <0.001 |
| Oxygen flow rate on diagnosis, median (IQR), L/min | 2 (1–4) | 2 (1–3) | 3 (2–4) | 0.04 |
| Laboratory variable on admission, median (IQR) | ||||
| Haemoglobin, g/dL | 10.7 (9.7–11.6) | 10.8 (9.8–11.8) | 10.3 (9.3–11.6) | 0.23 |
| Platelets, G/L | 166 (124–226) | 166 (123–228) | 167 (129–220) | 0.60 |
| Leucocytes, G/L | 4.9 (3.7–7.3) | 4.6 (3.7–6.4) | 5.9 (4.1–8.7) | 0.10 |
| Neutrophils, G/L | 3.6 (2.6–5.5) | 3.2 (2.5–4.7) | 4.7 (3.0–7.2) | 0.03 |
| Lymphocytes, G/L | 0.78 (0.46–1.09) | 0.90 (0.61–1.18) | 0.60 (0.40–0.86) | <0.001 |
| Eosinophil, G/L | 0.03 (0.00–0.10) | 0.04 (0.00–0.11) | 0.01 (0.00–0.10) | 0.30 |
| Monocytes, G/L | 0.42 (0.29–0.70) | 0.40 (0.25–0.60) | 0.50 (0.30–0.80) | 0.09 |
| CRP, mg/L | 47.5 (15.6–95.1) | 25.4 (11.6–75.0) | 68.5 (32.1–132.0) | 0.006 |
| Albumin, g/L | 34.5 (30.0–37.5) | 35.0 (31.0–38.6) | 32.4 (29.9–37.0) | 0.19 |
| LDH, UI/L | 282 (217–394) | 252 (211–388) | 305 (265–421) | 0.42 |
| Ferritin, µg/L | 771 (469–1609) | 754 (446–1514) | 958 (527–1937) | 0.48 |
| Fibrinogen, g/L | 5.1 (4.4–6.7) | 4.8 (4.2–5.7) | 5.9 (4.8–7.3) | 0.02 |
| D-Dimer, µg/mL | 1.43 (0.81–2.76) | 1.06 (0.66–1.63) | 2.32 (1.26–3.77) | 0.02 |
| Troponins T, ng/mL | 108 (61–192) | 92 (52–142) | 140 (98–307) | 0.43 |
| Hepatic cytolysis, n (%) | 29 (25) | 13 (18) | 16 (38) | 0.02 |
| PCR SARS-CoV-2 positive, n (%) | 113 (93) | 72 (94) | 41 (91) | 0.46 |
| Chest CT scan on admission | ||||
| Chest CT scan realized, n (%) | 89 (73) | 53 (69) | 36 (80) | 0.21 |
| Pathological, n (% of CT scan) | 71 (80) | 42 (79) | 29 (81) | 0.99 |
| Ground-glass opacity, n (% of CT scan) | 65 (92) | 39 (74) | 26 (72) | 0.99 |
| Crazy paving, n (% of CT scan) | 12 (18) | 7 (13) | 5 (14) | 0.75 |
| Consolidation, n (% of CT scan) | 29 (41) | 13 (26) | 16 (44) | 0.02 |
| Pleural effusion, n (% of CT scan) | 10 (14) | 2 (4) | 8 (22) | 0.005 |
| Lesions extension degree <50%, n (% of CT scan) | 53 (61) | 32 (60) | 21 (59) | 0.84 |
| Lesions extension degree >50%, n (% of CT scan) | 15 (17) | 8 (15) | 7 (19) | |
| Lesions extension degree not precise, n (% of CT scan) | 3 (4) | 2 (4) | 1 (3) | |
Quantitative data are expressed in median (IQR 25–75% quartile).
SBP, systolic blood pressure; G, giga.
Forty-five percent of patients required oxygen therapy on diagnosis, and median flow rate was 2 L/min (IQR = 1–3). Oxygen therapy on diagnosis was more frequent in the critical-evolution group (67% versus 32%; P < 0.001), and the oxygen flow rate was also significantly higher in that group [3 (IQR = 2–4) versus 2 (IQR = 1–3) L/min; P = 0.04].
Laboratory results and radiological characteristics
Laboratory results are detailed in Table 2. Median neutrophil count, CRP, fibrinogen, D-Dimer and hepatic cytolysis were significantly higher in the critical-evolution group. Conversely, lymphocyte median count was significantly lower in the critical-evolution group [0.60 G/L (IQR = 0.40–0.86) versus 0.90 G/L (IQR = 0.61–1.18); P < 0.001]. The baseline radiologic characteristics are presented in Table 2.
Treatment of COVID-19
Drugs administrated to patients to treat COVID-19 prior to ICU admission are presented in Table 3. The AZT/HCQ combination was administered in 46% of the cases, antiretroviral therapy in 20%, IL-1 and/or IL-6 inhibitors in 3%, preventive heparin in 45%, curative heparin in 13% and antibiotics in 90%, (cephalosporin in 81%, quinolone in 28% and macrolide in 57% of patients). The administration of curative heparin was significantly more frequent in the critical-evolution group (29% versus 4%; P < 0.0001), as was the administration of antibiotics (98% versus 86%; P = 0.03).
Table 3.
Therapeutics and outcomes
| Characteristics | All patients (n = 122) | Non-critical evolution (n = 77) | Critical evolution (n = 45) | P-value |
|---|---|---|---|---|
| Therapeutics used before ICU, n (%) | ||||
| AZT/HCQ combination, n (%) | 56 (46) | 39 (51) | 17 (38) | 0.17 |
| IL-1 and/or IL-6 inhibitors, n (%) | 4 (3) | 1 (1) | 3 (7) | 0.11 |
| Antiretroviral therapy (next generation), n (%) | 25 (20) | 17 (22) | 8 (18) | 0.57 |
| Corticoid, n (%) | 31 (25) | 17 (22) | 14 (31) | 0.29 |
| Preventive heparin, n (%) | 55 (45) | 35 (45) | 20 (44) | 0.91 |
| Curative heparin, n (%) | 16 (13) | 3 (4) | 13 (29) | <0.0001 |
| Antibiotics, n (%) | 110 (90) | 66 (86) | 44 (98) | 0.03 |
| Cephalosporin, n (%) | 99 (81) | 60 (78) | 39 (87) | 0.33 |
| Quinolone, n (%) | 34 (28) | 21 (27) | 13 (29) | 0.84 |
| Macrolide, n (%) | 69 (57) | 45 (58) | 24 (53) | 0.7 |
| Clinical outcomes | ||||
| Hospitalization, n (%) | 99 (81) | 62 (81) | 37 (82) | 0.6 |
| Ambulatory, n (%) | 23 (19) | 15 (21) | 8 (18) | |
| Do not resuscitate, n (%) | 66 (54) | 40 (52) | 26 (58) | 0.43 |
| Oxygen therapy, n (%) | 91 (75) | 47 (61) | 44 (98) | <0.0001 |
| Oxygen therapy maximal flow rate, L/min | 4 (3–15) | 3 (2–4) | 12 (4–15) | <0.0001 |
| Hospitalization duration, median (IQR), days | 11 (7–14) | 14 (10–17) | 5 (4–11) | – |
| Transfer to ICU, n (%) | 19 (16) | – | – | – |
| Death, n (%) | 34 (28) | – | – | – |
| Transfer to ICU or death, n (%) | 45 (37) | – | – | – |
| Time between first symptoms or hospitalization and ICU admission or death, median (IQR) days | 7 (4–11) | |||
Quantitative data are expressed in median (IQR 25–75% quartile).
Clinical outcomes
Clinical outcomes are presented in Table 3. Patients were hospitalized in 81% of cases. On admission, after diagnosis of COVID-19, resuscitation status was ‘do not resuscitate’ in 54% of cases. Seventy-five percent of patients required oxygen therapy at one time during the disease, with a median maximal flow rate of 4 L/min (IQR 3–15). Median length of hospital stays was 11 days (IQR = 7–14). Sixteen percent were transferred to ICU (with 42% mortality). In total, 28% of patients died; 7% of this 28% died after transfer to ICU. Four patients (21% of patients in ICU) remained in ICU with a follow-up superior to 28 days. The median time between onset of symptoms and critical evolution was 7 days (IQR = 4–11). Figure 3 shows oxygen therapy was required for 75% of patients (61% for the non-critical evolution group and 98% for the critical evolution group, P < 0.0001).
FIGURE 3:
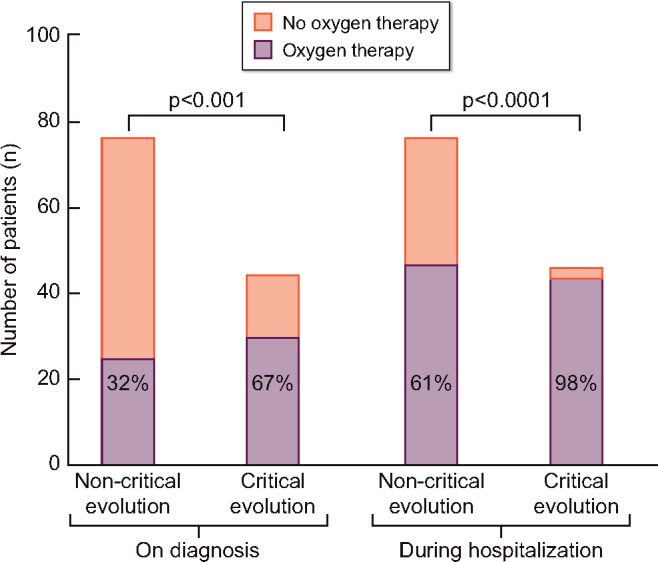
Oxygen requirement on diagnosis and during hospitalization according to patient outcome.
Figure 4 shows the cause of death: respiratory for 65% of non-survival patients, cardiovascular for 20%, sepsis for 9% and other for 6%.
FIGURE 4:
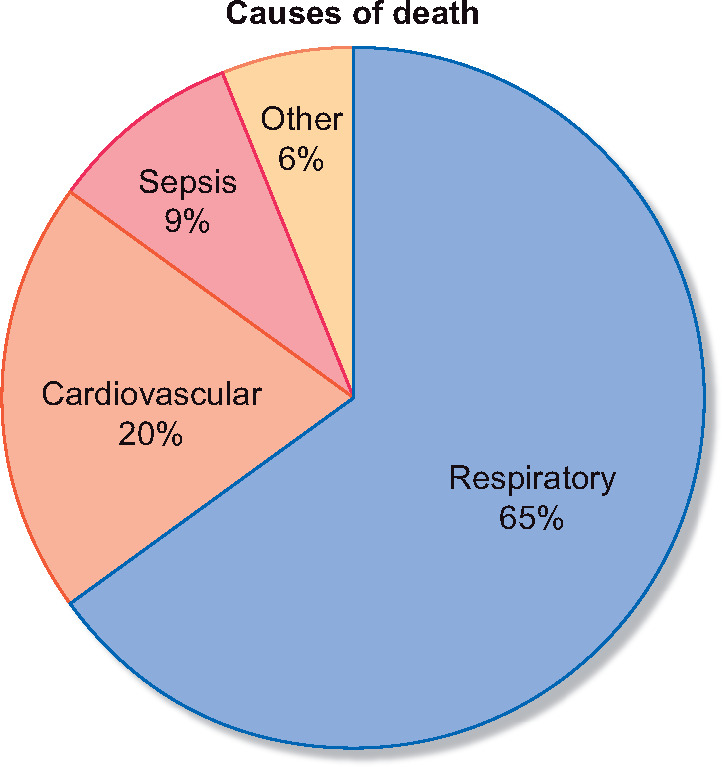
Cause of death.
Occurrence of the composite outcome and death according to severity of disease parameters and treatment
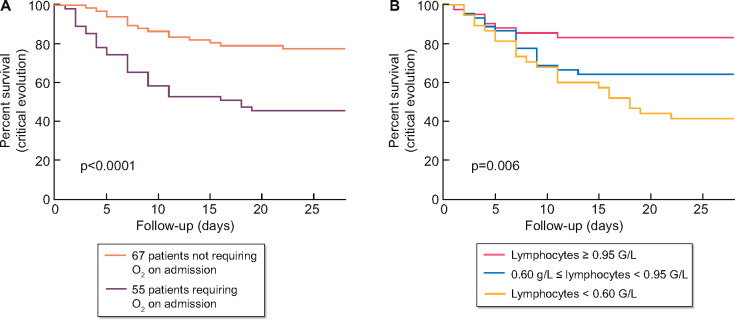
Two models of multivariate analysis were presented in Table 4 (critical evolution) and Table 5 (mortality). The need for oxygen therapy on diagnosis was independently associated with the composite outcome critical evolution [odds ratio (OR) estimate = 3.28, 95% confidence interval (CI) 1.396–7.97; P = 0.007]. Figure 5A shows the survival curve comparing the group of patients without oxygen therapy on diagnosis (67 patients, 55%) and the group of patients with oxygen therapy on diagnosis (55 patients, 45%). Low lymphocyte count on admission was also associated with the composite outcome critical evolution (OR = 0.186, 95% CI 0.057–0.530; P = 0.003). Figure 5B shows the survival curve comparing three groups of patients determined by tertiles of lymphocyte count. Elevated CRP was associated with critical outcome (OR = 1.006, 95% CI 1.001–1.013; P= 0.002). Oxygen therapy on diagnosis and lymphocyte count were also associated with mortality, with OR = 5.386 (95% CI 2.057–15.35), P < 0.001 and OR = 0.195 (95% CI 0.049–0.625), P = 0.01, respectively. Among the non-critical-evolution group, five patients needed oxygen therapy >6 L/min during hospitalization, revealing serious respiratory failure, but they were not transferred to ICU and they were not dead at the end of the 28 days follow-up. The combination AZT/HCQ was not associated in univariate and in multivariate analyses with critical evolution or death (Tables 3 and 4, Model 2, Table 5, Model 2; Supplementary data, Figure S2). In the subgroup of patients not requiring oxygen therapy on diagnosis (67 patients), the combination AZT/HCQ was not associated with critical evolution (Supplementary Figure S3).
Table 4.
Multivariate linear regression analysis to evaluate the relation between severity of disease parameters and treatment and critical evolution outcomes (45 events)
| Variable | OR estimate (95% CI) | P-value |
|---|---|---|
| Model 1 | ||
| Age | 1.002 (0.965–1.043) | 0.90 |
| Oxygen therapy on diagnosis | 3.281 (1.396–7.97) | 0.007 |
| Lymphocytes | 0.186 (0.057–0.530) | 0.003 |
| CRP | 1.006 (1.001–1.013) | 0.02 |
| AZT/HCQ combination | 0.475 (0.188–1.116) | 0.11 |
| Model 2 | ||
| Age | 1.012 (0.980–1.048) | 0.46 |
| Congestive heart failure (LVEF <45%) | 1.665 (0.452–6.481) | 0.44 |
| Atrial fibrillation | 1.838 (0.751–4.481) | 0.13 |
| Peripheral vascular disease | 2.192 (0.884–5.554) | 0.15 |
| ARBs (current medication) | 0.342 (0.085–1.110) | 0.08 |
LVEF, left ventricular ejection fraction.
Table 5.
Multivariate linear regression analysis to evaluate the relation between severity of disease parameters and treatment and mortality (34 events)
| Variable | OR estimate 95%CI | P–value |
|---|---|---|
| Model 1 | ||
| Age | 1.043 (0-997–1.097) | 0.08 |
| Oxygen therapy on diagnosis | 5.386 (2.057–15.35) | <0.001 |
| Lymphocytes | 0.195 (0.049–0.625) | 0.01 |
| CRP | 1.005 (1.001–1.012) | 0.07 |
| AZT/HCQ combination | 0.578 (0.208–1.536) | 0.28 |
| Model 2 | ||
| Age | 1.041 (1.001–1.089) | 0.07 |
| Congestive heart failure (LVEF < 45%) | 1.222 (0.309–4.649) | 0.77 |
| Atrial fibrillation | 1.406 (0.519–3.707) | 0.49 |
| Peripheral vascular disease | 2.905 (1.088–7.928) | 0.03 |
| ARBs (current medication) | 0.093 (0.005–0.540) | 0.03 |
LVEF, left ventricular ejection fraction.
FIGURE 5:
Occurrence of the composite outcome critical evolution (ICU admission or death) according to oxygen therapy on diagnosis and lymphopaenia. (A) Critical evolution according to oxygen therapy on diagnosis: five events (22% of patients) occurred in the group without oxygen therapy on diagnosis and 30 events (54% of patients) in the group without oxygen therapy on diagnosis. (B) Critical evolution according to the tertile of lymphocytes count: 22 events (26% of patients) occurred in group with lymphocytes <0.60 G/L, 16 events (21% of patients) in the group with 0.60 G/L ≤ lymphocytes < 0.95 G/L and 7 events (9% of patients) in the group with lymphocytes ≥0.95 G/L.
Occurrence of the composite outcome and death according to treatments demographic and pre-existant factors
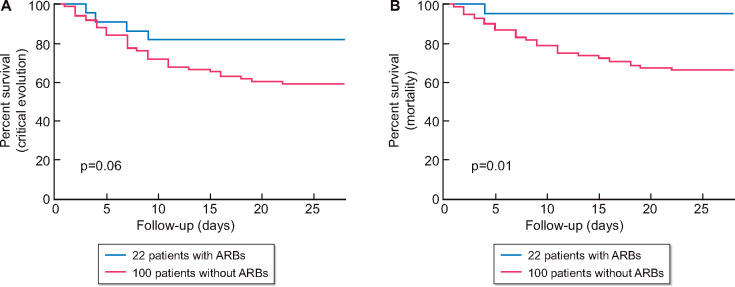
In our multivariable analysis models, peripheral vascular disease is positively associated with mortality (OR = 2.905, 95% CI 1.088–7.928; P = 0.03). Moreover, chronic use of ARBs was significantly associated with a protective effect against mortality (OR = 0.093, 95% CI 0.005–0.540; P = 0.03) (Table 5, Model 1). We did not found association with the composite critical evolution outcomes (OR = 0.342, 95% CI 0.085–1.110; P = 0.08) (Table 4, Model 1). Supplementary data, Table S1 compares variables of interest between groups of patients with or without ARBs in their usual medication. Patients with ARBs were younger (67 versus 74 years; P = 0.02) and had less atrial fibrillation (25% versus 49%; P = 0.01). Figure 6 shows the survival curve comparing critical evolution (Figure 5A) and mortality (Figure 5B) of patients with ARBs and patients without. In the subgroup of patients requiring oxygen on diagnosis (55 patients), chronic use of ARBs was significantly associated with a protective effect against the composite critical evolution outcomes (OR estimate = 0.088, 95% CI 0.004–0.580; P = 0.03) (Supplementary data, Table S2 and Figure S1).
FIGURE 6:
Occurrence of the composite outcome critical evolution (ICU admission or death) and death according to chronic ARBs treatment. (A) Critical evolution: four events (18% of patients) occurred in group with ARBs and 41 events (41%) in group without ARBs. (B) Mortality: one event (5% of patients) occurred in group with ARBs and 33 events (33%) in group without ARBs.
DISCUSSION
The COVID-19 pandemic has had enormous consequences for ESRD patients. Of the 2336 patients on chronic haemodialysis in our units, we studied 129 who had fallen ill with COVID-19 between 5 March and 8 May 2020, corresponding to an incidence of 5.5%. The hospitalization rate was 81%, those who stayed home came three times a week to specially designated dialysis units. Both the incidence and hospitalization rates are higher than in the general population (incidence estimate around 4.4%, with 3.6% hospitalization to date) [13]. Our multicentre study composed of ESRD patients from two opposite regions of France: the Champagne region in the North East and Marseille in the South showed no difference in the incidence of COVID-19, whereas in the general population of France, the incidence is higher in the ‘Grand Est’ region compared with the Southern region [13]. However, as in the general population, the first cases of COVID-19 in ESRD patients appeared in the Grand Est region. We also observed a heterogeneity between the different units in the same region, which could be explained by clusters of disease in some dialysis units. Nevertheless, these data should be interpreted with caution because screening strategies for the disease could influence the incidence rate of COVID-19. For example, in the Marseille region, only the Bouchard private hospital performed systematic screening of all haemodialysis patients in their centre. Five patients were diagnosed there although they had no symptoms, explaining Bouchard’s higher incidence rate (8.3%).
Our global incidences are lower compared with the first published haemodialysed patient cohorts from Brescia, Italy (15%) [8] and Madrid, Spain (13%) [10], but higher compared with the cohort from Wuhan, China (2.5%) [9]. More recently, new cohorts from Italy [14] and Spain [15] report similar results, with higher incidences (26 and 24%, respectively) compared with the second published cohort from Wuhan, China (3.5%, despite a large screening of asymptomatic by chest CT) [16]. Canadian [17] and Turkish [18] cohorts reported incidences of 4.6 and 1.1%, respectively.
Among the 122 cases at the end of the 28 follow-up days, 77 (63%) were alive, and 16% were transferred to ICU (with 42% mortality). In total 28% of patients died, but only 7% of the 28% died after transfer to ICU. A total of 45 (37%) had a critical evolution outcome (ICU admission or death before 28 days after diagnosis). The mortality rate in our study, while very much higher than that observed in the general population [4], is comparable to cohorts from Wuhan (31%) [9], Brescia (28%) [8] and Madrid (30%) [10]. The recently published cohorts reported a mortality of 43% in China [16], 25% in Italy [14] and 10% in Spain [15], and no deaths have been reported from Canada [17] and Turkish [18] cohorts. The small numbers of COVID-19 cases in these studies may explain this heterogeneity concerning mortality. A strength of our study is that all patients still alive were discharged or had 28 days of follow-up, compared with continuing hospitalization of some patients from the other cohorts.
In the general population, a meta-analysis has already identified many clinical and biological factors associated with severe forms of the COVID-19 [5]. In our study, two variables have independent and robust prediction for the occurrence of the event ‘critical evolution’ and for global mortality. First, the necessity of oxygen therapy on diagnosis was 2-fold more frequent in what became the critical-evolution group (67%) than in the non-critical-evolution group (32%), and the multivariate analysis confirmed this independent association. To our knowledge, it is the first study to demonstrate this association in ESRD Stage 5D patients, oxygen therapy needed at admission appears to be more significant than other respiratory disorder signals such as dyspnoea. Secondly, the decrease in the lymphocyte count. In fact, median lymphocyte count was 0.3-fold lower in the critical-evolution group (0.6 G/L) than in the non-critical-evolution group (0.9 G/L), and the multivariate analysis also confirmed this independent association. Lymphopaenia has already been shown to be associated with severe disease during COVID-19 infection in dialysis patients [10] and in the general population [19]. In our study, lymphocyte count appears to be a better predictive marker for poor outcomes compared with other classical markers such as CRP [8] or LDH [10]. On the other hand, this effect was not found in a subgroup analysis of patients with severe initial symptoms (requiring oxygen therapy on admission). We did not find independent associations between the composite outcome ‘critical evolution’ or mortality and risk factors previously identified in the general population, such as age, obesity, diabetes and cardiovascular disease. However, since the number of cases in our cohort is relatively small, and since most of dialysis patients are old and have high rate of comorbidity, our study may not have enough power to identify these variables as risk factors.
We were interested in studying the treatment of COVID-19 using the AZT/HCQ combination. This treatment was largely used by the Marseille team of nephrologists [20]. In our cohort, 46% of patients received this treatment (86% in Marseille). There was no difference in the univariate analysis (51% of treatment in the non-critical-evolution group versus 38% in the critical-evolution group; P = 0.17). Survival curves and multivariate analyses showed no statistical association. Since Gautret et al. argue that efficacy of this combination therapy depends on its early initiation [20], we studied the effect of this treatment in a subgroup of patients not requiring oxygen therapy on diagnosis, but we still did not find any association with a clinical benefit. This result suggests that a prospective study to test the effectiveness of AZT/HCQ treatment is necessary. The administration of other treatments such as corticoids, antiretroviral therapy and preventive heparin had no effect on either group. Antibiotics were largely administered (90% of all patients) to prevent bacterial infection. The more frequent need for treatments such as curative heparin in the critical-evolution group probably reflects a higher level of pro-inflammatory and pro-coagulant markers (neutrophil, CRP and fibrinogen), which are significantly more elevated within this category of patients.
We studied the potential effect of the use of ARBs as current medication on the composite outcomes of critical evolution and mortality. Multivariable analysis showed a trend for a protective effect on the composite outcome, and a protective effect on global mortality (1% in group with ARBs versus 33% for the group without ARBs). In a subgroup of patients requiring oxygen therapy on diagnosis (excluding asymptomatic patients), we found a protective effect of chronic ARBs on global mortality, but not on composite outcome, probably due to a lack of power. Our results must be interpreted with caution due to the observational design of our work, and ARBs usual prescription seems to concern younger patients with less atrial fibrillation. Recent studies showed that prior use of ARBs was not associated with COVID-19 diagnosis or with the severity of the disease [21–23]. The protective association in our study is in line with results observed in other studies in non-dialysis patients [24, 25]. SARS-CoV-2 enters host cells through binding of the S protein virus to the ACE2 [26]. ACE2 receptor has proinflammatory properties [27]. Pharmacological intervention in this pathway, like the use of recombinant ACE2 [27, 28] or ARBs [27, 29], could improve the outcomes of patients with COVID-19 [28], particularly in those who are critically ill [30]. Several randomized controlled trials are in progress to evaluate ACEIs and ARBs for treatment of COVID-19 [23, 27]. ESRD patients, who experience frequent hypertension, cardiovascular disease and inflammatory disorders, might be good candidates for the beneficial albeit hypothetical protective effect of ARBs in COVID-19.
To conclude, COVID-19 disease in patients on chronic haemodialysis seems to be more frequent than in the general population. It is a severe disease with poor prognosis in patients with ESRD with high rate of mortality (28%). The requirement for oxygen therapy on diagnosis and lymphopaenia are critical prognosis factors for poor outcomes. Usual treatment with ARBs seems to be protective and therefore should be continued in patients under this medication. There is no evidence of clinical benefit with the combination of AZT/HCQ.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank all of the medical and paramedical team involved in this work.
AUTHORS’ CONTRIBUTIONS
G.L., A.B., T.R. and P.R. conceived and designed the study, and drafted the manuscript. S.Bataille, G.C., J.M.-F., B.G., P.Bindi, M.N., J.M., P.H., B.L., E.C., K.G., I.K., N.N., A.W., A.D., N.J.-C., V.M., R.V., V.S., M.B., M.G., T.L., M.P., M.S., S.Burtey and P.Brunet were involved in data collection analysis and provided guidance. All authors critically revised the manuscript for important intellectual content and approved the final version for publication.
CONFLICT OF INTEREST STATEMENT
All the authors declare that they have no conflict of interest.
REFERENCES
- 1. Huang C, Wang Y, Li X. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet 2020; 395: 497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu C, Chen X, Cai Y. et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Int Med 2020; 180: 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wiersinga WJ, Rhodes A, Cheng AC. et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020; 324: 782. [DOI] [PubMed] [Google Scholar]
- 4. Grasselli G, Zangrillo A, Zanella A. et al. ; for the COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020; 323: 1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zheng Z, Peng F, Xu B. et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect 2020; 81: e16–e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Henry BM, Lippi G.. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol 2020; 52: 1193–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. D’Marco L, Puchades MJ, Romero-Parra M. et al. Coronavirus disease 2019 in chronic kidney disease. Clin Kidney J 2020; 13: 297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alberici F, Delbarba E, Manenti C. et al. A report from the Brescia renal COVID task force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int 2020; 98: 20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xiong F, Tang H, Liu L. et al. Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan, China. J Am Soc Nephrol 2020; 31: 1387–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goicoechea M, Sánchez Cámara LA, Macías N. et al. COVID-19: clinical course and outcomes of 36 maintenance hemodialysis patients from a single center in Spain. Kidney Int 2020; 98: 27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Basile C, Combe C, Pizzarelli F. et al. Recommendations for the prevention, mitigation and containment of the emerging SARS-CoV-2 (COVID-19) pandemic in haemodialysis centres. Nephrol Dial Transplant 2020; 35: 737–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bataille S, Pedinielli N, Bergounioux JP.. Could ferritin help the screening for COVID-19 in hemodialysis patients? Kidney Int 2020; 98: 235–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salje H, Tran Kiem C, Lefrancq N. et al. Estimating the burden of SARS-CoV-2 in France. Science 2020; 369: 208–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. La Milia V, Bacchini G, Bigi MC. et al. COVID-19 outbreak in a large hemodialysis center in Lombardy, Italy. Kidney Int Rep 2020; 5: 1095–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stock da Cunha T, Gomá-Garcés E, Avello A. et al. The spectrum of clinical and serological features of COVID-19 in urban hemodialysis patients. J Clin Med 2020; 9: 2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang R, He H, Liao C. et al. Clinical outcomes of hemodialysis patients infected with severe acute respiratory syndrome coronavirus 2 and impact of proactive chest computed tomography scans. Clin Kidney J 2020; 13: 328–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yau K, Muller MP, Lin M. et al. COVID-19 outbreak in an urban hemodialysis unit. Am J Kidney Dis 2020; doi: 10.1053/j.ajkd.2020.07.001 [15 July 2020, Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arslan h, Musabak U, Ayvazoglu Soy EH. et al. Incidence and immunologic analysis of coronavirus disease (COVID-19) in hemodialysis patients: a single-center experience. Exp Clin Transplant 2020; 18: 275–283 [DOI] [PubMed] [Google Scholar]
- 19. Zhao Q, Meng M, Kumar R. et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a systemic review and meta-analysis. Int J Infect Dis 2020; 96: 131–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gautret P, Lagier J-C, Parola P. et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med Infect Dis 2020; 34: 101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mancia G, Rea F, Ludergnani M. et al. Renin–angiotensin–aldosterone system blockers and the risk of covid-19. N Engl J Med 2020; 382: 2431–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fosbøl EL, Butt JH, Østergaard L. et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA 2020; 324: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mackey K, King VJ, Gurley S. et al. Risks and impact of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers on SARS-CoV-2 infection in adults: a living systematic review. Ann Int Med 2020; 173: 195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang P, Zhu L, Cai J. et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 2020; 126: 1671–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang X, Yu J, Pan L. et al. ACEI/ARB use and risk of infection or severity or mortality of COVID-19: a systematic review and meta-analysis. Pharmacol Res 2020; 158: 104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoffmann M, Kleine-Weber H, Schroeder S. et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–280.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Michaud V, Deodhar M, Arwood M. et al. ACE2 as a therapeutic target for COVID-19; its role in infectious processes and regulation by modulators of the RAAS system. J Clin Med 2020; 9: 2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alhenc-Gelas F, Drueke TB.. Blockade of SARS-CoV-2 infection by recombinant soluble ACE2. Kidney Int 2020; 97: 1091–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sparks MA, South A, Welling P. et al. Sound science before quick judgement regarding RAS blockade in COVID-19. Clin J Am Soc Nephrol 2020; 15: 714–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang H, Penninger JM, Li Y. et al. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 2020; 46: 586–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.