Extended reality healthcare applications are becoming increasingly affordable, less complex to implement, and more performant, resulting in the rapid expansion of applications in cardiology for patient and medical student education, patient rehabilitation, and real-time intraprocedural use1. We describe the first-in-human prospective use of the Enhanced ELectrophysiology Visualization and Interaction System (ĒLVIS) implemented in an Electrophysiology (EP) laboratory, providing the electrophysiologist with a real-time, intraprocedural 3-dimensional digital image of the patient’s electroanatomic maps, along with real-time catheter locations. (see Supplemental Video #1).
The ĒLVIS system combines the HoloLens headset (Microsoft, Redmond, WA) with proprietary SentEP software (SentiAR, St Louis, MO) to display images from an electroanatomic mapping system (EAMS, EnSite Velocity, St Jude Medical, St Paul, MN)2. We hypothesized that providing the physician with these real-time, 3D data would improve point navigation and accuracy.
To assess and quantify point navigation and accuracy, a protocol was designed in which physicians were asked to carry out a series of navigation tasks during the post-ablation waiting phase of the EP study (EPS) under 2 conditions, 1) using ĒLVIS, and 2) using current standard of care. The study coordinator randomized the order of the conditions was randomized through blinded envelope. Physicians were permitted to use the ĒLVIS system throughout the procedure. During the post-ablation waiting phase, after generating a geometry of a cardiac chamber chosen by the physician (5-minute allotment per condition for geometry creation), 5 target markers were placed within the geometry using the EAMS. The sequence of navigation and anatomical location of the targets were ordered so that the physician had to move to disparate locations in the anatomy, rather than making small movements. Physicians were then asked to navigate to the markers sequentially under both conditions (60 second allotment per marker, per condition), resulting in matched data sets for analysis. The physicians alone determined when they were at the optimal navigation site, and a marker was placed at that site in the EAMS. (See Supplemental Video #2) If physicians were not able to navigate to and mark the target within the time frame under either condition, the result was noted, and the point pair was excluded from the analysis. The navigated location x, y, z coordinates were then compared to the target x, y, z ground truth coordinates as measured and recorded by the EAMS. A blinded reviewer calculated the Euclidean distance, a straight line, between the coordinates to determine the accuracy of the SentEP system. A 20-minute training session was completed by the operating EPs prior to first case enrollment.
After obtaining approval from the Western Institutional Review Board, patients age 6-21 years who were scheduled for an EPS were identified and screened by the study team and informed consent and assent were obtained. Two board-certified EPs (operator #1 n=7; operator #2 n=9) completed a total 16 cases that completed the protocol with the following diagnoses: atrioventricular nodal reentrant tachycardia n=7, accessory pathway mediated tachycardia n=7, premature ventricular contractions n=2. There was no noted difference in navigation times point navigation (ELVIS 31±14 sec versus EAMS 28±15 sec, p=0.174). From these 16 patients, a total of 75 paired points (150 total points) were available for analysis. Five paired points (10 total points) were discarded for violation of the 60 second time limit. Wilcoxon Signed-Rank testing demonstrated a significant accuracy improvement with ĒLVIS, with an error of 2.99±1.91 mm vs 4.50±3.74 mm (p<0.005). (See Figure 1)
Figure 1 |. Navigation Accuracy.
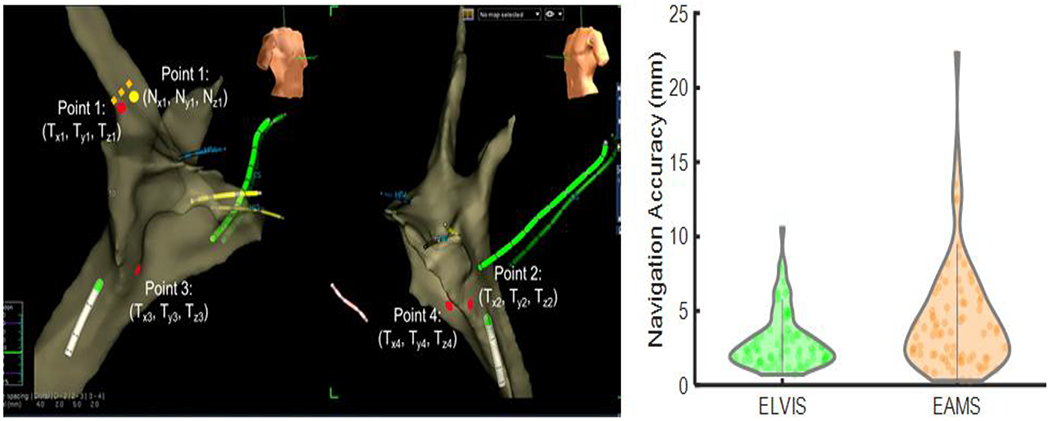
Left: During a first-in-human clinical study, physicians were asked to navigate to five points during the waiting phase of an ablation procedure. The error between the target Point 1 (Tx1, Ty1, Tz1) and the marked catheter location Point 1 (Nx1, Ny1, Nz1), is indicated by the dotted orange line. Right: Physicians using the ĒLVIS system were significantly more accurate, 2.99±1.91 mm (green) vs standard of care (EAMS) 4.50±3.74 mm (orange), p<0.005, Wilcoxon Signed-Rank test.
These data are the first to demonstrate that point navigation accuracy is significantly improved with a mixed reality display. Our study shows that improved visualization of existing information can amplify physician skill. This improvement in accuracy is likely to be clinically impactful as ablation lesions typically have a diameter of 6 mm3. Without the use of the ĒLVIS 3D display, a significant fraction of lesions (34%) would be delivered outside of the target area, as opposed to 6% with ĒLVIS 3D display. For anatomic ablations such as pulmonary vein isolation for the treatment of atrial fibrillation4, we expect that this improved lesion delivery will improve patient outcomes and potentially reduce the need for repeat procedures.
This enabling technology will provide a more intuitive visualization of currently displayed 2D images in a 3D, stereoscopic display. The ability to place and remove dynamic 3D digital objects conveniently will provide further enhanced access to and control of information within the clinical space. Future potential improvements including additional modes of sterile interaction, access to cloud computing resources and remote consultation may amplify physician control and skill, and ultimately to improved patient access to care. Given the widespread promise of this technology, mixed reality has the potential to overtake and aggregate current displays in the cardiac catheterization lab.
Supplementary Material
Acknowledgments
Financial Support: This work has been supported by the National Heart, Lung and Blood Institute (National Institute of Health) R44 HL140896. Additional funding for this project was provided by the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital CH-II-2017-575.
Abbreviations:
- EP
electrophysiology
- ĒLVIS
Enhanced Electrophysiology Visualization and Interaction System
- EAMS
electoanatomic mapping system
- EPS
electrophysiology study
References:
- 1.Silva JNA, Southworth M, Raptis C & Silva J Emerging Applications of Virtual Reality in Cardiovascular Medicine. JACC Basic Transl Sci 3, 420–430, doi: 10.1016/j.jacbts.2017.11.009 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silva J & Silva J (Google Patents, 2019). [Google Scholar]
- 3.Ames A & Stevenson WG Cardiology patient page. Catheter ablation of atrial fibrillation. Circulation 113, e666–668, doi: 10.1161/CIRCULATIONAHA.105.613083 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Anter E, Contreras-Valdes FM, Shvilkin A, Tschabrunn CM & Josephson ME Acute pulmonary vein reconnection is a predictor of atrial fibrillation recurrence following pulmonary vein isolation. J Interv Card Electrophysiol 39, 225–232, doi: 10.1007/s10840-013-9864-9 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


