INTRODUCTION:
Compared with endoscopic variceal ligation (EVL), cap-assisted endoscopic sclerotherapy (CAES) improves efficacy in the treatment of small esophageal varices (EVs) but has not been evaluated in the management of medium EVs. The aim of this study was to compare CAES with EVL in the long-term management of patients exhibiting cirrhosis with medium EVs and a history of esophageal variceal bleeding (EVB), with respect to variceal eradication and recurrence, adverse events, rebleeding, and survival.
METHODS:
Cirrhotic patients with medium EVs and a history of EVB were divided randomly into EVL and CAES groups. EVL or CAES was repeated each month until variceal eradication. Lauromacrogol was used as a sclerosant. Patients were followed up until 1 year after eradication.
RESULTS:
In total, 240 patients (age: 51.1 ± 10.0 years; men: 70.8%) were included and randomized to the EVL and CAES groups. The recurrence rate of EVs was much lower in the CAES group than in the EVL group (13.0% vs 30.7%, P = 0.001). The predictors for variceal recurrence were eradication by EVL (hazard ratio [HR]: 2.37, P = 0.04), achievement of complete eradication (HR: 0.27, P < 0.001), and nonselective β-blocker response (HR: 0.32, P = 0.003). There was no significant difference in the rates of eradication, rebleeding, requirement for alternative therapy, and mortality or the incidence of complications between groups.
DISCUSSION:
CAES reduces the recurrence rate of EVs with comparable safety to that of EVL in the long-term management of patients presenting cirrhosis with medium EVs and a history of EVB.
INTRODUCTION
Esophageal variceal bleeding (EVB) is a serious complication of cirrhosis with a 6-week mortality of approximately 15%–20% and a 1-year rebleeding rate of approximately 60% in cirrhotic patients without prophylactic treatment (1). The combination of endoscopic variceal ligation (EVL) and nonselective β-blockers (NSBBs) is recommended to prevent rebleeding in cirrhosis (1–5). However, EVL has some limitations in the treatment of esophageal varices (EVs), such as a high variceal recurrence rate and fatal post-EVL bleeding ulcers (6–8). The high recurrence rate of EVs is perhaps because eradication of deeper EVs, esophageal perforating veins (EPVs), and residual small varices (RSVs) is not achieved by EVL (9). No such problems arise in the endoscopic injection of sclerotherapy (EIS). The sclerosant can drain into deeper EVs, EPVs, and RSVs to prevent the recurrence and rebleeding of EVs.
Most guidelines and meta-analyses have shown that repeated EVL is superior to repeated EIS in the control of acute variceal bleeding (AVB) (1–5,10,11) and that the combination of EIS with EVL does not improve clinical outcomes but increases the rate of esophageal stricture in the secondary prophylaxis of AVB (11,12). The advantages of repeated EVL over sequential therapy of EIS after EVL in the secondary prophylaxis of EVB are not well supported. It has been reported that the incidence rate of EIS-induced complications can be reduced with intravariceal or small-volume injection of EIS (13,14). Some studies have demonstrated that EIS may be better than EVL for treating EVs with small volumes (6,7,13). The effectiveness of EIS is believed to depend on the duration of contact between the vascular endothelial cells and the sclerosant. Cap-assisted endoscopic sclerotherapy (CAES) is a technique involving the placement of a transparent cap on the tip of a gastroscope. This approach can help expose target EVs and make intravariceal injection of the sclerosant easier. Instant compression by the cap after EIS injection would not only control instant bleeding but also prevent outflow of the sclerosant and increase the duration of contact of the sclerosant with endothelial cells. It was reported that compared with conventional EIS, CAES can improve the hemostatic efficiency and reduce adverse effects in the treatment of EVs (15–17).
Presently, there is a lack of trials comparing the efficacy and safety of CAES and EVL in the long-term management of patients with medium EVs and a history of EVB. We hypothesized that compared with EVL, CAES could reduce the recurrence rate of EVs and incidence rate of endoscopic treatment-induced complications. The aim of this study was to compare the efficacy and safety of CAES with those of EVL in the long-term management of cirrhotic patients with medium EVs and a history of EVB.
METHODS
Study population
Cirrhotic patients (aged between 18 and 65 years) with a history of EVB admitted to the First Affiliated Hospital of Nanchang University were considered for enrollment. Patients were included when EVs were reduced to medium size by repeated EVL for prevention of rebleeding. The exclusion criteria included the following: (i) noncirrhotic causes of varices; (ii) previous endoscopic therapy other than EVL, a previous surgery or transjugular intrahepatic portosystemic shunt (TIPS) for portal hypertension, liver transplantation (LT), and presence or history of malignancy including hepatocellular carcinoma; (iii) presence of gastrointestinal bleeding symptoms (including melena, hematemesis, or/and hematochezia) within 120 hours before entry; (iv) concomitance with gastroesophageal varices 2, isolated gastric varices (GVs), or ectopic varices; (v) debilitating illness or severe systemic disease in the heart, brain, lung, or kidney; total bilirubin >170 μmol/L; Child-Pugh classification (CPC) score >13; or hepatic encephalopathy grade 2–4; (vi) intolerance to endoscopy or refusal to participate in the study; (vii) allergies to rubber, propofol, or lauromacrogol; and (viii) American Society of Anesthesiologists class IV and V or sleep apnea. Women were required to be nonpregnant and nonlactating and to maintain effective contraception if they had child-bearing potential. Cirrhosis was diagnosed through clinical, radiological, or laboratory parameters and/or liver biopsy. The diagnosis and classification of gastroesophageal varices were based on previous studies (8,18).
Study protocol
This study protocol was approved by the Institutional Ethical Committee of our hospital (approval no. 2016-017; date of review: January 13, 2016). Informed consent was obtained from all patients. The study followed the Consolidated Standards of Reporting Trials (CONSORT) guidelines and was registered at www.chictr.org.cn on February 22, 2016 (Identifier: ChiCTR-IIR-16007964).
Randomization
An independent statistician (H.-Q.Y.) uninvolved with the study conduct generated the allocation list using a computer-generated randomization schedule and had opaque coverings over the assignments. Randomization was performed using block randomization with a block size of 4. On the day of entry, patients were randomly assigned with concealed allocation in a 1:1 ratio to receiving either repeated CAES (the CAES group) or EVL (the EVL group).
Study design
The clinical characteristics of the patients, including their CPCs, were evaluated on the day of randomization. All patients underwent propofol sedation for endoscopic assessment and treatment by an electronic endoscope GIF-Q260J (Olympus Optical Corporation, Tokyo, Japan). The endoscopic assessment was made at each session according to AASLD 2007 guidelines (18). Briefly, small varices were defined as minimally elevated veins above the esophageal mucosal surface. Medium varices were defined as tortuous veins occupying less than one-third of the esophageal lumen, and large varices were defined as those occupying more than one-third of the esophageal lumen. All endoscopic procedures were performed by endoscopists with over 5 years of experience in caring for such patients. Endoscopists with ≥10 years of EVL or sclerotherapy experience were defined as senior endoscopists. The varices were evaluated by 2 endoscopists (J.W. and B.-M.L.) during the withdrawal of the endoscope, with adequate air insufflation of the esophagus and stomach. Multiple endoscopic images for EV grade assessment were recorded at the endoscopy session site. When assessment disagreements occurred, the recorded images were retrieved and reviewed by a third endoscopist (Y.Y.).
For patients in the EVL group, ligation was performed with a standard technique as previously described (8). For patients in the CAES group, lauromacrogol in a small volume was administered to thrombose the main variceal channels and thereby eradicate varices (as previously described (13,15)) and was used as sclerosant. Briefly, patients received an injection of lauromacrogol along with a transparent cap, which was placed in front of the endoscope. An intravariceal injection of no more than 20 mL per session of 1% lauromacrogol (10 mg/mL; Tianyu Pharmaceutical, Shanxi, China) was administered through the 23-g injection needle (Boston Scientific Interject-M00518301; Boston Scientific, Spencer, IN). An intravariceal puncture was confirmed by the suction of blood through a transparent tube connected to a needle. The initial injections were administered 2–3 cm above the cardioesophageal junction and continued until all the EVs were treated. Each injection contained different volumes (1.0–4.0 mL) as needed, and each EV was injected with lauromacrogol (at least 8 mL volume). Compression by the transparent cap on each puncture site for 2–3 minutes was the routine procedure in case of bleeding of the injection site or overflow of lauromacrogol.
For both groups of patients, salvage hemostasis methods, including instant balloon tamponade or injection of cyanoacrylate, were used if bleeding during the procedure was uncontrollable. For patients with ulceration, the treatment was delivered to an area without ulceration. Patients were blinded to the endoscopic intervention allotted. As the interval for endoscopic treatment of EVs is debatable (1,19), EVL or CAES was arbitrarily chosen to be repeated at an interval of 1 month until variceal eradication. NSBB therapy with propranolol (Jinshi Pharma, Shantou, China) was regularly administered to all patients unless contraindicated or patients refused to take it. For the sake of safety, patients with refractory ascites, systolic blood pressure <90 mm Hg, serum creatinine >1.5 mg/dL, or hyponatremia <130 mmol/L were not administered NSBBs. As the hepatic venous pressure gradient was not routinely measured in clinical practice, NSBBs were titrated according to the heart rate. Patients were defined as NSBB responders if their heart rate was between 55 and 60 beats per minute or showed a 25% decrease compared with the baseline values through titration of the propranolol dose within 1 month from entry. Treatments targeting the etiology and complications of cirrhosis were also performed (e.g., alcohol abstinence for alcohol-related cirrhosis, anti–hepatitis B virus (HBV)/hepatitis C virus therapy for HBV/hepatitis C virus–related cirrhosis, albumin transfusion, and diuretics for ascites).
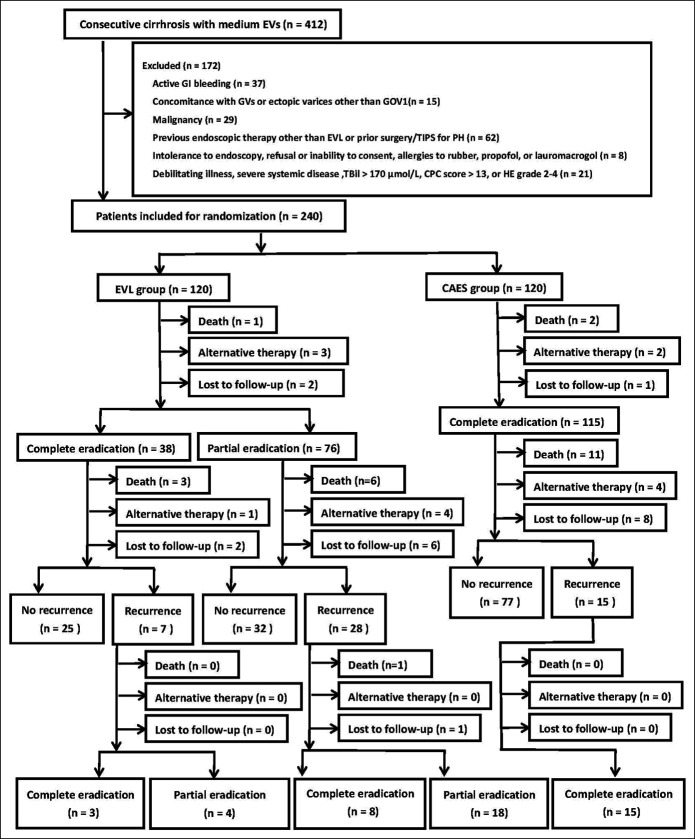
After the initial eradication of varices, patients in both groups were followed up as outpatients at every 3-month interval thereafter. Alternative therapy, including TIPS, surgery, or LT, was performed during follow-up when necessary. Rebleeding, recurrence of EVs, mortality, transfer to alternative therapy, and endoscopic treatment-induced complications were recorded. All patients were followed up until death, 1 year after eradication of EVs, or transfer to alternative therapy. Patients with recurrence of medium/large EVs or small EVs with red color signs during follow-up were managed with a repeat of their initial technique and follow-up at 1-month intervals until re-eradication was achieved. The study design and patient flow are shown in Figure 1.
Figure 1.
Flow chart for enrolled patients. CAES, cap-assisted endoscopic sclerotherapy; CPC, Child-Pugh classification; EVs, esophageal varices; EVL, endoscopic variceal ligation; GI, gastrointestinal; GOV, gastroesophageal varices; GV, gastric varices; HE, hepatic encephalopathy; PH, portal hypertension; TBil, total bilirubin; TIPS, transjugular intrahepatic portosystemic stent.
Endpoints
The primary endpoint was the recurrence of EVs, defined as the presence of small or greater EVs after eradication. The secondary endpoints included the eradication of EVs, rebleeding, mortality, the use of the salvage hemostasis method during the procedure, the use of alternative therapy, and the development of endoscopic treatment-induced complications. Eradication was defined as no clear evidence of EVs (complete eradication). Patients in the EVL group with RSVs difficult to aspirate into the ligation chamber were defined as having partial eradication (6,7). All rebleeding events were defined based on gastrointestinal bleeding symptoms. The origins of the bleeding were subsequently confirmed by endoscopy, as defined in a previous study (8). The clinical endpoints, including rebleeding, eradication and recurrence of EVs, and complications of endoscopic treatments, were evaluated by physicians (J.-W.Z. and G.-H.G.) who were blinded to the patient assignments.
Statistical analyses
Statistical analyses were performed by SPSS 23.0 for Windows (SPSS, Chicago, IL). The statistical analysis was performed according to an intention-to-treat strategy. Patients were censored at the time of death/LT, loss to follow-up, or the last visit before study closure. Descriptive statistics are reported as the mean ± SD for continuous variables with a normal distribution, which were compared using the Student t test. The Mann-Whitney (U) test was used for comparison of quantitative variables between 2 groups of nonnormally distributed data. Qualitative data were compared using the χ2 test or Fisher exact test when appropriate. Kaplan-Meier survival analysis was performed to estimate the cumulative overall rates of survival, rebleeding, eradication, and recurrence of EVs, and the log-rank test was used to compare them. Potential predictors for recurrence were analyzed with univariate analysis. All tested variables with P values <0.1 were entered into a stepwise Cox proportional hazards regression analysis. Two-sided P values <0.05 were considered significant.
Sample size determination
According to previous studies, we estimated that the 1-year recurrence rate of EVs was 10% in the CAES group and 25% in the EVL group after eradication (6,7). At least 97 patients with eradication of EVs in each group were needed for an alpha of 0.05 and a power of 80%. As a 90% eradication rate achieved by EVL/CAES and a 10% drop-out rate were assumed, 240 patients with medium EVs and a history of EVB at minimum had to be included. The sample size was calculated using the Power and Sample Size Calculation program (Nashville, TN. Available at: http://biostat.mc.vanderbilt.edu/PowerSampleSize).
RESULTS
Patient demographics
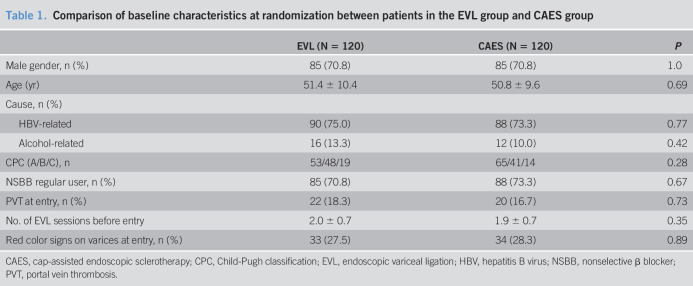
Between April 1, 2016, and October 31, 2018, a total of 412 cirrhotic patients were considered for enrollment. After 172 patients were excluded, the 240 included patients were randomized to the EVL group (n = 120) and CAES group (n = 120) (Figure 1). The age of the included patients was 51.1 ± 10.0 years, and 70.8% were men. Most patients were categorized as having HBV-related cirrhosis (74.2%). Only 33 patients (13.8%) classified as CPC-C consented to participation in this study because most patients with CPC-C underwent TIPS for the secondary prophylaxis of bleeding. The baseline of the included patients is listed in Table 1. The median follow-up period was 14 months (range: 1–17 months). A total of 11 patients in the EVL group and 9 patients in the CAES group (11/120 vs 9/120, P = 0.64) were lost to follow-up.
Table 1.
Comparison of baseline characteristics at randomization between patients in the EVL group and CAES group
| EVL (N = 120) | CAES (N = 120) | P | |
| Male gender, n (%) | 85 (70.8) | 85 (70.8) | 1.0 |
| Age (yr) | 51.4 ± 10.4 | 50.8 ± 9.6 | 0.69 |
| Cause, n (%) | |||
| HBV-related | 90 (75.0) | 88 (73.3) | 0.77 |
| Alcohol-related | 16 (13.3) | 12 (10.0) | 0.42 |
| CPC (A/B/C), n | 53/48/19 | 65/41/14 | 0.28 |
| NSBB regular user, n (%) | 85 (70.8) | 88 (73.3) | 0.67 |
| PVT at entry, n (%) | 22 (18.3) | 20 (16.7) | 0.73 |
| No. of EVL sessions before entry | 2.0 ± 0.7 | 1.9 ± 0.7 | 0.35 |
| Red color signs on varices at entry, n (%) | 33 (27.5) | 34 (28.3) | 0.89 |
CAES, cap-assisted endoscopic sclerotherapy; CPC, Child-Pugh classification; EVL, endoscopic variceal ligation; HBV, hepatitis B virus; NSBB, nonselective β blocker; PVT, portal vein thrombosis.
Eradication
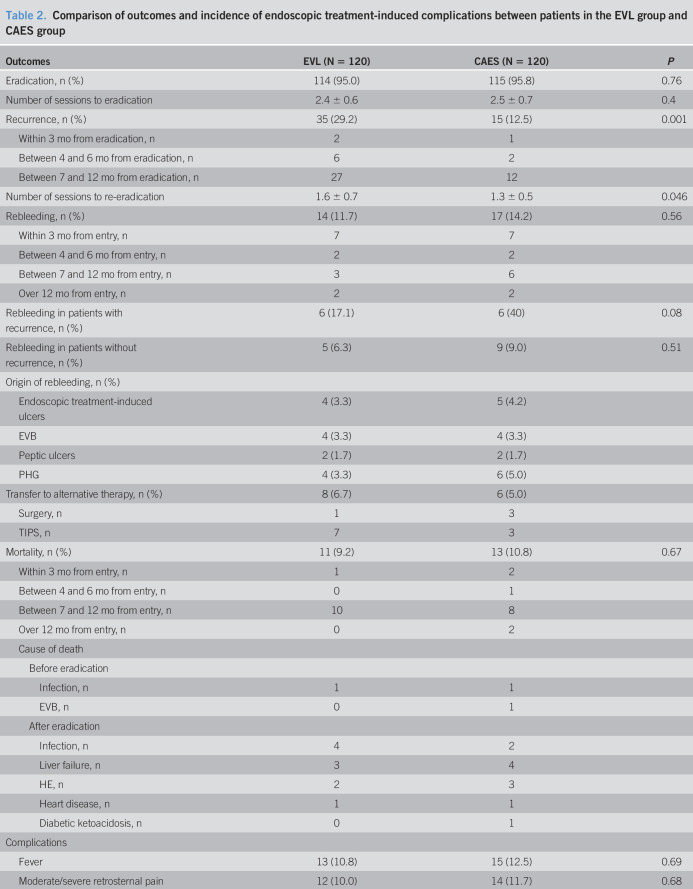
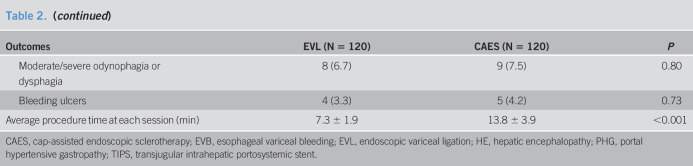
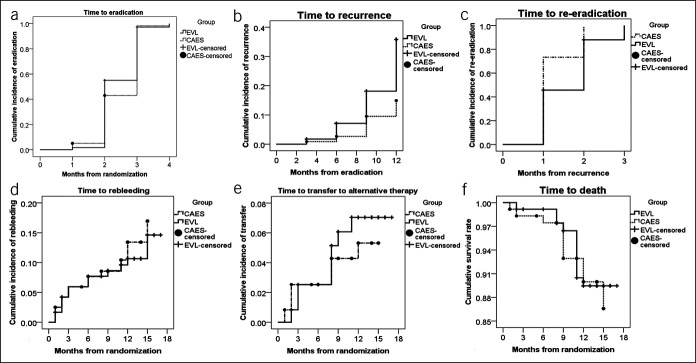
Eradication of EVs was achieved in 114 EVL patients (95.0%) and 115 CAES patients (95.8%) (Table 2). The mean time necessary for eradication was 2.4 ± 0.6 months. There was no significant difference in the eradication rate between these 2 groups (log-rank test, P = 0.21) (Figure 2a). Notably, more patients in the CAES group achieved complete eradication than those in the EVL group (95.8% (115/120) in the CAES group vs 31.7% (38/120) in the EVL group, P < 0.001).
Table 2.
Comparison of outcomes and incidence of endoscopic treatment-induced complications between patients in the EVL group and CAES group
| Outcomes | EVL (N = 120) | CAES (N = 120) | P |
| Eradication, n (%) | 114 (95.0) | 115 (95.8) | 0.76 |
| Number of sessions to eradication | 2.4 ± 0.6 | 2.5 ± 0.7 | 0.4 |
| Recurrence, n (%) | 35 (29.2) | 15 (12.5) | 0.001 |
| Within 3 mo from eradication, n | 2 | 1 | |
| Between 4 and 6 mo from eradication, n | 6 | 2 | |
| Between 7 and 12 mo from eradication, n | 27 | 12 | |
| Number of sessions to re-eradication | 1.6 ± 0.7 | 1.3 ± 0.5 | 0.046 |
| Rebleeding, n (%) | 14 (11.7) | 17 (14.2) | 0.56 |
| Within 3 mo from entry, n | 7 | 7 | |
| Between 4 and 6 mo from entry, n | 2 | 2 | |
| Between 7 and 12 mo from entry, n | 3 | 6 | |
| Over 12 mo from entry, n | 2 | 2 | |
| Rebleeding in patients with recurrence, n (%) | 6 (17.1) | 6 (40) | 0.08 |
| Rebleeding in patients without recurrence, n (%) | 5 (6.3) | 9 (9.0) | 0.51 |
| Origin of rebleeding, n (%) | |||
| Endoscopic treatment-induced ulcers | 4 (3.3) | 5 (4.2) | |
| EVB | 4 (3.3) | 4 (3.3) | |
| Peptic ulcers | 2 (1.7) | 2 (1.7) | |
| PHG | 4 (3.3) | 6 (5.0) | |
| Transfer to alternative therapy, n (%) | 8 (6.7) | 6 (5.0) | |
| Surgery, n | 1 | 3 | |
| TIPS, n | 7 | 3 | |
| Mortality, n (%) | 11 (9.2) | 13 (10.8) | 0.67 |
| Within 3 mo from entry, n | 1 | 2 | |
| Between 4 and 6 mo from entry, n | 0 | 1 | |
| Between 7 and 12 mo from entry, n | 10 | 8 | |
| Over 12 mo from entry, n | 0 | 2 | |
| Cause of death | |||
| Before eradication | |||
| Infection, n | 1 | 1 | |
| EVB, n | 0 | 1 | |
| After eradication | |||
| Infection, n | 4 | 2 | |
| Liver failure, n | 3 | 4 | |
| HE, n | 2 | 3 | |
| Heart disease, n | 1 | 1 | |
| Diabetic ketoacidosis, n | 0 | 1 | |
| Complications | |||
| Fever | 13 (10.8) | 15 (12.5) | 0.69 |
| Moderate/severe retrosternal pain | 12 (10.0) | 14 (11.7) | 0.68 |
| Moderate/severe odynophagia or dysphagia | 8 (6.7) | 9 (7.5) | 0.80 |
| Bleeding ulcers | 4 (3.3) | 5 (4.2) | 0.73 |
| Average procedure time at each session (min) | 7.3 ± 1.9 | 13.8 ± 3.9 | <0.001 |
CAES, cap-assisted endoscopic sclerotherapy; EVB, esophageal variceal bleeding; EVL, endoscopic variceal ligation; HE, hepatic encephalopathy; PHG, portal hypertensive gastropathy; TIPS, transjugular intrahepatic portosystemic stent.
Figure 2.
Time from randomization to outcomes by using the Kaplan-Meier curve. (a) Time from randomization to eradication (log-rank χ2 = 1.59, df = 1, P = 0.21). (b) Time from eradication to recurrence (log-rank χ2 = 10.62, P = 0.001). (c) Time from recurrence to re-eradication (log-rank χ2 = 4.15, df = 1, P = 0.04). (d) Time from randomization to rebleeding (log-rank χ2 = 0.24, df = 1, P = 0.62). (e) Time from randomization to transfer to alternative therapy (log-rank χ2 = 0.28, P = 0.60). (f) Time from randomization to death (log-rank χ2 = 0.09, df = 1, P = 0.76).
Primary endpoint
A total of 50 patients (male 78.0%, age 54.2 ± 5.6 years, CPC-A/B/C 8/31/11) had recurrence of EVs during follow-up. Compared with patients without recurrence, those with recurrence were older and more likely to have portal vein thrombosis, exhibit red colors on their EVs, have worse liver function according to the CPC, and undergo EVL for eradication at entry. In addition, more patients in the recurrence group had partial eradication and rebleeding and did not respond to NSBBs during the follow-up (Table 3). The mean time until recurrence was 10.5 ± 2.5 months. One year after variceal eradication, the recurrence rate of EVs was 29.2% in the EVL group and 12.5% in the CAES group (log-rank test, P = 0.001) (Figure 2b). There was no significant difference in the 3- or 6-month rate of recurrence between the 2 groups (Table 2).
Table 3.
Comparison of clinical characteristics between patients with and without recurrence after the first eradication
| Patients with recurrence (N = 50) | Patients without recurrence (N = 179)a | P | |
| Male gender, n (%) | 39 (78.0) | 125 (69.8) | 0.26 |
| Age (yr) | 54.2 ± 5.6 | 50.1 ± 10.8 | <0.001 |
| CPC-A, n (%) | 8 (16.0) | 107 (59.8) | <0.001 |
| NSBB responders, n (%) | 10 (20.0) | 84 (46.9) | 0.001 |
| PVT at entry, n (%) | 15 (30.0) | 27 (15.1) | 0.02 |
| Procedure by senior endoscopists, n (%) | 39 (78.0) | 123 (68.7) | 0.20 |
| Red color signs on varices at entry, n (%) | 23 (46.0) | 44 (24.6) | 0.003 |
| EVL for eradication, n (%) | 35 (70.0) | 79 (44.1) | 0.001 |
| Complete eradication, n (%) | 22 (44.0) | 136 (76.0) | <0.001 |
| Rebleeding, n (%) | 12 (24.0) | 14 (7.8) | 0.001 |
| Rebleeding before eradication, n (%) | 2 (4.0) | 6 (3.4) | 0.69 |
| Rebleeding after eradication, n (%) | 10 (20.0) | 8 (4.5) | <0.001 |
CPC, Child-Pugh classification; EVL, endoscopic variceal ligation; HBV, hepatitis B virus; NSBB, nonselective β blocker; PVT, portal vein thrombosis.
Eleven patients died, were transferred to alternative therapies, or lost to follow-up before they achieved eradication of EVs.
Other secondary endpoints
After the eradication of EVs, EVL was performed again on 35 patients in the EVL group at intervals of every month to eradicate recurrent EVs, while CAES was performed on 15 patients in the CAES group. All except 2 patients (1 lost to follow-up and 1 who died from liver failure) achieved re-eradication. The re-eradication rate was higher in the CAES group than in the EVL group (100% vs 94.3%, log-rank test, P = 0.04) (Figure 2c). The number of sessions needed for re-eradication was greater in the EVL group than in the CAES group (1.6 ± 0.7 vs 1.3 ± 0.5, P = 0.046). All patients in the CAES group but only 11 patients in the EVL group achieved complete re-eradication (100% (15/15) vs 31.4% (11/35), P < 0.001). The proportion of patients with complete eradication achieved at the second attempt by EVL was similar to that achieved at the first attempt (31.4% [11/35] vs 31.7% [38/120], P = 0.98).
The rebleeding rate was similar between the 2 groups (11.7% [14/120] in the EVL group vs 14.2% [17/120] in the CAES group, log-rank test, P = 0.62) (Figure 2d and Table 2). Although patients with recurrence of EVs had a higher rebleeding rate than those without recurrence (24.0% vs 7.8%, P = 0.001) (Table 3), a significant difference existed only when rebleeding occurred after the eradication of EVs (20.0% vs 4.5%, P < 0.001). When rebleeding occurred, 3 patients underwent TIPS for AVB (1 in EVL and 2 in CAES), and 5 patients underwent another endoscopic procedure to control AVB. Bleeding from treatment-induced ulcers, active peptic ulcers, and portal hypertensive gastropathy was successfully resolved within 3–5 days after the administration of proton pump inhibitors. Salvage hemostasis methods were not used for instant hemostasis during the endoscopic procedure in either group. No patients had bleeding after recurrence.
Transfer to alternative therapy occurred in 14 patients (6.7% (8/120) in the EVL group vs 5.0% (6/120) in the CAES group, log-rank test, P = 0.60) (Figure 2e and Table 2). No patients underwent LT during the study period.
In total, 24 patients died (9.2% (11/120) in the EVL group vs 10.8% (13/120) in the CAES group, log-rank test, P = 0.76) during follow-up (Figure 2f). The causes of death are listed in Table 2.
The average dose of sclerosant per session in the CAES group was 13.2 ± 2.7 mL. The average procedure duration at each session was significantly longer for patients in the CAES group due to compression by the cap (Table 2).
Adverse events
No serious adverse events (SAEs) in the form of perforation, chest empyema, pericardial effusion or esophageal strictures, or deterioration of portal hypertensive gastropathy and presence of GVs were observed in any group in the observed periods. The endoscopic treatment-induced AEs that occurred during the first series of endoscopic sessions for eradication are listed in Table 2. All these AEs disappeared within 3–5 days after treatment and did not show significant differences in incidence. During the second series of endoscopic sessions for re-eradication, 1 patient in the CAES group had a fever with a temperature lower than 38°C, which resolved spontaneously within 3 days after the treatment.
Independent predictors for recurrence
The results of the univariate analysis and Cox proportional hazards regression analysis of predictors for recurrence of EVs are shown in Table 4. After adjusting for age, gender, and CPC, the independent predictors for recurrence included EVL for eradication (hazard ratio [HR]: 2.37, 95% confidence interval [CI] 1.06–5.30, P = 0.04), achievement of complete eradication (HR: 0.27, 95% CI 0.13–0.56, P < 0.001), and NSBB response (HR: 0.32, 95% CI 0.15–0.68, P = 0.003).
Table 4.
Predictors for recurrence of EVs in univariate and multivariate analysis
| Variables | Univariate analysis | Multivariate analysis | ||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Age <40 yr | 0.42 | 0.06–3.06 | 0.39 | |||
| Female gender | 0.68 | 0.35–1.32 | 0.25 | |||
| CPC-B or C | 7.36 | 3.45–15.71 | <0.001 | |||
| Procedure by senior Endoscopists | 1.42 | 0.73–2.77 | 0.31 | |||
| PVT at entry | 2.45 | 1.33–4.50 | 0.004 | |||
| Red color signs on varices at entry | 2.17 | 1.24–3.78 | 0.006 | |||
| Rebleeding | 3.98 | 2.07–7.66 | <0.001 | |||
| NSBB responders | 0.21 | 0.10–0.44 | <0.001 | 0.32 | 0.15–0.68 | 0.003 |
| EVL for eradication | 2.52 | 1.38–4.62 | 0.003 | 2.37 | 1.06–5.30 | 0.04 |
| Complete eradication | 0.30 | 0.17–0.52 | <0.001 | 0.27 | 0.13–0.56 | <0.001 |
CI, confidence interval; CPC, Child-Pugh classification; EVs, esophageal varices; EVL, endoscopic variceal ligation; NSBB, nonselective β blocker; HR, hazard ratio; PVT, portal vein thrombosis.
DISCUSSION
In this randomized double-blinded controlled trial, cirrhotic patients were randomized to compare CAES with EVL in the long-term management of patients presenting cirrhosis with medium EVs and a history of EVB. The recurrence rate of EVs in the CAES group was significantly lower than that in the EVL group, but CAES was similar to EVL with respect to the eradication rate, the rebleeding rate, the mortality rate, and the rates of transfer to alternative therapy and AEs. Our results suggest that CAES is more efficient than EVL in preventing the recurrence of EVs and has safety comparable with that of EVL in treating medium EVs in the long term. To the best of our knowledge, our study represents the largest single-center, randomized double-blinded study performed to date that compares EVL and EIS regarding efficacy and safety in the long-term management of medium EVs in cirrhosis.
Two studies (6,7) found that EIS performed after EVs were reduced to a small size by EVL prevented the recurrence of EVs with similar incidence rates of mortality and complications. The recurrence rates of EVs in the EVL and sequential groups in these 2 studies were 26%–43% and 14%, respectively. However, these 2 studies had conflicting results with regard to rebleeding. One study showed that sequential therapy reduced the rebleeding rate (7), while the other study did not (6). In our study, EIS was performed after EVL when EVs were reduced to medium size for secondary prophylaxis of bleeding. Our results showed that the recurrence rate of EVs was similar to that in the above studies and that EIS reduced the recurrence rate of EVs, albeit with comparable rates of rebleeding and complications. However, Lo et al. (7) found that EIS after EVL significantly reduced the rebleeding rate compared with that after EVL alone. The enrollment of patients with GVs in that particular study may be one of the reasons. The injected sclerosant could drain from EVs to GVs through collaterals to prevent rebleeding from both EVs and GVs, while EVL alone could only prevent rebleeding from EVs. Moreover, 2 randomized controlled trials (20,21) showed that combined sclerotherapy and EVL (known as scleroligation) achieved a lower rate of recurrence because scleroligation could result in a longer retention of sclerosant to enhance the efficacy of sclerosant in the treatment of EVs. In our study, compression by a transparent cap played a similar role as ligation in localizing the sclerosant. Further studies are warranted to compare the efficacy and safety of CAES and scleroligation in the long-term management of EVs.
Technically, it is difficult to perform an intravariceal injection of sclerosant when EVs are reduced to a small size. CAES can fix targeted EVs to increase the possibility of intravascular injection of the sclerosant. In our study, CAES performed on medium EVs would be much easier than CAES performed on small or minimal EVs. Moreover, transparent cap compression could be used to control instant bleeding during injection. A recent randomized controlled trial with a small sample size (15) enrolled patients with small EVs. The results showed that CAES reduced the number of injection sites, the endoscopic therapy cost, and the use of the salvage hemostasis method during injection with a similar rebleeding rate during the 6-month follow-up compared with that in the conventional EIS group. However, the study did not evaluate the recurrence rate of EVs or the incidence of EIS-induced complications. Another recent retrospective study compared the efficacy and safety of CAES vs direct EIS in the long-term management of EVs graded as F2 or F3 in patients with cirrhosis. Although these patients developed complications with a high incidence (44%–66.7%), which may be due to the large volume of sclerosant (<40 mL of lauromacrogol) per session, CAES resulted in lower rates of EV recurrence and rebleeding than those after conventional EIS (16).
The clinical implications of RSVs by EVL have seldom been discussed. A previous study demonstrated that RSVs with red color signs could cause delayed rebleeding (22). Our results found that the presence of RSVs was a predictor for the recurrence of EVs. However, we did not identify the risk of rebleeding of RSVs, which may be attributed to the low incidence of rebleeding rate and the small number of patients exhibiting RSVs with red color signs.
Compared with EVL alone, the combination of endoscopic therapy and NSBB seems to reduce the rebleeding rate and improve survival with increased adverse effects in patients with secondary prophylaxis of bleeding (1,3,4). A critical review (23) found that the rate of variceal recurrence was reported to be 14%–68% in the combination therapy group but 26%–97% in the group receiving endoscopic therapy alone. Our results also showed that NSBB responders were protected against recurrence of EVs. Notably, our study excluded patients who may have exhibited systemic hemodynamic depressive effects of NSBBs. Therefore, the benefits of NSBBs might be overrated in our study compared with real clinical practice. The use of NSBBs in clinical practice should be based on a critical risk/benefit evaluation, especially in patients with refractory ascites or signs of systemic circulatory dysfunction.
The main concern about EIS for treating EVs is the incidence of AEs caused by sclerosant. A review summarized the incidence of complications of EIS, including transient dysphagia (70%); retrosternal chest discomfort (65%); low-grade fever (6%–10%); esophageal stricture (8%–10%); esophageal perforation (0.5%); systemic embolization such as pulmonary embolism, portal thrombosis, and splenic thrombosis (0.5%–3%); esophageal ulceration of the injection site (60%); and bleeding ulcer (20%–30%) (24). Severe EIS-induced AEs due to extensive wall necrosis are mostly caused by incorrect injection techniques, an excessive amount of sclerosant injected, or the use of a highly concentrated sclerosant (13,20). However, EIS with a small-volume injection of sclerosant at each session could reduce the incidence rate of complications. Previous studies demonstrated that EIS with a small volume when EVs were reduced to a small size had a similar incidence of AEs as EVL (6,7). Our study also showed that EIS with a small volume of EVs of medium size had comparable safety to EVL. The incidence rate of AEs was also similar to those in the above studies. Furthermore, none of the patients in our study had extensive esophageal ulcers or esophageal strictures, while the results of the other studies showed that the rate of esophageal stricture was approximately 2.7%–4.5%. In addition to injection with a small volume of sclerosant, a transparent cap could perhaps further improve the safety of EIS.
There are some limitations to the current study. First, our study was limited to a single center in 1 geographical area where portal hypertension is mainly postviral in cause. The conclusions could be further confirmed in multicenter studies enrolling patients with cirrhosis of various etiologies. Second, as patients with poor liver function (such as total bilirubin >170 μmol/L, CPC score >13, hepatocellular carcinoma, or hepatic encephalopathy grade 2–4) had a high risk of transfer to alternative therapy or of death before EV recurrence, the conclusions of our study were not generalized to these patients. Furthermore, as current guidelines recommend that early TIPS be considered in selected patients with CPC-C, CAES should not be recommended as a priority for patients with CPC-C. Third, we did not measure the hepatic venous pressure gradient because this technique is expensive and not routinely performed in China.
In conclusion, CAES reduces the recurrence rate of EVs and is comparable with EVL in the rates of eradication, rebleeding, mortality, and complications. Patients receiving CAES for eradication, patients achieving complete eradication, and NSBB responders after eradication are at a lower risk of recurrence of EVs. Although CAES could not reduce the rebleeding rate, it had comparable safety to EVL in treating EVs. The beneficial effects of preventing recurrence could also reduce the number of endoscopic sessions necessary to treat recurrent EVs and improve the quality of life of patients. In addition to EVL + NSBB, CAES + NSBB could be suggested for the secondary prophylaxis of bleeding in cirrhotic patients with medium EVs.
CONFLICTS OF INTEREST
Guarantor of the article: Xuan Zhu, MD, PhD, and Bi-Min Li, MD, PhD.
Specific author contributions: A.-J.W.: wrote the manuscript. X.-L.Z., H.-Y.Z., and Y.G.: statistical analysis. H.-Q.Y. and N.G.: performed the randomization. J.W., J.-B.H., Y.Y., B.-S.X., A.-J.W., and B.-M.L.: performed the endoscopic procedures. J.-W.Z. and G.H.G.: followed up the patients. X.Z., B.-M.L., and A.-J.W.: designed the study and critically edited the manuscript.
Financial support: This study was financially supported by the Project in the Science and Technology Pillar Program of Jiangxi Provincial Department of Science and Technology (No. 20161BBG70166), the National Natural Science Fund of China (No. 81460122) and the Science Fund For Distinguished Young Scholars of Jiangxi Province (No. 20171BCB23085).
Potential competing interests: None to report.
Declaration: Ethics approval and consent to participate: The study protocol conformed to the Declaration of Helsinki and was approved by the Institutional Ethics Committee of the First Affiliated Hospital of Nanchang University (file number 2016017) on January 13, 2016. The study followed the CONSORT guidelines and was registered at www.chictr.org.cn (Identifier: ChiCTR-IIR-16007964). All the patients signed informed written consent.
Study Highlights.
WHAT WAS KNOWN
✓ Cap-assisted endoscopic sclerotherapy (CAES) reduces the recurrence rate of esophageal varices (EVs) and is comparable to endoscopic variceal ligation in the rates of eradication, rebleeding, mortality and complications.
WHAT IS NEW HERE
✓ Patients receiving CAES for eradication, patients achieving complete eradication and non-selective β blocker (NSBB) responders after eradication are at a lower risk for recurrence of EVs.
TRANSLATIONAL IMPACT
✓ In addition to endoscopic variceal ligation + NSBB, CAES + NSBB could be suggested for the secondary prophylaxis of bleeding in cirrhotic patients with medium EVs.
References
- 1.Tripathi D, Stanley AJ, Hayes PC, et al. Clinical Services and Standards Committee of the British Society of Gastroenterology. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut 2015;64:1680–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Franchis R, Faculty BV. Expanding consensus in portal hypertension: Report of the Baveno VI consensus workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63:743–52. [DOI] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69(2):406–60. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2017;65(1):310–35. [DOI] [PubMed] [Google Scholar]
- 5.De Franchis R, Baveno V. Faculty revising consensus in portal hypertension: Report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2010;53:762–8. [DOI] [PubMed] [Google Scholar]
- 6.Cheng YS, Pan S, Lien GS, et al. Adjuvant sclerotherapy after ligation for the treatment of esophageal varices: A prospective, randomized long-term study. Gastrointest Endosc 2001;53(6):566–71. [DOI] [PubMed] [Google Scholar]
- 7.Lo GH, Lai KH, Cheng JS, et al. The additive effect of sclerotherapy to patients receiving repeated endoscopic variceal ligation: A prospective, randomized trial. Hepatology 1998;28(2):391–5. [DOI] [PubMed] [Google Scholar]
- 8.Wang AJ, Wang J, Zheng XL, et al. Sedated second-look endoscopy-guided therapy prevents early rebleeding after variceal ligation for acute variceal bleeding. J Dig Dis 2020;21:170–8. [DOI] [PubMed] [Google Scholar]
- 9.Irisawa A, Saito A, Obara K, et al. Endoscopic recurrence of esophageal varices is associated with the specific EUS abnormalities: Severe periesophageal collateral veins and large perforating veins. Gastrointest Endosc 2001;53:77–84. [DOI] [PubMed] [Google Scholar]
- 10.Dai C, Liu WX, Jiang M, et al. Endoscopic variceal ligation compared with endoscopic injection sclerotherapy for treatment of esophageal variceal hemorrhage: A meta-analysis. World J Gastroenterol 2015;21:2534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onofrio FQ, Pereira-Lima JC, Valença FM, et al. Efficacy of endoscopic treatments for acute esophageal variceal bleeding in cirrhotic patients: Systematic review and meta-analysis. Endosc Int Open 2019;7(11):E1503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karsan HA, Morton SC, Shekelle PG, et al. Combination endoscopic band ligation and sclerotherapy compared with endoscopic band ligation alone for the secondary prophylaxis of esophageal variceal hemorrhage: A meta-analysis. Dig Dis Sci 2005;50:399–406. [DOI] [PubMed] [Google Scholar]
- 13.Kong DR, Wang JG, Chen C, et al. Effect of intravariceal sclerotherapy combined with esophageal mucosal sclerotherapy using small-volume sclerosant for cirrhotic patients with high variceal pressure. World J Gastroenterol 2015;21(9):2800–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarin SK, Nanda R, Sachdev G, et al. Intravariceal versus paravariceal sclerotherapy: A prospective, controlled, randomised trial. Gut 1987;28(6):657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma L, Huang X, Lian J, et al. Transparent cap-assisted endoscopic sclerotherapy in esophageal varices: A randomized-controlled trial. Eur J Gastroenterol Hepatol 2018;30(6):626–30. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Zhang X, Zhao S. Transparent cap-assisted endoscopic injection sclerotherapy for the treatment of patients with esophageal varices. Medicine (Baltimore) 2020;99(24):e20721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Fu J, Zhou G, et al. Transparent cap-assisted endoscopic sclerotherapy for esophageal variceal hemorrhage compared with conventional sclerotherapy: A retrospective case-control study. Turk J Gastroenterol 2017;28:542–3. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Tsao G, Sanyal AJ, Grace ND, et al. ; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis, AASLD practice guidelines. Hepatology 2007;46(3):922–38. [DOI] [PubMed] [Google Scholar]
- 19.Hwang JH, Shergill AK, Acosta RD, et al. ; American Society for Gastrointestinal Endoscopy. The role of endoscopy in the management of variceal hemorrhage. Gastrointest Endosc 2014;80(2):221–7. [DOI] [PubMed] [Google Scholar]
- 20.Mansour L, El-Kalla F, El-Bassat H, et al. Randomized controlled trial of scleroligation versus band ligation alone for eradication of gastroesophageal varices. Gastrointest Endosc 2017;86:307–15. [DOI] [PubMed] [Google Scholar]
- 21.Harras F, Sheta el S, Shehata M, et al. Endoscopic band ligation plus argon plasma coagulation versus scleroligation for eradication of esophageal varices. J Gastroenterol Hepatol 2010;25(6):1058–65. [DOI] [PubMed] [Google Scholar]
- 22.Baroncini D, Milandri GL, Borioni D, et al. A prospective randomized trial of sclerotherapy versus ligation in the elective treatment of bleeding esophageal varices. Endoscopy 1997;29:235–40. [DOI] [PubMed] [Google Scholar]
- 23.Xu X, Guo X, Tacke F, et al. Use of nonselective β blockers after variceal eradication in cirrhotic patients undergoing secondary prophylaxis of esophageal variceal bleeding: A critical review of current evidence. Ther Adv Chronic Dis 2019;10:2040622319862693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Khazraji A, Curry MP. The current knowledge about the therapeutic use of endoscopic sclerotherapy and endoscopic tissue adhesives in variceal bleeding. Expert Rev Gastroenterol Hepatol 2019;13(9):893–7. [DOI] [PubMed] [Google Scholar]