Abstract
The publication of reproducible, replicable, and translatable data in studies utilizing animal models is a scientific, practical, and ethical necessity. This requires careful planning and execution of experiments and accurate reporting of results. Recognition that numerous developmental, environmental, and test-related factors can affect experimental outcomes is essential for a quality study design. Factors commonly considered when designing studies utilizing aquatic animal species include strain, sex, or age of the animal; water quality; temperature; and acoustic and light conditions. However, in the aquatic environment, it is equally important to consider normal species behavior, group dynamics, stocking density, and environmental complexity, including tank design and structural enrichment. Here, we will outline normal species and social behavior of 2 commonly used aquatic species: zebrafish (Danio rerio) and Xenopus (X. laevis and X. tropicalis). We also provide examples as to how these behaviors and the complexity of the tank environment can influence research results and provide general recommendations to assist with improvement of reproducibility and replicability, particularly as it pertains to behavior and environmental complexity, when utilizing these popular aquatic models.
Keywords: behavior, enrichment, husbandry, neurobehavior, replicability, reproducibility, xenopus; zebrafish
Introduction
Scientific research should be conducted in a responsible manner (utilizing consistent, objective evaluation, detailed reporting of experimental design, processes, and results) and be reproducible when attempting to test a given hypothesis. In biomedical research, there is also the important ethical goal to ensure that animals are used responsibly to meet these objectives. Several factors may reduce reproducibility of reported research. Indeed, poor study design, including insufficient knowledge about an animal model, failure to control variables that have the potential to influence results, or failure to recognize the degree of influence these variables may have, will influence research quality. The lack of statistical power and failure to report all experimental conditions thoroughly and accurately are other factors that often influence data replicability and reproducibility. Here, we review ways in which individual and social behavior and environmental complexity have the potential to influence research results as they relate to aquatic vertebrate models, with an emphasis on 2 commonly used aquatic animal models: zebrafish (Danio rerio) and Xenopus (X. laevis and X. tropicalis).
Zebrafish (Danio rerio)
Overview and Natural History
The zebrafish is an important biomedical research model organism whose use in research continues to grow. A recent literature search resulted in over 13 000 publications on zebrafish in the past 5 years alone [1]. Zebrafish are used in studies of developmental biology, toxicology, neurobiology, infectious diseases, tissue regeneration, cancer, metabolic diseases, and others. It is important to know the natural history of zebrafish and the factors that can influence how they react to their environment to understand how behavior, social interactions, and tank complexity may influence research results.
In the wild, zebrafish are omnivorous, opportunistic feeders that consume zooplankton, aquatic and terrestrial insects, algae, plant material, and larval fish [2–6]. Zebrafish are freshwater fish native of South Asia, inhabiting small rivers and streams, shallow ponds, and rice fields (Figure 1). The natural feeding behavior of fish is affected by multiple sensory systems intervening to detect a food item: vision, olfaction, acoustic, lateral line organ, electroreception, mechanoreception, and taste [7]. Foraging zebrafish larvae respond to moving stimuli by targeted movement toward and alignment with the stimuli once detected [8–10]. Mechanosensory systems also play a role at this stage, and zebrafish larvae are able to forage successfully in the dark but not when superficial neuromasts are ablated experimentally [11]. As adults, the fish continue to become more efficient foragers and are capable of feeding at all levels of the water (surface, water column, and substratum) [12]. They utilize various sensory systems to detect and discriminate among prey items, but there is evidence that they rely primarily on vision to feed effectively [4]. Adult zebrafish prefer red food items over blue, green, and yellow feeds even when they have been conditioned to feed on other colors [13].
Figure 1.

Striking differences between the natural habitat in Bangladesh (A) and laboratory habitat (B). (A) Water varies from clear to murky with vegetation in shallow, slow-moving, or stagnant ponds and streams. (B) A 3.5-L holding tank of zebrafish in clear water with no vegetation and constant incoming flow of treated water. Photograph (A) courtesy of Dr. Gregory Paull.
The natural habitat range of the zebrafish in South Asia includes some of the most species-rich freshwater habitats on the planet [14], and they consequently cohabitate with an extremely wide range of other fishes [2, 3, 5, 6, 15, 16]. Their ecological relationships with these species, as well as with organisms from other taxa that live in these habitats, are complex and may take a number of different classical forms [17]. The most important for understanding their implications on the animals’ artificial laboratory environment (Figure 1) are competition and predation.
Zebrafish are typically classified as “upper water column” or “surface dwelling” fish and are often among the most abundant species in the habitats where they occur [3, 5, 15]. There are numerous sympatric smaller-bodied fishes in these habitats that fall within the same or a similar ecological guild as the zebrafish, including other danioids (eg, Danio sp., Devario sp.) and other small cyprinids, such as minor carp (Puntius shalynius), hill trout (Barilius sp.), and flying barb (Esomus danricus) [2, 3, 5, 15, 16]. Interestingly, field observations of aggressive interactions between zebrafish and these other fishes are rare. It is not uncommon to find zebrafish in mixed-species shoals, especially, for example, with flying barbs (Esomus danricus), a close relative to the genus Danio [2, 16].
Wild zebrafish are also subject to predation by fish and several other organisms. Predation is an extremely powerful selective force that shapes behavioral, life history, and morphologic traits [18–20]. Zebrafish display remarkable aversion to various sympatric predators, some of which (eg, Indian leaf fish, Nandus nandus) have become a useful tool to evoke experimental stress in zebrafish neurobehavioral studies [21]. Predation pressure dramatically influences zebrafish affective and social behavior. For example, wild-caught zebrafish are highly anxious compared with their laboratory-raised counterparts [22]. Zebrafish from habitats with fast-flowing water and more predatory fishes tend to be bolder and more aggressive than individuals collected from slower-moving or still waters where predators are less abundant [23]. Domestication is a key factor in animal research [24] and has been well-documented in zebrafish and other fishes. Marked differences between wild and laboratory environments (Figure 1) may contribute to behavioral differences between wild and domesticated animals, since food delivery is always from the surface and there is a lack of predators under laboratory conditions. Thus, adaptation to laboratory environments may affect surface orientation and startle or fear response in zebrafish. Indeed, wild and domesticated zebrafish differ markedly in surface orientation; as an example, the wild-derived Nadia strain stay further from the surface of the water than domesticated TM1 fish [25]. In addition, wild fish display significantly higher latency to enter the stimulus (light exposure) zone compared with laboratory fish as well as less time spent in the stimulus zone [26]. Shoaling tendency also significantly decreases in wild fish [26], and laboratory fish are much bolder than their wild counterparts. Thus, the utilization of wild caught vs domesticated fish is an extremely important factor to consider in neurobehavioral research using this aquatic species [27].
Individual and Social Behavior of Zebrafish
Ethology examines animal behavior under natural conditions by carefully observing and describing what subjects do in the wild and in captivity. Behavior patterns performed by animals are often aimed at specific goals, such as locomotion, hunting, prey avoidance, exploration, reproduction, and territory protection [28]. Social behavior, defined broadly as behavior that affects or is in response to conspecifics, is complex and is influenced by intrinsic factors (eg, age, sex, hormonal state), individual differences (eg, personality, experiences), and extrinsic factors (eg, intra- and inter-group interactions, shoal/colony sizes, food availability, seasonality, or time of day) [29]. Identifying relevant units for objectively measuring behavior can be challenging and requires knowledge of the circumstances in which the behavior typically occurs, factors that may influence the behavior, and a determination of what specific behaviors are measurable and how to quantify them.
Analyzing zebrafish behavior presents several technical challenges related to capturing biological movement complexity for quantitative analysis. Video tracking is widely used to monitor fish behavior in both 2D and 3D [30, 31]. For this, adult fish are often placed in novel open tanks and filmed from above, side, or below, allowing tracking of location and orientation, tail curvature, horizontal eye position, and pectora fin motions [32, 33]. Larval fish are often placed in smaller, 96-well plates, and their activity recorded from the top or below in a high-throughput manner [34]. A comprehensive list of zebrafish behavioral phenotypes has recently been compiled by the International Zebrafish Neuroscience Research Consortium [35], illustrating the complexity of fish phenomics in motor, social, affective, cognitive, reward, and other neurobehavioral domains. A complete review of all zebrafish behaviors is beyond the scope of this manuscript; however, several specific aspects of behavior will be discussed with respect to how they may interfere with research design and study results.
Olfactory communication between zebrafish can have a significant influence on behavior. Zebrafish show behavioral responses to pheromones used in reproduction [36–38] as well as alarm pheromones [39] and amino acids [40–42] (which serve as both olfactory and gustatory stimulants) [43]. Freshwater fish, including zebrafish, contain a specialized cell type called club cells that have no external opening to the outside world, but when the skin is damaged they release alarm pheromone [44]. The olfactory system unambiguously contributes to social behavior in zebrafish. For example, male zebrafish are attracted to pheromones secreted by the ovaries of the females and react to these odors by initiating spawning behaviors. In addition, females can be induced to ovulate by exposure to water previously containing male fish. Analysis of the water supports the possibility that hormones and their metabolites (eg, steroid gluconides) from the “male” water may function as ovulation-inducing pheromones [40]. Analysis of neuronal activity measured in the olfactory bulb of both male and female adult zebrafish demonstrated that the olfactory sensory system responds to prostaglandin F2 and 17,20-dihydroxy-4-pregnene-3-one-20 sulfate [45]. Finally, zebrafish form and retain olfactory memories collated with genomic changes in the olfactory epithelium [46]. These examples demonstrate that the presence, or prior presence, of other fish can have a direct influence on zebrafish physiologic responses and behavior and must be kept in mind when conducting studies evaluating reproduction, behavior, or neurobiology.
Zebrafish are oviparous animals with external fertilization and no parental care. Their fecundity is typically high, and a single female may release clutches of several hundred eggs in a single spawning session. The presence of males stimulates ovulation in females and oviposition [47]. Females require several hours to enter the spawning state and typically ovulate overnight, stimulated by the male gonad pheromones [48]. Although olfactory cues are critical for synchronizing fish breeding behavior and for kin recognition [48–49], visual stimuli and behavioral cues also participate in the selection of an individual mating partner. For example, specific phenotypic traits (eg, male coloration, stripe patterns, and symmetry of caudal fin pattern) may influence mate choice in zebrafish [50]. The effect of visual stimuli and morphology on mate choice can potentially be confounded by social interactions, since dominant individuals generally behave aggressively toward subordinate individuals [50], whereas mating behavior within zebrafish social hierarchies is affected by both intrasexual competition and female mate preferences. As with many other behaviors, these observations suggest that the fish respond and adjust their activities in accordance with the environment and social situations. Evaluations of reproductive success must consider these variables as they may influence the parameters studied, such as breeding success, clutch size, and fertilization rates.
Adult zebrafish, like many other fishes, are highly social and are a shoaling species [50–55]. A “shoal” is a term to describe an aggregation of fish, analogous to a flock of birds or a herd of mammals [56]. These groups occur because animals choose to remain with others of their own kind to gain some benefit. In fishes, the most common advantages gained by shoaling are increased foraging efficiency [57] and predator avoidance [58]. In the real (or even the experimental) world, there are other forces that influence the decision to aggregate (eg, mating), and it is important to consider that some situations promote social aggregation while others do not. Zebrafish shoaling behavior develops gradually between 1 and 3 weeks post fertilization [59–61] and changes throughout life [51, 53, 62, 63]. For example, shoals consisting of young fish are dispersed, whereas adult shoals are more compact [64]. The term “school” refers to a specific pattern sometimes present in shoals: the coordinated, polarized (the degree to which individuals are moving in the same direction) nature of fish group swimming [65]. Interestingly, when fish were subjected to novel, stressful environments, they exhibited polarized (schooling) and more cohesive behavior, but, once acclimated, switched to nonpolarized (shoaling) and looser group swimming [66]. This pattern aligns with observations of zebrafish in nature, where they tend to school in environments with higher levels of unpredictability and stress [16, 23]. For instance, wild-caught fish subjected to different flow regimes with or without structural enrichment form tighter shoals in barren, still environments, lending support to the idea that the behavior is context specific [67]. Laboratory zebrafish also display “heightened-shoaling” behavior, representing a spontaneous onset of polarized shoaling with no signs of distress, thus likely serving as a form of social bonding or communication [68]. Collectively, these observations indicate that zebrafish behavior may not be defined narrowly but is rather plastic and dependent upon the environment, including factors such as flow rates [67], holding tank size [69], tank wall color [66], sex [70], predator presence/absence [23], age and relatedness of the fish [71], group size and activity level [63], phenotype/learned visual cues [72], nutritional status [73], and new surroundings. For example, the distance among animals in a shoal increases with time in a novel tank and decreases in the presence of a predator [74]. Zebrafish choose to shoal with conspecifics over an empty compartment, also showing a preference for larger shoal sizes [75] and fish of similar appearance [76]. Shoaling can also be altered by stress, as fish exposed to chronic 7 or 14 days of stress increase or decrease shoal cohesion, respectively [74]. Importantly, such social behaviors may markedly affect neurobehavioral results since even a short bout of social isolation from a group (eg, in a beaker during individual drug treatment, as commonly happens in most pharmacological assays in zebrafish) evokes robust behavioral and endocrine (cortisol) stress responses in zebrafish [77]. This, in turn, may affect, mask, or prevent the examined phenotype in question from being fully expressed and/or experimentally detected [77], thereby perturbing results and potentially leading to inaccurate conclusions.
As in other fish species, antagonistic behavioral interactions during the establishment of territoriality and the formation of dominance hierarchies contribute to stress in zebrafish, with plasma cortisol levels being higher in subordinate than in dominant individuals [78–82]. In some coral reef fish species, local population density can strongly affect the economic defensibility of a mating territory [83]. This may also apply to captive or cultured fish populations, since territorial interactions in captivity should be highest at lower to intermediate densities, in part because as densities increase above a threshold level territories become impossible to defend [84]. Zebrafish readily establish social hierarchies in captivity [78, 85–87], and so these same dynamics likely apply, suggesting a minimal density threshold for zebrafish in captivity below which chronic aggression related to these normal behaviors becomes problematic for long-term welfare [12, 88].
Stocking density is important to consider in terms of its effects on zebrafish behavior and physiology in the laboratory. However, the relationship between fish number per unit of water volume, water flow, and stress may be neither direct nor intuitive. Multiple studies have explored the effects of housing zebrafish in pairs or in social isolation, and the general consensus is that social isolation, even for a short time, is negative and stressful [77, 89]. For a shoaling fish, this represents an unnatural state, but in the laboratory it may be common [12, 90]. Likewise, there is also evidence that pair-housing is also stressful and potentially detrimental to fish welfare. Indeed, pair-housed zebrafish rapidly form dominant-subordinate relationships that are mediated by aggressive behaviors [91] and may increase mortality rates [90]. Social isolation also promotes aggression in males, as males subjected to social isolation are more aggressive in dyadic fights with other males than group-housed controls [92]. Fish maintained in isolation show higher fear response and a longer time to recovery after a fear-inducing event (eg, exposure to alarm pheromone) than fish maintained in groups [93, 94], whereas singly-housed animals recover more slowly from welfare challenges (eg, anesthesia, handling, or fin-clipping) than group-housed fish [95]. Interestingly, reports on cortisol levels in chronically isolated fish are variable, including both reduced [96–101] and increased levels [90]. Isolation in early life also alters fish behavior and physiology, as larval fish reared in isolation display decreased locomotor activity compared with siblings reared in groups [102]. These effects can also last into adulthood, as fish socially isolated early in life show hyperlocomotion and lower shoal cohesion as adults, in parallel with aberrant dopamine responses to social stimuli [103]. These variances highlight the need to carefully interpret cortisol results (eg, looking carefully at timing, social context, and mode of collection) and to employ complementary methods to assay stress (eg, neurochemical changes). Finally, socially isolating females without exposure to males promotes egg retention and egg-associated inflammation [104], which may negatively affect future reproductive success. These examples demonstrate that the effects of isolation are complicated and can be profound for welfare and management of fish in captivity. This may also have critical implications for research reproducibility. For example, the effects of caffeine on singly-housed adult zebrafish are reduced when maintained with nontreated conspecifics [105]. Moreover, as already mentioned, even a short 15-minute period of social isolation may evoke robust behavioral and endocrine stress responses in zebrafish [77]. This situation is not uncommon in the majority of zebrafish drug studies, which often involve short-term (eg, 20-minute) individual exposure of fish to a drug in a relatively small (eg, 0.5- to 1-L) beaker [77]. Collectively, these findings highlight how even seemingly subtle differences in social environment can impact research endpoints in zebrafish assays.
There are also problems associated with elevated rearing or holding densities in zebrafish. For example, maintaining larval and juvenile fish in high-density conditions during sexual development may lead to the masculinization and environmental sex reversal [106]. Adult fish subjected to acute and chronic high-density holding conditions show elevated plasma cortisol levels [88, 107]. Periods of acute stress elicited by intermittent crowding increase cortisol secretion in zebrafish and reduce their capacity to regenerate cardiac tissue after injury [108]. This last example is also instructive in terms of demonstrating how background stress may influence research endpoints. Generally, reduction of animal numbers among zebrafish housed initially at high densities correlates with lower cortisol levels [88, 107]. However, cortisol levels will also increase as density decreases, likely because of rising stress (eg, due to reduced social stability and/or lesser security of the members in the smaller groups) [88]. Complicating this further, the literature is sometimes unclear as to what constitutes “high” or “low” densities under laboratory conditions. For example, in one study evaluating rearing density and zebrafish sexual development, “low density” groups were 9–19 while “high density” groups were 37–74 fish/L, respectively [106]. In other studies, housing of <2 fish/L [69] or 0.25 fish/L [109] are described as “high-density” conditions. This problem persists across the literature and highlights the need to interpret these results carefully when developing standards for density guidelines in laboratories.
All these factors have important implications for laboratory populations, especially group size, housing density, impacts of shoaling behavior, and social dynamics. From studies of the fish in their natural environments, it is clear that zebrafish group size varies widely depending on several environmental factors, and this, in turn, will markedly influence behaviors [2, 3, 16, 23, 109].
Behavioral Variance
While neurobehavioral phenotypes are the most complex biological responses, the complexity of a biological system increases the variability of its responses. Indeed, like rodent and human behaviors, zebrafish behavior is highly variable [110, 111], and this intraspecies variance may factor into neurobehavioral analyses and their data reliability. As mentioned previously, there are behavioral differences in wild vs domesticated zebrafish, but sex differences and genetic variations between strains can also influence behavior [112–114]. For example, AB and Tupfel long-fin strains differ in expression of neuro-development related genes and in behavioral responses to inhibitory avoidance and light/dark motor behavior tests and habituation to acoustic/vibrational stimuli [113, 114].
There is also considerable individual variation in zebrafish behaviors [110, 111], which is evolutionarily beneficial as it increases fitness and helps fish to adjust to rapid environmental changes. Variation may be high in zebrafish populations because they are usually outbred and highly polymorphic. Paralleling behavioral variation observed in other animal populations, they likely arise from genetic and environmental differences and interactions [115], and there are individual neurochemical differences in zebrafish as well [116]. Individual behavioral differences in zebrafish have been extensively studied in several key domains, including boldness, aggressiveness, sociability, exploration, and locomotion. These differences may also cluster together, forming stable “behavioral syndromes” [52, 117] such as patterns of social dominance or stress responsivity [118]. Although individual differences in behavior may at first be considered unwanted noise, they have multiple biological causes that may be valuable to study. Thus, rather than attempting to reduce such noise, individual differences in zebrafish behaviors are valuable to carefully study. One strategy for this can be to conduct studies that directly address individual differences between fish and their physiological, neurochemical, and genomic correlates [110, 111]. Perhaps more feasible, practical, and 3R-compliant is a neurophenomics-based approach. This involves routine collection of large amounts of biological and behavioral data (eg, during routine husbandly logging) for covariant-based analyses to reveal novel biological factors that drive key behaviors and their variance [110, 111].
Finally, it is important to consider that health status may influence behavior, particularly if fish are infected with pathogens that infect the central nervous system. For example, zebrafish infected with the microsporidian Pseudoloma neurophilia displayed a distinct behavioral phenotype when compared with controls and uninfected cohorts when tested for startle response habituation [119]. Infection with P. neurophilia has also been associated with altered shoaling behavior [120]. Other infectious organisms or disease states could potentially alter swimming behavior or social interactions; thus, knowledge of the health status of test subjects is important to consider.
Experimenter Identity Effects on Zebrafish Behavior
Experimenter identity can be an important variable in most neurobehavioral assays. While complete automation of such testing may eventually solve the problem, it remains a distant reality as behavioral tests are currently run by human experimenters who may differ markedly in their performance and physical characteristics, even within the same laboratory. Rodent behavioral models are sensitive to individual experimenters’ identity. For example, experimenters differentially affected behavioral test scores of 8 inbred mouse strains under the influence of ethanol [121]. Rats and mice have been shown to display higher stress when handled by male compared with female experimenters [122]. In human clinical trials, experimenter gender also skews findings due to significant experimenter biases that may influence study results [123]. Do similar factors affect zebrafish behavior? Surprisingly, and unlike rodent models, experimenter identity seems to have relatively little effect on zebrafish performance in the most sensitive anxiety model, the novel tank test [124]. Corroborating these findings, the Kalueff laboratory (A. Kalueff, unpublished data) used 2 experimenters with distinct physical characteristics (a 25-year-old male and a 23-year-old female) to perform the novel tank and light dark anxiety testing in the same population of wild-type short-fin laboratory zebrafish. Both sensitive anxiety tests produced similar results regardless of experimenter identity (Table 1), suggesting that zebrafish models may be more resilient than rodents to variation in experimenter identity, further supporting the growing value of zebrafish models for neurobehavioral stress research.
Table 1.
Effects of experimenter identity on zebrafish behavior
| Endpoints | Female experimenter | Male experimenter | P, U-test (male vs female observer) |
|---|---|---|---|
| Novel tank test | |||
| No. of top entries | 7.6 ± 1.9 | 7.8 ± 1.6 | P > .05, NS |
| Time spent in top, seconds | 66 ± 15 | 70 ± 14 | P > .05, NS |
| Light-dark box test | |||
| No. of light entries | 14 ± 1.6 | 11 ± 1.4 | P > .05, NS |
| Time spent in light, seconds | 127 ± 20 | 97 ± 17 | P > .05, NS |
In this pilot study, the Kalueff laboratory (unpublished data) showed that experimenter identity does not affect zebrafish behavioral responses in 2 sensitive anxiety models, the novel tank test (n = 24 per group) and the light-dark box (n = 17–18). A male experimenter (25-year-old male) and a female experimenter (23-year-old female) tested adult zebrafish in parallel in 2 common behavioral tests (novel tank test, n = 24, and light-dark box, n = 17–18), generating highly consistent similar results regardless of the experimenter identity (P > .05, U-test). NS = no significant differences.
Handling and Husbandry Effects on Zebrafish Behavior
Handling is known to be stressful for laboratory animals and may influence their behavior [125]. Handling fish, which usually entails removing them from the water by net, is probably the greatest single acute stressor for laboratory fish. Such handling represents a combined (net chasing + water removal) stressor that may strongly influence fish’s behavioral phenotype (eg, making them more anxious). Indeed, zebrafish exhibit rapid cortisol elevation and subsequent recovery from acute netting stress [126]. Fish handling in a laboratory setting also often involves other stressful events, such as exposure to pathogens or chemicals by immersion, gavage, or various routes of injection. Fish housing can also contribute to stress reactivity, as animals held in recirculating aquaculture systems might experience elevated stress because of exposure to metabolites, cortisol, and alarm substances in intensively stocked systems [126].
Environmental Complexity
Multiple environmental factors affect the zebrafish’s response to external stimuli including, but not limited to, tank design, water flow, tank substrate, structures within the tank, and the environment surrounding the tank. The impact of environmental complexity is often evaluated by measuring physiological or behavioral parameters under differing conditions of environmental complexity; however, this must also be evaluated in various social and environmental contexts, as they may also influence responses [89, 127].
Tank Material, Shape, Size, and Color
Zebrafish housing tanks come in various shapes and sizes. However, there is currently no research on which size and shape of tank may be ideal for housing zebrafish [128]. Tanks are manufactured from multiple different materials, including glass, fiberglass, polysulfone, and polycarbonate. Some of these materials can potentially be detrimental to fish. For example, plasticizers, a component of plastics, are not covalently bound and low levels of plasticizers can leach into the aquatic environment [129]. Thermoplastics consist of polymerized chemicals, such as bisphenol-A, a known endocrine disruptor and toxin; therefore, consideration should be given to minimizing exposure to these compounds due to their potential impact on zebrafish physiology and behavior [128].
Zebrafish vision is tetrachromatic and they perceive color wavelengths from ultraviolet to red [130]. Innate and learned color preferences have been investigated in larval and adult zebrafish. Tests in larvae suggest they prefer white to gray environments and prefer orange and green to red, yellow, black, and blue. Changes in lighting conditions (continuous dark vs continuous light) can also modulate response to preferred colors [131]. For example, when raised in high light levels (3000 lux), 5-dpf larvae preferred blue over red, green, and yellow [132]. Adult zebrafish were shown to prefer red and green to yellow and blue in some studies [133, 134] but preferred blue and green and avoided yellow and red in another study [135]. Since the majority of zebrafish aquatic systems are made with green or blue elements (eg, Figure 2), the influence of light level and color preferences must be considered. While red and green seem to be innately preferred colors, zebrafish raised in other colors learn to respond to those as well, demonstrating that rearing environment may play an important role in zebrafish response to testing paradigms as adults [13]. The influence of color on behavior and behavioral tests is also important because color can be used (with or without food) to hasten learning in learning and memory tasks. For instance, red color paired with a food reward accelerates learning in a T-maze task [134]. While zebrafish also have an innate preference for red diets to blue, green, or white diets, if habituated to another color diet, they will also react strongly to that color as well [13].
Figure 2.
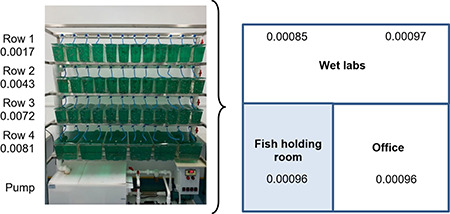
Example of intra-lab variability in vibration. The 200-m2 Kalueff laboratory at Southwest University includes office space, wet laboratories, and a fish holding room housing 1000–2000 zebrafish in a 4-row benchtop system (Jin Shui Ocean Company, Shandong, China). Background vibration levels were tested in each location using the VibSensor accelerometer recorder iPad application (Now Instruments + Software, Inc., Poway, CA) and expressed as the integrated spectrum density (ISD). Vibrations are present in the fish holding room due to the aquatic system’s pump, and there are subtle variations of vibrations within the laboratory. Note marked variance in vibration levels between the rows in the aquatic system with the top row, most distant from the pump, vibrating 4-fold less than the bottom row, closest to the pump (P < 0.0005, U-test) when the pump is on. All rows showed similar vibration levels, as did the testing rooms (Wet labs), when the pump is off (P > 0.05; data not shown).
In addition to color, alterations of tank depth and transparency elicit different responses in depth preference and scototaxis (dark/light preference) assays [136]. Zebrafish are scototaxic (preferring black/dark) and also prefer transparent testing areas to white [137–139]. Note, however, that transparent tanks—in fact, the majority of behavioral apparatuses currently used for fish testing—may elicit greater anxiety in zebrafish than either white or black opaque tanks [136]. Furthermore, zebrafish preferred well-lit areas and darker backgrounds and avoid cave-like environments [140]. There are several possible reasons for these discrepancies: different light levels between laboratories, heterogeneous light intensity across all areas of the testing apparatus, transparency of the tank, movement of experimenters (either over the apparatus or through transparent tanks), background shade, and openness of the environment [139, 140]. Importantly, the difference between the environmental characteristics of the fish holding room vs the fish testing room—the so-called delta—may be a major factor contributing to experimental variance between and within laboratories.
In general, animal behavior can also be altered by variation in the spatial dimension of the experimental enclosures. Enclosures that are too small may trigger repetitive and invariant behaviors [141]. In contrast, too much space, or large enclosures with extended and potentially unsafe areas, may trigger fearful and anxious states that can disrupt behavioral responses [142–144]. In fish (eg, mosquitofish [Gambusia holbrooki]), tank size influences activity levels and risk-taking behavior during different periods of ontogeny. For example, mean velocity increases significantly with tank size, independently of the ontogenetic stage. In contrast, velocity variance is independent of tank size but is age-dependent since juvenile fish exhibit the highest, and adults the lowest, velocity variance. Interestingly, fish spent more time within the sheltered area (eg, covered by a Plexiglas cover) when tested in the small tank, with juvenile fish hiding consistently longer than adult fish [145].
Water Flow
The rate of water flow into housing tanks varies considerably within and between facilities and has been shown to impact aggression. In barren tanks, higher water flow increases aggression between conspecifics [67, 146], suggesting that the water flow rate is an important variable to consider in aquatic behavioral tests, such as in aggression models (eg, the mirror test).
Structural Enrichment
Environmental enrichment in the form of structural enrichment refers to interventions in the housing environment that contribute to improving the welfare of laboratory animals and attempts to resemble their natural habitat. This is an important variable to consider for ensuring reproducible and valid data [147]. The provision of structural enrichment provides exposure to sensory stimuli (visual, motor, cognitive, and somatosensory), which stimulates several brain regions [148]. Enriched environments promote neuroprotection and neurogenesis and affect multiple behaviors (eg, drug self-administration and response to stress [149]) by modulating brain reward circuits, mainly in the mesolimbic dopamine system [150, 151].
Like rodents, zebrafish prefer structured to barren environments [152, 153], and anxiety-like behaviors are often seen in fish housed singly in a barren environment [147]. In contrast, structural enrichment reduces zebrafish stress and anxiety [89, 100, 154, 155]. In learning and memory tests, zebrafish raised as juveniles in enriched environments learned to navigate a maze faster than fish housed in barren conditions [156, 157], demonstrating that the larval rearing environment may influence zebrafish behavior in adulthood. Interestingly, longitudinal analyses show that 24-month-old fish do not alter anxiety-like behaviors even after exposure to environmental enrichment, suggesting that fish age may also play a role in behavioral responses [155]. Housing of fish in enriched vs barren tanks resulted in a protective effect against oxidative stress when exposed to a unpredictable chronic stress protocol [158].
Responses to structural enrichment are strongly influenced by the social environment; thus, it is difficult to look at these variables separately. For example, single-housed fish in barren tanks and group-housed zebrafish with or without enrichment spent more time near conspecifics than near plant enrichment devices. However, single-housed fish maintained in enriched tanks display no preference between conspecifics or plant enrichment [89]. Zebrafish raised in enriched environments show increased exploration with reduced anxiety-like and inhibitory avoidance behavior as adults [155] and group housing promotes zebrafish risk-taking in unfamiliar settings [159]. Objects within the tank can lead to aggression and monopolization of resources. For instance, objects that obstruct the view of conspecifics can reduce aggression by providing subordinates with a place to hide from the dominant fish [90, 160]. In support of this, physical structures reduced aggression and death in pair-housed zebrafish maintained together for over 24 hours [90]; however, adding vertical glass rods produces little effects on zebrafish anxiety and aggression [161]. Objects in the tank may also allow dominant fish to establish a safe and secure territory, which it may defend, leading to increased aggression [146, 153, 154] and causing growth retardation and delays in subordinate fish. Finally, structural enrichment may affect drug actions in zebrafish. While acute stress increases cortisol in group-housed zebrafish maintained in a barren environment, single-housed zebrafish display blunted cortisol responses to stress. Both structural enrichment and anxiolytic drugs (eg, diazepam and fluoxetine) blunt stress responses in isolated and group-housed zebrafish [100], demonstrating structural enrichment as an alternative and/or complementary approach to reduce stress and promote animal welfare (see Volgin et al. for a recent comprehensive review [147]). In general, the influence of structural enrichment on reproduction is believed to be positive. While plastic plants in breeding tanks stimulate increased embryo production [162], larvae reared in enriched environments have greater survivorship than those raised in barren tanks [154].
Several studies have described zebrafish preference for gravel as a tank substrate [153]. Gravel allows fish to display natural behaviors (eg, foraging and exploring) and also represents a preferred substrate for oviposition [163]. However, in reality, few facilities employ gravel in standard housing, as it is difficult to sanitize, can be ingested by the fish, and may provide a substrate that harbors various pathogens [164]. Interestingly, zebrafish also prefer images of gravel placed on the bottom of tanks [153], and while this approach offers a reasonable enrichment alternative, it remains unclear whether images of gravel similarly affect other zebrafish behaviors as does real gravel.
Varied response to enrichment may reflect the plasticity of the fish’s adaptations to changes in their environment [146, 160]. Further research is required to elucidate how and when enrichment may lead to changes in zebrafish behavior. In mice, responses to enrichment vary by strain [165], and similar responses are likely between strains or between wild caught vs captive reared zebrafish. For example, collective behaviors of 2 morphologically different strains of zebrafish (Tupfel long-fin and AB) differed when evaluated in the same environment [166]. Regardless of what type of structural enrichment is added to a tank, it is important to consider both the chemical and physical composition of the structure. They should not leach chemicals (eg, plasticizers), should be sanitizable (but free of residual cleaning chemicals), and should be evaluated for ingestion risk. In addition to these health and safety concerns, each of these factors can influence research results. Although significant questions remain regarding the zebrafish’s housing environment and the subsequent influence on research results, it is imperative to accurately describe all structural enrichment when publishing scientific data.
External (to the Tank) Environment
Although the influence of the macroenvironment is discussed elsewhere in this issue, there are several key considerations as it pertains to aquatic species. For example, while light intensity in housing and experimental areas is rarely reported in manuscripts, this variable may affect the level of aversiveness in certain tests such as the light/dark test. A sudden switch from complete darkness to light may elicit a startle response. Phased lighting, mimicking sunrise and sunset, is preferred to reduce this response [167]. There are no published data on the light intensity experienced by zebrafish housed on the different rows in a housing rack [140]. To address this, the Kalueff laboratory based at Southwest University (Chongqing, China) used a free Galactica Luxmeter iPhone application (Flint Soft Ltd., Moscow, Russia) to measure light intensity in 4 different rows of their benchtop aquatic system (data not shown). Indeed, while the top row of tanks (row 1; closest to the ceiling-mounted lamp in the holding room) predictably showed the highest light intensity levels, row 2 received nearly half the light of row 1 (P < .0005, U-test), and rows 3 and 4 (closest to the floor) received 3 times less light than row 1 (P < .05). There was also a considerable variation in light levels between the fish-holding and behavioral testing room (740 ± 56 vs 1117 ± 84 lux), which could affect zebrafish behaviors when testing fish housed on different rows in the rack, on different racks in a multi-rack facility, or when moving fish from the holding to the testing rooms for acclimation and behavioral experiments. The ambient lighting must also provide an appropriate light-dark cycle and wavelength spectrum for all life stages of zebrafish. The presence/absence of a light-dark cycle and the wavelength of ambient lighting influences larval growth, development, behavior, and gene expression in zebrafish [168, 169]. In particular, it is important to consider that light-emitting diode bulbs are available in a wide variety of emission spectrums, some of which may be not be in the optimal spectrum for zebrafish. This may influence behavior, physiology, and development. Similarly, the sound of air and water pumps in the animal facilities as well as the vibration they produce can also be a factor to consider, as fish may be susceptible to stress and hearing loss due to chronic exposure to them. Do background sound and vibration differ in various parts of the laboratory? The Kalueff laboratory at Southwest University found only slight differences in background vibration in different locations of their 200-m2 laboratory but measured marked differences in vibration in fish-holding tanks on different rows of the benchtop aquatic system (Figure 2) when the pump was running, whereas vibration levels in all rows were similar to other rooms when the pump was off. Differences in vibration are likely to affect zebrafish behaviors when testing fish housed on different rows and when fish are moved from the holding to the testing rooms for acclimation and behavioral experiments. Utilizing another free iPhone application (Decibel Meter Sound Detector, SkyPaw Co Ltd., Hanoi, Vietnam), the Kalueff laboratory measured noise levels in various parts of the laboratory as well in various rows of a benchtop system. While similar levels of background noise (10–32 ± 2.4 dB) were detected in several laboratory testing rooms, the fish holding room had significantly higher noise levels (75 ± 0.2, P < .005, U-test) when the aquatic system pump was on, while it was similar to other rooms (25 + 0.6 dB) when the pump was off. Unlike lighting and vibration levels, there was no considerable difference in noise levels between the rows (73–77 dB) when the pump was working. While such differences in background noise may not impact testing of fish housed on different rows, they are likely to affect zebrafish behaviors when fish are moved from the “noisy” holding room to the “quiet” testing rooms for acclimation and behavioral experiments. Larger multi-rack or multi-system aquatic facilities may have higher levels of vibration and noise, which may influence both fish welfare and behavioral performance.
Xenopus
Overview and Natural History
Amphibians are frequently used animal models in developmental and cell biology, regeneration, genetics, and toxicology for their conserved cellular developmental and genomic organization and due to their ability to produce abundant numbers of large oocytes and eggs, which tolerate extensive manipulation and can be used for high-throughput biochemical or toxicology studies. Amphibians also tolerate extensive manipulation [170, 171]. Several species are useful for understanding unique immune structures and functions due to their production of defensive skin secretions. Adult urodele amphibians, such as Ambystoma sp. and newts, can regenerate large sections of their bodies, including limbs, jaws, and ocular tissues after damage or transection [172], making them important models for regenerative studies. Given the importance of these animal models, it is critical to understand their natural habitat and behaviors to minimize the introduction of research variables that may result from manipulation or lack of understanding of these traits. This manuscript will focus on the 2 species most commonly used in the laboratory: X. laevis and X. tropicalis.
X. laevis, also known as the South-African Clawed Frog, is native to South Africa but is a highly adaptable invasive species found in wild populations around the globe, including Asia, Europe, and the Americas [173, 174]. X. tropicalis, also known as the Western or Tropical Clawed Frog, has more stringent habitat requirements and is restricted primarily to the warm tropical lowlands of West Africa, preferring warmer water temperatures of 24–26°C [175–178]. In the wild, Xenopus are typically found in large groups or colonies in still, muddy-bottomed pools of warm fresh water, either man-made or natural. X. laevis may also be found in active water (rivers/streams) or salt water at a wide variety of temperatures and do well in clear water in both the wild and in laboratories [175, 176]. Although Xenopus are fully aquatic for all life stages, when unfavorable environmental conditions arise, they may either aestivate and remain dormant for more than a year [174, 175] or migrate overland between water bodies [175, 179]. Xenopus have eyes on the dorsal aspect of their head to detect food and predators on the surface of the water and nostrils at the end of snout for detecting food in water and air. Their eyes have a convex cornea designed for vision in air [180, 181] and are most accurate at detecting objects nearby and directly in front of the animal [182, 183]. Given their typically murky water habitats in the wild, this is not thought to be a disadvantage; however, utilizing a behavioral testing paradigm in the laboratory that requires the use of distal visual cues or lateral visual acuity may not appropriate for this species. Despite their visual shortcomings, they have a well-developed sense of smell and a mechanoreceptor and electroreceptor system known as the lateral line, consisting of rows of sensory receptors (neuromasts) on the head and body, to detect water movements and electric currents. These senses are utilized to detect prey and predators appearing from any direction and to assess the animal's position in relation to conspecifics, and they are involved in current detection and current-related postural adjustments [180, 182, 184, 185]. Anything in the environment that either stimulates or damages the cells of the lateral line has the ability to significantly affect many aspects of behavior. For example, exposure of X. laevis tadpoles to streptomycin, which damages cilia in superficial neuromasts, resulted in changes in schooling behaviors and body position in the water column [185, 186]. Thus, researchers must be careful if chemicals are added to the water as part of experiments and must consider how this may impact the movement or social interactions of the animals and, potentially, their ability to locate and ingest food.
Individual and Social Behavior of Xenopus
Social behavior, particularly interaction among conspecifics, is important for individual and species survival and is often developmentally acquired [187]. When considering the behavior of Xenopus, it is important to understand that behavior in tadpoles may differ from that of adults, and it may be of benefit to think of the 2 as different organisms [188]. Conspecific recognition among Xenopus begins in the larval stage, when tadpoles are social and form stationary conspecific schools in which they are oriented parallel to each other, maintaining a nonrandom fixed difference from each other, with their heads facing downwards. Both vision and neuromasts in their lateral line are used to assess their orientation and distance from their neighbors while avoiding direct contact [186–188]. Recognition of kin is mediated by water-borne olfactory cues received by receptor cells in the vomeronasal organ, and response to olfactory cues changes throughout life whereby the non-kin aversion response in tadpoles raised with kin is replaced by a preference to a non-kin odor [187, 189] as adults for mating. Adult Xenopus do not demonstrate schooling behavior, do not maintained a fixed distance from one another, and tend to rest horizontally at the bottom of the tank when not breathing at the water’s surface. In other species of anurans (ie, Pipa pipa), it was shown that water previously occupied by receptive females excited males [180]. In addition to the use of olfactory cues for mating, they can also be utilized for prey detection. Given the sensitivity of their sense of smell, it is important to consider its influence on their behavior when designing experiments, particularly if frogs are being moved out of their home environment for manipulation or testing. Xenopus also utilize an auditory communication system, primarily for communication of dominance and fertility, with calling even being suppressed in less dominant males [190, 191]. The presence or absence of female X. tropicalis affects male vocalizations and is further influenced by the administration of hormones such as HCG or arginine vasotocin. For example, a social stimulus is necessary to illicit male vocalization, but hormonal manipulation influences both male vocalization and behavior [192]. Therefore, sensory and social stimuli, including odor, sound, the presence/absence of other animals, and colony hierarchy, should be considered when evaluating neuroendocrine system responses in this species.
While large-scale scientific studies evaluating social behaviors of Xenopus in the laboratory are lacking, field observations and limited laboratory studies suggest that they are territorial and develop a hierarchical system [176, 193]. A small study noted individual differences in aggression of X. laevis females, as measured by the numbers of nips, pushes, or approaches inflicted, which was inversely related to the number of those behaviors that were received. Individual behaviors were conserved in these individuals regardless of their housing in pairs, their partners, or being housed in a large group. The animals displaying more aggressive behavior tended to weigh more, albeit without a direct correlation with food consumption. The behavior pattern of the aggression was like the normal feeding behavior of Xenopus in that the nipping that occurred was visually similar to the behavior leading up to the consumption of food. There is also more frequent aggressive behavior when food is present, and the amount of food consumed varies directly with the number of frogs housed together. This suggests that the more sweeping motions being made by more frogs in the presence of food increases the chances that a frog will come into contact with both food and a limb of a tank mate [194]. Individual frogs establish dominance in a colony in which new individuals are frequently being introduced or removed and reintroduced [195]. Nondominant animals may have less opportunity to feed or may be noted to be smaller or have wounds from fighting. Thus, this practice has the potential to constantly challenge the hierarchy of a colony and result in more aggressive behavior [196]. This could result in not only behavioral changes within the tank and in behavioral tests, but also in changes of weight or growth due to access to food. It also would be expected to be stressful, resulting in additional behavioral or physiological changes associated with stress. When evaluating social behaviors in this species, it is important to remember that tank stocking density, or the availability of space or water per frog, may also play a role. An optimal stocking density for Xenopus in the laboratory has not been defined but is likely to be dependent on several different environmental and social factors. Indeed, body weight and length vary markedly when frogs are housed in the same tanks but with a density of 22 (14 L/frog) vs 37 (8.6 L/frog) [197]. Stocking density has also been shown to influence the frequency of aggressive acts and bite wounds [198].
It is not uncommon for 3 or 4 species of Xenopus to share the same habitat in the wild, and hybrids have been noted [175, 199]. More commonly, however, behavioral interactions of Xenopus with other aquatic animals revolve around their role as either predators or prey. Xenopus are carnivorous, cannibalistic, and prefer live feed (fish, birds, slugs, worms, aquatic invertebrates); however, they readily adapt to non-live feed in the laboratory. Their hind feet have sharp black claws to dig insects from the mud and shred food, and they use their forelimbs to shovel potential food objects into their mouth. They are indiscriminant feeders and will try to eat whatever they sense in the water around their mouths [176]. Detrimental effects of inadvertent consumption of toxic, indigestible, unpalatable objects can be avoided by their ability to regurgitate their stomach and use their forelimbs to wipe the mucosal lining free of the unwanted substance. Knowledge of this behavior is important, because the frogs can easily reject orally administered experimental compounds. Another important aspect of their feeding behavior is that the presence of food in a group of frogs often triggers a feeding frenzy. As a result, it is common to observe 1 frog attempting to consume a limb of the other during a feeding episode. It is unclear if this is inadvertent or due to aggression. It is a concern, however, because this behavior can result in fight wounds with the potential for secondary bacterial or fungal infections [200]. To minimize these types of injuries, it has been recommended that small pieces of food be provided frequently, in adequate amounts, to promote quick consumption of food and decrease the potential for competitive injuries [199, 200]. These antagonistic interactions, however, may also be influenced by social behavior (ie, dominance) or factors of environmental complexity, such as stocking density and the presence/absence of a refuge. All these factors should be taken into consideration to minimize fight wounds and the impact of secondary infections or animal morbidity/mortality may have on research.
For most amphibians, their natural predators are birds, fish, aquatic mammals, reptiles, or humans. Their main defense against these predators is escape by fleeing or hiding or a combination of both. Xenopus may hide in the muddy bottom of a lake or find cover under rocks or behind plants. In the laboratory setting, humans may be perceived as predators; therefore, it is important to be aware of their mechanisms of defense. Xenopus use their strong hind limbs to contort their bodies and propel themselves either forward or backwards away from a predator, including gloved hands during manual restraint. Their instinct to flee is quite strong, and they can propel themselves over a great distance, resulting in damage when they land. This is 1 reason why it is imperative to have lids (with appropriate modifications to allow for air exchange) on tanks or any other enclosure that is used for housing, transport, or testing. Another defense mechanism used by Xenopus is toxic secretions in their slime layer. Serous glands on the head and shoulder secrete peptides such as caeruleins (homologous to mammalian cholecystokinin), xenopsin (homologous to mammalian neurotensin), xenoxin (similar to neurotoxins and cytotoxins found in snake venom), and others with antimicrobial (magainins) and anti-viral properties [178, 201–203]. This results in a protective slime that serves as a barrier against predators, pathogens, and abrasions. Damage to this slime layer can result in opportunistic infections. Stressed animals or wild-caught animals that are not acclimated to the laboratory may release a white mucus that causes oral dyskinesia in predators in the wild [204]. This highlights 1 of several possible variables of behavior and physiology that may be introduced when using wild-caught vs laboratory-reared frogs.
Stress causes overt behavioral and physiologic changes in various amphibian species and should be considered when performing husbandry procedures and designing and conducting experimental studies using Xenopus. Increased levels of water-borne corticosterone and decreased weight have been documented in X. laevis exposed to stressful conditions, such as a variety of methods of transportation [205]. In this study, water-borne corticosterone levels remained elevated for at least 7 days and body mass remained lower than baseline levels for up to 35 days. Since body mass is directly correlated with the mass of the ovary, this is not desirable for research dependent on large numbers of oocytes or eggs [206]. Similar to other vertebrates, the release of corticosterone in Xenopus is a result of the activation of the hypothalamic-pituitary-interrenal axis and changes in the central nervous system [207]. Similar to what is seen in other species, exposure to elevated levels of glucocorticoids or corticosterone results in a blood cell differential reflective of a stress response in Xenopus, including increased and active peripheral neutrophils with decreased peripheral lymphocytes [208]. Xenopus may also respond to stress by everting their stomach. If stressed after eating, they may regurgitate, [176, 209] which may result in animals losing nutrition, hydration, and body condition if it occurs frequently. In addition to causing stress, human handling of these animals can result in attempts to escape and resultant physical trauma to the animal’s body and damage to the slime layer. Husbandry procedures, such as moving frogs to other tanks or cleaning tanks while frogs are present, have the potential to cause stress. In other species, differences in handling techniques or handlers have been associated with variability in behavioral research results, and it is likely that similar problems could occur with the handling of amphibians [210]. Other aspects of the environment, such as social stress, stocking density, environmental enrichment devices, and tank design, can also influence stress levels and will be discussed below. To counteract responses to stress from human manipulation, handling of animals should be minimized, and, if needed, frogs should be acclimated and only handled by properly trained individuals. If handled without sedation or anesthesia, the use of a net to catch the frog while covering the top of net with moist, powder-free non-latex gloves is recommended [211]. Overall, minimization of stress and associated behaviors and physiologic changes should be strived for to avoid introduction of variables into research and animal welfare concerns.
Environmental Complexity
Tank Material, Shape, Size, and Color
Components of environmental complexity for amphibians include tank design (shape, size, color, material), water flow through the tank (static/continuous/interrupted flow, flow rate, etc.), structures within the tank, and the environment immediately external to the tank. As mentioned previously, the optimal stocking density for these animals has not yet been determined. However, the number of frogs in a single enclosure should not only be thought of in relation to an amount of water per frog, but should also consider the shape of the tank, which can influence depth of the water, the amount of floor space or water surface area per animal, and vision of and frequency of direct contact with conspecifics. To allow for normal behaviors, it has been recommended that tanks provide a depth of at least 8 inches of water and allow enough space and water for frogs to move or swim around and turn fully in any direction without impediment from tank walls or contact with other animals [209]. All of these factors can influence interactions with conspecifics and potentially growth, stress, and associated physiologic changes, frequency of injury, and behaviors.
Xenopus are nocturnal and most active at night [162]. This is important to consider if conducting studies evaluating activity levels and when considering their micro- and macro-environmental conditions. Specific aspects of the macroenvironment of aquatics species, including light cycles and light perception, will be covered elsewhere in this journal. The physical appearance of Xenopus can be influenced by tank color and transparency. Xenopus’ chromatophores (cells containing pigment) can respond to hormones as well as change to adapt to habitat. For example, if housed in white or opaque tanks in the laboratory, frogs are typically a pale green compared with a dark green or black if housed in a darker tank. Background preferences for X. laevis appear to change from white as early-stage tadpoles to black at metamorphosis [212]. In another study, when adult X. laevis were placed into tanks with a nonecologically relevant white opaque background for 48 hours, higher corticosterone release rates were found when compared with placement in tanks with a black opaque background. Greater body mass loss and increased active behaviors in both sexes were found following placement in tanks with a white vs a black background. More walling behavior (repeated swimming against tank walls) was also noted upon placement into a tank with a white background [213]. It is unclear if these responses are temporary, as another study comparing frogs in black, gray, or white tanks for up to 7 months observed that frogs kept in black tanks developed in body length fastest, grey tanks intermediate, and white tanks slowest during the first month. However, there were no significant differences in either body weight or length between the groups housed in tanks of different colors at the end of the study [197]. The visual characteristics of these species should also be kept in mind when designing their housing and experimental environments. X. laevis’ vision is believed to have a peak absorbance sensitivity between 519 nm and 522 nm and only 1 visual pigment and thus may not be able to perceive color at longer wavelengths, such as reds and some in the blue range [214]. X. tropicalis is thought to be similar, and in a study evaluating refuge preferences this species utilized refuge with a transparent red cover similar to that of the same refuge with a black cover [214]. This is a reminder that not only opacity but specific colors of the tanks or objects within the tanks can influence behavior in these species, and this can have an impact on preference tests and other experiments. In addition, tank opacity will influence visibility of adjacent tanks and may influence colony hierarchical behavior or may cause the animals to react to what may be perceived as predatory movement outside of the tank. Thus, the tank color and opacity have the potential to influence studies evaluating growth, hormone levels, and behavior.
Water Flow
Xenopus may be housed in a variety of water flow conditions in the laboratory ranging from static water tanks on one end of the spectrum and continuous large-volume flow-through systems on the other. Intermediate conditions of smaller volume continuous flow in recirculating systems or intermittent flow are more common in commercially available housing systems. Water flow, both within housing tanks and in testing tanks, can have a significant impact on the behavior of amphibians and is an important aspect of tank complexity to be considered. It is important to understand normal behavior in response to water movement to recognize abnormal responses throughout the course of research. Responses to water movement in both fish and frogs are thought to be primarily mediated by neuromasts in the lateral line system. These responses vary by developmental stage because the lateral line system reorganizes at metamorphosis, [184, 185, 215] resulting in changes of location and distribution of groups of neuromasts. Rheostasis, orientation with respect to water current, varies in tadpoles and adult Xenopus with tadpoles showing more pronounced responses. This is postulated to be associated with the location of neuromasts in tadpoles, which are more superficially located than those in adults. For example, Xenopus tadpoles between the stages of 47 and 56 exhibit positive rheotaxis, turning to face an oncoming current at a rate of 1 cm s−1, whereas adults showed no response to the same current [185]. In addition, tadpoles in these stages were shown to hang suspended in the water column at a pronounced body tilt in static water with a reduction in the angle once current is introduced, whereas no significant changes are noticed in adults primarily remaining at the bottom of the tank. Tadpoles were unable to maintain their body position in higher flow conditions of 2 cm s−1, an important consideration if maintaining animals with water flow at this life stage. Adult X. laevis demonstrate positive rheotaxis at stronger currents, such as surface waves [216]. Their sensitivity to respond to water movement has the potential to cause severe stress if not controlled. For example, vibrations from nearby construction resulted in multiple deaths of laboratory X. laevis due to overstimulation of the mechanoreceptive function of the lateral line system and regurgitation, and eversion of the stomach and distal esophagus into the oral cavity with subsequent suffocation due to airway obstruction [217]. Location of water entry and exit points and associated volumes and velocities should also be taken into consideration when working with these animals as these variables have the potential to alter behavior or cause stress. Because of the intimate relationship between water current and the lateral line of amphibians, they must be highly sensitive to waves, vibrations, and water current, and any changes to these have the potential to impact behavior, appetite, and overall welfare.
Structural Enrichment
Amphibians generally avoid being exposed; [218] thus, the provision of a hiding place is recommended to allow them to display this natural behavior from predators. Scientific studies on the effect of environmental complexity of Xenopus are minimal, often utilize small sample sizes, and conclusions are variable. Studies directly correlating environmental complexity to research results are even less common; however, changes in the tank environment can influence behavioral repertoires, stress levels, and physical health in Xenopus,[127, 197, 198, 219, 220] and alterations to the housing environment have been shown to influence physical brain structure in other species, including mice, rats, and zebrafish [147, 221, 222]. Therefore, the influence of the tank environment and possible influences on the animals and research must be considered. Looking specifically at Xenopus, 1 study showed the introduction of pipes into a densely stocked tank decreased bite wounds [198]. Several studies demonstrated alterations in activity and feeding patterns when shelter was provided in the tanks [127, 197, 220]. Conclusions from studies evaluating the impact of shelter on growth rates and egg production/egg quality are variable or utilized small sample sizes [197, 219, 220, 223], and the amount of time X. tropicalis adults spend in a refuge has been shown to vary by the characteristics of the refuge [214]. One other aspect to consider is that some species or life stages may require the addition of objects to the tank to ensure the animals’ well-being. For example, young X. tropicalis froglets may drown from exhaustion when trying to breath and thus may require objects on the water’s surface on which to cling to and rest [178]. These examples demonstrate that it is important to be cognizant of the impact of environmental structures, as they may alter social and feeding behavior, stress and associated physiologic changes, activity levels, and physical and morphologic parameters.
As with to other aquatic species, it is important to be mindful that objects placed into a tank may support the growth of a biofilm, and biofilms have the potential to harbor infectious organisms [164]. Thus, these objects should be cleaned on a regular basis. Because the skin of Xenopus is very permeable to chemicals or toxicants in the water [200], it is important that no residual chemicals from cleaning be introduced into the tank. As for fish, only devices made from inert materials should be placed in the tank. Plasticizers that can act as endocrine disruptors reduce survivability, impair development, and impair spermatogenesis in X. laevis [224, 225]. Injury is another possible adverse effect associated with the placement of objects in the tank. Xenopus may get stuck in devices with small openings or may not be able to find their way to the surface (C. Lieggi, personal observation). Therefore, it is important that their path to the surface of the water, with enough surface area to accommodate all frogs in the tank, is not obstructed. This is important when using enrichment devices near or floating on the surface of the water. Objects intended for use on the bottom of the tank should be sinkable and heavy enough that they do not move around and block drains or other devices and that they cannot be moved into position to assist frogs with leaping out of the tank. As with other species, tanks and objects placed in the tanks should not have any sharp edges. Substrate is not typically used for Xenopus in laboratory animal environments, and the most appropriate substrate to allow for natural behaviors would be a deep layer of mud. Due to the natural indiscriminate feeding behavior of this species, considerations for any type of substrate would be the digestibility of the substrate and the potential for harboring pathogens. In addition, substrate could interfere with the observation of eggs, uneaten food, feces, etc.
General Considerations and Recommendations: Reducing Variability and Improving Replicability
In most cases, the ultimate goal of animal experiments and using animal models is to generalize the findings to humans and/or a species other than the one studied or in the same species to conditions different from those under which the study was performed [226, 227]. Standardization can control the undesirable effects of factors that may affect results and that challenge replicability from study to study. Additional aims of standardization are to reduce the number of subjects and to increase the comparability of results within and between laboratories. The parameters that require standardization to achieve these purposes depend on the experimental objections, the sensitivity of the readouts, and the possible impact of standardization on animal welfare. However, a strictly standardized study may fail to detect the effects of experimental manipulations, either because the environmental and developmental conditions and/or the test environment may not promote the expression of a phenotype, or because the test environment provides conditions hardly ever seen in replication but under which an experimental outcome becomes visible. Therefore, as already noted, a systematic inventory of the factors that act during an animal’s life, which may affect its behavior, is required [228].
As highlighted here, numerous developmental, environmental, and test-related factors can affect experimental outcome, including strain, sex, or age of the animal; group dynamics; tank environment; climate; or acoustic and light conditions. Controlled conditions are rarely standardized across laboratories and hence may mask a contribution to the treatment results. Here, we will provide general recommendations to assist with improvement of reproducibility and replicability, particularly as it pertains to behavior and environmental complexity, when utilizing popular aquatic models.
Study Design
Appropriate study design for experiments utilizing aquatic animal models requires knowledge of factors that may influence research results. Inadequate knowledge of the research subject’s biology, physiology, behavior, and responses to the environment and how those variables may influence research results will result in a poor study design. Depending on the type of research, factors to consider could include age, sex, reproductive status, diet, day length, time of day of testing, season, cage environment, sexual and social experience, and husbandry and handling procedures [29]. Unlike rodent models, which are primarily nocturnal and fed ad libitum, aquatic species are often fed a defined amount at various times throughout the day. Consider the time of day that the feeding occurs; are they being fed at the same time each day and how does this relate to experiments being conducted? For example, it may be a good idea to avoid direct handling of Xenopus sp. shortly after feeding due to the risk of regurgitation or avoid testing zebrafish after feeding, as they may be more excitable after these events. Tank environments must be scrutinized for potential influencers, including evaluation of the physical aspects of the tank (shape, size, color, water flow), social housing conditions (single, pair, or group-housing, hierarchical influences), and the presence or absence of structural enrichment. In regard to structural enrichment, it must be the same for all subjects and either be present or absent at all times throughout the course of the study. Animals should be acclimated appropriately to surroundings, handling, and procedures/tests that will be used as part of the experiment. How an animal is caught and transported may influence how it will behave during behavioral tests. Thus, habituation sessions may be helpful [229] when conducted appropriately, but if they are not done properly they may confound results by adding to anxiety. Resources for planning animal experiments are available and their use is encouraged. For example, the PREPARE guidelines: Planning Research and Experimental Procedures on Animals: Recommendations for Excellence [230], provide a checklist of factors to consider when planning animal-based studies.
Unfortunately, with both aquatic and mammalian models, we do not know everything. There are limitations to our knowledge of all factors and how they may influence research results. It is important to recognize these shortcomings as a reason for the inability to fully replicate or reproduce experimental data, especially between laboratories. It is also important that confounding variables be reported when discovered and systematic studies on the subject continue to be performed.
Reporting Results
Appropriate reporting is a critical aspect of scientific inquiry. Both the Institute for Laboratory Animal Research Guidance for the description of animal research in scientific publications and the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines emphasize the importance of reporting environmental conditions, including cage complexity (enrichment), tank shape, and housing paradigms [231, 232, 233]. These aspects of environmental conditions are frequently absent from publications involving aquatic species with the description of environmental conditions when present, being limited to more obvious environmental variables such as lighting, temperature, water quality parameters, and diet. Furthermore, as described above, social aspects of housing, including housing densities, group sizes, sex ratios, and other factors, are important to consider. All of these factors combined are critical when designing a study and may help elucidate whether differences observed in manuscripts are due to social or environmental factors. Thus, these details should be included in manuscripts when reporting research results.
Conclusion
Overall, more research is required to dissect the full impact of individual and social behavior and environmental complexity and how these factors influence the results generated utilizing aquatic animal models. Thorough knowledge of the natural history, biology, and behavior of the research subject and knowledge of previously reported influencers on research must be considered during the experimental design process. At the same time, we recognize that not all potential variables are known, and thus rigorous attention to detail and keen observations should be employed when conducting research. Finally, it is important to completely report all details about the animals and their environment (including testing environments) when publishing scientific results.
Acknowledgments
A.V.K. research was supported by the Russian Science Foundation grant 19-15-00053. He is the Chair of the International Zebrafish Neuroscience Research Consortium (ZNRC). This collaboration was supported, in part, through the NIH/NCI Cancer Center Support Grant P30 CA008748. The authors would like to thank Gregory Paull for sharing his photographs and insight into the natural habitat of zebrafish in Bangladesh.
References
- 1. National Center for Biotechnology Information, U.S. National Library of Medicine PubMed. https://www.ncbi.nlm.nih.gov/pubmed. Pubmed Advanced Search Builder (zebrafish [Title/Abstract]) AND ("04/01/2014"[Date - Publication]: "3000"[Date - Publication]). Filtered to published in the last 5 years, accessed July 22, 2019.
- 2. Arunachalam M, Raja M, Vijayakumar C, et al. Natural history of Zebrafish (Danio Rerio) in India. Zebrafish. 2013; 10(1):1–14. [DOI] [PubMed] [Google Scholar]
- 3. Engeszer RE, Patterson LB, Rao AA, et al. Zebrafish in the wild: a review of natural history and new notes from the field. Zebrafish. 2007; 4(1):21–40. [DOI] [PubMed] [Google Scholar]
- 4. Howe HB, McIntyre PB, Wolman MA. Adult Zebrafish primarily use vision to guide Piscivorous foraging behavior. Behavioural Processes. 2018; 157:230–7. [DOI] [PubMed] [Google Scholar]
- 5. McClure MM, McIntyre PB, McCune AR. Notes on the natural diet and habitat of eight Danionin fishes, including the Zebrafish Danio Rerio. Journal of Fish Biology. 2006; 69(2):553–70. [Google Scholar]
- 6. Spence R, Fatema MK, Ellis S, et al. Diet, growth and recruitment of wild zebrafish in Bangladesh. Journal of Fish Biology. 2007; 71(1):304–9. [Google Scholar]
- 7. Morais S The physiology of taste in fish: potential implications for feeding stimulation and gut chemical sensing. Reviews in Fisheries Science and Aquaculture. 2017; 25(2):133–49. [Google Scholar]
- 8. Bianco IH, Kampff AR, Engert F. Prey capture behavior evoked by simple visual stimuli in larval Zebrafish. Frontiers in systems neuroscience. 2011; 5:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Filosa A, Barker AJ, Dal Maschio M, et al. Feeding state modulates behavioral choice and processing of prey stimuli in the Zebrafish Tectum. Neuron. 2016; 90(3):596–608. [DOI] [PubMed] [Google Scholar]
- 10. McElligott MB, O’Malley DM. Prey tracking by larval Zebrafish: axial kinematics and visual control. Brain, Behavior and Evolution. 2005; 66(3):177–96. [DOI] [PubMed] [Google Scholar]
- 11. Carrillo A, McHenry MJ. Zebrafish learn to forage in the dark. Journal of Experimental Biology. 2016; 219(4):582–9. [DOI] [PubMed] [Google Scholar]
- 12. Harper C, Lawrence C. The Laboratory Zebrafish. Boca Raton, FL: CRC Press; 2010. [Google Scholar]
- 13. Rowena S, Smith C. Innate and learned colour preference in the Zebrafish. Danio rerio. Ethology. 2008; 114(6):582–8. [Google Scholar]
- 14. Myers N, Mittermeier RA, Mittermeier CG, et al. Biodiversity hotspots for conservation priorities. Nature 2000; 403:853–8. [DOI] [PubMed] [Google Scholar]
- 15. Spence R, Fatema MK, Reichard M, et al. The distribution and habitat preferences of the Zebrafish in Bangladesh. Journal of Fish Biology. 2006; 69(5):1435–48. [Google Scholar]
- 16. Suriyampola PS, Shelton DS, Shukla R, et al. Zebrafish social behavior in the wild. Zebrafish. 2016; 13(1):1–8. [DOI] [PubMed] [Google Scholar]
- 17. Lidicker WZ 2006. A clarification of interactions in ecological systems. BioScience. 1979; 29(8):475–7. [Google Scholar]
- 18. Herczeg G, Gonda A, Merilä J. Predation mediated population divergence in complex behaviour of nine-spined stickleback (Pungitius Pungitius). Journal of Evolutionary Biology. 2009; 22(3):544–52. [DOI] [PubMed] [Google Scholar]
- 19. Magurran AE 2005. Oxford Series in Ecology and Evolution Evolutionary Ecology: The Trinidadian Guppy. New York, NY: Oxford University Press; 2005. [Google Scholar]
- 20. Thomson JS, Phillip CW, Pottinger TG, et al. Plasticity of boldness in rainbow trout, Oncorhynchus Mykiss: do hunger and predation influence risk-taking behaviour? Hormones and Behavior. 2012; 61(5):750–7. [DOI] [PubMed] [Google Scholar]
- 21. Gaikwad S, Stewart A, Hart P, et al. Acute stress disrupts performance of zebrafish in the cued and spatial memory tests: the utility of fish models to study stress-memory interplay. Behav. Processes. 2011; 87(2):224–30. [DOI] [PubMed] [Google Scholar]
- 22. Kalueff AV, Echevarria DJ, Homechaudhuri S, et al. Zebrafish neurobehavioral phenomics for aquatic neuropharmacology and toxicology research. Aquat Toxicol. 2016; 170:297–309. [DOI] [PubMed] [Google Scholar]
- 23. Roy T, Rohitashva S, Bhat A. Risk-taking during feeding: between- and within-population variation and repeatability across contexts among wild Zebrafish. Zebrafish. 2017; 14(5):393–403. [DOI] [PubMed] [Google Scholar]
- 24. Pan ZY, He XY, Wang XY, et al. Selection signature in domesticated animals. Yi Chuan. 2016; 38(12):1069–80. [DOI] [PubMed] [Google Scholar]
- 25. Robison BD, Rowland W. A potential model system for studying the genetics of domestication: behavioral variation among wild and domesticated strains of zebra danio (Danio rerio). Canadian Journal of Fisheries and Aquatic Sciences. 2005; 62(9):2046–54. [Google Scholar]
- 26. Wright D, Nakamichi R, Krause J, et al. QTL analysis of behavioral and morphological differentiation between wild and laboratory zebrafish (Danio rerio). Behav Genet. 2006; 36(2):271–84. [DOI] [PubMed] [Google Scholar]
- 27. Kalueff AV, Echevarria DJ, Homechaudhuri S, et al. Zebrafish neurobehavioral phenomics for aquatic neuropharmacology and toxicology research. Aquat Toxicol. 2016; 170:297–309. [DOI] [PubMed] [Google Scholar]
- 28. Burghardt GM Environmental enrichment and cognitive complexity in reptiles and amphibians: concepts, review, and implications for captive populations. Appl Anim Behav Sci. 2013; 147:286–98. [Google Scholar]
- 29. Wersinger SR, Martin LB. Optimization of laboratory conditions for the study of social behavior. ILAR Journal. 2009; 50(1):64–80. [DOI] [PubMed] [Google Scholar]
- 30. Dell AI, Bender JA, Branson K, et al. Automated image-based tracking and its application in ecology. Trends Ecol Evol. 2014; 29:417–28. [DOI] [PubMed] [Google Scholar]
- 31. Cachat J, Stewart A, Utterback E, et al. Three-dimensional neurophenotyping of adult zebrafish behavior. PLoS One 2011; 6(3): e17597. doi: 10.1371/journal.pone.0017597 Accessed May 4, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Easter SS, Nicola GN. The development of eye movements in the zebrafish (Danio rerio). Developmental Psychobiology. 1997; 31(4):267–76. [DOI] [PubMed] [Google Scholar]
- 33. Liu KS, Fetcho JR. Laser ablations reveal functional relationships of segmental hindbrain neurons in zebrafish. Neuron. 1999; 23:325–35. [DOI] [PubMed] [Google Scholar]
- 34. Stewart AM, Gerlai R, Kalueff AV. Developing highER-throughput zebrafish screens for in-vivo CNS drug discovery. Front Behav Neurosci. 2015; 9:14. doi: 10.3389/fnbeh.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kalueff AV, Gebhardt M, Stewart AM, et al. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish. 2013; 10(1):70–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rvd H, Lambert J. Ovarian steroid glucuronides function as sex pheromones for male zebrafish. Brachydanio rerio. Can J Zool. 1983; 61(11):2381–7. [Google Scholar]
- 37. Chen L-C, Martinich RL. Pheromonal stimulation and metabolite inhibition of ovulation in the zebrafish, Brachydanio rerio. Environ Biol Fishes. 1975; 73(4):889–93. [Google Scholar]
- 38. Bloom HD, Perlmutter A. A sexual aggregating pheromone system in the zebrafish, Brachydanio rerio (Hamilton-Buchanan). J Exp Zool. 1977; 199(2):215–26. [DOI] [PubMed] [Google Scholar]
- 39. Hall D, Suboski MD. Visual and olfactory stimuli in learned release of alarm reactions by zebra danio fish (Brachydanio rerio). Neurobiol Learn Mem. 1995; 63(3):229–40. [DOI] [PubMed] [Google Scholar]
- 40. Steele CW, Owens DW, Scarfe AD. Attraction of zebrafish, Brachydanio rerio, to alanine and its suppression by copper. J Fish Biol. 1990; 36(3):341–52. [Google Scholar]
- 41. Vitebsky A, Reyes R, Sanderson MJ, et al. Isolation and characterization of the laure olfactory behavioral mutant in the zebrafish, Danio rerio. Dev Dyn. 2005; 234(1):229–42. [DOI] [PubMed] [Google Scholar]
- 42. Michel W, Lubomudrov L. Specificity and sensitivity of the olfactory organ of the zebrafish, Danio rerio. J Comp Physiol A. 1995; 177(2):191–9. [DOI] [PubMed] [Google Scholar]
- 43. Hara TJ Olfaction and gustation in fish: an overview. Acta Physiol Scand. 1994; 152(2):207–17. [DOI] [PubMed] [Google Scholar]
- 44. Speedie N, Gerlai R. Alarm substance induced behavioral responses in zebrafish (Danio rerio). Behav Brain Res. 2008; 188(1):168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Friedrich RW, Korsching SI. Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron. 1997; 18(5):737–52. [DOI] [PubMed] [Google Scholar]
- 46. Whitlock KE The sense of scents: olfactory behaviors in the zebrafish. Zebrafish. 2006; 3(2):203–13. [DOI] [PubMed] [Google Scholar]
- 47. Hisaoka KK, Firlit CF. Ovarian cycle and egg production in the zebrafish. Brachydanio rerio. Copeia. 1962; 4:788–92. [Google Scholar]
- 48. Rvd H, Resink J. male reproductive system as sex pheromone producer in teleost fish. J Exp Zool. 1992; 261:204–13. [Google Scholar]
- 49. Rvd H, Lambert JGD. Ovarian steroid glucuronides function as sex pheromones for male zebrafish. Brachydanio rerio. Can J Zool. 1983; 61:2381–7. [Google Scholar]
- 50. Pritchard VL Behaviour and morphology of the zebrafish, Danio rerio [PhD thesis] Leeds: University of Leeds; 2001. [Google Scholar]
- 51. Engeszer RE, Barbiano LA, Ryan MJ, Parichy DM. An analysis of shoaling preference in the Zebrafish, Danio Rerio. In: Integrative and Comparative Biology Conference: Annual Meeting of the Society-for-Integrative-and-Comparative-Biology; January 4–8, 2005; San Diego, CA Abstract 2.5. [Google Scholar]
- 52. Moretz JA, Martins EP, Robison BD. 2007. Behavioral syndromes and the evolution of correlated behavior in Zebrafish. Behav Ecol. 2007; 18(3):556–62. [Google Scholar]
- 53. Spence RG, Gerlach G, Lawrence C, et al. The behaviour and ecology of the Zebrafish, Danio Rerio. Biol Rev Camb Philos Soc. 2008; 83(1):13–34. [DOI] [PubMed] [Google Scholar]
- 54. Wright D, Rimmer LB, Pritchard VL, et al. Inter and intra-population variation in shoaling and boldness in the Zebrafish (Danio Rerio). Naturwissenschaften. 2003; 90(8):374–7. [DOI] [PubMed] [Google Scholar]
- 55. Wright D, Krause J. Repeated measures of shoaling tendency in Zebrafish (Danio Rerio) and other small teleost fishes. Nat Protoc. 2006; 1(4):1828–31. [DOI] [PubMed] [Google Scholar]
- 56. Pitcher T Shoaling and schooling behavior in fishes In: Greenberg G, Haraway M, eds. Comparative Psychology: A Handbook. New York, NY: Routledge; 1998. p. 748–60. [Google Scholar]
- 57. Pitcher TJ, Magurran AE, Winfield IJ. Fish in larger shoals find food faster. Behav Ecol Sociobiol. 1982; 10:149–51. [Google Scholar]
- 58. Dawkins R, Krebs JR. 1979. Arms races between and within species. In: Proceedings of the Royal Society of London. Series B. Biological Sciences. 1979; 205:489–511 10.1098/rspb.1979.0081. Accessed November 14, 2019. [DOI] [PubMed] [Google Scholar]
- 59. Dreosti E, Lopes G, Kampff AR, et al. Development of social behavior in young zebrafish. Front Neural Circuits. 2015; 18:39 10.3389/fncir.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Engeszer RE, Barbiano LA, Ryan MJ, et al. Timing and plasticity of shoaling behaviour in the zebrafish. Danio rerio. Anim Behav. 2007; 74(5):1269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mann KD, Turnell ER, Atema J, et al. Kin recognition in juvenile Zebrafish (Danio Rerio) based on olfactory cues. Biol Bull. 2003; 205:224–5. [DOI] [PubMed] [Google Scholar]
- 62. Miller NY, Gerlai R. Shoaling in zebrafish: what we don’t know. Rev Neurosci. 2011; 22(1):17–25. [DOI] [PubMed] [Google Scholar]
- 63. Pritchard VL, Lawrence J, Butlin RK, et al. Shoal choice in zebrafish, Danio Rerio: The influence of shoal size and activity. Anim Behav. 2001; 62(6):1085–8. [Google Scholar]
- 64. Miller NY, Gerlai R. Oscillations in shoal cohesion in zebrafish (Danio rerio). Behav Brain Res. 2008; 193(1):148–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pitcher TJ Functions of shoaling behaviour in teleosts In: Pitcher TJ, ed. Behaviour of Teleost Fishes. Boston, MA: Springer; 1986. p. 294–337. [Google Scholar]
- 66. Miller N, Gerlai R. From schooling to shoaling: patterns of collective motion in zebrafish (Danio Rerio). PLoS ONE. 2012; 7(11):e48865 10.1371/journal.pone.0048865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Suriyampola PS, Delawrence JS, Khemka A, et al. Water flow impacts group behavior in zebrafish (Danio rerio). Behav Ecol. 2017; 28(1):94–100. [Google Scholar]
- 68. Franks B, Graham C, von MAG. Is heightened-shoaling a good candidate for positive emotional behavior in zebrafish? Animals (Basel). 2018; 8(9):152. doi: 10.3390/ani8090152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hutter S, Penn DJ, Magee S, et al. Reproductive behaviour of wild zebrafish (Danio Rerio) in large tanks. Behaviour. 2010; 147:641–60. [Google Scholar]
- 70. Etinger A, Lebron J, Palestis BG. Sex-assortative shoaling in zebrafish (Danio Rerio). BIOS. 2009; 80(4):153–8 10.1893/011.080.0402. [DOI] [Google Scholar]
- 71. Gerlach G, Hodgins-Davis A, MacDonald B, et al. Benefits of kin association: related and familiar zebrafish larvae (Danio Rerio) show improved growth. Behav Ecol Sociobiol. 2007; 61(11):1765–70. [Google Scholar]
- 72. Engeszer RE, Ryan MJ, Parichy DM. Learned social preference in zebrafish. Curr Biol. 2004; 4(10):881–4. [DOI] [PubMed] [Google Scholar]
- 73. Krause J, Hartmann N, Pritchard VL. The influence of nutritional state on shoal choice in zebrafish. Danio Rerio. Animal Behav. 1999; 57:771–5. [DOI] [PubMed] [Google Scholar]
- 74. Miller N, Gerlai R. Quantification of shoaling behaviour in zebrafish (Danio rerio). Behav Brain Res. 2007; 184(2):157–66. [DOI] [PubMed] [Google Scholar]
- 75. Arganda S, Pérez-Escudero A, de Polavieja GG. A common rule for decision making in animal collectives across species. Proceedings of the National Academy of Sciences. 2012; 109(50):20508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Snekser JL, Ruhl N, Bauer K, et al. The influence of sex and phenotype on shoaling decisions in zebrafish. International Journal of Comparative Psychology 2010; 23(1):70–81. https://escholarship.org/uc/item/8n68z3pf#article_main. Accessed March 23, 2019. [Google Scholar]
- 77. Kalueff AV, Echevarria DJ, Stewart AM. Gaining translational momentum: more zebrafish models for neuroscience research. Prog Neuropsychopharmacol Biol Psychiatry. 2014; 55:1–6. [DOI] [PubMed] [Google Scholar]
- 78. Filby AL, Paull GC, Bartlett EJ, et al. Physiological and health consequences of social status in Zebrafish (Danio Rerio). Physiol Behav. 2010; 101(5):576–87. [DOI] [PubMed] [Google Scholar]
- 79. Fox HE, White SA, Kao MHF, et al. Stress and dominance in a social fish. J Neurosci. 1997; 7(16):6463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. North BP, Turnbull JF, Ellis T, et al. The impact of stocking density on the welfare of rainbow trout (Oncorhynchus Mykiss). Aquaculture. 2006; 225:466–79. [Google Scholar]
- 81. Pottinger TG, Pickering AD. The influence of social interaction on the acclimation of rainbow trout, Oncorhynchus Mykiss (Walbaum) to chronic stress. J Fish Biol. 1992; 41:435–47. [Google Scholar]
- 82. Tea J, Alderman SL, Gilmour KM. Social stress increases plasma cortisol and reduces forebrain cell proliferation in subordinate male Zebrafish (Danio Rerio). J Exp Biol 2018; 222(Pt 2). pii: jeb194894. doi: 10.1242/jeb.194894. [DOI] [PubMed] [Google Scholar]
- 83. Warner RR, Hoffman SG. Population density and the economics of territorial defense in a coral reef fish. Ecology. 2006; 61(4):772–80. [Google Scholar]
- 84. Pickering AD Rainbow trout husbandry: management of the stress response. Aquaculture. 1992; 100:125–39. [Google Scholar]
- 85. Larson ET, O’Malley DM, Melloni RH Jr. Aggression and vasotocin are associated with dominant-subordinate relationships in zebrafish. Behav Brain Res. 2006; 67:94–102. [DOI] [PubMed] [Google Scholar]
- 86. Larson ET, O’Malley DM, Melloni RH. Aggression and vasotocin are associated with social rank in zebrafish. Hormones and Behavior Conference: 9th Annual Meeting of the Society-for-Behavioral-Neuroendocrinology. 2005; 48(143). [Google Scholar]
- 87. Lesley R, Summers CH, Larson ET, et al. Development of aggressive phenotypes in zebrafish: interactions of age, experience and social status. Anim Behav. 2013; 86(2):242–52. [Google Scholar]
- 88. Pavlidis M, Digka N, Theodoridi A, et al. Husbandry of zebrafish, Danio Rerio, and the cortisol stress response. Zebrafish. 2013; 10(4):524–31. [DOI] [PubMed] [Google Scholar]
- 89. Collymore C, Tolwani RJ, Rasmussen S. The behavioral effects of single housing and environmental enrichment on adult zebrafish (Danio rerio). J Am Assoc Lab Anim Sci. 2015; 54(3):280–5. [PMC free article] [PubMed] [Google Scholar]
- 90. Keck VA, Edgerton DS, Hajzadeh S, et al. Effects of habitat complexity on pair-housed zebrafish. J Am Assoc Lab Anim Sci. 2015; 54(4):378–83. [PMC free article] [PubMed] [Google Scholar]
- 91. Miller TH, Clements K, Ahn S, et al. Social status–dependent shift in neural circuit activation affects decision making. J Neurosci. 2017; 37(8):2137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Oliveira R, Silva JF, Simões JM. Fighting zebrafish: characterization of aggressive behavior and winner–loser effects. Zebrafish. 2011; 8(2):73–81. [DOI] [PubMed] [Google Scholar]
- 93. Mathuru A, Schirmer A, Tabitha NPY, et al. Familiarity with companions aids recovery from fear in Zebrafish. bioRxi 2017. 10.1101/098509. [DOI] [Google Scholar]
- 94. Faustino A, Tacão-Monteiro A, Oliveira RF. Mechanisms of social buffering of fear in zebrafish. Scientific Reports. 2017; 7:44329. 10.1038/srep44329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. White L, Thomson JS, Pounder KC, et al. The impact of social context on behaviour and the recovery from welfare challenges in zebrafish. Danio Rerio. Anim Behav. 2017; 132:189–99 10.1016/j.anbehav.2017.08.017. [DOI] [Google Scholar]
- 96. Mohammad ND, Safari O, Boiti C. Effects of social isolation on growth, stress response, and immunity of Zebrafish. Acta Ethologica. 2017; 20(3):255–61. [Google Scholar]
- 97. Parker MO, Millington ME, Combe FJ, et al. Housing conditions differentially affect physiological and behavioural stress responses of Zebrafish, as well as the response to anxiolytics. PLoS ONE. 2012; 7(4):e34992. doi: 10.1371/journal.pone.0034992 [Epub 2012 Apr 11]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shams S, Seguin D, Facciol A, et al. Effect of social isolation on anxiety-related behaviors, cortisol, and monoamines in adult zebrafish. Behav Neurosci. 2017; 131(6):492–504. doi: 10.1037/bne0000220. [DOI] [PubMed] [Google Scholar]
- 99. Shams S, Chatterjee D, Gerlai R. Chronic social isolation affects thigmotaxis and whole-brain serotonin levels in adult zebrafish. Behav Brain Res. 2015; 292:283–7. [DOI] [PubMed] [Google Scholar]
- 100. Giacomini ACVV, Abreu MS, Zanandrea R, et al. Environmental and pharmacological manipulations blunt the stress response of zebrafish in a similar manner. Sci Rep. 2016; 6:28986 10.1038/srep28986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Giacomini AC, de Abreu MS, Koakoski G, al. 2015. My stress, our stress: blunted cortisol response to stress in isolated housed zebrafish. Physiol Behav . 2015;139:182-187. doi: 10.1016/j.physbeh.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 102. Zellner D, Padnos B, Hunter DL, et al. Rearing conditions differentially affect the locomotor behavior of larval zebrafish, but not their response to valproate-induced developmental neurotoxicity. Neurotoxicol Teratol. 2011; 33(6):674–9. [DOI] [PubMed] [Google Scholar]
- 103. Shams S, Amlani S, Buske C, et al. Developmental social isolation affects adult behavior, social interaction, and dopamine metabolite levels in zebrafish. Dev Psychobiol. 2018; 60(1):43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Matthews JL Common diseases of laboratory zebrafish. Methods in Cell Biology. 2004; 77:617–43. [DOI] [PubMed] [Google Scholar]
- 105. Neri D, Ruberto T, Mwaffo V, et al. Social environment modulates anxiogenic effects of caffeine in zebrafish. Behav pharmacol. 2019; 30(1):45–58. [DOI] [PubMed] [Google Scholar]
- 106. Ribas L, Valdivieso A, Díaz N, et al. Appropriate rearing density in domesticated zebrafish to avoid masculinization: links with the stress response. J Exp Biol. 2017; 220:1056–64. doi: 10.1242/jeb.144980. [DOI] [PubMed] [Google Scholar]
- 107. Ramsay JM, Feist GW, Varga ZM, et al. Whole-body cortisol is an indicator of crowding stress in adult zebrafish. Danio Rerio. Aquaculture. 2006; 258(1–4):565–74. [Google Scholar]
- 108. Sallin P, Pauline JA. Acute stress is detrimental to heart regeneration in zebrafish. Open Biol. 2016; 6(3160012). doi: 10.1098/rsob.160012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Spence R, Jordan WC, Smith C. Genetic analysis of male reproductive success in relation to density in the zebrafish. Danio Rerio. Front Zool. 2006; 3(1):5. doi: 10.1186/1742-9994-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Demin KA, Lakstygal AM, Alekseeva PA, et al. The role of intraspecies variation in fish neurobehavioral and neuropharmacological phenotypes in aquatic models. Aquat Toxicol. 2019; 210:44–55. [DOI] [PubMed] [Google Scholar]
- 111. Volgin AD, Yakovlev OA, Demin KA, et al. Zebrafish models for personalized psychiatry: insights from individual, strain and sex differences, and modeling gene x environment interactions. J Neurosci Res. 2019; 97(4):402–13. [DOI] [PubMed] [Google Scholar]
- 112. Genario R, de Abreu MS, Giacomini ACVV, et al. Sex differences in behavior and neuropharmacology of zebrafish. Eur J Neruosci. 2019. doi: 10.1111/ejn.14438 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 113. van den R, Mes W, Galligani P, et al. Further characterisation of differences between TL and AB zebrafish (Danio rerio): gene expression, physiology and behaviour at day 5 of the larval stage. PLoS ONE. 2017; 12(4):e0175420. doi: 10.1371/journal.pone.0175420 eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gorissen M, Manuel R, Pelgrim TNM, et al. Differences in inhibitory avoidance, cortisol and brain gene expression in TL and AB zebrafish. Genes Brain Behav. 2015; 14:428–38 https://onlinelibrary.wiley.com/doi/full/10.1111/gbb.12220. Accessed April 2, 2019. [DOI] [PubMed] [Google Scholar]
- 115. Gerlai R, Csányi V. Genotype-environment interaction and the correlation structure of behavioral elements in paradise fish (Macropodus opercularis). Physiol Behav. 1990; 47(2):343–56. [DOI] [PubMed] [Google Scholar]
- 116. Oswald ME, Drew RE, Racine M, et al. Is behavioral variation along the bold-shy continuum associated with variation in the stress axis in zebrafish? Physiological and Biochemical Zoology. 2012; 85(6):718–28. [DOI] [PubMed] [Google Scholar]
- 117. Dahlbom SJ, Lagman D, Lundstedt-Enkel K, et al. Boldness predicts social status in zebrafish (Danio rerio). PLoS ONE. 2011; 6(8):e23565 10.1371/journal.pone.0023565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pantoja C, Hoagland A, Carroll EC, et al. Neuromodulatory regulation of behavioral individuality in zebrafish. Neuron. 2016; 91(3):587–601. doi: 10.1016/j.neuron.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Spagnoli S, Xue L, Kent ML. The common neural parasite Pseudoloma neurophilia is associated with altered startle response habitation in adult zebrafish (Danio rerio): implications for the zebrafish as a model organism. Behav Brain Res. 2015; 291:351–60. doi: 10.1016/j.bbr.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Spagnoli S, Sanders J, Kent ML. The common neural parasite Pseudoloma neurophilia causes altered shoaling behavior in adult laboratory zebrafish (Danio rerio) and its implications for neurobehavioral research. J Fish Dis. 2017; 40(3):443–6. doi: 10.1111/jfd.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bohlen M, Hayes ER, Bohlen B, et al. Experimenter effects on behavioral test scores of eight inbred mouse strains under the influence of ethanol. Behav Brain Res. 2014; 272:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sorge U, Martin LJ, Isbester KA, et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014; 11:629–32. doi: 10.1038/nmeth doi:10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- 123. Chapman CD, Benedict C, Schiöth HB. Experimenter gender and replicability in science. Science Advances. 2018; 4(1):e1701427. doi: 10.1126/sciadv.1701427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Stewart AM, Kaluyeva AA, Poudel MK, et al. Building zebrafish neurobehavioral phenomics: effects of common environmental factors on anxiety and locomotor activity. Zebrafish. 2015; 12(5):339–48. [DOI] [PubMed] [Google Scholar]
- 125. Mertens S, Vogt MA, Gass P, et al. Effect of three different forms of handling on the variation of aggression-associated parameters in individually and group-housed male C57BL/6NCrl mice. PLoS One. 2019; 14(4):e0215367.Published 2019 Apr 12. doi: 10.1371/journal.pone.0215367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Martins CI, Eding EH, Verreth JA. Stressing fish in recirculating aquaculture systems (RAS): does stress induced in one group of fish affect the feeding motivation of other fish sharing the same RAS? Aquac Res. 2011; 42(9):1378–84. [Google Scholar]
- 127. Archard GA Refuge use affects daily activity patterns in female Xenopus laevis. Appl Anim Behav Sci. 2013; 145:123–8. https://www.sciencedirect.com/science/article/pii/S0168159113000658. Accessed April 2, 2019. [Google Scholar]
- 128. Lawrence C, Mason T. Zebrafish housing systems: a review of basic operating principles and considerations for design and functionality. ILAR J. 2012; 53(2):179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Mathieu-Denoncourt J, Wallace SJ, de Solla R, et al. Plasticizer endocrine disruption: highlighting developmental and reproductive effects in mammals and non-mammalian aquatic species. Gen Comp. Endocrinol. 2015; 219:74–88. [DOI] [PubMed] [Google Scholar]
- 130. Meier A, Nelson R, Connaughton VP. Color processing in zebrafish retina. Front Cell Neurosci. 2018; 12:327. doi: 10.3389/fncel.2018.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ahmad F Zebrafish Embryos and Larvae as a Complementary Model for Behavioural Research . [PhD Thesis]. Leiden, The Netherlands: University of Leiden; 2014. [Google Scholar]
- 132. Park J-S, Ryu J-H, Choi T-I, et al. Innate color preference of zebrafish and its use in behavioral analyses. Mol Cells. 2016; 39(10):750–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Avdesh A, Martin-Iversion MT, Mondal A, et al. Evaluation of color preference in zebrafish for learning and memory. J Alzheimers Dis. 2012; 28:459–69. [DOI] [PubMed] [Google Scholar]
- 134. Kim Y-H, Lee KS, Park AR, et al. Adding preferred color to a conventional reward method improves the memory of zebrafish in the T-maze behavior model. Anim Cells Sys. 2017; 21(6):374–81. doi: 10.1080/19768354.2017.1383938. [DOI] [Google Scholar]
- 135. Oliveira J, Silveira M, Chacon D, et al. The zebrafish world of colors and shapes: preference and discrimination. Zebrafish. 2015; 12(2):166–73. [DOI] [PubMed] [Google Scholar]
- 136. Blaser RE, Rosemberg DB. Measures of anxiety in zebrafish (Danio rerio): dissociation of black/white preference and novel tank test. PLoS ONE. 2012; 7(5):e36931. doi: 10.1371/journal.pone.0036931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Rosemberg DB, Rico EP, Mussulini BHM, et al. Differences in spatio-temporal behavior of zebrafish in the open tank paradigm after a short-period confinement into dark and bright environments. PLoS ONE. 2011; 6(5):e19397 https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0019397. Accessed April 2, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Maximino C, de Brito TM, Colmanetti R, et al. Parametric analyses of anxiety in zebrafish scototaxis. Behav Brain Res. 2010; 210(1):1–7. [DOI] [PubMed] [Google Scholar]
- 139. Stewart A, Maximino C, Marques de Brito T, et al. Neurophenotyping of adult zebrafish using the scototaxis paradigm In: Kalueff AV, Cachat JM, eds. Zebrafish Neurobehavioral Protocols. New York, NY: Humana Press; 2011. p. 157–67. [Google Scholar]
- 140. Gerlai R Reproducibility and replicability in zebrafish behavioral neuroscience research. Pharmacol Biochem Behav. 2019; 178:30–8. [DOI] [PubMed] [Google Scholar]
- 141. Mason G Stereotypies: a critical review. Anim Behav. 1991; 4:1015–37. [Google Scholar]
- 142. Manosevitz M, Pryor JB. Cage size as a factor in environmental enrichment. J Comp Physiol Psychol. 1975; 89(6):648–54. 10.1037/h0077426. [DOI] [Google Scholar]
- 143. Davidson LP, Chedester AL, Cole MN. Effects of cage density on behavior in young adult mice. Comp Med. 2007; 57(4):355–9. [PubMed] [Google Scholar]
- 144. Ennaceur A, Michalikova S, Chazot PL. Models of anxiety: responses of rats to novelty in an open space and an enclosed space. Behav Brain Res. 2006; 171(1):26–49. doi: 10.1016/j.bbr.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 145. Polverino G, Bierbach D, Killen SS, et al. Body length rather than routine metabolic rate and body condition correlates with activity and risk-taking in juvenile zebrafish. Danio rerio. J Fish Biol. 2016; 89:2251–67. doi: 10.1111/jfb.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Bhat A, Greulich MM, Martins EP. Behavioral plasticity in response to environmental manipulation among zebrafish (Danio rerio) populations. PLoS ONE. 2015; 10(4):e0125097. doi: 10.1371/journal.pone.0125097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Volgin AD, Yakovlev OV, Demin KA, et al. Understanding the role of environmental enrichment in zebrafish neurobehavioral models. Zebrafish. 2018; 15(5):425–32. [DOI] [PubMed] [Google Scholar]
- 148. Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006; 7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- 149. Bardo MT, Klebaur JE, Valone JM. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl). 2001; 155(3):278–84. [DOI] [PubMed] [Google Scholar]
- 150. Bezard E, Dovero S, Belin D, et al. Enriched environment confers resistance to 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine and cocaine: involvement of dopamine transporter and trophic factors. J Neurosci. 2003; 23:10999–1007 10.1523/JNEUROSCI.23-35-10999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zhu J, Apparsundaram S, Bardo MT, et al. Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J Neurochem. 2005; 93(6):1434–43. [DOI] [PubMed] [Google Scholar]
- 152. Kistler C, Hegglin D, Wurber H, et al. Preference for structured environment in zebrafish (Danio rerio) and checker barbs (Puntius oligolepis). Appl Anim Behav Sci. 2011; 135:318–27. [Google Scholar]
- 153. Schroeder P, Jones S, Young IS, et al. What do zebrafish want? Impact of social grouping, dominance and gender on preference for enrichment. Lab Anim. 2014; 48(4):328–37. [DOI] [PubMed] [Google Scholar]
- 154. Lee CJ, Paull GC, Tyler CR. Effects of environmental enrichment on survivorship, growth, sex ratio and behaviour in laboratory maintained zebrafish. J Fish Biol. 2018; 94:86–95. [DOI] [PubMed] [Google Scholar]
- 155. Manuel R, Gorissen M, Stokkermans M, et al. The effects of environmental enrichment and age-related differences on inhibitory avoidance in zebrafish (Danio rerio Hamilton). Zebrafish. 2015; 12(2):152–65. [DOI] [PubMed] [Google Scholar]
- 156. DePasquale C, Neuberger T, Hirrlinger AM, et al. The influence of complex and threatening environments in early life on brain size and behaviour. Proc Biol Sci. 2016; 283(1823):20152564 10.1098/rspb.2015.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Spence R, Magurran AE, Smith C. Spatial cognition in zebrafish: the role of strain and rearing environment. Anim Cogn. 2011; 14(4):607–12. doi: 10.1007/s10071-011-0391-8 Epub 2011 Mar 1. [DOI] [PubMed] [Google Scholar]
- 158. Marcon M, Mocelin R, Sachett A, et al. Enriched environment prevents oxidative stress in zebrafish submitted to unpredictable chronic stress. PeerJ. 2018; 6:e5136. doi: 10.7717/peerj.5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Kareklas K, Elwood RW, Holland RA. Grouping promotes risk-taking in unfamiliar settings. Behav Processes. 2018; 148:41–5. [DOI] [PubMed] [Google Scholar]
- 160. Hamilton IM, Dill LM. Monopolization of food by zebrafish (Danio rerio) increases in risky habitats. Can J Zool. 2002; 80:2164–9. [Google Scholar]
- 161. Wilkes L, Owen SF, Readman GD, et al. Does structural enrichment for toxicology studies improve zebrafish welfare? Appl Anim Behav Sci. 2012; 139(1–2):143–50. https://www.sciencedirect.com/science/article/pii/S0168159112000949?via%3Dihub. Accessed April 2, 2019. [Google Scholar]
- 162. Wafer LN, Behrana Jensen V, Whitney JC, et al. Effects of environmental enrichment on the fertility and fecundity of zebrafish (Danio rerio). J Am Assoc Lab Anim Sci. 2016; 55(3):291–4. [PMC free article] [PubMed] [Google Scholar]
- 163. Spence R, Ashton R, Smith C. Oviposition decisions are mediated by spawning site quality in wild and domesticated zebrafish, Danio rerio. Behaviour. 2007; 144(8):953–66 10.1163/156853907781492726. [DOI] [Google Scholar]
- 164. Chang CT, Lewis J, Whipps CM. Source or sink: examining the role of biofilms in transmission of mycobacterium spp. in laboratory zebrafish. Zebrafish. 2019; 16(2):197–206. doi: 10.1089/zeb.2018.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Bailoo JD, Murphy E, Boada-Saña M, et al. Effects of cage enrichment on behavior, welfare and outcome variability in female mice. Front Behav Neurosci. 2018; 12:232. doi: 10.3389/fnbeh.2018.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Séguret A, Collignon B, Halloy J. Strain differences in the collective behaviour of zebrafish (Danio rerio) in heterogeneous environment. R Soc Open Sci. 2016; 3(10):160451. doi: 10.1098/rsos.160451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Collymore C, Lawrence C, Lieggi C. Enrichment for laboratory zebrafish (Danio rerio). LASPro. 2014; 2(3):51–4. https://www.aalas.org/articles/2014/09/01/enrichment-for-laboratory-zebrafish-danio-rerio. Accessed April 2, 2019. [Google Scholar]
- 168. Di V, Frigato E, López-Olmeda JF, et al. The light wavelength affects the ontogeny of clock gene expression and activity rhythms in Zebrafish larvae. PLoS ONE 2015; 10(7):e0132235. doi: 10.1371/journal.pone.0132235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Villamizar N, Vera LM, Foulkes NS, et al. Effect of lighting conditions on zebrafish growth and development. Zebrafish. 2014; 11(2):173–81. doi: 10.1089/zeb.2013.0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Liu LS, Zhao LY, Wang SH, et al. Research proceedings on amphibian model organisms. Dongwuxue Yanjiu. 2016; 37(4):237–45. doi: 10.13918/j.issn.2095-8137.2016.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Karimi K, Fortriede JD, Lotay VS, et al. Xenbase: a genomic, epigenomic and transcriptomic model organism database. Nucleic Acids Research. 2018; 46(D1):D861–8. doi: 10.1093/nar/gkx936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Brockes JP Amphibian limb regeneration: rebuilding a complex structure. Science. 1997; 276(5309):81–7. http://science.sciencemag.org/content/276/5309/81.long. Accessed March 23, 2019. [DOI] [PubMed] [Google Scholar]
- 173. Tinsley RC, McCoid MJ. Feral populations of Xenopus outside Africa In: Tinsley RC, Kobel HR, eds. The Biology of Xenopus. New York, NY: Oxford University Press; 1996. p. 81–94. [Google Scholar]
- 174. Tinsley RC, Stott LC, Viney ME, et al. Extinction of an introduced warm-climate alien species, Xenopus laevis, by extreme weather events. Biol Invasions. 2015; 17(11):3183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Tinsley RC, Loumont C, Kobel HR. Geographical distribution and ecology In: Tinsley RC, Kobel HR, eds. The Biology of Xenopus. New York, NY: Oxford University Press; 1996. p. 35–56. [Google Scholar]
- 176. Green SL Important biological features In: The Laboratory Xenopus sp. Boca Raton, FL: CRC Press; 2009. p. 1–18. [Google Scholar]
- 177. Jafkins A, Abu-Daya A, Noble A, Zimmerman LB, Guille M. Husbandry of Xenopus tropicalis. In: Hoppler S, Vize PD, eds. Xenopus Protocols. Methods in Molecular Biology (Methods and Protocols). 2nd ed Totowa, NJ: Humana Press; 2012:17–31. https://link.springer.com/protocol/10.1007%2F978-1-61779-992-1_2. [DOI] [PubMed] [Google Scholar]
- 178. Chum H, Felt S, Garner J, et al. Biology, behavior, and environmental enrichment for the captive African clawed frog (Xenopus spp). Appl Anim Behav Sci. 2013; 143(2–4):150–6. [Google Scholar]
- 179. Measey J Overland movement in African clawed frogs (Xenopus laevis): a systematic review. PeerJ. 2016; 4:e2474. doi: 10.7717/peerj.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Elepfandt A Sensory perception and the lateral line system in the clawed frog In: Tinsley RC, Kobel HR, eds. The Biology of Xenopus. New York, NY: Oxford University Press; 1996. p. 97–120. [Google Scholar]
- 181. Schultz TW, Dawson DA. Housing and husbandry of Xenopus for oocyte production. Lab Anim (NY). 2003; 32(2):34–9. [DOI] [PubMed] [Google Scholar]
- 182. Claas B, Dean J. Prey-capture in the African clawed toad (Xenopus laevis): comparison of turning to visual and lateral line stimuli. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006; 192(10):1021–36 10.1007/s00359-006-0137-2. [DOI] [PubMed] [Google Scholar]
- 183. Measey GJ Terrestrial prey capture in Xenopus Laevis. Copeia. 1998; 1998(3):787–91. www.jstor.org/stable/1447816. [Google Scholar]
- 184. Shelton PM The structure and function of the lateral line system in larval Xenopus laevis. J. Exp. Zool. 1971; 178:211–31. doi: 10.1002/jez.1401780207. [DOI] [PubMed] [Google Scholar]
- 185. Simmons AM, Costa LM, Gerstein HB. Lateral line-mediated rheotactic behavior in tadpoles of the African clawed frog (Xenopus laevis). J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004; 190(9):747–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Lum M, Wassersug RJ, Potel MJ, et al. Schooling behavior of tadpoles: a potential indicator of ototoxicity. Pharmacol Biochem Behav. 1982; 17(2):363–6. [DOI] [PubMed] [Google Scholar]
- 187. Gliksberg M, Levkowitz G. Smells familiar: pheromone-induced neurotransmitter switching mediates social discrimination. Neuron. 2017; 95(6):1229–31 10.1016/j.neuron.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 188. Wassersug R The biology of Xenopus tadpoles In: Tinsley RC, Kobel HR, eds. The Biology of Xenopus. New York, NY: Oxford University Press; 1996. p. 196–211. [Google Scholar]
- 189. Dulcis D, Lippi G, Stark CJ, Do LH, Berg DK, Spitzer NC. Neurotransmitter switching regulated by miRNAs controls changes in social preference. Neuron . 2017;95(6):1319–1333.e5. 10.1016/j.neuron.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Elepfandt A Underwater acoustics and hearing in the clawed frog, Xenopus In: Tinsley RC, Kobel HR, eds. The Biology of Xenopus. New York, NY: Oxford University Press; 1996. p. 177–93. [Google Scholar]
- 191. Tobias ML, Barnard C, O'Hagan R, et al. Vocal communication between male Xenopus laevis. Anim Behav. 2003; 67(2):353–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Miranda RA, Searcy BT, Propper CR. Arginine vasotocin induces calling behavior with a female social stimulus and interacts with gonadotropins to affect sexual behaviors in male Xenopus tropicalis. Physiol Behav. 2015; 151:72–80. 10.1016/j.physbeh.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 193. Yager DD Sound production and acoustic communication in Xenopus borealis In: Tinsley RC, Kobel HR, eds. The Biology of Xenopus. New York, NY: Oxford University Press; 1996. p. 121–41. [Google Scholar]
- 194. Haubrich R Hierarchical behaviour in the South African clawed frog, Xenopus laevis Daudin. Anim Behav. 1961; 9:71–6. [Google Scholar]
- 195. Hayes MP, Jennings MR, Mellen JD. Beyond mammals. Environmental enrichment for amphibians and reptiles In: Shepherdson DJ, Mellen JD, Hutchins M, eds. Second Nature: Environmental Enrichment for Captive Animals. Washington, D.C.: Smithsonian Institution Press; 1998. p. 205–34. [Google Scholar]
- 196. Green SL Husbandry In: The Laboratory Xenopus sp. Boca Raton, FL: CRC Press; 2009. p. 19–59. [Google Scholar]
- 197. Hilken G, Dimigen J, Iglauer F. Growth of Xenopus laevis under different laboratory rearing conditions. Lab Anim. 1995; 29(2):152–62. [DOI] [PubMed] [Google Scholar]
- 198. Torreilles SL, Green SL. 2007. Refuge cover decreases the incidence of bite wounds in laboratory South African clawed frogs (Xenopus laevis). J Am Assoc Lab Anim Sci. 2007; 46:33–6. [PubMed] [Google Scholar]
- 199. Kobel HR, Loumont C, Tinsley RC. The extant species In: Tinsley RC, Kobel HR, eds. The Biology of Xenopus. New York, NY: Oxford University Press; 1996. p. 9–33. [Google Scholar]
- 200. Green SL Veterinary Care In: The Laboratory Xenopus sp. Boca Raton, FL: CRC Press; 2009. p. 69–123. [Google Scholar]
- 201. Kreil G Skin secretion of Xenopus laevis. In: Tinsley RC, Kobel HR, eds. The Biology of Xenopus. New York. NY: Oxford University Press 1996; 1996:263–77. [Google Scholar]
- 202. Kolbe HV, Huber A, Cordier P, et al. Xenoxins, a family of peptides from dorsal gland secretion of Xenopus laevis related to snake venom cytotoxins and neurotoxins. J Biol Chem. 1993; 22:16458–64. [PubMed] [Google Scholar]
- 203. Zasloff M Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987; 84(15):5449–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Barthalmus GT Neuroleptic modulation of oral dyskinesias induced in snakes by Xenopus skin mucus. Pharmacol Biochem Behav. 1989; 30:957–9. [DOI] [PubMed] [Google Scholar]
- 205. Holmes AM, Emmans CJ, Coleman R, et al. Effects of transportation, transport medium and re-housing on Xenopus laevis (Daudin). Gen Comp Endocrinol. 2018; 266:21–38. [DOI] [PubMed] [Google Scholar]
- 206. Archard GA Effect of enrichment on the behavior and growth of juvenile Xenopus laevis. Appl Anim Behav Sci. 2012; 139:264–70. [Google Scholar]
- 207. Yao M, Westphal NJ, Denver RJ. Distribution and acute stressor-induced activation of corticotrophin-releasing hormone neurones in the central nervous system of Xenopus laevis. J. Neuroendocrinol. 2004; 16:880–93. [DOI] [PubMed] [Google Scholar]
- 208. Falso PG, Noble CA, Diaz JM, et al. The effect of long-term corticosterone treatment on blood cell differentials and function in laboratory and wild-caught amphibian models. Gen Comp Endocrinol. 2015; 212:73–83. [DOI] [PubMed] [Google Scholar]
- 209. Reed BT Guidance on the Housing and Care of the African Clawed Frog Xenopus laevis. London: Royal Society of the Prevention of Cruelty to Animals, Research Animals Department; 2005. [Google Scholar]
- 210. Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999; 284(5420):1670–2. [DOI] [PubMed] [Google Scholar]
- 211. Green SL Experimental Methodology In: The Laboratory Xenopus sp. Boca Raton, FL: CRC Press; 2009. p. 125–44. [Google Scholar]
- 212. Moriya T, Kito K, Miyashita Y, et al. Preference for background color of the Xenopus laevis tadpole. J Exp Zool. 1996; 276:335–44. doi: 10.1002/(SICI)1097-010X(19961201)276:5<335::AID-JEZ4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 213. Holmes AM, Emmans CJ, Jones N, et al. Impact of tank background on the welfare of the African clawed frog, Xenopus laevis (Daudin). Appl Anim Behav Sci. 2016; 185:131–6. [Google Scholar]
- 214. Cooke GM Use of a translucent refuge for Xenopus tropicalis with the aim of improving welfare. Lab Anim. 2018; 52(3):304–7. [DOI] [PubMed] [Google Scholar]
- 215. Mohr C, Görner P. Innervation patterns of the lateral line stitches of the clawed frog, Xenopus laevis, and their reorganization during metamorphosis. Brain Behav Evol. 1996; 48:55–69. [DOI] [PubMed] [Google Scholar]
- 216. Claas B, Münz H, Görner P. Reaction to surface waves by Xenopus laevis Daudin: are sensory systems other than the lateral line involved? J Comp Physiol A. 1993; 172:759–65. [DOI] [PubMed] [Google Scholar]
- 217. Felt SA, Cowan AM, Luong R, et al. Mortality and morbidity in African clawed frogs (Xenopus laevis) associated with construction noise and vibrations. J Am Assoc Lab Anim Sci. 2012; 51(2):253–2456. [PMC free article] [PubMed] [Google Scholar]
- 218. Pough FH Amphibian biology and husbandry. ILAR J. 2007; 48(3):203–13. [DOI] [PubMed] [Google Scholar]
- 219. Brown MJ, Nixon RM. Enrichment for a captive environment: the Xenopus laevis. Anim Tech Welfare. 2004; 3:87–95. [Google Scholar]
- 220. Gouchie GM, Roberts LF, Wassersug RJ. Effects of available cover and feeding schedule on the behavior and growth of the juvenile African clawed frog (Xenopus laevis). Lab Anim (NY). 2008; 37(4):165–9. doi: 10.1038/laban0408-165. [DOI] [PubMed] [Google Scholar]
- 221. van H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000; 1(3):191–8. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 222. Juraska JM, Fitch JM, Henderson C, et al. Sex differences in the dendritic branching of dentate granule cells following differential experience. Brain Res. 1985; 333:73–80. [DOI] [PubMed] [Google Scholar]
- 223. Harr JC, Coyne L, Chaudry A, et al. A study of the impact of environmental enrichment on Xenopus laevis oocytes. Anim. Welf. Inst. Quart. 2008; 57:25 https://awionline.org/awi-quarterly/2008-summer/study-impact-environmental-enrichment-xenopus-laevis-oocytes. Accessed March 23, 2019. [Google Scholar]
- 224. Lee SK, Owens GA, Veeramachaneni DN. Exposure to low concentrations of Di-n-butyl phthalate during embryogenesis reduces survivability and impairs development of Xenopus Laevis frogs. J Toxic Environ Health A. 2005; 68(10):763–72. doi: 10.1080/15287390590930243. [DOI] [PubMed] [Google Scholar]
- 225. Lee SK, Veeramachaneni DN. Subchronic exposure to low concentrations of Di-n-butyl phthalate disrupts spermatogenesis in Xenopus laevis frogs. Toxicol Sci. 2005; 84(2):394–407. 10.1093/toxsci/kfi087. [DOI] [PubMed] [Google Scholar]
- 226. Arndt SS, Surjo D. Methods for the behavioural phenotyping of mouse mutants. How to keep the overview. Behav Brain Res. 2001; 125(1–2):39–42. [DOI] [PubMed] [Google Scholar]
- 227. van der Staay FJ Animal models of behavioral dysfunctions: basic concepts and classifications, and an evaluation strategy. Brain Res. Rev. 2006; 52:131–59. [DOI] [PubMed] [Google Scholar]
- 228. Hendrick C. Replication, strict replications, and conceptual replications: are they important? In: Neuliep JW, ed. Handbook of replication research in the behavioral and social sciences . [Special Issue.] Journal of Social Behavior and Personality 1990;5(4): 41–49. [Google Scholar]
- 229. Sison M, Gerlai R. Associative learning performance is impaired in zebrafish (Danio rerio) by the NMDA-R antagonist MK-801. Neurobiol Learn Mem. 2011; 96:230–7 10.1016/j.nlm.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Smith AJ, Clutton RE, Lilley E, et al. PREPARE: Guidelines for planning animal research and testing. Lab Anim. 2017; 52(2):135–41. doi: 10.1177/0023677217724823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. National Research Council (US) Institute for Laboratory Animal Research (ILAR) The Research Animal Environment (Study Conditions) In: Guidance for the Description of Animal Research in Scientific Publications. Washington, DC: National Academies Press; 2011. Available from: https://www.ncbi.nlm.nih.gov/books/NBK84203/. [PubMed] [Google Scholar]
- 232. Kilkenny C, Browne W, Cuthill IC, et al. NC3Rs reporting guidelines working group. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010; 160(7):1577–9. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2019: updated guidelines for reporting animal research. bioRxiv 2019:703181. doi: 10.1101/703181. [DOI] [Google Scholar]


