Abstract
Background:
There is high risk of transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in congregate settings, including shelters. This study describes a coronavirus disease 2019 (COVID-19) outbreak and corresponding reported symptomatology at a shelter in Toronto.
Methods:
This clinical and epidemiologic analysis focuses on a COVID-19 outbreak at a dedicated refugee shelter in downtown Toronto. All adult residents on site at the shelter were offered SARS-CoV-2 testing on Apr. 20, 2020. At the time of testing, residents were screened for 3 typical COVID-19 symptoms (fever, cough and shortness of breath). Among those who tested positive, a more comprehensive clinical assessment was conducted 1 day after testing and a standardized 15-item symptom screen was administered by telephone 14 days after testing. We report rates of positive test results and clinical symptoms with each assessment interval.
Results:
Of the 63 adult residents on site at the shelter, 60 agreed to be tested. Among those tested, 41.7% (n = 25) were positive for SARS-CoV-2 infection. Of those who tested positive (n = 25), 20.0% (n = 5) reported fever, cough or shortness of breath at the time of testing. On more detailed assessment 1 day later, 70.8% (17/24) reported a broader range of symptoms. During the 14 days after testing, 87.5% (21/24) reported symptoms of infection.
Interpretation:
We found a high rate of SARS-CoV-2 infection in this shelter population. Our study underscores the high risk of SARS-CoV-2 transmission in congregate living settings and the importance of mobilizing timely testing and management of symptomatic, paucisymptomatic and asymptomatic residents in shelters.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can spread rapidly within congregate living settings.1–4 Homeless shelters, similar to long-term care facilities and other congregate settings, are densely populated environments, posing difficulties for physical distancing and intensifying risks for infectious disease outbreaks. Toronto, like numerous other cities, has experienced coronavirus disease 2019 (COVID-19) outbreaks in homeless shelters.5
About 235 000 individuals experience homelessness in Canada annually, though the actual number is likely much higher.6 According to the nationally coordinated “Point-in-Time” count of homelessness in Canadian communities in 2018, 14% of homeless individuals identified as coming to Canada at some point in their life as immigrants, refugees or refugee claimants.7 In Toronto, a Street Needs Assessment point-in-time count estimated that 8715 people were experiencing absolute homelessness on Apr. 26, 2018, although this is acknowledged as an underestimate, as it does not reflect a more comprehensive definition of homelessness.8 Among those surveyed, 52% reported coming to Canada as immigrants, refugees or refugee claimants.8 Refugee claimants, elsewhere known as asylum seekers, arrive to Canada seeking safety from persecution. In 2019, 58 378 claimants sought refuge in Canada.9 Most refugee claimants spend several months in shelters while they seek independent housing.
We report the clinical and epidemiologic features of a COVID-19 outbreak at one of the largest refugee shelters in Toronto. The outbreak occurred during the height of the first wave of COVID-19 in Toronto. At the time of testing, on Apr. 20, 2020, there were 230 new COVID-19 cases reported in Toronto and a total of 5753 cumulative cases (including 693 deaths), according to Toronto Public Health.10
Methods
Setting
We describe the clinical features and epidemiology of a COVID-19 outbreak that occurred at a downtown Toronto shelter in April 2020. At the time of the study, an outbreak within a congregate living setting was defined by the Ontario Ministry of Health and Long-term Care as 1 or more laboratory-confirmed cases in a resident or staff member.11 The affected shelter provides temporary housing exclusively to refugee claimants and refugees (primarily the former) aged 16 years and older. The average length of stay at the emergency shelter is about 3 months; however, shelter stays have been longer during the COVID-19 pandemic. The shelter can accommodate about 90 people across 2 of its linked emergency shelter sites. Residents live in shared rooms with 2 to 6 people, which include shared bathrooms, and eat prepared meals in a shared dining room.
The Crossroads Clinic at the Women’s College Hospital (WCH) has a long-standing partnership with this shelter, including operating an on-site shelter-based primary care clinic. Refugee claimants receive health care coverage through the Interim Federal Health Program, which provides comprehensive coverage for basic medical services, including physician consultations, laboratory tests, diagnostic imaging and hospital services, similar to Canadian public provincial health insurance, and supplementary coverage for medications and other services and devices, similar to coverage through provincial social assistance programs.12 Of note, in March 2020, the Ontario government expanded health care coverage to all individuals during the COVID-19 pandemic, regardless of health insurance or immigration status.13
In response to a COVID-19 outbreak at this refugee shelter, the Crossroads Clinic and WCH’s mobile COVID-19 testing team were invited to support on-site SARS-CoV-2 testing and posttesting management for shelter residents.
Participants
Adults aged 18 years and older residing at the shelter were eligible for SARS-CoV-2 testing with a nasopharyngeal swab. Residents who were off site in isolation facilities at the time of testing were excluded from the study.
Outbreak clinical response and outcome measures
On Apr. 20, 2020, nasopharyngeal swabs were performed for participating shelter residents by 3 WCH physicians (including V.R.) and sent for SARS-CoV-2 polymerase chain reaction (PCR) testing. At the time of SARS-CoV-2 testing, the clinicians screened each participant for the presence of 3 typical COVID-19 symptoms: fever, cough and shortness of breath. This limited symptom screen was based on what were considered classic symptoms of COVID-19 at the time and consistent with symptom screening used by many shelters and other health facilities at the time of the study.
On Apr. 21, 2020, members of the clinical team (V.R. and V.W.) conducted a more detailed clinical assessment for those individuals who had tested positive for SARS-CoV-2, including measurement of vital signs and open-ended questioning regarding COVID-19 symptoms and date of symptom onset, as well as medical history, current medications, allergies, substance use history and required behavioural supports. Responses were recorded on referral forms for the isolation facility, and forms were subsequently scanned to patient electronic medical record (EMR) charts. Individuals who had tested positive were transferred to a COVID-19 isolation facility with on-site medical support for 14-day isolation. Two participants who had tested positive were not available on site for clinical assessment on Apr. 21, 2020; 1 was contacted at a later date for symptom review (reflecting Apr. 21 clinical status) and 1 individual could not be reached.
Fourteen days after testing, individuals who had tested positive were telephoned by members of the clinical team (V.R. and M.R.) for reassessment, including a standardized screen for the presence of 15 symptoms (fever, cough, shortness of breath, chills, myalgias, headache, sore throat, new loss of smell, new loss of taste, new nasal congestion, diarrhea, malaise, dizziness, nausea and/or vomiting, and chest pain and/or tightness) at any time during the preceding 2 weeks and on day 14 posttesting. The 15-item symptom screen was based on a list of COVID-19-related symptoms from the Centers for Disease Control and Prevention,14 as well as other symptoms emerging in the literature at the time of assessment.4 Responses were documented in patients’ EMR charts using a standardized template (Appendix 1, available at www.cmajopen.ca/content/8/4/E819/suppl/DC1). One individual could not be reached for reassessment.
Clinical encounters were conducted with professional medical interpreters via telephone when needed (i.e., for any patient who could not communicate in English or who requested an interpreter).
Data sources and extraction
Data were manually extracted from the paper-based record of shelter residents who underwent testing and from review of EMR charts of patients who had tested positive for SARS-CoV-2 infection. The initial symptom screen (fever, cough and shortness of breath) and SARS-CoV-2 test results were derived from paper records. Data regarding symptoms day 1 posttesting were extracted from free text documented on the clinical assessment forms (scanned into patient EMR charts); symptoms were categorized according to the 15-item symptom list. Data from the day 14 posttesting clinical reassessment were derived from a standardized 15-item symptom checklist template in the patients’ EMR charts (Appendix 1). Selected variables included basic demographic factors (age and sex), SARS-CoV-2 PCR test results, presence of 3 symptoms at the time of testing (fever, cough and shortness of breath), and symptoms 1 day after testing and on reassessment 14 days posttesting (15-item symptom list). Data were entered into an Excel spreadsheet by V.R. and a research assistant.
Statistical analysis
Descriptive statistics, including counts, proportions, means, and standard deviations, were calculated using Microsoft Excel software.
Ethics approval
This study was approved by the WCH Research and Ethics Board, with a waiver of informed consent.
Results
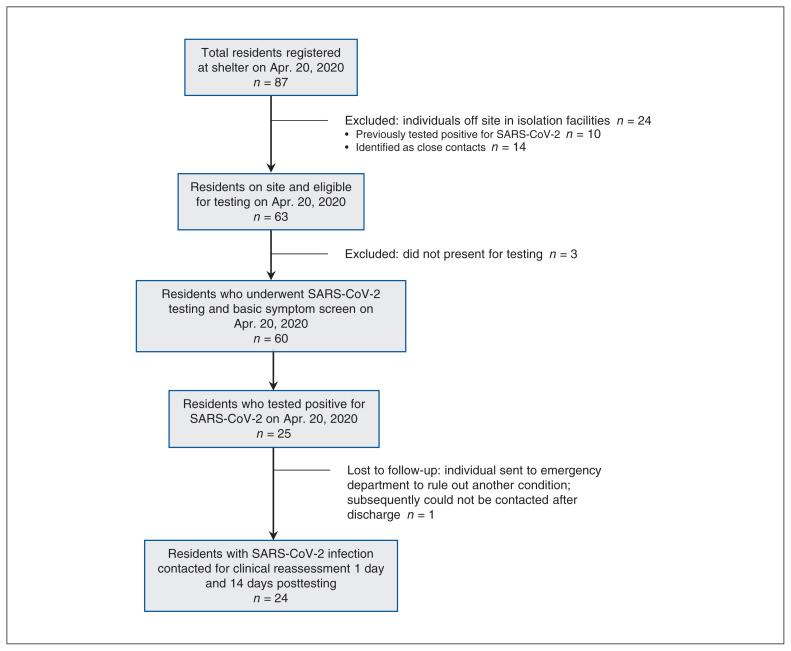
Twenty-four of the total 87 shelter residents were off site in isolation facilities at the time of testing and were excluded from this study, including 10 individuals previously diagnosed with COVID-19 and 14 individuals identified as close contacts. All residents at the time of the outbreak were refugee claimants and aged 18 or older. No shelter residents had arrived in Canada in the 14 days before testing. Of the 63 residents on site at the shelter, 60 underwent SARS-CoV-2 testing and basic symptom screening on Apr. 20, 2020 (Figure 1). The mean age of participants was 36.0 years, and 80.0% (n = 48) were male (Table 1). A total of 25 individuals (41.7%) tested positive for SARS-CoV-2 infection. At the time of testing, 6 individuals screened positive for fever, cough or shortness of breath that same day. Five of the 6 individuals who reported symptoms were found to have positive SARS-CoV-2 tests.
Figure 1:
Flow chart showing study participants included in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing, and participants who tested positive for SARS-CoV-2 infection and were followed for clinical reassessment posttesting (day 1 and day 14).
Table 1:
Characteristics and initial symptoms of shelter residents who underwent SARS-CoV-2 testing on Apr. 20, 2020
| Characteristic | No. (%) of residents | ||
|---|---|---|---|
| Total n = 60 |
Positive SARS-CoV-2 test n = 25 |
Negative SARS-CoV-2 test n = 35 |
|
| Age, mean ± SD, yr | 36.0 ± 10.0 | 38.7 ± 11.0 | 34.1 ± 8.8 |
| Sex | |||
| Male | 48 (80.0) | 22 (88.0) | 26 (74.3) |
| Female | 12 (20.0) | 3 (12.0) | 9 (25.7) |
| Symptoms at time of testing | |||
| Fever | 3 (5.0) | 2 (8.0) | 1 (2.9) |
| Cough | 3 (5.0) | 3 (12.0) | 0 |
| Shortness of breath | 0 | 0 | 0 |
Note: SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, SD = standard deviation.
On Apr. 21, 2020, on more detailed clinical assessment of those who tested positive and could be contacted, 17 of 24 (70.8%) reported at least 1 symptom consistent with SARS-CoV-2 infection. Headache (33.3%, n = 8) and fever (29.2%, n = 7) were the most commonly reported symptoms (Table 2).
Table 2:
Clinical symptoms of shelter residents who tested positive for SARS-CoV-2 on Apr. 20, 2020 (n = 24)*
| Clinical symptom | No. (%) of residents | ||
|---|---|---|---|
| On day 1 posttesting | Any time during 14 days posttesting | On day 14 posttesting | |
| Asymptomatic | 7 (29.2) | 3 (12.5) | 15 (62.5) |
| Any symptoms | 17 (70.8) | 21 (87.5) | 9 (37.5) |
| Fever | 7 (29.2) | 8 (33.3) | 0 |
| Cough | 6 (25.0) | 5 (20.8) | 4 (16.7) |
| Shortness of breath | 0 | 1 (4.2) | 0 |
| Chills | 2 (8.3) | 3 (12.5) | 0 |
| Myalgias | 6 (25.0) | 6 (25.0) | 2 (8.3) |
| Headache | 8 (33.3) | 14 (58.3) | 0 |
| Sore throat | 6 (25.0) | 6 (25.0) | 0 |
| New loss of taste | 1 (4.2) | 10 (41.7) | 3 (12.5) |
| New loss of smell | 1 (4.2) | 7 (29.2) | 1 (4.2) |
| New nasal congestion | 1 (4.2) | 6 (25.0) | 1 (4.2) |
| Diarrhea | 1 (4.2) | 3 (12.5) | 0 |
| Malaise | 3 (12.5) | 5 (20.8) | 1 (4.2) |
| Dizziness | 1 (4.2) | 4 (16.7) | 1 (4.2) |
| Nausea and/or vomiting | 0 | 2 (8.3) | 1 (4.2) |
| Chest pain/tightness | 1 (4.2) | 4 (16.7) | 0 |
| Other | 3 (12.5) | 7 (29.2) | 2 (8.3) |
Note: SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
One individual of the 25 who tested positive could not be contacted after initial testing.
During the 2 weeks after testing, 21 of 24 (87.5%) individuals reported experiencing at least 1 symptom consistent with SARS-CoV-2 infection. The most commonly reported symptoms were headache (58.3%, n = 14), loss of taste (41.7%, n = 10) and fever (33.3%, n = 8). On day 14 posttesting, 9 (37.5%) reported the presence of at least 1 symptom: cough (16.7%, n = 4) and loss of taste (12.5%, n = 3) were most common. We could not reach 1 individual until day 15 posttesting, but responses reflected symptoms over the preceding 14 days.
One patient with documented fever and headache was sent to the emergency department at the time of testing to rule out another possible cause for their symptoms. The test for SARS-CoV-2 subsequently returned positive and the patient was discharged from the emergency department, but we were unable to reach the patient for reassessment on day 1 and day 14 posttesting. A single hospital admission was related to isolation requirements rather than clinical severity; the patient was subsequently discharged to a COVID-19 isolation facility. There were no cases of intensive care unit admission, intubation or death. All residents who tested positive for SARS-CoV-2 infection recovered after 14 days of isolation in the COVID isolation facility with low acuity symptomatic management.
Interpretation
We found a high incidence of SARS-CoV-2 infection (41.7%, 25/60) with universal testing of on-site shelter residents. The overall estimated positivity rate among all 87 residents is likely higher, accounting for individuals who tested positive before Apr. 20, 2020, and thereafter.5 Although a minority (20%, 5/25) of individuals with SARS-CoV-2 infection reported a narrow range of symptoms at the time of testing, most (87.5%, 21/24) developed mild symptoms during the subsequent 14 days.
These findings are consistent with high infection rates found in other shelter settings where testing followed identification of a cluster of COVID-19 cases: 36% of shelter residents tested positive for SARS-CoV-2 in a shelter in Boston, 66% in a shelter in San Francisco and 17% across 3 shelters in Seattle.1,2 Common public health interventions to contain SARS-CoV-2 spread, such as physical distancing, hand hygiene, timely testing, contact tracing, isolation and use of personal protective equipment, may be challenging to implement among many people experiencing homelessness, given physical constraints of shelter settings, limited access to supplies, and concurrent mental and physical health conditions.15 Proactive measures, including increased physical spacing between shelter beds, decreased density within each shelter through opening of additional accommodation facilities, isolation centres for homeless individuals testing positive for SARS-CoV-2, and on-site assessment and testing with rapid turnaround of test results, have been shown to help mitigate the risk of COVID-19 outbreaks in other shelter settings.16 Collaboration among public health units, local government, shelter operators and health providers is key.16
Few individuals with SARS-CoV-2 infection in our study filled the criteria for prior, narrowly defined COVID-19 symptoms (fever, cough and shortness of breath) at the time of testing. This is consistent with findings from a shelter outbreak in Boston, in which a minority of shelter residents with SARS-CoV-2 infection had fever (0.7%), cough (7.5%) or shortness of breath (1.4%) on testing.1 However, most individuals with SARS-CoV-2 infection in our study (87.5%) subsequently developed a range of symptoms consistent with infection during the 14-day period after testing, which were identified on more detailed clinical assessment. Similarly, a study of a COVID-19 outbreak at a call centre in South Korea showed that only 4.1% of 97 infected individuals remained completely asymptomatic during a 14-day posttesting period,17 which is much lower than the previously estimated asymptomatic ratio of 30.8% based on earlier modelling. 18 Another study in a long-term care skilled nursing facility in Seattle found that, among 13 residents who tested positive for SARS-CoV-2 and were asymptomatic at the time of testing, 10 went on to develop symptoms 1 week after testing. 19 These findings underscore the value of enhanced testing strategies for SARS-CoV-2 infection in high-risk settings, such as shelters, particularly in light of mounting evidence of paucisymptomatic and presymptomatic spread.4,19–21 Our study also emphasizes the importance of evaluating for diverse symptoms suggestive of infection.
Most shelter residents in our study had mild to moderate clinical courses, which may reflect the relatively young age of this sample and low prevalence of pre-existing comorbidities. In the general homeless population, many individuals are older and have underlying health conditions, increasing their risk of severe COVID-19-related complications.15,22,23
Our findings also highlight the importance of ensuring timely access to testing and medical treatment for all, regardless of immigration or health insurance status. This is particularly pertinent as we witness SARS-CoV-2 outbreaks in other forms of congregate settings, including among migrant farm workers who are often working and living in crowded conditions and have precarious employment, immigration and health insurance status.24–26
Limitations
This study describes SARS-CoV-2 test results and individuals’ clinical courses at a single homeless shelter. Not all residents were present at the time of SARS-CoV-2 testing, and subsequent test results completed after Apr. 20, 2020, were not available to the research team. Rates of SARS-CoV-2 infection and clinical courses may be highly variable across different shelter settings and homeless populations.
Although we conducted limited symptom screening on day 1, comprehensive clinical assessment on day 2 and retrospective systematic symptom evaluation on day 14, daily comprehensive symptom screening may have provided a more accurate depiction of symptom emergence and evolution during the study period, and the day-14 symptom screen is prone to recall bias. In particular, we may have identified more individuals with a diverse range of symptoms at the time of testing if they were screened using the expanded standardized symptom checklist (such as the 15-item symptom screen used on day 14). However, the nonspecific nature of some of the symptoms (e.g., malaise) may have limited clinical utility as a symptom screen.
Conclusion
We found a high incidence of SARS-CoV-2 infection with universal testing of on-site shelter residents. Our study underscores the high risk of SARS-CoV-2 transmission in congregate living settings. We also found that most individuals with SARS-CoV-2 infection developed compatible symptoms of COVID-19, although most had mild symptoms. Our findings highlight the importance of mobilizing timely testing and management of all residents of shelters where infection is present, including symptomatic, paucisymptomatic and asymptomatic residents, to account for the potential of presymptomatic transmission. Tailored strategies are critical to respond to the unique needs of homeless and refugee populations to decrease risks of transmission and manage cases of infection in congregate settings. Alongside these interventions for COVID-19 mitigation, there is a pressing need for upstream action to address the root causes of homelessness.
Supplementary Material
Acknowledgements
The authors are grateful to shelter management and staff for their leadership and dedication. They also thank Shivani Chandra for assistance with data entry.
Footnotes
Competing interests: Vanessa Redditt is on the volunteer community board of directors of the shelter described in this study. Isaac Bogoch has consulted to BlueDot, a social benefit company that tracks emerging infectious diseases.
This article has been peer reviewed.
Contributors: All authors were involved in the conception of the study and study design. Vanessa Redditt collected, analyzed and interpreted the data, and drafted the manuscript. All of the authors contributed to data interpretation, revised the manuscript critically for important intellectual content, approved the final version to be published, and agreed to be accountable for all aspects of the work.
Funding: No funding was received for this study. Isaac Bogoch is supported by a grant from the Canadian Institutes of Health Research (02179-000).
Data sharing: A deidentified data set from the study may be made available to other researchers who provide a detailed study proposal clearly describing the use of the data and that is approved by an independent review committee. Researchers who wish to access the study data may contact the corresponding author, Vanessa Redditt, at vanessa.redditt@wchospital.ca.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/8/4/E819/suppl/DC1.
References
- 1.Baggett TP, Keyes H, Sporn N, et al. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA. 2020;323:2191–2. doi: 10.1001/jama.2020.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosites E, Parker EM, Clarke KEN, et al. COVID-19 Homelessness Team. Assessment of SARS-CoV-2 infection prevalence in homeless shelters: four U.S. cities, March 27–April 15, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:521–2. doi: 10.15585/mmwr.mm6917e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tobolowsky FA, Gonzales E, Self JL, et al. COVID-19 outbreak among three affiliated homeless service sites: King County, Washington, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:523–6. doi: 10.15585/mmwr.mm6917e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arons MM, Hatfield KM, Reddy SC, et al. Public Health–Seattle; King County and CDC COVID-19 Investigation Team. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–90. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVID-19 — status of cases in Toronto. Toronto Public Health; [accessed 2020 May 6]. Active COVID-19 outbreaks in Toronto in shelters and respite centres. Available: www.toronto.ca/home/covid-19/covid-19-latest-city-of-toronto-news/covid-19-status-of-cases-in-toronto. [Google Scholar]
- 6.Gaetz S, Dej E, Richter T, et al. The state of homelessness in Canada 2016. Toronto: The Canadian Observatory on Homelessness/Homeless Hub; 2019. [accessed 2020 June 27]. Available: www.homelesshub.ca/SOHC2016. [Google Scholar]
- 7.Everyone counts 2018: highlights — report. Ottawa: Employment and Social Development Canada; [accessed 2020 June 27]. Newcomers to Canada. modified 2020 Aug 31 Available: www.canada.ca/en/employment-social-development/programs/homelessness/reports/highlights-2018-point-in-time-count.html#3.5. [Google Scholar]
- 8.Street needs assessment: 2018 — results report. Toronto: City of Toronto; 2018. [accessed 2020 Aug. 31]. Available: www.toronto.ca/wp-content/uploads/2018/11/99be-2018-SNA-Results-Report.pdf. [Google Scholar]
- 9.Refugee protection claims (new system) statistics. Ottawa: Immigration and Refugee Board of Canada; [accessed 2020 June 27]. modified 2020 Sept. 9. Available: https://irb-cisr.gc.ca/en/statistics/protection/Pages/RPDStat.aspx. [Google Scholar]
- 10.Toronto Public Health. COVID-19: status of cases in Toronto. Toronto: City of Toronto; [accessed 2020 Aug. 31]. Available: www.toronto.ca/home/covid-19/covid-19-latest-city-of-toronto-news/covid-19-status-of-cases-in-toronto. [Google Scholar]
- 11.COVID-19 guidance: congregate living for vulnerable populations. Version 1 — May 28, 2020. Ontario Ministry of Health; [accessed 2020 Nov. 18]. Available: http://health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/2019_congregate_living_guidance.pdf. [Google Scholar]
- 12.Interim Federal Health Program: summary of coverage. Ottawa: Immigration, Refugees and Citizenship Canada; [accessed 2020 June 27]. modified 2019 Dec 2. Available: www.canada.ca/en/immigration-refugees-citizenship/services/refugees/help-within-canada/health-care/interim-federal-health-program/coverage-summary.html. [Google Scholar]
- 13.INFOBulletin: Keeping health care providers informed of payment, policy or program changes, Bulletin No. 4749. Toronto: Ministries of Health and Long-Term Care; 2020. [accessed 2020 June 27]. Available: www.health.gov.on.ca/en/pro/programs/ohip/bulletins/4000/bul4749.aspx. [Google Scholar]
- 14.Symptoms of coronavirus. Atlanta: Centers for Disease Control and Prevention; [accessed 2020 Apr. 27]. updated 2020 May 13. Available: www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. [Google Scholar]
- 15.Tsai J, Wilson M. COVID-19: a potential public health problem for homeless populations. Lancet Public Health. 2020;5:e186–7. doi: 10.1016/S2468-2667(20)30053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodkin C, Mokashi V, Beal K, et al. Pandemic planning in homeless shelters: a pilot study of a COVID-19 testing and support program to mitigate the risk of COVID-19 outbreaks in congregate settings. Clin Infect Dis. 2020 Jun 8; doi: 10.1093/cid/ciaa743. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SY, Kim Y-M, Yi S, et al. Coronavirus disease outbreak in call center, South Korea. Emerg Infect Dis. 2020;26:1666–70. doi: 10.3201/eid2608.201274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishiura H, Kobayashi T, Miyama T, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int J Infect Dis. 2020;94:154–5. doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimball A, Hatfield KM, Arons M, et al. CDC COVID-19 Investigation Team. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility: King County, Washington, March 2020. MMRW Morb Mortal Wkly Rep. 2020;69:377–81. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huff HV, Singh A. Asymptomatic transmission during the COVID-19 pandemic and implications for public health strategies. Clin Infect Dis. 2020 May 28; doi: 10.1093/cid/ciaa654. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furukawa NW, Brooks JT, Sobel J. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg Infect Dis. 2020;26:e201595. doi: 10.3201/eid2607.201595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fazel S, Geddes JR, Kushel M. The health of homeless people in high-income countries: descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet. 2014;384:1529–40. doi: 10.1016/S0140-6736(14)61132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aldridge RW, Story A, Hwang SW, et al. Morbidity and mortality in homeless individuals, prisoners, sex workers, and individuals with substance use disorders in high-income countries: a systematic review and meta-analysis. Lancet. 2018;391:241–50. doi: 10.1016/S0140-6736(17)31869-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Temporary foreign worker dies due to COVID-19 as disease hits southwestern Ontario farms hard. CBC News Windsor. 2020. Jun 1, [accessed 2020 June 27]. Available: www.cbc.ca/news/canada/windsor/southwestern-ontario-farms-covid19-migrant-worker-dies-1.5593046.
- 25.Assessment centre for migrant workers opens in Leamington, following 2 COVID-19 deaths. [accessed 2020 June 27];CBC News Windsor. 2020 Jun 9; Available: www.cbc.ca/news/canada/windsor/assessment-centre-migrant-workers-covid19-windsor-essex-1.5603400. [Google Scholar]
- 26.Rodriguez S. Third Ontario migrant worker dies of COVID-19. CBC News London. 2020. Jun 21, [accessed 2020 June 27]. Available: www.cbc.ca/news/canada/london/third-ontario-migrant-worker-dies-of-covid-19-15621487.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.