Abstract
Chronic rhinosinusitis (CRS) is a chronic disease that involves long-term inflammation of the nasal cavity and paranasal sinuses. Bacterial biofilms present on the sinus mucosa of certain patients reportedly exhibit resistance against traditional antibiotics, as evidenced by relapse, resulting in severe disease. The aim of this study was to determine the killing activity of human cathelicidin antimicrobial peptides (LL-37, LL-31) and their D-enantiomers (D-LL-37, D-LL-31), alone and in combination with conventional antibiotics (amoxicillin; AMX and tobramycin; TOB), against bacteria grown as biofilm, and to investigate the biological activities of the peptides on human lung epithelial cells. D-LL-31 was the most effective peptide against bacteria under biofilm-stimulating conditions based on IC50 values. The synergistic effect of D-LL-31 with AMX and TOB decreased the IC50 values of antibiotics by 16-fold and could eliminate the biofilm matrix in all tested bacterial strains. D-LL-31 did not cause cytotoxic effects in A549 cells at 25 μM after 24 h of incubation. Moreover, a cytokine array indicated that there was no significant induction of the cytokines involving in immunopathogenesis of CRS in the presence of D-LL-31. However, a tissue-remodeling-associated protein was observed that may prevent the progression of nasal polyposis in CRS patients. Therefore, a combination of D-LL-31 with AMX or TOB may improve the efficacy of currently used antibiotics to kill biofilm-embedded bacteria and eliminate the biofilm matrix. This combination might be clinically applicable for treatment of patients with biofilm-associated CRS.
Introduction
Chronic rhinosinusitis (CRS) is defined as chronic infection and inflammation of the upper airways involving the nasal cavity and paranasal sinuses with a duration of at least 12 consecutive weeks [1]. This disease significantly impacts the quality of life of patients and imposes a socio-economic burden on the community [2]. CRS is now documented as an extremely widespread inflammatory disease with an estimated prevalence of 12% across the world [3]. CRS has been subdivided into 2 major categories: CRSwNP (chronic rhinosinusitis with nasal polyps present) and CRSsNP (chronic rhinosinusitis without nasal polyps) [1, 3].
The U.S. National Institutes of Health estimated that at least 65% of all microbial infections are associated with biofilms [4]. Recently, many bacterial biofilm-associated CRS cases have been reported [5, 6]. Of these, 80% were found to have micrographic indication of biofilms on the nasal mucosa after undergoing sinus surgery [7]. Consequently, the management of biofilm-associated infections is problematic as they are difficult to prevent, diagnose, and treat [8].
A dramatically reduced susceptibility to conventional antibiotics (up to 1000 times) and to host immune responses is typical of biofilm-associated infections [8, 9]. Biofilm-embedded bacteria in CRS patients exhibit resistance to various groups of antibiotics such as tobramycin (TOB), moxifloxacin and also beta-lactam antibiotics; cephalosporins, ampicillin and amoxicillin (AMX) [10, 11]. Biofilm is increasingly implicated in relapse, persistence and severity of certain CRS cases and in recalcitrant infections [12, 13]. To treat biofilm-based antibiotic resistance, there is an increasing need to design novel therapeutic agents and/or discover alternative agents that improve the bactericidal activity of the currently used antibiotics.
Antimicrobial peptides (AMPs) are host-defense peptides with a broad spectrum of biological activities against a wide range of microorganisms (e.g. bacteria, fungi and viruses) [14–16], which are regarded as promising additions to antibiofilm strategies. Human cathelicidin-derived peptide LL-37 is a 37-amino-acid cationic peptide generated by extracellular cleavage of the C-terminal end of the hCAP18 protein (18 kDa) by serine proteases [17]. This peptide exhibits strong antibiofilm capacity against various pathogens such as Candida albicans, Staphylococcus aureus, and Escherichia coli [18]. Also, a truncated form of LL-37, produced by organic chemical synthesis without the six C-terminus residues (LL-31), has shown efficacy against biofilm-forming Burkholderia pseudomallei [19]. The substitution of L- by D-amino acids in these peptides improves their stability, enhances their antimicrobial activity and reduces toxicity [20]. The latest research has also found that D-LL-31 exhibits high potency against B. pseudomallei biofilm in static and flow-cell systems without causing toxicity to human red blood cells (hRBCs) [15].
A patient with CRS generally exhibits an inflammatory immune response [1, 3]. Several studies have shown that elevated levels of pro-inflammatory cytokines, interleukin-4 (IL-4), IL-6 and interferon-gamma (IFN-γ) result in decreased specialized tight junctions, which could contribute to the defective function of a physical barrier in the mucosal tissue [21, 22]. In addition, recruitment of B cells by tumor necrosis factor alpha (TNF-α) has also been thought to play a role in the pathogenesis of CRS [23]. The pro-inflammatory milieu might function to impair the mucosal barrier and promote chronic inflammation by allowing microbes, antigens, and allergens across the mucosal tissue, where they can trigger or promote immune responses [21, 22].
The chronic inflammation is typical of CRS [1, 3]. An imbalance between the matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) was found to be involved in pathological tissue remodeling in CRS patients [24]. The elevated expression of MMP-2, -7 and -9 [24–26] and significant decrease of TIMP-1 and -2 [25, 27] have been frequently found in CRS patients. This imbalance results in excessive degradation of airway extracellular matrix (ECM), pseudocyst formation, albumin deposition and edema in patients with CRS [28]. Thus, the immunomodulatory effect of any new therapeutic agent should be investigated before clinical use.
In this study, the bactericidal effects of LL-37, LL-31 and their D-enantiomeric forms (D-LL-37 and D-LL31) against bacteria isolated from CRS patients grown as biofilm were investigated alone and in combination with conventional antibiotics. We found that D-LL-31 has a strong killing effect against all tested bacteria under biofilm-stimulating conditions. It also enhanced antimicrobial activity of the commonly used antibiotics to combat biofilm-embedded cells and to eradicate the biofilm matrix. Additionally, D-LL-31 does not cause the induction of the cytokines involved in immunopathogenesis of CRS on human lung epithelial cells.
Materials and methods
Peptide synthesis
The human cathelicidin peptides LL-37, LL-31 and D-enantiomers (D-LL-37 and D-LL-31) (Table 1) were synthesized by solid-phase peptide synthesis using fluoren-9-ylmethoxycarbonyl (Fmoc) chemistry with a Siro II synthesizer (Biotage, Uppsala Sweden) according to the manufacturer’s protocol. Labeling of D-LL-31 with 5,6-carboxytetramethylrhodamine (TAMRA) was carried out in-synthesis using an additional Fmoc-Ahx-OH (NovaBiochem) at the N-terminus. Peptides were purified to at least 95% purity by preparative reversed-phase HPLC on a Dionex Ultimate 3000 system (Thermo Scientific, Breda, the Netherlands). The authenticity was confirmed by mass spectrometry with a Microflex LRF MALDI-TOF (Bruker Daltonik GmbH, Bremen, Germany) essentially as described previously [29, 30]. Molar concentrations were calculated based on their weight.
Table 1. Sequences and characteristics of the peptides investigated.
| Peptidesa | Sequences | Mol. wt. | Chargeb |
|---|---|---|---|
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | 4494 | 6+ |
| D-LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | 4494 | 6+ |
| LL-31 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNL | 3824 | 6+ |
| D-LL-31 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNL | 3824 | 6+ |
aThe purity of peptides was at least 95% and the authenticity of the peptides was confirmed by MALDI-TOF mass spectrometry.
bCalculated net charge at neutral pH.
Ethics statement
All patients provided signed consent in accordance with the ethical principles relating to biomedical research involving human subjects as adopted by the 18th World Medical Association General Assembly, Helsinki (1964) and the ICH Good Clinical Practice Guidelines. This study was approved by the Center for Ethics in Human Research, Khon Kaen University, reference number: HE561075.
Sample collection and bacterial identification
Washings from sinus irrigation and sinus mucosa samples were collected from patients with chronic rhinosinusitis, defined according to the criteria of the 2003 Chronic Rhinosinusitis Task Force [31]. These patients were potential candidates for endoscopic sinus surgery in the otolaryngology clinic, Srinagarind Hospital, Khon Kaen University, Thailand.
The sinus lavage was cultured for aerobic and facultative anaerobic bacteria in Luria-Bertani broth (Himedia®, Mumbai, India) and Thioglycollate broth (Himedia®). Then, the bacteria were grown on 5% sheep-blood agar, chocolate agar or MacConkey agar (MAC) (Himedia®). Cultures on MAC and 5% sheep-blood agar were incubated at 37˚C for 24 h, whereas chocolate agar was kept under 5% CO2 at 37˚C and results observed after 24, 48 and 72 h. Afterward, a single colony of each sample was identified to genus and species according to standard bacteriological methods.
Biofilm observation on sinus mucosal tissues of CRS patients
Biofilm architecture on sinus mucosal tissues of CRS patients was observed using scanning electron microscopy (SEM). The tissue samples were taken from diseased maxillary or ethmoid sinuses during functional endoscopic sinus surgery. Tissues were fixed by 4% paraformaldehyde/1.25% glutaraldehyde in PBS with 4% sucrose, pH 7.2, and stored at 4°C. After three PBS washes, samples were post-fixed with 2% osmium tetroxide (OsO4) on a rotator for 1 h. The dehydration of the tissue samples was subsequently performed using a graded series of 70% to 100% ethanol concentrations. Samples were critical-point dried in CO2, mounted on scanning electron microscopy stubs, sputter coated with gold palladium (K500X sputter coater, EMITECH, Quorum technologies LTD, UK), and examined using a field emission gun scanning electron microscope (S-3000N, HITACHI, Japan).
Bacterial strains and growth conditions
Three clinical bacterial isolates, Klebsiella pneumoniae, Pseudomonas aeruginosa and Staphylococcus epidermidis, were selected from patients no. 1, 3 and 5, respectively (Table 2) according to the predominant bacterial species that commonly found in clinical studies on CRS [32–34] and displayed a biofilm-positive phenotype on the surface tissue from CRS patients in this study. P. aeruginosa ATCC 27853 was also included in this study as reference strain. Antimicrobial susceptibility test results of the bacterial isolates are shown in S1 Table. K. pneumoniae and P. aeruginosa were regularly cultured on MAC (Himedia®) while S. epidermidis was grown on Nutrient agar (NA) (Himedia®). Plates were incubated at 37˚C for 24 h. A single colony of K. pneumoniae and P. aeruginosa were cultured aerobically in modified Vogel and Bonner’s medium (MVBM) at 37˚C in a 200 rpm shaker incubator for 18 h. S. epidermidis was cultured overnight in brain heart infusion (BHI) (Himedia®) under the same conditions as mentioned above.
Table 2. Characteristics of the 20 patients with CRS.
| No. | Agea/genderb | Microorganisms isolated from maxillary and ethmoid sinusesc | Total No. of isolate | Biofilmd |
|---|---|---|---|---|
| 1 | 61/f | P. aeruginosa | 1 | + |
| 2 | 45/f | S. epidermidis | 1 | – |
| 3 | 58/f | S. epidermidis, Acinetobacter lwoffii | 2 | + |
| 4 | 63/m | Enterobacter sp., Neisseria sicca, Corynebacterium hoffmannii, Streptococcus viridians | 4 | + |
| 5 | 44/m | S. epidermidis, K. pneumoniae | 2 | + |
| 6 | 78/m | Escherichia coli | 1 | + |
| 7 | 64/f | NG | - | + |
| 8 | 81/m | Alcaligenes faecalis | 1 | + |
| 9 | 67/m | NG | - | + |
| 10 | 54/m | NG | - | + |
| 11 | 54/f | NG | - | – |
| 12 | 37/f | NG | - | + |
| 13 | 59/f | Haemophilus influenzae | 1 | – |
| 14 | 13/f | NG | - | + |
| 15 | 59/m | NG | - | + |
| 16 | 27/m | A. lowffii | 1 | + |
| 17 | 60/f | NG | - | – |
| 18 | 39/m | Enterobacter sp., H. influenzae | 2 | + |
| 19 | 48/m | NG | - | + |
| 20 | 52/f | NG | - | – |
aIn years.
bm, male; f, female.
cNG, No growth.
d(+), positive; (–), negative.
Antibiotic susceptibility testing
MICs and MBCs of conventional antibiotics were determined using a broth microdilution technique [35]. Briefly, two-fold serial dilutions of AMX (Sigma-Aldrich, St. Louis, MO) were prepared in Mueller Hinton broth (MHB) (Himedia®) at concentrations ranging from 17 to 34209 μM (6–12500 μg/ml) in 96-well microtiter plates (Nunclon™, Roskilde, Denmark) for antimicrobial susceptibility testing. Only P. aeruginosa was additionally tested for susceptibility to TOB (Sigma-Aldrich) (0.03–52 μM or 0.01–25 μg/ml). Bacterial suspensions (5 x 105 CFU/ml) were added to the microtiter plates, which were then incubated at 37˚C for 24 h. Antibiotic-free culture was used as a control. After incubation, MIC values were observed according to the guidelines of the Clinical & Laboratory Standards Institute (CLSI). The results were confirmed using a microplate reader at OD620 (Sunrise™, TECAN, Australia). The plate count technique was further used to determine the MBC values on Mueller Hinton agar (MHA) (Himedia®). Each experiment was performed independently three times in triplicate.
Antimicrobial activity of antibiotics and peptides against planktonic bacteria
Each bacterial suspension (5 x 105 CFU/ml) in 1 mM potassium phosphate buffer (PPB) (pH 7.0) was added to an equal volume of each antibiotic (AMX and TOB) and AMP (LL-37, LL-31, D-LL-37 and D-LL-31) to reach final concentrations of 1, 5 and 10 μM and incubated at 37°C for 1 and 2 h. A bacterial suspension without peptide served as a control. The samples were then serially diluted and cultivated on NA (Himedia®) and incubated for 24 h to allow colony counting. The percentage killing by each AMP was calculated using the formula [1 − (CFU sample/CFU control)] × 100% [15, 36]. Each experiment was performed independently three times in duplicate.
Determination of the biofilm-forming capacity
The biofilm-forming capacity of each strain was examined using the biofilm crystal violet staining assay (on 2-day biofilm) as previously described [37]. Two hundred microlitres of bacterial suspension (108 CFU/ml) were added into a sterile 96-well flat-bottom plate (Nunclon™) and the plate was incubated at 37˚C for 3 h. Cell-free medium was used as a control. Weakly adhered planktonic cells were removed and fresh medium was added, then the plate was incubated at 37˚C for 21 h. The biofilms were washed with sterile distilled water, fresh medium was added and the plate was incubated at 37˚C for 24 h. The adherent biofilms were then fixed with 99% (v/v) methanol, air dried and stained with 2% (w/v) crystal violet for 5 min. Subsequently, 2-day biofilms were solubilized with 33% (v/v) glacial acetic acid. The absorbance was spectrophotometrically measured at the wavelength of 620 nm using a microplate reader (Sunrise™). Each experiment was performed independently three times in 8 replicates.
Comparison of the antimicrobial activity of peptides and antibiotics against bacteria grown as biofilm
The effects of both conventional antibiotics and AMPs against bacteria under biofilm-stimulating condition were determined using a transferable solid phase (TSP) pin lid, which resembles the ‘Calgary’ biofilm device as previously described with some modifications [38]. Each strain (107 CFU/ml) was added to sterile 96-well plates (Nunclon™), then covered by a TSP pin lid (NUNC™, Roskilde, Denmark), and further incubated at 37˚C for 24 h. Following the period of incubation, biofilms formed on the TSP pin surfaces were washed by sterilized distilled water and challenged with AMX in a range from 134 to 34418 μM (50–25000 μg/ml) and 0.05 to 100 μM of LL-37, LL-31, D-LL-37 and D-LL-31 in 1 mM PPB. Additionally, the effect of TOB (2–836 μM or 1–391 μg/ml) against P. aeruginosa biofilm was also investigated. Then the plates were statically incubated at 37˚C for 24 h. A well free of antimicrobial agent was used as a control. After 24 h, bacterial survival was determined using the plate count technique on NA (Himedia®). The percentage killing of each antimicrobial agent was calculated using the formula [1 − (CFU sample/CFU control)] × 100% [15, 19]. The concentration of each agent required to kill 50% of cells in each tested bacterial strain (IC50 value) was determined by linear regression of plots. Each experiment was performed independently three times in duplicate.
The synergistic activity of D-LL-31 with antibiotics against bacteria grown as biofilm
After the IC50 value of each antimicrobial agent was calculated, D-LL-31 (the most effective peptide) was then selected to determine its synergistic activity with conventional antibiotics (AMX and TOB) using the broth microdilution checkerboard technique with some modification [15, 39]. Briefly, 24 h biofilms on TSP pin lids were mixed with the antibiotics (AMX or TOB) and D-LL-31 diluted in 1 mM PPB to final concentrations of IC50 down to 1/16 of the IC50 values of each agent and incubated at 37°C for 24 h (Table 4). Bacterial viability in each interaction was determined using the plate count technique. The fractional inhibitory concentration index (FICI) values were calculated and interpreted in terms of synergism/antagonism as previously described [40]. Each experiment was performed independently three times in duplicate.
Table 4. The IC50 values of antimicrobial peptides and antibiotics against bacterial isolated from CRS patients under biofilm-stimulating condition.
| Isolates | IC50 value (μM) | |||||
|---|---|---|---|---|---|---|
| LL-37 | LL-31 | D-LL-37 | D-LL-31 | AMX | TOBa | |
| K. pneumoniae | 0.8 | 0.7 | 0.8 | 0.6 | 7925 (2896 μg/ml) | ND |
| S. epidermidis | 2.4 | 1.8 | 1.3 | 0.5 | 33092 (12092 μg/ml) | ND |
| P. aeruginosa | 11.5 | 5.3 | 10.1 | 4 | 45163 (16502 μg/ml) | 4 (2 μg/ml) |
aND, not determined.
The effect of D-LL-31 alone and in combination with antibiotics on biofilm matrix
The in vitro Amsterdam Active Attachment Model (AAA-model) was used to determine the effect of peptides alone and in combination with conventional antibiotics on biofilm matrix [41]. Briefly, 1.5 ml of each overnight culture (108 CFU/ml) was added to a 24-well tissue culture plate (Nunclon™). The plate was covered with a sterile stainless-steel lid containing glass coverslips and aerobically incubated at 37°C for 24 h. After growing the biofilm, D-LL-31 or AMX alone (IC50 values; 0.6 μM D-LL-31 and 7925 μM AMX (2896 μg/ml)) and a combination of both (based on the synergy data; 0.07 μM D-LL-31 and 495 μM AMX (181 μg/ml)) were used in K. pneumoniae biofilm matrix challenge. For S. epidermidis biofilm matrix, IC50 values of D-LL-31 (0.5 μM) or AMX (33092 μM or 12092 μg/ml) and combination of D-LL-31/AMX (0.03 μM D-LL-31 and 2068 μM AMX (756 μg/ml)) were used. For P. aeruginosa biofilm matrix, IC50 values of D-LL-31 (4 μM) or AMX (45163 μM or 16502 μg/ml) or TOB (4 μM or 2 μg/ml) and combination of D-LL-31/AMX (0.25 μM D-LL-31 and 2823 μM AMX (1031 μg/ml)) or D-LL-31/TOB (0.5 μM D-LL-31 and 0.2 μM TOB (0.1 μg/ml)) were used. All tested conditions were incubated at 37°C for 24 h. A well free of antimicrobial agent was used as a control. Following incubation, biofilms were fixed with 2.5% glutaraldehyde (Sigma-Aldrich) at 25°C for 3 h. The fixed bacterial biofilms on glass coverslips were stained with 50 μg/ml Alexa Fluor® 488 (Invitrogen, Carlsbad, CA) for 30 min. This stain reacts with exopolysaccharide matrixes of the biofilm. The glass coverslips were then mounted with 80% glycerol and the stained cells were photographed using a confocal laser-scanning microscope (CLSM) (TCS SP8-Leica Microsystems, Wetzlar, Germany). The biofilm biomass was used as a quality-control parameter to confirm the reduction of biofilm matrix using COMSTAT analysis (BioCentrum-DTU, Lungby, Denmark). Each experiment was performed independently three times in duplicate.
The localization of peptide on biofilm matrix
The localization of TAMRA-labeled D-LL-31 on biofilm matrix was investigated using the AAA-model as mentioned above. One-day biofilm was fixed with 2.5% glutaraldehyde (Sigma-Aldrich) at 25°C for 3 h and stained with 50 μg/ml of Alexa Fluor® 488 (Invitrogen) for 30 min. Subsequently, TAMRA-labeled D-LL-31 at the IC50 concentration was added onto the biofilm architecture of each tested strain for 5 min, and then the biofilm was washed 3 times using sterile distilled water. The localization of D-LL-31 on biofilm matrix was visualized by CLSM (TCS SP8-Leica Microsystems). Each experiment was performed independently three times in duplicate.
Determination of the cytotoxicity of D-LL-31 for A549 cells
Human lung epithelial cells (A549, ATCC®, CCL-185™) were used to investigate the cytotoxicity of D-LL-31 as previously observed [42]. Briefly, A549 cells were maintained in RPMI-1640 (HyClone™, Marlborough, MA) with 10% fetal bovine serum (FBS) (HyClone™) and sub-cultured by standard methods using trypsin/EDTA (0.25% trypsin, 0.1% EDTA) (Corning™, Corning, NY). A standard curve of A549 cells (100–50000 cells/well) was constructed as previously described with some modification [43]. For MTT assay, cells were plated at a density of 2.5×104 cells/well in 96-well plates and grown overnight. D-LL-31 was diluted in PPB and added to culture medium to final concentrations ranging from 0.05 to 100 μM. Prepared A549 cells were then exposed to the diluted peptides for 24 h at 37°C, 5% CO2 atmosphere. Negative controls received either culture medium or PPB. Cells with 2% dimethyl sulfoxide (DMSO) (Sigma-Aldrich) were used as positive control. Cytotoxicity was assessed using MTT (0.5 mg/ml) according to the manufacturer's instructions (Sigma-Aldrich). Absorbance was measured at a wavelength of 570 nm with background subtraction at 630–690 nm. Then absorbance values from the MTT were converted to cells/well using a standard curve. Cell viabilities were calculated using the formula; [mean of cells in treated well]/[mean of cells in control well] × 100%. Each experiment was performed independently three times in triplicate.
Detection of cytokine release from D-LL-31-treated A549 cells
The immunomodulatory effect of D-LL-31 on A549 human lung epithelial cells was investigated using cytokine antibody arrays. A total of 5.5 × 106 A549 cells were seeded into a T25 tissue culture flask (Corning™) and grown overnight at 37°C, 5% CO2. The cells were incubated with 10 μM of D-LL-31 at 37°C, 5% CO2 for 24 h (This concentration spanned the IC50 values of the peptide against all tested bacterial strains grown as biofilm and showed no cytotoxicity in A549 cells). Cells in medium without peptide were used as a control. After incubation, the secreted cytokines in culture supernatant were detected using a human angiogenesis antibody array kit (AAH-ANG-1000-4, Norcross, GA) according to the manufacturer's instructions. The images were taken using a chemiluminescence imaging system and the densitometry data were calculated using ImageQuant TL Software version 8.1 (Amersham™ Imager 600, GE Healthcare Life Sciences, Chicago, IL). Each experiment was performed independently two times in duplicate.
Statistical analysis
The percentage of killing activity of all tested agents against bacteria grown as biofilm, biofilm biomass, cytotoxicity and densitometry data of cytokine expression are presented as mean ± standard deviation (SD). Comparisons between the average percentage killing activity of each AMP at the same concentration in all tested agents were analyzed using one-way ANOVA. The statistical significance of cell viabilities and pixel density ratio in each treatment compared with controls was determined by Student’s t test. All statistical testing was performed using the SPSS software, version 16.0 (Chicago, IL).
Results
Isolation and identification of bacteria from CRS patients
Characteristics of the 20 patients (ten males and ten females) with CRS are shown in Table 2. The ages ranged from 13 to 81 years (average 53 years). None was pregnant or had underlying diseases; diabetes mellitus, cancer or AIDS. Of 20 clinical specimens, ten yielded positive cultures on at least one of the media that were used. Sixteen microorganisms were isolated from maxillary or ethmoid sinus washings, 11 of which (69%) were Gram-positive and 5 were Gram-negative bacteria. About 75% (12/16) of the isolates were classified as facultative anaerobic bacteria and 25% (4/16) as obligate aerobes or aerobic bacteria.
The presences of biofilms on sinus mucosal tissues of CRS patients
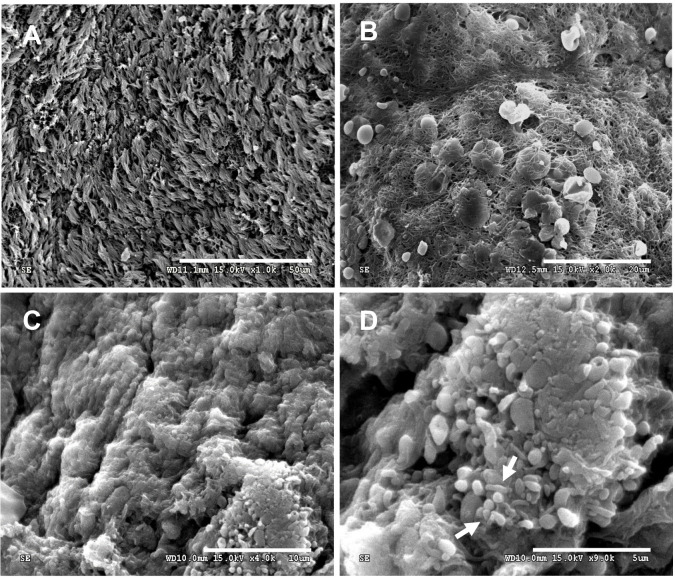
Investigation using SEM revealed evidence of biofilm formation in about 75% (15/20) of sinus mucosal tissues samples from CRS patients, as shown in Table 2. Scanning electron micrographs of mucosal tissues with the indication of biofilms are shown Fig 1. The presence of a biofilm was defined on the basis of visible bacterial aggregation located on the surface of the epithelium. Large biofilm aggregates were observed (Fig 1C and 1D) and are distinct from the appearance of ciliated epithelium from samples that yielded bacteria in culture but without evidence of biofilm (Fig 1A). Coccus- and bacillus-shaped elements were observed on the biofilm structure (Fig 1D, white arrow). Biofilm obtained from CRS mucosal tissues showed strands of extracellular materials between the cells, which might represent extracellular DNA (eDNA) from bacterial and human cells (Fig 1B).
Fig 1. Scanning electron micrographs of mucosal tissues from CRS patients.
(A) Maxillary sinus mucosa without evidence of biofilm but positive for bacteria when cultured; bar = 50 μm. (B) Wire-like structures seen on the sinus mucosal epithelium; bar = 20 μm. (C) Large biofilm aggregates; bar = 10 μm and (D) biofilm with coccus- and bacillus-shaped elements (white arrows); bar = 5 μm.
Antimicrobial activity of antibiotics and AMPs against all tested bacteria in planktonic form
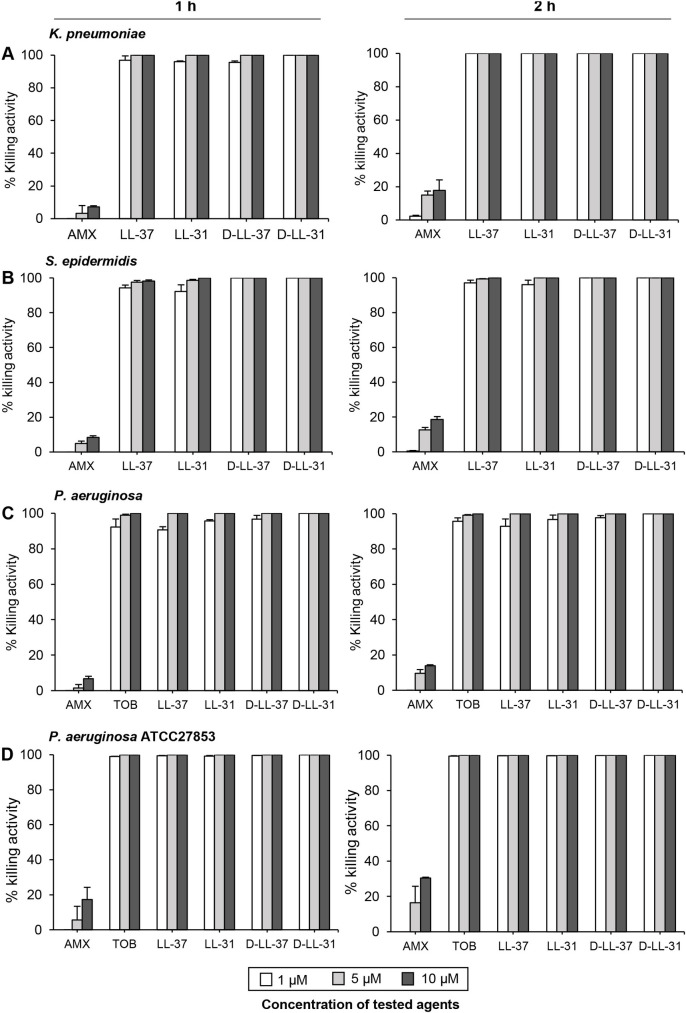
The MIC and MBC values of conventional antibiotics against bacteria isolated from CRS patients are shown in Table 3. Resistance to AMX was observed in all strains. P. aeruginosa in planktonic form was susceptible TOB. All tested peptides, at 1 μM, exhibited ≥ 95% killing activity against all tested isolates after 1 h of incubation (Fig 2). All peptides at 5 μM completely killed the initial inoculum after 2 h. All concentrations of AMX showed a low killing ability (about 0.5–18.5%) toward all bacterial strains in planktonic form during 1 and 2 h of incubation. TOB at concentrations of 1 and 5 μM showed ≥ 92% killing effect against the clinical isolate P. aeruginosa at 1–2 h, but 10 μM was required to kill all bacterial cells (Fig 2C) while only 1 μM TOB showed ≥ 98% killing effect against the reference strain P. aeruginosa ATCC 27853 at 1 h and both 5 and 10 μM killed all bacterial cells within 1 h (Fig 2D). Interestingly, only D-LL-31 (1 μM) caused rapid killing of all tested strains: bacterial cells at 5 x 105 CFU/ml were completely killed within 1 h of the peptide treatment.
Table 3. Antimicrobial susceptibility of antibiotics against bacterial isolated from CRS patients in planktonic form.
| Isolates | Antibiotics | MIC (μM/μg/ml) | MBC (μM/μg/ml) |
|---|---|---|---|
| K. pneumoniae | AMX | 4276/ 1562.5 | 8552/ 3125 |
| S. epidermidis | AMX | 4276/ 1562.5 | 17104/ 6250 |
| P. aeruginosa | AMX | 17105/ 6250 | 34209/ 12500 |
| TOB | 0.2/ 0.1 | 1/ 0.4 |
Fig 2. Antimicrobial activity of antibiotics and AMPs against planktonic culture.
Bacterial suspensions of (A) K. pneumoniae, (B) S. epidermidis and (C) P. aeruginosa (D) P. aeruginosa ATCC 27853 were incubated with 1, 5 and 10 μM of each peptide for 1 and 2 h. The results are presented as mean values ± SD of three independent experiments, carried out in duplicate.
The quantification of bacterial biofilm formation
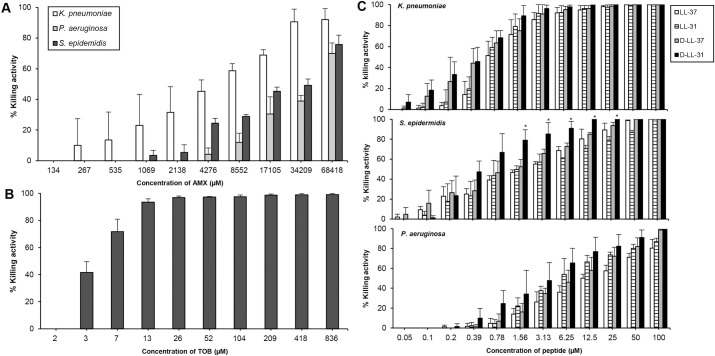
The crystal violet assay (using 2-day biofilm) was performed to determine the biofilm-formation capacity of each bacterial stain. P. aeruginosa showed the greatest tendency for biofilm formation [OD620 (mean ± SD); 0.376 ± 0.1], followed by S. epidermidis and K. pneumoniae, 0.273 ± 0.05 and 0.203 ± 0.04, respectively. Therefore, the biofilm-producing capacity of all bacterial strains was confirmed.
Killing effect of all tested agents against bacteria under biofilm-stimulating condition
All agents displayed a clear dose- and strain-dependent killing activity against all tested bacteria grown as biofilm (Fig 3). The highest concentration of AMX (68418 μM or 25000 μg/ml) exhibited 70%, 75%, and 90% killing activity against P. aeruginosa, S. epidermidis and K. pneumoniae biofilm, respectively (Fig 3A). Biofilm of P. aeruginosa was found to be sensitive to TOB (Fig 3B). Among all peptides, D-LL-31 exhibited the strongest killing activity against all bacterial strains (Fig 3C). The killing activities of D-LL-31 at concentrations 1.56–25 μM were significantly higher than all tested peptides (P < 0.05) against S. epidermidis biofilm. However, there were no statistically significant differences in killing activity of D-LL-31 against biofilm of K. pneumoniae and P. aeruginosa when compared to the other peptides.
Fig 3. The killing activity of AMPs and antibiotics against all tested bacterial strains grown as biofilm.
(A) Effect of AMX against all tested isolates and (B) TOB toward only P. aeruginosa biofilm. (C) The killing capacity of AMPs against all tested bacteria. The 24 h biofilm of each bacterial isolate was incubated with AMPs and antibiotics for 24 h. The results are presented as mean values ± SD of three independent experiments, carried out in duplicate. One-way ANOVA was used for determining the statistical significance of the killing activities of each antimicrobial peptide in different concentrations (*P<0.05 comparing between each antimicrobial peptide at the same concentration).
The IC50 values of each agent was further investigated (Table 4). D-LL-31 exhibited the lowest IC50 values against all bacteria in biofilm at concentration ranging from 0.5 to 4 μM. IC50 values of AMX against biofilm of P. aeruginosa, K. pneumoniae, and S. epidermidis were higher than those of D-LL-31 (about 11000-, 13000- and 66000-fold, respectively). In contrast, the IC50 values of TOB and D-LL-31 toward P. aeruginosa biofilm were similar. However, these findings also indicated that D-LL-31 was more effective than the L-form and the LL-37 variants against all bacteria grown as biofilm.
Synergistic effect of D-LL-31 with antibiotics
The most effective antimicrobial peptide, D-LL-31, showed synergistic interactions with all tested antibiotics against bacteria grown as biofilm based on FICI ≤ 0.5 (Table 5). The combination of D-LL-31 with antibiotics exhibited 50% killing against each bacterial strain at IC50 values that were at least 8- to 16-fold lower than those of either agent alone. A 16-fold reduction in IC50 values of both D-LL-31 and AMX were found against P. aeruginosa and S. epidermidis biofilm (FICI = 0.125). The interaction of D-LL-31 with AMX and TOB reduced the IC50 values of the antibiotics by 8- to 16-fold against K. pneumoniae and P. aeruginosa biofilm, respectively (FICI = 0.188).
Table 5. The synergistic interaction of D-LL-31 with conventional antibiotics against bacteria in biofilm condition.
| Isolates | Antimicrobial agent in concentration of combination (μM) | FICI values | Fold decrease of IC50 valuesb | ||||
|---|---|---|---|---|---|---|---|
| D-LL-31 | AMXa | TOBa | D-LL-31 | AMXa | TOBa | ||
| K. pneumoniae | 0.07 | 495 (181 μg/ml) | ND | 0.188 | 8 | 16 | ND |
| S. epidermidis | 0.03 | 2068 (756 μg/ml) | ND | 0.125 | 16 | 16 | ND |
| P. aeruginosa | 0.25 | 2823 (1031 μg/ml) | ND | 0.125 | 16 | 16 | ND |
| 0.5 | ND | 0.2 (0.1 μg/ml) | 0.188 | 8 | ND | 16 | |
aND, not determined.
bFold decrease of each antimicrobial agent were calculated from their IC50 value compared with concentration of combination (μM).
Effect of D-LL-31 alone and in combination with antibiotics against biofilm matrix under static conditions
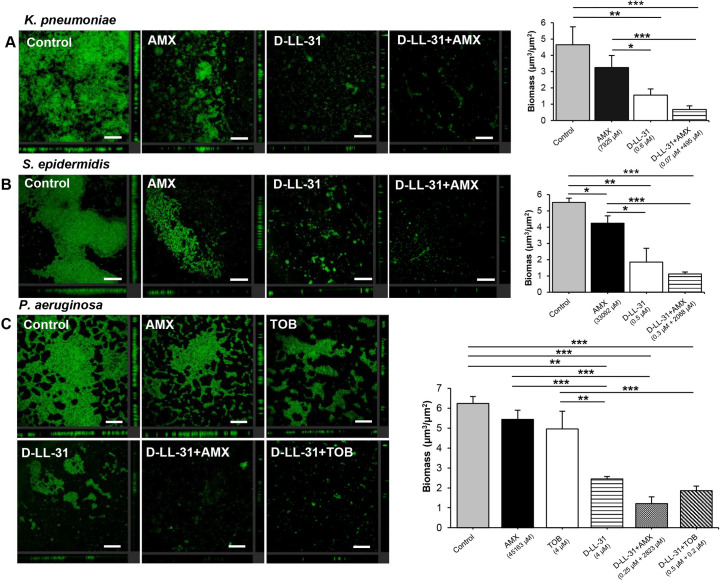
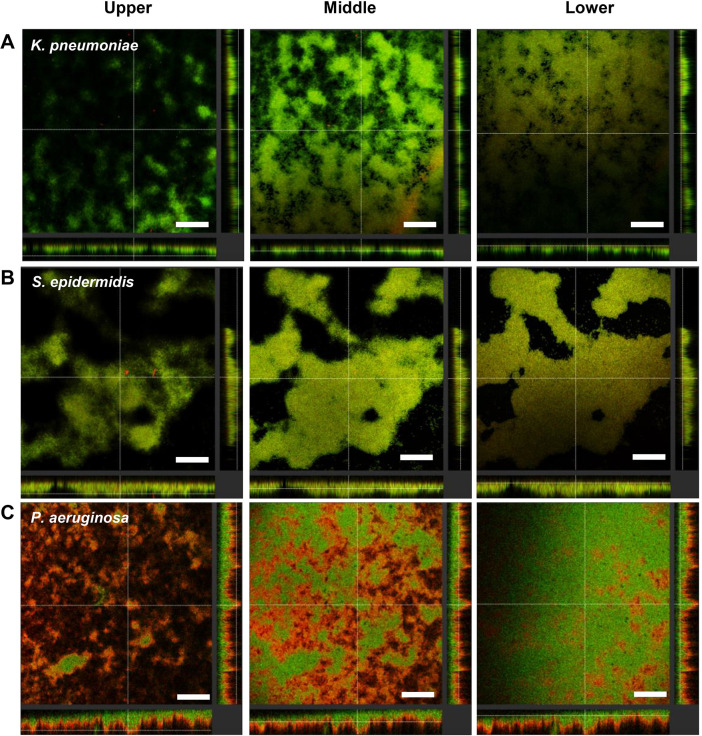
The green fluorescence of Alexa Fluor® 488 was used to represent the biofilm matrix of all tested bacteria after exposure to D-LL-31 alone or in combination with antibiotics (Fig 4). A combination of D-LL-31 with AMX led to a distinct decrease in biofilm matrix for all tested strains relative to controls. A statistically significant reduction of P. aeruginosa biofilm matrix occurred when treated with a combination of D-LL-31 and TOB (Fig 4C). Moreover, a greater reduction of biofilm matrix was observed in all tested strains after challenge with D-LL-31 alone than after challenge with antibiotic alone.
Fig 4. Effect of D-LL-31 alone and in combination with antibiotics on biofilm matrix.
The 24 h biofilm of (A) K. pneumoniae, (B) S. epidermidis and (C) P. aeruginosa were incubated with D-LL-31 alone and in combination with antibiotics (AMX or TOB) for 24 h. Biofilm were stained with Alexa Fluor® 488. Biomass was analyzed using COMSTAT and values are shown as mean ± SD in duplicate from three independent experiments. *P<0.05, **P<0.01 and ***P<0.001. Scale bar indicate 100 μm.
To confirm the biofilm-disrupting effect of the agents, biofilm biomass (μm3/μm2) was measured. Significant decreases of biomass were observed following D-LL-31 treatment alone (P < 0.01) and in combination with antibiotics (P < 0.001) relative to controls. There were no statistically significant effects of either antibiotic tested against K. pneumoniae and P. aeruginosa biofilm. However, AMX had a significant effect on S. epidermidis (P < 0.05).
Localization of D-LL-31 on biofilm matrix
The localization of the peptide on biofilm architecture was observed using TAMRA-labeled D-LL-31. Image slices were obtained at 2-μm increments moving from the top of the biofilm matrix through the substrate. The stacked sections of biofilm matrix of all tested bacterial strains in the presence of peptide are shown in Fig 5. A rapid binding of D-LL-31 to biofilm matrix of all tested bacteria was observed within 5 min of incubation. D-LL-31 could be detected in all stacks of K. pneumoniae and S. epidermidis biofilms (Fig 5A and 5B). The peptide accumulated mainly in the top layers of P. aeruginosa biofilm (Fig 5C), but some could also be seen in the middle layers.
Fig 5. The localization of TAMRA-labelled D-LL-31 on biofilm matrix.
The 24h biofilm of (A) K. pneumoniae, (B) S. epidermidis and (C) P. aeruginosa were stained with Alexa Fluor® 488 (green) then incubated with TAMRA-labeled D-LL-31 (red) for 5 min. Each z-stack image was visualized using CLSM. Scale bar indicate 100 μm.
Cytotoxicity of D-LL-31 on human lung epithelial cells
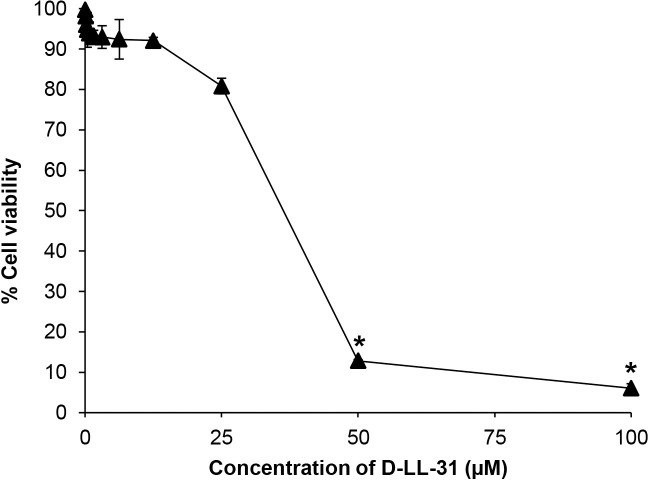
A cell-survival assay was performed to determine the cytotoxicity of D-LL-31 on human lung epithelial cells as shown in Fig 6. More than 90% of cells remained viable after treatment with 12.5 μM D-LL-31, and more than 80% after treatment with 25 μM, indicating that D-LL-31exhibited low cytotoxicity against A549 cells at concentrations that are effective against the bacteria grown as biofilm (IC90 values of D-LL-31 at concentration ranging from 1 to 25 μM). However, treatment with ≥ 50 μM D-LL-31 resulted in more than 90% cytotoxicity of A549 cells.
Fig 6. Cytotoxicity of D-LL-31 on human lung epithelial cells.
The cells were incubated with different concentrations of D-LL-31 (0.05–100 μM) for 24 h. The cell viability was assessed using the MTT assay. Data are presented as the mean ± SD (*P<0.001).
Expression of cytokines and angiogenesis-related proteins
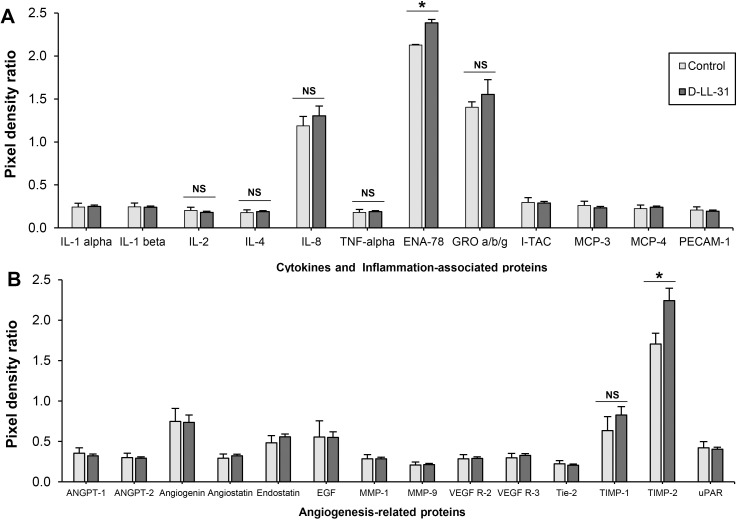
The expression of angiogenesis-related proteins and inflammatory cytokines in the presence of D-LL-31 was determined using a cytokine array membrane assay. The pixel density ratio of each identified protein is shown in Fig 7A and 7B. Epithelial-derived neutrophil-activating peptide 78 (ENA-78) was significantly secreted in the presence of D-LL-31 (P < 0.05) relative to controls (Fig 7A). There was no significant change in levels of cytokines that might impact CRS, such as IL-4, IL-8, TNF-α and growth-related oncogene-a, b and g (GRO-a, b and g). The expression of IFN-γ, an important cytokine in the immunopathogenesis of CRSsNP, was not found after incubation with D-LL-31. Moreover, treatment with D-LL-31 did not induce expression of IL-6, a marker of inflammation in CRSwNP. In addition, TIMP-2 was significantly elevated in culture supernatant with D-LL-31 (P < 0.05) (Fig 7B).
Fig 7. Expression of cytokines and angiogenesis-related proteins in the presence of D-LL-31.
The cells were incubated with 10 μM D-LL-31 for 24 h. (A) The cytokine and (B) angiogenesis-related protein were investigated using cytokine antibody arrays. The pixel density ratio of each identified proteins was calculated using ImageQuant TL Software and presented as mean ± SD. (*P<0.05).
Discussion
Bacterial infection play an important role in the pathogenesis, cause inflammation and prescribe antimicrobial therapy in CRS [44, 45]. Moreover, biofilm on mucosal tissues of CRS patients have demonstrated the significant role in the pathogenesis and disease progression of CRS [46] and its association with antibiotic resistance [10, 11]. In this study, the enhancement of biofilm-eradicating effect of currently used antibiotics for CRS treatment with cathelicidin-derived peptides and D-enantiomers were observed. Various bacterial species were isolated from maxillary and ethmoid sinus irrigation samples from CRS patients. Of these, S. epidermidis (a colonizer of the nasal cavity) was the most common, in agreement with previous reports [33]. Moreover, Gram-negative bacilli such as Enterobacter spp, E. coli, K. pneumoniae and P. aeruginosa which are considered as agents of CRS infection [47, 48] were also found in this study. The SEM micrographs revealed that 75% (15/20) of CRS patients had evidence of biofilms on their nasal mucosa, consistent with other studies [7, 33]. Seven of the 15 individuals that were biofilm-positive did not yield bacteria in culture. In these cases, biofilm might be a product of anaerobic bacteria and fungi. Anaerobic organisms are relatively uncommon in the nasal cavity [44] and fungi are found in only 1.6% of CRS patients [47]. Therefore, these groups of microorganisms were excluded in this study. In addition, using standard culture-based methods was the limitation in this study which may affect the exact number of isolated bacteria from CRS patients. Molecular technique should be used as addition tool to identify microorganisms in clinical samples. Finally, K. pneumoniae, P. aeruginosa and S. epidermidis were selected according to the known prevalence of these species in CRS patients with biofilm [47, 48] for further experiments.
AMX is a reasonable first-line antibiotic for treatment of sinus infections in many geographic areas [49]. In addition, TOB is recommended to treat P. aeruginosa infection [50]. Our antimicrobial susceptibility results showed that all bacterial strains isolated from CRS patients in planktonic form were resistant to AMX, as previously reported [51]. Despite their resistance to AMX, we observed that the bacteria were sensitive to all AMPs tested. We were able to demonstrate that the D-enantiomeric LL-31 peptide had a higher antibacterial potency against all tested bacteria in planktonic culture than the other AMPs (LL-37, LL-31 and D-LL-37) and currently used antibiotics (AMX and TOB).
Long-term antibiotic therapy in CRS patients may be one of the reasons of the antibiotic-resistance. Moreover, microbial biofilm can be up to 1000 times more resistant to antibiotic treatment than its planktonic form in CRS patients [34, 52]. Thus, we investigated the killing capacity of the tested agents against bacteria grown as biofilm. Our findings are consistent with Kifer D et al [53], given the greater resistance to AMX than their planktonic counterparts. However, all bacterial isolates grown as biofilm tended to be more sensitive to all peptides, especially D-LL-31. This protease-resistant peptide displayed a killing activity comparable to that of TOB against P. aeruginosa biofilm and greater biofilm-reduction properties than TOB. The mode of action of AMPs relies on their capacity to disrupt bacterial membranes, even in slow-growing or dormant biofilm-forming cells [8, 54]. Thus, AMPs are better able to combat bacterial cells during biofilm-stimulating conditions. Several studies have shown that AMPs may penetrate into the biofilm matrix and kill the persister cells, a great advantage over conventional antibiotics [8, 15].
The biofilm matrix accounts for up to 90% of the total biofilm mass and acts as a barrier for antibiotic diffusion [55], which largely explains why bacteria in biofilms are so resistant to antibiotics [8]. We have shown previously that D-LL-31 may disrupt the biofilm matrix leading to enhanced access by ceftazidime to kill bacterial cells, ultimately resulting in synergistic interaction [15]. Here, TAMRA-labelled D-LL-31 was used to observe the localization of the peptide before it exerted its effect. D-LL-31 was observed in all layers of K. pneumoniae and S. epidermidis biofilm matrix, whereas a small amount of peptide was seen in the mid-layers of P. aeruginosa biofilm (most being in the surface layer). Probably as a consequence, IC50 values against P. aeruginosa were higher than other tested bacterial strains. Bacterial species differ in their biofilm matrix components [56]. For instance, the ability of P. aeruginosa to synthesize large amounts of the anionic polysaccharide alginate and eDNA probably reduces its susceptibility to magainin II and cecropin P1 and might entrap AMPs (positively charged molecules) before they can reach their bacterial target [57]. Additionally, the positively charged exopolymers (Pel and Psl) in P. aeruginosa biofilm can also cause electrostatic repulsion of the cationic polypeptide antibiotics (polymyxin B and colistin) [58], as also observed for human β-defensin-3 (hBD-3) and LL-37 against S. epidermidis [59]. Nevertheless, our results still indicate the rapid penetration and strong killing activity of D-LL-31 against all tested isolates in a strain-, biofilm-forming ability-, and matrix component-dependent manner. We can conclude that consideration of the interaction capacity of AMPs with biofilm matrix and interference of biofilm-specific molecules will be an essential part of the development of peptide-based therapeutics.
There is much evidence that AMPs in combination with common antibiotics often enhance the efficacy of the antibiotic and decrease development of antibiotic resistance [60]. In addition, previous reports have shown that biofilm-related infections in CRS patients are more difficult to clear, prone to relapse, inherently resistant to antibiotics and might lead to repeated surgery [32, 34]. In our study, D-LL-31 showed synergistic effects with conventional antibiotics (AMX and TOB) against embedded bacterial cells within biofilm in all tested isolates, leading to greatly reduced IC50 values (as much as 16-fold lower) and restoration of the efficacy of the antibiotics. The lowest quantity of biofilm matrix was observed after incubation with a combination of D-LL-31 and antibiotics in all isolates tested. Indeed, D-LL-31 alone could reduce biofilm matrix to a greater extent than did either antibiotic. We hypothesize that the improved antibiotic activity in the presence of D-LL-31 is likely due to the interference with intracellular signals for biofilm formation [61], down-regulation of genes essential for biofilm development [62], reduction of biofilm matrix synthesis [63] and biofilm disruption by the AMPs [15, 64]. Consequently, these may recognize the possible mechanisms that perturbation of matrix synthesis and disruption of biofilm architecture caused by AMPs plays a key role in allowing increased access of currently used antibiotics toward biofilm-embedded bacteria, as also previously expected [15, 64]. These findings demonstrate that enhanced activities of antibiotic in combination with D-LL-31 to eradicate the biofilm matrix and increase the bactericidal efficacy against embedded cells within biofilm may reduce the relapse cases and number of patients for revision endoscopic sinus surgery.
CRS is an inflammatory disorder disease [1, 3]. Given that, we should avoid the use of any treatment that could induce expression of the major cytokines involved in immunopathogenesis of CRS [28]. Thus, the immunomodulatory effect of D-LL-31 was investigated. Previous research has indicated that elevated expression of IL-4, IFN-γ and IL-6 on airway epithelial cells of CRS patients resulted in decreased epithelial barrier function, a phenomenon that might account in part for the progression of CRS [21, 22]. We did not detect IFN-γ and IL-6 in the presence of D-LL-31, while low levels of IL-4 were detected both in the presence and absence of the peptide. Although the neutrophil-attracting chemokines, IL-8 and GRO-a, b and g, increased following D-LL-31 treatment, this increase was not statistically significant. We also found high levels of ENA-78 in the culture supernatants with D-LL-31. Rudack and colleagues recently noted that IL-8 and ENA-78 appear to be of secondary importance for the chemotaxis of neutrophils in CRS [65]. Another recent study found that LL-37 causes increased IL-6 and IL-8 release from human nasal cells [66]; while, IL-6 (a marker of inflammation in CRSwNP) was not observed after incubation with D-LL-31. In addition, TNF-α has been thought to play a role in the pathogenesis of CRS via the recruitment of B cells [23]. We did not detect TNF-α expression after treatment with D-LL-31. Based on our results, D-LL-31 does not appear to induce the cytokines that play a prominent role in ongoing inflammatory reactions in patients with CRS.
Several studies have shown that a balance between MMPs and their regulators (TIMPs) controls the remodeling and repairing of airway ECM [24]. The elevated expression of MMP-2, -7 and -9, important endopeptidases for degrading the ECM, has been considered to play important roles in the pathogenesis of nasal polyposis in CRS patients [24–26]. Significantly decreased expression of TIMP-1 and -2 has been found in patients with CRS, failing to counterbalance activity of MMPs [25, 27]. This imbalance contributes to the excessive degradation of ECM, formation of pseudocysts, albumin deposition and edema [28]. Interestingly, TIMP -2 was up-regulated in presence of D-LL-31. Perhaps D-LL-31 has a novel role in tissue remodeling, which may prevent the progression and development of nasal polyposis in patients with CRS.
Successful development of any pharmaceutical substance requires minimal or no toxicity. D-LL-31 exhibited no cytotoxicity on human lung epithelial cells after 24 h of incubation. Additionally, D-LL-31 exhibits only marginal hemolytic activity against hRBCs (less than 1%) [15].
Conclusion
In summary, our study demonstrated that D-LL-31 displayed strong broad-spectrum activity against bacteria in planktonic condition. Furthermore, this peptide exhibited a high potential to kill bacterial cells under biofilm-stimulating conditions when compared to all tested agents. Increased biofilm matrix eradication was observed when D-LL-31 was combined with conventional antibiotics. These findings suggested that D-LL-31 is an interesting candidate peptide for further development as an antibacterial or antibiofilm agent for use alone, or to enhance the efficacy of commonly used antibiotics to combat biofilm-related CRS. Co-treatment with this peptide may permit reduction in antibiotic concentrations, possible side effects and disease severity. D-LL-31 was not toxic to human lung epithelia cells. No cytokines involved in the immunopathogenesis of CRS were induced after treatment with D-LL-31. A role for D-LL-31 in the remodeling and repairing of airway extracellular matrix was also observed. Therefore, D-LL-31 is a feasible therapeutic supplement for applying to the biofilm-associated infection of CRS patients.
Supporting information
Bacterial suspensions were incubated with antibiotics for 24 h and the results were interpreted according to BD BBL™ Sensi-Disc™ antimicrobial susceptibility test discs.
(DOCX)
Acknowledgments
We would like to acknowledge Prof. David Blair for editing the MS via Publication Clinic KKU, Thailand.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
SK was supported by grant fund under Khon Kaen University, Thailand (61003402 and 6200021004) https://www.kku.ac.th/ SW was supported by grant fund under Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand https://www.md.kku.ac.th/ The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, Brook I, Ashok Kumar K, Kramper M, et al. 2015. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg 152:S1–S39. 10.1177/0194599815574247 [DOI] [PubMed] [Google Scholar]
- 2.Rudmik L, Smith TL, Schlosser RJ, Hwang PH, Mace JC, Soler ZM. 2014. Productivity costs in patients with refractory chronic rhinosinusitis. Laryngoscope 124:2007–12. 10.1002/lary.24630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akdis CA, Bachert C, Cingi C, Dykewicz MS, Hellings PW, Naclerio RM, et al. 2013. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol 131:1479–90. 10.1016/j.jaci.2013.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamal M, Ahmad W, Andleeb S, Jalil F, Imran M, Nawaz MA, et al. 2017. Bacterial biofilm and associated infections. J Chin Med Assoc. 10.1016/j.jcma.2017.07.012 [DOI] [PubMed] [Google Scholar]
- 5.Bezerra TFP, de Melo Padua FG, Gebrim EMMS, Saldiva PHN, Voegels RL. 2011. Biofilms in chronic rhinosinusitis with nasal polyps. Otolaryngol Head Neck Surg 144:612–6. 10.1177/0194599811399536 [DOI] [PubMed] [Google Scholar]
- 6.Ferguson BJ, Stolz DB. 2005. Demonstration of biofilm in human bacterial chronic rhinosinusitis. Am J Rhinol 19:452–7. 10.1177/194589240501900506 [DOI] [PubMed] [Google Scholar]
- 7.Sanclement JA, Webster P, Thomas J, Ramadan HH. 2005. Bacterial biofilms in surgical specimens of patients with chronic rhinosinusitis. Laryngoscope 115:578–82. 10.1097/01.mlg.0000161346.30752.18 [DOI] [PubMed] [Google Scholar]
- 8.Batoni G, Maisetta G, Esin S. 2016. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. BBA-Biomembranes 1858:1044–60. 10.1016/j.bbamem.2015.10.013 [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen TB, Givskov M. 2006. Quorum-sensing inhibitors as anti-pathogenic drugs. Int J Med Microbiol 296:149–61. 10.1016/j.ijmm.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 10.Antunes MB, Feldman MD, Cohen NA, Chiu AG. 2007. Dose-dependent effects of topical tobramycin in an animal model of Pseudomonas sinusitis. Am J Rhinol 21:423–7. 10.2500/ajr.2007.21.3046 [DOI] [PubMed] [Google Scholar]
- 11.Desrosiers M, Bendouah Z, Barbeau J. 2007. Effectiveness of topical antibiotics on Staphylococcus aureus biofilm in vitro. Am J Rhinol 21:149–53. 10.2500/ajr.2007.21.3007 [DOI] [PubMed] [Google Scholar]
- 12.Psaltis AJ, Weitzel EK, Ha KR, Wormald P-J. 2008. The effect of bacterial biofilms on post-sinus surgical outcomes. Am J Rhinol 22:1–6. 10.2500/ajr.2008.22.3119 [DOI] [PubMed] [Google Scholar]
- 13.Bendouah Z, Barbeau J, Hamad WA, Desrosiers M. 2006. Biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa is associated with an unfavorable evolution after surgery for chronic sinusitis and nasal polyposis. Otolaryngol Head Neck Surg 134:991–6. 10.1016/j.otohns.2006.03.001 [DOI] [PubMed] [Google Scholar]
- 14.Yu Y, Cooper CL, Wang G, Morwitzer MJ, Kota K, Tran JP, et al. 2020. Engineered human cathelicidin antimicrobial peptides inhibit Ebola virus infection. Iscience:100999 10.1016/j.isci.2020.100999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wongkaewkhiaw S, Taweechaisupapong S, Anutrakunchai C, Nazmi K, Bolscher JGM, Wongratanacheewin S, et al. 2019. D-LL-31 in combination with ceftazidime synergistically enhances bactericidal activity and biofilm destruction in Burkholderia pseudomallei. Biofouling 35:573–84. 10.1080/08927014.2019.1632835 [DOI] [PubMed] [Google Scholar]
- 16.Tsai P-W, Yang C-Y, Chang H-T, Lan C-Y. 2011. Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates. PloS one 6:e17755 10.1371/journal.pone.0017755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dürr UH, Sudheendra U, Ramamoorthy A. 2006. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. BBA-Biomembranes 1758:1408–25. 10.1016/j.bbamem.2006.03.030 [DOI] [PubMed] [Google Scholar]
- 18.Luo Y, McLean DT, Linden GJ, McAuley DF, McMullan R, Lundy FT. 2017. The naturally occurring host defense peptide, LL-37, and its truncated mimetics KE-18 and KR-12 have selected biocidal and antibiofilm activities against Candida albicans, Staphylococcus aureus, and Escherichia coli in vitro. Front Microbiol 8:544 10.3389/fmicb.2017.00544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanthawong S, Bolscher JG, Veerman EC, van Marle J, de Soet HJ, Nazmi K, et al. 2012. Antimicrobial and antibiofilm activity of LL-37 and its truncated variants against Burkholderia pseudomallei. Int J Antimicrob Agents 39:39–44. 10.1016/j.ijantimicag.2011.09.010 [DOI] [PubMed] [Google Scholar]
- 20.Molhoek EM, Van Dijk A, Veldhuizen EJ, Haagsman HP, Bikker FJ. 2011. Improved proteolytic stability of chicken cathelicidin-2 derived peptides by D-amino acid substitutions and cyclization. Peptides 32:875–80. 10.1016/j.peptides.2011.02.017 [DOI] [PubMed] [Google Scholar]
- 21.Soyka MB, Wawrzyniak P, Eiwegger T, Holzmann D, Treis A, Wanke K, et al. 2012. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-γ and IL-4. J Allergy Clin Immunol 130:1087–96. 10.1016/j.jaci.2012.05.052 [DOI] [PubMed] [Google Scholar]
- 22.Pothoven K, Norton J, Ocampo C, Suh L, Carter R, Hulse K, et al. 2014. Oncostatin M is elevated in chronic rhinosinusitis and decreases barrier function in human airway epithelium. J Allergy Clin Immunol 133 10.1016/j.jaci.2015.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R, et al. 2008. Evidence of a role for B cell–activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol 121:1385–92. e2. 10.1016/j.jaci.2008.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen YS, Langhammer T, Westhofen M, Lorenzen J. 2007. Relationship between matrix metalloproteinases MMP‐2, MMP‐9, tissue inhibitor of matrix metalloproteinases‐1 and IL‐5, IL‐8 in nasal polyps. Allergy 62:66–72. 10.1111/j.1398-9995.2006.01255.x [DOI] [PubMed] [Google Scholar]
- 25.Eyibilen A, Cayli S, Aladag I, Koç S, Gurbuzler L, Atay GA. 2011. Distribution of matrix metalloproteinases MMP-1, MMP-2, MMP-8 and tissue inhibitor of matrix metalloproteinases-2 in nasal polyposis and chronic rhinosinusitis. Histol Histopathol 26:615–21. 10.14670/HH-26.615 [DOI] [PubMed] [Google Scholar]
- 26.Watelet J-B, Bachert C, Claeys C, Van Cauwenberge P. 2004. Matrix metalloproteinases MMP‐7, MMP‐9 and their tissue inhibitor TIMP‐1: expression in chronic sinusitis vs nasal polyposis. Allergy 59:54–60. 10.1046/j.1398-9995.2003.00364.x [DOI] [PubMed] [Google Scholar]
- 27.Li X, Meng J, Qiao X, Liu Y, Liu F, Zhang N, et al. 2010. Expression of TGF, matrix metalloproteinases, and tissue inhibitors in Chinese chronic rhinosinusitis. J Allergy Clin Immunol 125:1061–8. 10.1016/j.jaci.2010.02.023 [DOI] [PubMed] [Google Scholar]
- 28.Schleimer RP. 2017. Immunopathogenesis of chronic rhinosinusitis and nasal polyposis. Annu Rev Pathol-Mech 12:331–57. 10.1146/annurev-pathol-052016-100401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolscher J, Nazmi K, van Marle J, van ‘t Hof W, Veerman E. 2012. Chimerization of lactoferricin and lactoferrampin peptides strongly potentiates the killing activity against Candida albicans. Biochem Cell Biol 90:378–88. 10.1139/o11-085 [DOI] [PubMed] [Google Scholar]
- 30.Puknun A, Bolscher JG, Nazmi K, Veerman EC, Tungpradabkul S, Wongratanacheewin S, et al. 2013. A heterodimer comprised of two bovine lactoferrin antimicrobial peptides exhibits powerful bactericidal activity against Burkholderia pseudomallei. World J Microbiol Biotechnol 29:1217–24. 10.1007/s11274-013-1284-6 [DOI] [PubMed] [Google Scholar]
- 31.Hwang PH, Irwin SB, Griest SE, Caro JE, Nesbit GM. 2003. Radiologic correlates of symptom-based diagnostic criteria for chronic rhinosinusitis. Otolaryngol Head Neck Surg 128:489–96. 10.1016/s0194-5998(02)23295-7 [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Adappa ND, Chiu AG, Doghramji LJ, Cohen NA, Palmer JN. 2015. Biofilm‐forming bacteria and quality of life improvement after sinus surgery. Int Forum Allergy Rhinol 5:643–9. 10.1002/alr.21505 [DOI] [PubMed] [Google Scholar]
- 33.Dlugaszewska J, Leszczynska M, Lenkowski M, Tatarska A, Pastusiak T, Szyfter W. 2016. The pathophysiological role of bacterial biofilms in chronic sinusitis. Eur Arch Otorhinolaryngol 273:1989–94. 10.1007/s00405-015-3650-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karunasagar A, Garag SS, Appannavar SB, Kulkarni RD, Naik AS. 2018. Bacterial biofilms in chronic rhinosinusitis and their implications for clinical management. Indian journal of otolaryngology and head and neck surgery: official publication of the Association of Otolaryngologists of India 70:43–8. 10.1007/s12070-017-1208-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.CLSI. Performance Standards for Antimicrobial Susceptibility Testing. Kristy L. Leirer M, editor. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 36.Kanthawong S, Nazmi K, Wongratanacheewin S, Bolscher JG, Wuthiekanun V, Taweechaisupapong S. 2009. In vitro susceptibility of Burkholderia pseudomallei to antimicrobial peptides. Int J Antimicrob Agents 34:309–14. 10.1016/j.ijantimicag.2009.05.012 [DOI] [PubMed] [Google Scholar]
- 37.Sawasdidoln C, Taweechaisupapong S, Sermswan RW, Tattawasart U, Tungpradabkul S, Wongratanacheewin S. 2010. Growing Burkholderia pseudomallei in biofilm stimulating conditions significantly induces antimicrobial resistance. PLoS One 5:e9196 10.1371/journal.pone.0009196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ceri H, Olson M, Stremick C, Read R, Morck D, Buret A. 1999. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol 37:1771–6. 10.1128/JCM.37.6.1771-1776.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia L. Synergism testing: broth microdilution checkerboard and broth macrodilution methods. LSG & Associates, Santa Monica, CA, USA: ASM Press; 2010. 512–23 p. [Google Scholar]
- 40.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1 10.1093/jac/dkg301 [DOI] [PubMed] [Google Scholar]
- 41.Deng DM, Hoogenkamp MA, Exterkate RA, Jiang LM, van der Sluis LW, Jacob M, et al. 2009. Influence of Streptococcus mutans on Enterococcus faecalis biofilm formation. J Endod 35:1249–52. 10.1016/j.joen.2009.05.038 [DOI] [PubMed] [Google Scholar]
- 42.Gordon YJ, Huang LC, Romanowski EG, Yates KA, Proske RJ, McDermott AM. 2005. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr Eye Res 30:385–94. 10.1080/02713680590934111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young FM, Phungtamdet W, Sanderson BJ. 2005. Modification of MTT assay conditions to examine the cytotoxic effects of amitraz on the human lymphoblastoid cell line, WIL2NS. Toxicol In Vitro 19:1051–9. 10.1016/j.tiv.2005.05.001 [DOI] [PubMed] [Google Scholar]
- 44.Ivanchenko O, Karpishchenko S, Kozlov R, Krechikova O, Otvagin I, Sopko O, et al. 2016. The microbiome of the maxillary sinus and middle nasal meatus in chronic rhinosinusitis. Rhinology 54:68–74. 10.4193/Rhin15.018 [DOI] [PubMed] [Google Scholar]
- 45.Brook I. 2016. Microbiology of chronic rhinosinusitis. Eur J Clin Microbiol Infect Dis 35:1059–68. 10.1007/s10096-016-2640-x [DOI] [PubMed] [Google Scholar]
- 46.Barshak MB, Durand ML. 2017. The role of infection and antibiotics in chronic rhinosinusitis. Laryngoscope Investig Otolaryngol 2:36–42. 10.1002/lio2.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mantovani K, Bisanha AA, Demarco RC, Tamashiro E, Martinez R, Anselmo-Lima WT. 2010. Maxillary sinuses microbiology from patients with chronic rhinosinusitis. Braz J Otorhinolaryngol 76:548–51. 10.1590/S1808-86942010000500002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foreman A, Boase S, Psaltis A, Wormald P-J. 2012. Role of bacterial and fungal biofilms in chronic rhinosinusitis. Curr Allergy Asthma Rep 12:127–35. 10.1007/s11882-012-0246-7 [DOI] [PubMed] [Google Scholar]
- 49.Pourmousa R, Dadashzadeh R, Ahangarkani F, Rezai MS. 2016. Frequency of bacterial agents isolated from patients with chronic sinusitis in northern iran. Glob J Health Sci 8:239 10.5539/gjhs.v8n5p239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suh JD, Kennedy DW. 2011. Treatment options for chronic rhinosinusitis. Proc Am Thorac Soc 8:132–40. 10.1513/pats.201003-028RN [DOI] [PubMed] [Google Scholar]
- 51.Goldstein EJ, Citron DM, Merriam CV. 1999. Comparative in vitro activities of amoxicillin-clavulanate against aerobic and anaerobic bacteria isolated from antral puncture specimens from patients with sinusitis. Antimicrob Agents Chemother 43:705–7. 10.1128/AAC.43.3.705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dworniczek E, Frączek M, Seniuk A, Kassner J, Sobieszczańska B, Adamski J, et al. 2009. Bacterial biofilms in patients with chronic rhinosinusitis. Folia Microbiol 54:559–62. 10.1007/s12223-009-0082-x [DOI] [PubMed] [Google Scholar]
- 53.Kifer D, Mužinić V, Klarić MŠ. 2016. Antimicrobial potency of single and combined mupirocin and monoterpenes, thymol, menthol and 1, 8-cineole against Staphylococcus aureus planktonic and biofilm growth. J Antibiot 69:689 10.1038/ja.2016.10 [DOI] [PubMed] [Google Scholar]
- 54.Mahlapuu M, Håkansson J, Ringstad L, Björn C. 2016. Antimicrobial peptides: an emerging category of therapeutic agents. Front Cell Infect Microbiol 6:194 10.3389/fcimb.2016.00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flemming H-C, Wingender J. 2010. The biofilm matrix. Nat Rev Microbiol 8:623 10.1038/nrmicro2415 [DOI] [PubMed] [Google Scholar]
- 56.Wiens JR, Vasil AI, Schurr MJ, Vasil ML. 2014. Iron-regulated expression of alginate production, mucoid phenotype, and biofilm formation by Pseudomonas aeruginosa. mBio 5:e01010–13. 10.1128/mBio.01010-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan C, Burrows LL, Deber CM. 2004. Helix induction in antimicrobial peptides by alginate in biofilms. J Biol Chem 279:38749–54. 10.1074/jbc.M406044200 [DOI] [PubMed] [Google Scholar]
- 58.Billings N, Millan MR, Caldara M, Rusconi R, Tarasova Y, Stocker R, et al. 2013. The extracellular matrix component Psl provides fast-acting antibiotic defense in Pseudomonas aeruginosa biofilms. PLoS Pathog 9:e1003526 10.1371/journal.ppat.1003526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, et al. 2004. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol 6:269–75. 10.1046/j.1462-5822.2004.00367.x [DOI] [PubMed] [Google Scholar]
- 60.Grassi L, Maisetta G, Esin S, Batoni G. 2017. Combination strategies to enhance the efficacy of antimicrobial peptides against bacterial biofilms. Front Microbiol 8:2409 10.3389/fmicb.2017.02409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De la Fuente-Núñez C, Reffuveille F, Haney EF, Straus SK, Hancock RE. 2014. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog 10:e1004152 10.1371/journal.ppat.1004152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Overhage J, Campisano A, Bains M, Torfs EC, Rehm BH, Hancock RE. 2008. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect Immun 76:4176–82. 10.1128/IAI.00318-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu C, Tan H, Cheng T, Shen H, Shao J, Guo Y, et al. 2013. Human β-defensin 3 inhibits antibiotic-resistant Staphylococcus biofilm formation. J Surg Res 183:204–13. 10.1016/j.jss.2012.11.048 [DOI] [PubMed] [Google Scholar]
- 64.Beaudoin T, Stone TA, Glibowicka M, Adams C, Yau Y, Ahmadi S, et al. 2018. Activity of a novel antimicrobial peptide against Pseudomonas aeruginosa biofilms. Sci Rep 8:14728 10.1038/s41598-018-33016-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rudack C, Sachse F, Alberty J. 2006. Primary role of growth‐related oncogene‐α and granulocyte chemotactic protein‐2 as neutrophil chemoattractants in chronic rhinosinusitis. Clin Exp Allergy 36:748–59. 10.1111/j.1365-2222.2006.02501.x [DOI] [PubMed] [Google Scholar]
- 66.Thomas AJ, Pulsipher A, Davis BM, Alt JA. 2017. LL-37 causes cell death of human nasal epithelial cells, which is inhibited with a synthetic glycosaminoglycan. PloS one 12:e0183542 10.1371/journal.pone.0183542 [DOI] [PMC free article] [PubMed] [Google Scholar]