Abstract
Extracellular adenosine, a danger signal, can cause hypothermia. We generated mice lacking neuronal adenosine A1 receptors (A1AR, encoded by the Adora1 gene) to examine the contribution of these receptors to hypothermia. Intracerebroventricular injection of the selective A1AR agonist (Cl-ENBA, 5'-chloro-5'-deoxy-N6-endo-norbornyladenosine) produced hypothermia, which was reduced in mice with deletion of A1AR in neurons. A non-brain penetrant A1AR agonist [SPA, N6-(p-sulfophenyl) adenosine] also caused hypothermia, in wild type but not mice lacking neuronal A1AR, suggesting that peripheral neuronal A1AR can also cause hypothermia. Mice expressing Cre recombinase from the Adora1 locus were generated to investigate the role of specific cell populations in body temperature regulation. Chemogenetic activation of Adora1-Cre-expressing cells in the preoptic area did not change body temperature. In contrast, activation of Adora1-Cre-expressing dorsomedial hypothalamus cells increased core body temperature, concordant with agonism at the endogenous inhibitory A1AR causing hypothermia. These results suggest that A1AR agonism causes hypothermia via two distinct mechanisms: brain neuronal A1AR and A1AR on neurons outside the blood-brain barrier. The variety of mechanisms that adenosine can use to induce hypothermia underscores the importance of hypothermia in the mouse response to major metabolic stress or injury.
Introduction
Extracellular adenosine, a product of excessive ATP breakdown such as from extreme metabolic demand or tissue injury, has been called a ‘retaliatory metabolite’ or danger signal [1]. Due to rapid (i.e., seconds) cellular uptake and metabolism, extracellular adenosine levels are typically low [2] and many of its effects occur locally. Adenosine elicits protective physiology to diminish metabolic demand, reduce injury, and orchestrate recovery from the instigating ‘extreme’ physiology [3]. The adenosine A1 receptor (A1AR) is one of the four adenosine G protein-coupled receptors and is primarily coupled to Gi/o [4]. Its structure has been solved [5]. A1AR is very widely expressed and agonism at this receptor has cardiovascular (bradycardia, protection from ischemia-reperfusion injury), nervous system (antinociception, anti-seizure, protection from ischemia injury, sleep), anti-inflammatory, antidiuretic, and tissue protective (lung, kidney) actions, among other effects [6].
Clinically, hypothermia is used to reduce injury after neonatal hypoxia or cardiopulmonary resuscitation [7]. The hypothermia is typically produced by physical cooling. However, a wide variety of pharmacologic agents can cause hypothermia (see [8]), including adenosine [9], and there is interest in using such agents clinically. This can be readily investigated using mice, as their small size makes them a sensitive, responsive model for exploring mammalian thermal physiology [10]. In the brain, the preoptic area (POA) plays a major integratory role in the control of body temperature [11]. For example, this region has neurons that can drive hypothermia/torpor [12], so this brain region is a prime candidate for pharmacologic actions.
A number of lines of evidence indicate a role for A1AR in regulation of core body temperature (Tb), particularly hypothermia. Classic pharmacologic studies identified agonism at brain A1AR as able to cause hypothermia [13] and there is interest in using A1AR agonists for therapeutic hypothermia [14]. Selective agonist microinjections and other localization studies suggested a role in hypothermia for A1AR in multiple brain regions, including the POA [15–18]. However, we and others have determined that agonists selective for each of the four adenosine receptors can cause hypothermia [19–22]. Also, while a role for A1AR in torpor has been proposed [23, 24], neither A1AR nor any of the other ARs is required for torpor [21, 25].
Understanding the role of A1AR in temperature regulation is complicated by the very wide distribution of the A1AR throughout the brain [6, 26] and its presence in both neurons and glia [27]. The highest brain A1AR concentrations are in portions of the hippocampus, thalamus, and cerebral and cerebellar cortex. Interestingly, A1AR levels are much lower in the hypothalamus, including the POA [26]. Here we investigated where A1AR agonists might act to cause hypothermia.
Materials and methods
Mice
Mice at 3–12 months of age were housed at 21–22°C with a 12:12-h dark:light cycle (lights on at 0600) in a clean, conventional facility with paper bedding (7099-TEK-fresh, Envigo, Indianapolis, IN) and ad libitum access to water and chow (7022, 15% kcal fat, Envigo Inc, Madison, WI). Experiments were approved by the NIDDK Institutional Animal Care and Use Committee (protocol K016-DEOB-20). Mice were studied >7 days after any operation or prior treatment, and singly housed after telemeter implantation. Reuse of mice tends to reduce physical activity levels during drug testing, presumably due to acclimatization to handling. No specific effort was made to acclimatize mice in individual experiments. Mice were euthanized by exposure to carbon dioxide followed by cervical dislocation.
Male C57BL/6J mice were purchased from Jackson Laboratories, Bar Harbor, ME (JAX; #000664). Ai6 mice carrying Cre-dependent fluorescent protein ZsGreen [28] were purchased from JAX (#007906). Male Adora1-/- mice on a C57BL/6J background were provided by Dr. Jurgen Schnermann, NIDDK [29] and genotyped as described [21]. Adora1fl/fl mice [30] were generously provided by Dr. Robert Greene, UTSW, and genotyped by PCR. Primers x692 (forward, 5'-CCACCATTATCTGGCTCCCAT) and x691 (reverse, 5'-GCTGAGTCACCACTGTCTTGT) produce 268-bp (wild-type allele) and 302-bp (flox allele) products. Primers x692 and x708 (reverse, 5'-GCTCCCCTGTCTGACTGAAG) produce a ~230-bp product from a deleted flox allele. Syn1-Cre mice expressing Cre in neurons [31] were purchased from JAX (#003966) and genotyped (~300 bp product) using primers x682 (forward, 5'-CTCAGCGCTGCCTCAGTCT) and x683 (reverse, 5'-GCATCGACCGGTAATGCA). Offspring (both sexes) of Adora1fl/fl x Adora1fl/+;Syn1-Cre/+ and Adora1del/fl x Adora1fl/+;Syn1-Cre/+ matings were used for neuronal deletion experiments.
Generation of Adora1-Cre mice
The Adora1-Cre mouse was made in a B6D2F1/J founder at the NHLBI Transgenic Core using CRISPR/Cas9 to insert a T2A peptide sequence and Cre recombinase at the Adora1 stop codon. The T2A sequence adds EGRGSLLTCGDVEENPG to the C terminus of A1AR and a proline to the N terminus of Cre. It is expected that the two proteins are translated in a 1:1 ratio [32] and that the added T2A sequence may reduce A1AR function [33, 34]. The founder was bred to C57BL/6J once. Presence of Adora1-Cre (553 bp) vs wild type (318 bp) alleles was determined by PCR using primers x617 (common forward, 5'-AAGTTCCGGGTCACCTTTCT), cre1 (Cre reverse, 5'-CCTGTTTTGCACGTTCACCG), and x630 (Adora1 reverse, 5'- ACTCCAAACCTCCTCCAGGT). While homozygous Adora1-Cre mice are fertile and appear grossly normal, only heterozygous Adora1-Cre mice were used in this study. The Adora1-Cre mouse is available from the corresponding author.
Compounds
Compounds (source; vehicle) were obtained as follows: Cl-ENBA [(±)-5'-chloro-5'-deoxy-N6-endo-norbornyladenosine, Tocris 3576; 10% DMSO], SPA [N6-(p-sulfophenyl)adenosine, Sigma S198; saline], CNO (clozapine-N-oxide, Sigma C0832; saline).
Quantitative PCR and RT-PCR
DNA and RNA were extracted (Allprep DNA/RNA micro Kit, Qiagen, Germantown, MD) from whole brain, excluding cerebellum and brain stem. RNA was reverse transcribed (Transcriptor High Fidelity cDNA Synthesis Kit, Roche, Indianapolis, IN). DNA and cDNA were quantified (QuantStudio 7 Flex Real-Time PCR System, Applied Biosystems, Waltham, MA) using SYBR green. Adora1 mRNA primers were x711 (forward, 5'-CATTGGGCCACAGACCTACT) and x713 (reverse, 5'-TGTACCGGAGAGGGATCTTG), normalized to 18S RNA using primers x530 (forward, 5'-AGTCCCTGCCCTTTGTACACA), and x531 (reverse, 5'-CGATCCGAGGGCCTCACTA).
Telemetric monitoring of body temperature and activity
Core body temperature (Tb) and physical activity were continuously measured by telemetry, using G2 E-Mitters implanted intraperitoneally, ER4000 energizer/receivers, and VitalView software (v 5.0 Starr Life Sciences, Oakmont, PA), with data collected each minute [35].
Surgical procedures
Mice were anesthetized with ketamine/xylazine (80/10 mg/kg, i.p.) and placed in a stereotaxic instrument (Digital Just for Mouse Stereotaxic Instrument, Stoelting). Ophthalmic ointment (Puralube, Dechra) was applied. Post-surgery mice received subcutaneous sterile saline injections to prevent dehydration and an analgesic (buprenorphine; 0.1 mg/kg, i.p.). G2 E-Mitters were implanted intraperitoneally as described [35].
Central infusions
Sterile guide cannulas (5.25 mm, 26 gauge; Plastics One, Roanoke, VA) were unilaterally implanted into the lateral ventricle (coordinates relative to bregma: -0.34 mm anterior, 1.0 mm lateral, 1.7 mm ventral) and fixed with dental cement (Parkell, Edgewood, NY). Compounds in 5 μl were infused (0.5 μl/min) through a 33-gauge cannula protruding 0.5 mm past the tip of the guide cannula using PE-50 tubing fitted to a 5 μl syringe (Hamilton, Reno, NV) on a dual syringe pump (KD Scientific, Holliston, MA).
Virus injections
All injections (200 nl) were done with pulled-glass pipettes (pulled 20–40 μm tip diameter; 0.275 ID, 1 mm OD, Wilmad Lab Glass) at a visually controlled rate of 50 nl per min with an air pressure system regulator (Grass Technologies, Model S48 Stimulator). The pipette was kept in place for 5 min after injection. The viruses AAV8-hSyn-DIO-hM3Dq-mCherry (gift from B. Roth; Addgene viral prep # 44361-AAV8 [36]) and AAV8-hSyn-DIO-hM4Di-mCherry (gift from B. Roth; Addgene viral prep # 44362-AAV8 [36]) were injected bilaterally into the preoptic area (POA, coordinates relative to bregma: 0.35 mm anterior, ±0.3 mm lateral, -5.25 mm ventral) or dorsomedial hypothalamus (DMH, coordinates relative to bregma: 1.85 mm posterior, ±0.25 mm lateral, -5.2 mm ventral) of Adora1-Cre mice. This batch of AAV8-hSyn-DIO-hM4Di-mCherry was functional in other experiments in our lab that were not part of this project. The hM4Di-mCherry is a fusion protein, so if mCherry is expressed, then hM4Di is also expressed.
Chemogenetics experimental procedure
hM3Dq and hM4Di were activated by CNO (1 mg/kg, i.p.) or saline vehicle. After completion of all experiments, mice were anesthetized (chloral hydrate, 500 mg/kg, i.p.), perfused transcardially with 0.9% saline followed by 10% neutral buffered formalin, the brain was removed, and reporter expression was visualized by immunohistochemistry [33]. Mice without hM3Dq or hM4Di expression in the target area on at least one side were excluded from analysis.
Experimental design and statistical analysis
Drug treatments were typically administered in crossover design using randomized treatment order. Hypothermia was assessed as the mean Tb from 0 to 60 minutes after dosing. Hyperthermia was assessed from 60 to 120 minutes after dosing to exclude the handling-induced Tb increase. Physical activity was assessed as the mean from 10 to 60 (hypothermia experiments to avoid the first 10 min after handling) or 60 to 120 (hyperthermia experiments) minutes after dosing. In crossover experiments, paired t-tests were used to compare the within mouse effect of drug vs vehicle. Unpaired t-tests were used to compare the within mouse drug vs vehicle effect between genotypes. Statistical significance was defined as 2-tailed P <0.05. Data are presented as mean ±SEM. No statistical methods were used to pre-determine sample size. Data, keyed to each figure, are available in the S1 Data.
Image capture and processing
Images were captured using an Olympus BX61 motorized microscope with Olympus BX-UCB hardware (VS120 slide scanner) and processed (including contrast adjustments) using OlyVIA software (Olympus).
Results
No effect on baseline body temperature from neuronal loss of A1AR
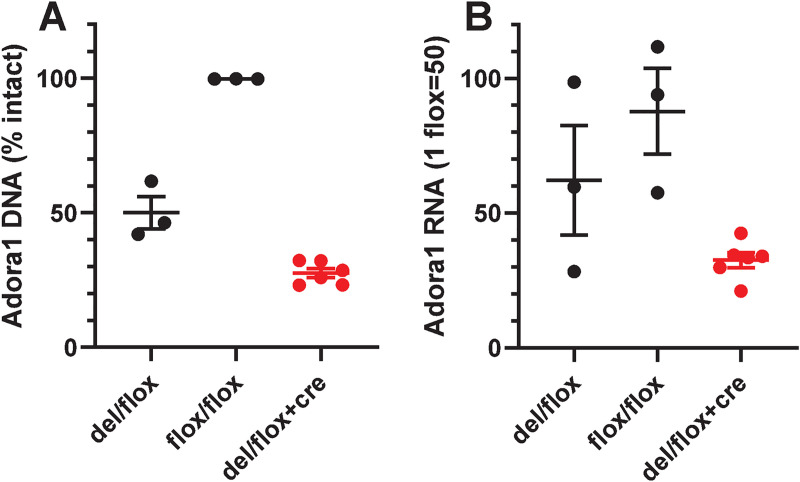
To distinguish between adenosine action on glia [27] and neurons, we produced mice carrying both floxed Adora1 and Syn1-Cre to selectively ablate A1AR function in neurons. The effect of neuronal deletion was studied in pooled Adora1fl/fl;Syn1-Cre and Adora1del/fl;Syn1-Cre mice (hereafter referred to collectively as Adora1fl;Syn1-Cre, where del indicates a germline-deleted allele). In whole brain, Syn1-Cre produced 45% deletion per flox allele (Fig 1A). Concomitantly, Syn1-Cre reduced Adora1 mRNA levels by 35% per flox allele (Fig 1B). Since Syn1-Cre is selectively expressed in differentiated neurons [31], these data are consistent with neuronal deletion of Adora1fl, with the remaining expression likely derived from non-Syn1-Cre-expressing (non-neuronal) cells.
Fig 1. Levels of Adora1 DNA and RNA in mouse brain.
(A) Adora1 DNA levels in brains from control (black, n = 3 del/flox and n = 3 flox/flox) and Syn1-Cre (red, n = 6 del/flox;Syn1-Cre) mice. The % intact is the % of the signal for two intact alleles, calculated assuming a level of 0% per deleted (del) allele and 50% per wild type or undeleted flox allele. The intact level of 27.6 ± 1.7% (different from 50% at P < 0.0001 by 1 sample t-test) in the del/flox;Syn1-Cre mice indicates 45% deletion of the flox alleles. (B) Adora1 RNA was measured in the same samples, assuming expression of 0% per del allele and 50% per wild type or undeleted flox allele. The RNA level of 32.6 ± 2.8% (different from 50% at P < 0.0001 by 1 sample t-test) in the del/flox;Syn1-Cre mice indicates 35% reduction per flox allele compared to wild type levels. DNA and RNA were extracted from one-half of the brain without cerebellum and brain stem and assayed as detailed in Methods. Data are mean ± SEM.
At baseline, there was no difference between control and Adora1fl;Syn1-Cre littermate mice, matched for sex, in body temperature (Tb) during light or dark phase, or in Tb variability (as Tb span, the difference between the 95th and 5th Tb percentiles). There was also no effect of neuronal deletion on the level of physical activity or its diurnal rhythm (Table 1).
Table 1. Deletion of neuronal A1AR has no effect on baseline body temperature or physical activity.
| Sex | Adora1fl Female | Adora1fl;Syn1-Cre Female | P | Adora1fl Male | Adora1fl;Syn1-Cre Male | P |
|---|---|---|---|---|---|---|
| N | 4 | 5 | 7 | 8 | ||
| Tb, light phase (°C) | 36.17 ±0.07 | 36.40 ±0.16 | 0.27 | 35.67 ±0.16 | 35.76 ±0.12 | 0.64 |
| Tb, dark phase (°C) | 37.37 ±0.17 | 37.39 ±0.16 | 0.94 | 36.77 ±0.13 | 36.93 ±0.05 | 0.25 |
| ΔTb, dark-light (°C) | 1.21 ±0.10 | 0.99 ±0.25 | 0.50 | 1.10 ±0.10 | 1.17 ±0.11 | 0.64 |
| Tb span (°C) | 2.58 ±0.15 | 2.09 ±0.45 | 0.38 | 2.28 ±0.08 | 2.49 ±0.14 | 0.23 |
| Activity, light phase (counts) | 6.8 ±0.5 | 8.1 ±0.6 | 0.18 | 5.4 ±0.3 | 6.4 ±0.4 | 0.09 |
| Activity, dark phase (counts) | 19.6 ±1.1 | 25.0 ±3.5 | 0.23 | 14.5 ±1.0 | 15.5 ±1.1 | 0.51 |
Data collected from 5-month old mice over a continuous 96-hour interval. Tb span is the 95th minus the 5th percentiles. Data are mean ± SEM. P values are from unpaired t-tests comparing genotype within sex.
A1AR agonist-induced hypothermia is mediated by both peripheral and central neurons
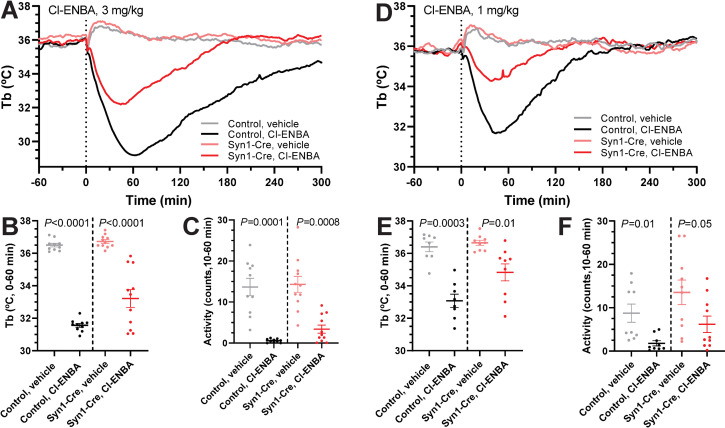
Cl-ENBA is a selective A1AR agonist. Peripherally-dosed Cl-ENBA (3 mg/kg, i.p.) caused robust hypothermia in Adora1fl controls (-4.93 ± 0.17°C vs vehicle at 0 to 60 minutes) and Adora1fl;Syn1-Cre (-3.50 ± 0.53°C vs vehicle) mice (Fig 2A–2C). The hypothermia in Adora1fl;Syn1-Cre mice was attenuated compared to controls (P = 0.02). Since Cl-ENBA at high doses can also cause hypothermia via activation of mast cell A3AR [21], we tested a lower Cl-ENBA dose (1 mg/kg; -3.33 ± 0.50°C in controls vs. -1.83 ± 0.55°C in Adora1fl;Syn1-Cre mice; P = 0.06) (Fig 2D–2F). The similar level of partial loss of Cl-ENBA-induced hypothermia at the two doses suggests that i.p. Cl-ENBA is causing hypothermia partially through neuronal A1AR.
Fig 2. Neuronal A1AR partially mediate Cl-ENBA induced hypothermia.
(A-C) Core body temperature (Tb) and physical activity response to Cl-ENBA (3 mg/kg, i.p.) or vehicle in Adora1fl (n = 10) and Adora1fl;Syn1-Cre (n = 11) mice. (D-F) Tb and physical activity response to Cl-ENBA (1 mg/kg, i.p.) or vehicle in Adora1fl(n = 8) and Adora1fl;Syn1-Cre (n = 9) mice. Data are mean ± SEM (SEM is omitted for visual clarity in A and D). P values calculated by paired t-test comparing drug vs vehicle within mouse.
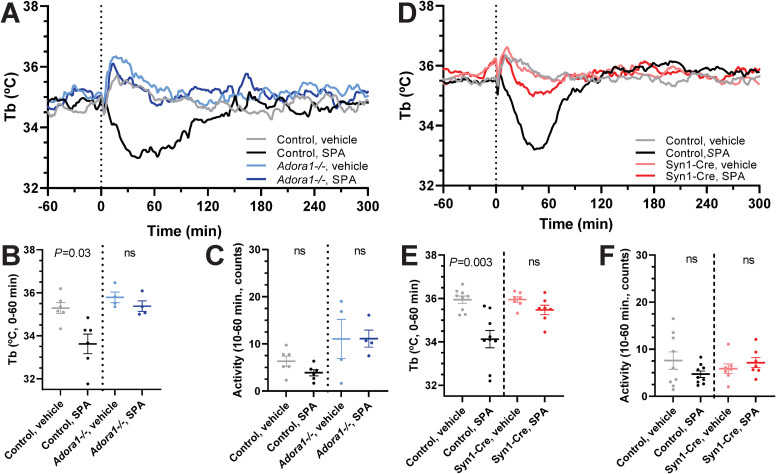
SPA is a non-brain penetrant selective A1AR agonist [37]. Peripherally dosed SPA (1 mg/kg, i.p.) caused hypothermia in wild-type mice that was lost in Adora1-/- mice (Fig 3A–3C). In Adora1fl;Syn1-Cre mice, SPA-induced hypothermia was greatly diminished and not statistically different from vehicle treatment (Fig 3D–3F). These results suggest that hypothermia can be elicited via A1AR expressed by neurons that are outside the blood-brain barrier.
Fig 3. SPA-induced hypothermia is lost in global Adora1-/- and attenuated in Adora1fl;Syn1-Cre mice.
(A-C) Tb and physical activity response to SPA (1 mg/kg, i.p.) in control (n = 6) and Adora1-/- (n = 4) mice. (D-F) Tb and physical activity response to SPA (1 mg/kg, i.p.) in Adora1fl (control, n = 9) and Adora1fl;Syn1-Cre (Syn1-Cre, n = 7) mice. Data are mean ± SEM (SEM is omitted for visual clarity in A and D). P values were calculated by paired t-test comparing drug vs vehicle within mouse.
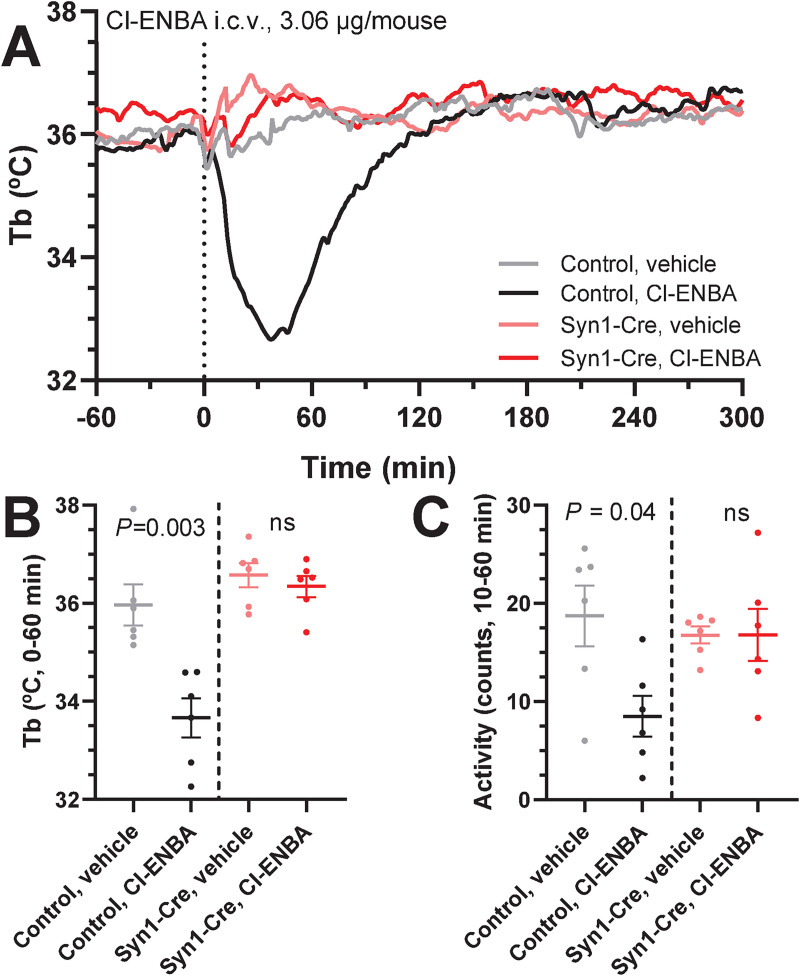
We next tested the ability of a low i.c.v. dose of A1AR agonist to produce hypothermia. Central administration of Cl-ENBA (3.06 μg/mouse; ~0.1 mg/kg) caused hypothermia in control but not Adora1fl;Syn1-Cre mice (-2.30 ± 0.43°C vs -0.23 ± 0.17°C; P = 0.001) (Fig 4A–4C). This demonstrates that A1AR agonists can cause hypothermia by acting directly on brain neurons.
Fig 4. Central administration of Cl-ENBA does not cause hypothermia in Adora1fl;Syn1-Cre mice.
(A-C) Tb and physical activity response to Cl-ENBA (3.06 μg/mouse, i.c.v.) or vehicle in Adora1fl (control, n = 6) and Adora1fl;Syn1-Cre (Syn1-Cre, n = 6) mice. Data are mean ± SEM (SEM is omitted for visual clarity in A). P values were calculated by paired t-test comparing drug vs vehicle within mouse.
Activation of Adora1-Cre-expressing neurons in the dorsomedial hypothalamus increased body temperature
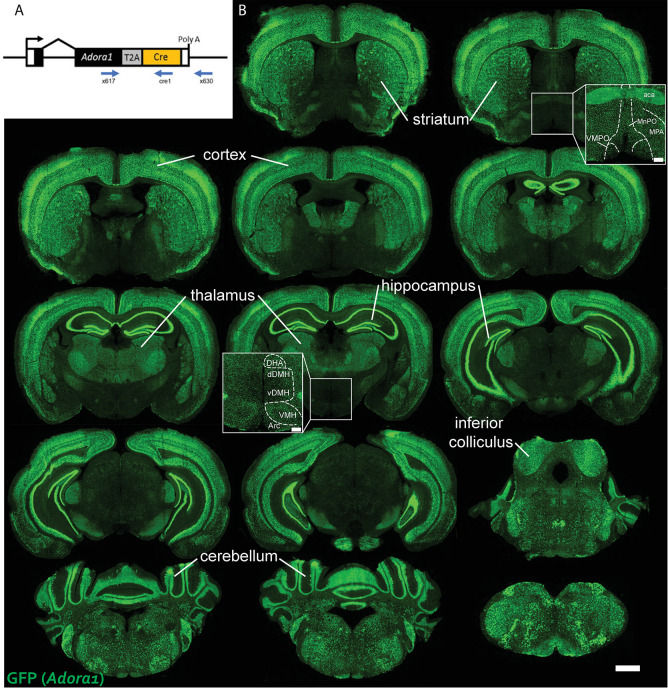
Since A1AR is widely distributed in the brain, we wished to probe specific regions for their potential role in driving A1AR agonist-induced hypothermia. Adora1-Cre mice were produced by targeted insertion of a Cre recombinase gene into the endogenous Adora1 locus, preserving the coding region (Methods, Fig 5A). The mice were bred with reporter mice expressing GFP in a Cre-dependent manner. The resulting GFP expression was widespread and particularly high in the cortex, hippocampus, and thalamus (Fig 5B). This GFP reporter expression pattern matches that reported for A1AR ligand binding activity [26].
Fig 5. Expression pattern of Adora1-Cre in mouse brain.
(A) Schematic representation of the Adora1-Cre allele, showing the Adora1coding region in black, T2A peptide in gray, and Cre recombinase in orange. Primers for PCR-based genotyping are also indicated. Not to scale. (B) Representative brain sections showing Cre-dependent GFP expression in Adora1-Cre;Ai6 mouse brain. Scale bar is 1 mm. Inset scale bar is 200 μm. MnPO, median preoptic nucleus; MPA, medial preoptic area; VMPO, ventromedial preoptic area; DHA, dorsal hypothalamic area; dDMH, dorsal part of dorsomedial hypothalamus; vDMH, ventral part of dorsomedial hypothalamus; VMH, ventromedial hypothalamus.
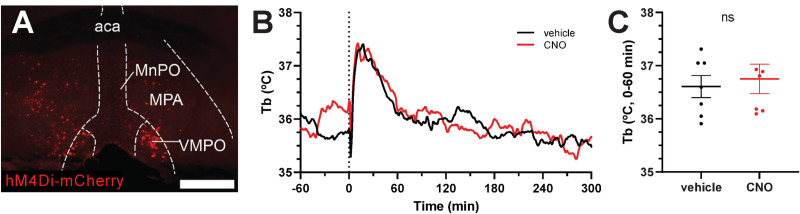
The preoptic area (POA) is a region of the brain with major control over Tb; other areas also contribute [38]. Local injection of A1AR agonist has been shown to produce hypothermia in hypothalamic sites, including POA and dorsomedial hypothalamus (DMH) [16], so we focused on these regions. A1AR is coupled to Gi/o, therefore we used chemogenetics to selectively target Adora1-Cre neurons with viral expression of a Gi-coupled designer receptor exclusively activated by designer drug (DREADD), hM4Di, to mimic the endogenous inhibitory A1AR signaling. Mice with virally-delivered hM4Di in the POA were treated with DREADD agonist, clozapine-N-oxide (CNO), or vehicle. No effect of CNO on Tb was observed (Fig 6A–6C). All mice had at least unilateral virus expression in the medial preoptic area, and some mice had virus expression also in the medial septal nuclei and/or diagonal band of Broca. Thus, agonism of an inhibitory DREADD in POAAdora1 neurons did not cause hypothermia.
Fig 6. Chemogenetic inhibition of POAAdora1 neurons has no effect on Tb.
(A) Example of hM4Di-mCherry expression in Adora1-Cre mouse injected with AAV8-hSyn-DIO-hM4Di-mCherry in the POA. Scale bar is 500 μm. (B) Tb response to CNO (1 mg/kg, i.p.) vs. vehicle in Adora1-Cre mice with hM4Di-mCherry in POA (n = 7). (C) Mean Tb at 0 to 60 minutes after dosing. Data are mean ± SEM (SEM is omitted for visual clarity in B). P value was calculated by paired t-test comparing drug vs vehicle within mouse. Aca, anterior commissure; MnPO, median preoptic area; MPA, medial preoptic area; VMPO, ventromedial preoptic area.
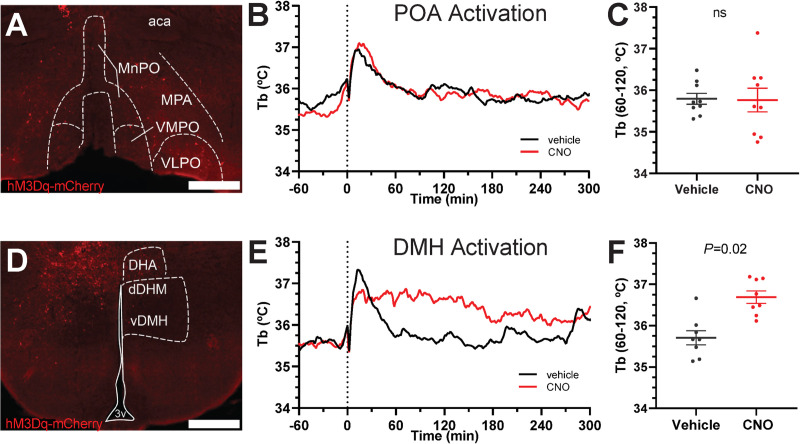
We next tested activation of an excitatory Gq-coupled DREADD, hM3Dq, in Adora1-Cre-expressing cells. As endogenous A1AR signaling is inhibitory, an increase in Tb due to chemogenetic activation of stimulatory Gq would be a concordant observation. Activation of POAAdora1 neurons with CNO had no effect on Tb (Fig 7A–7C). All mice had unilateral or bilateral virus expression in the medial preoptic area, with virus expression in varying extent throughout the anterior hypothalamus. In contrast, activation of DMHAdora1 neurons expressing hM3Dq with CNO increased Tb (Fig 7D–7F). All mice had unilateral or bilateral virus expression restricted to the DMH/dorsal hypothalamic area. Thus, the DMH is a potential candidate region for neurons driving A1AR agonist-induced hypothermia.
Fig 7. Chemogenetic activation of DMHAdora1, but not POAAdora1 neurons increases Tb.
A-C tests activation of the POA. (A) Example of hM3Dq-mCherry expression in Adora1-Cre mouse injected with AAV8-hSyn-DIO-hM3Dq-mCherry in the POA. (B) Tb response to CNO (1 mg/kg, i.p.) vs. vehicle in Adora1-Cre mice with hM3Dq-mCherry in POA (n = 11). (C) Mean Tb at 60 to 120 minutes after dosing with CNO (1 mg/kg, i.p.) vs. vehicle. D-F tests activation of the DMH. (D) Example of hM3Dq-mCherry expression in Adora1-Cre mouse injected with AAV8-hSyn-DIO-hM3Dq-mCherry in the DMH. (E) Tb response to CNO (1 mg/kg, i.p.) vs. vehicle in Adora1-Cre mice with hM3Dq-mCherry in DMH (n = 8). (F) Mean Tb at 60 to 120 minutes after dosing with CNO (1 mg/kg, i.p.) vs. vehicle (n = 8). Data are mean ± SEM (SEM is omitted for visual clarity in B and E). P values were calculated by paired t-test comparing drug vs vehicle within mouse. Scale bar is 500 μm. aca, anterior commissure; dDMH, dorsomedial hypothalamus, dorsal part; DHA, dorsal hypothalamic area; MnPO, median preoptic area; MPA, medial preoptic area; vDMH, dorsomedial hypothalamus, ventral part; VMPO, ventromedial preoptic area; VLPO, ventrolateral preoptic area.
Discussion
The brain controls Tb. Thus, we had anticipated identifying an A1AR-expressing neuron population, possibly in the POA, as the driver of A1AR agonist-induced hypothermia. However, our results suggest that A1AR agonism can cause hypothermia acting at neuronal A1AR both inside and outside the brain, demonstrating that adenosine can cause hypothermia via more than one A1AR neuronal mechanism.
Central mechanisms of A1AR hypothermia
While A1AR is found in microglia, oligodendrocytes, astrocytes, and neurons [39], the loss-of-function experiments demonstrate that neurons (Syn1-Cre-positive cells) are required for hypothermia caused by central administration of an A1AR agonist. Activation of different defined populations of POA neurons can increase or decrease Tb [12, 40–44]. In addition, local A1AR agonist administration to this region causes hypothermia [16]. Thus, we had expected that chemogenetic inhibition or activation of POAAdora1 neurons would affect Tb, but this was not observed. The effect on Tb from stimulation of POAAdora1 neurons may be more variable than in controls, suggesting that there may be multiple POAAdora1 neuron populations with differing effects on Tb.
Chemogenetic activation of Adora1-Cre-expressing neurons of the DMH, another known thermoregulatory center, uniformly increased Tb, consistent with reports that disinhibition and activation of DMH neurons increase Tb [33, 40, 45]. One interpretation of our results is that DMHAdora1 neurons are driving A1AR agonist-induced hypothermia, as activation of these neurons (opposing the inhibitory signaling from endogenous A1AR activation) increased Tb. Another interpretation is that the chemogenetically manipulated neurons have a role in thermoregulation, but this is not directly related to pharmacologic A1AR agonist-induced hypothermia.
Our results do not rule out the possibility of a single, specific nucleus mediating central A1AR hypothermia. However, with widespread expression throughout the brain, we hypothesize that expression of A1AR may not identify a discrete neuronal population that drives hypothermia. Rather, adenosine acting on A1AR may be a general mechanism to reduce local neuronal activity, with the effect on Tb depending on the particular neuronal subpopulation’s direct or indirect contributions to thermoregulation.
Peripheral mechanisms of A1AR hypothermia
Our results indicate A1AR agonist-induced hypothermia can be caused by neuronal A1AR outside the central nervous system. The hypothermic effect of the non-brain penetrant, peripherally-restricted A1AR agonist SPA was lost in Adora1fl;Syn1-Cre mice, indicating that peripheral A1AR agonist-induced hypothermia is mediated by cells expressing synapsin-1 (i.e. peripheral nerves). A1AR is found throughout the peripheral nervous system (PNS): in motor and sensory nerve terminals of rats, as well as sympathetic and parasympathetic nerves [46–49]. Like central A1AR, PNS A1AR play a role in regulating numerous physiological processes (nociception, vascular tone, etc.) by providing an inhibitory tone on neurotransmission. Therefore, the effect of PNS A1AR agonism on Tb depends on the function of the peripheral neuron being inhibited.
A1AR is present in many non-neuronal cells (smooth muscle, epithelial, immune cells) and tissues (heart, adipose tissue, pancreas, kidney) that have a very wide variety of functions (reviewed in [1]). Agonism of non-neural A1AR can produce physiologic effects such as hypotension that could secondarily decrease Tb. The lack of significant hypothermia produced by the peripheral A1AR agonist SPA in Adora1fl;Syn1-Cre mice suggests that while non-neuronal cells may contribute, neuronal A1AR are required for hypothermia by this mechanism. Further research will be needed to identify the specific neuronal populations involved.
Adenosine as a physiologic and general danger signal
Adenosine can act at each of the four adenosine receptors to cause hypothermia. Activation of A3AR on peripheral mast cells causes hypothermia via histamine release, followed by activation of H1 receptors [22, 50, 51]. Agonism at peripheral A2AAR or central A2BAR both cause hypothermia [20, 52]. However, the specific cells that elicit hypothermia when directly activated by A2AAR and A2BAR agonists have not been identified. Including A1AR on central and on peripheral neurons, there are five identified paths by which adenosine agonism elicits hypothermia. When hypothermia is studied in sufficient detail, it is accompanied by hypometabolism, decreased physical activity, and hypotension. While hypometabolism is likely required to achieve hypothermia, different driving physiology (eg., hypotension vs torpor) can produce hypothermia.
Adenosine is a ubiquitous molecule that acts locally to signal metabolic stress or injury. The distinct mechanism(s) by which activation of each AR causes hypothermia suggests that adenosine signaling is harnessed to minimize damage, regardless if the signaling occurs centrally or peripherally. Hypothermia can be due to direct adenosine agonism on thermoregulatory neurons, or a secondary effect of other adenosine-driven physiology. Taken with previous studies, these results demonstrate that at least five distinct mechanisms of hypothermia mediated by adenosine receptors exist. The redundancy in AR signaling highlights the importance of hypothermia as a component of protective response mechanisms.
Supporting information
(XLSX)
Acknowledgments
We thank Alice Franks, Naili Liu, Yuning Huang, and Zhenzhong Cui for experimental support, and Chengyu Liu of the NHLBI Transgenic Core for collaborating in generating the Adora1-Cre mouse.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (ZIA DK075062 and ZIA DK075063 to MLR and ZIA DK070002 to OG). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol Rev. 2018;98(3):1591–625. Epub 2018/06/01. 10.1152/physrev.00049.2017 . [DOI] [PubMed] [Google Scholar]
- 2.Moser GH, Schrader J, Deussen A. Turnover of adenosine in plasma of human and dog blood. Am J Physiol. 1989;256(4 Pt 1):C799–806. 10.1152/ajpcell.1989.256.4.C799 . [DOI] [PubMed] [Google Scholar]
- 3.Fredholm BB. Adenosine—a physiological or pathophysiological agent? J Mol Med (Berl). 2014;92(3):201–6. 10.1007/s00109-013-1101-6 . [DOI] [PubMed] [Google Scholar]
- 4.Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—an update. Pharmacol Rev. 2011;63(1):1–34. Epub 2011/02/10. 10.1124/pr.110.003285 pr.110.003285 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glukhova A, Thal DM, Nguyen AT, Vecchio EA, Jorg M, Scammells PJ, et al. Structure of the Adenosine A1 Receptor Reveals the Basis for Subtype Selectivity. Cell. 2017;168(5):867–77 e13. Epub 2017/02/25. 10.1016/j.cell.2017.01.042 . [DOI] [PubMed] [Google Scholar]
- 6.Varani K, Vincenzi F, Merighi S, Gessi S, Borea PA. Biochemical and Pharmacological Role of A1 Adenosine Receptors and Their Modulation as Novel Therapeutic Strategy. Adv Exp Med Biol. 2017;1051:193–232. Epub 2017/07/06. 10.1007/5584_2017_61 . [DOI] [PubMed] [Google Scholar]
- 7.Arrich J, Holzer M, Havel C, Mullner M, Herkner H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2016;2:CD004128 Epub 2016/02/16. 10.1002/14651858.CD004128.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark WG. Changes in body temperature after administration of antipyretics, LSD, delta 9-THC and related agents: II. Neurosci Biobehav Rev. 1987;11(1):35–96. Epub 1987/01/01. S0149-7634(87)80003-9 [pii]. 10.1016/s0149-7634(87)80003-9 . [DOI] [PubMed] [Google Scholar]
- 9.Bennet DW, Drury AN. Further observations relating to the physiological activity of adenine compounds. J Physiol. 1931;72(3):288–320. 10.1113/jphysiol.1931.sp002775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skop V, Guo J, Liu N, Xiao C, Hall KD, Gavrilova O, et al. Mouse Thermoregulation: Introducing the Concept of the Thermoneutral Point. Cell Rep. 2020;31(2):107501 Epub 2020/04/16. 10.1016/j.celrep.2020.03.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison SF, Nakamura K. Central Mechanisms for Thermoregulation. Annu Rev Physiol. 2019. Epub 2018/09/27. 10.1146/annurev-physiol-020518-114546 . [DOI] [PubMed] [Google Scholar]
- 12.Takahashi TM, Sunagawa GA, Soya S, Abe M, Sakurai K, Ishikawa K, et al. A discrete neuronal circuit induces a hibernation-like state in rodents. Nature. 2020. Epub 2020/06/13. 10.1038/s41586-020-2163-6 . [DOI] [PubMed] [Google Scholar]
- 13.Anderson R, Sheehan MJ, Strong P. Characterization of the adenosine receptors mediating hypothermia in the conscious mouse. Br J Pharmacol. 1994;113(4):1386–90. Epub 1994/12/01. 10.1111/j.1476-5381.1994.tb17151.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drew KL, Romanovsky AA, Stephen TK, Tupone D, Williams RH. Future approaches to therapeutic hypothermia: a symposium report. Temperature (Austin). 2015;2(2):168–71. 10.4161/23328940.2014.976512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ticho SR, Radulovacki M. Role of adenosine in sleep and temperature regulation in the preoptic area of rats. Pharmacol Biochem Behav. 1991;40(1):33–40. Epub 1991/09/01. 10.1016/0091-3057(91)90317-u . [DOI] [PubMed] [Google Scholar]
- 16.Shintani M, Tamura Y, Monden M, Shiomi H. Characterization of N(6)-cyclohexyladenosine-induced hypothermia in Syrian hamsters. J Pharmacol Sci. 2005;97(3):451–4. 10.1254/jphs.sc0040178 . [DOI] [PubMed] [Google Scholar]
- 17.Tupone D, Madden CJ, Morrison SF. Central activation of the A1 adenosine receptor (A1AR) induces a hypothermic, torpor-like state in the rat. J Neurosci. 2013;33(36):14512–25. 10.1523/JNEUROSCI.1980-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frare C, Jenkins ME, McClure KM, Drew KL. Seasonal decrease in thermogenesis and increase in vasoconstriction explain seasonal response to N(6) -cyclohexyladenosine-induced hibernation in the Arctic ground squirrel (Urocitellus parryii). J Neurochem. 2019;151(3):316–35. Epub 2019/07/06. 10.1111/jnc.14814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Gimenez-Llort L, et al. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci U S A. 2001;98(16):9407–12. Epub 2001/07/27. 10.1073/pnas.161292398 161292398 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlin JL, Jain S, Duroux R, Suresh RR, Xiao C, Auchampach JA, et al. Activation of adenosine A2A or A2B receptors causes hypothermia in mice. Neuropharmacology. 2018;139:268–78. Epub 2018/03/20. 10.1016/j.neuropharm.2018.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlin JL, Jain S, Gizewski E, Wan TC, Tosh DK, Xiao C, et al. Hypothermia in mouse is caused by adenosine A1 and A3 receptor agonists and AMP via three distinct mechanisms. Neuropharmacology. 2017;114:101–13. 10.1016/j.neuropharm.2016.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlin JL, Tosh DK, Xiao C, Pinol RA, Chen Z, Salvemini D, et al. Peripheral Adenosine A3 Receptor Activation Causes Regulated Hypothermia in Mice That Is Dependent on Central Histamine H1 Receptors. J Pharmacol Exp Ther. 2016;356(2):474–82. Epub 2015/11/27. 10.1124/jpet.115.229872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iliff BW, Swoap SJ. Central adenosine receptor signaling is necessary for daily torpor in mice. Am J Physiol Regul Integr Comp Physiol. 2012;303(5):R477–84. Epub 2012/07/13. 10.1152/ajpregu.00081.2012 ajpregu.00081.2012 [pii]. . [DOI] [PubMed] [Google Scholar]
- 24.Silvani A, Cerri M, Zoccoli G, Swoap SJ. Is Adenosine Action Common Ground for NREM Sleep, Torpor, and Other Hypometabolic States? Physiology (Bethesda). 2018;33(3):182–96. Epub 2018/04/05. 10.1152/physiol.00007.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao C, Liu N, Jacobson KA, Gavrilova O, Reitman ML. Physiology and effects of nucleosides in mice lacking all four adenosine receptors. PLoS Biol. 2019;17(3):e3000161 Epub 2019/03/02. 10.1371/journal.pbio.3000161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fastbom J, Pazos A, Palacios JM. The distribution of adenosine A1 receptors and 5'-nucleotidase in the brain of some commonly used experimental animals. Neuroscience. 1987;22(3):813–26. 10.1016/0306-4522(87)92961-7 . [DOI] [PubMed] [Google Scholar]
- 27.Boison D, Chen JF, Fredholm BB. Adenosine signaling and function in glial cells. Cell Death Differ. 2010;17(7):1071–82. Epub 2009/09/19. 10.1038/cdd.2009.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–40. Epub 2009/12/22. 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, et al. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci U S A. 2001;98(17):9983–8. Epub 2001/08/16. 10.1073/pnas.171317998 98/17/9983 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scammell TE, Arrigoni E, Thompson MA, Ronan PJ, Saper CB, Greene RW. Focal deletion of the adenosine A1 receptor in adult mice using an adeno-associated viral vector. J Neurosci. 2003;23(13):5762–70. Epub 2003/07/05. 10.1523/JNEUROSCI.23-13-05762.2003 [pii]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu Y, Romero MI, Ghosh P, Ye Z, Charnay P, Rushing EJ, et al. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 2001;15(7):859–76. Epub 2001/04/12. 10.1101/gad.862101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szymczak-Workman AL, Vignali KM, Vignali DA. Design and construction of 2A peptide-linked multicistronic vectors. Cold Spring Harb Protoc. 2012;2012(2):199–204. Epub 2012/02/04. 10.1101/pdb.ip067876 . [DOI] [PubMed] [Google Scholar]
- 33.Pinol RA, Zahler SH, Li C, Saha A, Tan BK, Skop V, et al. Brs3 neurons in the mouse dorsomedial hypothalamus regulate body temperature, energy expenditure, and heart rate, but not food intake. Nat Neurosci. 2018;21(11):1530–40. Epub 2018/10/24. 10.1038/s41593-018-0249-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao C, Liu N, Province H, Pinol RA, Gavrilova O, Reitman ML. BRS3 in both MC4R- and SIM1-expressing neurons regulates energy homeostasis in mice. Mol Metab. 2020;36:100969 Epub 2020/04/02. 10.1016/j.molmet.2020.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lute B, Jou W, Lateef DM, Goldgof M, Xiao C, Pinol RA, et al. Biphasic effect of melanocortin agonists on metabolic rate and body temperature. Cell Metab. 2014;20(2):333–45. Epub 2014/07/02. 10.1016/j.cmet.2014.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. The Journal of clinical investigation. 2011;121(4):1424–8. Epub 2011/03/03. 10.1172/JCI46229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobson KA, Nikodijevic O, Ji XD, Berkich DA, Eveleth D, Dean RL, et al. Synthesis and biological activity of N6-(p-sulfophenyl)alkyl and N6-sulfoalkyl derivatives of adenosine: water-soluble and peripherally selective adenosine agonists. J Med Chem. 1992;35(22):4143–9. 10.1021/jm00100a020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan CL, Knight ZA. Regulation of Body Temperature by the Nervous System. Neuron. 2018;98(1):31–48. Epub 2018/04/06. 10.1016/j.neuron.2018.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hackett TA. Adenosine A1 Receptor mRNA Expression by Neurons and Glia in the Auditory Forebrain. Anat Rec (Hoboken). 2018;301(11):1882–905. Epub 2018/10/14. 10.1002/ar.23907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan CL, Cooke EK, Leib DE, Lin YC, Daly GE, Zimmerman CA, et al. Warm-Sensitive Neurons that Control Body Temperature. Cell. 2016;167(1):47–59 e15. 10.1016/j.cell.2016.08.028 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao ZD, Yang WZ, Gao C, Fu X, Zhang W, Zhou Q, et al. A hypothalamic circuit that controls body temperature. Proc Natl Acad Sci U S A. 2017;114(8):2042–7. Epub 2017/01/06. 10.1073/pnas.1616255114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Machado NLS, Bandaru SS, Abbott SBG, Saper CB. EP3R-Expressing Glutamatergic Preoptic Neurons Mediate Inflammatory Fever. J Neurosci. 2020;40(12):2573–88. Epub 2020/02/23. 10.1523/JNEUROSCI.2887-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hrvatin S, Sun S, Wilcox OF, Yao H, Lavin-Peter AJ, Cicconet M, et al. Neurons that regulate mouse torpor. Nature. 2020. Epub 2020/06/13. 10.1038/s41586-020-2387-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang KX, D'Souza S, Upton BA, Kernodle S, Vemaraju S, Nayak G, et al. Violet-light suppression of thermogenesis by opsin 5 hypothalamic neurons. Nature. 2020. Epub 2020/09/04. 10.1038/s41586-020-2683-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res. 2002;928(1–2):113–25. Epub 2002/02/15. 10.1016/s0006-8993(01)03369-8 . [DOI] [PubMed] [Google Scholar]
- 46.Sebastiao AM, Stone TW, Ribeiro JA. The inhibitory adenosine receptor at the neuromuscular junction and hippocampus of the rat: antagonism by 1,3,8-substituted xanthines. Br J Pharmacol. 1990;101(2):453–9. Epub 1990/10/01. 10.1111/j.1476-5381.1990.tb12729.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lima FO, Souza GR, Verri WA Jr., Parada CA, Ferreira SH, Cunha FQ, et al. Direct blockade of inflammatory hypernociception by peripheral A1 adenosine receptors: involvement of the NO/cGMP/PKG/KATP signaling pathway. Pain. 2010;151(2):506–15. Epub 2010/09/04. 10.1016/j.pain.2010.08.014 . [DOI] [PubMed] [Google Scholar]
- 48.Sangsiri S, Dong H, Swain GM, Galligan JJ, Xu H. Impaired function of prejunctional adenosine A1 receptors expressed by perivascular sympathetic nerves in DOCA-salt hypertensive rats. J Pharmacol Exp Ther. 2013;345(1):32–40. Epub 2013/02/12. 10.1124/jpet.112.199612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Searl TJ, Dynda DI, Alanee SR, El-Zawahry AM, McVary KT, Silinsky EM. A1 Adenosine Receptor-Mediated Inhibition of Parasympathetic Neuromuscular Transmission in Human and Murine Urinary Bladder. J Pharmacol Exp Ther. 2016;356(1):116–22. Epub 2015/11/05. 10.1124/jpet.115.228882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salvatore CA, Tilley SL, Latour AM, Fletcher DS, Koller BH, Jacobson MA. Disruption of the A(3) adenosine receptor gene in mice and its effect on stimulated inflammatory cells. J Biol Chem. 2000;275(6):4429–34. Epub 2000/02/08. 10.1074/jbc.275.6.4429 . [DOI] [PubMed] [Google Scholar]
- 51.Jain S, Panyutin A, Liu N, Xiao C, Pinol RA, Pundir P, et al. Melanotan II causes hypothermia in mice by activation of mast cells and stimulation of histamine 1 receptors. Am J Physiol Endocrinol Metab. 2018;315(3):E357–E66. Epub 2018/05/31. 10.1152/ajpendo.00024.2018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eisner C, Kim S, Grill A, Qin Y, Hoerl M, Briggs J, et al. Profound hypothermia after adenosine kinase inhibition in A1AR-deficient mice suggests a receptor-independent effect of intracellular adenosine. Pflugers Arch. 2017;469(2):339–47. 10.1007/s00424-016-1925-3 . [DOI] [PubMed] [Google Scholar]