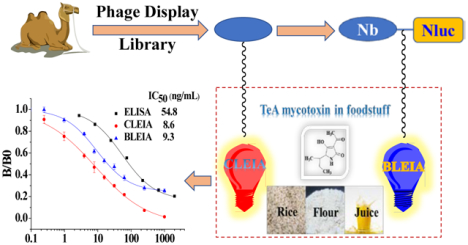
Abstract
The isolation of nanobodies (Nbs) from phage display libraries is an increasingly effective approach for the generation of new biorecognition elements, which can be used to develop immunoassays. In this study, highly specific Nbs against the Alternaria mycotoxin tenuazonic acid (TeA) were isolated from an immune nanobody phage display library using a stringent biopanning strategy. The obtained Nbs were characterized by classical enzyme-linked immunosorbent assay (ELISA), and the best one Nb-3F9 was fused with nanoluciferase to prepare an advanced bifunctional fusion named nanobody-nanoluciferase (Nb-Nluc). In order to improve the sensitivity and reduce the assay time, two different kinds of luminescent strategies including chemiluminescent enzyme immunoassay (CLEIA) and bioluminescent enzyme immunoassay (BLEIA) were established respectively on the basis of the single Nb and the fusion protein Nb-Nluc for TeA detection. The two-step CLEIA was developed on the basis of the same nanobody as ELISA only with simple substrate replacement from 3,3’,5,5’-tetramethylbenzidine (TMB) to luminol. In contrast with CLEIA, the novel BLEIA was conducted in one-step new strategy on the basis of Nb-Nluc and bioluminescent substrate coelenterazine-h (CTZ-h). Their half maximal inhibitory concentration (IC50) values were similar to 8.6 ng/mL for CLEIA and 9.3 ng/mL for BLEIA, which was a 6-fold improvement in sensitivity compared with ELISA (IC50 of 54.8 ng/mL). Both of the two assays provided satisfactory recoveries ranging from 80.1%−113.5% in real samples, which showed better selectivity for TeA analogues and other common mycotoxins. These results suggested that Nbs and Nb-Nluc could be used as useful reagents for immunodetection and that the developed CLEIA/BLEIA have great potential for TeA analysis.
Keywords: tenuazonic acid, nanobody, nanobody-nanoluciferase, chemiluminescent immunoassay, bioluminescent immunoassay
Graphical Abstract
Introduction
Due to the improper storage of cereal grains, fruits, and their products, the contamination of mycotoxins has become very common. The growth of molds in foods can produce kinds of toxic secondary metabolites, such as alternaria toxins, aflatoxins, trichothecenes, deoxynivalenol, fumonisins, zearalenone, and ochratoxin.1–3 The significant adverse effects of mycotoxins have already been verified in some studies, and even very low concentrations of the toxins could be extremely harmful to human health.4, 5 Alternaria alternata is one of the most important species in agricultural products, particularly cereal and fruit-based products. Tenuazonic acid (TeA) is a unique tetramic acid derivative, and it is considered to have the highest toxicity and exposure among the Alternaria toxins.6, 7 Many studies have reported the risk of TeA,8, 9 the contamination of TeA in foods has widely been a concern for food safety control.
Immunology-based assays have been widely used because of their unique advantages, especially in detection of small molecule contaminants in foods. The pollutants mainly include some common pesticide residues, veterinary drug residues, and mycotoxins. Up to now, there are only a few immunoassay reports on TeA, and the assay performance also showed some deficiencies.10–14 Antibodies serve as core reagents in immunodetection, which mostly plays a great role in the analytical performance. The reported studies have attempted to prepare specific antibodies, but results indicated that the developed antibodies were specific to TeA or its derivatives with only low sensitivity, which limited the application and expansion of immunoassays for TeA analysis in real samples. The reported studies were mostly based on polyclonal antibodies (pAbs) and monoclonal antibodies (mAbs), which also had shortcomings in terms of thermal stability and resistance to organic solvents. In contrast to the traditional pAbs and mAbs, nanobodies (Nbs) are ~15 kDa protein fragments derived from camelid heavy chain antibodies and they often exhibit superior thermal and conformational stability.15, 16 Nbs-based immunoassays are extremely suitable for the detection of analytes extracted with organic solvent from foodstuff. Besides, since Nbs are new kind of biorecognition elements empowered by phage-display library technology, the binding features of nanobodies could be optimized by a stringent biopanning strategy, especially the specificity and sensitivity. Recently, from nonimmune or immune antibody libraries including camel, llama, and alpaca, nanobodies with desired specificity and high sensitivity were isolated and used for analysis of small pollutants in foodstuff.17–19 Nbs-based immunoassays might meet the requirement of trace analysis of TeA in food matrix, but few reports have been published on the generation of nanobodies against TeA until now. As Nb is a recombinant binder, it is convenient to recreate various functional nanobody fusions by antibody engineering technology. Recently, nanoluciferase (Nluc) was reported as a new tracer in luminescence assay and offered excellent performance with over 150-fold increases in signal amplification compared with that of a classical tracer. In addition, Nluc has been used as a fusion partner to prepare several fusion bifunctional tracers including peptidomimetic-Nluc, green fluorescence protein (GFP)-Nluc and Nb-Nluc.20–23 Few reports have been published on the application of Nb-Nluc fusion for anti-TeA immunoassay development, and it seems to be a promising strategy to exploit its advantages in signal amplification to improve the detection sensitivity for tracing TeA in foodstuff.
In this study, a phage display library derived from an immunized camel was constructed for the isolation of anti-TeA nanobodies. In order to achieve efficient and precise biopanning, a stringent procedure with the combination of acid elution and competitive elution was performed. The specific Nbs were isolated with desired specificity and high sensitivity against TeA, and then the best clone (Nb-3F9) was fused with the Nluc for generation of a bifunctional fusion protein. Chemiluminescent enzyme immunoassay (CLEIA) and bioluminescent enzyme immunoassay (BLEIA) were developed, respectively, on the basis of the single Nb and Nb-Nluc, which were both ultrasensitive immunoassays for the detection of trace TeA in foodstuff. The analytical performance of two different luminescent assays was investigated and validated by liquid chromatography-tandem mass spectrometry (LC-MS/MS) for analysis of spiked samples from local supermarkets.
Materials and Methods
Reagents and Materials
TeA and alternariol monomethyl ether (AME) were purchased from Toronto Research Chemicals (Ontario, Canada). Alternariol (AOH), deoxynivalenol (DON), and zearalenone (ZEN) were purchased from Aladdin Chemical Technology Co., Ltd. (Shanghai, China). Iso-tenuazonic acid (ITeA, 97.64% purity) was prepared and identified in our lab. The keyhole limpet hemocyanin (KLH), bovine serum albumin (BSA), ovalbumin (OVA), Freundś complete adjuvants, and Freundś incomplete adjuvants were purchased from Sigma (St. Louis, MO). The TeA-CMO-KLH and TeA-CMO-OVA (the conjugates of hapten TeA-CMO with carrier protein KLH or OVA) were prepared by our laboratory (Figure S1). Horseradish peroxidase-labeled goat anti-HA tag (HRP-anti-HA-Tag) was obtained from Roche Molecular Systems, Inc. (Indianapolis, IN). The total RNA extraction kit was from Guangzhou Gbcbio Technologies Inc. (Guangzhou, China) The first strand cDNA synthesis kit was purchased from TaKaRa (Dalian, China). The gel extraction and PCR purification kit were purchased from QIAGEN (Dusseldorf, Germany). The molecular biology reagents, including helper phage M13K07, restriction enzymes, T4 DNA ligase and others, were obtained from New England Biolabs. Primer synthesis and DNA sequencing were conducted by the Guangzhou branch of Beijing Ruibiotech Co, Ltd. (Guangzhou, China), Integrated DNA Technologies, Inc. (Coralville, IA) and DNA Sequencing Faciliity in UC Davis. Coelenterazine substrate CTZ-h was purchased from NanoLight Technology (Pinetop, AZ). All other organic solvents and chemicals were of analytical grade from Sigma (St. Louis, MO) and Thermo Fisher Scientific.
Apparatus
The absorbance value was measured on a Multiskan MK3 microplate reader (Thermo Labsystems). Luminescence values were measured using a Tecan Infinite M1000-Pro plate reader (Männedorf, Switzerland). Microplates were washed by a Multiskan MK2 microplate washer (Thermo Scientific) and AquaMax Microplate Washer (Bethesda, MA). LC-MS/MS analysis was carried out on an Agilent SL liquid chromatograph system connected to a 4000 Qtrap mass spectrometer (Agilent Technologies, Santa Clara, CA).
Construction and Identification of Nanobody Library
The three-year-old male Bactrian camel was raised in Tangshan, China, and all the procedures involving camels were approved and performed in accordance with the relevant protective and administrative guidelines of China. The construction method of the nanobody library was previous described with slight modification.24 It is described in details in the Supporting Information.
Biopanning and Characterization of Nanobodies against TeA
The biopanning method for nanobody isolation was conducted on the basis of phage display technology by immobilizing the coating antigen onto 96-well plates as the solid phase. The procedures used were operated as described before with minor modifications.24 Specifically, the biopanning was conducted with four rounds. At the beginning of every round, 1 μg/mL KLH, BSA, and OVA and a appropriate concentration of TeA-CMO-OVA were coated overnight in different wells of plates at 37 °C. The concentrations of TeA-CMO-OVA presented a trend of decreasing gradient with 12.5, 2.5, 0.5 and 0.1 μg/mL in the four rounds. Four kinds of blocking buffers (1% fish gelatin, 3% skimmed milk, 1% fish gelatin and 3% skimmed milk in PBS buffer) were used in different rounds to decrease the nonspecific binding. After washing two times with washing buffer, the wells were blocked with 120 μL/well blocking buffer and incubated at 37 °C for 3 h. Plates were dried at 37 °C for 1 h after discarding the solution.
In the first round, 100 μL/well nanobody phage library was added into the KLH-coated wells and incubated at 37 °C for 1 h on a microplate shaker. Then the nonbound phages were successively transferred to different BSA-coated wells, OVA-coated wells, and antigen-coated wells with the same time as incubation. After that, the wells were washed 5 times with PBST (0.1% Tween 20) and another 15 times with PBS buffer. Finally, the specific phages were eluted at 37 °C for 10 min by 100 μL/well of 0.1 mol/L glycine-HCl buffer (pH 2.2) and immediately neutralized with 50 μL/well of 1 mol/L Tris-HCl (pH 8.0). Ten microliters of eluted specific phages was used for assessment of output titer, and others were amplified for the following round of biopanning. The incubation was first done with different wells of carrier protein (KLH, BSA, and OVA) in every round. The content of Tween 20 in PBST increased from 0.1% to 0.2%, 0.3%, and 0.4% from the second to the fourth round. However, the competitive elution methods were used with different concentrations (1000, 100 and 10 ng/mL) of TeA for incubation at 37 °C for 1 h from the second to the fourth round. After four rounds of biopanning, a total of 50 clones were picked up from the output plates of the second to fourth round and cultivated in the deep 96-well plate overnight. Then, the overnight cultured bacteria were induced with 1 mM isopropylthio-β-D-galactoside (IPTG) for production of nanobody at 37 °C. The supernatants were analyzed by indirect competitive enzyme-linked immunosorbent assay (ic-ELISA), as previous described.24 The positive clones were extracted and sequenced for further research.
Expression, Purification, and Identification of Nbs
Three candidate Nbs named Nb-3G2, Nb-4D11, and Nb-3F9 were chosen to express in E. coli BL21(DE3), and the performances were evaluated. In general, the recombinant plasmid Nb-3G2-pComb3XSS, Nb-4D11-pComb3XSS, and Nb-3F9-pComb3XSS were transformed into E. coli BL21(DE3). A single clone was picked and cultivated in the 2×YT medium with 100 μg/mL ampicillin at 37 °C and 250 rpm overnight. Then, the overnight medium was used for expanding cultivation in 2×YT medium containing ampicillin at 37 °C and 250 rpm. IPTG was added with a final concentration of 1 mM until the OD600 value of culture medium reached approximately 0.8. Then the cells were needed another 16 h at 37 °C. After centrifugation at 10 000 rpm for 20 min, the cells were harvested and extracted with bacterial protein extraction reagent (B-PER) for 30 min at room temperature. After centrifugation, the supernatant was collected by centrifugation at 4 °C for 20 min at 15 000 rpm. The three kinds of Nbs were purified with HisPur Ni-NTA resin and identified by SDS-PAGE according to the standard protocols. The activity and sensitivities of Nb-3G2, Nb-4D11, and Nb-3F9 were assessed by ic-ELISA (details in the Supporting Information).
Construction, Expression, Purification, and Identification of Fusion Protein
The modified pET22b containing nanoluciferase gene was prepared before,22 and a flexible linker of -GGGGSGGGGS- was designed between the nanobody gene and nanoluciferase gene. The forward primer 5′-GGCCATGGCCGAGGTGCAGCT-3′ and reverse primer 5′-TCGGATCCTGAGGAGACGGTG-3′ were used to amplify the nanobody 3F9 gene, and then it was subcloned into the pET22b vector using the restriction sites NcoI and BamHI. The recombinant plasmid Nb-3F9-Nluc-pET22b was transformed into E. coli Top10 competent cells, and several randomly clones were selected for sequencing. The correct recombinant plasmid Nb-3F9-Nluc-pET22b was transformed into E. coli Rosetta-gami2(DE3) for Nb-3F9-Nluc expression. The process of expression was similar to that of Nbs. The protein Nb-3F9-Nluc was identified by SDS-PAGE and tests of nanoluciferase catalytic activity, nanobody binding activity, and specific TeA inhibition activity.
Development of CLEIA/BLEIA Based on Nb or Nb-Nluc Fusion Protein
CLEIA
The CLEIA method was conducted on the basis of the chemiluminescent substrate luminol and an enhancer 4-iodophenol as reported with slight modification.25 The optimal concentrations of coating antigen TeA-CMO-OVA and Nb-3F9 were determined by a checkerboard method on high binding white 96-well microplates. The procedures were similar to that of ELISA. After coating overnight at 37 °C, the plates were blocked with 3% BSA in PBS after three times of washing and were incubated at 37 °C for 1 h. Diluted Nb-3F9 (50 μL/well) and 50 μL/well of gradient concentration of TeA were added. The plates were incubated for 40 min, washed for three times, and supplied with 100 μL/well of HRP-anti-HA-Tag with the concentration of 9.1 ng/mL for another 40 min of reaction. After another three times of washing, the substrate replacement from TMB to luminol was conducted as described before.24 The plates were incubated with 100 μL/well of fresh luminol solution and read at the luminescence detection mode on an Infinite M1000 PRO.
BLEIA
After evaluation of the optimal concentration of coating antigen and Nb-3F9-Nluc, the plates were processed with a series of procedures including coating, blocking, and incubation. Diluted Nb-3F9-Nluc (50 μL/well) and 50 μL/well of gradient concentration of TeA were added and incubated for 1 h. After three times of washing steps, the plates were supplied with 100 μL/well of fresh coelenterazine substrates CTZ-h solution and read at the luminescence detection mode on an Infinite M1000 PRO.
Optimization, Sensitivity, and Selectivity of CLEIA/BLEIA
The sensitivity of immunoassay was greatly affected by reaction conditions. Thus the parameters were investigated including different kinds and pHs of dilution buffers and suitable concentrations of methanol in buffer. The maximum relative light unit (RLUmax), a half inhibitory concentration (IC50), and RLUmax/IC50 were used to evaluate the factors as previously described.24 Under the optimal conditions, the standard curves were established by evaluating the data with a four-parameter logistic equation using the Origin 8.0 software (Origin Lab, Northhampton, ME). The limit of detection was defined as the 10% inhibitory concentration value (IC10) calculated from the curves. The selectivity of assays was essential to be studied, and it was determined by evaluating the cross-reactivity (CR) of Nb/Nb-3F9-Nluc with other analogues and other common mycotoxins. It was calculated as follows: CR (%) = (IC50 of TeA/IC50 of analogues or other mycotoxins)×100.
Analysis and Validation of Spiked Samples by CLEIA, BLEIA, and LC-MS/MS
Different samples from local markets were used for recovery assessment. For rice and flour samples, 5.0 g of homogenized samples was precisely weighed into 50 mL tubes and fortified with several gradient concentrations of TeA, respectively. The samples were extracted with 10 mL of 50 % (V/V) methanol/H2O. For apple juice, 5 mL of juice was fortified with TeA and used for extraction with 5 mL of 50 % (V/V) methanol/H2O. The mixtures were thoroughly mixed for 5 min on a vortex and centrifuged at 20 000 rpm for 30 min. The supernatants were filtered and diluted 10 fold with H2O for sample analysis by both CLEIA and BLEIA.
For LC-MS/MS validation, it was operated as described before with minor modifications.26, 27 Extracted supernatants samples (5.0 mL) were filtered and analyzed on Agilent SL system with a Kinetex C18 column (30mm × 4.6 mm, 2.6 μm) at 50 °C. The mobile phases were operated at a flow rate of 0.4 mL/min, which was gradient mixtures of mobile phase A of 5.0 mmol/L NH3-CH3COONH4 with pH 9.0 and mobile phase B of methanol: 0–0.5 min, 5% B; 0.5–1.0 min, 5%−20% B; 1.0–3.0 min, 20%−40% B; 3.0–4.0 min, 40%−60% B; 4.0–4.1 min, 60%−5% B; 4.1–5.0 min, 5% B. The contents of TeA in samples were observed with an AB Sciex 4000 Qtrap mass spectrometer in negative ESI mode with both Q1 multiple ions scan (Q1MI) and multiple reaction monitoring (MRM) scanning modes.
Results and Discussion
Library Construction and Nanobody Isolation
Using nanobody gene amplification by two-step nested PCR, a library with a capacity of 3.6×108 cfu/mL was developed. After amplification with helper phage M13K07, the titer of nanobody phage display library was 5.2×1012 pfu/mL. After four rounds of panning, 10 different sequences of nanobodies with the same length were obtained (Figure 1 A). By multiple alignment sequence analysis of variable/constant domains, all nanobodies were exactly same in the complementarity determining regions (CDRs) with a slight difference in framework region 1 (FR1) and framework region 4 (FR4) (Figure 1 B).
Figure 1.
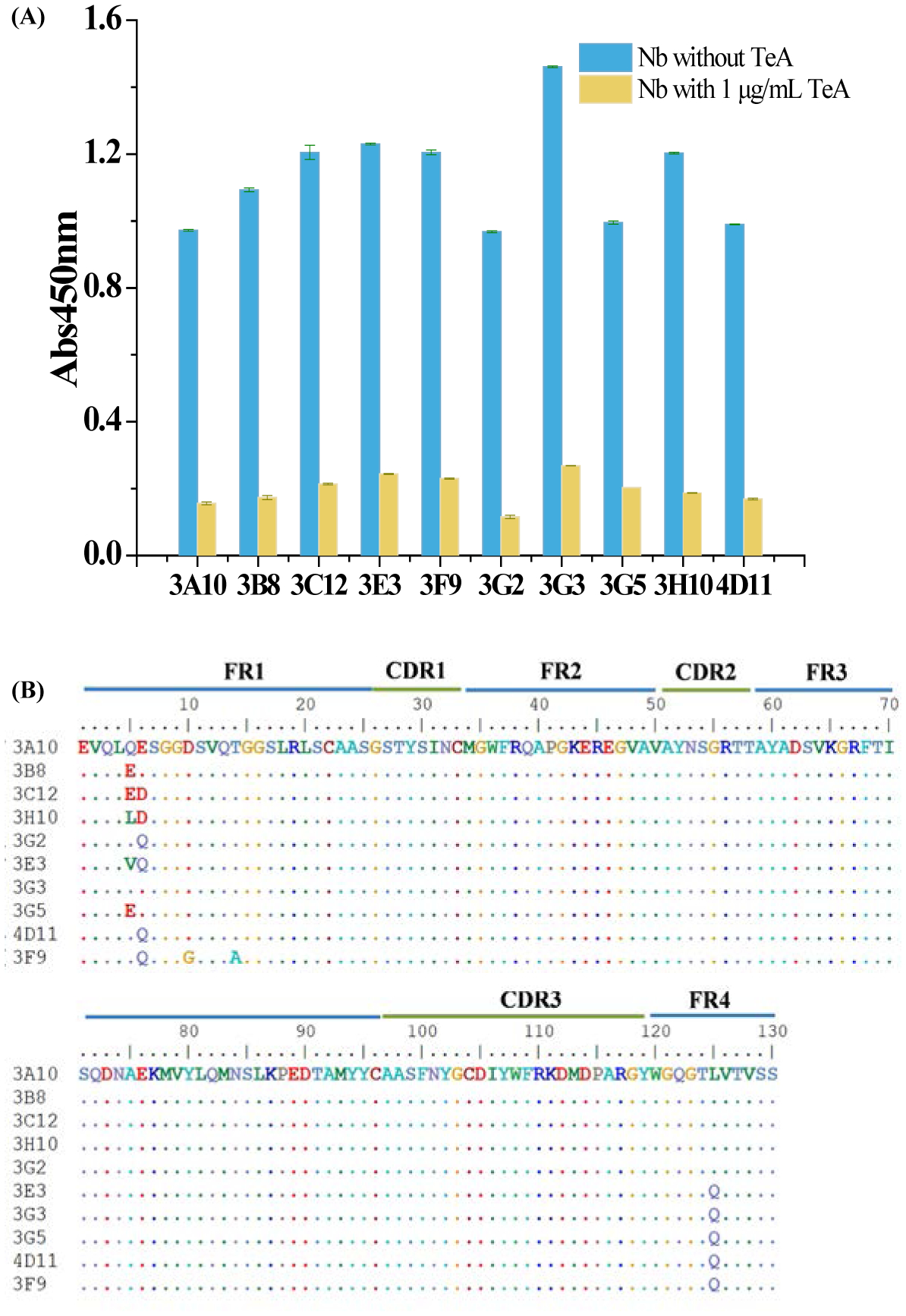
(A) Positive clones identified by ELISA through biopanning. (B) Amino acid sequences of the selected nanobodies.
Expression, Purification, and Identification of the Nb and Nb-Nluc Fusion Protein
To evaluate the performance of candidate nanobodies, Nb-3G2, Nb-4D11 and Nb-3F9 were chosen for further expression in E.coli BL21(DE3). After purification, the three nanobodies were evaluated by SDS-PAGE and ic-ELISA, and then standard curves were used to assess sensitivities. As shown in Figure 2 A, the purified nanobody from three clones all had characteristic bands at approximately 18 kDa, and Nb-3F9 had the strongest band among them. This result indicated that Nb-3F9 with different residues (D10G and T14A) in FR1 was the reason why it was easier to express in E.coli host than the other clones. In addition, the sensitivity of ic-ELISA based on Nb-3F9 showed the highest sensitivity (Figure 2 B), which demonstrated that the sequences in FR could also affect antigen-antibody binding activity although these sequences were universally conserved28, 29. On the basis of the results above, nanobodies against TeA with high sensitivity were isolated by phage display technology along with the stringent biopanning. Moreover, the best anti-TeA clone Nb-3F9 was cloned into the modified pET22b vector containing Nluc gene to generate biofunctional nanobody fusion. The Nb-3F9-Nluc fusion protein was successfully expressed in the host E. coli Rosetta-gami2(DE3) with the expected size of ~40 kDa in SDS-PAGE (Figure 2 C). This biofunctional fusion protein was also analyzed to have nanoluciferase catalytic activity, nanobody binding activity, and specific TeA inhibition activity (Figure 2D). The performance of Nb-3F9-Nluc and the original Nb-3F9 was evaluated using two-step ELISA with HRP-anti-6×His tag secondary antibody. The IC50 value of ELISA based on Nb-3F9-Nluc was 80.3 ng/mL, and that for Nb-3F9 was 89.5 ng/mL (Figure S2). It was indicated that the Nb-3F9-Nluc fusion protein had similar performance to that of original Nb-3F9.
Figure 2.

(A) SDS-PAGE of Nb-3G2, Nb-4D11 and Nb-3F9. (B) Standard curves of ic-ELISA based on Nb-3G2, Nb-4D11, and Nb-3F9. (C) SDS-PAGE of Nb-3F9-Nluc, (D) Evaluation of nanoluciferase catalytic activity, nanobody binding activity, and specific TeA inhibition activity of Nb-3F9-Nluc. The nanoluciferase catalytic activity of Nb-3F9-Nluc was determined in the presence or absence of bioluminescent substrate CTZ-h, the nanobody binding activity of Nb-3F9-Nluc was determined in the presence or absence of coating antigen by direct noncompetitive BLEIA, and the specific TeA inhibition activity of Nb-3F9-Nluc was determined in the presence or absence of TeA by direct competitive BLEIA.
Development and Optimization of CLEIA/BLEIA
To obtain higher analytical sensitivity of assays, CLEIA based on Nb-3F9 and BLEIA based on Nb-3F9-Nluc were developed. First of all, the concentrations of coating antigen and Nb-3F9/Nb-3F9-Nluc were obtained by checkerboard titration in CLEIA and BLEIA, respectively. After optimization, the concentrations of TeA-CMO-OVA and Nb-3F9 were 1.0 and 0.54 μg/mL for CLEIA, while the optimal concentrations for BLEIA was 0.5 μg/mL coating antigen and 5.35 μg/mL of Nb-3F9-Nluc. The process parameters of immunoassays were optimized through single factor experiment, and results are listed in Figure S 3 and Figure S 4. It was indicated that the best dilution buffer was 10 mmol/L PBS buffer solution, which is commonly used in immunoassay. The neutral buffer with relatively lower ionic strength of PBS was more suitable than acidic or alkaline solution for antigen-antibody binding. Organic solvents were commonly used in foodstuff sample preparation, and a certain concentration of organic solvents could increase the solubility of TeA without affecting the assay sensitivity or even increase the sensitivity in some immunodetection studies.22, 30 In the study, methanol (MeOH) was used to evaluate the organic solvent resistance of the methods. It was found that no obvious effects on CLEIA could been observed under 10% MeOH in PBS buffer. However, the BLEIA assay was more sensitive to the concentration of MeOH due to its influence on the enzymatic properties of Nb-3F9-Nluc. However, the developed BLEIA was not affected by low concentration of 5% MeOH, which was the final concentration of MeOH in foodstuff extraction solution after dilution treatment.
Under the optimal assay conditions, the IC50 and limit of detection (LOD) value of CLEIA were 8.6 and 0.3 ng/mL and those of BLEIA were 9.3 ng/ml and 1.1 ng/mL, respectively (Figure 3). The two different luminescent methods had similar IC50, but CLEIA could enable higher detection limit probably because of the signal amplification effect of enzyme-labeled secondary antibody. Nevertheless, the IC50 values of CLEIA and BLEIA both had about 6-fold improvement than that of classical two-step ELISA. Thus, the ultrasensitive CLEIA/BLEIA have great potential to detect the trace TeA in foodstuffs, which would compensate for the loss of sensitivity during sample extraction and dilution. Moreover, the one-step strategy of BLEIA was much simpler than two-step ELISA/CLEIA as it reduced operation time by nearly half. The performances of these two developed methods were listed and compared with that of the reported immunoassays (Table S1).
Figure 3.
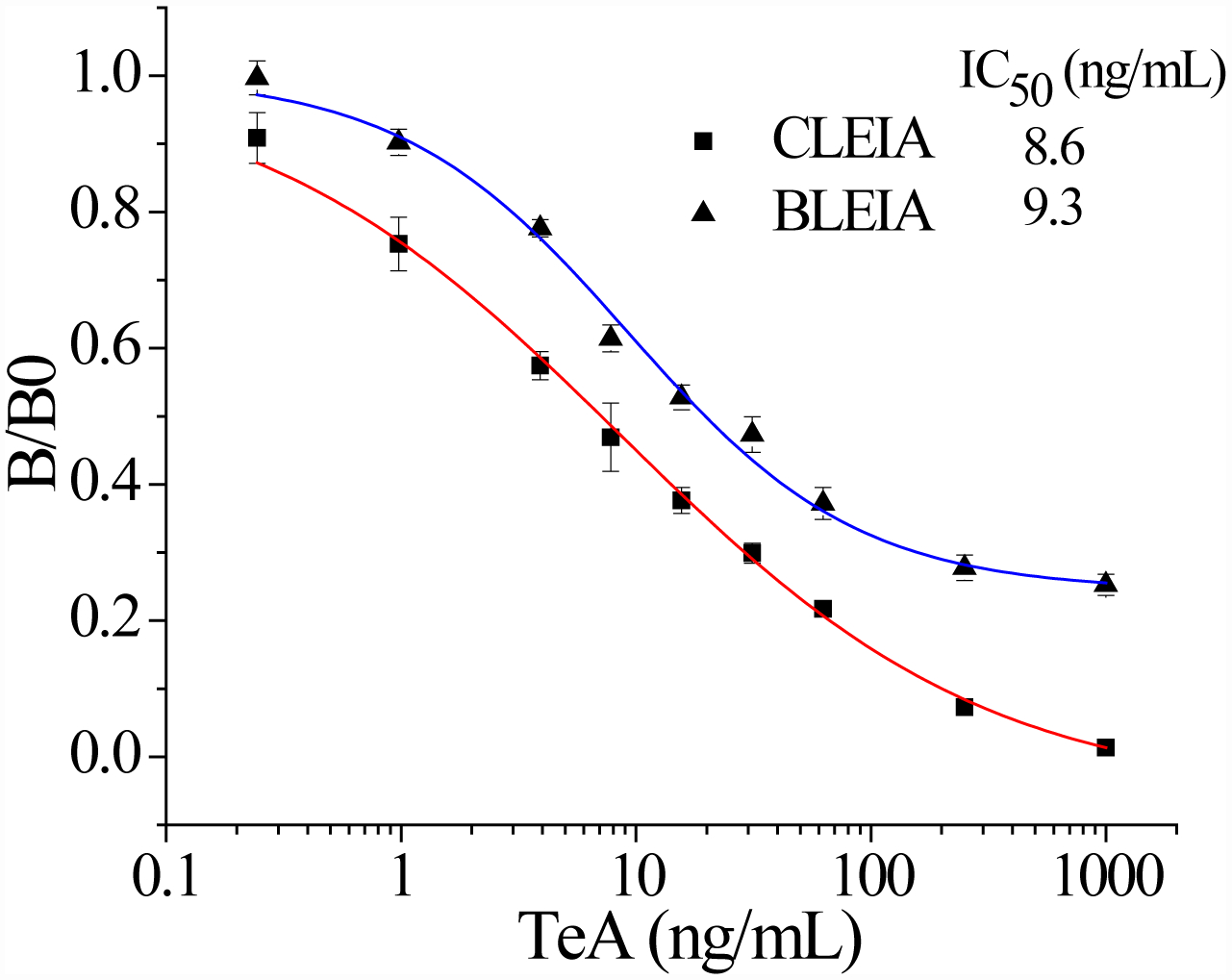
Standard curves of CLEIA and BLEIA respectively based on Nb-3F9 and Nb-3F9-Nluc. The value of “B/B0” was used to characterize the binding ability of nanobodies with the coating antigen on the microplate, where B and B0 represented the maximum RLUmax values in the presence or absence of TeA, respectively.
Selectivity of CLEIA/BLEIA
To study selectivity of two developed methods, several compounds were used to assess the CR values including the Alternaria mycotoxins (ITeA, AOH and AME) and other common mycotoxins (DON and ZEN). As listed in Table 1, there was no obvious CR with AOH, AME, DON, and ZEN, likely because TeA had a great difference in structures with these four mycotoxins, Though the ITeA has high similar structure as TeA only with a slight difference on the position of the methyl group, there was only low CR less than 3% for ITeA. These results indicated that the nanobody against TeA with desired specificity could be discriminated and isolated under the stringent biopanning procedure, even though there are only differences in potential distribution on the surface of ITeA and TeA.13
Table 1.
Assay Selectivity of CLEIA and BLEIA
| Analytes | Structures | CLEIA | BLEIA | ||
|---|---|---|---|---|---|
| IC50 (ng/mL) | CR | IC50 (ng/mL) | CR | ||
| TeA | 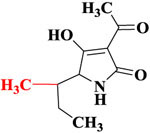 |
8.6 | 100% | 9.3 | 100% |
| ITeA | 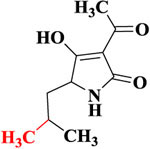 |
623.1 | 1.38% | 412.5 | 2.25% |
| AOH | 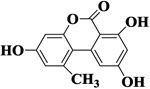 |
>1000 | <1% | >1000 | <1% |
| AME | 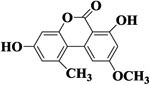 |
>1000 | <1% | >1000 | <1% |
| DON | 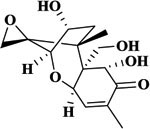 |
>1000 | <1% | >1000 | <1% |
| ZEN |  |
>1000 | <1% | >1000 | <1% |
Recovery Analysis of TeA in Spiked Samples and Method Validation by LC-MS/MS
In this study, three kinds of foodstuffs were used for recovery evaluation in samples, including rice, flour, and apple juice. As a startpoint, in order to minimize the interference of sample matrix on assay analysis, several standard curves applying a series of dilutions of sample matrix extracts were developed and compared with the original curve in assay buffer (Figure S5). The matrix of rice, flour, and apple juice was conducted with a 10-fold dilution, which largely eliminated the matrix effects. Rice and flour samples were spiked with three different concentrations of TeA (20, 80, and 200 ng/g), and apple juice proceeded in the same way with 20, 80, and 200 ng/mL of TeA. The sample extractions were analyzed by CLEIA and BLEIA under optimal reaction conditions. Results in Table 2 indicated that the recoveries in spiked samples for CLEIA were 80.1%−105.2%, and the recoveries for BLEIA were 86.4%−113.5%, with coefficient of variation (CV) below 15% for both CLEIA and BLEIA. Analytical performance of two developed luminescence methods was validated and compared with that of LC-MS/MS in spiked samples. Excellent correlations between CLEIA/BLEIA and LC-MS/MS analysis were shown in Figure S6. These results indicated that the CLEIA/BLEIA exhibited excellent performance for quantitative analysis, and both of the two assays were reliable to be used for immunodetection of TeA in samples.
Table 2.
Recovery Analysis of TeA in Spiked Samples by CLEIA and BLEIA
| CLEIA | BLEIA | ||||||
|---|---|---|---|---|---|---|---|
| Samples | Spiked level (ng/g or ng/mL) | Found ± SD (ng/g or ng/mL) | Recovery (%) | CV (%) | Found ± SD (ng/g or ng/mL) | Recovery (%) | CV (%) |
| Rice | 20 | 16.8±1.2 | 84.0 | 6.0 | 20.7±0.8 | 103.5 | 4.0 |
| 80 | 74.9±9.8 | 93.6 | 12.3 | 83.4±9.3 | 104.3 | 11.6 | |
| 200 | 210.4±9.0 | 105.2 | 4.5 | 181.0±12.8 | 90.5 | 6.4 | |
| Flour | 20 | 17.3±2.3 | 86.5 | 11.5 | 17.4±1.3 | 87.0 | 6.5 |
| 80 | 65.0±1.5 | 81.3 | 1.9 | 71.1±9.1 | 88.9 | 11.4 | |
| 200 | 201.5±15.3 | 100.8 | 7.7 | 172.7±12.2 | 86.4 | 6.1 | |
| Apple juice | 20 | 16.5±1.0 | 82.5 | 5.0 | 22.7±1.5 | 113.5 | 7.5 |
| 80 | 65.7±5.7 | 82.1 | 7.1 | 74.0±9.0 | 92.5 | 11.3 | |
| 200 | 160.1±2.9 | 80.1 | 1.5 | 172.7±3.5 | 86.4 | 1.8 | |
Conclusions
In this study, highly specific nanobodies isolated from a camel immunized nanobody library and the bifunctional fusion protein named nanobody-nanoluciferase were applied to establish two different luminescent assays based on chemiluminescent and bioluminescent strategies for the detection of TeA mycotoxin. The anti-TeA Nbs were generated through the novel phage display technology through stringent combined panning methods; acid elution was first used to increase the output of first round, followed by competitive elution to obtain antibodies against TeA. The selected anti-TeA nanobodies exhibited desired specificity and high sensitivity in classical two-step ELISA. Furthermore, the novel CLEIA was developed on the basis of the best clone Nb-3F9 with simple substrate replacement from TMB to luminol compared with ELISA. To further reduce assay time, Nb-3F9-Nluc fusion protein was efficiently used as a bifunctional tracer for one-step BLEIA. Both of the two assays provided high sensitivity and satisfactory recoveries in real samples, which showed good selectivity for TeA over its analogues and other mycotoxins. Therefore, the preparation of nanobodies could provide new detection reagents for immunodetection, and the development of chemiluminescent and bioluminescent assays have great potential application value for original innovation in immunoassay for TeA analysis. The research could be applied for the combination of nanobodies and new functionalized nanoparticles and development of the label-free immunoassays.31–34
Supplementary Material
Acknowledgments
This work was supported by Key-Area Research and Development Program of Guangdong Province (2019B020211002), Natural Science Foundation of China (31972157), Science and Technology Planning Project of Guangzhou City (201804020077), National Key R&D Program of China (2016YFE0106000), Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2017), International Cooperation Program of SCAU (2019SCAUGH03), the NIEHS Superfund Research Program (P42 ES04699), and the NIEHS RIVER Award (R35 ES030443-01). F.W. is an awardee of International Training Program for Outstanding Young Scientists in Universities of Guangdong Province (2018YQGP_BS006).
Footnotes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.analchem.0c02338.
Discussions of protocols for construction and identification of nanobody library and identification of Nbs by ic-ELISA, figures of structures of the conjugates of hapten TeA-CMO with carrier protein, Performance evaluation of Nb-3F9-Nluc and the original Nb-3F9, parameters optimization of CLEIA and BLEIA, studies of sample matrix extracts developed by CLEIA and BLEIA, correlations analysis for spiked samples between CLEIA and LC-MS/MS, and BLEIA and LC-MS/MS, table of analytical performance of these two developed methods (PDF).
Ethical Approval
All procedures involving animals were approved and performed in accordance with the relevant protective and administrative guidelines for laboratory animals of China.
References
- (1).Abrunhosa L; Morales H; Soares C; Calado T; Vila-Chã AS; Pereira M; Venâncio A A review of mycotoxins in food and feed products in Portugal and estimation of probable daily intakes. Crit. Rev. Food Sci. Nutr 2016, 56 (2), 249–265. [DOI] [PubMed] [Google Scholar]
- (2).Gruber-Dorninger C; Novak B; Nagl V; Berthiller F Emerging mycotoxins: Beyond traditionally determined food contaminants. J. Agric. Food Chem 2017, 65 (33), 7052–7070. [DOI] [PubMed] [Google Scholar]
- (3).Khaneghah AM; Fakhri Y; Gahruie HH; Niakousari M; Sant’Ana AS Mycotoxins in cereal-based products during 24 years (1983–2017): a global systematic review. Trends Food Sci. Technol 2019, 91, 95–105. [Google Scholar]
- (4).Ostry V; Malir F; Toman J; Grosse Y Mycotoxins as human carcinogens-the IARC Monographs classification. Mycotoxin Res. 2017, 33 (1), 65–73. [DOI] [PubMed] [Google Scholar]
- (5).Lee HJ; Ryu D Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: public health perspectives of their co-occurrence. J. Agric. Food Chem 2017, 65 (33), 7034–7051. [DOI] [PubMed] [Google Scholar]
- (6).Solfrizzo M Recent advances on Alternaria mycotoxins. Curr. Opin. Food Sci 2017, 17, 57–61. [Google Scholar]
- (7).Li FQ, Yoshizawa T Alternaria mycotoxins in weathered wheat from China. J. Agric. Food Chem 2000, 48 (7), 2920–2924. [DOI] [PubMed] [Google Scholar]
- (8).European Food Safety Authority; Arcella D, Eskola M; Gómez Ruiz JA Dietary exposure assessment to Alternaria toxins in the European population. EFSA J. 2016, 14 (12), e04654. [Google Scholar]
- (9).Rychlik M; Lepper H; Weidner C; Asam S Risk evaluation of the Alternaria mycotoxin tenuazonic acid in foods for adults and infants and subsequent risk management. Food Control 2016, 68, 181–185. [Google Scholar]
- (10).Gross M; Curtui V; Ackermann Y; Latif H; Usleber E Enzyme immunoassay for tenuazonic acid in apple and tomato products. J. Agric. Food Chem 2011, 59 (23), 12317–12322. [DOI] [PubMed] [Google Scholar]
- (11).Yang XX; Liu XX; Wang H; Xu ZL; Shen YD; Sun YM Development of an enzyme-linked immunosorbent assay method for detection of tenuazonic acid. Chin. J. Anal. Chem 2012, 40 (9), 1347–1352. [Google Scholar]
- (12).Kong DZ; Liu LQ; Song SS; Zheng QK; Wu XL; Kuang H Rapid detection of tenuazonic acid in cereal and fruit juice using a lateral-flow immunochromatographic assay strip. Food Agric. Immunol 2017, 28 (6), 1293–1303. [Google Scholar]
- (13).Wang F; Cai J; Eremin SA; Xiao ZL; Shen YD; Tian YX; Xu ZL; Yang JY; Lei HT; Sun YM; Wang H Fluorescence polarization immunoassay for Alternaria mycotoxin tenuazonic acid detection and molecular modeling studies of antibody recognition. Food Anal. Method 2018, 11, 2455–2462 [Google Scholar]
- (14).Gross M; Asam S; Rychlik M Evaluation of an enzyme immunoassay for the detection of the mycotoxin tenuazonic acid in sorghum grains and sorghum-based infant food. Mycotoxin Res. 2017, 33 (1), 75–78. [DOI] [PubMed] [Google Scholar]
- (15).Muyldermans S Nanobodies: natural single-domain antibodies. Annu. Rev. Biochem 2013, 82, 775–797. [DOI] [PubMed] [Google Scholar]
- (16).De Meyer T; Muyldermans S; Depicker A Nanobody-based products as research and diagnostic tools. Trends Biotechnol. 2014, 32 (5), 263–270. [DOI] [PubMed] [Google Scholar]
- (17).Wang J; Mukhtar H; Ma L; Pang Q; Wang XH VHH antibodies: reagents for mycotoxin detection in food products. Sensors 2018, 18 (2), 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).He T; Zhu J; Nie Y; Hu R; Wang T, Li PW; Zhang Q; Yang YH Nanobody technology for mycotoxin detection: current status and prospects. Toxins 2018, 10 (5), 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Bever CS; Dong JC; Vasylieva N; Barnych B; Cui YL; Xu ZL; Hammock BD; Gee SJ VHH antibodies: emerging reagents for the analysis of environmental chemicals. Anal. Bioanal. Chem 2016, 408 (22), 5985–6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Hall MP; Unch J; Binkowski BF; Valley MP; Butler BL; Wood MG; Otto P; Zimmerman K; Vidugiris G; Machleidt T; Robers MB; Benink HA; Eggers CT; Slater MR; Meisenheimer PL; Klaubert DH; Fan F; Encell LP; Wood KV Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem. Biol 2012, 7 (11), 1848–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Ding Y; Hua XD; Chen H; Liu FQ; González-Sapien G; Wang MH Recombinant peptidomimetic-nano luciferase tracers for sensitive single-step immunodetection of small molecules. Anal. Chem 2018, 90 (3), 2230–2237. [DOI] [PubMed] [Google Scholar]
- (22).Ren WJ; Li ZF; Xu Y; Wan DB; Barnych B; Li YP; Tu Z; He QH; Fu JH; Hammock BD One-Step Ultrasensitive bioluminescent enzyme immunoassay based on nanobody/nanoluciferase fusion for detection of aflatoxin B1 in cereal. J. Agric. Food Chem 2019, 67 (18), 5221–5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Setoh YX; Periasamy P; Peng NYN; Amarilla AA; Slonchak A; Khromykh AA Helicase domain of West Nile virus NS3 protein plays a role in inhibition of type I interferon signalling. Viruses 2017, 9 (11), 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Zhang YQ; Xu ZL; Wang F; Cai J; Dong JX; Zhang JR; Si R; Wang CL; Wang Y; Shen YD; Sun YM; Wang H Isolation of Bactrian camel single domain antibody for parathion and development of one-step dc-FEIA method using VHH-alkaline phosphatase fusion protein. Anal. Chem 2018, 90 (21), 12886–12892. [DOI] [PubMed] [Google Scholar]
- (25).Huo JQ; Barnych B; Li ZF; Wan DB; Li DY; Vasylieva N;. Knezevic SZ; Osipitan OA; Scott JE; Hammock BD Hapten synthesis, antibody development, and a highly sensitive indirect competitive chemiluminescent enzyme immunoassay for detection of dicamba. J. Agric. Food Chem 2019, 67 (20), 5711–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Fraeyman S; Devreese M; Broekaert N; De Mil T; Antonissen G; De Baere S; De Backer P; Rychlik M; Croubels S Quantitative determination of tenuazonic acid in pig and broiler chicken plasma by LC-MS/MS and its comparative toxicokinetics. J. Agric. Food Chem 2015, 63 (38), 8560–8567. [DOI] [PubMed] [Google Scholar]
- (27).Puntscher H; Cobankovic I; Marko D; Warth B Quantitation of free and modified Alternaria mycotoxins in European food products by LC-MS/MS. Food Control, 2019, 102, 157–165. [Google Scholar]
- (28).Henry KA; Hussack G; Kumaran J; Gilbert M; MacKenzie CR; Sulea T; Arbabi‐Ghahroudi M Role of the non‐hypervariable FR3 D‐E loop in single‐domain antibody recognition of haptens and carbohydrates. J. Mol. Recognit 2019, 32 (11), e2805. [DOI] [PubMed] [Google Scholar]
- (29).Abskharon R; Wang F; Wohlkonig A; Ruan JX; Soror S; Giachin G; Pardon E; Zou WQ; Legname G; Ma JY; Steyaert J Structural evidence for the critical role of the prion protein hydrophobic region in forming an infectious prion. PLoS Pathog. 2019, 15 (12), e1008139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Liu X; Xu Y; Wan DB; Xiong YH; He ZY; Wang XX; Shirley JG; Ryu D; Hammock BD Development of a nanobody-alkaline phosphatase fusion protein and its application in a highly sensitive direct competitive fluorescence enzyme immunoassay for detection of ochratoxin a in cereal. Anal. Chem 2015, 87 (2), 1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Ryan S; Kell AJ; Van Faassen H; Tay LL; Simard B; Mackenzie R; Gilbert M; Tanha J Single-domain antibody-nanoparticles: promising architectures for increased staphylococcus aureus detection specificity and sensitivity[J]. Bioconjugate Chem. 2009, 20 (10), 1966–1974. [DOI] [PubMed] [Google Scholar]
- (32).Ren X; Yan JR; Wu D; Wei Q; Wan YK Nanobody-Based Apolipoprotein E Immunosensor for Point-of-Care Testing. ACS Sens. 2017, 2 (9), 1267–1271. [DOI] [PubMed] [Google Scholar]
- (33).Zhang DG; Cai LJ; Bian FK; Kong TT; Zhao YJ Label-free quantifications of multiplexed mycotoxins by g-quadruplex based on photonic barcodes. Anal. Chem 2020, 92 (4), 2891–2895. [DOI] [PubMed] [Google Scholar]
- (34).Li HN; Mu YW; Yan JR, Cui DM, Ou WJ, Wan YK, Liu SQ Label-free photoelectrochemical immunosensor for neutrophil gelatinase-associated lipocalin based on the use of nanobodies. Anal. Chem 2015, 87 (3), 2007–2015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


