Abstract
The zona pellucida (ZP) is an extracellular matrix (ECM) that surrounds all mammalian oocytes, eggs, and embryos and plays vital roles during oogenesis, fertilization, and preimplantation development. The mouse and human ZP is composed of three or four unique proteins, respectively, called ZP1–4, that are synthesized, processed, and secreted by oocytes during their growth phase. All ZP proteins have a zona pellucida domain (ZPD) that consists of ≈270 amino acids and has 8 conserved Cys residues present as four intramolecular disulfides. Secreted ZP proteins assemble into long fibrils around growing oocytes with ZP2-ZP3 dimers located periodically along the fibrils. The fibrils are cross-linked by ZP1 to form a thick, transparent ECM to which sperm must first bind and then penetrate during fertilization of eggs. Inactivation of mouse ZP1, ZP2, or ZP3 by gene targeting affects both ZP formation around oocytes and fertility. Female mice with eggs that lack a ZP due to inactivation of either ZP2 or ZP3 are completely infertile, whereas inactivation of ZP1 results in construction of an abnormal ZP and reduced fertility. Results of a large number of studies of infertile female patients strongly suggest that gene sequence variations (GSV) in human ZP1, ZP2, or ZP3 due to point, missense, or frameshift mutations have similar deleterious effects on ZP formation and female fertility. These findings are discussed in light of our current knowledge of ZP protein synthesis, processing, secretion, and assembly.
Keywords: mouse, human, oogenesis, zona pellucida, ZP genes, ZP proteins, ZP domain, gene targeting, mouse fertility, gene sequence variations, human fertility
1. Introduction
The term “zona pellucida” was adopted by the Baltic German embryologist Karl Ernst von Baer (1792–1876) when describing human eggs for the first time in 1827 (Greek word zone, meaning belt or girdle; Latin word pellucida, meaning to shine or be transparent) [1]. By the 1840s the term zona pellucida (ZP) had gained widespread use among embryologists to describe the relatively thick transparent zone surrounding all mammalian eggs.
The ZP is a relatively thick extracellular matrix (ECM) that surrounds all mammalian oocytes, eggs, and preimplantation embryos and plays various roles during oogenesis, fertilization, and preimplantation development [2–5]. It supports the health and growth of mammalian oocytes and follicles during the final stages of oogenesis, regulates species-restricted fertilization of eggs by sperm, and protects preimplantation embryos as they traverse the female reproductive tract toward the uterus. A ZP remains around preimplantation embryos until the expanded blastocyst stage when they hatch from the ZP and implant in the uterus. Shortly after eggs are fertilized by a single sperm, the ZP undergoes physical and biological changes that assist in both the prevention of polyspermy and protection of preimplantation embryos in the female reproductive tract.
The ZP is a viscoelastic, fibrillar ECM that is permeable to large macromolecules, such as antibodies, enzymes, and small viruses [3] (Figure 1). The width of the ZP varies relatively little, from ≈2 to ≈22 μm, whereas the diameter of eggs varies enormously among different animal species. ECM of most animal cells is constructed of proteoglycans, such as hyaluronic acid, heparin-, chondroitin-, and keratin-sulfate, and fibrous proteins, such as collagens, elastins, fibronectins, and laminins [6]. On the other hand, the ZP of mammalian eggs consists of either three (e.g., mouse) or four (e.g., human) glycosylated proteins, called ZP1–4, each with a unique polypeptide chain that includes a large ZP domain (ZPD) [3–5]. The ZPD consists of ≈270 aa, has 8 conserved cysteine (Cys) residues, and has two subdomains, called ZP-N and ZP-C, as well as a short linker region connecting the two subdomains [5, 7]. Eggs from fish, amphibia, reptiles, and birds are also surrounded by a ZP or vitelline envelope that consists of several ZPD proteins, all closely related to ZP1–4 [3, 5, 8].
Figure 1.
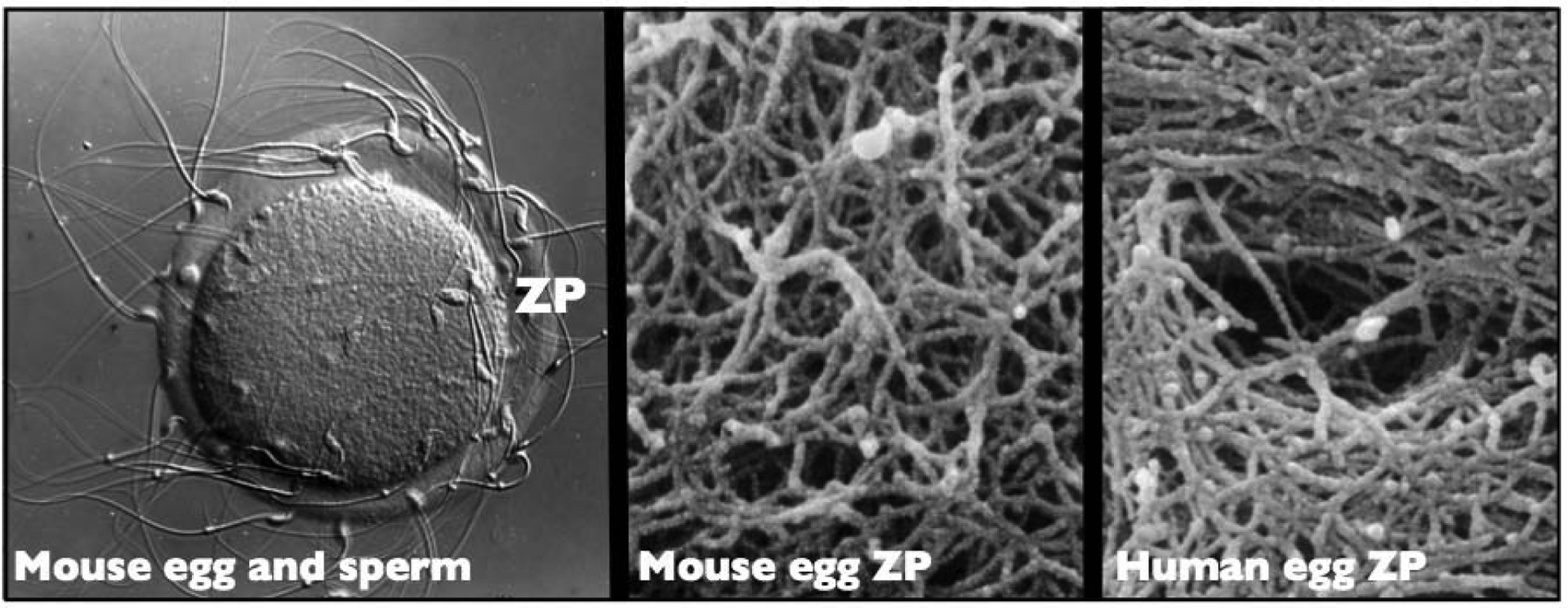
Photographic images of the mouse and human egg zona pellucida (ZP). Left: A light micrograph (Nomarski differential interference contrast) of an unfertilized mouse egg incubated in the presence of free-swimming sperm. Sperm are shown bound to the ZP (≈550 × magnification). Middle: Scanning electron micrograph of the outer surface of the ZP of a mouse oocyte showing its fibrillar organization (≈50,000 × magnification). Right: Scanning electron micrograph of the outer surface of the ZP of a human oocyte showing its fibrillar organization and the presence of a pore (≈50,000 × magnification). The fibrils are 0.1–0.4 μm long and 10–14 nm wide. The latter two micrographs (Middle and Right) were obtained with samples treated with saponin-ruthenium red-osmium thiocarbohydrazide to reveal mouse and human ZP fibrils and are included here with the permission of G. Familiari at the University of Rome, Italy [72].
Mouse ZP fibrils are polymers constructed of ZP2-ZP3 dimers and the long fibrils are interconnected by ZP1 to produce a thick ECM [7]. The ZP can be dissolved under conditions that do not result in breaking of covalent bonds, such as at low pH and elevated temperatures, suggesting that the structural integrity of the ZP is stabilized by non-covalent interactions between ZP proteins. Mechanical properties of the ZP, such as its viscosity and stiffness, change markedly after fertilization and is seen as an increased resistance of the ZP to dissolution by various agents (i.e., a hardening of the ZP) and an inability of free-swimming sperm to bind to the ZP of fertilized eggs [2, 4].
Mouse ZP proteins serve as structural proteins during assembly of the ZP around growing oocytes and as species-restricted receptors for sperm during fertilization [4, 9, 10]. Experiments involving gene targeting to produce ZP1, ZP2, and ZP3 homozygous and heterozygous null mice suggest that inactivation or modification of ZP genes can have a significant effect on female fertility [4, 10]. In this context, results of a large number of relatively recent studies with infertile female patients suggest that gene sequence variations (GSV) in human ZP1, ZP2, or ZP3 due to point, missense, or frameshift mutations can result in oocytes or eggs with either an abnormal or no ZP and in infertility. Here we provide some background information about ZP genes and proteins and discuss particular molecular aspects of GSV in human ZP1, ZP2, or ZP3 that affect female fertility.
2. Mouse and human ZP genes and proteins
2.1. Mouse and human ZP genes
The mammalian egg ZP generally consists of either three or four proteins called ZP1–4. In mice and humans, ZP1–4 are encoded by single-copy genes located on different chromosomes (Table 1). Mouse ZP1, ZP2, and ZP3 are located on chromosomes 19, 7, and 5, respectively, vary in length from 6.5 (ZP1) to 18.5 (ZP2) kb, and contain 12 (ZP1), 18 (ZP2), and 8 (ZP3) exons. Mouse ZP4 is a pseudogene located on chromosome 13 [11–13]. Human ZP1, ZP2, ZP3, and ZP4 are located on chromosomes 11, 16, 7, and 1, respectively, vary in length from 8.1 (ZP1) to 18.3 (ZP3) kb, and contain 12 (ZP1), 19 (ZP2), 8 (ZP3), and 12 (ZP4) exons [14].
Table 1.
Mouse (m) and human (h) ZP genes.
| ZP gene | Chromosome location (No.) | Gene length (kb) | Number exons |
|---|---|---|---|
| mZP1 | 19 | 6.5 | 12 |
| hZP1 | 11 | 8.1 | 12 |
| mZP2 | 7 | 18.5 | 18 |
| hZP2 | 16 | 14 | 19 |
| mZP3 | 5 | 8.6 | 8 |
| hZP3 | 7 | 18.3 | 8 |
| mZP4 | 13 | - | - |
| hZP4 | 1 | 17 | 12 |
2.2. Expression of ZP genes
ZP genes are expressed by oocytes during the latter stages of oogenesis when oocytes, arrested in first meiotic prophase, undergo tremendous growth (Figure 2). For example, mouse oocytes grow ≈300-fold, from ≈0.9 to ≈270 picoliters in volume, over 2–3 weeks [15]. Non-growing mouse oocytes, ≈12 μm in diameter, lack a ZP, whereas fully-grown oocytes, ≈80 μm in diameter, have a ZP that is ≈6.2 μm thick and contains ≈3.5 nanograms of protein. Consistent with the pattern of ZP1–3 synthesis, mRNA encoding mouse ZP proteins is undetectable in non-growing oocytes (i.e., <1,000 copies/cell), increases to hundreds-of-thousands of copies in mid-stage growing to fully-grown oocytes, and decreases to undetectable levels in fertilized eggs [16]. ZP genes are expressed only by growing oocytes, consequently, only by females, and is an example of gender-specific gene expression. Transgenic experiments, using a firefly luciferase reporter gene, strongly suggest that cis-acting sequences which restrict expression of ZP genes to growing oocytes are located very close to the transcription start-site [17, 18]. In addition, an E-box sequence (CANNTG) located about 200 bp upstream of the transcription start-site is apparently involved in oocyte-specific expression of ZP genes when E12/FIGα heterodimers bind to E-boxes [19, 20]. It should be noted that in fish and birds there are two sites of ZP protein synthesis, the ovary and the liver, and ZP precursor proteins synthesized in the liver are transported via circulating blood to the oocyte for uptake [5, 8].
Figure 2.
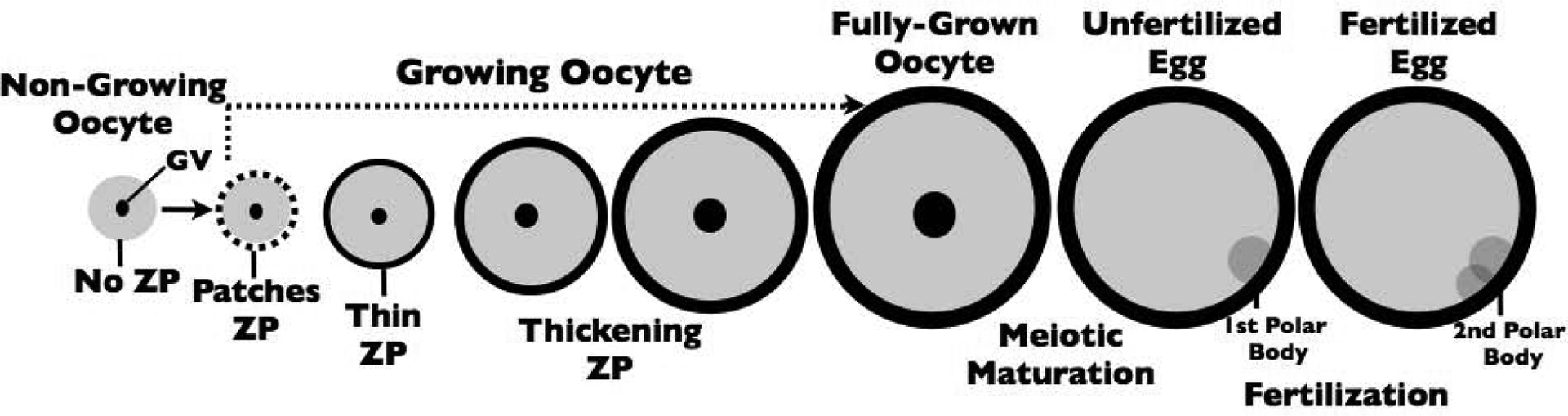
Schematic representation of zona pellucida (ZP) production during oocyte growth in mice (2–3 weeks). Non-growing oocytes lack a ZP, but as soon as they begin to grow they lay down patches of ZP fibrils (black dashes). The fibrils coalesce into a ZP matrix (black) relatively early in oocyte growth and the ZP (black) continues to thicken throughout oocyte growth. The ZP remains around fully-grown oocytes [germinal vesicle (GV) present, black], unfertilized eggs (dissolution of the GV and emission of the first polar body, dark gray), and fertilized eggs (emission of second polar body, dark gray). The ZP remains around the early embryo until the expanded blastocyst stage when the embryo hatches from the ZP and implants in the uterus.
2.3. Mouse and human ZP proteins
The mouse (m) ZP consists of three proteins, mZP1–3, and the human (h) ZP consists of four proteins, hZP1–4 (Figure 3). Nascent ZP proteins are processed by removal of an N-terminal signal sequence (SS) as proteins move from the endoplasmic reticulum to the Golgi and by removal of a C-terminal propeptide (CTP) at the oocyte’s plasma membrane. The unprocessed polypeptides of mZP1, mZP2, and mZP3 are 623, 713, and 424 amino acids (aa) in length, respectively, and the processed polypeptides are 526 (≈120 kD MW), 599 (≈120 kD MW), and 329 (≈83 kD MW) aa in length, respectively [4, 15]. The unprocessed polypeptides of hZP1, hZP2, hZP3, and hZP4 are 638, 745, 424, and 540 aa in length, respectively, and the processed polypeptides are 528 (≈100 kD MW), 602 (≈75 kD MW), 328 (≈55 kD MW), and 445 (≈65 kD MW) aa in length, respectively [10, 21, 22] (Table 2). The polypeptides are heterogeneously glycosylated with asparagine-linked (N-linked) and serine/threonine-linked (O-linked) oligosaccharides that may be sialylated and sulfated [15]. As a consequence of these modifications, mouse and human ZP proteins are acidic (ave. pI <5.5) and migrate as very broad bands on SDS-PAGE.
Figure 3.
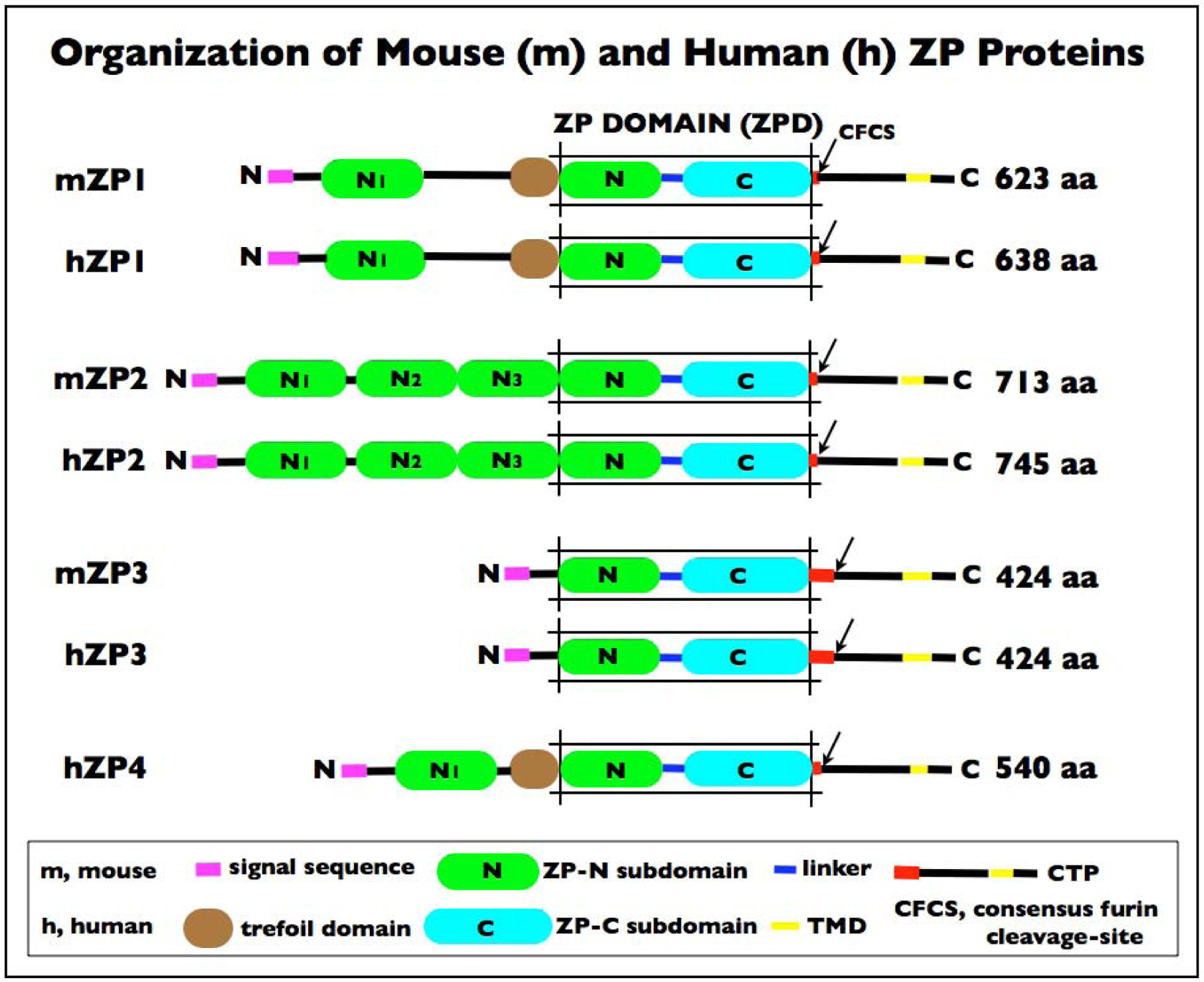
Schematic representation of the organization of mouse (m) zona pellucida (ZP) proteins mZP1–3 and human (h) ZP proteins hZP1–4. Linker region (blue); concensus furin cleavage-site (CFCS; arrow); transmembrane domain (TMD; yellow); and cytoplasmic tail (black) in the C-terminus. In each case, the polypeptide has a signal sequence (SS; magenta) at the N-terminus; a ZP domain (ZPD) consisting of two subdomains, ZP-N (green) and ZP-C (cyan), and a C-terminal propeptide (CTP). mZP1 (623 aa), hZP1 (638 aa), and hZP4 (540 aa) also have a trefoil domain (TD; brown) adjacent to the ZPD. mZP2 (713 aa) and hZP2 (745 aa) have 3 additional ZP-N subdomains (N1-N3; green) between the N-terminus of the polypeptide and the ZPD. mZP1, hZP1, and hZP4 have one additional ZP-N subdomain (N1; green) between the N-terminus of the polypeptide and the TD. mZP3 (424 aa) and hZP3 (424 aa) are the smallest of the ZP proteins and consist primarily of a ZPD (mZP3 260 aa; hZP3 259 aa). Notably, mZP1 and 2 and hZP1, 2, and 4 have only 3 to 5 aa between the ZPD and the CFCS (red), whereas mZP3 has 47 aa and hZP3 has 45 aa between the ZPD and the CFCS which includes the sperm combining-site [9, 59] and represents a region of positive Darwinian selection during evolution [15, 27]. This region of the ZP3 polypeptide also includes 4 conserved Cys residues in close proximity to one another (mZP3, Cys-320, −322, −323, and −328; hZP3, Cys-319, −321, −322, and −327) that could form two intramolecular disulfides.
Table 2.
Primary structure of mouse (m) and human (h) ZP proteins.
| ZP protein | Polypeptide length (aa) | Signal sequence (aa) | ZP domain (aa) | Consensus furin cleavage-site (aa) | Transmembrane domain (aa) | Trefoll domain (aa) |
|---|---|---|---|---|---|---|
| mZP1 | 623 | 1–20 | 271–542 | 545–548 | 591–611 | 225–266 |
| hZP1 | 638 | 1–25 | 279–549 | 552–555 | 602–622 | 234–274 |
| mZP2 | 713 | 1–34 | 364–630 | 632–635 | 684–703 | - |
| hZP2 | 745 | 1–38 | 372–637 | 639–642 | 717–736 | - |
| mZP3 | 424 | 1–22 | 45–304 | 350–353 | 388–408 | - |
| hZP3 | 424 | 1–22 | 45–303 | 349–352 | 387–409 | - |
| hZP4 | 540 | 1–19 | 188–462 | 463–466 | 505–526 | 141–183 |
Mouse and human ZP2 and ZP3 are monomers present in roughly equimolar amounts in the ZP and account for ≈80% of the mass of the ZP. ZP1 and ZP4 are homologous proteins and their genes are paralogous. ZP1 has a single intermolecular disulfide that supports the ZP1 dimer, whereas ZP4 does not have an intermolecular disulfide and in humans is a monomer [14, 23]. Unlike ZP2 and ZP3, ZP1 and ZP4 polypeptides possess a trefoil domain (TD), a three-loop compact structure with 6 Cys residues present as three intramolecular disulfides [24]. ZP1 serves as a cross-linker for the long fibrils that make up the ZP matrix. Mouse and human ZP1 have a proline (Pro)-rich N-terminus (100 aa, 17–21% Pro) that may provide the flexibility that contributes to the documented elasticity of the ZP prior to fertilization [4].
The primary structures of mouse and human ZP1–3 are well conserved having ≈64% identity and ≈84% similarity (Table 3) and human ZP1 and ZP4 are ≈43% identical and ≈72% similar. In this context, transgenic mouse lines have been established in which human ZP1–3 replaced the endogenous mouse ZP proteins [25, 26]. Mouse and human ZP polypeptides exhibit several regions characteristic of ZP proteins from jellyfish to humans, representing more than 600 million years of evolution [27]. These regions include an N-terminal SS (≈25 aa) that targets nascent ZP protein to the secretory pathway, followed by a long ZPD (≈270 aa) and a CTP required for secretion of nascent protein. The CTP includes a consensus furin cleavage-site (CFCS; ≈4 aa), an intervening sequence, a hydrophobic transmembrane domain (TMD; ≈20 aa), and a C-terminal tail (Figure 3; Table 2).
Table 3.
Mouse (m) and human (h) ZP proteins.
| ZP protein | % Similarity | % Identity |
|---|---|---|
| mZP1/hZP1 | 84 | 67 |
| mZP2/hZP2 | 80 | 58 |
| mZP3/hZP3 | 87 | 68 |
2.4. Structure of the ZPD
The ZPD, which has 8 conserved Cys residues present as four intramolecular disulfides, is found in all ZP proteins as well as in hundreds of other proteins of diverse functions, from receptors to mechanical transducers, from a wide variety of tissues and organs in all multicellular organisms [4, 5, 7]. The ZPD is a bipartite structure consisting of two subdomains, an N-terminal ZP-N (≈100 aa) and a C-terminal ZP-C (≈135–150 aa) subdomain, separated from each other by a short protease-sensitive region (≈25–30 aa). ZP1 and ZP4 have one extra ZP-N subdomain and ZP2 has three extra ZP-N subdomains as extensions at the N-terminus of their polypeptides [28]. ZP-N is used for polymerization of nascent ZP proteins and other extracellular ZPD proteins, such as tectorin and uromodulin, into long fibrils [29, 30].
The three-dimensional structure of the ZPD has been determined by X-ray diffraction and revealed that both the ZP-N and ZP-C subdomains adopt immunoglobulin (Ig)-like folds [4, 31, 32]. The ancestral gene for the Ig superfamily may have originated about 750 million years ago in sponges as a primitive sandwich-like fold used in vertebrate extracellular recognition systems [33]. The ZP-N fold consists of an antiparallel sandwich of two β-sheets made up of eight strands of polypeptide that enclose a hydrophobic core of buried residue side-chains, with two buried disulfides that clamp both sides of the sandwich. The ZP-C fold also consists of a β-sandwich comprising two stacked β-sheets, one with four strands and the other with six strands, that approximate a Greek-key like motif characteristic of Ig-like domains [34]. The strong structural similarity between the ZP-N and ZP-C subdomains suggests that the ZPD may have arisen by duplication of an ancestral gene encoding a protein containing a single ZP-N subdomain.
2.5. Structure of ZP fibrils
In mice the ZP consists of cross-linked fibrils 7–8 nm in width and several μm in length with a ZP2-ZP3 dimer located every 14–15 nm along the fibrils [35–37]. The structural periodicity of fibrils can be visualized as protuberances along fibrils in electron micrographs of solubilized ZP, as well as in micrographs of solubilized ZP preparations decorated with monoclonal antibodies against either ZP2 or ZP3. Fibrils in the inner and outer layers of the ZP are oriented perpendicular and parallel, respectively, to the oolemma, whereas fibrils in the intervening layer are oriented randomly [4, 38–40]. It has been proposed that ZP fibrils have properties analogous to amyloid proteins that self-aggregate and form cross-β-sheet fibrillar structures [4, 41, 42]. However, ZP fibrils consist of heteromeric aggregates (i.e., ZP2-ZP3) rather than the homomeric aggregates typical of amyloids [43]. It has been suggested that the plasticity (rigid or flexible) of the linker between ZP-N and ZP-C subdomains of ZPD proteins determines whether the proteins form homo- or hetero-polymers [44].
3. Mouse ZP genes and female fertility
Results of experiments in which antisense oligonucleotides directed against either ZP2 or ZP3 mRNAs were injected into growing mouse oocytes suggest that ZP2 and ZP3 are dependent upon each other for incorporation into the ZP [45]. To extend these observations, gene targeting was used to establish mouse lines in which ZP genes were inactivated by either homologous recombination or insertional mutagenesis and the fertility of the mice was assessed. Results of the gene targeting experiments in mice are summarized below and in Table 4.
Table 4.
Phenotypes of ZP1, 2, 3 null female mice.
| Genotype | Fertility | Zona pellucida |
|---|---|---|
| Wild-type | Fertile | Normal |
| ZP1−/− | Reduced fertility | Abnormal |
| ZP2−/− | Infertile | None |
| ZP3−/− | Infertile | None |
| ZP3+/− | Fertile | Thin |
3.1. Wild-type male mice
Male mice that are homozygous nulls for ZP1, ZP2, or ZP3 are as fertile as wild-type males. ZP genes are only expressed in female mice.
3.2. ZP2 and ZP3 homozygous null female mice
Female mice that are homozygous nulls for either ZP2 (ZP2−/−) or ZP3 (ZP3−/−) produce eggs that lack a ZP and the females are infertile [46–48]. The infertility is due to a scarcity of growing oocytes in ovaries and ovulated eggs in oviducts of homozygous null mice. This suggests that the presence of both ZP2 and ZP3 is absolutely required for assembly of a ZP around growing oocytes and these findings are consistent with results of antisense experiments mentioned above. The paucity of growing oocytes and follicles in ovaries of ZP3−/− mice is reflected in the weight difference of ovaries from wild-type females (20 days-of-age, 1.0 ± 0.17 mg/ovary) and ovaries from ZP3−/− females (20 days-of-age, 0.26 ± 0.1 mg/ovary) [49]. In the absence of a ZP, formation of gap junctions between oocytes and surrounding follicle cells is severely reduced, thereby compromising transfer of nutrients, metabolites, and other molecules essential for oocyte and follicle growth. The latter finding is consistent with the phenotype of female mice that are homozygous nulls for gap junction proteins, such as connexin-37 and −43, that are deficient in multi-layered follicles and growing oocytes and are infertile [50, 51].
3.3. ZP3 heterozygous null female mice
Female mice that are heterozygous nulls for ZP3 (ZP3+/−) are as fertile as wild-type females, but their eggs have a thin ZP (ave. width 2.7 ± 1.2 μm) compared to the ZP of eggs from wild-type females (ave. width 6.2 ± 1.9 μm) [52]. The thin ZP contains about one-half the amount of ZP2 and ZP3 found in ZP of eggs from wild-type mice. These observations suggest that the width of the ZP is not a critical parameter for either binding of free-swimming sperm to the ZP or fertilization of eggs.
3.4. ZP1 homozygous null female mice
Female mice that are homozygous nulls for ZP1 (ZP1−/−) are fertile, but exhibit reduced fertility compared to wild-type mice due to early loss of preimplantation embryos in the oviduct [53]. This loss is attributable to a ZP that is insufficiently cross-linked and, consequently, extremely fragile as cleavage-stage embryos traverse the reproductive tract on their way to the uterus. The presence of ZP2 and ZP3 in growing oocytes of ZP1−/− mice supports formation of heterodimers that can assemble into long fibrils. However, in the absence of ZP1 the fibrils are not properly cross-linked, creating an unusually porous ZP matrix that even permits follicle cells to enter the perivitelline space between the oocyte’s ZP and plasma membrane. New insights into the structural basis of ZP1/ZP4 crosslinking of human ZP have recently been obtained [54].
4. Human ZP genes and female fertility
Some early evidence suggested that there might be a causal relationship between gene sequence variations (GSV) in human ZP genes and female fertility [55–57]. For example, it was found that there was ≈1.5-times more GSV in ZP1 and ZP3 of women who were unsuccessful in IVF trials compared to women with proven fertility [55]. This finding has now been extended by a large number of studies carried out to assess whether GSV in human ZP1–3 have an effect on female fertility. Results of these experiments with human patients are summarized below and in Table 5.
Table 5.
ZP1, 2, and 3 mutations in infertile human patients.
| Designation: hZP1 mutations | Location of mutation | Status of zona pellucida | Reference |
|---|---|---|---|
| G57Dfs*9 | exon-1, SC in N1 before TD | none | [68] |
| R61C | exon-1, N1 before TD | none (?) | [67] |
| W83R | exon-2, N1 before TD | abnormal/none | [66] |
| R109H | exon-3, N1 before TD | none | [64] |
| H170Ifs*52 | exon-3, SC between N1 and TD | none | [69] |
| Q292→X | exon-5, SC in ZP-N | none | [65] |
| I386→X | exon-7, SC between ZP-N and ZP-C (linker) | none | [65] |
| I390fs404X | exon-7, SC between ZP-N and ZP-C (linker) | none | [58, 63] |
| I390Tfs*16 | exon-7, SC between ZP-N and ZP-C (linker) | none | [67, 68] |
| R410W | exon-7, SC between ZP-N and ZP-C (linker) | none | [69] |
| W471→X | exon-8, SC in ZP-C | abnormal/none | [66] |
| C478→X | exon-9, SC in ZP-C | none | [69] |
| V570M | exon-11, between CFCS and EHP | none | [69] |
| D592Gfs*29 | exon-12, SC between CFCS and TMD | none | [69] |
| hZP2 mutations | |||
| C372S | exon-11, ZP-N | thin/none | [69] |
| R533S | exon-15, ZP-C | abnormal/none | [66] |
| C566R | exon-16, ZP-C | abnormal/none | [66] |
| R698→X | exon-19, SC between CFCS and TMD | very thin/none | [70] |
| hZP3 mutations | |||
| A134T | exon-2, ZP-N | none | [64, 71] |
| R255G | exon-5, ZP-C | none | [69] |
| R349L→X | exon-8, SC at CFCS | very thin/none | [70] |
Abbreviations: CFCS, concensus furin cleavage-site; EHP, external hydrophobic patch; h, human; SC, stop-codon; TD, trefoil domain; TMD, transmembrane domain; ZPD, zona pellucida domain; ZP-C, ZPD carboxy-terminal subdomain; ZP-N, ZPD amino-terminal subdomain.
4.1. GSV in human ZP1
One study revealed a homozygous frameshift deletion of eight bp in ZP1 of women who were infertile and whose eggs lacked a ZP [58]. The deletion was predicted to result in a premature stop-codon (SC) in ZP1 and synthesis of a truncated form of ZP1; Ile390fs404X, a 404 aa polypeptide versus a 638 aa polypeptide in wild-type ZP1. Truncated ZP1 had the N-terminal SS, TD, and first half of the ZPD, but was missing the CTP essential for protein secretion [59–62]. Since oocytes from ZP1 homozygous null mice have a ZP, albeit a very loose and porous ZP, it was surprising that oocytes from these women lacked a ZP. However, subsequently it was reported that accumulation of truncated ZP1 in the oocyte’s cytoplasm apparently interfered with secretion of nascent ZP3 and ZP4 and thereby prevented assembly of a ZP around growing ocytes [63]. An alternative explanation for the observation has recently been put forward that does not involve interference with secretion of nascent ZP proteins by truncated ZP1, but rather by affecting the cross-linking function of ZP1 [54].
Other studies also have attributed female infertility to GSV in ZP1. A heterozygous missense mutation in exon-3 of ZP1 was identified in an infertile patient whose oocytes lacked a ZP [64]. The mutation resulted in His replacing Arg109 at the N-terminus of ZP1. Similarly, a compound heterozygous mutation consisting of a point mutation and deletion in ZP1 was identified in an infertile woman whose oocytes lacked a ZP [65]. The mutation in exon-5 resulted in synthesis of ZP1 stopping at Gln292 and a two basepair deletion in exon-7 also resulted in a premature SC and ZP1 synthesis stopping at Ile386.
Several additional studies also led to identification of GSV in ZP1 in infertile females who had abnormal oocytes. For example, a missense mutation in exon-2, Trp83Arg, was found in a patient with degenerated oocytes and an abnormal or no ZP, and in another patient a nonsense mutation with a premature SC in exon-8, Trp471→X, had a similar phenotype [66]. A compound heterozygous mutation, Arg61Cys and Ile390Thrfs*16, was found to be associated with abnormal oocytes and no ZP since replacement of Arg61 with Cys was predicted to be deleterious to ZP1 and the frameshift mutation introducing an SC in exon-7 (Ile390Thrfs404X) resulted in a 234 aa deletion at the C-terminus of ZP1 [67, 68]. In another case, two frameshift mutations in ZP1 resulted in premature SCs in exon-1 (Gly57Aspfs*9) and exon-7 (Ile390Thrfs*16) and apparently disrupted interactions between ZP proteins and caused degeneration of oocytes [68]. Missense mutations, Val570Met and Arg410Trp, were identified in two infertile females that had no oocytes or oocytes lacking a ZP [69]. Similarly, a compound heterozygous mutation with premature SCs in exon-9 (Cys478→X) and exon-12 (Asp592Glyfs*29) and a frameshift mutation in exon-3, His170Ilefs*52, were identified that possibly resulted in a truncated ZP1 that interfered with ZP formation [69].
In many instances GSV in ZP1 affected its ZPD (Figure 4), a region of all ZP proteins considered critical for proper secretion of nascent ZP proteins and proper assembly of a ZP around growing oocytes [4, 7, 29, 30, 61].
Figure 4.

Primary structure of human (h) ZP1 (NCBI Reference Sequence: NP_997224.2) using the single letter amino acid code. The polypeptide consists of 638 aa and has a signal sequence (SS; aa 1–25), a ZP-N1 (aa 43–135) with 5 Cys residues (bold), a proline-rich region (aa 136–235), a trefoil domain (TD; aa 236–275) with 6 Cys residues (bold), a ZPD (aa 279–546) consisting of a ZP-N (aa 279–381) with 4 Cys residues (bold), a ZP-C (aa 409–546) with 6 Cys residues (bold), and a linker region (aa 382–408; internal hydrophobic patch, aa 402–408), a concensus furin cleavage-site (CFCS; aa 552–555), an d a transmembrane domain (TMD; aa 602–622). The SS is italicized, ZP-N1 is in bold, the TD is indicated by a dashed line, ZP-N and ZP-C are in bold, the linker region is indicated by a dotted line, the CFCS (bold; aa 552–555), and the TMD is indicated by a dotted line. Three black arrows indicate the positions of gene sequence variations (GSV) that result in changes in ZP1 that are proposed to cause female infertility. One arrow indicates the replacement of Arg-109 with His (R109H; exon-3, N1 before TD) and the two other arrows indicate the insertion of an SC that results in the synthesis of truncated ZP1 that lacks the ZP-C (aa 409–546) and CTP (aa 547–638).
4.2. GSV in human ZP2 and ZP3
GSV in human ZP2 and ZP3 of infertile women can also result in synthesis of ZP proteins that are unable to undergo normal secretion and assembly during oocyte growth. An infertile woman was found to have a heterozygous missense mutation in ZP2 (exon-19 Arg698→X) with insertion of a SC at aa 698 and a heterozygous frameshift mutation in ZP3 (exon-8 Arg349Leu→X) followed by a SC [70]. Both mutations resulted in the synthesis of truncated ZP proteins, ZP2 lacking a TMD and ZP3 lacking a CTP. Three other cases of GSV in ZP2 have been described in which Cys372 was changed to Ser, Arg533 to Ser, and Cys566 to Arg [66, 69]. All these changes occurred at conserved aa residues in the ZPD of ZP2, aa 371–637. A heterozygous missense variant in exon-2 of ZP3 also was identified as a change of Ala134 to Thr; a change proposed to cause empty follicle syndrome and female infertility [64, 71]. A similar missense mutation in exon-5 of ZP3, Arg255 to Gly, was found in a female with primary infertility [69]. In both cases the mutations occurred in the ZPD of ZP3, aa 45–304.
5. Final comments
ZP1–4 are structural proteins essential for constructing a proper ZP around mammalian oocytes during their growth phase [3, 15, 37]. ZP2-ZP3 dimers polymerize to form the long fibrils cross-linked by ZP1 that constitute the thick ECM. ZP genes in mice and humans are single-copy genes located on different chromosomes and are expressed only in growing oocytes. Gene targeting of ZP1–3 in mice [46–48, 52] and analysis of GSV in ZP1–3 in human patients [58, 63–71] have revealed that female fertility can be severely affected by the absence of ZP1, ZP2, or ZP3 or by the presence of mutated forms of ZP1, ZP2, or ZP3 during oocyte growth. Failure to assemble a proper ZP around growing oocytes can result in infertility.
Inactivation of mouse ZP1, ZP2, or ZP3 by gene targeting results in infertility (ZP2−/− or ZP3−/−) or reduced fertility (ZP1−/−) in mice and strongly suggests that both ZP2 and ZP3 must be present to construct a proper ZP. The presence of mouse ZP1, a cross-linker protein, is not required since a loose ZP can be constructed from ZP2 and ZP3. ZP1−/− female mice are fertile, but a reduced number of preimplantation embryos survive in their reproductive tract due to a labile ZP. The thickness of the ZP does not appear to affect fertility since heterozygous null mice (ZP3+/−) with a thin ZP, about one-half the width of a wild-type ZP, are as fertile as wild-type mice.
GSV in human ZP1, ZP2, or ZP3 as point, missense, or frameshift mutations also can result in infertility in humans. In many instances the mutations affect secretion of nascent ZP1–3 by growing oocytes due to insertion of premature SCs in ZP genes and thereby prevent construction of a proper ZP and causes infertility. The extent of female infertility has increased dramatically (≈15%) over the past 25 years, such that today it is estimated that ≈10% of married women worldwide are infertile. It is likely that many more mutations in human ZP1–3 and ZP4 will be identified in the future that account for female infertility. Correction of these mutations by genetic engineering, perhaps by using CRISPR/Cas9 technology, remains an interesting and tempting possibility.
ACKNOWLEDGEMENTS
We are grateful to all members of our laboratory over the years who contributed to our research on the zona pellucida. We respectfully acknowledge the late Jeffrey David Bleil (1952-2014) who initiated our research on the zona pellucida as a graduate student at Harvard Medical School more than forty years ago. We thank Giuseppe Familiari at the University of Rome, Italy, for kindly providing images from his publications for our mini-review. Our research was supported in part by the NIH (NICHD) and F. Hoffmann-La Roche AG.
ABBREVIATIONS
- aa
amino acid
- N-terminus
amino-terminus
- bp
basepairs
- CTP
carboxy-terminal propeptid
- C-terminus
carboxy-terminus
- CFCS
concensus furin cleavage-site
- Cys
cysteine
- ECM
extracellular matrix
- GSV
gene sequence variations
- hZP1–4
human ZP1–4
- pI
isoelectric point
- kD
kilodaltons
- MW
molecular weight
- mZP1–3
mouse ZP1–3
- SC
stop-codon
- SS
signal sequence
- TD
trefoil domain
- TMD
transmembrane domain
- ZP
zona pellucida
- ZP-C
ZPD carboxy-terminal subdomain
- ZPD
zona pellucida domain
- ZP-N
ZPD amino-terminal subdomain
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no disclosures.
REFERENCES
- 1.von Baer KE 1827, De ovi mammalium et hominis genesi [On the Genesis of the Ovum of Mammals and of Man]. Leopold Voss, Leipzig, Germany. [Google Scholar]
- 2.Wassarman PM 1988, Annu. Rev. Biochem, 57, 415–442. [DOI] [PubMed] [Google Scholar]
- 3.Litscher ES and Wassarman PM (Eds.) 2018, Extracellular Matrix and Egg Coats. Academic Press/Elsevier, Oxford, UK. [Google Scholar]
- 4.Litscher ES and Wassarman PM 2020, Annu. Rev. Biochem, 89, 695–715. [DOI] [PubMed] [Google Scholar]
- 5.Litscher ES and Wassarman PM 2015, A Guide to Zona Pellucida Domain Proteins. John Wiley and Sons, Hoboken. [Google Scholar]
- 6.Franz C, Stewart KM and Weaver VM 2010, J. Cell Sci, 123, 4195–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jovine L, Darie CC, Litscher ES and Wassarman PM 2005, Annu. Rev. Biochem, 74, 83–114. [DOI] [PubMed] [Google Scholar]
- 8.Litscher ES and Wassarman PM 2014, Trends Dev. Biol, 8, 65–76. [PMC free article] [PubMed] [Google Scholar]
- 9.Wassarman PM 1987, Science, 235, 553–560. [DOI] [PubMed] [Google Scholar]
- 10.Avella MA, Xiong B and Dean J 2013, Mol. Hum. Reprod, 19, 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang L, Chamow SM and Dean J 1990, Mol. Cell. Biol, 10, 1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang L-F and Dean J 1993, Dev. Biol, 156, 399–408. [DOI] [PubMed] [Google Scholar]
- 13.Epifano O, Liang L-F and Dean J 1995, J. Biol. Chem, 270, 27254–27258. [DOI] [PubMed] [Google Scholar]
- 14.Hughes DC and Barratt CL 1999, Biochem. Biophys. Acta, 1447, 303–306. [DOI] [PubMed] [Google Scholar]
- 15.Wassarman PM and Litscher ES 2018, Curr. Top. Dev. Biol, 130, 331–356. [DOI] [PubMed] [Google Scholar]
- 16.Roller RJ, Kinloch RA, Hiraoka BY, Li SS and Wassarman PM 1989, Development, 106, 251–261. [DOI] [PubMed] [Google Scholar]
- 17.Lira SA, Kinloch RA, Mortillo S and Wassarman PM 1990, Proc. Natl. Acad. Sci. USA, 87, 7215–7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lira SA, Schickler M and Wassarman PM 1993, Mol. Reprod. Dev, 36, 494–499. [DOI] [PubMed] [Google Scholar]
- 19.Liang L-F, Soyal SM and Dean J 1997, Development, 124, 4939–4947. [DOI] [PubMed] [Google Scholar]
- 20.Soyal SM, Amleh A and Dean J 2000, Development, 127, 4645–4654. [DOI] [PubMed] [Google Scholar]
- 21.Lefievre L, Conner SJ, Salpekar A, Olufowobi O, Ashton P, Pavlovic B, Lenton W, Afnan M, Brewis IA, Monk M, Hughes DC and Barratt CL 2004, Hum. Reprod, 19, 1580–1586. [DOI] [PubMed] [Google Scholar]
- 22.Gupta SK 2018, Curr. Top. Dev. Biol, 130, 379–411. [DOI] [PubMed] [Google Scholar]
- 23.Connor SJ, Lefievre L, Hughes DC and Barratt CL 2005, Hum. Reprod, 20, 1148–1152. [DOI] [PubMed] [Google Scholar]
- 24.Thim L 1997, Cell. Mol. Life Sci, 53, 888–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rankin TL, Coleman JS, Epifano O, Hoodbhoy T, Turner SG, Castle PE, Lee E, Gore-Langton R and Dean J 2003, Dev. Cell, 5, 33–43. [DOI] [PubMed] [Google Scholar]
- 26.Baibakov B, Boggs NA, Yauger B, Baibakov G and Dean J 2012, J. Cell Biol, 197, 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Killingbeck EE and Swanson WJ 2018, Curr. Top. Dev. Biol, 130, 443–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Callebaut I, Mornon JP and Monget P 2007, Bioinformatics, 23, 1871–1874. [DOI] [PubMed] [Google Scholar]
- 29.Jovine L, Qi H, Williams Z, Litscher E and Wassarman PM 2002, Nature Cell Biol, 4, 457–461. [DOI] [PubMed] [Google Scholar]
- 30.Jovine L, Janssen WG, Litscher ES and Wassarman PM 2006, BMC Biochem, 7, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raj I, Sadat Al Hosseini H, Dioguardi E, Nishimura K, Han L, Villa A, de Sanctis D and Jovine L 2017, Cell, 169, 1315–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bokhove M and Jovine L 2018, Curr. Top. Dev. Biol, 130, 413–442. [DOI] [PubMed] [Google Scholar]
- 33.Williams AF and Barclay AN 1988, Annu. Rev. Immunol, 6, 381–405. [DOI] [PubMed] [Google Scholar]
- 34.Hutchinson EG and Thornton JM 1993, Protein Eng, 6, 233–245. [DOI] [PubMed] [Google Scholar]
- 35.Greve JM and Wassarman PM 1985, J. Mol. Biol, 181, 253–264. [DOI] [PubMed] [Google Scholar]
- 36.Wassarman PM and Mortillo S 1991, Intl. Rev. Cytol, 130, 85–110. [DOI] [PubMed] [Google Scholar]
- 37.Wassarman PM, Liu C and Litscher ES 1996, J. Cell Sci, 109, 2001–2004. [DOI] [PubMed] [Google Scholar]
- 38.Keefe D, Tran P, Pellegrini C and Oldenbourg R 1997, Hum. Reprod, 12, 1250–1252. [DOI] [PubMed] [Google Scholar]
- 39.Pelletier C, Keefe DL and Trimarchi JR 2004, Fertil. Steril, 81, 850–856. [DOI] [PubMed] [Google Scholar]
- 40.El-Mestrah M, Castle PE, Borossa G and Kan FWK 2002, Biol. Reprod, 66, 866–876. [DOI] [PubMed] [Google Scholar]
- 41.Egge N, Muthusubramanian A and Cornwall GA 2015, PLoS One, 10, e129907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Louros NN, Iconomidou VA, Giannelou P and Hamodrakas SJ 2013, PLoS One, 8, e73258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eisenberg DS and Sawaya MR 2017, Annu. Rev. Biochem, 86, 69–95. [DOI] [PubMed] [Google Scholar]
- 44.Bokhove M, Nishimura K, Brunati M, Han L, de Sanctis D, Rampoldi L and Jovine L 2016, Proc. Natl. Acad. Sci. USA, 113, 1552–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tong Z-B, Nelson LM and Dean J 1995, J. Biol. Chem, 270, 849–853. [DOI] [PubMed] [Google Scholar]
- 46.Liu C, Litscher ES, Mortillo S, Sakai Y, Kinloch RA, Stewart CL and Wassarman PM 1996, Proc. Natl. Acad. Sci. USA, 93, 5431–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rankin T, Familiari M, Lee E, Ginsberg A, Dwyer N, Blanchette-Mackie J, Drago J, Westphal H and Dean J 1996, Development, 122, 2903–2910. [DOI] [PubMed] [Google Scholar]
- 48.Rankin T, O’Brien M, Lee E, Wigglesworth K, Eppig J and Dean J 2001, Development, 128, 1119–1126. [DOI] [PubMed] [Google Scholar]
- 49.Wassarman PM and Litscher ES 2012, Intl. J. Dev. Biol, 56, 833–839. [DOI] [PubMed] [Google Scholar]
- 50.Simon AM, Goodenough DA, Li E and Paul DL 1997, Nature, 385, 525–529. [DOI] [PubMed] [Google Scholar]
- 51.Ackert CL, Gittens JE, O’Brien MJ, Eppig JJ and Kidder GM 2001, Dev. Biol, 233, 258–270. [DOI] [PubMed] [Google Scholar]
- 52.Wassarman PM, Qi H and Litscher ES 1997, Proc. Roy. Soc. London, Biol. Sci, 264, 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rankin TL, Talbot P, Lee E and Dean J 1999, Development, 126, 3847–3855. [DOI] [PubMed] [Google Scholar]
- 54.Nishimura K, Dioguardi E, Nishio S, Villa A, Han L, Matsuda T and Jovine L 2019, Nature Comm, 10, 3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Männikkö M, Törmälä RM, Tuuri T, Haltia A, Martikainen H, Ala-Kokko L, Tapanainen JS and Lakkakorpi JT 2005, Hum. Reprod, 20, 1578–1585. [DOI] [PubMed] [Google Scholar]
- 56.Pökkylä RM, Lakkakorpi JT, Noujua-Huttunen SH and Tapanainen JS 2011, Fertil. Steril, 95, 2669–2672. [DOI] [PubMed] [Google Scholar]
- 57.Margalit M, Paz G, Yavetz H, Yogev L, Amit A, Hevlin-Schwartz T, Gupta SK and Kleiman SE 2012, Eur. J. Obstet. Gynecol. Reprod. Biol, 165, 70–76. [DOI] [PubMed] [Google Scholar]
- 58.Huang H-L, Lv C, Zhao Y-C, Li W, He X-M, Li P, Sha A-G, Tian X, Papasian CJ, Deng H-W, Lu G-X and Xiao H-M 2014, New Engl. J. Med, 370, 1220–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams Z and Wassarman PM 2001, Biochemistry, 40, 929–937. [DOI] [PubMed] [Google Scholar]
- 60.Qi H, Williams Z and Wassarman PM 2002, Mol. Biol. Cell, 13, 530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jovine L, Qi H, Williams Z, Litscher ES and Wassarman PM 2004, Proc. Natl. Acad. Sci. USA, 101, 5922–5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jimenez-Movilla M and Dean J 2011, J. Cell Sci, 124, 940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lv C, Huang H-L, Wang Y, Peng T-L, Tan H-J, Zeng MH, Quan R-P, Deng H-W and Xiao H-M 2019, BioRxiv, 825018. [Google Scholar]
- 64.Cao Q, Zhao C, Zhang X, Zhang H, Lu Q, Wang C, Hu Y, Ling X, Zhang J and Huo R 2020, J. Cell. Mol. Med, 2020, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Z, Shangguan T, Li Y-Y and He W 2020, Reprod. Dev. Med, 2, 183–186. [Google Scholar]
- 66.Yang P, Luan X, Peng Y, Chen T, Su S, Zhang C, Wang Z, Cheng L, Zhang X, Wang Y, Chen Z-J and Zhao H 2017, Fertil. Steril, 107, 1364–1369. [DOI] [PubMed] [Google Scholar]
- 67.Yuan P, Li R, Li D, Zheng L, Ou S, Zhao H, Zhang Q and Wang W 2019, J. Assist. Reprod. Genet, 36, 741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun L, Fang X, Chen Z, Zhang H, Zhang Z, Zhou P, Xue T, Peng X, Zhu Q, Yin M, Liu C, Deng Y, Hu H and Li N 2019, Hum. Mut, 40, 2001–2006. [DOI] [PubMed] [Google Scholar]
- 69.Zhou Z, Ni C, Wu L, Chen B, Xu Y, Zhang S, Mu J, Li B, Yan Z, Fu J, Wang W, Zhao L, Dong J, Sun X, Kuang Y, Sang Q and Wang L 2019, Hum. Genet, 138, 327–337. [DOI] [PubMed] [Google Scholar]
- 70.Liu W, Li K, Bai D, Yin J, Tang Y, Chi F, Zhang L, Wang Y, Pan J, Liang S, Guo Y, Ruan J, Kou X, Zhao Y, Wang H, Chen J, Teng X and Gao S 2017, Hum. Genet, 136, 1822–1827. [DOI] [PubMed] [Google Scholar]
- 71.Chen T, Bian Y, Liu X, Zhao S, Wu K, Yan L, Li M, Yang Z, Liu H, Zhao H and Chen Z-J 2017, Amer. J. Hum. Genet, 101, 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Familiari G, Relucenti M, Heyn R, Micara G and Correr S 2006, Microsc. Res. Tech, 69, 415–426. [DOI] [PubMed] [Google Scholar]


