Abstract
The global spread of COVID-19 has created an urgent need for a safe and effective vaccine. However, in the United States, the politicization of the vaccine approval process, including which public figures are endorsing it, could undermine beliefs about its safety and efficacy and willingness to receive it. Using a pair of randomized survey experiments, we show that announcing approval of a COVID-19 vaccine one week before the presidential election compared to one week after considerably reduces both beliefs about its safety and efficacy and intended uptake. However, endorsement by Dr. Anthony Fauci increases confidence and uptake among all partisan subgroups. Further, an endorsement by Dr. Fauci increased uptake and confidence in safety even if a vaccine receives pre-election approval. The results here suggest that perceptions of political influence in COVID-19 vaccine approval could significantly undermine the viability of a vaccine as a strategy to end the pandemic.
Keywords: COVID-19, Vaccine hesitancy, Endorsements, Politicization
1. Introduction
The true value of a vaccine in controlling an infectious disease is a combination of the vaccine’s efficacy and the willingness of individuals to vaccinate. If enough individuals refuse to receive a vaccine, the protection afforded to the population at large is considerably diminished. Past research has documented the challenges that vaccine hesitancy poses for the medical community [1], [2], [3]. COVID-19 presents a unique challenge given that the need to rapidly develop a vaccine could broadly undermine the public’s beliefs that one will be safe. Recent survey evidence has shown that only 51% of US adults indicate they are likely to accept a COVID-19 vaccine with only 19% of the public having a “great deal” of confidence that the process to create a vaccine will produce one that is safe and effective [4].
Hesitancy to receive a COVID-19 vaccine may also be driven, in part, by public concerns that political considerations are affecting when a vaccine is approved and whether it is approved before proven to be safe and effective. A majority of Americans report that they are worried that political pressure could cause a vaccine to be approved before it is safe and effective and a majority of both Republicans and Democrats express concerns that approval of the vaccine will be based more on politics than science [5], [6]. Further, in the 2020 Vice Presidential Debate, Senator Kamala Harris stated that she would not receive a vaccine if it was endorsed by President Trump, but she would receive it if NIAID (National Institute of Allergy and Infectious Diseases) director Dr. Anthony Fauci advised that it was safe and effective [7]. Taken together, this fits into a broader pattern of the politicization of the COVID-19 pandemic and responses to it among political elites and in mass public opinion [8], [9], [10].
While approval of a COVID-19 vaccine prior to the November presidential election could affect the outcome, it is not known whether the timing of a COVID-19 vaccine approval coinciding with the presidential election would undermine the public confidence that the vaccine is safe and effective and willingness to receive it. Further, we also do not know whether endorsements of the vaccine by politicians, like President Donald Trump and House Speaker Nancy Pelosi, and health experts, like Dr. Anthony Fauci, would have consequences for beliefs about safety and efficacy and individuals’ willingness to receive a vaccine.
Prior to the 2020 United States presidential election, President Trump was the most visible Republican elected official who had expressed skepticism about the severity of the COVID-19 pandemic, while simultaneously highlighting his administration’s role in the rapid development of a vaccine. On several occasions during the pandemic, President Trump touted unproven treatments for COVID-19 and publicly contradicted guidance provided by scientific experts, including Dr. Fauci. In contrast, Speaker Pelosi was the highest ranking elected Democrat and had been a notable critic of President Trump’s touting of unproven therapeutic treatments and the handling of the COVID-19 pandemic more generally. Given their acrimonious relationship, President Trump and Speaker Pelosi jointly endorsing a COVID-19 vaccine could therefore have a powerful effect on broadly increasing uptake and public confidence. More generally, understanding the politicization of COVID-19 vaccines has important implications for strategies to increase vaccination among the general public.
2. Design
We use two randomized, controlled experiments fielded on a nationally representative sample of adult U.S. residents (N = 5014) to examine how timing and elite endorsement effect public opinion about a COVID-19 vaccine. Both experiments were embedded in the same survey that was fielded by the survey vendor YouGov between September 9 and September 22, 2020. The experiments were fielded under an IRB exemption granted by the Yale University IRB.
Prior to our randomized treatments, respondents were asked about their background, including a generic vaccine confidence battery [11]. The first experiment was a vignette in which respondents were asked to consider a vaccine with a particular approval date: “Suppose a COVID-19 vaccine receives approval from the FDA on [DATE].” DATE was randomly assigned to be “October 27, 1 week before the election”, “November 10, 1 week after the election”, or “December 15.” Respondents were then asked their likelihood of getting the vaccine (“How likely would you be to get this COVID-19 vaccine within the first 3-months of it becoming available to you?” measured on a 5-point scale running from 0 [Extremely unlikely] to 1 [Extremely likely]) and their confidence in its safety and efficacy (“How confident are you that this COVID-19 vaccine would be safe and effective?” measured on a 4-point scale running from 0 [Not confident at all] to 1 [Extremely confident]). For subsequent analyses, we dichotomized the measure of the likelihood that respondents would receive the vaccine, such that likely and extremely likely were coded 1 and the other responses were coded 0.
The second experiment followed the first and held fixed the date of approval but added a third-party statement about the approved vaccine’s safety and efficacy. The statement was randomly assigned to one of six values, (1) a positive or (2) negative statement by Dr. Anthony Fauci, (3) a positive or (4) negative statement by President Trump, (5) a joint positive statement by Trump and Speaker of the House Nancy Pelosi, or (6) a positive Trump statement and a negative Pelosi statement. Respondents were then asked the same outcome questions. The full text of the endorsements is displayed in Table 1 .
Table 1.
Experiment 2, endorsement conditions.
| (1) Fauci Positive | Dr. Anthony Fauci, the director of the National Institute of Allergy and Infectious Diseases, says that he believes the vaccine is safe and effective |
| (2) Fauci Negative | Dr. Anthony Fauci, the director of the National Institute of Allergy and Infectious Diseases, says that he is not convinced the vaccine is safe and effective |
| (3) Trump Positive | President Trump says that he believes the vaccine is safe and effective |
| (4) Trump Negative | President Trump says that he is not convinced the vaccine is safe and effective |
| (5) Trump and Pelosi Positive | both President Trump and Democratic Speaker of the House Nancy Pelosi say that they believe the vaccine is safe and effective |
| (6) Trump Positive, but Pelosi Negative | President Trump says that he believes the vaccine is safe and effective but Democratic Speaker of the House Nancy Pelosi says that she is not convinced the vaccine is safe and effective |
We use OLS regression with robust Huber-White standard errors to estimate the treatment effects that are presented below. For analyses focusing on a specific group, e.g. Republicans, we restrict the sample to the subgroup and re-estimate the model. Differences in effect sizes are calculated using linear combination of coefficient tests.
3. Results
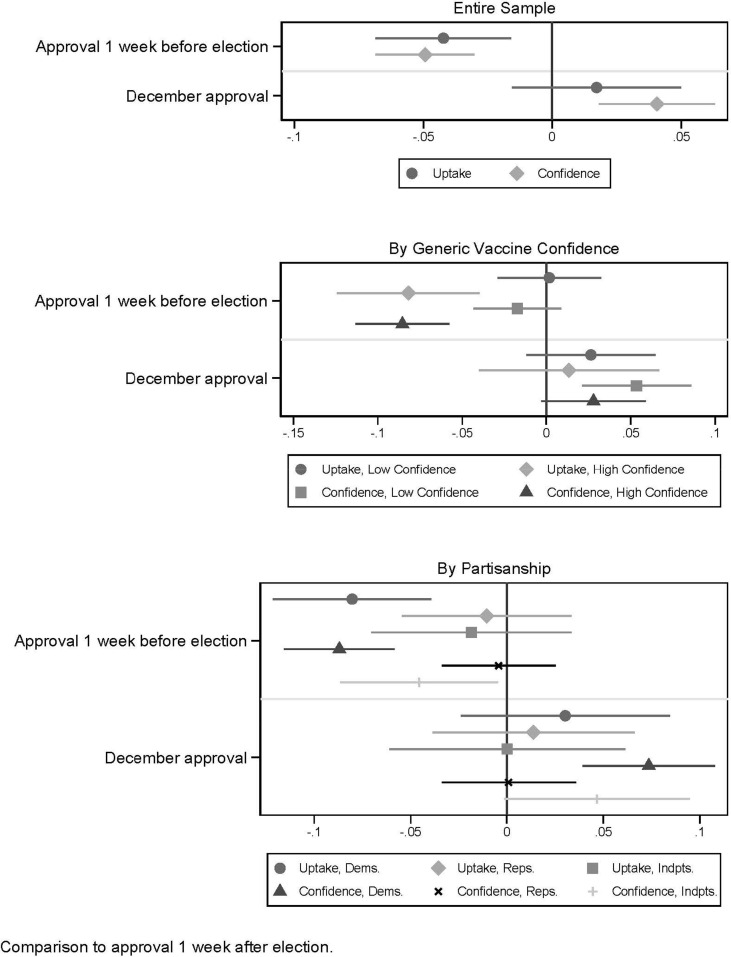
Using the data from the first experiment, Fig. 1 shows how vaccine timing affects stated willingness to vaccinate and confidence. Panel A shows the results for the overall sample. Compared to a baseline announcement one week after the election, as well as an approval in December, approval before the election reduced willingness to vaccinate and confidence. An announcement of approval one week prior to the election was estimated to decrease the reported likelihood of receiving a COVD-19 vaccine within the first three months of availability by 4.2 percentage points (95% C.I. = −1.6 to −6.9, p < .01), a 14% reduction from reported intentions to vaccinate if announced one week after the election (0.042/0.299 = 0.14). Respondents were also less confident that the vaccine would be safe and effective if approved before the election (difference = −0.049 for scale outcome ranging from 0 to 1, 95% C.I. = −0.030 to -0.069, p < .001). A vaccine approved in December compared to the week after the election increased willingness to vaccinate by 1.7 percentage points (95% C.I. = −1.5 to 5.0, p = .30) and confidence by 0.41 units (95% C.I. = 0.18 to 0.63, p < .001).
Fig. 1.
Experiment 1, effect of vaccine timing on uptake and confidence. Panels show ordinary least squares (OLS) regression coefficient estimates with 95% confidence intervals for the entire sample (Panel A, top), by generic vaccine confidence (Panel B, middle) and by political partisanship (Panel C, bottom). Full model estimates shown in Table S1.
The subsequent panels of Fig. 1 show that the effect of the politicized context is based on a strong response among respondents with high general vaccine confidence (Panel B, for high confidence respondents the early announcement reduced uptake intentions by 8.2 percentage points (95% C.I. = −4.0 to −12.4, p < .001) and confidence by 0.085 units (95% C.I. = −0.058 to −0.113, p < .001) and was heavily concentrated among Democrats (Panel C, uptake reduced by 8 percentage points (95% C.I. = −3.9 to −12.1, p < .001) and confidence by −0.086 units (95% C.I. = −0.058 to -0.116, p < .001); Effects for Republicans and Independents smaller and not statistically significant).
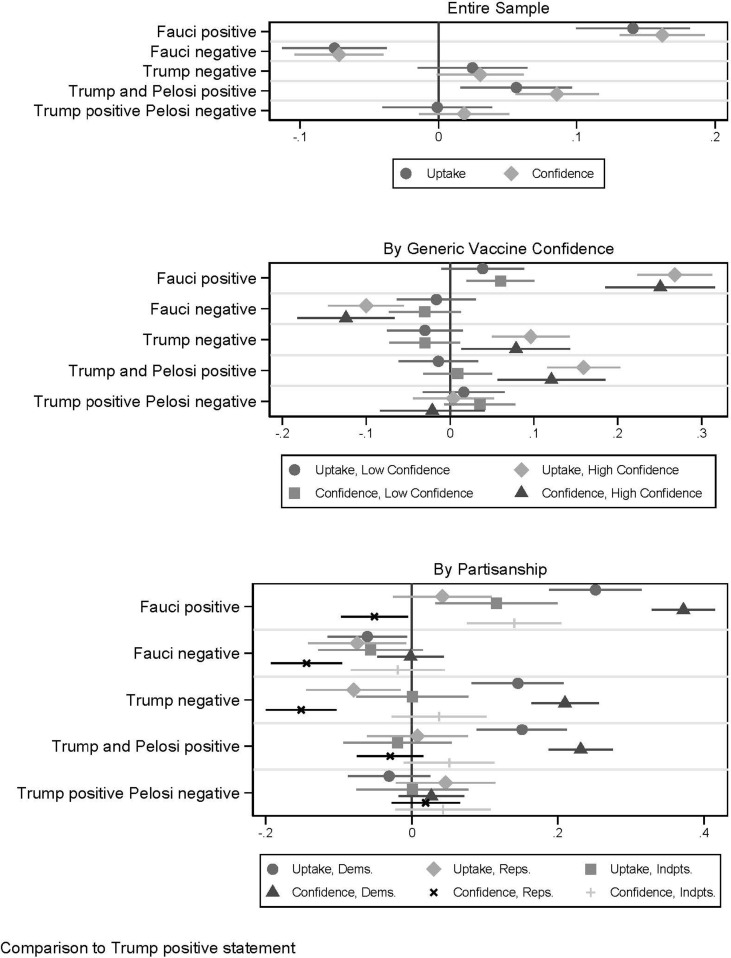
For the second experiment, compared to the baseline condition of a positive statement by President Trump, Dr. Fauci’s statements had dramatic effects on public reactions (Fig. 2 ). For vaccine uptake and confidence, respectively, the effects of a positive rather than negative endorsement by Dr. Fauci were very large, approximately 21.6 percentage points (95% C.I. = 17.6 to 25.5, p < .001) and 0.234 units (95% C.I. = 0.204 to 0.264, p < .001). President Trump’s statement in favor rather than opposed was not statistically significant for either outcome. Speaker Pelosi’s co-endorsement with President Trump versus contradicting the President with a negative statement had effects approximately one-third to one half as large as that of Dr. Fauci (vaccine uptake difference = 5.7 percentage points, 95% C.I. = 1.7 to 9.8, p < . 001; confidence difference = 0.067, 95% C.I. = 0.037 to 0.098, p < .001).
Fig. 2.
Experiment 2, effect of vaccine endorsement on uptake and confidence. Panels show ordinary least squares (OLS) regression coefficient estimates with 95% confidence intervals for the entire sample (Panel A, top), by generic vaccine confidence (Panel B, middle) and by political partisanship (Panel C, bottom). Full model estimates shown in Table S2.
The effects of Fauci and Pelosi were concentrated among those with a high vaccine confidence (Fig. 2), although there was a positive effect from a positive rather than negative statement from Dr. Fauci among both groups. In contrast, the overall null effect of President Trump’s positive rather than negative statement is shown to be a combination of a positive (not significant) increase in confidence among those low in baseline confidence and a negative (significant) effect among those high in confidence.
All groups indicated more willingness to receive a vaccine if Dr. Fauci supported it rather than opposed it, but the effect was 4 times larger for Democrats than Republicans, with the effect for Independents in between. In contrast, President Trump had a polarized effect; his statement in support of versus opposition to vaccine approval raised vaccine confidence among Republicans about as much as Dr. Fauci, but lowered confidence among Democrats and had no effect among independents. Speaker Pelosi’s impact was concentrated among Democratic respondents, with effects near zero for both Republicans and independents.
Given the sensitive nature of pre-election approval, we more closely examine the effect of endorsement by public figures of an approval one week prior to the election (Table S3). Even in the most politicized window for approval, Dr. Fauci’s support increased reported uptake intentions and confidence in safety and efficacy compared to Dr. Fauci opposing a vaccine (vaccine uptake difference = 15.4 percentage points, 95% C.I. = 9.2 to 21.6, p < .001; confidence difference = 0.201 units, 95% C.I. = 0.152 to 0.251, p < .001). Notably, endorsement by political figures does not appear to move vaccine uptake or confidence in safety and efficacy in the pre-election window.
4. Conclusion
There are several key conclusions from our experiments. First, public confidence in a COVID-19 vaccine is significantly affected by the political context of vaccine approval. Second, and consistent with our evidence about the importance of political context, endorsements of the vaccine by political leaders have a polarized response, increasing confidence among co-partisans while being ignored or undermining confidence among respondents affiliated with the other party. In contrast, Dr. Fauci’s endorsement (versus skepticism) increases confidence among Democrats, Republicans, and Independents, but the effect is greatest for Democrats and smallest for Republicans. Third, those who have a high level of baseline vaccine confidence appear to be especially sensitive to political context and endorsements suggesting that the politicization of a COVID-19 vaccine may be particularly detrimental to achieving a high rate of take-up.
The observation that those who are most confident in vaccines are most responsive to political endorsements suggests that the increased politicization of vaccination may be undercutting uptake among those who are most likely to vaccinate at baseline. This may also mean that the best strategy for encouraging vaccination against COVID-19 might be to find people who are not political actors, such as public health experts or prominent members of the community, to make endorsements because it would not risking alienating an opposing partisan subgroup. Alternatively, people who are high in vaccine confidence may share other characteristics that make them more susceptible to persuasion by political figures. For example, respondents in our sample who reported that they follow what is happening in government and public affairs most of the time scored significantly higher in vaccine confidence than people who reported paying less attention to the news. Those who are highest in vaccine confidence are seemingly more aware about conflicts between political parties. This suggests that future research is necessary to determine what causes people to be responsive to cues from political elites regarding their decisions to vaccinate. It also means that more effort is needed to identify messages that are effective among those who are more skeptical of vaccines.
Prior research has found that public health organization, like the Center for Disease Control and the World Health Organization, increase COVID-19 vaccination intentions among American adults relative to endorsement by President Trump [12]. Our work build on this prior work by considering how the timing of vaccine approval relative to an election and the effects of elite endorsement differ by an individual’s partisanship. Further, Experiment 2 tests whether bipartisan endorsement by President Trump and Speaker Pelosi increases intentions to get vaccinated and confidence in the vaccine’s safety and efficacy. Notably, the effect of bipartisan endorsement was considerably smaller than endorsement by Dr. Fauci, which suggests that partisan unity is not a substitute for expertise.
In sum, these experiments demonstrate that public opinion toward the efficacy and safety of the COVID-19 vaccine is responsive to perceptions of political motivation and endorsements. While it is common for politicized issues to display polarized beliefs, the evidence that vaccine approval has become politicized suggests that there is great value in understanding how to communicate factual information about vaccine safety and efficacy, including the importance of independent public figures who are not perceived in partisan terms. Further research is needed to develop strategies to provide accurate information that is not ignored or exaggerated due to the political dispositions of the public and their perceptions of the political motives of those overseeing this key public medical and public health issue.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Notes and Acknowledgments
The authors declare that there are no conflicts of interest related to this work. We would like to acknowledge the generous support of the Tobin Center for Economic Policy, the Center for the Study of American Politics, the Institution for Social and Policy Studies at Yale University and the Yale Institute for Global Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2020.12.048.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.MacDonald N.E. Vaccine hesitancy: Definition, scope, and determinants. Vaccine. 2015;33:4161–4164. doi: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 2.Salmon D.A., Dudley M.Z., Glanz J.M., Omer S.B. Vaccine hesitancy: Causes, consequences, and a call to action. Vaccine. 2015;33:D66–D71. doi: 10.1016/j.vaccine.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 3.Dubé E., Gagnon D., MacDonald N.E. Strategies intended to address vaccine hesitancy: Review of published reviews. Vaccine. 2015;33:4191–4203. doi: 10.1016/j.vaccine.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 4.Tyson A, Johnson C, & Funk C. U.S. public now divided over whether to get COVID-19 vaccine. Pew Research Center. https://www.pewresearch.org/science/2020/09/17/u-s-public-now-divided-over-whether-to-get-covid-19-vaccine/; 2020 [accessed 16 October 2020].
- 5.Hamel, L. Kearny, A. Kirzinger, A. Lopes, L. Munana, C. & Brodie, M. KFF health tracking poll- September 2020: top issues in 2020 election, The role of misinformation, and views on a potential coronavirus vaccine. Kaiser Family Foundation. https://www.kff.org/coronavirus-covid-19/report/kff-health-tracking-poll-september-2020/; 2020 [accessed 15 October 2020].
- 6.Silverman, E. Poll: Most Americans believe the COVID-19 vaccine approval process is driven by politics, not science. STAT news. https://www.statnews.com/pharmalot/2020/08/31/most-americans-believe-the-covid-19-vaccine-approval-process-is-driven-by-politics-not-science/; 2020 [accessed 15 October 2020].
- 7.Facher, L. Harris and Pence square off on whether- and when- to trust a COVID-19 vaccine. STAT news. https://www.statnews.com/2020/10/07/harris-pence-vaccine-trump-covid-19/; 2020 [accessed 15 October 2020].
- 8.Kreps S.E., Kriner D.L. Model uncertainty, political contestation, and public trust in science: Evidence from the COVID-19 pandemic. Sci Adv. 2020;eabd4563 doi: 10.1126/sciadv.abd4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green J., Edgerton J., Naftel D., Shoub K., Cranmer S.J. Elusive consensus: Polarization in elite communication on the COVID-19 pandemic. Sci Adv. 2020;6(eabc2717) doi: 10.1126/sciadv.abc2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hart P.S., Chinn S., Soroka S. Politicization and polarization in COVID-19 news coverage. Sci Commun. 2020;1075547020950735 doi: 10.1177/1075547020950735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larson H.J., Schulz W.S., Tucker J.D., Smith D.M. Measuring vaccine confidence: introducing a global vaccine confidence index. PLoS currents. 2015;7 doi: 10.1371/currents.outbreaks.ce0f6177bc97332602a8e3fe7d7f7cc4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreps S., Prasad S., Brownstein J.S., Hswen Y., Garibaldi B.T., Zhang B., et al. Factors associated with US adults’ likelihood of accepting COVID-19 vaccination. JAMA Network Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.25594. e2025594 e2025594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.